1. Introduction
The skin, which also participates in the
hydro-electrolytic balance by limiting water loss, is the largest
organ of the human body and it is a general protective barrier
against external aggression, but this protection also includes
important immunological functions. Thus, protection against
external agents, as it is conferred by the tegument, also involves
the active and integrated part of the immune system that is present
here. The skin's immune system creates a permanent balance between
the pro-inflammatory and the anti-inflammatory response. Structural
and functional integrity of the skin are imperative conditions for
it to be able to fulfill its many roles.
Epidermis, the most superficial layer of the skin,
is the skin compartment that provides the barrier function. It
consists of 4-5 cell layers (basal layer, cornified layers,
granular layer, clear or translucent layer (present only in the
skin of the palms and plantar part of the feet), and the spinous
layer (1). The epidermis acts as
three lines of defense: physical barrier that limits mechanical
aggression and penetration of pathogens; chemical barrier,
including antimicrobial role; barrier against water and electrolyte
loss (2).
Keratinocytes have an essential role in the
formation and maintenance of the skin barrier function. These cells
traverse all layers of the epidermis. Through a proliferation and
differentiation process, in the cornified layer they turn into
corneocytes, a process which is under the strict control of
cytokines (3,4), produced by keratinocytes and by other
resident cells of the skin. The perturbation of the physiological
process of keratinocyte differentiation, caused by the disturbance
of cytokine gene expression, modifications of the lipid composition
of the keratinocyte membrane and corneocyte desquamation defects,
lead to altered quality of the skin barrier. Thus, by increasing
the permeability of this barrier, a number of
substances/microorganisms penetrate the body, which promotes and
perpetuates the inflammatory processes at this level and triggers
immunological reactions. Defects in skin barrier formation have
been observed in a number of inflammatory skin diseases, such as
atopic dermatitis (5), psoriasis,
ichthyosis and urticaria (6,7).
On the surface of the skin there is also a multitude
of commensal bacteria, which form the cutaneous microbiota and
which have a role in protection against invasion by pathogens. The
skin has a powerful mechanism to repair its integrity after traumas
or aggression by ultraviolet radiation. This repair function also
involves the immune cells present in the skin. Cutaneous
homeostasis (8) is maintained by
permanent coordination and communication between epithelial cells
and immune cells in the skin. Any disruption of the skin microbiota
and/or of the skin repair mechanism leads to inflammatory cutaneous
processes, to an imbalance between proinflammatory and
anti-inflammatory factors and to the occurrence of inflammatory and
allergic dermatological diseases. In recent years there has been an
increase in autoimmune skin diseases. Autoimmune skin disease
affects the immune system and results in autoantibodies, i.e.
antibodies against self-antigens. The concept of the immune system
of the skin, can be seen like a complex relation between immune
cells, epithelial cells, and the external environment.
Epidemiological data show that psoriasis, a
significant public health challenge, affects ~125 million people
globally (9). Prevalence estimates
within adult populations range from 0.91% in the USA to 8.5% in
Norway (10). Another autoimmune
skin disease, vitiligo affects ~1% of the general population
(11), the risk of a patient's
sibling developing the disease is 6%, and for an identical twin it
is 23%, while pemphigus is estimated to affect ~0.5-30 cases per
million globally (12).
2. Skin barrier formation
The process of keratinization
Cornification or keratinization is the complex
process of normal formation of the stratum corneum, through which
keratinocytes suffer important morphological and structural
changes, eventually transforming into corneocytes. An intact
cornified layer is essential for the skin to form an impenetrable
barrier (13). Keratinocytes, cells
that are permanently restored, undergo mitosis and proliferation in
the basal layer. Differentiated and mature keratinocytes, pass
through all layers of skin, lose their nuclei and cellular
organelles, begin to secrete keratin, and turn into corneocytes in
the cornified layer.
During the process, a membrane is formed that
surrounds the keratinocytes at the periphery. The membrane is rich
in proteins and lipids. The membrane formation process involves the
binding of proteins, especially loricrine and involucrine, to
keratinocyte filaments, when they transit from the granule layer to
the stratum corneum (14). An
important role in the stabilization of these proteins is the
cross-linking of filagrin. The lipid barrier is located externally
from the corneous membrane and is composed especially of ceramides,
which bind covalently. The lipid layer limits hydro-electrolytic
losses through the epidermis. The dead and flattened corneocytes,
united by corneodesmosomes (modified desmosomal structures), form
the stratum corneum, which confers resistance to mechanical
stimuli.
