Introduction
Irritable bowel syndrome (IBS) is one of the most
frequent and common functional gastrointestinal disorders (1,2). IBS is
defined by the association of pain or abdominal discomfort with a
disturbed bowel transit (3).
Although it is not a life threatening condition, IBS is a chronic
debilitating disease that impairs the quality of life (4,5).
According to Rome criteria, IBS patients are divided
into subtypes: IBS with constipation (IBS-C), IBS with diarrhea
(IBS-D), mixed IBS (IBS-M) and unsubtyped IBS (3). IBS can be triggered by a precedent
gastrointestinal infection, post-infectious IBS (PI-IBS) or by
other causes (non-PI-IBS) (6,7).
Diagnosis still relies on symptom-based criteria
(1-3).
Therefore, the need for a reliable test or marker that could help
improve diagnosis strategy for IBS is reflected in a high number of
studies addressing IBS.
Several studies have stressed the importance of a
reliable test or marker to improve the knowledge and the management
of IBS. Intestinal inflammation has been proposed as a potential
mechanism since 1960s (8), later
microscopic inflammation was considered a strong candidate in the
pathogenesis (9). Studies have shown
the role of inflammation (8-10),
confirming a persistent state of inflammation especially in PI-IBS
patients. To date, the multifactorial pathogenesis of IBS includes
altered gastrointestinal motility, brain-gut interactions, visceral
hypersensitivity, bacterial overgrowth, perturbation of microbiota,
and food sensitivity (11-16).
A chemotactic cytokine, named in 1989 as monocyte
chemoattractant protein-1 (MCP-1) (17,18) is
one of the members of the CC chemokine subfamily that regulates the
migration and recruitment of leukocytes to inflammatory regions
(19,20). It has been shown that MCP-1 recruits
leukocytes (monocytes or macrophages) to inflammatory sites in
several conditions such as: interstitial lung disease (21), and atherosclerosis (22). Its potential role in IBS pathogenesis
has been hypothesized (20) but its
value, as a serological marker it is not established.
An inflammatory cascade that begins with an
infiltration of inflammatory cells in the mucosa and the release of
pro-inflammatory mediators such as reactive oxygen metabolites,
which provides support for the relation between oxidative stress
and inflammation has been cited (23). The role of oxidative stress in IBS
etiopathogenesis is suggested also by another study (24).
Free and protein-bound tyrosine residues react with
nitrating/nitrosating agents leading to nitrotyrosine (NT), which
was proposed as a marker for nitrosation and nitration (22). Detection of NT provides evidence for
generation of nitrogen species (23).
The aim of the present study was to evaluate two
biomarkers, of two different putative pathways of the pathogenesis
of IBS as potential serologic biomarkers for IBS.
Patients and methods
Subject selection
A total number of 42 consecutive patients that
fulfilled Rome III criteria were prospectively included. IBS
patients were recruited from a tertiary care center. Any other
confounding condition (gastrointestinal disorders, inflammatory
processes) was ruled out. Standard laboratory workout, including
inflammation markers: C-reactive protein (CRP), erythrocyte
sedimentation rate (ESR) and fibrinogen were assessed in order to
exclude active inflammation.
Patients with IBS were also subdivided into PI-IBS
and non-PI-IBS, according to previous methodology (8,25) by
asking patients about their medical history over the year before
the onset of IBS. If patients recognized or described that IBS
symptoms occurred after a triggering event consisting of an acute
episode of gastroenteritis (nausea, vomiting and diarrhea), they
were assigned to PI-IBS group analysis. Thirty-five consecutive
controls were recruited by similar approach, including local
advertising. For both groups, body mass index (BMI) was calculated
using the formula: weight (kg) / [height (m)]2. BMI
below 18.5 kg/m2 is considered underweight, a BMI
ranging between 18.5 and 24.9 kg/m2 represents a
normoponderal status while a BMI higher than 25 kg/m2 is
referred as overweight. Exclusion criteria for all subjects were
alcohol and substance abuse or dependence, presence of severe
organic disorders, use of antioxidants and antibiotics in the
previous month or anti-inflammatories in the week prior to
inclusion.
Ethical considerations
The study was approved by the Ethics Committee of
the ‘Iuliu Haţieganu’ University of Medicine and Pharmacy and was
carried out in accordance with the Declaration of Helsinki. All
participants were informed about the study protocol and all
subjects signed an informed consent prior to inclusion in the
study.
Assessment of biomarkers
Subsequent to overnight fasting, a whole venous
blood sample of 5 ml volume was collected. Samples were centrifuged
immediately 10 min at 2,000 x g at room temperature (21˚C) and
separated serum frozen at -80˚C until use. Serum levels of the
biomarkers were measured using solid-phase sandwich enzyme-linked
immunosorbent assays (ELISA). ELISA is a widely used approach that
allows quantitative measurement of proteins in biological
specimens, including serum (25).
