Introduction
Chronic rhinosinusitis (CRS) is a common rhinology
disease; the reported prevalence of Staphylococcus aureus
(S. aureus) in CRS patients is 61% (1). Bacterial biofilms are considered as a
common and important cause of persistent infections; biofilms
require up to 1,000× higher antibiotic doses for effective
treatment compared to planktonic cells, thus hindering eradication
(2,3). Long-term use of antibiotics and
emerging resistant bacteria pose a great threat to human health.
Thus, a new drug delivery system needs to be developed to overcome
the antibiotic resistance and to eliminate biofilm.
Healthy individuals present high concentrations of
nitric oxide (NO) in the sinuses (4), NO plays a role in antimicrobial and
antiviral effects, keeping the sinuses relatively sterile, while
enhancing the clearance function of mucosa cilia (5,6). CRS
patients have significantly lower levels of sinonasal NO (7). Some studies have demonstrated that high
NO concentrations suppress S. aureus biofilm growth
(8,9). Isosorbide mononitrate (ISMN), widely
used as a NO-donor in the trials, has been shown to be safe for
various applications (10,11). Different types of liposomes, which
can reduce drug toxicity, have also been certified for clinical use
(12). A new type of liposome,
immunoliposomes (antibody-conjugated liposomes), have attracted
increasing attention owing to their potential use as targeted drug
delivery systems (13). Currently,
immunoliposomes are extensively used for treating cancer cells.
Targeted delivery of drugs encapsulated in nanoparticles can
increase drug accumulation at the tumor site and slow down drug
elimination in blood circulation (14).
The S. aureus α-hemolysin (HLA) is an
important virulence factor, which can also promote bacterial
biofilm formation. The potential role of anti-HLA antibodies in
targeting molecules for the functionalization of anti-biofilm
drug-loaded nanovectors has not been studied to date. Thus, we
combined the anti-HLA antibodies with ISMN liposomes to treat
infectious diseases caused by S. aureus biofilms. The
anti-Staphylococcus aureus alpha-toxin (anti-S.
aureus α-toxin) monoclonal antibody can neutralize S.
aureus exotoxins, as well as target the nanoparticles to the
biofilm.
Materials and methods
Liposome preparation
ISMN liposomes were prepared using the film
dispersion method. Egg lecithin and cholesterol were mixed at a
weight ratio of 3:1 and then dissolved in 5 ml chloroform. The
chloroform was slowly removed under reduced pressure using a rotary
evaporator to deposit a thin film of dry lipid on the inner wall of
the flask. The dry lipid film was hydrated with 10 ml
phosphate-buffered saline (PBS) solution containing 45 mg/ml ISMN
for 30 min to obtain the liposomes. The resultant mixture was then
successively filtered through 0.45 µm membranes. The prepared
liposomes were stored at 4°C until used.
The study was approved by the Ethics Committee of
the First Affiliated Hospital of Zhengzhou University (Zhengzhou,
China).
Construction of the pET28a-Hla
recombinant plasmid and expression of the HLA protein
HLA genes were PCR amplified using S. aureus
(ATCC25923) genomic DNA as the template with the following
conditions: at 95.0°C for 5 min; 30 cycles at 95.0°C for 30 sec, at
58.0°C for 30 sec, and at 72.0°C for 1 min; and 72.0°C for 5 min.
The HLA PCR product was cloned into linearized pET28a using the
Fast-Fusion Cloning Kit (GeneCopoeia, Inc.), resulting in
recombinant plasmid pET28a-Hla, which was verified by PCR and
restriction enzyme analysis. pET28a-Hla was transformed into
Escherichia coli BL21 (DE3) sensory cells and HLA protein
expression was induced with 0.4 mM Isopropyl β-D-thiogalactoside at
20°C. The bacterial cells were resuspended in buffer (20 mM
Tris-HCl, 0.5 M NaCl, and 20 mM imidazole, pH 8.0) and the HLA
recombinant protein was obtained by Ni2+-affinity
chromatography.
Preparation of monoclonal
antibody
The purified HLA recombinant protein was used as an
antigen to immunize BALB/c mice. Freund's complete adjuvant
(Sigma-Aldrich; Merck KGaA) was used to emulsify the antigen. A
suspension of spleen cells from the immunized mice was fused with
myeloma SP2/0 cells to screen for hybridoma cells that could stably
secrete the antibody. The hybridoma cell was inoculated into the
abdomen of mice and the hydroperitoneum was collected and purified
using the octanoic acid-ammonium sulfate method to obtain the
monoclonal antibody against HLA. Western blot analysis was used to
determine its specificity.
Preparation of ISMN
immunoliposomes
Twenty-five microliters of 25% glutaraldehyde were
added to 1 ml ISMN liposomes and the mixture was maintained at 25°C
for 10 min. The excess glutaraldehyde was removed by saline
dialysis for 3 h. Subsequently, the anti-S. aureus α-toxin
monoclonal antibody was conjugated with liposomes that reacted with
glutaraldehyde (overnight at 4°C with shaking). Immunoliposomes
were stored at 4°C until used.
Encapsulation percentage
determination
ISMN immunoliposomes were centrifuged at 2,035 × g
for 20 min at 4°C using an ultrafiltration tube (EMD Millipore).
The ultrafiltrate was diluted with PBS and then analyzed by
ultraviolet spectrophotometry (210 nm) to determine the amount of
free ISMN(mfree drug), the mtotal drug was
450 mg. An ISMN standard curve was generated using ISMN solutions
of known concentrations. Encapsulation efficiency (En %) =
mtotal drug-mfree drug/mtotal
drug.
