Introduction
Colon cancer is a common malignant tumor of the
digestive tract that can improve with lifestyle and diet
modifications (1). Previously, there
has been a decline in the incidence of CRC in adults older than 50
years in the United States; however, the number of new cases is
expected to increase among young adults aged 20–49 years by 2030
(2). The 5-year survival rate for
colon cancer is relatively high, however, the high recurrence rate
and metastasis of the tumor to distant organs is the primary cause
of mortality in patients with this disease (3,4). In
recent years, colon cancer has become one of the main causes of
cancer-associated mortality (5).
Treatments used for colon cancer include surgery, radio- and
chemotherapy. With the development of technology, these traditional
methods have undergone a number of improvements (6,7).
However, these improvements are insufficient in meeting clinical
needs, including prolonging survival, preventing cancer metastasis,
and reducing recurrence rates (4).
To achieve an increased survival rate and improved treatment
efficacy, the development and evaluation of phytochemicals that
exhibit anticancer properties is urgently required.
Luteolin (3′,4′,5,7-tetrahydroxy-flavone) is an
important flavonoid that can be found in honeysuckle,
chrysanthemum, nepeta and Prunella vulgaris (8). Celery, sweet pepper, Chinese cabbage,
cauliflower and Camellia sinensis also contain large
quantities of luteolin (9). Luteolin
has been revealed to exhibit anti-inflammatory, antioxidative and
anticancer properties (9). Luteolin
has also been reported to decrease serum glucose and exhibit a
number of other pharmacological activities (8,10,11).
Studies have demonstrated that luteolin can provide resistance
against oncogenic stimulation in vivo and in vitro,
inhibit cell proliferation, and induce cell cycle arrest and
apoptosis by stimulating or inhibiting intracellular and
extracellular signaling pathways (12,13).
Furthermore, the efficacy of luteolin in treating colon cancer has
been previously reported (14,15).
Luteolin has been indicated to induce apoptosis in colon cancer
cells by arresting the cell cycle at the G2/M phase (11,13).
Recent research has revealed that the molecular mechanisms
underlying the luteolin-induced apoptosis of colon cancer cells is
associated with the inhibition of the Wnt/β-catenin/glycogen
synthase kinase-3β (16) and
phosphatidylinositol 3-kinase/Akt signaling pathways (17), reduction of antioxidant capacity
(16) and the induction of changes
in the ceramide/sphingosine-1-phosphate ratio (18).
A variety of drugs exert an anticancer effect by
increasing the level of reactive oxygen species (ROS) and
activating the mitochondrial apoptosis pathway (19,20). It
has been indicated that the nuclear factor erythroid 2-related
factor 2 (Nrf2)/antioxidant responsive element (ARE) signaling
pathway is an important pathway during the cellular antioxidant
response (21). Furthermore, it has
been demonstrated that the regulation of antioxidant enzymes and
phase II detoxification enzymes via this signaling pathway can
result in the scavenging of ROS and other harmful substances
(22).
The current study was performed to investigate
whether luteolin induces mitochondrial apoptosis in the colon
cancer cell line HT29 by inhibiting the Nrf2/ARE signaling
pathway.
Materials and methods
Cells and reagents
HT29 cells were purchased from the Cell Bank of Type
Culture Collection of Chinese Academy of Sciences.
Luteolin was purchased from Dalian Meilun Biology
Technology Co., Ltd. MTT (cat. no. 298-93-1), DMSO (cat. no.
68-67-5), FBS and high-glucose DMEM were purchased from Beijing
Solarbio Science & Technology Co., Ltd.
Dichloro-dihydro-fluorescein diacetate (DCFH-DA, cat. no. d6883)
was obtained from Sigma-Aldrich; Merck KGaA. RIPA buffer (cat. no.
P0013C), SDS-PAGE gel preparation kit (cat. no. P0012A) and
Mitochondrial Membrane Potential Detection kit (cat. no. C2006)
were purchased from Beyotime Institute of Biotechnology. RNAiso
Plus (cat. no. 9108; Takara Biotechnology Co., Ltd.), the
PrimeScript RT Reagent kit (cat. no. rr047a) and TB Green Premix Ex
Taq II kit (cat. no. rr820l) were obtained from Takara Bio, Inc.,
rabbit anti-cytochrome c (cyt C) monoclonal antibody (1:2,500; cat.
no. ab133504), rabbit anti-caspase-3 monoclonal antibody (1:500;
cat. no. ab197202), rabbit anti-p47phox monoclonal
antibody (1:2,500; cat. no. ab181090), rabbit
anti-p22phox monoclonal antibody (1:2,000; cat. no.
ab191512), rabbit β-actin antibody (1:1,000; cat. no. bs-0061R),
goat anti-rabbit IgG-HRP (H+L) secondary antibody (1:1,000; cat.
no. E030120), rabbit anti-Nrf2 monoclonal antibody (1:250; cat. no.
ab62352) and Alexa Fluor® 647-labeled goat anti-rabbit
fluorescent secondary antibody (1:500; cat. no. ab150079) was
purchased from Abcam. The primers used in the RT-qPCR were as
follows: Bax forward, 5′-CATGGAGCTGCAGAGGATGA-3′ and reverse,
5′-CTCCCGGAGGAAGTCCAAT-3′ (NG_012191; length 318); Bcl-2 forward,
5′-AGGATTGTGGCCTTCTTTGAGT-3′ and reverse,
5′-ACTGCTTTAGTGAACCTTTTGCAT-3′ (NG_009361; length 335) and β-actin
forward, 5′-CGCGAGAAGATGACCCAGAT-3′ and reverse,
5′-GCACTGTGTTGGCGTACAGG-3′ (NG_007992; length 550).
MTT assay
Cells in the log growth phase were digested to
obtain a single-cell suspension with a density of
1.5×105 cells/ml. Subsequently, 100 µl cell suspension
cultured in high-glucose DMEM containing 10% FBS was added to each
well of a 96-well plate, after which the plate was incubated for 24
h (37°C; 5% CO2). Luteolin was added to the wells at a
final concentration of 10, 20, 40, 80 or 160 µM, with six parallel
wells for each group. 100 µl of solvent (0.1% DMSO) was added to
the blank control cells. Cells were treated for 24, 48 or 72 h. A
total of 20 µl MTT solution (5 mg/ml) was subsequently added to
each well, after which the plate was incubated at 37°C for an
additional 4 h. The supernatant was discarded and the MTT in each
well was dissolved in 150 µl DMSO. Optical density (OD) was
measured at 490 nm and inhibition rate (IR) was calculated as
follows: IR(%)=[OD(blank control)-OD(experimental group)]/OD(blank
control) × blank. The half-maximal inhibitory concentration
(IC50) values of luteolin on HT29 cells were determined
by a plotting dose-response curve.
