Introduction
The introduction of the human programmed death-1
(PD-1) immune checkpoint inhibitor Nivolumab has changed the
therapeutic strategy for metastatic renal cell carcinoma (mRCC).
Nivolumab has shown to prolong the overall survival of mRCC
patients in second line after vascular endothelial growth factor
receptor tyrosine kinase inhibitors (VEGFR TKIs) failure (1). Nevertheless, the efficacy of subsequent
therapies that are considered after VEGFR TKIs and immunotherapy
failure is still unclear and additional therapeutic strategy is
limited. The abscopal effect is a rare phenomenon that was first
described over half a century ago (2), in which tumor regression occurs outside
the irradiated sites through activation of the immune system.
Recently, the efficacy of cancer immunotherapy combined with
radiotherapy (RT) has been suggested (3). We experienced a case of a patient with
mRCC who demonstrated the abscopal effect during nivolumab
treatment after palliative radiotherapy. This patient had a unique
treatment course after the abscopal effect. Furthermore,
pathological re-examination of the primary specimen showed unique
pathological findings. The unique treatment course with Nivolumab
combined with RT and the appearance of abscopal effect might be
related to the unique pathological findings.
Case report
A 40-year-old woman who had never been diagnosed
with any other disease and malignancy presented with lumbar pain.
Computed tomography (CT) showed a left renal tumor with a maximum
diameter of 8.2 cm, without distant metastases. She underwent
radical nephrectomy, and pathological examination showed a clear
cell renal cell carcinoma (ccRCC), stage pT2aN0M0, Fuhrman grade 2.
Three months after surgery, she developed two lung metastases.
During the following two years, she received various systemic
therapies, including interferon-α (3 months), axitinib (9 months),
everolimus (3 months), and pazopanib (9 months). However, their
effects were transient, and follow-up CT showed progression of lung
metastases with pleural effusion and new lesions (right
supraclavicular and para-aortic lymph node swellings).
Because nivolumab received government approval in
Japan, it was started at 3 mg/kg intravenously every 2 weeks. After
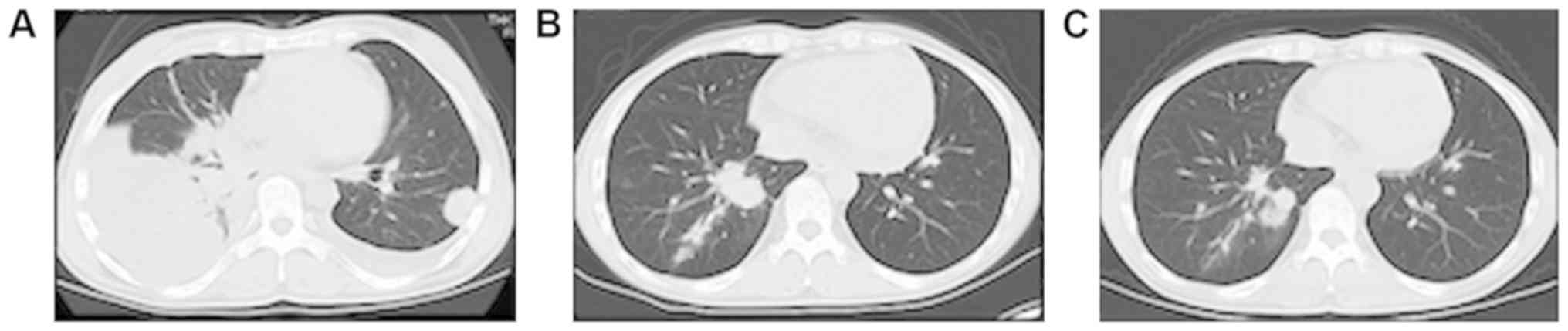
26 cycles, most of the lung nodules had shrunk, and the pleural