Coupling rate determination
Immunoliposomes were centrifuged at 11,300 × g for
30 min at 4°C and the supernatant (free protein) was carefully
removed to another tube. The immunoliposomes were resuspended in an
equal volume of PBS. The concentration of free protein was
determined using the BCA kit (Solarbio). Coupling rate =
1-mfree protein/mtotal protein.
Suspension preparation
Biofilm forming strain S. aureus ATCC25923
was used in this study. Bacterial cultures were established as
previously described (3). Briefly,
bacterial strains (frozen glycerol stock) were inoculated onto
nutrient agar (Oxoid) plates and incubated overnight at 37°C for
recovery. A bacterial suspension of 1 McFarland unit in 0.9% saline
prepared using single colonies from the plate was used for
subsequent experiments.
Effect of immunoliposomes on biofilm
formation
The immunoliposomes were resuspended in tryptose
phosphate broth (TPB) (Sigma-Aldrich; Merck KGaA) and ISMN was
dissolved in TPB at a concentration of 45 mg/ml. The bacterial
suspension was diluted, 15 µl of bacterial suspension was mixed
with 135 µl of the different drug dosage forms (immunoliposomes,
liposomes, and ISMN), transferred into 96-well microplates (Corning
Life Sciences Plastic), and incubated for 48 h at 37°C under 5%
CO2 and 90% humidity.
The 96-well microplate was rinsed twice in saline to
remove planktonic bacteria and then dried for 10 min at 25°C. Next,
200 µl of methyl alcohol (Hengxing, Tianjin, China) was added to
each well and incubated at 25°C for 15 min. Then, 200 µl/well of
the crystal violet was added and incubated statically at 25°C for
15 min, distilled water was applied to remove the extra dye
following decanting, and 250 µl/well of 95% ethyl alcohol was added
and incubated at 25°C for 1 h. Finally, the absorbance of the
samples was measured using a microplate reader at 560 nm.
Effect of immunoliposomes on formed
biofilms
For biofilm growth, the S. aureus suspension
was diluted 1:10 in TPB and 150 µl of the dilution was pipetted
into the wells of 96-well clear-bottom microplates (Corning Life
Sciences Plastic, NY, USA) and incubated at 37°C under 5%
CO2 and 90% humidity for 48 h (3,15).
The prepared biofilm-coated 96-well microplate was
rinsed twice with saline to remove planktonic bacteria and 200 µl
of ISMN immunoliposomes, ISMN liposomes and ISMN, respectively,
were added to the wells for 24 h at 37°C, followed by another two
washes. The alamarBlue assay (Invitrogen; Thermo Fisher Scientific,
Inc.) was performed to test the viability of the challenged biofilm
according to the manufacturer's instructions; 250 µl/well of the
alamarBlue (1:10 dilution in TPB) was added and incubated
statically at 37°C for 1 h. Finally, the fluorescence intensity of
the samples was measured with a FLUOstar OPTIMA plate reader
(excitation, 530 nm; emission, 590 nm; BMG Labtech). PBS was used
as the negative control and wells that did not contain biofilms
were stained as the background. All treatments were carried out in
triplicate and the experiments were repeated twice.
To observe the biofilms following different
intervention formulations, 300 µl of the bacterial suspension was
added to CLSM, 8-well culture slides (BD Biosciences) and incubated
at 37°C for 24 h to form the S. aureus biofilm. Next, the
medium was carefully replaced with fresh TPB and the slide was
incubated at 37°C under 5% CO2 and at 90% humidity for
another 48 h to allow further biofilm formation. Once the biofilm
formed, different drug dosages and concentrations were added to the
wells.
The slide was rinsed twice with 0.9% NaCl to remove
planktonic bacteria (15,16) and 300 µl/well of immunoliposomes,
liposomes, and ISMN at different concentrations was added for 24 h.
PBS was applied as the non-treatment control. Following
intervention, the samples were fixed with 300 µl/well of 5%
glutaraldehyde (Zhiyuan) for 30 min prior to staining. Next, 300
µl/well of the SYTO9+PI (Invitrogen; Thermo Fisher Scientific,
Inc.) mixture in saline was added to each well and incubated in the
dark for 15 min at room temperature. All steps were followed by two
saline washes. After removing the upper chamber of the culture
slide, the samples were sealed with glycerol (Dingguo) and examined
using a Nikon (Nikon Microsystems) confocal laser scanning
microscopy with 60× objective and 0.25 µm for laser scanning step
size; PI, excitation 561 nm and emission 610 nm; SYTO9, excitation
476 nm and emission 510 nm. For each sample, the full thickness of
the biofilm was scanned along the z-stacks and the experiments were
repeated three times. Image processing and analysis were performed
using Nikon NIS-Elements (Nikon) and ImageJ 1.43 (Wayne Rasband,
National Institutes of Health).
Statistical analysis
Parametric data are expressed as mean ± standard
deviation. Depending on the type of data, significant differences
between multiple groups were confirmed by the Kruskal-Wallis test
or one-way analysis of variance (ANOVA); pairwise comparisons were
conducted using the Bonferroni method. Difference were considered
statistically significant at P<0.05.
Results
Amplification of target gene
The HLA gene was PCR amplified using S.
aureus genomic DNA. The obtained amplified fragment was of the
expected size, 960 bp.
Preparation of HLA monoclonal
antibody
The pET28a-Hla recombinant plasmid was successfully
transfected into cells and the resulting purified HLA recombinant
protein was used as an antigen to immunize BALB/c mice. Hybridoma
cells secreting anti-HLA monoclonal antibodies were obtained and
the antibodies were successfully prepared.