Measurement of intracellular ROS
level
A cell suspension containing 5×105
cells/ml was prepared from HT29 cells in the log growth phase. A
total of 5×104 cells/well were inoculated into a 6-well
plate and cultured overnight. After the cells adhered to the plate,
20 or 40 µM luteolin was added, and an equal volume of DMSO was
added to the blank control cells. After 48 h, the culture medium
(high-glucose DMEM containing 10% FBS) was aspirated. A total of 10
µmol/l DCFH-DA serum-free medium was then added to the culture. The
mixture was lightly agitated until all the cells were covered,
followed by incubation at 37°C for 20 min. Cells were then rapidly
washed three times with serum-free medium to remove DCFH-DA that
did not enter the cells. Finally, a total of 1×103
cells/field of view were imaged using a fluorescence microscope
under ×20 magnification. The fluorescence intensity was measured
(Image Pro Plus 6.0; Media Cybernetics) to determine the level of
intracellular ROS.
Measurement of mitochondrial membrane
potential
HT29 cells were treated as aforementioned. After 48
h of treatment with luteolin, the culture medium was aspirated and
the cells were washed once with PBS. A total of 1 ml culture medium
(high-glucose DMEM containing 10% FBS) and 1 ml JC-1 stain working
solution were successively added to the suspension, which was then
mixed thoroughly. Cells were incubated at 37°C for 20 min and
digested using trypsin. A multifunctional spectrophotometer was
used to measure absorbance at excitation and emission wavelengths
of 525 and 590 nm, respectively.
Immunofluorescence
HT29 cells in the log growth phase were digested
with trypsin to obtain a single-cell suspension containing
1×104 cells/ml. A total of 2 ml suspension was
subsequently placed in each well of a six-well plate with
coverslips at the bottom of each well. After adhesion, cells were
treated for 48 h with 20 or 40 µM luteolin. Cells were subsequently
fixed with 4% paraformaldehyde at 37°C for 30 min, permeabilized
with 0.3% Triton X-100 for 15 min at room temperature, blocked in
PBS containing 1% BSA for 1 h at 37°C, incubated with primary
antibodies against Nrf2 (1:250) overnight at 4°C, washed, treated
with Alexa Fluor® 647-labeled goat anti-rabbit
fluorescent secondary antibody (1:500) for 30 min at 37°C, washed
and imaged using a fluorescence microscope under ×200
magnification.
RT-qPCR
HT29 cells in the log growth phase were treated for
48 h with 20 or 40 µM luteolin or with an equal volume of vehicle
as the blank control. A total of 1.5 ml TRIzol reagent was
subsequently added to the suspension, which was then triturated on
ice, left to stand for 5 min and centrifuged at 13,000 × g for 5
min at 4°C. Supernatant was collected and 200 µl chloroform was
added. The mixture was left to stand for 5 min and then centrifuged
at 13,000 × g for 10 min at 4°C. The supernatant was collected and
400 µl isopropyl alcohol was added. The mixture was left to stand
and subsequently centrifuged at 13,000 × g for 10 min at 4°C. The
supernatant was discarded and the precipitate obtained was washed
with 75% ethanol. The mixture was then centrifuged to remove the
ethanol and the supernatant was discarded. The precipitate was
dried using super-clean bench at room temperature for 2 min, and
dissolved in 20 µl nuclease-free water at room temperature. Genomic
DNA removal and RT were performed using a reaction kit with the
following thermocycling conditions: 25°C for 5 min, 42°C for 30 min
and 85°C for 5 min. During qPCR, denaturation was performed at 95°C
for 10 min and amplification was performed at 95°C for 15 sec, 60°C
for 15 sec and 72°C for 30 sec for 40 cycles. Data were analyzed
using the 2−ΔΔCq method (23) and normalized to the internal
reference gene β-actin.
Western blot analysis
HT29 cells in the log growth phase were treated for
48 h with 20 or 40 µm luteolin or with an equal volume of vehicle
that was used as the blank control. RIPA buffer was added to the
cells on ice and cells were ground. The lysate was kept at 4°C for
30 min and subsequently centrifuged at 13,000 × g for 10 min at
4°C. Supernatant was collected and a BCA assay kit was used to
measure protein concentration. Afterwards, 4X loading buffer was
added the remaining supernatant, and the 30 µg of protein sample
was denatured and added to a 4% stacking/10% resolving gel. Samples
were then blotted onto a PVDF membrane, blocked with 5% skim milk
in TBS containing 0.05% Tween-20 (TBS-T) for 1 h at room
temperature, incubated with primary antibodies against cyt C
(1:2,500), caspase-3 (1:500), p47phox (1:2,500),
p22phox (1:2,000) and β-actin (1:1,000) overnight at 4°C
and incubated with secondary antibodies goat anti-rabbit IgG-HRP
(H+L) (1:1,000) for 1 h at room temperature. Enhanced
chemiluminescence, X-ray development, gel imaging (Bio-Rad ChemiDoc
XRS+ 170-8625; Bio-Rad Laboratories, Inc.) and image analysis
(ImageJ 1.8; National Institutes of Health) were subsequently
performed.
Statistical analysis
SPSS (version 21.0; IBM Corp.) was used for data
analysis. One-way ANOVA was used for comparison among multiple
groups with LSD post-hoc tests. P<0.05 was considered to
indicate a statistically significant result. Data are presented as
the mean ± SEM.
Results
The cytotoxicity of luteolin on HT29
cells
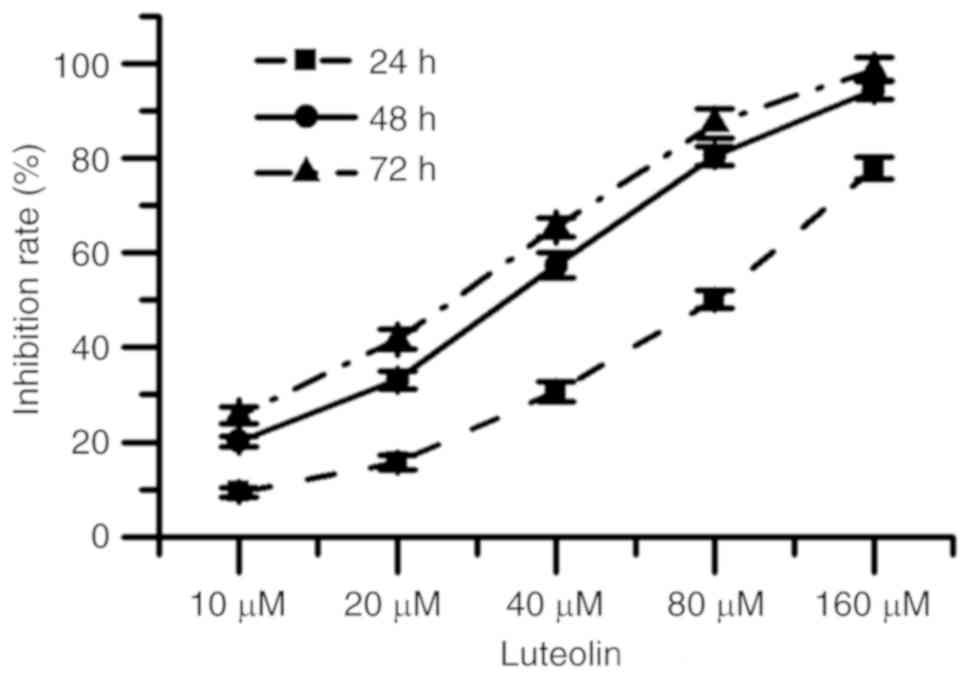
Fig. 1 indicated that
for each exposure period, as the concentration of luteolin was
increased, the cytotoxicity of luteolin on HT29 cell gradually
increased. The half-maximal inhibitory concentration
(IC50) values of luteolin were calculated as 69.66±3.42,
28.94±2.37 and 22.30±3.05 µM when cells were treated for 24, 48 and
72 h, respectively. Furthermore, for each concentration, the
cytotoxicity of luteolin gradually increased for the different
incubation periods.