Old corneocytes are removed from the surface of the
epidermis by desquamation. Increasing the calcium concentration in
the stratum corneum is the trigger factor of this process. A series
of specific proteases that degrade cornodesmozomal proteins are
activated, and thus the bonds of the cells of the stratum corneum
become unstable and the cells are eliminated. The keratinization
process involves a series of enzymatic reactions and is dependent
on structural proteins, fatty acids and lipids, whose gene
expression and function are controlled by cytokines and
intracellular signal molecules. Also, as they differentiate,
keratinocytes secrete a series of fatty acids (15) and antimicrobial peptides (RNASE 7,
psoriasin and calprotectin) that attach to the lipid membrane
(16). Keratinocytes respond to
inflammation caused by the pathogenesis of crackles of the skin
barrier by catechidyl LL-37 secretion and defensins, two peptides
with antimicrobial properties (17).
The keratohyaline granules
The keratohyaline granules are present in the
granular layer of the epidermis and contain keratin filaments and
proteins associated with these filaments: loricrine, filagrine and
tricohyaline (18,19). Depending on location, the
keratohyaline granules are either present in the globular form (the
oral mucosa epithelium) or in the stellate form (the normal
epidermis) (20). These granules are
insoluble in water and play an important role in the formation of
corneocyte envelope. The keratohyaline granules contain ribosomes
that are involved in initiating the formation of intermediate
keratin filaments and in the proteins assembling process (20). The granules grow progressively as
they move from the spinous layer to the stratum corneum (21) and participate in the homogenization
of the keratin matrix (22).
Profilagrin, the predominant content of keratohyaline granules, is
the protein precursor of filagrin (23). Profilagrin, in the course of
epidermal differentiation, turns into free monomers of filagrin,
which interact with keratin filaments. The keratohyaline granules
have been observed in the thymus medulla, in the cytoplasm of the
Hassal corpuscles. They appear to be involved in the development of
T lymphocytes because they produce IL-4 and IL-7(24).
3. Immune cells of the skin
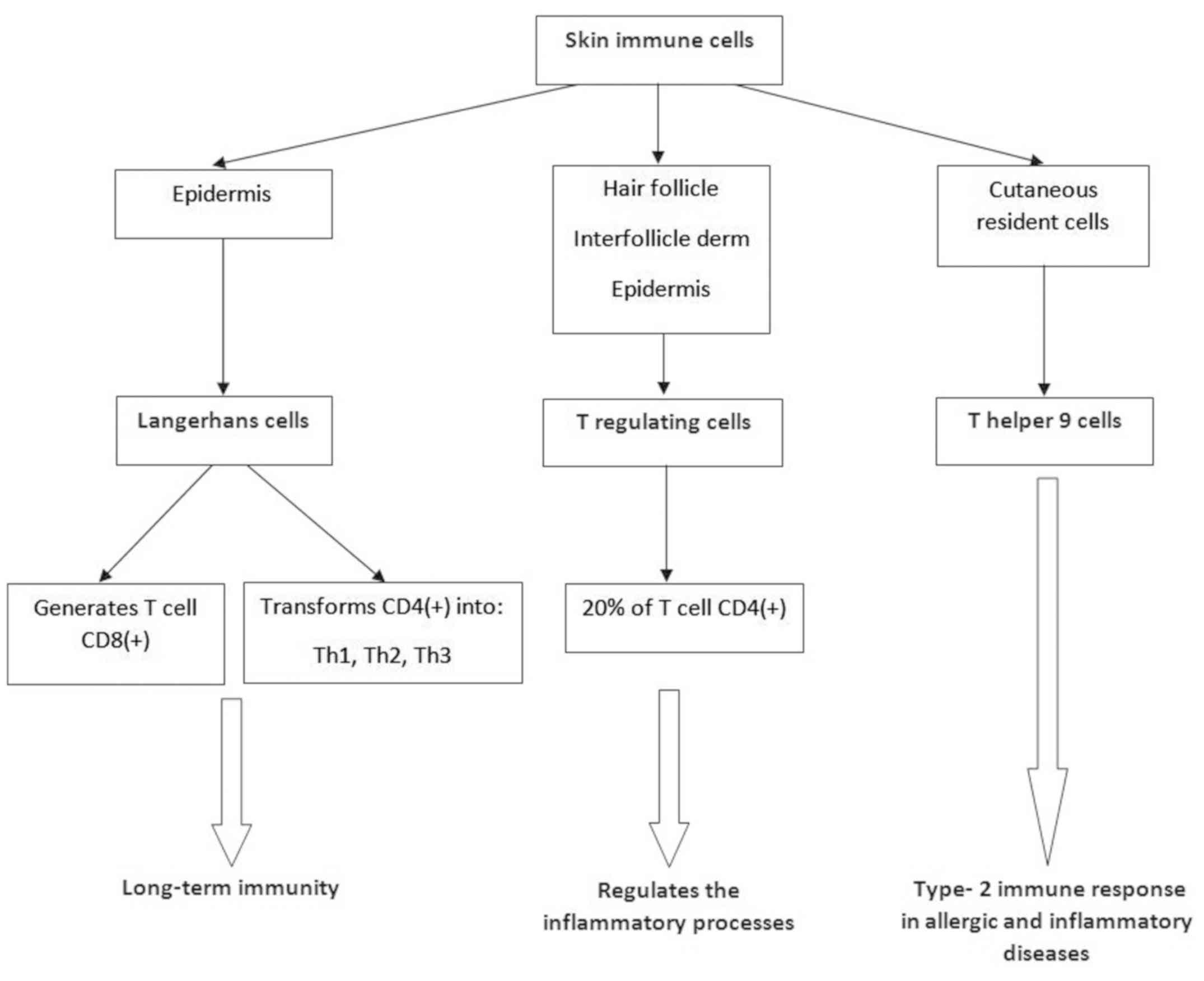
To summarize the overall picture of the skin as an
immune organ, as described hereafter, a general scheme is presented
in Fig. 1.