Immunoliposome characterization
ISMN immunoliposomes formed a milky white suspension
on gross examination. ISMN liposomes remained stable without
changing their appearance, hydrodynamic diameter and encapsulation
percentage (P<0.05) for one month at 4°C.
The physicochemical properties of the samples,
namely hydrodynamic diameter and ζ-potential, were obtained using
dynamic light scattering. The average hydrodynamic diameter of the
ISMN immunoliposomes was 175.7 nm (PDI=0.235) and the ζ potential
was −1.18 mV. We found that the produced liposomes were negatively
charged particles, regardless of whether they contained the
drug.
The ISMN immunoliposome encapsulation percentage was
17.6%, much higher than previously published (3). The coupling rate of the anti-S.
aureus α-toxin monoclonal antibody to ISMN immunoliposomes was
32.86%.
Effect of immunoliposomes on biofilm
formation
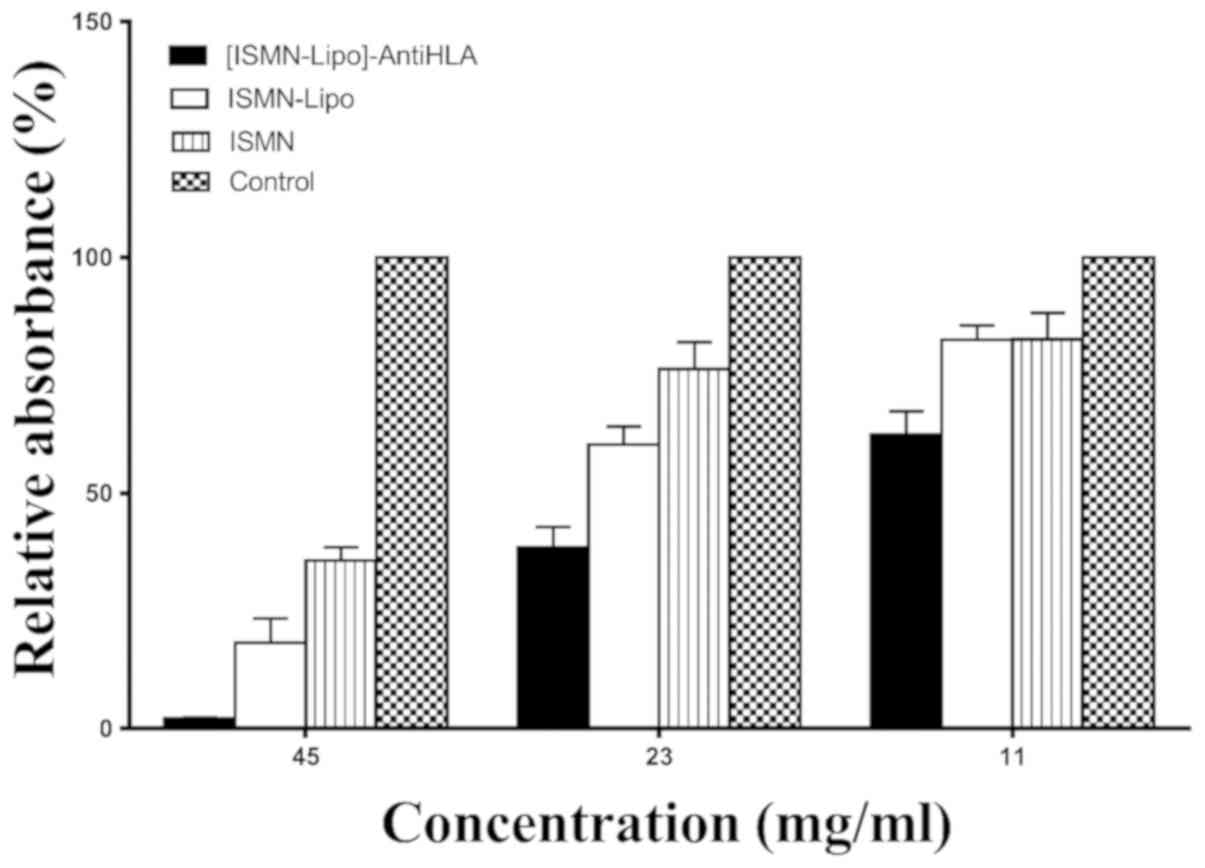
The effects of different drug dosage forms
(immunoliposomes, liposomes, and free ISMN) with ISMN
concentrations of 45, 23 and 11 mg/ml were examined. ISMN
immunoliposomes intervention reduced biofilm formation to
2.16±0.22, 38.42±4.44 and 62.38±5.01%, respectively; ISMN liposomes
intervention reduced biofilm formation to 18.26±5.21, 58.31±3.15
and 82.50±3.10%, respectively; and ISMN intervention reduced
biofilm formation to 35.7±2.87, 76.37±5.65 and 82.61±5.68%,
respectively. At these drug concentrations, the inhibitory effect
of ISMN immunoliposomes on biofilm formation was greater than that
of ISMN liposomes and ISMN (P<0.05). At drug concentrations of
45 and 23 mg/ml, the inhibitory effect of ISMN liposomes on biofilm
formation was stronger than ISMN (P<0.05), while at 11 mg/ml,
the inhibitory effect of ISMN liposomes on biofilm formation was
the same as ISMN (P>0.05; Table I
and Fig. 1).
 | Table I.Relative absorbance following
different interventions in S. aureus biofilm formation. |
Table I.