effusion had disappeared completely (Fig. 1). However, several lung nodules and
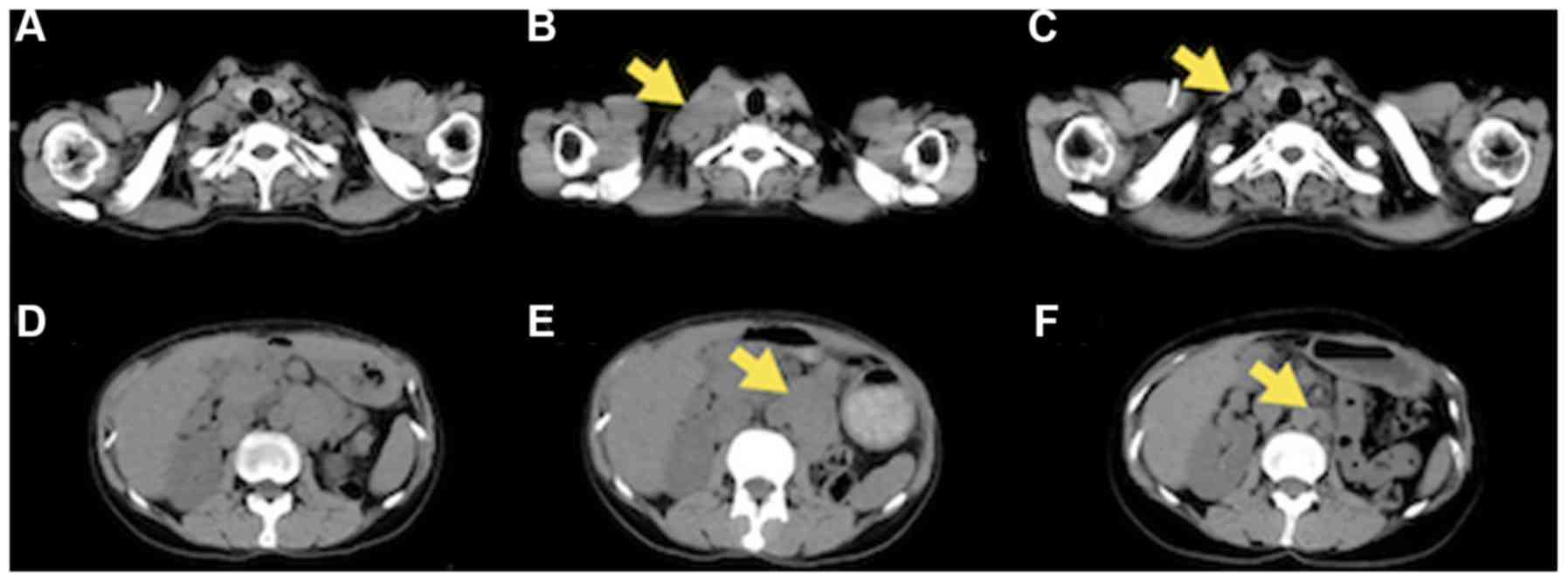
the right supraclavicular and para-aortic lymph nodes were still
growing (Fig. 2). The patient also
complained of lumbar pain, probably due to nerve compression by
metastatic nodes, and her Karnofsky Performance Status (KPS)
deteriorated to 50. Thereafter, palliative radiotherapy (RT) was
performed to the right supraclavicular and para-aortic lymph nodes
(30 Gy/10 Fr and 40 Gy/20 Fr, respectively). After the RT,
nivolumab was resumed. Follow-up CT showed the decrease in size of
both irradiated lesions (Fig. 2),
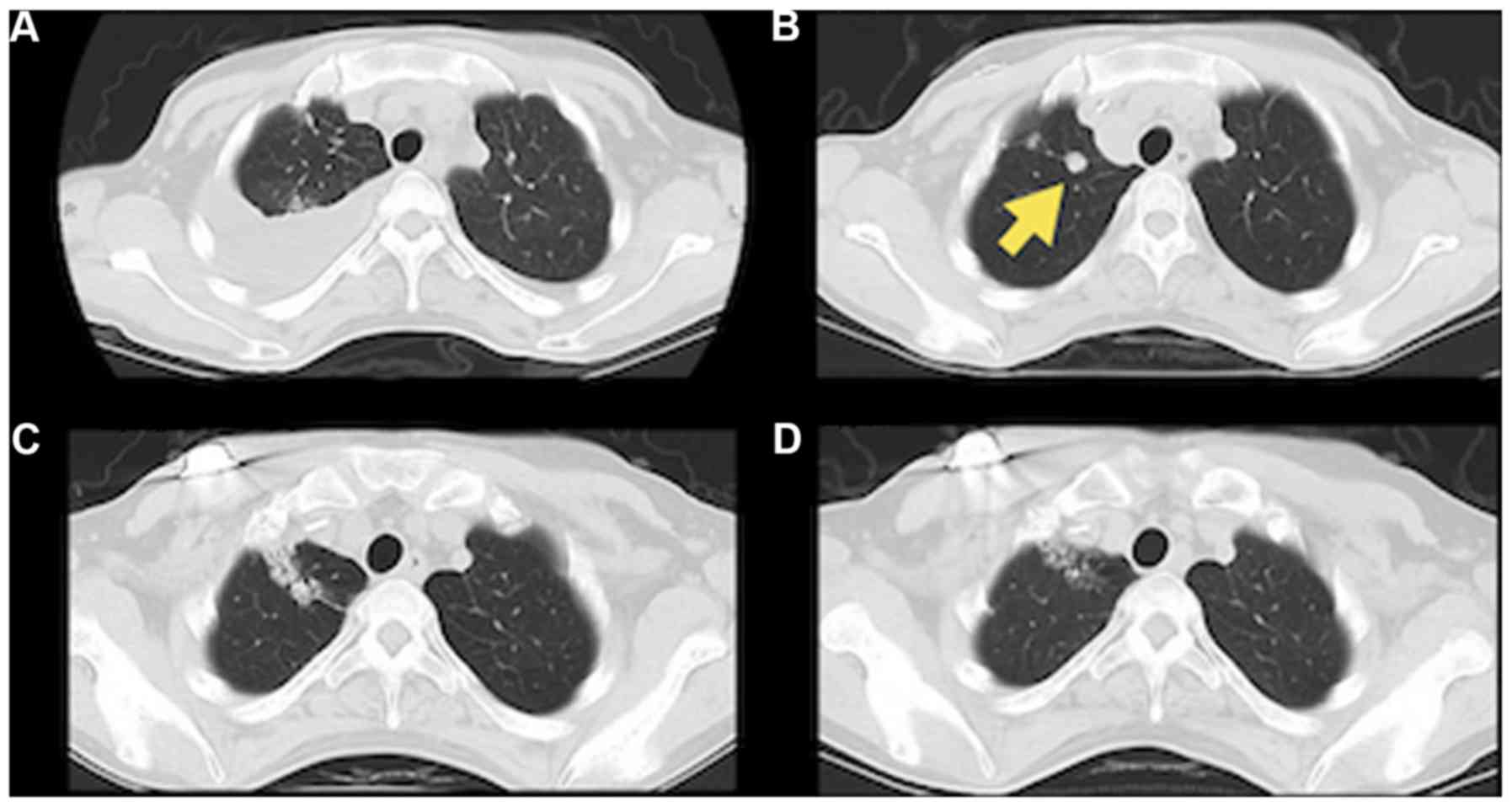
and, interestingly, the nivolumab-resistant lung nodules also
appeared to be decreasing after RT (Fig.
3), probably due to the abscopal effect. The patient's
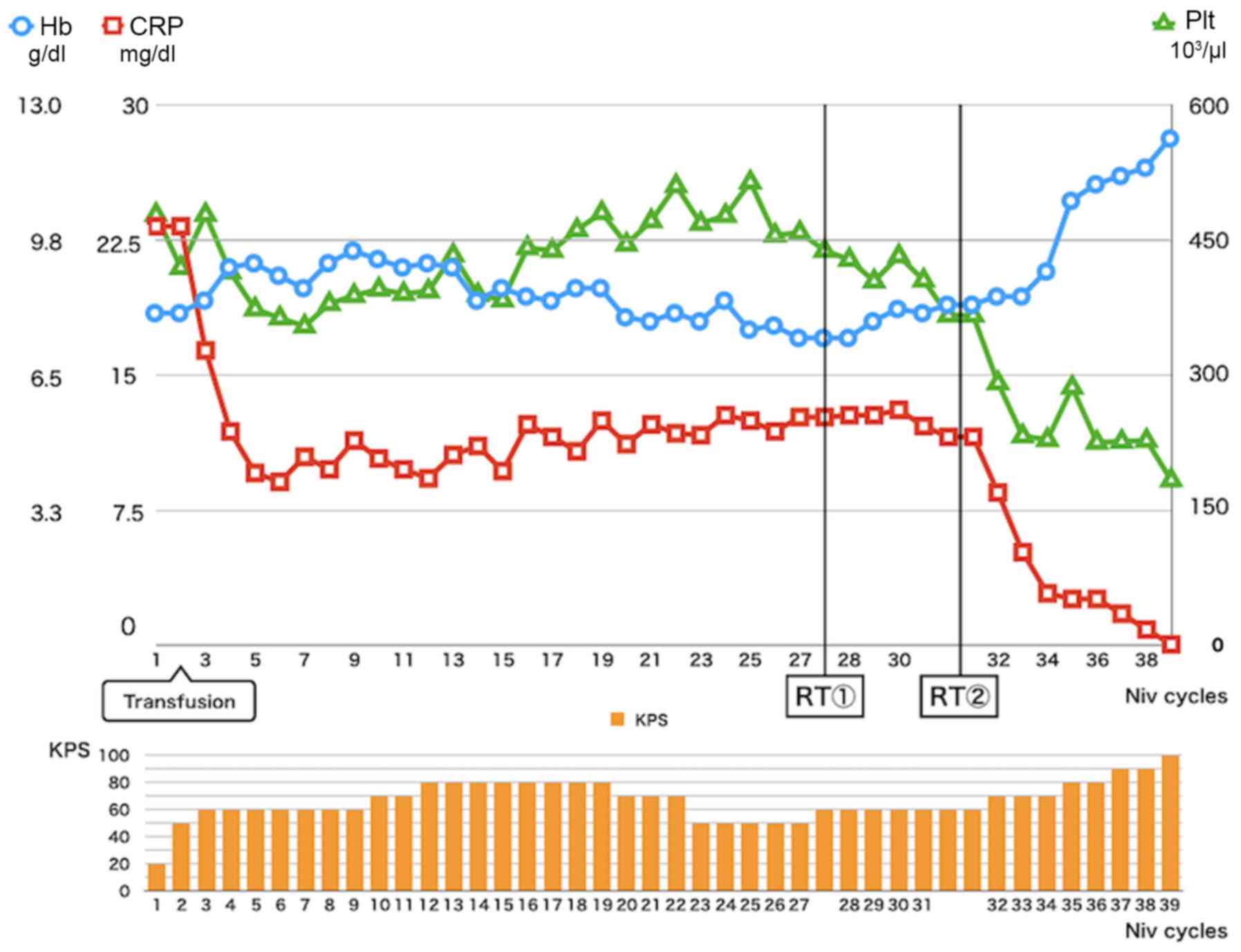
laboratory data also normalized, as shown in Fig. 4, and her KPS improved from 50 to 100.
Her laboratory data and KPS have remained excellent and she has
been received 33 cycles of niv after RT (total 64 cycles from
induction).
Repeat pathological examination of the tumor
specimen was performed, including immunohistochemical (IHC)