Langerhans cutaneous cells
Langerhans cells are antigen-presenting dendritic
cells (25,26) that play a role in long-term immunity.
They were discovered in 1970 by Ralph Steinman and Zanvil Cohn and
are involved in the generation of CD8+ lymphocytes and
in the transformation of CD4+ lymphocytes into various
cell subtypes (Th1, Th2, Th17) (21). Skin dendritic cells have a protective
or suppressive role in skin disorders (27) and are found in the epidermis. These
cells have myeloid origin and have characteristics close to
monocytes (28). At the skin level,
the dendritic cells are inserted between the keratinocytes and
create tight junctions with them. When the epidermis is stimulated
by mechanical stimuli, Langerhans cells increase the mobility of
dendrites. The integrity of the barrier is monitored this way.
Moreover, dendritic cells provide immunity without a real
infection, against a series of antigens that have not penetrated
the cutaneous barrier (29). The
presence of important dendritic cells at the skin level makes the
skin an important place for vaccination and creation of long-term
immunity (30). In atopic
dermatitis, skin dendritic cells play a role in the recruitment of
eosinophils (31). The lichen may
also involve autoimmune aspects (32,33),
including an increased Th1/Th2 ratio.
T regulating cells of the skin
(Treg)
An intact skin barrier also requires the integrity
of its attachments: hair follicle, sebaceous glands and sweat
glands. Treg cells (34) represent a
subset of CD4+ lymphocytes, which are predominantly
found in the hair follicle, especially in regions with high
follicular density. They have also been identified, but in small
amounts, in the interfollicular dermis and epidermis (35). These represent 20% of CD4+
T cells in the skin. Treg lymphocytes play a role in maintaining
immunological homeostasis of the skin (36) by regulating inflammatory processes at
this level. These cells come from either the thymus, after mature
T-lymphocytes (they become capable of recognizing their own
antigens), or formed from the naive CD4+ T lymphocytes
with peripheral antigens (37). Treg
cells offer protection against skin cancer (38) because they secrete IL-10 and TGF-β,
cytokines that suppress immune response and inflammation (39). Treg cells actively maintain the skin
microparticle homeostasis (40),
which provides protection against bacterial and parasitic agents,
hair follicle regeneration and skin stimulation of cell
differentiation processes and mucosal cell regeneration. IL-7 is
essential for the normal function of Treg and IL-2 is important for
their normal development in the thymus (41).
T helper 9 (Th9)
A subset of IL-9 secreting CD4+ cells,
Th9 lymphocytes are cutaneous resident cells. These, by regulating
the synthesis of pro-inflammatory cytokines (42), are involved in triggering the type 2
immune response from allergic and inflammatory diseases (43). In vitro, IL-9 increases the
synthesis of IL-8 and vascular endothelial growth factor (VEGF)
(44-46).
4. Inflammatory skin diseases
Psoriasis - an immune-mediated
inflammatory disease
World Health Organization (WHO) has recently stated
that psoriasis is a serious, chronic, disfiguring, disabling,
noncommunicable disease (47). The
WHO report highlights the need for data collection documenting
prevalence and incidence of psoriasis in order to estimate
accurately the social and economic burden of the disease (48). The etiology of the disease is unclear
but genetic and environmental factors (49) are thought to be the reason behind the
abnormal immune response in psoriasis patients. Epidemiological
studies in psoriasis have reported a significantly increased risk
of inflammatory comorbid conditions (50), including psoriatic arthritis,
depression, obesity, diabetes, liver disease, metabolic syndrome
and cardiovascular disease (CVD).
Psoriasis is a T cell mediated autoimmune disease,
characterized by keratinocyte proliferation, while psoriasin
(S100A7) and koebnerisin (S100A15) are proinflammatory proteins
that are upregulated in psoriasis and act as chemoattractants for
the immune cells. A protein group called antimicrobial peptides
(AMP) has been well studied concerning the immune mechanism of
psoriasis. They are highly expressed, especially cathelicidin,
β-defensins and S100 proteins (51).