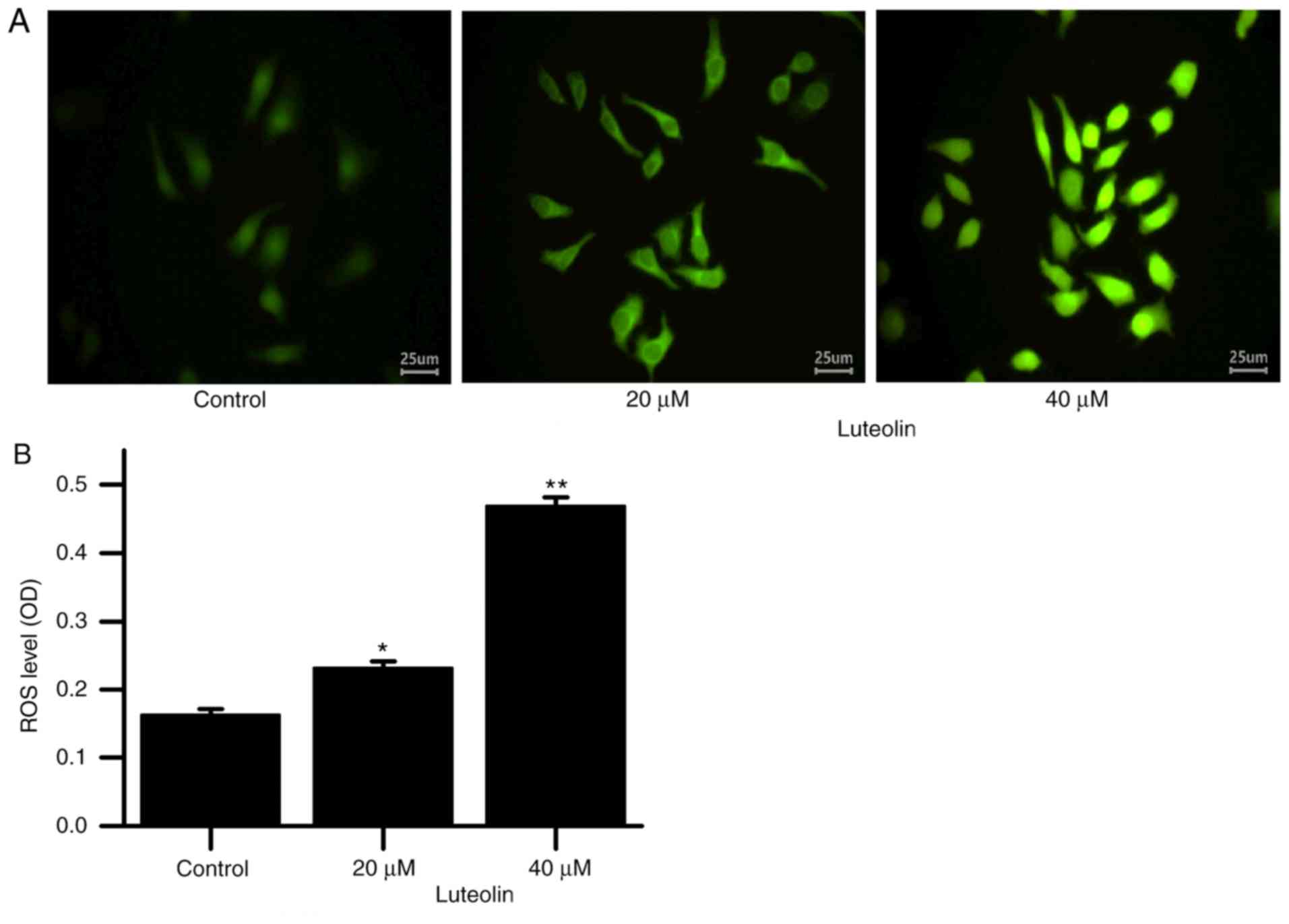
Effect of luteolin in HT29 cells
Fig. 2 demonstrated
that as the luteolin concentration was increased, the ROS level in
the HT29 cells increased.
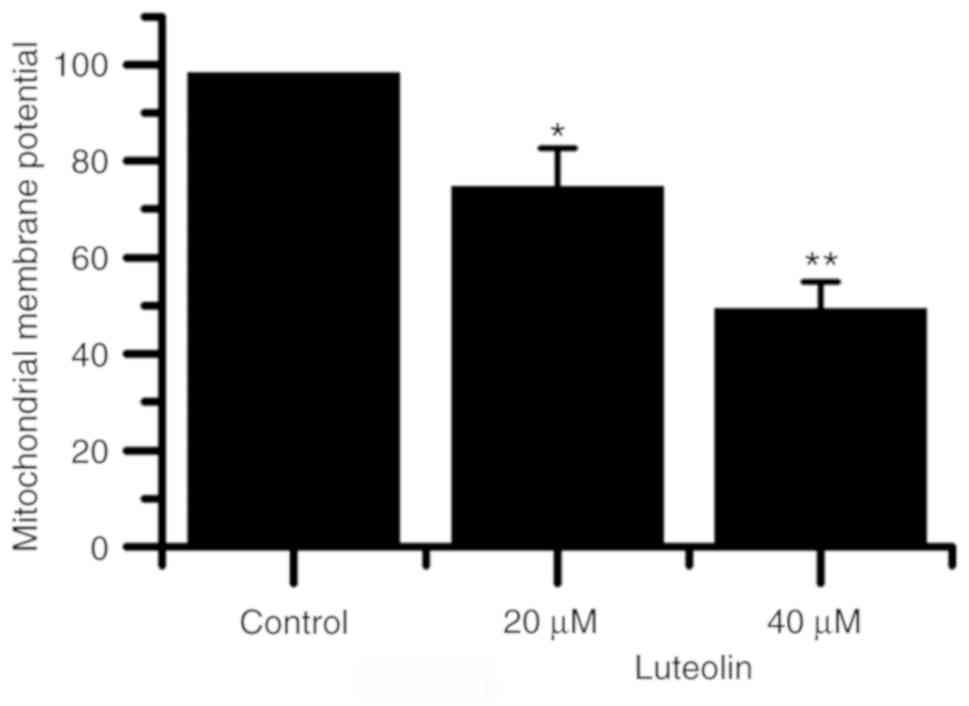
Fig. 3 indicated that
as the luteolin concentration was increased, the mitochondrial
membrane potential of HT29 cells decreased. The mitochondrial
membrane potential was indicated to be significantly lower in cells
treated with 20 and 40 µM luteolin compared with blank control
cells.
