Introduction
Direct dental pulp capping and vital pulpotomy are
the two most commonly used vital pulp preservative treatment
methods for permanent teeth without irreversible pulpitis. Such
dental procedures aim to preserve dental pulp tissue, maintain the
physiological function of the dentine-pulp complex and guarantee
root development in immature teeth. During vital pulp therapy, an
adequate pulp-capping agent is used and placed directly on the
dental pulp tissue; therefore, biocompatibility and cytotoxicity
require to be fully evaluated prior to use of a novel material as a
pulp-capping agent.
Calcium hydroxide (Ca(OH)2) has been used
as a pulp-capping agent for decades and is the most popular
material for vital pulp therapy (1).
Mineral trioxide aggregate (MTA) is a bioactive material with a
high sealing ability, superior antibacterial properties and
excellent biocompatibility (2).
iRoot BP plus (Innovative BioCeramix Inc.) is a convenient,
ready-to-use nanoparticulate bioceramic. Despite recently being
applied as a pulp-capping agent (3),
iRoot BP has not been widely used in vital pulp therapy and the
supporting evidence for its use is relatively less than that for
the use of MTA and Ca(OH)2. Previous research has
indicated that iRoot BP exhibited good biocompatibility with pulp
tissue and induced proliferation and reparative dentin bridge
formation in dental pulp cells (4),
suggesting that iRoot BP may be used as a pulp-capping agent.
Platelet-rich fibrin (PRF), which belongs to the
second generation of platelet concentrate products, has favorable
properties, including osteogenic ability, simple preparation and no
added biological agents. PRF was demonstrated to promote cell
proliferation and osteogenic differentiation in human dental pulp
cells (HDPCs) (5). Concentrated
growth factors (CGF) are also derived from autologous blood and
produced using a centrifuge device (Medifuge Silfradent srl). The
different centrifugation speeds permit the isolation of fibrin
matrix that is markedly larger, denser and richer in growth
factors, as compared with past-generation platelet concentrate
products (6). CGF contains numerous
growth factors with advantages including osteogenic ability, simple
preparation process, good biological properties and lack of added
biological agents. Although it was previously hypothesized that PRF
and CGF may be effective pulp-capping agents (5), evidence support this remains
insufficient.
To the best of our knowledge, no previous study has
compared these materials on dental pulp capping simultaneously.
Prior to in vivo experiments, the influence of these
materials on HDPCs requires to be investigated in vitro.
Therefore, the aim of the present study was to investigate and
compare the effect of Ca(OH)2, MTA, iRoot BP, PRF and
CGF on the proliferation, viability, cell cycle, apoptosis and
mineralization of HDPCs.
Materials and methods
Isolation and culture of HDPCs
The present study was approved by the Ethics
Committee of Chongqing Medical University (Chongqing, China) and
written informed consent was obtained from each donor.
Freshly-extracted human impacted third molars were collected from
the Stomatological Hospital of Chongqing Medical University
(Chongqing, China). The pulp tissue was isolated from the teeth,
cut into 1-mm3 pieces, enzymatically digested for 50 min
at 37°C with 3 mg/ml Type I collagenase (Gibco; Thermo Fisher
Scientific, Inc.) and then supplemented with fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.). The digested tissue
was pelleted by centrifugation at 201 × g for 5 min at room
temperature. The cells were then resuspended in 1.5 ml α-Minimum
Essential Medium (α-MEM; Hyclone; GE Healthcare Life Sciences)
containing 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin,
transferred into a 25-cm2 culture flask (cat. no.
430372; Corning Inc.), and then cultured at 37°C, 20% O2
and 5% CO2. After 24 h of incubation, 2.5 ml culture
medium was added, and the medium was changed every 3–4 days. When
passaging the cells, a mixture of 0.25% trypsin and 0.01% EDTA was
used. The expression of CD markers in these HDPCs was analyzed and
published in a previous study by our group (7). For all experiments, the HDPCs were in
passages 3–6.
Preparation of RPF and CGF
Venous blood was drawn from healthy volunteers and
divided into 10-ml sterile vacuum blood tubes (Sanli) without
anti-coagulants. To prepare PRF, blood samples were immediately
placed in a centrifuge (5810R; Eppendorf) for centrifugation. The
program was run for 10 min at 1,811 × g and the fresh whole blood
was divided into three layers. The intermediate filament protein
gel layer was collected. For the preparation of CGF, blood samples
were immediately placed in a centrifuge (Medifuge Silfradent srl)
for centrifugation. The in-built program was run as follows: 30 sec
for acceleration, 2 min at 408 × g, 4 min at 323 × g, 4 min at 408
× g, 3 min at 1,811 × g and 36 sec for deceleration and stop. All
centrifugation procedures were performed at room temperature. The
fresh whole blood was divided into three layers and the
intermediate layer was collected.