Psoriasis is a T cell mediated autoimmune disease and it is
hypothesized that the effector T cells accumulate in lymph nodes
and finally they migrate into the skin via the blood. Moreover,
psoriatic skin is shown to be another source for inflammatory T
cells (52). In psoriatic patients
various proinflammatory cytokines are in higher plasma
concentration, such as IL-6, IL-2, IL-10, IFN-γ, IL-17(53), IL-21, IL-22, IL-23, IL-9, while low
concentrations were found for IL-4 and IL-27 (54-56).
Vitiligo - immunologic mechanisms
Vitiligo is a common, disfiguring autoimmune
disorder that negatively influences patients self-esteem and
quality of life. The characteristic of this disease is that
epidermal melanocytes are targeted and destroyed in various ways
and the consequence is the appearance of irregular depigmentation
of the skin. Numerous studies show that melanocytes from vitiligo
patients have intrinsic defects that reduce their capacity to
manage cellular stress (57). The
pathogenic factors of vitiligo include CD8+ T
lymphocyte/T helper 1 infiltrates in lesional skin (58,59),
with increased expression of IFN-γ (60) and tumor necrosis factor-α (61-64),
decreased transforming growth factor-β, and circulating
autoantibodies against tyrosine hydroxylase (65). Additionally, several studies have
found a high prevalence of antecedent psychological stressors in
vitiligo patients, suggesting that specific stressors may trigger
or exacerbate vitiligo (66-69).
Studies reveal that CXCL16 expression increased and showed a
positive correlation with oxidative stress levels in serum and
lesions of patients with vitiligo. CXCL16 produced by stressed
keratinocytes induced migration of CXCR6+CD8+
T cells derived from patients with vitiligo.
CXCR6+CD8+ T cell skin infiltration is
accompanied by melanocyte loss in lesions of patients with vitiligo
(70). It is assumed that
infiltration of the cytotoxic CD8 T cells might be responsible for
the melanocyte destruction in vitiligo.
Pemphigus - immunologic network
base
Pemphigus and bullous pemphigoid are
autoantibody-mediated blistering skin diseases, in which IgG
autoantibodies are produced mainly against autoantigens
Desmoglein-1 (DSG1) and Desmoglein-3 (DSG3). In pemphigus the
keratinocytes in epidermis and mucous membranes lose cell-cell
adhesion, while in pemphigoid the basal keratinocytes lose adhesion
to the basement membrane (71,72).
Also, in pemphigus patients, activated B cells act as pathogenic
regulators by secreting anti-DSG3 autoantibodies. B regulatory
cells (B reg) are able to down regulate immune responses in mice
and humans by secreting IL-10, TGF-β and expressing FOXP3 (73,74). An
early cytokine of Th1 type, osteopontin (OPN), augments production
of IFN-γ, IL-12 and decreases IL-10 production (75). In pemphigus, as in other autoimmune
disorders, OPN production is elevated, which indicates a Th1
response (76).
Atopic dermatitis – immune disorder in
correlation with microbiota
Atopic dermatitis (AD) is a chronic Th2 type
inflammatory skin disease associated with cutaneous hyper-reactivity
to environmental triggers (74). A
chronic relapsing skin disease, AD has a characteristic phenotype,
with typically distributed skin lesions, that often render its
diagnosis very simple and clear-cut (75). Staphylococcus aureus is found
in >90% of the patients with chronic AD skin lesions (76). Acute exudative skin lesions can
contain over 10 million of these organisms per square centimeter,
and increased numbers have been found even in normal skin and the
nasal vestibula or intertriginous areas of patients with AD.
Scratching is an important factor, enhancing the binding of
bacteria, by disturbing the skin barrier and exposing extracellular
matrix molecules known to act as adhesins for S. aureus
(fibronectin, collagens, fibrinogen, elastin, laminin) (77). In addition, bacterial binding seems
to be higher at skin sites with Th2-mediated inflammation and the
respective cytokine medium, due to induction of an enhanced
production of these adhesins and downregulation of antimicrobial
peptides needed to control the replication of S. aureus
(78,79). IgE autoreactivity is an immune
pathogenic factor in AD. In addition, molecular analysis of
allergens has revealed striking similarities between environmental
allergens and human proteins, leading to the hypothesis that
autoimmune reactions also might play a role (80,81).