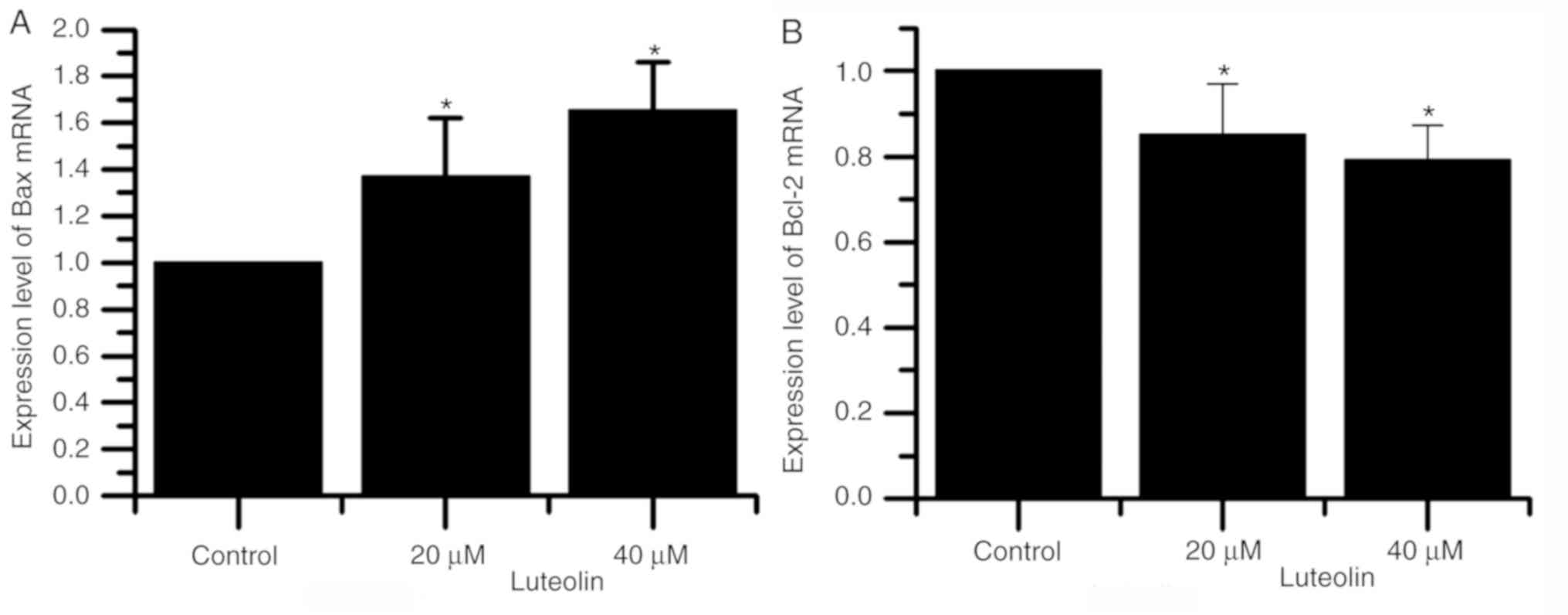
Fig. 4 demonstrated
that as the luteolin concentration was increased, mRNA expression
of Bax increased and of Bcl-2 decreased. These results indicated
that Bcl-2 can prevent multiple signaling pathways from blocking
cell apoptosis and prolonging cell survival. Bax is a mitochondrial
membrane protein that can promote or mediate apoptosis (24,25).
Additionally, the mRNA expression of Bax and Bcl-2 were
