AMP-activated protein kinase (AMPK) is a master
regulator of energy metabolism and forms a heterotrimer consisting
of a catalytic α-subunit and two regulatory subunits (β and γ).
AMPK is activated via the phosphorylation at Thr172 of the
α-subunit by liver kinase B1 (LKB1) through the interaction of
adenosine monophosphate (AMP) with the AMPK γ-subunit under
nutrient starvation (1–3). The other mechanism of AMPK activation
is the calcium-dependent phosphorylation at Thr172 of the α subunit
by calcium/calmodulin-dependent protein kinase kinase β (CaMKKβ)
(2,3). AMPK contributes to several cellular
events, such as protein synthesis, lipid metabolism, glucose
metabolism, anti-inflammation, redox regulation and anti-aging
(1–3). In addition, AICAR, an AMPK activator,
has been shown to suppress several diseases, such as acute and
relapsing colitis (4), autoimmune
encephalomyelitis (5) and acute lung
injury (6). Therefore, the
activation of AMPK may ameliorate inflammatory diseases.
Chronic inflammation has been reported to be a key
event in the development and progression of several diseases
(7–10) and is triggered by multiple immune
cells, including macrophages, T lymphocytes and mast cells
(11). Damage-associated molecular
patterns (DAMPs), such as proteins, peptides, fatty acids and
lipoproteins derived from dead cells are recognized by
pattern-recognition receptors (PRRs) expressed on the immune cell
surface and induce the secretion of inflammatory molecules,
including tumor necrosis factor-α (TNF-α), C-C motif chemokine
2/monocyte chemoattractant protein-1 (CCL2/MCP-1) and C-reactive
protein (CRP) (12–14). These molecules damage various tissues
and induce chronic inflammatory diseases, such as atherosclerosis
and type 2 diabetes.
In the present review, we summarize the
AMPK-activating effects of AGE and its components, and discuss the
association between AMPK activation and the ameliorating effects of
AGE on several inflammatory diseases.
AGE has been shown to trigger the activation of AMPK
in the liver, adipose tissues and gastrocnemius muscles in a model
of type 2 diabetes (34). In
addition, AGE has been shown to induce the phosphorylation of AMPK
in the liver in a mouse model of atherosclerosis (23). Accordingly, researchers have
demonstrated that AGE enhances AMPK activity in various tissues and
animal models. However, the mechanisms underlying AGE-induced AMPK
activation are not yet fully understood. Several natural compounds
have been shown to activate AMPK. Resveratrol from red grapes,
curcumin from the turmeric, and berberine from Coptis
chinensis trigger the phosphorylation of Thr172 of the AMPK
α-subunit by increasing the AMP/ATP ratio (38,39).
These compounds decrease ATP production through various mechanisms.
Resveratrol and curcumin inhibit the production of ATP by
suppressing mitochondrial F1F0-ATPase/ATP synthase, whereas
berberine decreases ATP production by blocking respiratory chain
complex I (38,39). SAMC has been shown to increase the
phosphorylation of LKB1 in the liver (37), whereas SAC promotes the activation of
CaMKKβ in human hepatocyte HepG2 cells (35). Therefore, both SAMC and SAC are major
constituents of AGE (15,16), and they are involved in the induction
of the phosphorylation of AMPK by increasing the AMP/ATP ratio
and/or intracellular Ca2+ concentration. Consequently,
AGE containing these components may induce the phosphorylation of
AMPK through these pathways.