5. Conclusion
The skin is important both as barrier and as
connection between the environment and the body. It is well known
that the skin provides a receptor function, translating to the
organism substantial information from the environment, in many
different ways. The skin immunological function could be regarded
as an extremely multifaceted and intricate interplay between signal
processing and the defense reactions. This is further complicated
by many autoimmune aspects. The continuous huge progress in
immunology clearly impacts our understanding of the immunological
function of the skin, with multiple insights to various
etyopathogenic entities. This review is an invitation to the wide
area of roles and mechanisms involved in the immunology of the
healthy and diseased skin.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
All the authors substantially contributed to each of
the following aspects of this review paper: Conception and design
(mainly MAM, LLH, DCB, DNS and ILS) and selection, analysis and
interpretation of cited references (all the authors, but mainly
MAM, LLH, DCB, DEB and ND). Moreover, all the authors were involved
in drafting of the manuscript (mainly MAM, LLH and DCB) and in
revising it critically for important intellectual content (mainly
DNS, DEB, ND and ILS). All the authors have given their final
approval of the version to be published. Thus, each author has
participated sufficiently in the work and takes public
responsibility for appropriate portions of the content, so that the
authors agree to be accountable for all aspects of the work in
ensuring that questions related to the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maranduca MA, Branisteanu D, Serban DN,
Branisteanu DC, Stoleriu G, Manolache N and Serban IL: Synthesis
and physiological implications of melanic pigments. Oncol Lett.
17:4183–4187. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hänel KH, Cornelissen C, Lüscher B and
Baron JM: Cytokines and the skin barrier. Int J Mol Sci.
14:6720–6745. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ilie MA, Caruntu C, Tampa M, Georgescu SR,
Matei C, Negrei C, Ion RM, Constantin C, Neagu M and Boda D:
Capsaicin: Physicochemical properties, cutaneous reactions and
potential applications in painful and inflammatory conditions. Exp
Ther Med. 18:916–925. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Branisteanu D, Caruntu C, Negrei C, Ghita
MA, Caruntu A, Badarau AI, Buraga I, Boda D and Albu A: Capsaicin,
a hot topic in skin pharmacology and physiology. Farmacia.
63:487–491. 2015.
|
|
5
|
Căruntu C, Boda D, Musat S, Căruntu A and
Mandache E: Stress-induced mast cell activation in glabrous and
hairy skin. Mediators Inflamm. 2014(105950)2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Guttman-Yassky E, Nograles KE and Krueger
JG: Contrasting pathogenesis of atopic dermatitis and psoriasis -
part I: Clinical and pathologic concepts. J Allergy Clin Immunol.
127:1110–1118. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Caruntu C, Boda D, Musat S, Caruntu A,
Poenaru E, Calenic B, Savulescu-Fiedler I, Draghia A, Rotaru M and
Badarau AI: Stress effects on cutaneous nociceptive nerve fibers
and their neurons of origin in rats. Rom Biotechnol Lett.
19:9517–9530. 2014.
|
|
8
|
Lupu M, Caruntu A, Caruntu C, Papagheorghe
LML, Ilie MA, Voiculescu V, Boda D, Constantin C, Tanase C, Sifaki
M, et al: Neuroendocrine factors: The missing link in non melanoma
skin cancer (Review). Oncol Rep. 38:1327–1340. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
International Federation of Psoriasis
Associations: World Psoriasis Day, 2015. Available at: https://ifpa-pso.com/2015/10/29/press-world-psoriasis-day-2015-brings-hope.
|
|
10
|
Parisi R, Symmons DP, Griffiths CE and
Ashcroft DM: Identification and Management of Psoriasis and
Associated ComorbidiTy (IMPACT) project team. Global epidemiology
of psoriasis: A systematic review of incidence and prevalence. J
Invest Dermatol. 133:377–385. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Taïeb A and Picardo M: Clinical practice.
Vitiligo. N Engl J Med. 360:160–169. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Alkhateeb A, Fain PR, Thody A, Bennett DC
and Spritz RA: Epidemiology of vitiligo and associated autoimmune
diseases in Caucasian probands and their families. Pigment Cell
Res. 16:208–214. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Proksch E, Brandner JM and Jensen JM: The
skin: An indispensable barrier. Exp Dermatol. 17:1063–1072.
2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Freeman SC and Sonthalia S: Histology,
Keratohyalin Granules. StatPearls Publishing, Treasure Island, FL,
2019.
|
|
15
|
Harder J, Schröder JM and Gläser R: The
skin surface as antimicrobial barrier: Present concepts and future
outlooks. Exp Dermatol. 22:1–5. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gallo RL and Hooper LV: Epithelial
antimicrobial defence of the skin and intestine. Nat Rev Immunol.
12:503–516. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Manabe M and O'Guin WM: Keratohyalin,
trichohyalin and keratohyalin-trichohyalin hybrid granules: An
overview. J Dermatol. 19:749–755. 1992.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nithya S, Radhika T and Jeddy N: Loricrin
- an overview. J Oral Maxillofac Pathol. 19:64–68. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Westerhof W and Dingemans KP: The
morphology of keratohyalin granules in orthokeratotic and
parakeratotic skin and oral mucosa. Int J Dermatol. 26:308–313.