significantly different in the cells treated with 20 and 40 µM
compared with the respective blank control cells.
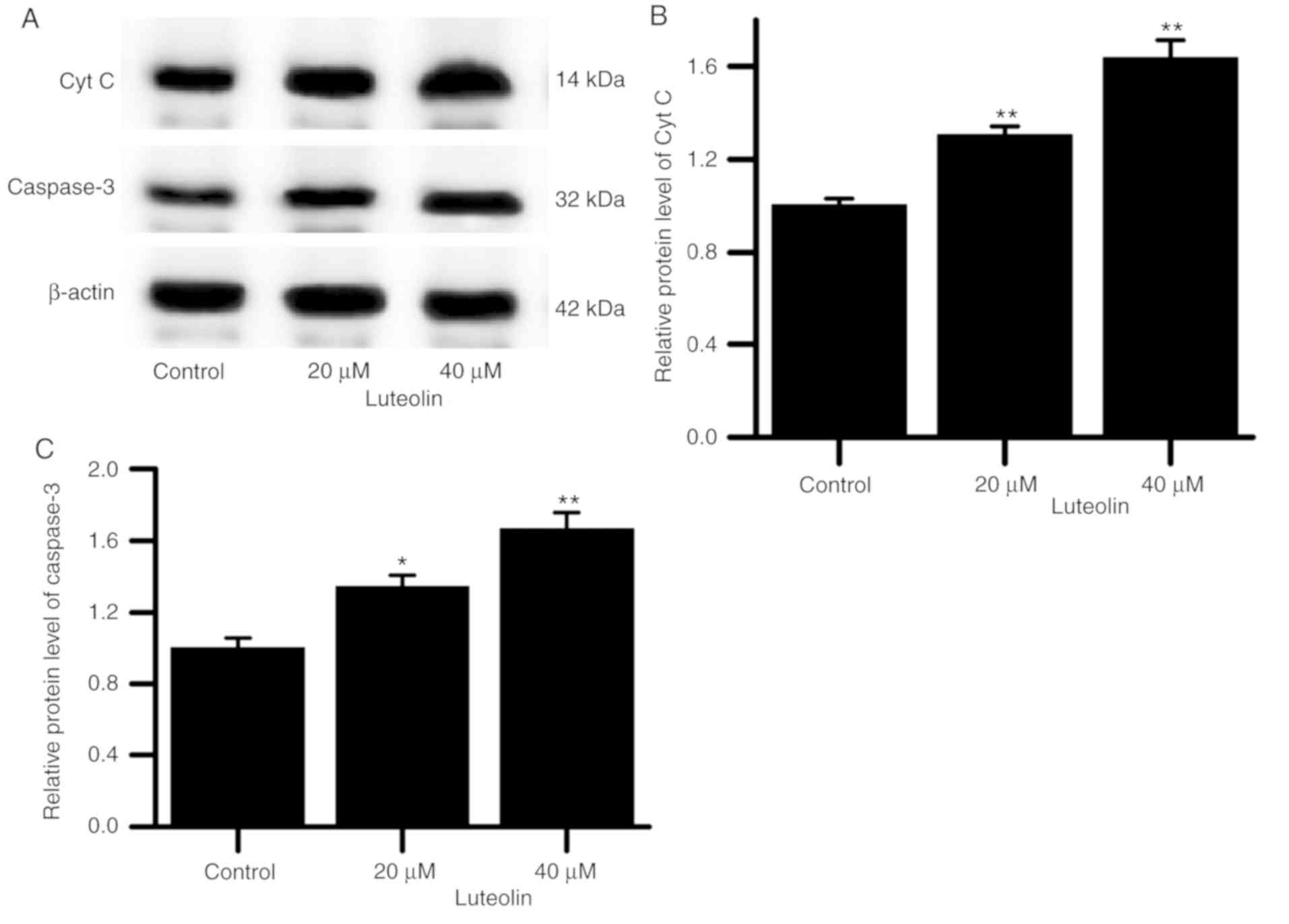
The results indicated that as the luteolin
concentration was increased, the protein expression of cyt C and
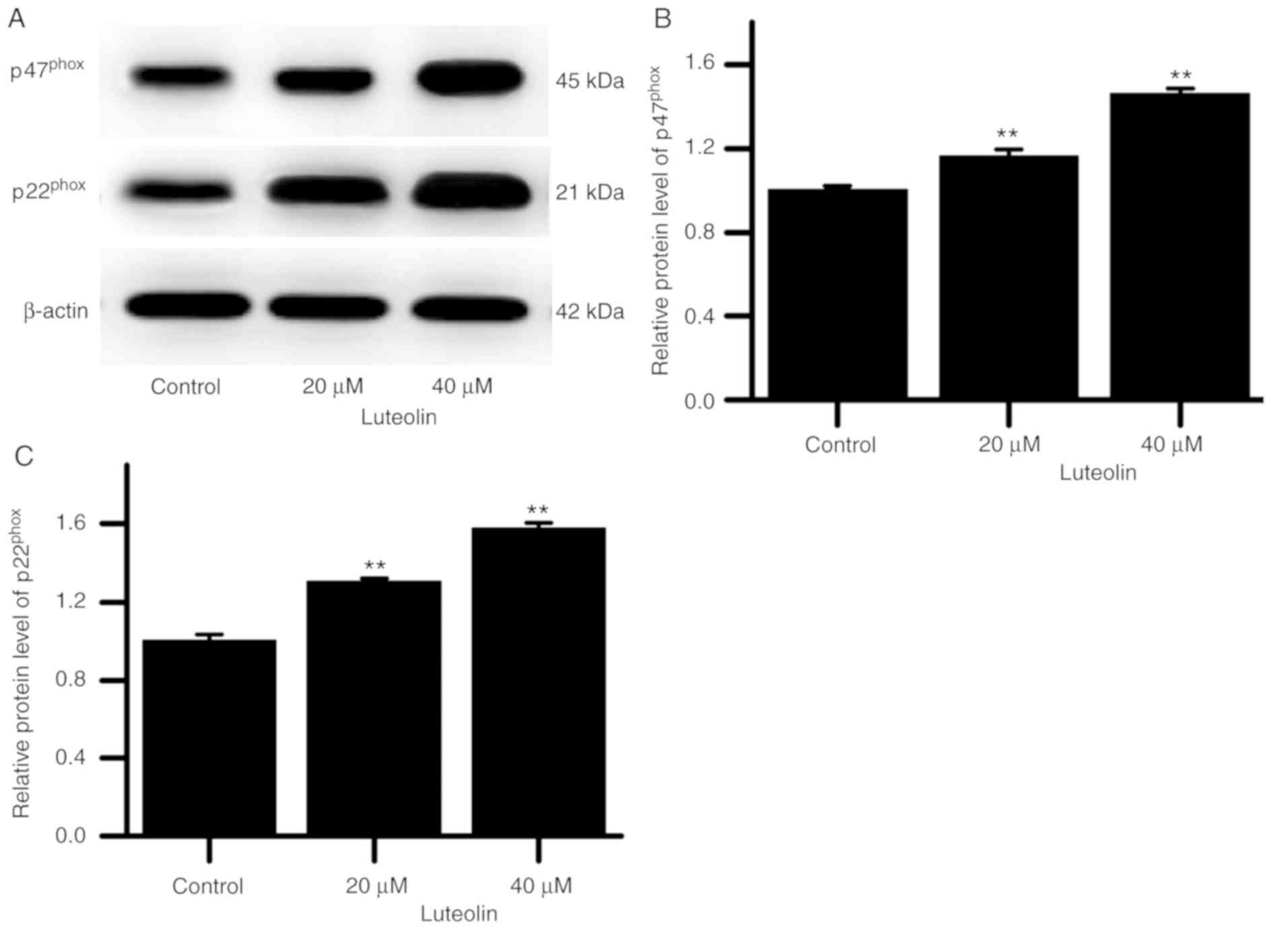
caspase-3 in the HT29 cells gradually increased (Fig. 5). Additionally, p47phox
and p22phox protein levels in the cells gradually
increased (Fig. 6).