For MCP-1 human MCP-1 ELISA kit (OmniKine™) was used
with 50 µl of serum diluted 1:5, based on the recommended protocol.
For NT the OxiSelect™ Nitrotyrosine ELISA kit (Cell Biolabs Inc.)
was used, according to product specifications, using 100 µl of
serum. The absorbance values from each serum were plotted against
the standard curve obtained for each kit and the results were
extrapolated by representative extrapolation model using GraphPad
Prism software.
Statistical analysis
We used descriptive statistics to characterize the
groups. Comparison of parametric data was performed with the
Mann-Whitney test and of the non-parametric data with Spearman’s
rank correlation. In order to evaluate the association between the
expression of the serum biomarkers as quantitative variables, a
bivariate correlation was performed, with Spearman’s correlation
test by using GraphPad Prism 6 (GraphPad Software, Inc.). Simple
and multiple linear regression analysis were performed using SPSS
version 15.0 (SPSS Inc.). For all tests, P≤0.05 was considered to
indicate a statistically significant difference.
Results
IBS group consisted of 42 patients, 30 females, 12
males: mean age, 55±14 years with a sex ratio of 1.4. Regarding IBS
types there were 21 patients (50%) IBS-C, 14 patients (33.33%)
IBS-D and 7 patients (16.66%) IBS-M. Control group included 35
individuals, 18 females, 17 males; mean age, 50±16 years. Mean
serum levels for the two biomarkers are displayed in Table I.
 | Table IMean values in IBS and controls. |
Table I
Mean values in IBS and controls.
| Baseline data | Control (n=35) | IBS (n=42) |
P-valuea |
|---|
| Age |
| Mean ± SD | 49.73±16.31 | 55.39±14.15 | |
|
Females | 49. 88±15.63 | 55.17±14.57 | |
|
Males | 48.05±16.94 | 55.92±13.07 | |
|
P-valueb | 0.749 | 0.882 | 0.072 |
| BMI |
|
<18.5
kg/m2 | 5 | 8 | |
|
18.5-24.9
kg/m2 | 14 | 17 | |
|
>25
kg/m2 | 16 | 17 | |
| MCP-1 (pg/ml) |
|
Mean ±
SD | 174.26±13.39 | 203.65±129.99 | |
|
Females | 164.58±78.18 | 201.94± 141.79 | |
|
Males | 184.51±73.07 | 211.23±92.08 | |
|
P-valueb | 0.455 | 0.895 | 0.311 |
| NT (nM) |
|
Mean ±
SD | 34.93±14.39 | 30.36±11.65 | |
|
Females | 34.98±15.32 | 31.87±10.64 | |
|
Males | 34.86±12.86 | 26.58±13.11 | |
|
P-valueb | 0.980 | 0.192 | 0.050 |
All the samples evaluated had values within the
detection limits of the MCP-1 kit. The concentration interval for
the determination of MCP-1 in samples was between 15.6-1,000 pg/ml.
The serum levels of MCP-1 were higher in the IBS group, but not
statistically significant (204±130 vs. 174±73 pg/ml; P=0.311).
NT, a marker of RNS, has a detection limit between
20 and 8,000 nM accordingly to producer’s specifications. In our
study NT showed statistically significant lower levels (P=0.050)
for the IBS group (average, 30±12 nM) than for the control group
(average, 35±15 nM).
Bioinformatics analysis did not show a statistically
significant difference of the parameters analyzed in relation to
sex in the control group versus IBS patients: age, MCP-1, and NT
(Table II). Data regarding BMI in
the IBS group and in controls is listed in Table I.
 | Table IIStatistical analysis of IBS-D
(PI-IBS, non-PI-IBS) patients and controls. |
Table II
Statistical analysis of IBS-D
(PI-IBS, non-PI-IBS) patients and controls.
| Mean and
P-value | MCP-1 | NT |
|---|
| Mean values ±
SD |
|
PI-IBS | 343.89±215.00 | 27.48±15.66 |
|
Non
PI-IBS | 167.94±76.75 | 28.03±17.79 |
|
Control | 174.26±73.39 | 34.93±14.39 |
| P-value |
|
PI-IBS vs.
non-PI-IBS | 0.064 | 0.952 |
|
Control vs.
non-PI-IBS | 0.376 | 0.271 |
|
Control vs.