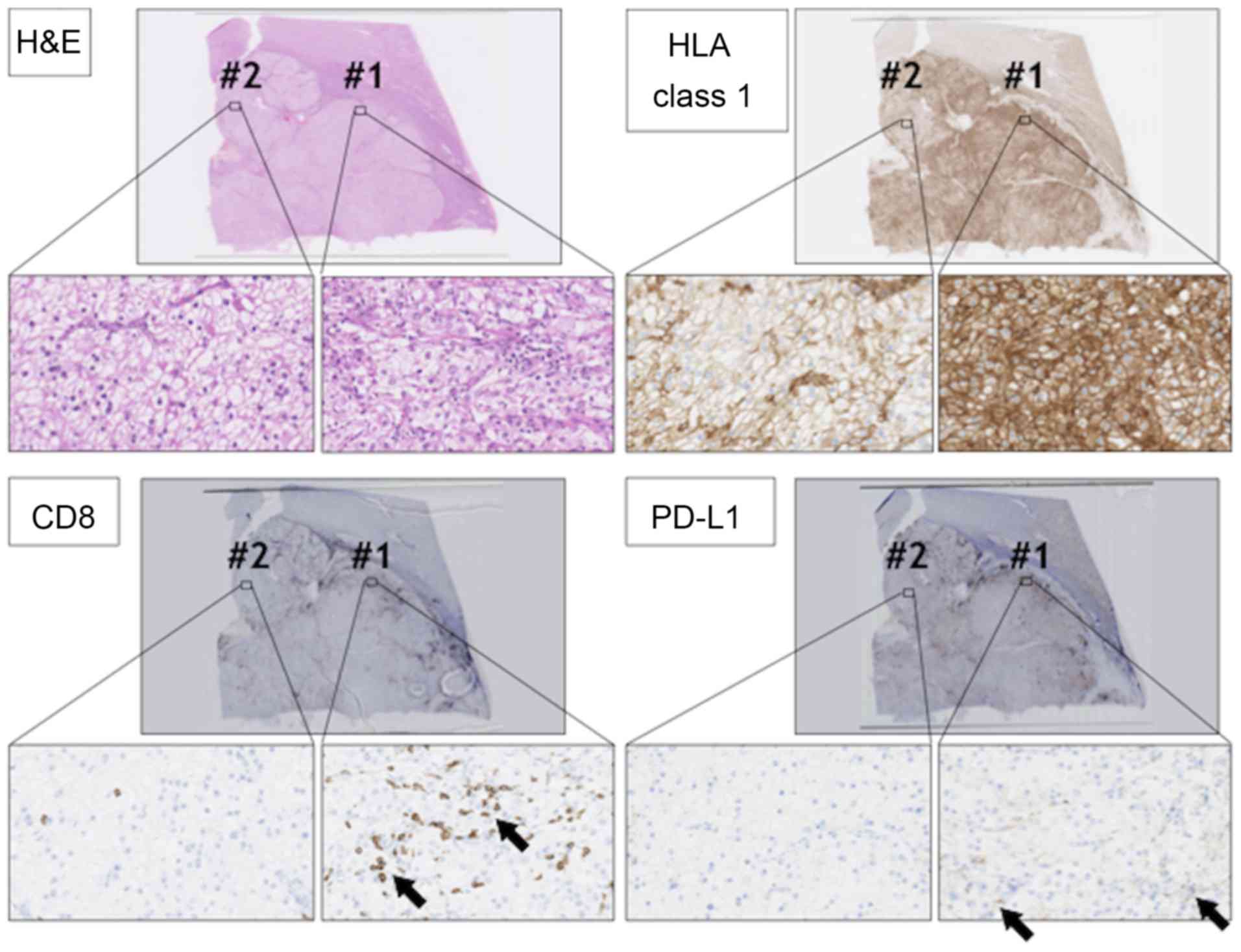
staining. IHC staining using anti-human leukocyte antigen (HLA)
class 1 (clone: EMR8-5) showed heterogeneity of the tumor with two
staining patterns (components #1 and #2) in the RCC lesion
(Fig. 5). In component #1, RCC cells
showed strong membrane-positive staining for anti-HLA class 1
antibody. On the other hand, in component #2, RCC cells showed
relatively weak HLA class 1 staining. Infiltration of CD8-positive
cytotoxic T lymphocytes (CTLs) also showed heterogeneity. There
were many CTLs in component #1, whereas CD8-positive CTLs were few
in component #2, suggesting that component #1 RCC was an inflamed
tumor, and component #2 RCC was an immune desert tumor (3). Programmed death-ligand 1 (PD-L1)
staining using anti-PD-L1 antibody (clone E1L3N) showed that PD-L1
was expressed in component #1 RCC cells, but not in component #2
RCC cells.
Discussion
Several prognostic markers have been reported in
RCCs (4,5), and high C-reactive protein (CRP), low
hemoglobin, and thrombocythemia were also reported to be related to
poorer prognosis in RCCs (6,7). In the present case, the patient showed
a relatively good response to nivolumab, and CRP improved
partially, whereas anemia and thrombocythemia did not improve with
PD1 blockade, even after most of the metastatic RCC lesions had
shrunk. Interestingly, after the RT to the metastatic nodes, the
nivolumab-resistant lung lesion shrank, probably due to the
abscopal effect, and the patient's laboratory data also normalized.
High CRP has also been shown to be related to poorer prognosis in
melanoma patients treated by cytotoxic T-lymphocyte-associated
protein 4 (CTLA-4) blockade (8). CRP
is a product of IL-6 stimulation, and so high-CRP level indicates
type 2 helper dominancy in the patient. Surprisingly, laboratory
data normalization including CRP indicates the improvement of
systematic type 2 helper dominancy that might help to induce new
cytotoxic T lymphocytes.
Histological re-examination showed the heterogeneity
of the primary RCC lesions in this case, with two components, #1
and #2. In component #1, ccRCC cells strongly expressed HLA class 1
molecule, and many infiltrating CD8+ CTLs were observed
in the intra-tumoral region even before anti-PD1 treatment,
indicating that the ccRCC cells in component #1 were
immunogenic.