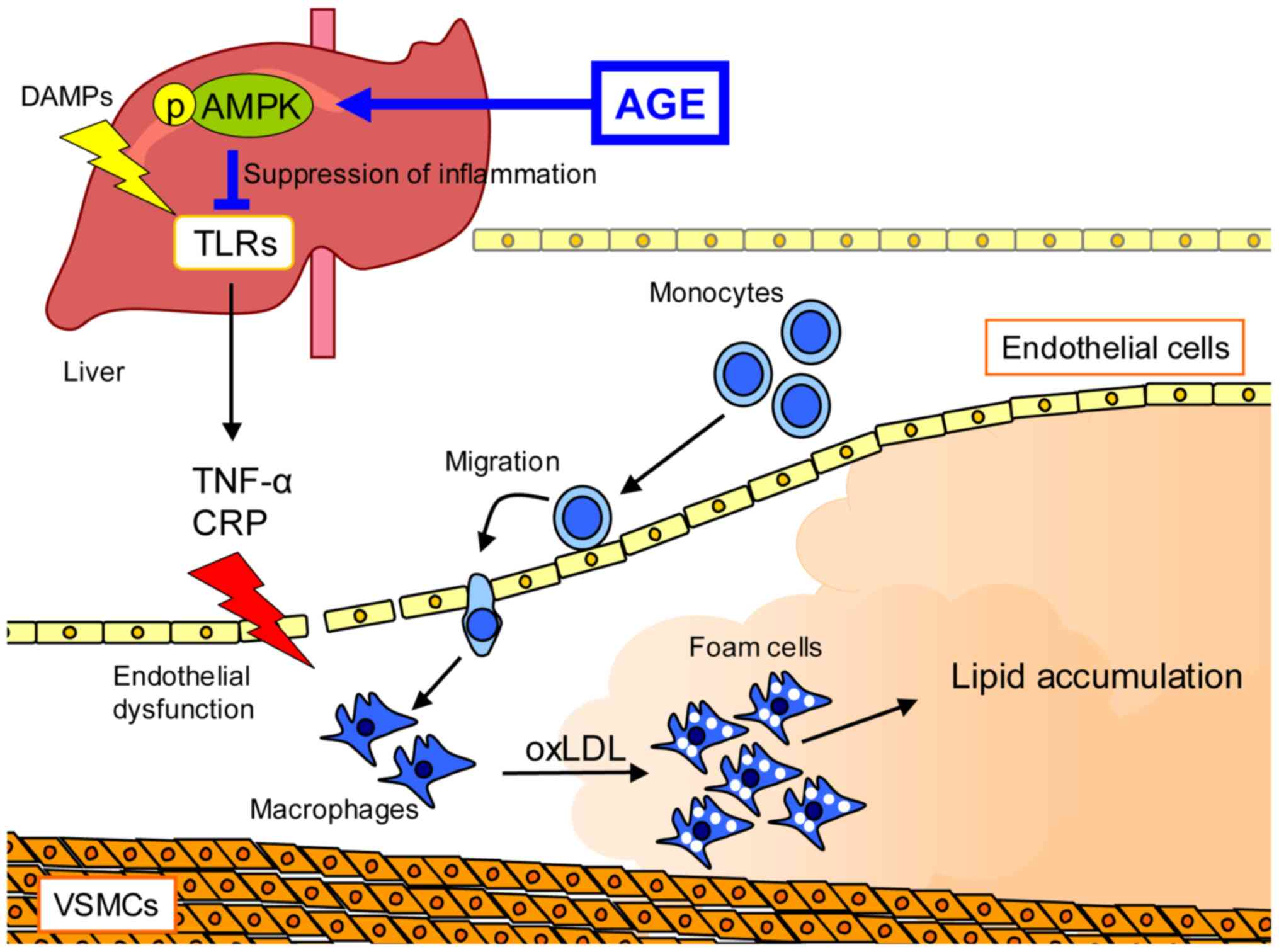
Atherosclerosis is a vascular inflammatory disease,
which causes macrophage infiltration, atherosclerotic lesion
formation and defective efferocytosis (40). AMPK regulates cell apoptosis,
inflammation, cholesterol efflux and efferocytosis via autophagy;
however, its function is impaired in atherosclerosis (41–44). AGE
has been shown to enhance the phosphorylation of AMPK in the livers
of apolipoprotein E knockout (ApoE-KO) mice, a mouse model of
atherosclerosis (23). It has
previously been suggested that the activation of AMPK improves
several processes in atherosclerosis. Li et al reported that
the administration of the AMPK activator, S17834, reduced
lipogenesis and the atherosclerotic plaque region by inhibiting
sterol regulatory element-binding protein 1c (SREBP-1c) cleavage
and nuclear translocation in low-density lipoprotein receptor
(LDLR)-knockout mice (45). In
addition, xanthohumol derived from the hop plant lowers plasma
lipids and inflammatory chemokine production by activating AMPK in
the liver (46). Furthermore,
berberine has been shown to suppress plaque formation by inhibiting
oxidative stress and vascular inflammation through the activation
of the AMPK-nuclear respiratory factor 1 signaling pathway
(47). These findings suggest that
AMPK inhibits plaque formation by modulating lipid metabolism, as
well as by exerting antioxidant and anti-inflammatory effects.
AGE has been shown to suppress lipid deposition in
the aorta and to prevent the elevation of serum CRP levels in
ApoE-KO mice (22,23). CRP secreted from hepatocytes is a
marker of chronic inflammation and systemic inflammation induced by
pro-inflammatory cytokines (48,49).