PI-IBS | 0.041 | 0.224 |
Biomarker levels in relation to IBS
subtypes
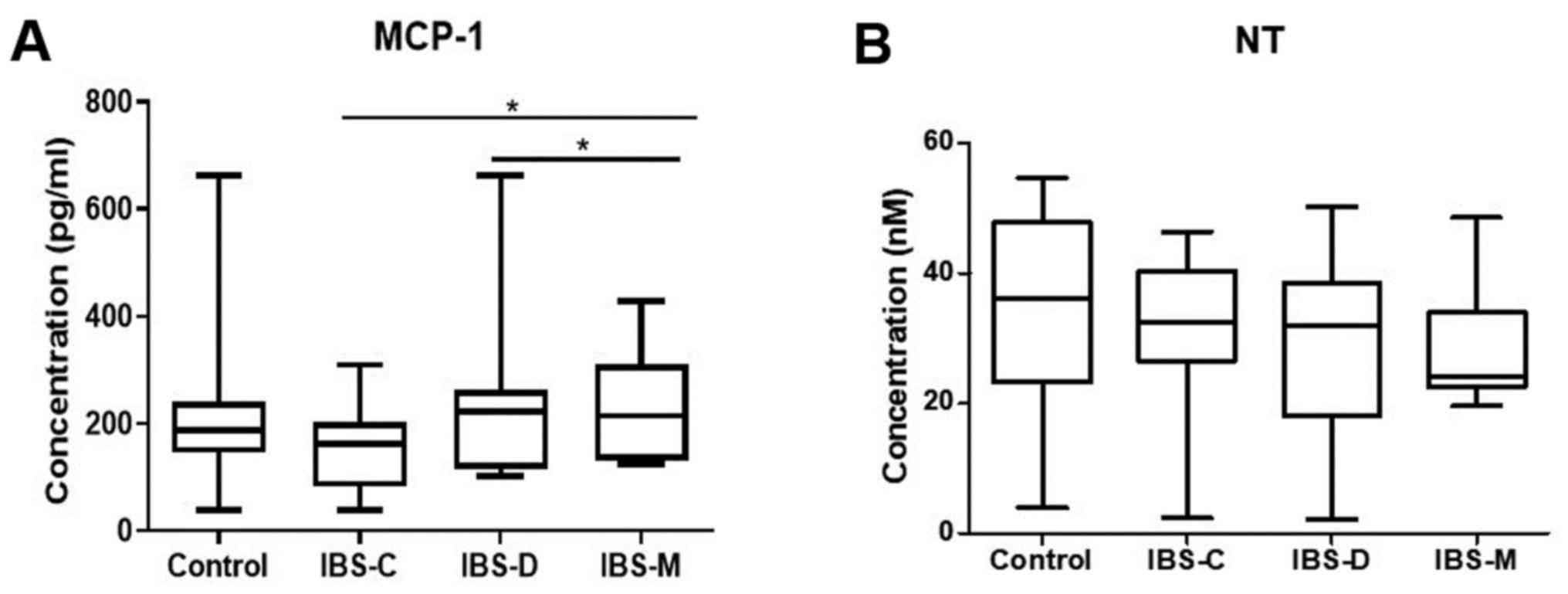
Fig. 1 presents the
serum levels for MCP-1 and NT in IBS subtypes and controls. For
MCP-1 serum levels were statistically significantly higher in IBS-D
patients (167±165 pg/ml; P=0.032) and IBS-M patients (236±92 pg/ml;
P=0.040) when compared with IBS-C (168±80 pg/ml).
Serum concentrations for NT had similar values in
the IBS subtypes (IBS-C, 33±33 nM; IBS-D, 29±15 nM; IBS-M, 30±11
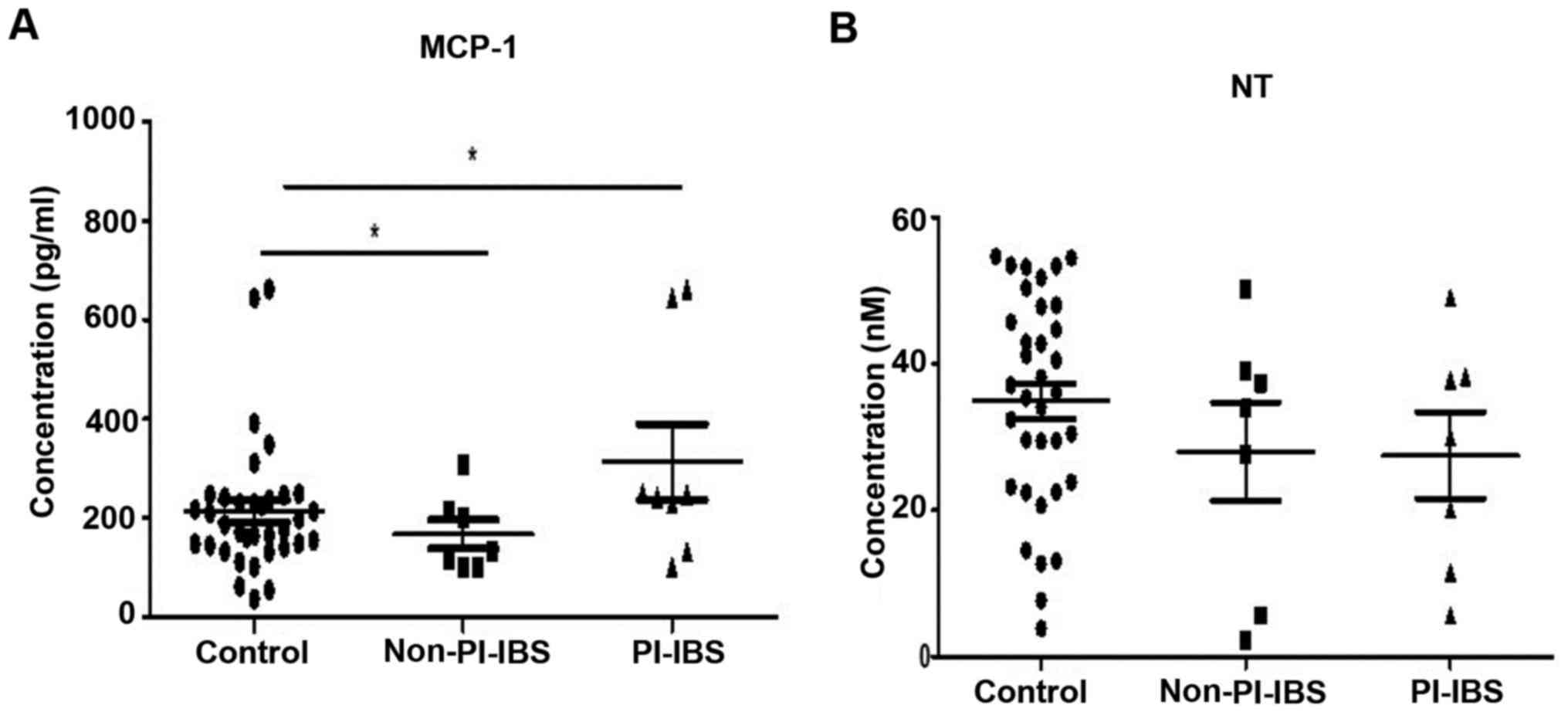
nM). Related to PI-IBS status, of the 42 patients with IBS, 8
patients (19%) were included as PI-IBS, of whom five were females.
Seven patients of the PI-IBS group were IBS-D (87.5%) and one
(12.5%) IBS-C. In the group of non-PI-IBS of 34 patients, there
were 7 patients with IBS-D, 20 patients with IBS-C and 7 patients
with IBS-M. MCP-1 had statistically significantly higher values
(P=0.004) in PI-IBS versus non-PI-IBS (310±211 vs. 163±89 pg/ml)
(Fig. 2).
Biomarkers, age and metabolic
syndrome
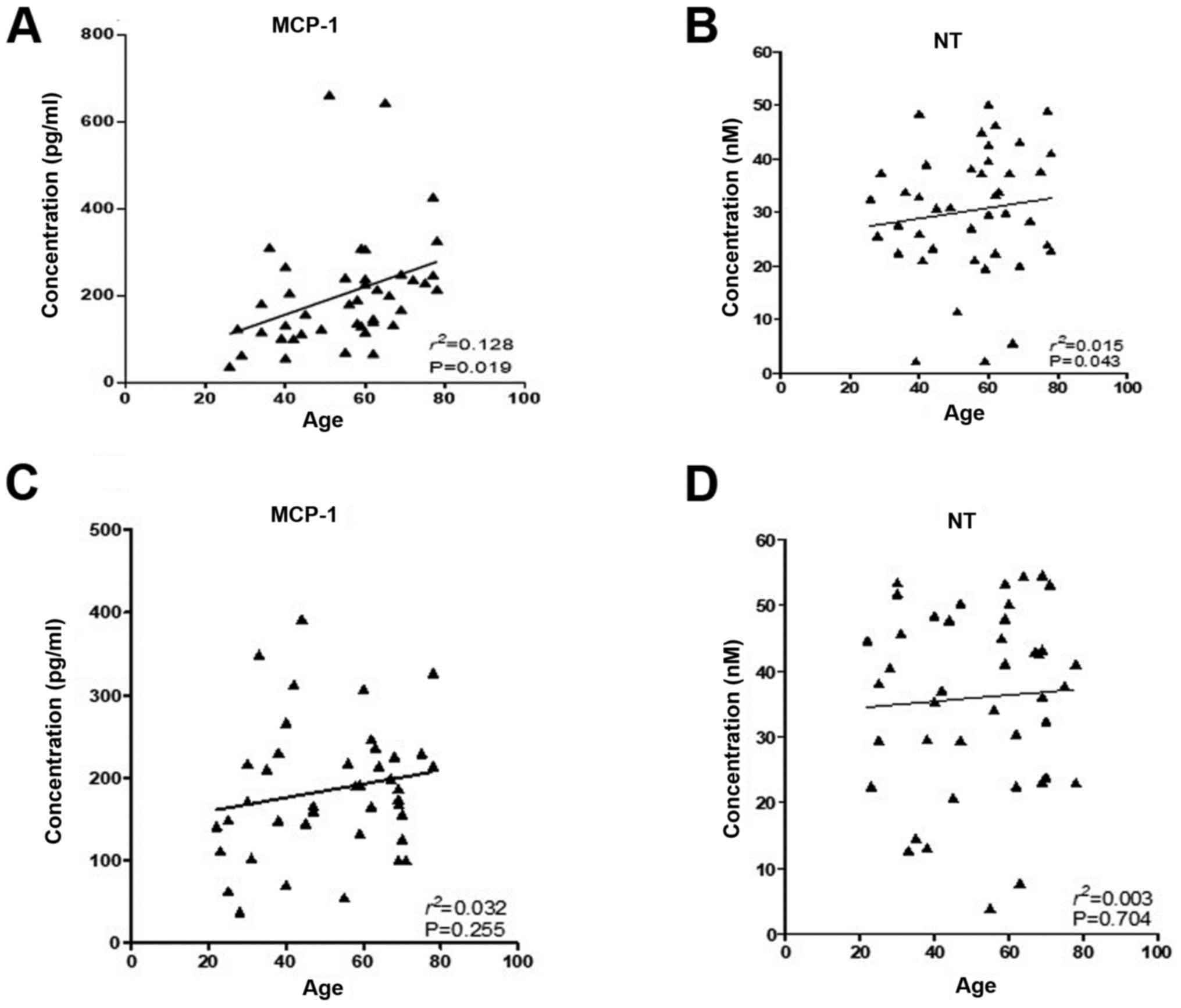
Fig. 3 presents
correlation of the biomarkers analyzed in relation with age in IBS
and control group, and statistical data are displayed in Table S1. Only MCP-1 concentrations were
statistically significantly correlated with age (P=0.019,
R2=0.128) in the IBS group. Spearman’s correlation for
serum biomarker expression and P-values are listed in Table S2.
MCP-1 levels were higher in IBS patients with
metabolic syndrome vs. IBS patients without metabolic syndrome
(239±153 vs. 168±120 pg/ml; P=0.948), controls with metabolic