1987.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yousef H, Alhajj M and Sharma S: Anatomy,
Skin (Integument), Epidermis. StatPearls Publishing, Treasure
Island, FL, 2019.
|
|
21
|
Westerhof W and Dingemans KP: The
morphological details of globular keratohyalin granules. J Cutan
Pathol. 13:375–382. 1986.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dinh MH, McRaven MD, Kelley Z, Penugonda S
and Hope TJ: Keratinization of the adult male foreskin and
implications for male circumcision. AIDS. 24:899–906.
2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Takahashi M, Horiuchi Y and Tezuka T:
Hematoxylin-stainability of keratohyalin granules is due to the
novel component, fibrinogen γ-chain protein. Arch Dermatol Res.
302:679–684. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fukuyama K and Epstein WL: Heterogeneous
ultrastructure of keratohyalin granules: A comparative study of
adjacent skin and mucous membrane. J Invest Dermatol. 61:94–100.
1973.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nwabudike LC, Elisei AM, Buzia OD,
Miulescu M and Tatu AL: Statins: A review on structural
perspectives, adverse reactions and relations with non-melanoma
skin cancer. Rev Chim Buchar. 69:2557–2562. 2018.
|
|
26
|
Cioplea M, Caruntu C, Zurac S, Bastian A,
Sticlaru L, Cioroianu A, Boda D, Jugulete G, Nichita L and Popp C:
Dendritic cell distribution in mycosis fungoides vs. inflammatory
dermatosis and other T-cell skin lymphoma. Oncol Lett.
17:4055–4059. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Steinman RM and Cohn ZA: Identification of
a novel cell type in peripheral lymphoid organs of mice I.
Morphology, quantitation, tissue distribution. J Exp Med.
137:1142–1162. 1973.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rajesh A, Wise L and Hibma M: The role of
Langerhans cells in pathologies of the skin. Immunol Cell Biol.
97:700–713. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nirschl CJ and Anandasabapathy N: Duality
at the gate: Skin dendritic cells as mediators of vaccine immunity
and tolerance. Hum Vaccin Immunother. 12:104–116. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Teunissen MBM, Haniffa M and Collin MP:
Insight into the immunobiology of human skin and functional
specialization of skin dendritic cell subsets to innovate
intradermal vaccination design. Curr Top Microbiol Immunol.
351:25–76. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Scharschmidt TC, Vasquez KS, Pauli ML,
Leitner EG, Chu K, Truong HA, Lowe MM, Sanchez Rodriguez R, Ali N,
Laszik ZG, et al: Commensal microbes and hair follicle
morphogenesis coordinately drive treg migration into neonatal skin.
Cell Host Microbe. 21:467–477.e5. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Brănişteanu DE, Pintilie A, Andreş LE,
Dimitriu A, Oanţă A, Stoleriu G and Brănişteanu DC: Ethiopatogenic
hypotheses in lichen planus. Rev Med Chir Soc Med Nat Iasi.
120:760–767. 2016.PubMed/NCBI
|
|
33
|
Brănişteanu DE, Brănişteanu DC, Stoleriu
G, Ferariu D, Voicu CM, Stoica LE, Căruntu C, Boda D,
Filip-Ciubotaru FM, Dimitriu A, et al: Histopathological and
clinical traps in lichen sclerosus: A case report. Rom J Morphol
Embryol. 57 (Suppl 2):817–823. 2016.PubMed/NCBI
|
|
34
|
Sakaguchi S, Yamaguchi T, Nomura T and Ono
M: Regulatory T cells and immune tolerance. Cell. 133:775–787.
2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tang L and Wang K: Chronic inflammation in
skin malignancies. J Mol Signal. 11(2)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Suwanpradid J, Holcomb ZE and MacLeod AS:
Emerging skin T-cell functions in response to environmental
insults. J Invest Dermatol. 137:288–294. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ali N and Rosenblum MD: Regulatory T cells
in skin. Immunology. 152:372–381. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Boda D: Cellomics as integrative omics for
cancer. Curr Proteomics. 10:237–245. 2013.
|
|
39
|
Sakaguchi S, Vignali DA, Rudensky AY, Niec
RE and Waldmann H: The plasticity and stability of regulatory T
cells. Nat Rev Immunol. 13:461–467. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
40
|
Schlapbach C, Gehad A, Yang C, Watanabe R,
Guenova E, Teague JE, Campbell L, Yawalkar N, Kupper TS and Clark
RA: Human TH9 cells are skin-tropic and have autocrine and
paracrine proinflammatory capacity. Sci Transl Med.