PRRs, including TLRs trigger the production of pro-inflammatory
cytokines by recognizing not only foreign pathogens, but also
cellular components, such as proteins, peptides, fatty acid and
lipoprotein, that are derived from dead cells. Accordingly, chronic
inflammation is caused by the continuous stimulation of these
components (12–14). AGE has been shown to inhibit the TLR
signaling pathway by reducing the phosphorylation of interleukin-1
receptor-associated kinase 4 (IRAK4), which is a key regulator of
TLR signaling, in ApoE-KO mice (23). The activation of AMPK also
contributes to the inhibition of the TLR signaling pathway via
several mechanisms (6,50,51).
S1PC induces the degradation of myeloid differentiation primary
response 88 (MyD88) by activating AMPK-mediated autophagy (19). SAC increases cholesterol efflux by
the induction of ATP-binding cassette protein A1 (ABCA1) expression
in THP-1 cells (52). In addition,
the pharmacological or genetic activation of AMPK increases ABCA1
expression through transcriptional activator liver X receptor α,
and promotes cholesterol efflux (53). Therefore, the activation of AMPK is
an important event for the amelioration of atherosclerosis and may
be a therapeutic target for this disease (Fig. 1).
Type 2 diabetes is characterized by hyperglycemia
and dyslipidemia associated with insulin resistance (54–56). In
addition, inflammation contributes to the disruption of insulin
signaling and the destruction of adipose homeostasis (57,58).
Furthermore, monocytes and macrophages infiltrate into adipose
tissues and produce inflammatory cytokines, such as TNF-α,
interleukin (IL)-1β and IL-18 (59–62).
AMPK is a key molecule for the prevention and/or amelioration of
type 2 diabetes and insulin resistance (39,63).
Metformin has been used for the therapy of diabetes through the
activation of AMPK by inhibiting mitochondrial complex I of the
respiratory chain (64,65). Treatment with metformin has been
shown to reduce blood glucose levels, inhibit hepatic
gluconeogenesis and improve insulin sensitivity (66,67). In
addition, resveratrol has been shown to increase glucose uptake and
mitochondrial biogenesis via the activation of AMPK (68–70).
Furthermore, berberine has been shown to improve glucose
intolerance, reduce body weight, increase the expression of the
insulin receptor and LDLR, lower total and LDL cholesterol levels,
and reduce triglyceride levels (71,72).
Therefore, the activation of AMPK helps ameliorate type 2
diabetes.
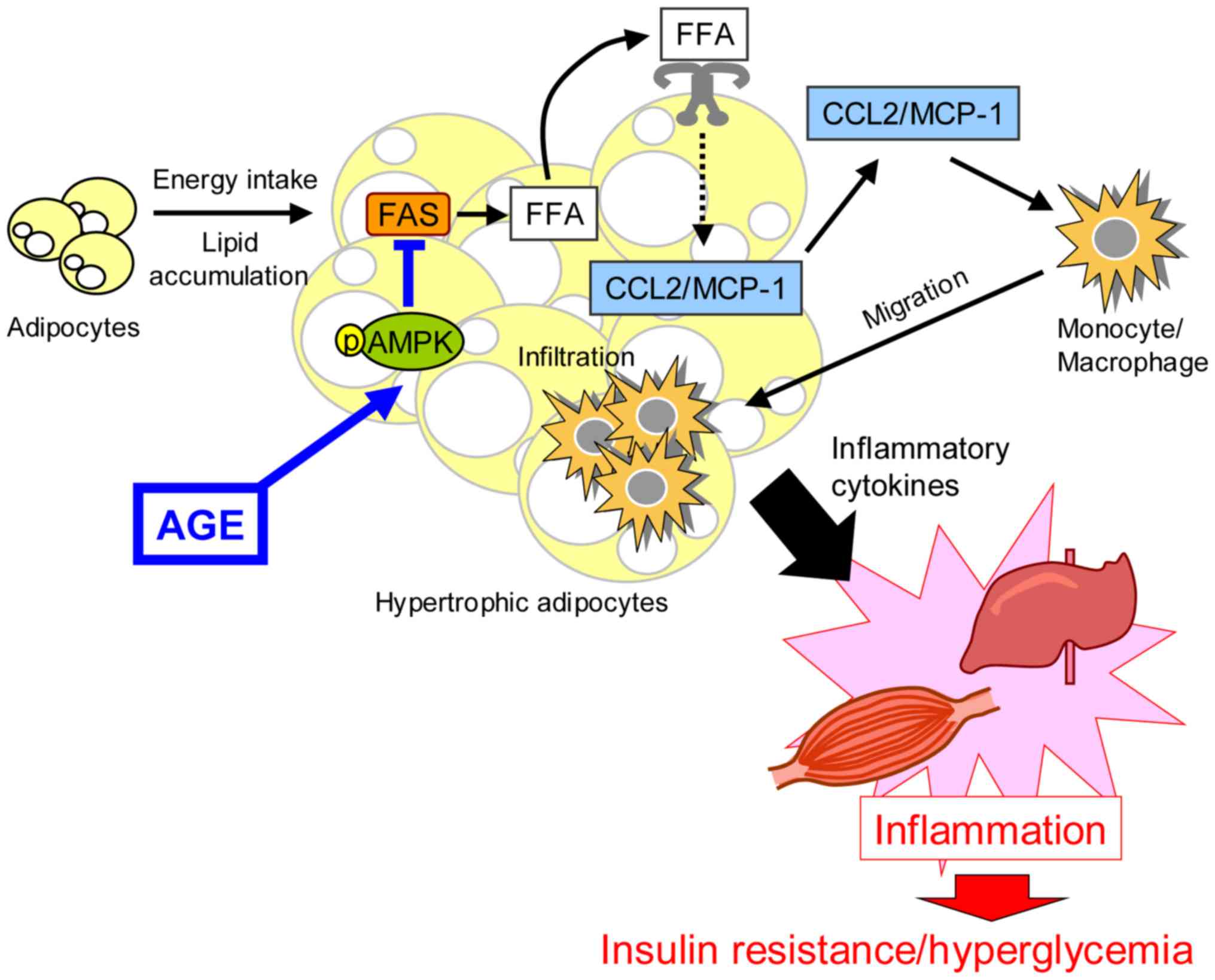