syndrome (174±56 pg/ml) or controls without metabolic syndrome
(157±89 pg/ml). MCP-1 serum levels were statistically significantly
higher in IBS patients with metabolic syndrome than in controls
(239±153 vs. 157±89 pg/ml; P=0.037). NT levels were statistically
significantly lower in IBS patients without metabolic syndrome than
in controls without metabolic syndrome (30±11 vs. 38±11 nM;
P=0.004).
Discussion
IBS is a complex functional gastrointestinal
disorder, highly prevalent worldwide, with most of the studies
indicating a female predominance (3). Although there are many studies
addressed to its pathogenesis, it still remains incompletely
unraveled, and there is a need for a reliable diagnostic
biomarker.
The available biomarkers or the ones investigated in
IBS have recently been reviewed (26,27). In
our study, we looked at two biomarkers: MCP-1 and NT. They were
chosen to define as possible biomarkers, due to paucity of data
involving them in IBS. There are scarce data in literature
concerning MCP-1 in IBS and also concerning oxidative stress and
its relation to IBS, indicating that patients with IBS have higher
levels of oxidative stress. Regarding NT, to our knowledge, there
are no studies assessing NT, as a marker of reactive oxygen or
nitrogen species in IBS patients.
The first study that investigated MCP-1 serum levels
found that the levels were elevated in IBS patients (20). However, MCP-1 levels were not
significantly different between idiopathic IBS and PI-IBS (20). Data suggest that even if the