Relative absorbance following
different interventions in S. aureus biofilm formation.
| Groups | 45 mg/ml | 23 mg/ml | 11 mg/ml |
|---|
| Control | 100% | 100% | 100% |
| ISMN | 35.70±2.87% | 76.37±5.65% | 82.61±5.68% |
| ISMN-Lipo | 18.26±5.21% | 60.31±3.83% | 82.50±3.10% |
|
[ISMN-Lipo]-AntiHLA | 2.16±0.22% | 38.42±4.44% | 62.38±5.01% |
The effect of immunoliposomes on
formed biofilms
The inhibition ratio was calculated following
normalization to the mean fluorescent intensity of the control
wells (biofilms without any intervention) to compare the
differences between the different interventions.
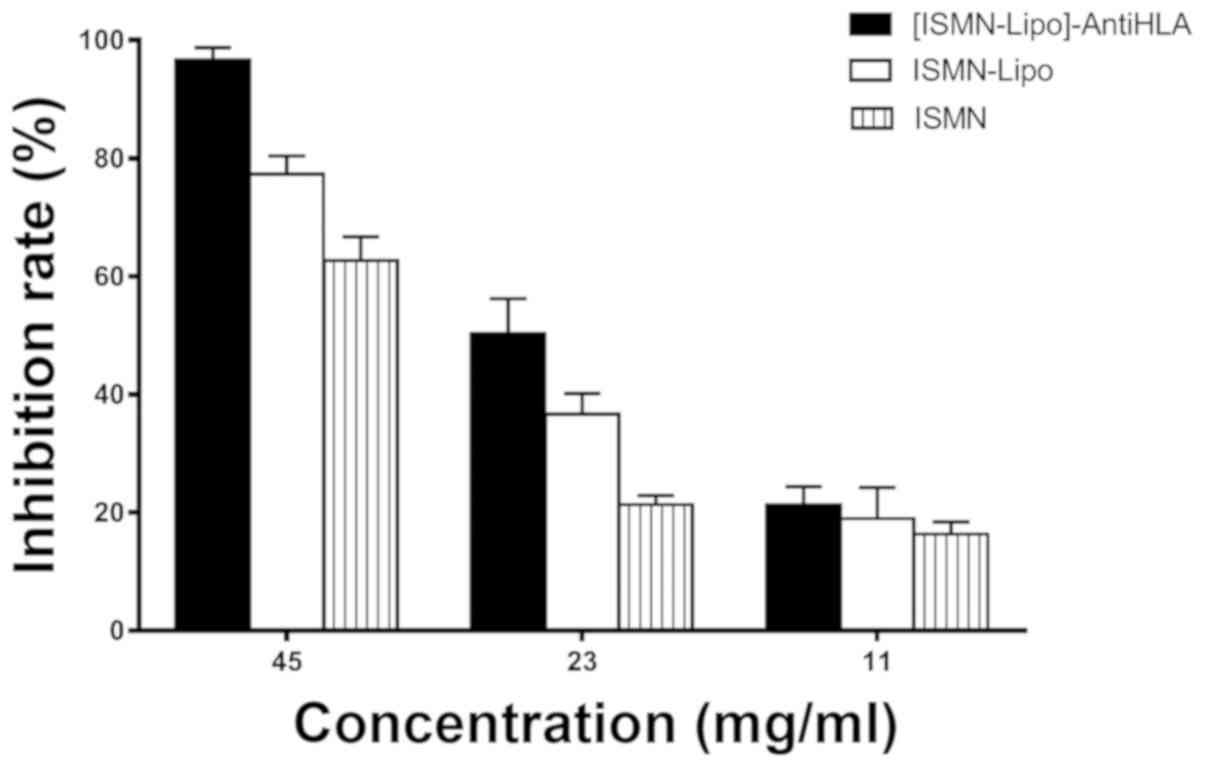
The inhibition rate with ISMN concentrations of 45,
23 and 11 mg/ml was 96.67±2.08, 50.33±5.86 and 21.33±3.06%,
respectively, for ISMN immunoliposomes; 77.33±3.06, 36.67±3.51 and
19.00±5.29%, respectively, for ISMN liposomes; and 62.67±4.04,
21.33±1.53 and 16.33±2.08%, respectively, for ISMN. At drug
concentrations of 45 and 23 mg/ml, the inhibitory effect of ISMN
immunoliposomes on formed biofilms was stronger than ISMN liposomes
and ISMN (P<0.05) and the inhibitory effect of ISMN liposomes
was stronger than ISMN (P<0.05). At 11 mg/ml, ISMN
immunoliposomes, ISMN liposomes, and ISMN had the same effect on
formed biofilms (P>0.05; Table
II and Fig. 2).
 | Table II.Inhibition rate after following
different interventions in S. aureus biofilms. |
Table II.
Inhibition rate after following
different interventions in S. aureus biofilms.