AGE has been shown to increase the phosphorylation
of AMPK in Tsumura Suzuki Obese Diabetes (TSOD) mice, a mouse model
of type 2 diabetes (34). In
addition, AGE suppresses the plasma glycated albumin level and
improves glucose intolerance in TSOD mice. The level of glycated
albumin is proportional to the amount of glucose in plasma as
glucose nonenzymatically reacts with the amino group of proteins in
plasma (73,74). Blood glucose is taken up via glucose
transporter 4 (GLUT4) in skeletal muscle and adipose tissue
(75,76). However, the production of GLUT4 is
impaired through the inhibition of insulin signaling by
pro-inflammatory cytokines in diabetes (75,76).
Accordingly, inflammation induces insulin resistance and
hyperglycemia. Previous studies have suggested that AGE suppresses
inflammation in clinical trials and animal studies (23,28,29,34). AGE
has been shown to inhibit the expression of
Ccl2/Mcp-1 mRNA in adipose tissue and liver of TSOD
mice (34). PRRs, including TLRs
trigger the production of inflammatory cytokines and chemokines by
recognizing antigens, whereas PRRs have been reported to recognize
not only foreign pathogens, but also cellular components (12–14).
Saturated free fatty acids (FFA) from adipose tissues have been
reported to activate TLR4 and trigger the production of
inflammatory cytokines in immune cells (57,58). The
level of FFA is regulated by fatty acid synthase (FAS), and AMPK
negatively regulates the expression of Fas mRNA by
modulating SREBP-1c (45,77). AGE has inhibited the expression of
Fas mRNA via the activation of AMPK (34). Therefore, AGE can ameliorate
hyperglycemia and insulin resistance by anti-inflammatory effect
via the activation of AMPK in TSOD mice (Fig. 2).
AGE ameliorates atherosclerosis and type 2 diabetes
through the suppression of inflammation. In addition, AGE and its
components induce the activation of AMPK in several tissues and
cells. AMPK plays an important role in regulating the inflammatory
response through the inhibition of the TLR signaling pathway. Thus,
AMPK is considered as a possible therapeutic target for
inflammatory-related diseases. Therefore, AGE, which has a
promoting effect on AMPK activation, may prove to be a useful
preparation for the prevention and improvement of various diseases
associated with chronic inflammation.
The authors would like to thank Dr Takami Oka of
Wakunaga Pharmaceutical Co., Ltd. for his helpful advice,
encouragement and critical reading of the manuscript.
No funding was received.
Not applicable.