etiopathogenesis is different, both forms of IBS, PI-IBS and
idiopathic IBS respectively, present similar phenotypes (20). In a previous study, Tülübaş et
al (28) found that MCP-1 levels
were significantly higher (P=0.000) in the IBS group compared with
the control group, concordant with the study of Darkoh et al
(20). Our data are consistent with
these studies, with MCP-1 serum higher in the IBS group, but we
found lower levels than the two previous studies (20,28). A
previous study also reported that plasmatic concentrations of MCP-1
did not exhibit differences in IBS patients with vestibulodinia
versus healthy controls (29). Lower
levels in healthy controls than those reported by Darkoh et
al (20) and Tülübaş et
al (28) were found also by
other studies (29,30).
There are no data available regarding MCP-1 levels
in IBS subgroups (D, C and M) our data indicate statistically
significant higher serum levels in IBS-D patients (P=0.032) and
IBS-M patients (P=0.040) when compared with IBS-C. Regarding age
and MCP-1 levels, a previous study found that MCP-1 levels increase
with age in healthy individuals (31) supporting the hypothesis that MCP-1
concentration could attest existence of atherosclerosis as
suggested by other literature data (31). Same authors also observed a sex
difference, higher levels being found in males (31). In another study, MCP-1 levels were
not related to age or sex (30). In
our study, MCP-1 levels increased with age (P=0.001), which might
explain similar serum levels in controls and IBS, higher MCP-1
serum levels being observed in elderly (Fig. 3).