Preparation of PRF and CGF
exudates
A sterile pre-fabricated mold (depth, 2 mm;
diameter, 5 mm) was used to measure the same volume of five
materials. The preparation of PRF and CGF exudate was similar to
the methods described by Masuki et al (8). The same volume of PRF and CGF clots was
placed on dry gauze to eliminate excessive serum, transferred to
freezing tubes and stored at −80°C. The frozen clots were ground
using a sample grinder (Tissuelyser-48; Digital China Jinxin
Technology Co., Ltd.), resuspended in 3 ml α-MEM, fully mixed using
a shaker and centrifuged at 453 × g for 5 min at 4°C to obtain PRF
and CGF exudates. The exudates were filtered using a 0.45-µm
sterile syringe filter (EMD Millipore) and stored at −80°C.
Preparation of the Ca(OH)2,
MTA and iRoot BP exudates
Ca(OH)2 (DYCAL®; Dentsply) and
MTA (ProRoot MTA) were mixed according to the manufacturers'
instructions. Prior to solidification, Ca(OH)2, MTA and
iRoot BP were also used to fill the pre-fabricated mold to ensure
that the same volume of each material was used. The same volume of
solidified Ca(OH)2, MTA and iRoot BP was ground using a
sample grinder (Tissuelyser-96; Digital China Jinxin Technology
Co., Ltd) containing 3 ml α-MEM, fully mixed using a shaker and
then centrifuged at 453 × g for 5 min at room temperature to obtain
exudates. All exudates from the three materials were filtered using
a 0.45-µm sterile syringe filter (EMD Millipore; Merck KGaA) and
stored at 4°C for no more than one month.
Cell viability
The effect of the five materials on the viability of
HDPCs was assessed by trypan blue dye exclusion. HDPCs were seeded
into 12-well plates (1×104 cells/well), cultured
overnight and treated with culture medium containing 10% exudate
from Ca(OH)2, MTA, iRoot, PRF or CGF for 1, 3 or 7 days.
α-MEM only was used for the control group. The number of viable
cells relative to that in the control group was calculated
following 0.4% trypan blue dye staining. The cells that stained
blue were considered non-viable. The assays were performed in
triplicate.
Cell proliferation assay
The effect of the five materials on HDPC
proliferation was assessed using a Cell Counting Kit-8 (CCK-8;
Dojindo Molecular Technologies, Inc.). Cells were transferred into
a 96-well plate (1×103 cells/well) and cultured for 4 h.
The wells were divided into 6 groups and a total of 10 µl
Ca(OH)2, MTA, iRoot BP, PRF, CGF exudate (all dissolved
in α-MEM) or α-MEM without serum (control) was added to the
corresponding wells. On days 1, 3 or 7, the culture medium was
discarded and 100 µl culture medium plus 10 µl CCK-8 solution was
added to each well. The plates were incubated in the dark for 2 h
and the absorbance at 450 nm was measured using a microplate reader
(Thermo Fisher Scientific, Inc.). The assays were performed in
triplicate.
Cell apoptosis
The effect of the five materials on apoptosis of
HDPCs was assessed using an Annexin V/propidium iodide (PI) assay.
HDPCs were seeded in 6-well plates (3×104 cells/well),
cultured overnight and treated with culture medium containing 10%
exudate from Ca(OH)2, MTA, iRoot, PRF or CGF for 1, 3 or
7 days. α-MEM only was used for the control. The relative number of
apoptotic HDPCs was calculated following Annexin V/PI binding. In
brief, the medium was removed and the cells incubated with Alexa
Fluor 488 annexin V/PI (Invitrogen™; Thermo Fisher Scientific,
Inc.) to label apoptotic cells, according to the manufacturer's
protocol. Subsequently, the cells were analyzed on a flow cytometer
(BD Influx™; BD Biosciences) using BD FACS™ software (version
1.0.0.650; BD Biosciences). The assays were performed in
triplicate.
Cell cycle
HDPCs were seeded in 6-well plates (3×104
cells/well), cultured overnight and treated with culture medium
containing 10% exudate from Ca(OH)2, MTA, iRoot, PRF or
CGF. The same volume of culture medium was used for the control. On
days 1, 3 and 7, the cells were trypsinized, transferred into
sampling tubes, and reagent A, B and C was added successively,
according to the instructions of the Cell Cycle Analysis kit
(NewMed Cytomics, Co. Ltd.). Cells were then analyzed on a flow
cytometer using BD FACS™ software (version 1.0.0.650; BD
Biosciences). The data were analyzed using Modfit software 3.1
(Verity Software House).