SM, JIS and NM conceived this review. SM, KK and JIS
analyzed the relevant literature. SM wrote the manuscript and
constructed the figures. JIS, KK and NM critically revised the
manuscript. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Hardie DG, Ross FA and Hawley SA: AMPK: A
nutrient and energy sensor that maintains energy homeostasis. Nat
Rev Mol Cell Biol. 13:251–262. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mihaylova MM and Shaw RJ: The AMPK
signalling pathway coordinates cell growth, autophagy and
metabolism. Nat Cell Biol. 13:1016–1023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garcia D and Shaw RJ: AMPK: Mechanisms of
Cellular Energy Sensing and Restoration of Metabolic Balance. Mol
Cell. 66:789–800. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bai A, Ma AG, Yong M, Weiss CR, Ma Y, Guan
Q, Bernstein CN and Peng Z: AMPK agonist downregulates innate and
adaptive immune responses in TNBS-induced murine acute and
relapsing colitis. Biochem Pharmacol. 80:1708–1717. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nath N, Giri S, Prasad R, Salem ML, Singh
AK and Singh I: 5-aminoimidazole-4-carboxamide ribonucleoside: A
novel immunomodulator with therapeutic efficacy in experimental
autoimmune encephalomyelitis. J Immunol. 175:566–574. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao X, Zmijewski JW, Lorne E, Liu G, Park
YJ, Tsuruta Y and Abraham E: Activation of AMPK attenuates
neutrophil proinflammatory activity and decreases the severity of
acute lung injury. Am J Physiol Lung Cell Mol Physiol.
295:L497–L504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lorenzatti AJ and Servato ML: New evidence
on the role of inflammation in CVD risk. Curr Opin Cardiol.
34:418–423. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Geovanini GR and Libby P: Atherosclerosis
and inflammation: Overview and updates. Clin Sci (Lond).
132:1243–1252. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu CH, Abrams ND, Carrick DM, Chander P,
Dwyer J, Hamlet MRJ, Macchiarini F, PrabhuDas M, Shen GL, Tandon P,
et al: Biomarkers of chronic inflammation in disease development
and prevention: Challenges and opportunities. Nat Immunol.
18:1175–1180. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arulselvan P, Fard MT, Tan WS, Gothai S,
Fakurazi S, Norhaizan ME and Kumar SS: Role of Antioxidants and
Natural Products in Inflammation. Oxid Med Cell Longev.
2016:52761302016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Conti P and Shaik-Dasthagirisaeb Y:
Atherosclerosis: A chronic inflammatory disease mediated by mast
cells. Cent Eur J Immunol. 40:380–386. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Piccinini AM and Midwood KS: DAMPening
inflammation by modulating TLR signalling. Mediators Inflamm.
2010:6723952010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vénéreau E, Ceriotti C and Bianchi ME:
DAMPs from Cell Death to New Life. Front Immunol. 6:4222015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Patel S: Danger-Associated Molecular
Patterns (DAMPs): The Derivatives and Triggers of Inflammation.
Curr Allergy Asthma Rep. 18:632018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsutomo T and Kodera Y: Development of
an Analytic Method for Sulfur Compounds in Aged Garlic Extract with
the Use of a Postcolumn High Performance Liquid Chromatography
Method with Sulfur-Specific Detection. J Nutr. 146:450S–455S. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kodera Y, Ushijima M, Amano H, Suzuki JI
and Matsutomo T: Chemical and biological properties of
S−1-propenyl-l-cysteine in aged garlic extract. Molecules.
22:E5702017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nantz MP, Rowe CA, Muller CE, Creasy RA,
Stanilka JM and Percival SS: Supplementation with aged garlic
extract improves both NK and γδ-T cell function and reduces the
severity of cold and flu symptoms: A randomized, double-blind,
placebo-controlled nutrition intervention. Clin Nutr. 31:337–344.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu C, Mathews AE, Rodrigues C, Eudy BJ,
Rowe CA, O'Donoughue A and Percival SS: Aged garlic extract
supplementation modifies inflammation and immunity of adults with
obesity: A randomized, double-blind, placebo-controlled clinical
trial. Clin Nutr ESPEN. 24:148–155. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suzuki JI, Kodera Y, Miki S, Ushijima M,
Takashima M, Matsutomo T and Morihara N: Anti-inflammatory action
of cysteine derivative S−1-propenylcysteine by inducing
MyD88 degradation. Sci Rep. 8:141482018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kyo E, Uda N, Kasuga S and Itakura Y:
Immunomodulatory effects of aged garlic extract. J Nutr. 131((3s)):
1075S–1079S. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morihara N, Ide N and Weiss N: Aged garlic
extract inhibits CD36 expression in human macrophages via
modulation of the PPARgamma pathway. Phytother Res. 24:602–608.