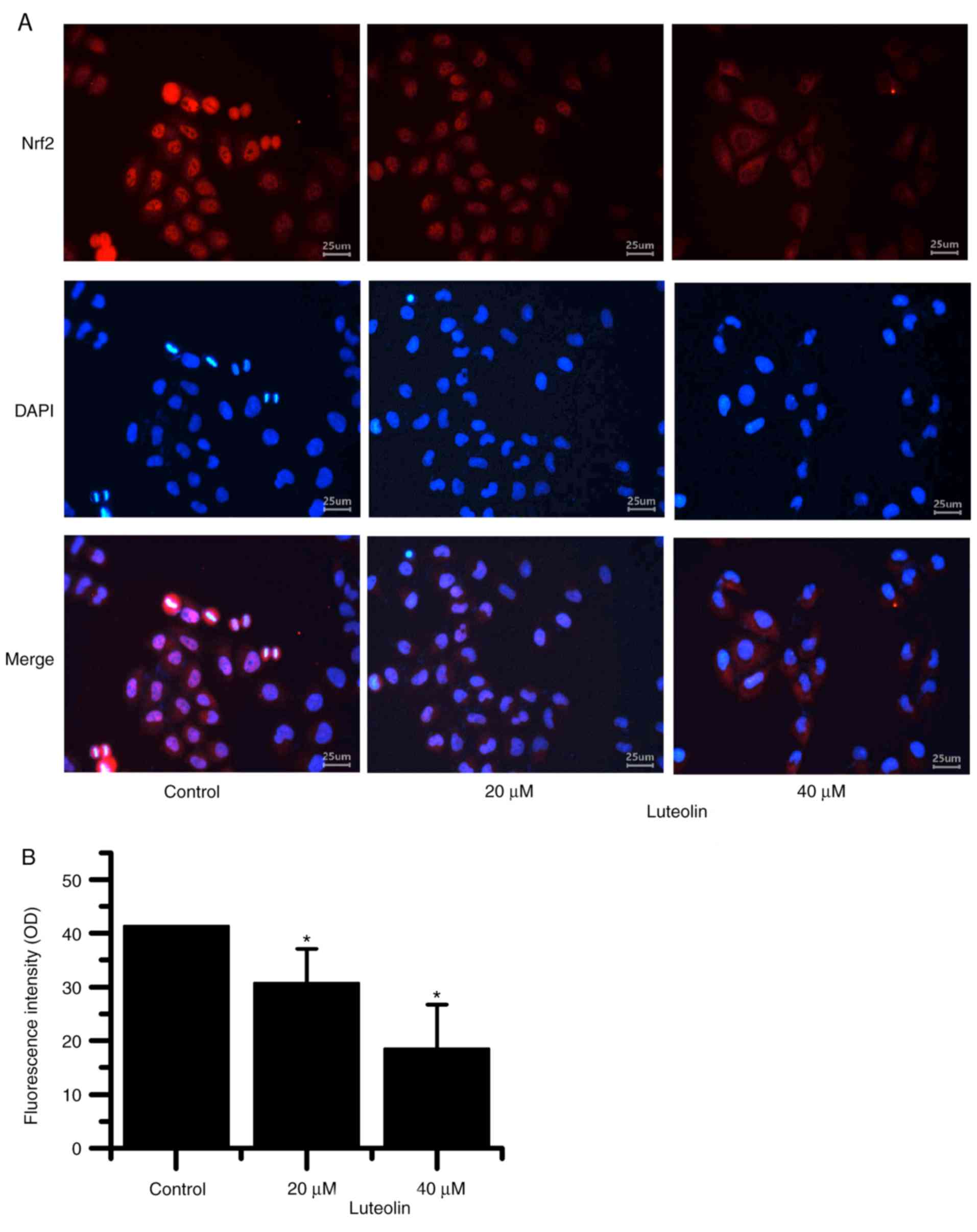
Fig. 7A and B
demonstrated that as the luteolin concentration increased, nuclear
Nrf2 localization in HT29 cells decreased.
Discussion
Dysregulated cell proliferation is a characteristic
of a large number of cancer types (26). Therefore, the induction of apoptosis
is a beneficial characteristic for anticancer drugs to exhibit.
Previous studies have demonstrated that resveratrol, matrine,
quercetin and a number of other phytochemicals induce apoptosis and
inhibit the proliferation of liver, stomach, oral, skin and colon
cancers (27–29). Luteolin is a flavonoid that has been
indicated to arrest the cell cycle and induce apoptosis in a
variety of different cancer types (13,14,27). In
the present study, the results of the MTT assay demonstrated that
luteolin exhibited cytotoxicity on HT29 cell in a concentration-
and time-dependent manner, which is consistent with the results of
other studies (17,30).
Signaling networks, including pathways associated
with cell proliferation, are modified in a number of cancer types,
which makes cells unable to regulate their properties (31). Dysregulated proliferation is the
basis of the development and progression of cancer, and it has been
revealed that apoptosis and cell cycle signaling pathways, and
proteins associated with these processes, are directly associated
with cancer cell proliferation (32,33).
Apoptosis may occur via the mitochondrial or death receptor pathway
(34). The majority of tumors avoid
intrinsic and extrinsic apoptosis by modifying the expression or
structure of proteins associated with apoptosis, which causes
cancer cells to exhibit higher error tolerance and resistance
(33). Therefore, the induction of
apoptosis is an important anticancer mechanism that can be
identified in chemotherapeutic agents.
During the initiation of apoptosis, an abnormal
increase in ROS occurs, which is an important stimulus for
mitochondrial apoptosis (35). It
has been demonstrated that a number of drugs induce apoptosis of
cancer cells by increasing ROS levels (36,37). In
the present study, it was indicated that luteolin induced an
increase in ROS level in HT29 cells. Changes in cellular ROS levels
are directly associated with changes in mitochondrial function,
with abnormalities in the electron permeability of the
mitochondrial membrane leading to significant changes in cellular
ROS levels (38). Additionally,
mitochondria are most vulnerable to ROS attack (39,40). In
the present study, luteolin decreased the mitochondrial membrane
potential in HT29 cells. Therefore, the luteolin-induced increase
in mitochondrial membrane permeability may result in increased ROS
levels in HT29 cells. However, the current study did not use normal
human colon cells as a control, which may lead to the inability of
the present study to directly determine whether luteolin induces
increased ROS in normal colon cells, which would allow for the
specificity and side effects of luteolin to be assessed accurately.
Therefore, future studies will aim to determine the effect of
luteolin on the levels of ROS and apoptosis in normal human colon
cells.
Changes in mitochondrial membrane permeability are
determined by the relative levels of Bcl-2 and Bax. In the
mitochondrial apoptotic pathway, Bax promotes apoptosis by damaging
mitochondrial membrane integrity, whereas Bcl-2 inhibits apoptosis
by maintaining the integrity of the mitochondrial membrane
(41). The results of the present
study indicated that luteolin increased Bax mRNA expression and
downregulated Bcl-2 mRNA expression. As mitochondrial membrane
permeability changes, the movement of cyt C from the mitochondria
to the cytoplasm is important in the initiation of the caspase
apoptosis cascade (42). The results
of the current study indicated that luteolin increased the
expression of cyt C and caspase-3 proteins in HT29 cells, and the
increase of cyt C and caspase-3 protein levels make HT29 cells more
sensitive to the intrinsic apoptotic pathway (43). The results of the current study
revealed that luteolin induced apoptosis of HT29 cells by
stimulating the mitochondrial apoptotic pathway. However, the
present study only demonstrated that luteolin induced mitochondrial
apoptosis in HT29 cells through the detection of mitochondrial
permeability and mitochondrial apoptosis-associated proteins and
did not identify the modification of mitochondria. The modification
of mitochondria can directly reflect the occurrence of
mitochondrial apoptosis (44).
Therefore, future studies may assess the modification of
mitochondria.
Luteolin can increase ROS levels in HT29 cells.
Increased endogenous cellular ROS levels are associated with ROS
production and clearance pathways (45). NADPH oxidase (NOX) is a membrane
protein that is widely distributed in tissues and organs (46). NOX reduces oxygen molecules in the
body to superoxide anions through NADPH-dependent electron transfer
(47). Furthermore, NOX has been
indicated to be responsible for ROS generation in the body
(48). NOX exhibits little catalytic
activity and binds to a number of regulatory subunits to form a
stable complex before it can exert any catalytic activity (49), including p47phox and
p22phox, which are important cofactors that are required
for the stability of NOX. In the present study, luteolin was
revealed to promote p47phox and p22phox
expression in HT29 cells. This result suggested that luteolin may
increase ROS levels in HT29 cells by increasing NOX stability.