A possible explanation for the difference
encountered in various studies might be variations in methodology
(including type of kit) used or mainly genetic polymorphisms,
hypothesis supported by literature data regarding MCP-1
polymorphism that has already been investigated in several
conditions (32,33). A genetic polymorphism of MCP-1 and
the risk of inflammatory bowel disease development have been
reported. This opens the perspectives to investigate this
polymorphism also in IBS.
Since some of the patients exclude certain food
groups (such as fermentable oligo-, di-, monosaccharides and
polyols) they replace them with other groups such as lipids, which
may be related to their BMI. Other studies also found an important
percentage of the study group to be overweight (29% of healthy
controls and 27% of IBS patients) (34). It is possible that overweight status
might influence some symptoms or their persistence in IBS (34). Also, higher BMI was in some studies
associated with reduced psychological well-being (35).
PI-IBS, accounted for 19% of IBS subjects in this
study, our data indicate statistically significant higher MCP-1
values in PI-IBS versus non-PI-IBS (P=0.004). Also for the subgroup
of PI-IBS in IBS-D we found a statistically significant difference
when IBS-D were compared to control group (P=0.001), supporting
infection-inflammation pathway in IBS etiopathogenesis and
confirming their value as biomarkers.
A role for inflammatory, oxidative and nitrosative
stress in inducing psychosomatic symptoms have been found in
chronic fatigue, somatization disorders (36), ROS being involved in many
inflammatory conditions, including those of the gastrointestinal
tract (37).
The first study that investigated oxidative stress
species in IBS found higher levels of malondialdehyde and nitric
oxide in IBS patients versus control in plasma (P<0.01
respectively P<0.05) (24). The
explanation for our results, with lower values of NT in IBS and
also the NT levels statistically significantly lower in IBS without
metabolic syndrome than in controls without metabolic syndrome
(P=0.004) group might be the results of diet and lifestyle adopted
by patients with IBS, either self-imposed, or as a medical
recommendation, which may lead subsequently to lower levels of
reactive oxygen species.
Biomarkers have been studied in serum. The study of
Yu et al (38) showed that
even if serum and plasma concentrations of biomarkers are in
general similar, they found higher metabolite concentrations in
serum, suggesting that serum would provide more sensitive results
in biomarker detection.
Data regarding inflammatory status were not
available for the previous study (24). In our group of patients, active
inflammation was a rule out criteria, based on current inflammatory
markers. However, both our and Mete et al (24) studies were conducted on a small
number of cases, therefore it is possible that in a relatively
small IBS patient group significant differences might not be
visible.
Literature data regarding MCP-1 in IBS patients are
limited (28), and there is no data
regarding NT in IBS patients, most of these studies being conducted
on small number of patients.
Analyzing the levels of the two serum biomarkers, we
found that lipid profile does not correlate with MCP-1 or NT. Also,
other study did not find a correlation between NT plasma levels and
oxidized low-density lipoprotein in the patient group (Alzheimer’s
disease) (39). Though it was not
the primary aim of our study we found a statistically significant
difference with higher MCP-1 levels in IBS patients with metabolic
syndrome versus controls without metabolic syndrome (P=0.037),
which supports previous data that showed that elevated MCP-1 levels
contribute to the development of certain pathologies associated
with hyperinsulinemia and obesity (40), such as in our case the metabolic
syndrome.
In conclusion, MCP-1 levels were significantly
higher in IBS patients with metabolic syndrome than in controls,
while nitrotyrosine levels were significantly lower in the IBS
patients, suggesting multiple factors being involved, particularly
the diet and its relation with the metabolic syndrome. MCP-1 levels
increase with age, suggesting that MCP-1 could represent a marker
for subclinical atherosclerosis. Low-grade inflammation that might
be related to lipid peroxidation or oxidative stress could play an
underestimated role in the pathogenesis of IBS. We consider that
nutritional status and diet should be more frequently assessed in
studies that investigate FGID and biomarkers in order to detect
other potential liaisons.
Supplementary Material
Simple and multiple linear regression
results for predictors of MCP-1 and NT.
Correlations and values established
between the serum biomarkers in IBS and control groups..
Acknowledgements
Not applicable.
Funding
The European Social Fund Human Resources Development
Operational Programme 2007-2013, funded this study, project no.
POSDRU/159/1.5/S/138776 TRANSCENT. The funders had no role in study
design, data collection and analysis, decision to publish, or
preparation of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available in part also from the Supplementary files and
from the corresponding author on reasonable request.
Authors’ contributions
AC and RIC drafted the manuscript. AC and DLD
designed the study. CB and LB performed the biomarker assessment
and part of the analysis and interpretation of data under the
supervision of IBN. AC and RIC acquired part of data and performed
part of data analysis and interpretation. RIC, IBN and DLD
critically revised the manuscript. All the authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the ‘Iuliu Haţieganu’ University of Medicine and Pharmacy,
(Cluj-Napoca, Romania). Signed informed consent was obtained from
the patients for the inclusion in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ford AC, Moayyedi P, Lacy BE, Lembo AJ,
Saito YA, Schiller LR, Soffer EE, Spiegel BM and Quigley EM: Task
Force on the Management of Functional Bowel Disorders: American
College of Gastroenterology monograph on the management of
irritable bowel syndrome and chronic idiopathic constipation. Am J
Gastroenterol. 109 (Suppl 1):S2–S26; quiz S27. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Longstreth GF: Definition and
classification of irritable bowel syndrome: Current consensus and
controversies. Gastroenterol Clin North Am. 34:173–187.