2010.PubMed/NCBI
|
|
22
|
Morihara N, Hino A, Yamaguchi T and Suzuki
J: Aged garlic extract suppresses the development of
atherosclerosis apolipoprotein E-knockout mice. J Nutr.
146:460S–463S. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morihara N, Hino A, Miki S, Takashima M
and Suzuki JI: Aged garlic extract suppresses inflammation in
apolipoprotein E-knockout mice. Mol Nutr Food Res. 61:17003082017.
View Article : Google Scholar
|
|
24
|
Matsumoto S, Nakanishi R, Li D, Alani A,
Rezaeian P, Prabhu S, Abraham J, Fahmy MA, Dailing C, Flores F, et
al: Aged Garlic Extract Reduces Low Attenuation Plaque in Coronary
Arteries of Patients with Metabolic Syndrome in a Prospective
Randomized Double-Blind Study. J Nutr. 146:427S–432S. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Budoff M: Aged garlic extract retards
progression of coronary artery calcification. J Nutr. 136
(Suppl):741S–744S. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ried K, Frank OR and Stocks NP: Aged
garlic extract reduces blood pressure in hypertensives: A
dose-response trial. Eur J Clin Nutr. 67:64–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsutomo T, Ushijima M, Kodera Y,
Nakamoto M, Takashima M, Morihara N and Tamura K: Metabolomic study
on the antihypertensive effect of S−1-propenylcysteine in
spontaneously hypertensive rats using liquid chromatography coupled
with quadrupole-Orbitrap mass spectrometry. J Chromatogr B Analyt
Technol Biomed Life Sci. 1046:147–155. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ried K, Travica N and Sali A: The effect
of aged garlic extract on blood pressure and other cardiovascular
risk factors in uncontrolled hypertensives: The AGE at Heart trial.
Integr Blood Press Control. 9:9–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ried K, Travica N and Sali A: The Effect
of Kyolic Aged Garlic Extract on Gut Microbiota, Inflammation, and
Cardiovascular Markers in Hypertensives: The GarGIC Trial. Front
Nutr. 5:1222018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hiramatsu K, Tsuneyoshi T, Ogawa T and
Morihara N: Aged garlic extract enhances heme oxygenase-1 and
glutamate-cysteine ligase modifier subunit expression via the
nuclear factor erythroid 2-related factor 2-antioxidant response
element signaling pathway in human endothelial cells. Nutr Res.
36:143–149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsuneyoshi T, Kunimura K and Morihara N:
S−1-Propenylcysteine augments BACH1 degradation and heme
oxygenase 1 expression in a nitric oxide-dependent manner in
endothelial cells. Nitric Oxide. 84:22–29. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thomson M, Al-Qattan KK, Js D and Ali M:
Anti-diabetic and anti-oxidant potential of aged garlic extract
(AGE) in streptozotocin-induced diabetic rats. BMC Complement
Altern Med. 16:172016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ushijima M, Takashima M, Kunimura K,
Kodera Y, Morihara N and Tamura K: Effects of
S−1-propenylcysteine, a sulfur compound in aged garlic
extract, on blood pressure and peripheral circulation in
spontaneously hypertensive rats. J Pharm Pharmacol. 70:559–565.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miki S, Inokuma KI, Takashima M, Nishida
M, Sasaki Y, Ushijima M, Suzuki JI and Morihara N: Aged garlic
extract suppresses the increase of plasma glycated albumin level
and enhances the AMP-activated protein kinase in adipose tissue in
TSOD mice. Mol Nutr Food Res. 61:16007972017. View Article : Google Scholar
|
|
35
|
Hwang YP, Kim HG, Choi JH, Do MT, Chung
YC, Jeong TC and Jeong HG: S-allylcysteine attenuates free
fatty acid-induced lipogenesis in human HepG2 cells through
activation of the AMP-activated protein kinase-dependent pathway. J
Nutr Biochem. 24:1469–1478. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu L, Di W, Dong X, Li Z, Xue X, Zhang J,
Wang Q, Xiao X, Han J, Yang Y, et al: Diallyl trisulfide exerts
cardioprotection against myocardial ischemia-reperfusion injury in
diabetic state, role of AMPK-mediated AKT/GSK-3β/HIF-1α activation.