Furthermore, cellular ROS levels determine the activity of the
antioxidant system (50). Recent
studies have demonstrated that the Nrf2/ARE signaling pathway is an
essential pathway during antioxidant response in cells (51). The regulation of antioxidant enzymes
and phase II detoxification enzymes by this signaling pathway can
result in the ROS scavenging, which can result in a detoxifying and
neutralizing effect (22).
The results of the present study also indicated that
luteolin inhibited Nrf2 activation, blocked nuclear localization of
Nrf2 and inhibited the expression of antioxidant enzymes.
Therefore, it was revealed that luteolin prevented Nrf2 activation
and promoted abnormal ROS level increases in HT29 cells by
modulating the expression of p47phox and
p22phox. ROS levels in cancer cells are high compared
with normal cells. Furthermore, in cancer cells that are adapted to
high ROS levels, further increases in ROS levels can promote cell
apoptosis.
However, other studies have demonstrated that
luteolin can induce the demethylation of the Nrf2 gene promoter
region, upregulate the Nrf2 gene expression, activate the Nrf2/ARE
pathway, increase the antioxidant capacity, inhibit the
transformation and promote apoptosis of colon cancer cells
(52,53), which is not supported by the results
of the present study. In the current study, it was speculated that
the antioxidant capacity served a different role at different
stages of cancer development. The increase of antioxidant capacity
reduces the level of ROS, protects cells from DNA damage caused by
oxidative stress, and inhibits further transformation of tumors.
Conversely, the improvement of antioxidant capacity exhibits an
increase drug resistance and oxidation resistance ability to tumor
cells, promoting the development of tumors.
In conclusion, luteolin induced apoptosis in HT29
cells by promoting ROS production and inhibiting ROS scavenging
through stimulating the mitochondrial apoptotic pathway. However,
the current study did not identify a useful luteolin inhibitor, and
the effects of luteolin on the proliferation, apoptosis and ROS
production of HT29 cells could not be fully identified. Future
studies will aim to identify a suitable luteolin inhibitor for use
in subsequent research.
Acknowledgements
Not applicable.
Funding
The current work was supported by the Scientific
Research Project of Gansu Health Industry (grant no.
GSWSKY2017-15).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FJX and NC designed the research. FJX performed the
MTT assay, measurement of intracellular ROS levels and measurement
of mitochondrial membrane potential. WLY performed
immunofluorescence experiments, RT-qPCR, and western blot analysis.
HY and BFL analyzed and interpreted the data and finalized the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and JEMAL A: Cancer
statistics, 2019. Ca Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bailey CE, Hu CY, You YN, Bednarski BK,
Rodriguez-Bigas MA, Skibber JM, Cantor SB and Chang GJ: Increasing
disparities in the age-related incidences of colon and rectal
cancers in the united states, 1975–2010. JAMA Surg. 150:17–22.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reddy BS: Metabolic epidemiology of colon
cancer. Oncology. 1991:88–98. 2015.
|
|
4
|
Aran V, Victorino AP, Thuler LC and
Ferreira CG: Colorectal cancer: Epidemiology, disease mechanisms
and interventions to reduce onset and mortality. Clin Colorectal
Cancer. 15:195–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cunningham D, Atkin W, Lenz HJ, Lynch HT,
Minsky B, Nordlinger B and Starling N: Colorectal cancer. Lancet.
375:1030–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang HF and Yang LL: Gamma-mangostin, a
micronutrient of mangosteen fruit, induces apoptosis in human colon
cancer cells. Molecules. 17:8010–8021. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scagliarini L, Anania G, Marino S,
Marchitelli I and Resta G: Treatment of colorectal cancer:
Multidisciplinay approach. Eur J Surg Oncol. 44:5552018. View Article : Google Scholar
|
|
8
|
Lin Y, Shi R, Wang X and Shen HM:
Luteolin, a flavonoid with potential for cancer prevention and
therapy. Current Cancer Drug Targets. 8:634–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lopez-Lazaro M: Distribution and
biological activities of the flavonoid luteolin. Mini Rev Med Chem.
9:31–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu T, Li D and Jiang D: Targeting cell
signaling and apoptotic pathways by luteolin: Cardioprotective role
in rat cardiomyocytes following ischemia/reperfusion. Nutrients.
4:2008–2019. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lim DY, Jeong Y, Tyner AL and Park JH:
Induction of cell cycle arrest and apoptosis in HT-29 human colon
cancer cells by the dietary compound luteolin. Am J Physiol
Gastrointest Liver Physiol. 292:G66–G75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu G, Li J, Yue J, Zhang S and Yunusi K:
Liposome encapsulated luteolin showed enhanced antitumor efficacy
to colorectal carcinoma. Mol Med Rep. 17:2456–2464. 2018.PubMed/NCBI
|
|
13
|
Chen Z, Zhang B, Gao F and Shi R:
Modulation of G2/M cell cycle arrest and apoptosis by
luteolin in human colon cancer cells and xenografts. Oncol Lett.
15:1559–1565. 2018.PubMed/NCBI
|
|
14
|
Meng X, Zhong WQ and Zhang XR: Luteolin
inhibits the colon cancer HT-29 cell proliferation, migration and
epithelial-mesenchymal transition: An experimental study. J Hainan
Med University. 23:5–8. 2017.
|
|
15
|
Chulenbayeva LE, Shaiken TE and Opekun AR:
Sa1967 The effect of flavonoids luteolin and quercetin upon colon
cancer cells in vitro; ‘So What's in Your Fiber’? Gastroenterology.
148:S–370. 2015. View Article : Google Scholar
|
|
16
|
Pandurangan AK, Dharmalingam P, Sadagopan
SK, Ramar M, Munusamy A and Ganapasam S: Luteolin induces growth
arrest in colon cancer cells through involvement of
Wnt/β-catenin/GSK-3β signaling. J Environ Pathol Toxicol Oncol.
32:131–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lim DY, Cho HJ, Kim J, Nho CW, Lee KW and
Park JH: Luteolin decreases IGF-II production and downregulates
insulin-like growth factor-I receptor signaling in HT-29 human
colon cancer cells. BMC Gastroenterol. 12:92012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abdelhadi L, Vito CD, Giussani P, Viani P
and Riboni L: Luteolin induces an alteration of the
Ceramide/Sphingosine-1-phosphate ratio leading to apoptosis in
human colon cancer cells. 2013.
|
|
19
|
Molavian HR, Goldman A, Phipps CJ,
Kohandel M, Wouters BG, Sengupta S and Sivaloganathan S:
Drug-induced reactive oxygen species (ROS) rely on cell membrane
properties to exert anticancer effects. Sci Rep. 6:274392016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen M, Zhou B, Zhong P, Rajamanickam V,
Dai X, Karvannan K, Zhou H, Zhang X and Liang G: Increased
intracellular reactive oxygen species mediates the anti-cancer
effects of WZ35 via activating mitochondrial apoptosis pathway in
prostate cancer cells. Prostate. 77:489–504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kobayashi M and Yamamoto M: Molecular
mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene
regulation. Antioxid Redox Signal. 7:385–394. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kundu JK and Surh Y: Nrf2-Keap1 signaling
as a potential target for chemoprevention of
inflammation-associated carcinogenesis. Pharm Res. 27:999–1013.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee MH, Hong SH, Park C, Kim GY, Leem SH,
Choi SH, Keum YS, Hyun JW, Kwon TK, Hong SH and Choi YH:
Hwang-Heuk-San induces apoptosis in HCT116 human colorectal cancer
cells through the ROS-mediated activation of caspases and the
inactivation of the PI3K/Akt signaling pathway. Oncol Rep.