Alkaline phosphatase (ALP)
activity
HDPC mineralization was evaluated by measuring the
ALP activity. HDPCs were seeded in 96-well plates (1×103
cells/well), cultured overnight and treated with
Ca(OH)2, MTA, iRoot, PRF or CGF exudates for 1, 3 or 7
days. α-MEM was used for the control. To quantitatively assess the
ALP activity of cells, an ALP Assay kit (Beyotime Institute of
Biotechnology) was used according to the manufacturer's protocol.
The absorbance at indicated time-points was measured at 405 nm
using a 96-well plate reader (Thermo Fisher Scientific, Inc.). Each
assay was performed in three wells and the experiments were
performed in triplicate.
Statistical analysis
SPSS 22.0 software (IBM Corp.). Statistical analysis
was performed using one-way analysis of variance followed by
Tukey's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cell viability and proliferation
The HDPCs exhibited a fibroblastic appearance and
epithelioid shapes (Fig. 1).
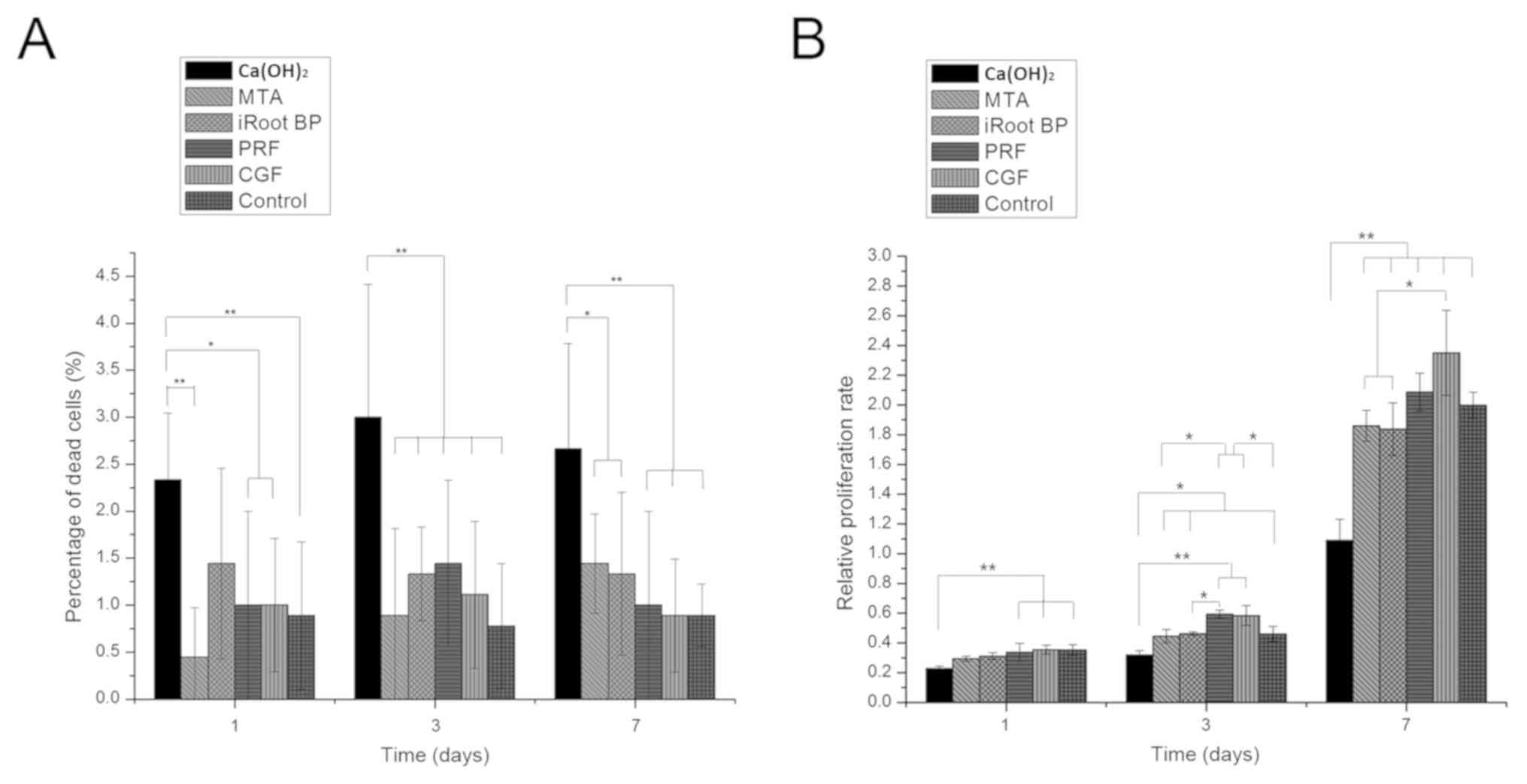
According to the results of the trypan blue exclusion assay, nearly
all detected materials except Ca(OH)2 displayed low
toxicity to HDPC. A higher percentage of dead cells was detected in
the Ca(OH)2 group as compared with that in the other
five groups on days 1, 3 and 7 (P<0.05; Fig. 2A). The results of the CCK-8 assay