| Groups | 45 mg/ml | 23 mg/ml | 11 mg/ml |
|---|
| ISMN | 62.67±4.04% | 21.33±1.53% | 16.33±2.08% |
| ISMN-Lipo | 77.33±3.06% | 36.67±3.51% | 19.00±5.29% |
|
[ISMN-Lipo]-AntiHLA | 96.67±2.08% | 50.33±5.86% | 21.33±3.06% |
Confocal laser scanning microscopy
(CLSM) analysis
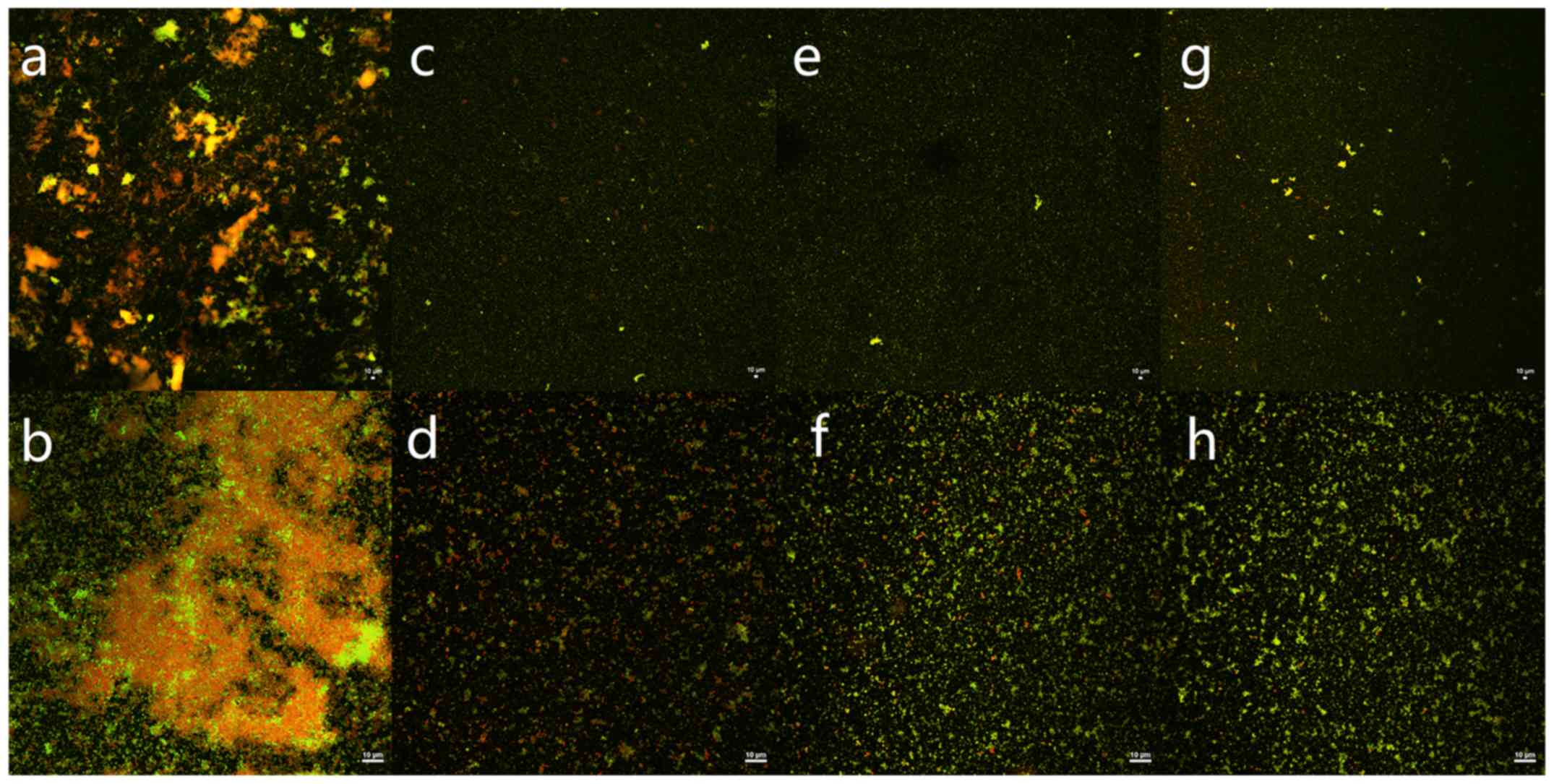
The killing effect of immunoliposomes, liposomes,
and free ISMN on biofilms was verified using CLSM with fluorescence
quantitative analysis. In this experiment, SYTO9 and PI interacted
with the nucleic acids of live and dead bacteria, which were
visualized as green and red, respectively. The S. aureus
biofilm in the control group appeared as a large cloud, in which
the dead and live bacteria and polysaccharide matrix were clustered
together. At a drug concentration of 45 mg/ml, the immunoliposomes
almost completely eradicated the biofilm; only a handful of
scattered bacteria remained (Fig.
3). At concentrations of 45 and 23 mg/ml, the green
fluorescence intensity of all intervention groups was lower than
that of the control group and the difference was statistically
significant between any two groups (P<0.05). At 11 mg/ml, the
green fluorescence intensity of all intervention groups was lower
than that of the control group and the difference was statistically
significant (P<0.05); however, there was no statistically
significant difference between any two dosage forms (P>0.05;
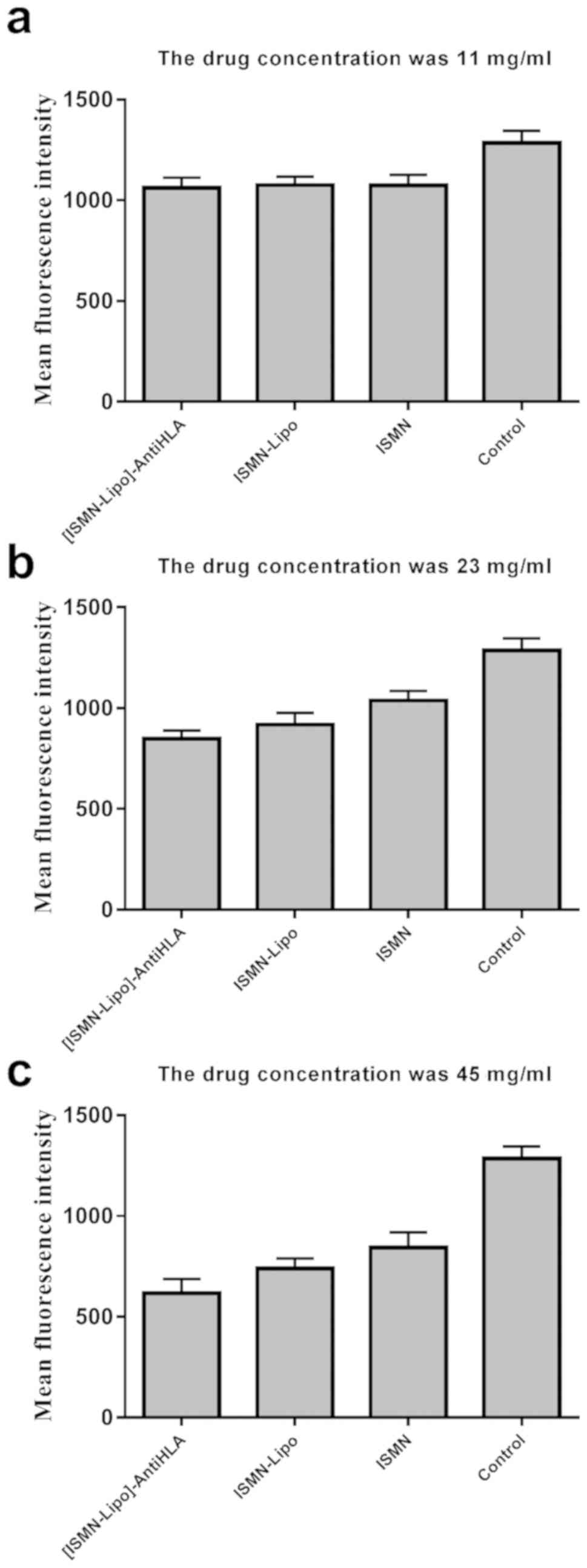
Table III and Fig. 4). Moreover, quantitative analysis of
green fluorescence intensity obtained from CLSM was consistent with
the alamarBlue assay results.
 | Table III.Mean green fluorescence intensity
following different interventions in S. aureus biofilms. |
Table III.
Mean green fluorescence intensity
following different interventions in S. aureus biofilms.
| Groups | 45 mg/ml | 23 mg/ml | 11 mg/ml |
|---|
| Control | 1,285.00±60.91 | 1,285.00±60.91 | 1,285.00±60.91 |
| ISMN | 843.90±76.01 | 1,038.00±47.54 | 1,077.00±52.41 |
| ISMN-Lipo | 739.60±49.18 | 918.6±57.97 | 1,075.00±41.33 |
|
[ISMN-Lipo]-AntiHLA | 616.30±70.14 | 848.20±41.07 | 1,062.00±49.66 |
Discussion
In this study, immunoliposomes were successfully
produced and the average hydrodynamic diameter of the ISMN
immunoliposomes was 175.7 nm (PdI=0.235). The encapsulation
percentage of ISMN immunoliposomes was 17.6%, which was much higher
than previously reported; the diameter was also smaller than
previously reported (3). Previous
studies have shown that the smaller the particle size of liposomes,
the better the liposomes are able to permeate biofilms (15). Encapsulation efficiency plays a vital
role in drug delivery to the biofilm. We used an orthogonal test to
determine the optimum dosage of egg lecithin and cholesterol; the
liposomes we obtained had a smaller diameter and higher
encapsulation efficiency.
The preliminary study confirms that 45 mg/ml ISMN
can be used to eradicate bacterial biofilms. Furthermore, we