The ccRCC cells in component #1 expressed PD-L1,
indicating that inhibitory receptor ligand PD-L1 was essential for
immunological escape for component #1 ccRCC cells. On the other
hand, component #2 ccRCC cells expressed lower levels of HLA class
1 and did not express PD-L1. CD8+ CTL infiltration was
small in component #2 of the ccRCC, indicating that ccRCC cells in
component #2 might be less immunogenic, and PD-L1 expression was
not necessary for immunological escape. After the abscopal effect,
almost all lesions shrank, suggesting that PD1 blockade therapy
also became effective in the less immunogenic component, component
#2. At this moment, we do not know the mechanisms for how RT
transformed ccRCC cells to become sensitive to PD1 blockade;
however, RT is known to induce anti-tumor immunity by releasing
tumor-associated antigens, over expression of MHC class 1, and
release danger signal molecules including High mobility group box 1
protein (HMGB1) and adenosine triphosphate (ATP) (9,10).
Furthermore, normalization of CRP highly suggest that type 2 helper
dominancy was improved to type 1 helper dominancy in this case as
described above. These improvements of immunological environment
might provoke a different anti-component #2 immunological reaction.
This case report has some limitations. we could not perform
genetical analysis such as Whole exome sequence in this case,
because only limited volume of formalin-fixed paraffin-embedded
specimen samples are available in this case.
In summary, this case had a unique treatment course
after an abscopal effect by RT. PD1 blockade combined with RT might
be an option for RCC patients in whom PD1 blockade monotherapy is
ineffective.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KH, TAo, NT, MM and KM designed the study, collected
and analysed the clinical data. KH and YH wrote the manuscript. YH,
HM and TT collected and analysed the pathological data. TAb and NS
contributed to the conception and design of the study, and revised
the manuscript.
Ethics approval and consent to
participate
Ethical approval was granted by The Kushiro City
General Hospital (approval no. 2019-2).
Patient consent for publication
Informed consent was obtained from the patient,
including for the publication of this report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mole RH: Whole body irradiation;
radiobiology or medicine? Br J Radiol. 26:234–241. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Formenti SC and Demaria S: Combining
radiotherapy and cancer immunotherapy: A paradigm shift. J Natl
Cancer Inst. 105:256–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galluzzi L, Chan TA, Kroemer G, Wolchok JD
and López-Soto A: The hallmarks of successful anticancer
immunotherapy. Sci Transl Med. 10:eaat78072018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun M, Shariat SF, Cheng C, Ficarra V,
Murai M, Oudard S, Pantuck AJ, Zigeuner R and Karakiewicz PI:
Prognostic factors and predictive models in renal cell carcinoma: A
contemporary review. Eur Urol. 60:644–661. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shinohara N and Abe T: Prognostic factors
and risk classifications for patients with metastatic renal cell
carcinoma. Int J Urol. 22:888–897. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xia L, Hu G and Guzzo TJ: Prognostic
significance of preoperative anemia in patients undergoing surgery
for renal cell carcinoma: A meta-analysis. Anticancer Res.
37:3175–3181. 2017.PubMed/NCBI
|
|
7
|
Bensalah K, Leray E, Fergelot P,
Rioux-Leclercq N, Tostain J, Guillé F and Patard JJ: Prognostic
value of thrombocytosis in renal cell carcinoma. J Urol.
175:859–863. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilgenhof S, Du Four S, Vandenbroucke F,
Everaert H, Salmon I, Liénard D, Marmol VD and Neyns B:
Single-center experience with ipilimumab in an expanded access
program for patients with pretreated advanced melanoma. J
Immunother. 36:215–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lugade AA, Moran JP, Gerber SA, Rose RC,
Frelinger JG and Lord EM: Local radiation therapy of B16 melanoma
tumors increases the generation of tumor antigen-specific effector
cells that traffic to the tumor. J Immunol. 174:7516–7523. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gameiro SR, Jammeh ML, Wattenberg MM,
Tsang KY, Ferrone S and Hodge JW: Radiation-induced immunogenic
modulation of tumor enhances antigen processing and calreticulin
exposure, resulting in enhanced T-cell killing. Oncotarget.
5:403–416. 2014. View Article : Google Scholar : PubMed/NCBI
|