suggested that Ca(OH)2 significantly inhibited HDPC
growth and proliferation as compared with the control group on days
1, 3 and 7 (P<0.05). In contrast, CGF and PRF significantly
promoted cell proliferation compared with the control group on days
3 (P<0.05). In the PRF group, the proliferation rate was higher
as compared with that in the MTA and iRoot BP groups on day 3
(P<0.05). However, no significant differences were identified
between MTA and iRoot BP or CGF and PRF on days 1, 3 and 7
(Fig. 2B).
Apoptotic rate
According to the results of the Annexin V assay,
there was a higher percentage of apoptotic cells in the
Ca(OH)2 group, as compared with that in the MTA, iRoot
BP and CGF groups on days 1, 3 and 7 (P<0.05).
Ca(OH)2 treatment also increased the apoptotic rate as
compared with PRF on days 3 and 7 (P<0.05). However, no
significant differences were identified among the iRoot BP, MTA,
CGF and PRF groups at any of the time-points (Fig. 3).
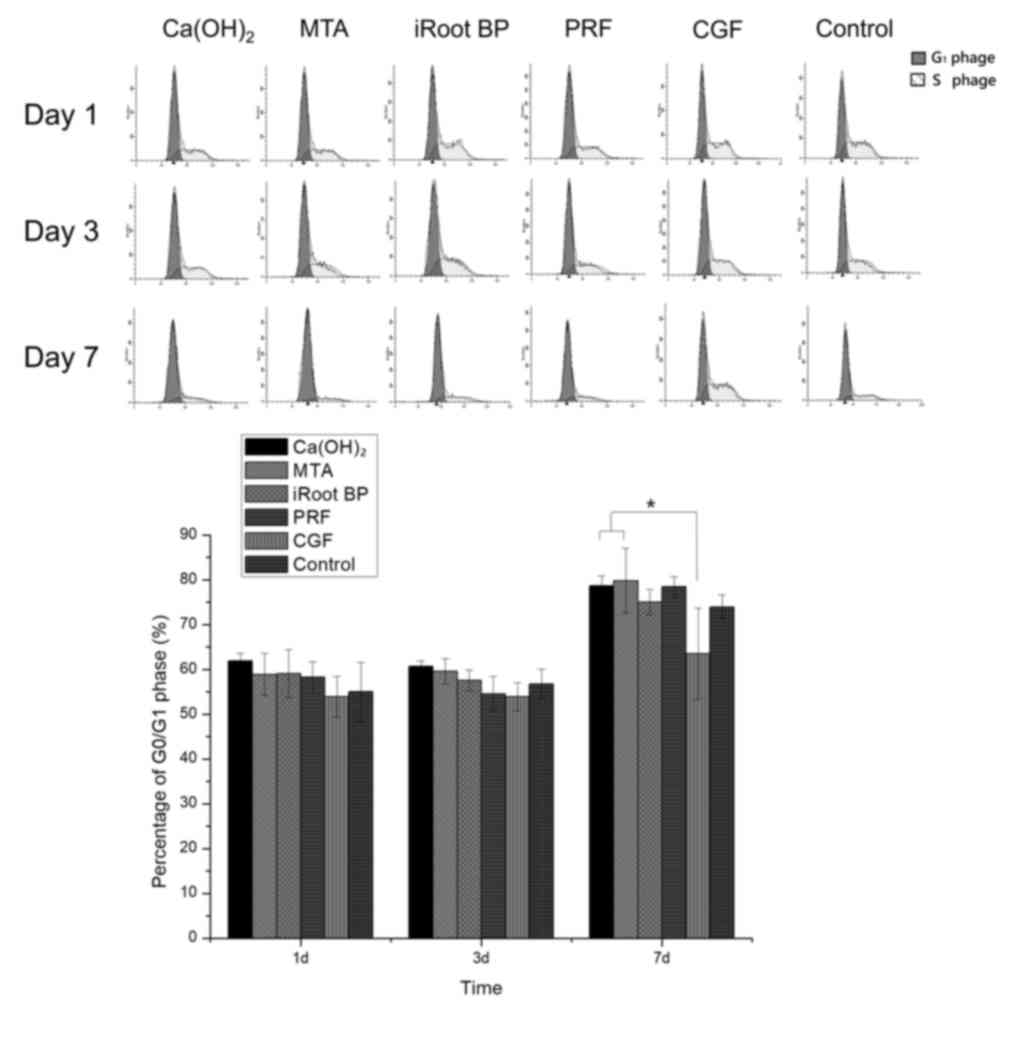
Cell cycle
The cell cycle analysis revealed that the CGF
groups, there were less cells in G0/G1-phase
as compared with those in the Ca(OH)2 and MTA groups on
day 7 (P<0.05). No significant differences were identified among
the six groups on days 1 and 3 (Fig.