Oncotarget. 8:74791–74805. 2017.PubMed/NCBI
|
|
37
|
Xiao J, Guo R, Fung ML, Liong EC, Chang
RC, Ching YP and Tipoe GL: Garlic-Derived
S-Allylmercaptocysteine Ameliorates Nonalcoholic Fatty Liver
Disease in a Rat Model through Inhibition of Apoptosis and
Enhancing Autophagy. Evid Based Complement Alternat Med.
2013:6429202013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim J, Yang G, Kim Y, Kim J and Ha J: AMPK
activators: Mechanisms of action and physiological activities. Exp
Mol Med. 48:e2242016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Coughlan KA, Valentine RJ, Ruderman NB and
Saha AK: AMPK activation: A therapeutic target for type 2 diabetes?
Diabetes Metab Syndr Obes. 7:241–253. 2014.PubMed/NCBI
|
|
40
|
Bäck M, Yurdagul A Jr, Tabas I, Öörni K
and Kovanen PT: Inflammation and its resolution in atherosclerosis:
Mediators and therapeutic opportunities. Nat Rev Cardiol.
16:389–406. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Salminen A, Hyttinen JM and Kaarniranta K:
AMP-activated protein kinase inhibits NF-κB signaling and
inflammation: Impact on healthspan and lifespan. J Mol Med (Berl).
89:667–676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Salminen A and Kaarniranta K:
AMP-activated protein kinase (AMPK) controls the aging process via
an integrated signaling network. Ageing Res Rev. 11:230–241. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ou H, Liu C, Feng W, Xiao X, Tang S and Mo
Z: Role of AMPK in atherosclerosis via autophagy regulation. Sci
China Life Sci. 61:1212–1221. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu-Bryan R: Inflammation and
intracellular metabolism: New targets in OA. Osteoarthritis
Cartilage. 23:1835–1842. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li Y, Xu S, Mihaylova MM, Zheng B, Hou X,
Jiang B, Park O, Luo Z, Lefai E, Shyy JY, et al: AMPK
phosphorylates and inhibits SREBP activity to attenuate hepatic
steatosis and atherosclerosis in diet-induced insulin-resistant
mice. Cell Metab. 13:376–388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Doddapattar P, Radović B, Patankar JV,
Obrowsky S, Jandl K, Nusshold C, Kolb D, Vujić N, Doshi L, Chandak
PG, et al: Xanthohumol ameliorates atherosclerotic plaque
formation, hypercholesterolemia, and hepatic steatosis in
ApoE-deficient mice. Mol Nutr Food Res. 57:1718–1728.
2013.PubMed/NCBI
|
|
47
|
Wang Q, Zhang M, Liang B, Shirwany N, Zhu
Y and Zou MH: Activation of AMP-activated protein kinase is
required for berberine-induced reduction of atherosclerosis in
mice: The role of uncoupling protein 2. PLoS One. 6:e254362011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Van Gaal LF, Mertens IL and De Block CE:
Mechanisms linking obesity with cardiovascular disease. Nature.
444:875–880. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shoelson SE, Lee J and Goldfine AB:
Inflammation and insulin resistance. J Clin Invest. 116:1793–1801.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rameshrad M, Maleki-Dizaji N, Soraya H,
Toutounchi NS, Barzegari A and Garjani A: Effect of A-769662, a
direct AMPK activator, on Tlr-4 expression and activity in mice
heart tissue. Iran J Basic Med Sci. 19:1308–1317. 2016.PubMed/NCBI
|
|
51
|
Mancini SJ, White AD, Bijland S,
Rutherford C, Graham D, Richter EA, Viollet B, Touyz RM, Palmer TM
and Salt IP: Activation of AMP-activated protein kinase rapidly
suppresses multiple pro-inflammatory pathways in adipocytes
including IL-1 receptor-associated kinase-4 phosphorylation. Mol
Cell Endocrinol. 440:44–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Malekpour-Dehkordi Z, Javadi E, Doosti M,
Paknejad M, Nourbakhsh M, Yassa N, Gerayesh-Nejad S and Heshmat R:
S-Allylcysteine, a garlic compound, increases ABCA1
expression in human THP-1 macrophages. Phytother Res. 27:357–361.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kemmerer M, Wittig I, Richter F, Brüne B
and Namgaladze D: AMPK activates LXRα and ABCA1 expression in human
macrophages. Int J Biochem Cell Biol. 78:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Olokoba AB, Obateru OA and Olokoba LB:
Type 2 diabetes mellitus: A review of current trends. Oman Med J.