investigated the effect of different concentrations of
immunoliposomes, liposomes, and free ISMN on formed biofilms and
biofilm formation. Our results show that immunoliposomes with 45
mg/ml ISMN had the greatest anti-biofilm effects (including formed
biofilms and biofilm formation) compared with the same
concentration of free ISMN and ISMN liposomes. Further mechanism of
how the immunoliposomes affect the delivery of the NO donor to the
biofilm is needed.
At a concentration of 11 mg/ml, immunoliposomes had
a significantly stronger anti-biofilm effect (biofilm formation)
than the corresponding concentration of liposomes and free drug and
the difference was statistically significant (P<0.05). The
biofilm formation process is divided into several stages, namely
adhesion, colony formation, maturation, and aging. The effect of
ISMN immunoliposomes on biofilms began at the adhesion stage or
during colony formation. Thus, 11 mg/ml ISMN immunoliposomes may
intervene more effectively with biofilm formation than the same
concentration of ISMN liposomes and free ISMN; the higher local
drug concentration could effectively inhibit and interfere with
biofilm formation.
In addition, 11 mg/ml ISMN immunoliposomes had a
stronger anti-biofilm effect (formed biofilm) than the
corresponding concentration of liposomes and free drug; however,
the difference was not statistically significant (P>0.05). The
reason for this may be that the biofilm was aging or mature;
although the immunoliposomes targeted the drug to the biofilm, the
drug concentration might have been too low to effectively eliminate
a mature stage biofilm. Thus, once the biofilm was formed, it was
difficult to eradicate, especially at low drug concentrations.
Therefore, the advantages of immunoliposomes were less obvious
compared with ISMN liposomes and free ISMN. In contrast, at early
stages of biofilm formation low drug concentrations were able to
effect biofilm formation during the 48 h interaction period.
There are several reasons explaining the increased
antibiotic resistance of biofilms compared with free bacteria: i)
The extracellular matrix secreted by bacteria in the biofilm can
form a physiological and metabolic barrier (17), which can prevent or greatly reduce
the entry of antibiotics into cells (18,19). ii)
The microenvironment in the biofilm is complex and there may be
some regional differences in oxygen concentration, osmotic
pressure, and pH, resulting in different susceptibility to
antibiotics and other drugs. iii) The specific growth patterns of
the biofilm can enable bacteria to escape the host immune system
(20,21). iv) Nutritional limitation: the
accumulation of metabolites in biofilms and the consumption of
oxygen and nutrients causes bacteria in the biofilm to starve and
enter a non-vegetative state, resulting in fewer sensitive cells in
the biofilm (22–24). v) Bacteria in the biofilm may exhibit
specific biological phenotypes, which are controlled by specific
genes.