2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Longstreth GF, Thompson WG, Chey WD,
Houghton LA, Mearin F and Spiller RC: Functional bowel disorders.
Gastroenterology. 130:1480–1491. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gralnek IM, Hays RD, Kilbourne A, Naliboff
B and Mayer EA: The impact of irritable bowel syndrome on
health-related quality of life. Gastroenterology. 119:654–660.
2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Drossman DA: Review article: An integrated
approach to the irritable bowel syndrome. Aliment Pharmacol Ther.
13 (Suppl 2):3–14. 1999.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chaudhary NA and Truelove SC: The
irritable colon syndrome. A study of the clinical features,
predisposing causes, and prognosis in 130 cases. Q J Med.
31:307–322. 1962.PubMed/NCBI
|
|
7
|
McKendrick MW: Post Salmonella
irritable bowel syndrome - 5 year review. J Infect. 32:170–171.
1996.PubMed/NCBI View Article : Google Scholar
|
|
8
|
David LE, Surdea-Blaga T and Dumitrascu
DL: Semiquantitative fecal calprotectin test in postinfectious and
non-postinfectious irritable bowel syndrome: cross-sectional study.
Sao Paulo Med J. 133:343–349. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Spiller RC, Jenkins D, Thornley JP, Hebden
JM, Wright T, Skinner M and Neal KR: Increased rectal mucosal
enteroendocrine cells, T lymphocytes, and increased gut
permeability following acute Campylobacter enteritis and in
post-dysenteric irritable bowel syndrome. Gut. 47:804–811.
2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bashashati M, Moossavi S, Cremon C,
Barbaro MR, Moraveji S, Talmon G, Rezaei N, Hughes PA, Bian ZX,
Choi CH, et al: Colonic immune cells in irritable bowel syndrome: A
systematic review and meta-analysis. Neurogastroenterol Motil.
30(30)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Camilleri M: Peripheral mechanisms in
irritable bowel syndrome. N Engl J Med. 367:1626–1635.
2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jeffery IB, O’Toole PW, Öhman L, Claesson
MJ, Deane J, Quigley EM and Simrén M: An irritable bowel syndrome
subtype defined by species-specific alterations in faecal
microbiota. Gut. 61:997–1006. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shepherd SJ, Parker FC, Muir JG and Gibson
PR: Dietary triggers of abdominal symptoms in patients with
irritable bowel syndrome: Randomized placebo-controlled evidence.
Clin Gastroenterol Hepatol. 6:765–771. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lembo AJ, Neri B, Tolley J, Barken D,
Carroll S and Pan H: Use of serum biomarkers in a diagnostic test
for irritable bowel syndrome. Aliment Pharmacol Ther. 29:834–842.
2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Snape WJ Jr, Carlson GM, Matarazzo SA and
Cohen S: Evidence that abnormal myoelectrical activity produces
colonic motor dysfunction in the irritable bowel syndrome.
Gastroenterology. 72:383–387. 1977.PubMed/NCBI
|
|
16
|
Stasi C, Rosselli M, Bellini M, Laffi G
and Milani S: Altered neuro-endocrine-immune pathways in the
irritable bowel syndrome: The top-down and the bottom-up model. J
Gastroenterol. 47:1177–1185. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yoshimura T, Robinson EA, Tanaka S,
Appella E and Leonard EJ: Purification and amino acid analysis of
two human monocyte chemoattractants produced by
phytohemagglutinin-stimulated human blood mononuclear leukocytes. J
Immunol. 142:1956–1962. 1989.PubMed/NCBI
|
|
18
|
Yoshimura T, Yuhki N, Moore SK, Appella E,
Lerman MI and Leonard EJ: Human monocyte chemoattractant protein-1
(MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated
blood mononuclear leukocytes, and sequence similarity to mouse
competence gene JE. FEBS Lett. 244:487–493. 1989.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Goede V, Brogelli L, Ziche M and Augustin
HG: Induction of inflammatory angiogenesis by monocyte
chemoattractant protein-1. Int J Cancer. 82:765–770.
1999.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Darkoh C, Comer L, Zewdie G, Harold S,
Snyder N and Dupont HL: Chemotactic chemokines are important in the
pathogenesis of irritable bowel syndrome. PLoS One.