6(219ra8)2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kaplan MH, Hufford MM and Olson MR: The
development and in vivo function of T helper 9 cells. Nat Rev
Immunol. 15:295–307. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu J, Harberts E, Tammaro A, Girardi N,
Filler RB, Fishelevich R, Temann A, Licona-Limón P, Girardi M,
Flavell RA, et al: IL-9 regulates allergen-specific Th1 responses
in allergic contact dermatitis. J Invest Dermatol. 134:1903–1911.
2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ma L, Xue HB, Guan XH, Shu CM, Zhang JH
and Yu J: Possible pathogenic role of T helper type 9 cells and
interleukin (IL)-9 in atopic dermatitis. Clin Exp Immunol.
175:25–31. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
World Health Organization: Global report
on psoriasis. World Health Organization, Geneva,. 2016, ISBN 978 92
4 156518 9. Available at: https://apps.who.int/iris/handle/10665/204417.
|
|
45
|
Gelfand JM, Neimann AL, Shin DB, Wang X,
Margolis DJ and Troxel AB: Risk of myocardial infarction in
patients with psoriasis. JAMA. 296:1735–1741. 2006.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Solomon I, Voiculescu VM, Caruntu C, Lupu
M, Popa A, Ilie MA, Albulescu R, Caruntu A, Tanase C, Constantin C,
et al: Neuroendocrine factors and head and neck squamous cell
carcinoma: An affair to remember. Dis Markers.
2018(9787831)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Batycka-Baran A, Maj J, Wolf R and
Szepietowski JC: The new insight into the role of antimicrobial
proteins-alarmins in the immunopathogenesis of psoriasis. J Immunol
Res. 2014(628289)2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Dunphy SE, Sweeney CM, Kelly G, Tobin AM,
Kirby B and Gardiner CM: Natural killer cells from psoriasis
vulgaris patients have reduced levels of cytotoxicity associated
degranulation and cytokine production. Clin Immunol. 177:43–49.
2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Grigore O, Mihailescu AI, Solomon I, Boda
D and Caruntu C: Role of stress in modulation of skin neurogenic
inflammation. Exp Ther Med. 17:997–1003. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Witte E, Kokolakis G, Witte K, Philipp S,
Doecke WD, Babel N, Wittig BM, Warszawska K, Kurek A,
Erdmann-Keding M, et al: IL-19 is a component of the pathogenetic
IL-23/IL-17 cascade in psoriasis. J Invest Dermatol. 134:2757–2767.
2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Nograles KE, Zaba LC, Guttman-Yassky E,
Fuentes-Duculan J, Suárez-Fariñas M, Cardinale I, Khatcherian A,
Gonzalez J, Pierson KC, White TR, et al: Th17 cytokines interleukin
(IL)-17 and IL-22 modulate distinct inflammatory and
keratinocyte-response pathways. Br J Dermatol. 159:1092–1102.
2008.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chen W, Gong Y, Zhang X, Tong Y, Wang X,
Fei C, Xu H, Yu Q, Wang Y and Shi Y: Decreased expression of IL-27
in moderate-to-severe psoriasis and its anti-inflammation role in
imiquimod-induced psoriasis-like mouse model. J Dermatol Sci.
85:115–123. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Caruntu C, Boda D, Dumitrascu G,
Constantin C and Neagu M: Proteomics focusing on immune markers in
psoriatic arthritis. Biomarkers Med. 9:513–528. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Harris JE: Cellular stress and innate
inflammation in organ-specific autoimmunity: Lessons learned from
vitiligo. Immunol Rev. 269:11–25. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Goronzy J, Weyand CM and Waase I: T cell
subpopulations in inflammatory bowel disease: Evidence for a
defective induction of T8+ suppressor/cytotoxic T
lymphocytes. Clin Exp Immunol. 61:593–600. 1985.PubMed/NCBI
|
|
56
|
Ongenae K, Van Geel N and Naeyaert JM:
Evidence for an autoimmune pathogenesis of vitiligo. Pigment Cell
Res. 16:90–100. 2003.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Grimes PE, Morris R, Avaniss-Aghajani E,
Soriano T, Meraz M and Metzger A: Topical tacrolimus therapy for
vitiligo: Therapeutic responses and skin messenger RNA expression
of proinflammatory cytokines. J Am Acad Dermatol. 51:52–61.
2004.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Birol A, Kisa U, Kurtipek GS, Kara F,
Kocak M, Erkek E and Caglayan O: Increased tumor necrosis factor
alpha (TNF-alpha) and interleukin 1 alpha (IL1-alpha) levels in the
lesional skin of patients with nonsegmental vitiligo. Int J
Dermatol. 45:992–993. 2006.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Moretti S, Spallanzani A, Amato L,
Hautmann G, Gallerani I, Fabiani M and Fabbri P: New insights into
the pathogenesis of vitiligo: Imbalance of epidermal cytokines at
sites of lesions. Pigment Cell Res. 15:87–92. 2002.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zailaie MZ: Decreased proinflammatory
cytokine production by peripheral blood mononuclear cells from
vitiligo patients following aspirin treatment. Saudi Med J.