Immunoliposomes have been studied extensively in
tumor treatment. Immunoliposomes have significant advantages in
clinical use (25). Firstly, in
terms of ensuring drug effects, immunoliposomes not only reduce
drug dosage, but could also reduce or prevent the adverse effects
of drugs in the patient. Secondly, immunoliposomes could be used to
establish new medical approaches and improve the efficacy of
currently used hydrophilic (low membrane trespassing capacity)
macromolecules (26). Thirdly,
immunoliposomes can target tumors and easily control drug dosage.
Bacterial biofilm possesses a certain spatial structure and the
biofilm matrix may protect bacteria against drug permeability;
hence, it is difficult for common drugs to permeate and eradicate
the biofilm. Thus, we designed ISMN immunoliposomes comprised of
ISMN encapsulated within liposomes coupled to an anti-S.
aureus α-toxin monoclonal antibody. Encapsulating ISMN with
liposomes allowed the drug to permeate the biofilm more easily via
lipid fusion (27). Furthermore,
coupling with the anti-S. aureus α-toxin monoclonal antibody
ensured that ISMN could selectively act on the biofilm, which would
not only improve the efficacy of ISMN against biofilms, but could
also reduce drug dosage and decrease adverse drug reactions. The
hydrophobic properties of the bacterial cell wall have also been
shown to impact liposomes penetration and enhance drug mobility
through the biofilm matrix (28).
Compared with topical antimicrobials, which have difficulty
penetrating the biofilm matrix, immunoliposomes could easily
combine with and enter into bacterial biofilm; the retention of
immunoliposomes in the biofilm could facilitate drug release in
close proximity of the bacteria over extended periods of time
(22), which is extremely important
for effective biofilm eradication. This study focused on the S.
aureus biofilm in vitro, further studies are required to
confirm this and to investigate its safety and efficacy in the
animal model of CRS.
Immunoliposomes, which provide a potential new form
of dosage that is effective and safe, will hopefully be conducive
to decreasing antibiotic use and consequently reducing the risk of
developing drug resistance. The optimum concentration of
immunoliposomes selected here, which demonstrated the best
anti-biofilm effects, could provide a starting drug concentration
for future animal experiments.
In conclusion, the findings of this study indicate
that ISMN immunoliposomes were the most effective formulation for
eradicating bacterial biofilms. Furthermore, we highlight the
advantages of immunoliposomes as a novel drug delivery system for
biofilm eradication. Future in vivo studies are required to
determine their safety and efficacy prior to topical clinical
application.
Acknowledgements
Not applicable.
Funding
The National Natural Science Foundation of China
(Grant Number: 81570901). International cooperation project of
science and technology department of Henan province (172102410004);
Medical science project of Henan province (SBGJ2018034).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YaZ, YuZ, DD and XL led the conception and design of
this study. YaZ, YuZ, DD, ZL, SL and JW were responsible for the
data collection and analysis. YaZ, DD and XL were in charge of
interpreting the data and drafting the manuscript. YuZ and SL made
revision from critical perspective for important intellectual
content. The final version was read and adopted by all the
authors.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the First Affiliated Hospital of Zhengzhou University (Zhengzhou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boase S, Foreman A, Cleland E, Tan L,
Melton-Kreft R, Pant H, Hu FZ, Ehrlich GD and Wormald PJ: The
microbiome of chronic rhinosinusitis: Culture, molecular
diagnostics and biofilm detection. BMC Infect Dis. 13:2102013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ha KR, Psaltis AJ, Butcher AR, Wormald PJ
and Tan LW: In vitro activity of mupirocin on clinical isolates of
Staphylococcus aureus and its potential implications in
chronic rhinosinusitis. Laryngoscope. 118:535–540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jardeleza C, Rao S, Thierry B, Gajjar P,
Vreugde S, Prestidge CA and Wormald PJ: Liposome-encapsulated ISMN:
A novel nitric oxide-based therapeutic agent against
Staphylococcus aureus biofilms. PLoS One. 9:e921172014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abuzeid WM, Girish VM, Fastenberg JH,
Draganski AR, Lee AY, Nosanchuk JD and Friedman JM: Nitric
oxide-releasing microparticles as a potent antimicrobial
therapeutic against chronic rhinosinusitis bacterial isolates. Int
Forum Allergy Rhinol. 8:1190–1198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barraud N, Hassett DJ, Hwang SH, Rice SA,
Kjelleberg S and Webb JS: Involvement of nitric oxide in biofilm
dispersal of Pseudomonas aeruginosa. J Bacteriol.
188:7344–7353. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Workman AD, Carey RM, Kohanski MA, Kennedy
DW, Palmer JN, Adappa ND and Cohen NA: Relative susceptibility of
airway organisms to antimicrobial effects of nitric oxide. Int
Forum Allergy Rhinol. 7:770–776. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hariri BM, McMahon DB, Chen B, Freund JR,
Mansfield CJ, Doghramji LJ, Adappa ND, Palmer JN, Kennedy DW, Reed
DR, et al: Flavones modulate respiratory epithelial innate
immunity: Anti-inflammatory effects and activation of the T2R14
receptor. J Biol Chem. 292:8484–8497. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kishikawa H, Ebberyd A, Römling U, Brauner
A, Lüthje P, Lundberg JO and Weitzberg E: Control of pathogen
growth and biofilm formation using a urinary catheter that releases
antimicrobial nitrogen oxides. Free Radic Biol Med. 65:1257–1264.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Slomberg DL, Lu Y, Broadnax AD, Hunter RA,
Carpenter AW and Schoenfisch MH: Role of size and shape on biofilm
eradication for nitric oxide-releasing silica nanoparticles. ACS
Appl Mater Interfaces. 5:9322–9329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oelze M, Knorr M, Kröller-Schön S,
Kossmann S, Gottschlich A, Rümmler R, Schuff A, Daub S, Doppler C,
Kleinert H, et al: Chronic therapy with isosorbide-5-mononitrate
causes endothelial dysfunction, oxidative stress, and a marked
increase in vascular endothelin-1 expression. Eur Heart J.