9(e93144)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Iyonaga K, Takeya M, Saita N, Sakamoto O,
Yoshimura T, Ando M and Takahashi K: Monocyte chemoattractant
protein-1 in idiopathic pulmonary fibrosis and other interstitial
lung diseases. Hum Pathol. 25:455–463. 1994.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gerrity RG, Naito HK, Richardson M and
Schwartz CJ: Dietary induced atherogenesis in swine. Morphology of
the intima in prelesion stages. Am J Pathol. 95:775–792.
1979.PubMed/NCBI
|
|
23
|
Oran M, Tulubas F, Mete R, Aydin M,
Sarikaya HG and Gurel A: Evaluation of paraoxonase and arylesterase
activities in patients with irritable bowel syndrome. J Pak Med
Assoc. 64:820–822. 2014.PubMed/NCBI
|
|
24
|
Mete R, Tulubas F, Oran M, Yılmaz A, Avci
BA, Yildiz K, Turan CB and Gurel A: The role of oxidants and
reactive nitrogen species in irritable bowel syndrome: A potential
etiological explanation. Med Sci Monit. 19:762–766. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Stermer E, Lubezky A, Potasman I, Paster E
and Lavy A: Is traveler’s diarrhea a significant risk factor for
the development of irritable bowel syndrome?A prospective study.
Clin Infect Dis. 43:898–901. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Sood R, Gracie DJ, Law GR and Ford AC:
Systematic review with meta-analysis: The accuracy of diagnosing
irritable bowel syndrome with symptoms, biomarkers and/or
psychological markers. Aliment Pharmacol Ther. 42:491–503.
2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chira A and Dumitrascu DL: Serum
biomarkers for irritable bowel syndrome. Clujul Med. 88:258–264.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Tülübaş F, Oran M, Mete R, Turan F, Yilmaz
A, Yildiz ZD and Gürel A: Investigation of serum macrophage
migration inhibitor factor and monocyte chemotactic protein-1
levels in irritable bowel syndrome. Turk J Med Sci. 44:967–971.
2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hashimoto S, Nakayama T, Gon Y, Hata N,
Koura T, Maruoka S, Matsumoto K, Hayashi S, Abe Y and Horie T:
Correlation of plasma monocyte chemoattractant protein-1 (MCP-1)
and monocyte inflammatory protein-1alpha (MIP-1alpha) levels with
disease activity and clinical course of sarcoidosis. Clin Exp
Immunol. 111:604–610. 1998.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Arakelyan A, Petrkova J, Hermanova Z,
Boyajyan A, Lukl J and Petrek M: Serum levels of the MCP-1
chemokine in patients with ischemic stroke and myocardial
infarction. Mediators Inflamm. 2005:175–179. 2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Inadera H, Egashira K, Takemoto M, Ouchi Y
and Matsushima K: Increase in circulating levels of monocyte
chemoattractant protein-1 with aging. J Interferon Cytokine Res.
19:1179–1182. 1999.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen S, Deng C, Hu C, Li J, Wen X, Wu Z
and Li Y, Zhang F and Li Y: Association of MCP-1-2518A/G
polymorphism with susceptibility to autoimmune diseases: A
meta-analysis. Clin Rheumatol. 35:1169–1179. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Arakelyan A, Zakharyan R, Hambardzumyan M,
Petrkova J, Olsson MC, Petrek M and Boyajyan A: Functional genetic
polymorphisms of monocyte chemoattractant protein 1 and C-C
chemokine receptor type 2 in ischemic stroke. J Interferon Cytokine
Res. 34:100–105. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sadik R, Björnsson E and Simrén M: The
relationship between symptoms, body mass index, gastrointestinal
transit and stool frequency in patients with irritable bowel
syndrome. Eur J Gastroenterol Hepatol. 22:102–108. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Carr D and Friedman MA: Is obesity
stigmatizing? Body weight, perceived discrimination, and
psychological well-being in the United States. J Health Soc Behav.
46:244–259. 2005.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Maes M: Inflammatory and oxidative and
nitrosative stress pathways underpinning chronic fatigue,
somatization and psychosomatic symptoms. Curr Opin Psychiatry.
22:75–83. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Parks DA, Bulkley GB and Granger DN: Role
of oxygen-derived free radicals in digestive tract diseases.
Surgery. 94:415–422. 1983.PubMed/NCBI
|
|
38
|
Yu Z, Kastenmüller G, He Y, Belcredi P,
Möller G, Prehn C, Mendes J, Wahl S, Roemisch-Margl W, Ceglarek U,
et al: Differences between human plasma and serum metabolite
profiles. PLoS One. 6(e21230)2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhao Z, Zhou H, Peng Y, Qiu CH, Sun QY,
Wang F and Xie HN: Expression and significance of plasma 3-NT and
ox-LDL in patients with Alzheimer’s disease. Genet Mol Res.
13:8428–8435. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sartipy P and Loskutoff DJ: Monocyte
chemoattractant protein 1 in obesity and insulin resistance. Proc
Natl Acad Sci USA. 100:7265–7270. 2003.PubMed/NCBI View Article : Google Scholar
|