26:799–805. 2005.PubMed/NCBI
|
|
61
|
Basak PY, Adiloglu AK, Ceyhan AM, Tas T
and Akkaya VB: The role of helper and regulatory T cells in the
pathogenesis of vitiligo. J Am Acad Dermatol. 60:256–260.
2009.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Kemp EH, Emhemad S, Akhtar S, Watson PF,
Gawkrodger DJ and Weetman AP: Autoantibodies against tyrosine
hydroxylase in patients with non-segmental (generalised) vitiligo.
Exp Dermatol. 20:35–40. 2011.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Barisić-Drusko V and Rucević I: Trigger
factors in childhood psoriasis and vitiligo. Coll Antropol.
28:277–285. 2004.PubMed/NCBI
|
|
64
|
Manolache L and Benea V: Stress in
patients with alopecia areata and vitiligo. J Eur Acad Dermatol
Venereol. 21:921–928. 2007.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Papadopoulos L, Bor R, Legg C and Hawk JL:
Impact of life events on the onset of vitiligo in adults:
Preliminary evidence for a psychological dimension in aetiology.
Clin Exp Dermatol. 23:243–248. 1998.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Picardi A, Pasquini P, Cattaruzza MS,
Gaetano P, Melchi CF, Baliva G, Camaioni D, Tiago A, Abeni D and
Biondi M: Stressful life events, social support, attachment
security and alexithymia in vitiligo. A case-control study.
Psychother Psychosom. 72:150–158. 2003.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Li S, Zhu G, Yang Y, Jian Z, Guo S, Dai W,
Shi Q, Ge R, Ma J, Liu L, et al: Oxidative stress drives
CD8+ T-cell skin trafficking in patients with vitiligo
through CXCL16 upregulation by activating the unfolded protein
response in keratinocytes. J Allergy Clin Immunol. 140:177–189.e9.
2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Kasperkiewicz M, Ellebrecht CT, Takahashi
H, Yamagami J, Zillikens D, Payne AS and Amagai M: Pemphigus. Nat
Rev Dis Primers. 3(17026)2017.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Pollmann R, Schmidt T, Eming R and Hertl
M: Pemphigus: A comprehensive review on pathogenesis, clinical
presentation and novel therapeutic approaches. Clin Rev Allergy
Immunol. 54:1–25. 2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Fujimoto M: Regulatory B cells in skin and
connective tissue diseases. J Dermatol Sci. 60:1–7. 2010.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Mauri C and Bosma A: Immune regulatory
function of B cells. Annu Rev Immunol. 30:221–241. 2012.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Inoue M and Shinohara ML: Intracellular
osteopontin (iOPN) and immunity. Immunol Res. 49:160–172.
2011.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Baroni A, De Filippis A, Buommino E,
Satriano RA and Cozza V: Osteopontin, a protein with cytokine-like
properties: A possible involvement in pemphigus vulgaris. Arch
Dermatol Res. 304:237–240. 2012.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Leung DY and Bieber T: Atopic dermatitis.
Lancet. 361:151–160. 2003.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Spergel JM and Paller AS: Atopic
dermatitis and the atopic march. J Allergy Clin Immunol. 112
(Suppl):S118–S127. 2003.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Leung DY: Infection in atopic dermatitis.
Curr Opin Pediatr. 15:399–404. 2003.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Cho SH, Strickland I, Boguniewicz M and
Leung DY: Fibronectin and fibrinogen contribute to the enhanced
binding of Staphylococcus aureus to atopic skin. J Allergy
Clin Immunol. 108:269–274. 2001.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Gallo RL, Murakami M, Ohtake T and Zaiou
M: Biology and clinical relevance of naturally occurring
antimicrobial peptides. J Allergy Clin Immunol. 110:823–831.
2002.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Ong PY, Ohtake T, Brandt C, Strickland I,
Boguniewicz M, Ganz T, Gallo RL and Leung DY: Endogenous
antimicrobial peptides and skin infections in atopic dermatitis. N
Engl J Med. 347:1151–1160. 2002.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Ochs RL, Muro Y, Si Y, Ge H, Chan EK and
Tan EM: Autoantibodies to DFS 70 kd/transcription coactivator p75
in atopic dermatitis and other conditions. J Allergy Clin Immunol.
105:1211–1220. 2000.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Seiberler S, Natter S, Hufnagl P, Binder
BR and Valenta R: Characterization of IgE-reactive autoantigens in
atopic dermatitis. 2. A pilot study on IgE versus IgG subclass
response and seasonal variation of IgE autoreactivity. Int Arch
Allergy Immunol. 120:117–125. 1999.PubMed/NCBI View Article : Google Scholar
|