36:205–214. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoshyar R, Bathaie SZ and Sadeghizadeh M:
Crocin triggers the apoptosis through increasing the Bax/Bcl-2
ratio and caspase activation in human gastric adenocarcinoma, AGS,
cells. DNA Cell Biol. 32:50–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wood PA, Du-Quiton J, You S and Hrushesky
WJ: Circadian clock coordinates cancer cell cycle progression,
thymidylate synthase, and 5-fluorouracil therapeutic index. Mol
Cancer Ther. 5:2023–2033. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun Y, Liu P and Chen J: Traditional
Chinese Medicine constitution analysis as predictors for Breast
Cancer: A cross-sectional and case control study. Langmuir.
12:4404–4410. 2015.
|
|
28
|
Parekh HS, Liu G and Wei MQ: A new dawn
for the use of traditional Chinese medicine in cancer therapy. Mol
Cancer. 8:212009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Konkimalla VB and Efferth T: Anti-cancer
natural product library from Traditional Chinese medicine. Comb
Chem High Throughput Screen. 11:7–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kang KA, Piao MJ, Ryu YS, Hyun YJ, Park
JE, Shilnikova K, Zhen AX, Kang HK, Koh YS, Jeong YJ and Hyun JW:
Luteolin induces apoptotic cell death via antioxidant activity in
human colon cancer cells. Int J Oncol. 51:1169–1178. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xuemin C, Yi L, Wunier, et al: The diverse
roles of small Rho GTPases in cancer cell biology. Chinese J Cell
Biol. 2015.
|
|
32
|
Xu Y, So C, Lam HM, Fung MC and Tsang SY:
Apoptosis reversal promotes cancer stem cell-like cell formation.
Neoplasia. 20:295–303. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lowe SW and Lin AW: Apoptosis in cancer.
Carcinogenesis. 21:485–495. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dobrzycka B, Terlikowski SJ, Bernaczyk PS,
Garbowicz M, Niklinski J, Chyczewski L and Kulikowski M: Prognostic
significance of Smac/DIABLO in endometrioid endometrial cancer.
Folia Histochem Cytobiol. 48:678–681. 2010.PubMed/NCBI
|
|
35
|
Zhao Y, Qu T, Wang P, Li X, Qiang J, Xia
Z, Duan H, Huang J and Zhu L: Unravelling the relationship between
macroautophagy and mitochondrial ROS in cancer therapy. Apoptosis.
21:517–531. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Y, Yang B, Zhang L, Cong X, Liu Z, Hu
Y, Zhang J and Hu H: Ginkgolic acid induces interplay between
apoptosis and autophagy regulated by ROS generation in colon
cancer. Biochem Biophys Res Commun. 498:246–253. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pavithra PS, Mehta A and Verma RS:
Aromadendrene oxide 2, induces apoptosis in skin epidermoid cancer
cells through ROS mediated mitochondrial pathway. Life Sci.
197:19–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Babayev E, Wang T, Szigeti-buck K, Lowther
K, Taylor HS, Horvath T and Seli E: Reproductive aging is
associated with changes in oocyte mitochondrial dynamics, function,
and mtDNA quantity. Maturitas. 93:121–130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS
release. Physiol Rev. 94:909–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cho HD, Lee JH, Moon KD, Park KH, Lee MK
and Seo KI: Auriculasin-induced ROS causes prostate cancer cell
death via induction of apoptosis. Food Chem Toxicol. 111:660–669.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Opferman JT and Kothari A: Anti-apoptotic
BCL-2 family members in development. Cell Death Differ. 25:37–45.
2018. View Article : Google Scholar
|
|
42
|
Heidelberg SB: Mitochondria Apoptosis
Pathway (M). Springer; Berlin Heidelberg: 2008
|
|
43
|
Huang X, Lu Q, Shen N and Wang Y:
Inhibitory effects of Alkaline S. Chinenis polysaccharides on
proliferation and invasion abilities of colon cancer HT-29 cells in
vitro. J Jilin University Medicine Edition. 41:287–290. 2015.
|
|
44
|
Jeong DW, Kim TS, Cho IT and Kim IY:
Modification of glycolysis affects cell sensitivity to apoptosis
induced by oxidative stress and mediated by mitochondria. Biochem
Biophys Res Commun. 313:984–991. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rowe LA, Degtyareva N and Doetsch PW: DNA
damage-induced reactive oxygen species (ROS) stress response in
Saccharomyces cerevisiae. 45:1167–1177. 2008.PubMed/NCBI
|
|
46
|
Nauseef WM: Nox enzymes in immune cells.
Semin Immunopathol. 30:195–208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nauseef WM: Assembly of the phagocyte
NADPH oxidase. Histochem Cell Biol. 122:277–291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Spencer NY and Engelhardt JF: The basic
biology of redoxosomes in cytokine-mediated signal transduction and
implications for disease-specific therapies. Biochemistry.
53:1551–1564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Brandes RP, Weissmann N and Schröder K:
Nox family NADPH oxidases: Molecular mechanisms of activation. Free
Radic Biol Med. 76:208–226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kang SW, Lee S and Lee EK: ROS and energy
metabolism in cancer cells: Alliance for fast growth. Arch Pharm
Res. 38:338–345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cao LJ, Gong H, Yan M, Li HD and Sun L:
Research progress on Nrf2-ARE signaling pathway involved in liver
disease pathological mechanism. Chinese Pharmacological Bulletin.
31:1057–1061. 2015.
|
|
52
|
Kang KA, Piao MJ, Hyun YJ, Zhen AX, Cho
SJ, Ahn MJ, Yi JM and Hyun JW: Luteolin promotes apoptotic cell
death via upregulation of Nrf2 expression by DNA demethylase and
the interaction of Nrf2 with p53 in human colon cancer cells. Exp
Ther Med. 51:402019.
|
|
53
|
Zuo Q, Wu R, Xiao X, Yang C, Yang Y, Wang
C, Lin L and Kong AN: The dietary flavone luteolin epigenetically
activates the Nrf2 pathway and blocks cell transformation in human
colorectal cancer HCT116 cells. J Cell Biochem. 119:9573–9582.
2018. View Article : Google Scholar : PubMed/NCBI
|