4).
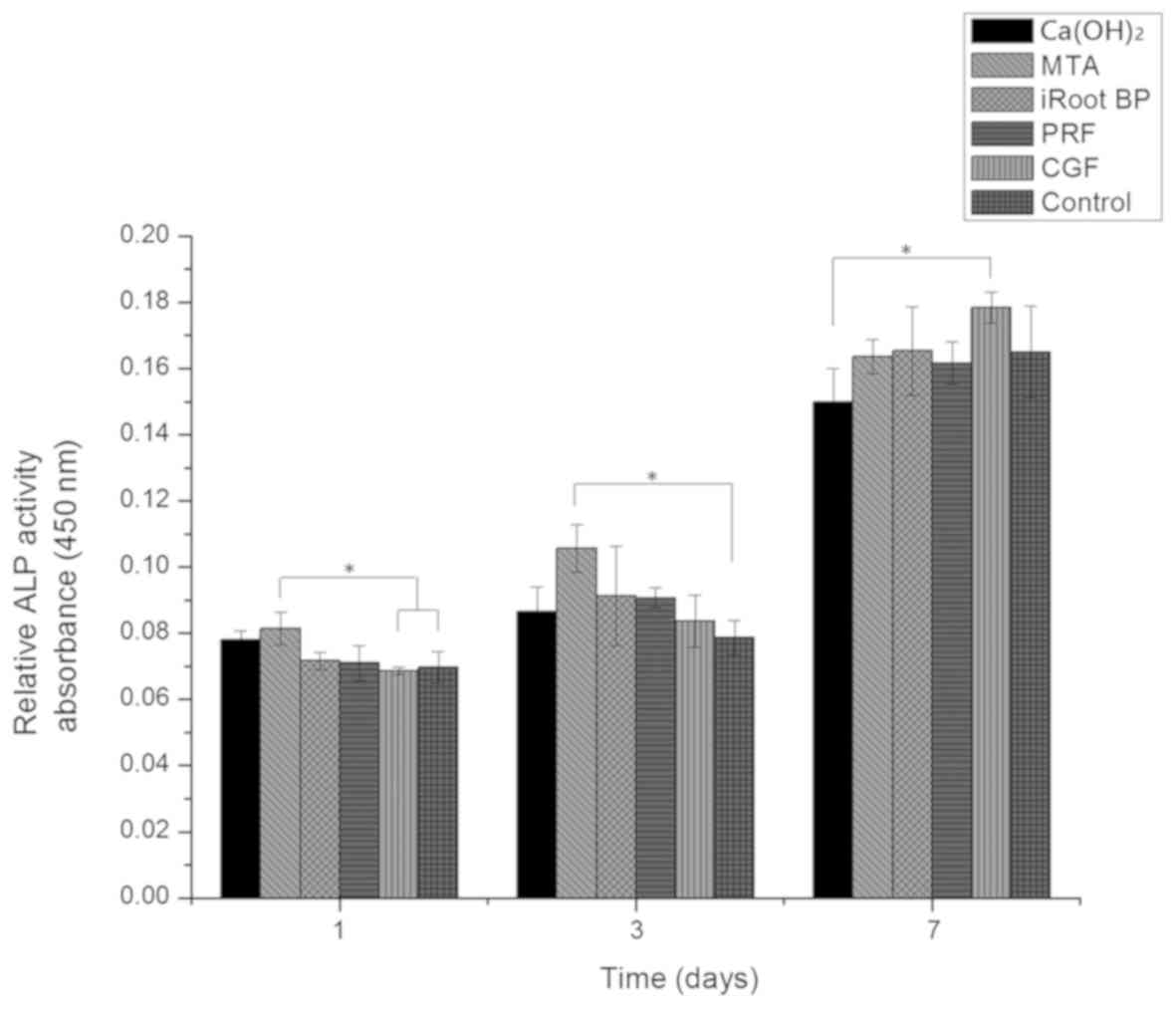
ALP activity
On day 1, the highest level of ALP activity was
observed in the MTA group; MTA significantly enhanced ALP activity
as compared with that in the CGF and control groups (P<0.05). On
day 3, the level of ALP activity in the MTA group was higher
compared with that in the control groups (P<0.05). On day 7, the
ALP activity in the CGF group increased and was significantly
higher than that in the Ca(OH)2 group (P<0.05;
Fig. 5). No significant differences
were observed between any other two groups.