34:3206–3216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Müller S, König I, Meyer W and Kojda G:
Inhibition of vascular oxidative stress in hypercholesterolemia by
eccentric isosorbide mononitrate. J Am Coll Cardiol. 44:624–631.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yaari Z, da Silva D, Zinger A, Goldman E,
Kajal A, Tshuva R, Barak E, Dahan N, Hershkovitz D, Goldfeder M, et
al: Theranostic barcoded nanoparticles for personalized cancer
medicine. Nat Commun. 7:133252016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anaya-Ruiz M, Bandala C, Landeta G,
Martínez-Morales P, Zumaquero-Rios JL, Sarracent-Pérez J and
Pérez-Santos M: Nanostructured systems in advanced drug targeting
for the cancer treatment: Recent patents. Recent Patents Anticancer
Drug Discov. 14:85–94. 2019. View Article : Google Scholar
|
|
14
|
Zheng Y, Tang L, Mabardi L, Kumari S and
Irvine DJ: Enhancing adoptive cell therapy of cancer through
targeted delivery of small-molecule immunomodulators to
internalizing or noninternalizing receptors. ACS Nano.
11:3089–3100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong D, Thomas N, Thierry B, Vreugde S,
Prestidge CA and Wormald PJ: Distribution and inhibition of
liposomes on Staphylococcus aureus and Pseudomonas
aeruginosa biofilm. PLoS One. 10:e01318062015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahmed K, Gribbon PN and Jones MN: The
application of confocal microscopy to the study of liposome
adsorption onto bacterial biofilms. J Liposome Res. 12:285–300.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tan Y, Ma S, Leonhard M, Moser D,
Haselmann GM, Wang J, Eder D and Schneider-Stickler B: Enhancing
antibiofilm activity with functional chitosan nanoparticles
targeting biofilm cells and biofilm matrix. Carbohydr Polym.
200:35–42. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Akanda ZZ, Taha M and Abdelbary H: Current
review - The rise of bacteriophage as a unique therapeutic platform
in treating peri-prosthetic joint infections. J Orthop Res.
36:1051–1060. 2018.PubMed/NCBI
|
|
19
|
Ivanova K, Fernandes MM, Francesko A,
Mendoza E, Guezguez J, Burnet M and Tzanov T: Quorum-quenching and
matrix-degrading enzymes in multilayer coatings synergistically
prevent bacterial biofilm formation on urinary catheters. ACS Appl
Mater Interfaces. 7:27066–27077. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pires DP, Melo L, Vilas Boas D,
Sillankorva S and Azeredo J: Phage therapy as an alternative or
complementary strategy to prevent and control biofilm-related
infections. Curr Opin Microbiol. 39:48–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Figueiredo AMS, Ferreira FA, Beltrame CO
and Côrtes MF: The role of biofilms in persistent infections and
factors involved in ica-independent biofilm development and gene
regulation in Staphylococcus aureus. Crit Rev Microbiol.
43:602–620. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Forier K, Raemdonck K, De Smedt SC,
Demeester J, Coenye T and Braeckmans K: Lipid and polymer
nanoparticles for drug delivery to bacterial biofilms. J Control
Release. 190:607–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martins M, McCusker MP, McCabe EM, O'Leary
D, Duffy G and Fanning S: Evidence of metabolic switching and
implications for food safety from the phenome(s) of Salmonella
enterica serovar Typhimurium DT104 cultured at selected points
across the pork production food chain. Appl Environ Microbiol.
79:5437–5449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fauvart M, De Groote VN and Michiels J:
Role of persister cells in chronic infections: Clinical relevance
and perspectives on anti-persister therapies. J Med Microbiol.
60:699–709. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang G and Yin B: Therapeutic effects of
long-circulating miR-135a-containing cationic immunoliposomes
against gallbladder carcinoma. Sci Rep. 7:59822017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marques J, Valle-Delgado JJ, Urbán P, Baró
E, Prohens R, Mayor A, Cisteró P, Delves M, Sinden RE, Grandfils C,
et al: Adaptation of targeted nanocarriers to changing requirements
in antimalarial drug delivery. Nanomedicine (Lond). 13:515–525.
2017. View Article : Google Scholar
|
|
27
|
Moles E, Moll K, Ch'ng JH, Parini P,
Wahlgren M and Fernàndez-Busquets X: Development of drug-loaded
immunoliposomes for the selective targeting and elimination of
rosetting Plasmodium falciparum-infected red blood cells. J Control
Release. 241:57–67. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ertem E, Gutt B, Zuber F, Allegri S, Le
Ouay B, Mefti S, Formentin K, Stellacci F and Ren Q: Core-shell
silver nanoparticles in endodontic disinfection solutions enable
long-term antimicrobial effect on oral biofilms. ACS Appl Mater
Interfaces. 9:34762–34772. 2017. View Article : Google Scholar : PubMed/NCBI
|