27:269–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kahn SE, Cooper ME and Del Prato S:
Pathophysiology and treatment of type 2 diabetes: Perspectives on
the past, present, and future. Lancet. 383:1068–1083. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
DeFronzo RA, Ferrannini E, Groop L, Henry
RR, Herman WH, Holst JJ, Hu FB, Kahn CR, Raz I, Shulman GI, et al:
Type 2 diabetes mellitus. Nat Rev Dis Primers. 1:150192015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
de Luca C and Olefsky JM: Inflammation and
insulin resistance. FEBS Lett. 582:97–105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mancuso P: The role of adipokines in
chronic inflammation. ImmunoTargets Ther. 5:47–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
McArdle MA, Finucane OM, Connaughton RM,
McMorrow AM and Roche HM: Mechanisms of obesity-induced
inflammation and insulin resistance: Insights into the emerging
role of nutritional strategies. Front Endocrinol (Lausanne).
4:522013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
León-Pedroza JI, González-Tapia LA, del
Olmo-Gil E, Castellanos-Rodríguez D, Escobedo G and González-Chávez
A: Low-grade systemic inflammation and the development of metabolic
diseases: From the molecular evidence to the clinical practice. Cir
Cir. 83:543–551. 2015.(In Spanish). PubMed/NCBI
|
|
61
|
Lumeng CN and Saltiel AR: Inflammatory
links between obesity and metabolic disease. J Clin Invest.
121:2111–2117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Rehman K and Akash MS: Mechanisms of
inflammatory responses and development of insulin resistance: How
are they interlinked? J Biomed Sci. 23:872016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang BB, Zhou G and Li C: AMPK: An
emerging drug target for diabetes and the metabolic syndrome. Cell
Metab. 9:407–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hardie DG: AMPK: A target for drugs and
natural products with effects on both diabetes and cancer.
Diabetes. 62:2164–2172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rena G, Hardie DG and Pearson ER: The
mechanisms of action of metformin. Diabetologia. 60:1577–1585.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rojas LB and Gomes MB: Metformin: An old
but still the best treatment for type 2 diabetes. Diabetol Metab
Syndr. 5:62013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Setter SM, Iltz JL, Thams J and Campbell
RK: Metformin hydrochloride in the treatment of type 2 diabetes
mellitus: A clinical review with a focus on dual therapy. Clin
Ther. 25:2991–3026. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lagouge M, Argmann C, Gerhart-Hines Z,
Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P,
Elliott P, et al: Resveratrol improves mitochondrial function and
protects against metabolic disease by activating SIRT1 and
PGC-1alpha. Cell. 127:1109–1122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Price NL, Gomes AP, Ling AJ, Duarte FV,
Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro
JS, et al: SIRT1 is required for AMPK activation and the beneficial
effects of resveratrol on mitochondrial function. Cell Metab.
15:675–690. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhu X, Wu C, Qiu S, Yuan X and Li L:
Effects of resveratrol on glucose control and insulin sensitivity
in subjects with type 2 diabetes: Systematic review and
meta-analysis. Nutr Metab (Lond). 14:602017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Liang Y, Xu X, Yin M, Zhang Y, Huang L,
Chen R and Ni J: Effects of berberine on blood glucose in patients
with type 2 diabetes mellitus: A systematic literature review and a
meta-analysis. Endocr J. 66:51–63. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ,
Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, et al: Berberine, a natural
plant product, activates AMP-activated protein kinase with
beneficial metabolic effects in diabetic and insulin-resistant
states. Diabetes. 55:2256–2264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Welsh KJ, Kirkman MS and Sacks DB: Role of
Glycated Proteins in the Diagnosis and Management of Diabetes:
Research Gaps and Future Directions. Diabetes Care. 39:1299–1306.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Anguizola J, Matsuda R, Barnaby OS, Hoy
KS, Wa C, DeBolt E, Koke M and Hage DS: Review: Glycation of human
serum albumin. Clin Chim Acta. 425:64–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Leto D and Saltiel AR: Regulation of
glucose transport by insulin: Traffic control of GLUT4. Nat Rev Mol
Cell Biol. 13:383–396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Huang S and Czech MP: The GLUT4 glucose
transporter. Cell Metab. 5:237–252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bijland S, Mancini SJ and Salt IP: Role of
AMP-activated protein kinase in adipose tissue metabolism and
inflammation. Clin Sci (Lond). 124:491–507. 2013. View Article : Google Scholar : PubMed/NCBI
|