Discussion
The results of the present study provided important
information on the biocompatibility and cytotoxicity of three
commercially available and two potential materials for vital pulp
capping. All materials except for Ca(OH)2 demonstrated
good biocompatibility with HDPCs. None of MTA, iRoot BP, PRF and
CGF significantly affected cell viability, cell death and
apoptosis, as compared with the control group. Of note, the
toxicity of Ca(OH)2 to HDPCs was relatively higher than
that of the other materials. Ca(OH)2 significantly
increased the number of dead or apoptotic cells and inhibited the
proliferation of HDPCs. With regard to cytotoxicity and
biocompatibility, MTA, iRoot BP, PRF and CGF qualify for direct
dental pulp-capping in vital pulp preservation. These results may
assist clinicians in applying and developing vital pulp
preservation therapies.
In the present study, three commercial dental
materials available in dental clinics throughout China, as well as
two potential materials for vital pulp capping were assessed.
Although PRF and CGF have not been clinically applied in direct
pulp capping or pulpotomy, they have potential as pulp-capping
agents. These materials are isolated from autologous blood and are
highly biocompatible. Their cytotoxicity is negligible and they
have previously been proved to be effective for bone repair
(9). As natural scaffolds, PRF and
CGF are degradable and may be replaced by newly-formed tissue
(10). Different from platelet-rich
plasma (PRP) as the 1st-generation platelet-derived product, the
addition of dissimilar thrombin and anti-coagulant was not
necessary for the preparation of PRF and CGF. The application of
PRF or CGF does not pose any risks for immune rejection and
transmission of infectious diseases. PRP was not considered a
potential pulp-capping material in the present study, since the
additive during PRP preparation may cause adverse effects (8). The biocompatibility of PRF and CGF on
HDPCs has been confirmed in the present in vitro study. PRF
and CGF were as effective as the widely-accepted MTA when in direct
contact with the cells, and were even superior to MTA in promoting
cell proliferation. Certain studies also indicated that PRF
increases HDPC proliferation and differentiation (5,11), which
is consistent with the results of the present study. PRF has been
applied in vital pulp therapy in a preclinical trial (12) and a case report (13), where good outcomes were suggested.
The application of CGF in vital pulp therapy is rare and the effect
of CGF on HDPC has remained to be determined. The present study
demonstrated that CGF exhibited perfect biocompatibility with HDPC
and compared CGF with other commercial pulp-capping materials. The
results of the present study supported the application potential of
CGF and PRF in vitro. Further in vivo studies are
required.
HDPC mineralization was evaluated by measuring ALP
activity. ALP activity is a vital test index for mineralization. A
higher level of ALP activity usually correlates with a higher
capacity for dentine bridge formation, which is important in direct
pulp capping (14). PRF and CGF have
a similar capacity to promote mineralization to that of MTA,
implying that PRF and CGF may have a positive effect on reparative
dentin formation. iRoot BP displayed an equal capacity to induce
mineralization as MTA, which was consistent with the results of
previous studies (4,14). The study by Zhang et al
(14) demonstrated that iRoot BP and
MTA optimized the mineralization ability of HDPC, while another
study reported on the use of iRoot BP to achieve favorable effects
on reparative dentin formation in vivo (4). iRoot BP is more convenient to use than
MTA and Ca(OH)2, and may be considered an alternative to
MTA as a pulp-capping agent.
In the present study, the cytotoxicity of five
pulp-capping materials was evaluated. Of note, HDPCs were treated
with the exudates of these materials, a method also adopted in
previous studies (15–17). Another study assessed the
cytotoxicity of pulp-capping materials by directly seeding the
cells onto the materials evaluating the cytotoxicity (14). In a preliminary experiment for the
present study, it was attempted to pack these materials at the
bottom of 96-well plates and seed the cells directly onto the
packed materials. However, due to the opaque nature of the
materials, it was impossible to observe the condition of the seeded
cells under the microscope. In addition, a large number of cells
appeared to die when in direct contact with Ca(OH)2.
Furthermore, it was considerably difficult to digest and collect
all cells in the dishes, particularly the cells that were partly
inside the materials. Under these circumstances, it was not
possible to accurately determine the total number of viable cells
and percentages of dead and apoptotic cells. Therefore, in the
present study, HDPCs were treated with exudates from different
materials, similar to previous studies (8,17).
Different from the calcium and silicon ions released
by MTA and iRoot BP, PRF and CGF are able to directly release a
variety of growth factors closely involved in the processes of cell
growth, proliferation, pro-inflammation and angiogenesis (18). The released factors contain
transforming growth factor-β, platelet-derived growth factor,
vascular endothelial growth factor, interleukin-1β and
interleukin-6 (8). When placed on
the exposed dental pulp tissue, PRF and CGF are expected to
function not only as a scaffolding material but also as a reservoir
to deliver certain growth factors and pro-inflammatory cytokines at
the implantation sites. In addition, PRF and CGF are collected from
autologous blood, which, in theory, means that they cannot cause
any rejective reactions.
Previous studies investigated the cytotoxicity of
MTA, Ca(OH)2 and iRoot BP to HDPCs, as well as the
effectiveness of these materials in vivo. Ca(OH)2
may cause necrosis and apoptosis of nearby cells and create an
alkaline environment to induce dentine bridge formation (19). MTA has a higher success rate and
results in a lesser pulpal inflammatory response and more
predictable hard dentin bridge formation than Ca(OH)2
(20,21). MTA and iRoot BP exhibited acceptable
biocompatibility to HDPC in vitro (14) and a similar efficacy in vital
pulpotomy treatment (22). The
present study compared these materials simultaneously and provided
data for dental clinicians.
There are certain limitations. In the present study,
a pre-fabricated mould was also designed to guarantee the identical
volume of different materials used. Although the concentrations of
different materials were consistent, it is still necessary to
compare the influence of different concentrations of materials.
Furthermore, the maximum observation time was 7 days, which
referred to certain previous studies (17,23).
Inevitable passaging of cells in vitro may limit the
evaluation time. In vitro study usually comprises evaluation
of the toxicity of these materials in the short term. In the
future, in vivo studies will be performed for long-term
evaluation.
In conclusion, under the experimental conditions of
the present study, all of the five materials except
Ca(OH)2 were indicated to be biocompatible with HDPC.
PRF and CGF are potential pulp-capping materials for vital pulp
therapy. Further study on the effectiveness of PRF and CGF as vital
pulp-capping agents in vivo is required.
Acknowledgements
Not applicable.
Funding
The study was supported by the National Natural
Science Foundation of China (grant nos. 81800958 and 31571508) and
Yuzhong District Science and Technology Commission in Chongqing
(grant no. 20180119).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DY conceived and designed the experiments; LD and QY
performed the experiments and analyzed the data; LD wrote the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Chongqing Medical University (Chongqing, China) and
written informed consent was obtained from each donor.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mente J, Hufnagel S, Leo M, Michel A,
Gehrig H, Panagidis D, Saure D and Pfefferle T: Treatment outcome
of mineral trioxide aggregate or calcium hydroxide direct pulp
capping: Long-term results. J Endod. 40:1746–1751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parirokh M and Torabinejad M: Mineral
trioxide aggregate: A comprehensive literature review-part I:
Chemical, physical, and antibacterial properties. J Endod.
36:16–27. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi S, Bao ZF, Liu Y, Zhang DD, Chen X,
Jiang LM and Zhong M: Comparison of in vivo dental pulp responses
to capping with iRoot BP Plus and mineral trioxide aggregate. Int
Endod J. 49:154–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu S, Wang S and Dong Y: Evaluation of a
bioceramic as a pulp capping agent in vitro and in vivo. J Endod.
41:652–657. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang FM, Yang SF, Zhao JH and Chang YC:
Platelet-rich fibrin increases proliferation and differentiation of
human dental pulp cells. J Endod. 36:1628–1632. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Durmuşlar MC, Balli U, Dede FÖ, Misir AF,
Bariş E, Kürkçü M and Kahraman SA: Histological evaluation of the
effect of concentrated growth factor on bone healing. J Craniofac
Surg. 27:1494–716. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dou L, Yan Q, Liang P, Zhou P, Zhang Y and
Ji P: iTRAQ-based proteomic analysis explore the influence of
hypoxia on the proteome of dental pulp stem cells under 3D culture.
Proteomics. 182018.
|
|
8
|
Masuki H, Okudera T, Watanebe T, Suzuki M,
Nishiyama K, Okudera H, Nakata K, Uematsu K, Su CY and Kawase T:
Growth factor and pro-inflammatory cytokine contents in
platelet-rich plasma (PRP), plasma rich in growth factors (PRGF),
advanced platelet-rich fibrin (A-PRF), and concentrated growth
factors (CGF). Int J Implant Dent. 2:192016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takeda Y, Katsutoshi K, Matsuzaka K and
Inoue T: The effect of concentrated growth factor on rat bone
marrow cells in vitro and on calvarial bone healing in vivo. Int J
Oral Maxillofac Implants. 30:1187–1196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Isobe K, Watanebe T, Kawabata H, Kitamura
Y, Okudera T, Okudera H, Uematsu K, Okuda K, Nakata K, Tanaka T and
Kawase T: Mechanical and degradation properties of advanced
platelet-rich fibrin (A-PRF), concentrated growth factors (CGF),
and platelet-poor plasma-derived fibrin (PPTF). Int J Implant Dent.
3:172017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen YJ, Zhao YH, Zhao YJ, Liu NX, Lv X,
Li Q, Chen FM and Zhang M: Potential dental pulp revascularization
and odonto-/osteogenic capacity of a novel transplant combined with
dental pulp stem cells and platelet-rich fibrin. Cell Tissue Res.
361:439–455. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Keswani D, Pandey RK, Ansari A and Gupta
S: Comparative evaluation of platelet-rich fibrin and mineral
trioxide aggregate as pulpotomy agents in permanent teeth with
incomplete root development: A randomized controlled trial. J
Endod. 40:599–605. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hiremath H, Saikalyan S, Kulkarni SS and
Hiremath V: Second-generation platelet concentrate (PRF) as a
pulpotomy medicament in a permanent molar with pulpitis: A case
report. Int Endod J. 45:105–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang S, Yang X and Fan M: BioAggregate
and iRoot BP Plus optimize the proliferation and mineralization
ability of human dental pulp cells. Int Endod J. 46:923–929. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Öncel Torun Z, Torun D, Demirkaya K, Yavuz
ST, Elçi MP, Sarper M and Avcu F: Effects of iRoot BP and white
mineral trioxide aggregate on cell viability and the expression of
genes associated with mineralization. Int Endod J. 48:986–993.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiu W, Sun B, He F and Zhang Y:
MTA-induced Notch activation enhances the proliferation of human
dental pulp cells by inhibiting autophagic flux. Int Endod J. 50
(Suppl 2):e52–e62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saeed MA, El-Rahman MA, Helal ME, Zaher AR
and Grawish ME: Efficacy of human platelet rich fibrin exudate vs.
fetal bovine serum on proliferation and differentiation of dental
pulp stem cells. Int J Stem Cells. 10:38–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qiao J, An N and Ouyang X: Quantification
of growth factors in different platelet concentrates. Platelets.
28:774–778. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luczaj-Cepowicz E, Marczuk-Kolada G,
Pawinska M, Obidzinska M and Holownia A: Evaluation of cytotoxicity
and pH changes generated by various dental pulp capping
materials-an in vitro study. Folia Histochem Cytobiol. 55:86–93.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Z, Cao L, Fan M and Xu Q: Direct Pulp
capping with calcium hydroxide or mineral trioxide aggregate: A
meta-analysis. J Endod. 41:1412–1417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kundzina R, Stangvaltaite L, Eriksen HM
and Kerosuo E: Capping carious exposures in adults: A randomized
controlled trial investigating mineral trioxide aggregate versus
calcium hydroxide. Int Endod J. 50:924–932. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Azimi S, Fazlyab M, Sadri D, Saghiri MA,
Khosravanifard B and Asgary S: Comparison of pulp response to
mineral trioxide aggregate and a bioceramic paste in partial
pulpotomy of sound human premolars: A randomized controlled trial.
Int Endod J. 47:873–881. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu T, Shi Z, Song H, Li Y and Li JH:
Cytotoxicity of local anesthetics on rabbit adipose-derived
mesenchymal stem cells during early chondrogenic differentiation.
Exp Ther Med. 16:2843–2850. 2018.PubMed/NCBI
|