Introduction
Despite dual anti-platelet and intensive statin
therapy, in-stent restenosis (ISR) remains the Achilles heel of
percutaneous coronary intervention (PCI) in the era of drug-eluting
stents (DES); it has been reported that ISR occurs in 12.2-14.6% of
patients with a drug-eluting stent, depending on patient-associated
factors, as well as lesion and procedure-associated
characteristics, including the stenting technique and stent type
(1,2). The pathophysiological mechanisms of ISR
have remained to be fully elucidated. The major trend of the
current viewpoint supports inflammatory mechanisms of adverse
responses to DES. It is suggested that the abnormal proliferation
and migration of vascular smooth muscle cells (VSMCs), as well as
extracellular matrix accumulation activated by various
pro-inflammatory mediators and growth factors in response to local
vascular injury, result in neointima formation and eventually lumen
loss (3-6).
Therefore, identifying a specific, sensitive and non-invasive blood
biomarker for predicting ISR is of clinical significance in order
to select tailored management strategies, including the use of
multiple stents or offering more prolonged anti-platelet therapy to
patients with coronary artery disease (CAD) with an elevated risk
of ISR.
S100 calcium-binding protein A12 (S100A12) is a
member of the S100 family of calcium-binding proteins, which are
predominantly expressed and secreted by myeloid-derived cells,
including neutrophils and monocytes (7). In the past 10 years, the roles of
S100A12 in inflammatory diseases have been gradually determined. It
has been indicated that the pro-inflammatory effects of S100A12 are
mediated through interaction with MOK protein kinase and Toll-like
receptor (TLR)4, which have important roles in mediating acute and
chronic inflammation and are relevant for atherosclerosis (8). Elevated serum levels of S100A12 have
been reported in a number of inflammatory disorders, including
chronic active inflammatory bowel disease, Kawasaki disease,
rheumatoid arthritis, Behcet's disease and glomerulonephritis
(9-12).
Recent studies suggest the biological and clinical
involvement of S100A12 in atherosclerosis. S100A12 was also
indicated to participate in atherosclerosis by mast cell and
monocyte recruitment as well as mast cell activation (13). Animal experiments in transgenic mice
with smooth muscle cell-targeted expression of S100A12 suggested
more severe atherosclerosis, an increased necrotic core, calcified
plaque area and reduced extracellular matrix in apolipoprotein E
(ApoE)-/- mice (14). In
fact, strong immunostaining for S100A12 has been detected in
advanced atherosclerosis, particularly at the rupture sites of
atherosclerotic plaques of subjects with sudden cardiac death,
suggesting a potential association of locally expressed S100A12
with plaque instability (15).
Mahajan et al (16) indicated
that S100A12 exacerbated atherosclerosis by promoting
pro-inflammatory cytokines in an autocrine, paracrine and endocrine
manner at the site of atherosclerotic lesions via activation of
RAGE signaling. Ligthart's study demonstrated the predictive value
of serum S100A12 for future myocardial infarction (MI) and
CAD-associated mortality in 839 healthy participants over a
10.6-year follow-up after adjusting for inflammatory markers and
traditional risk factors (17).
Another cross-sectional study analyzed 652 patients with stable CAD
who underwent PCI, revealing that the serum levels of S100A12 were
an independent factor for predicting major adverse cardiovascular
events, defined as a composite of events of congestive heart
failure, recurrence of angina pectoris, acute myocardial
infarction, stroke, critical arrhythmia, intervention on peripheral
arteries and cardiac death (18).
Given the pro-inflammatory characteristics of
S100A12 and its potential implications in the initiation and
progression of atherosclerosis and CAD, it was speculated that
increased serum levels of S100A12 at baseline may contribute to
restenosis in patients with stent implantation. To test this
hypothesis, the serum levels of S100A12 were compared between
consecutive patients with angiographically documented ISR and
patients with no ISR after DES-based PCI, and the predictive value
of circulating S100A12 levels for ISR was also assessed.
Patients and methods
Patient population
All of the patients (n=2,443) who underwent coronary
angiography (CAG) and subsequent DES-based PCI of de novo
lesions in native coronary arteries between October 2014 and June
2018 at the Department of Cardiology, Affiliated Provincial
Hospital, Shandong University (Jinan, China) were retrospectively
screened. Each patient was routinely treated with dual
anti-platelet therapy (aspirin for an indefinite period and a P2Y12
inhibitor for at least 1 month and up to 12 months) after stent
deployment. At ~1 year after the procedure, 642 patients were
subjected to follow-up CAG due to recurrent symptoms of abnormal
non-invasive test results for angina (either treadmill exercise
tests or myocardial perfusion scintigraphy), and ISR was detected
in 308 patients. Of these 308 patients, those who had concomitant
valvular disease (n=8), systematic inflammatory disease (n=16),
malignant tumor (n=10), severe liver disease (n=6), moderate to
severe chronic renal insufficiency [estimated glomerular filtration
rate (eGFR) <60 ml/min/1.73 m2; n=10] were excluded.
The remaining 258 patients with ISR constituted the population of
the present study. In addition, 258 age- and sex-matched patients
who had no ISR at the follow-up for CAG within the same study
period were randomly selected as the control group. The protocol
was approved by the institutional review board of Shandong
Provincial Hospital (Jinan, China).
CAG study
Coronary angiography was performed according to
standard Judkins techniques. Coronary cineangiography was
interpreted by two cardiologists who were blinded to each patient's
clinical characteristics and laboratory test results. Using the
outer diameter of the contrast-filled catheter as the calibration
standard, the minimal lumen diameter was measured in diastolic
frames from orthogonal projections. The reference vessel diameter
was averaged from user-defined, 5-mm, angiographically normal
segments proximal and distal to the lesion but between any major
side branches. The lesion was stented using a normal-to-normal
technique, usually including 5-mm, angiographically normal segments
proximal and distal to the lesion. A successful PCI was considered
as achieving a minimum stenosis diameter reduction to <30% in
the absence of any branch loss, dissection or thrombus formation.
ISR was defined as recurrence of luminal diameter narrowing by
>50% in a vessel of otherwise normal diameter, including 5 mm
proximal and distal to the stent edge.
Evaluation of risk factors
Demographic and clinical characteristics were
obtained by reviewing the hospital records of patients. Age, sex,
body mass index (BMI), family history of CAD, smoking status,
prevalence of concomitant disease, including hypertension, type 2
diabetes, hypercholesterolemia, as well as laboratory data,
including fasting blood glucose, cholesterol, high-/low-density
lipoprotein cholesterol and cardiac troponin T, were all recorded.
Hypertension, type 2 diabetes and hypercholesterolemia were
diagnosed in accordance with the European Society of
Hypertension/European Society of Cardiology guidelines for the
management of arterial hypertension (19), the criteria of the American Diabetes
Association and the Third Report of The National Cholesterol
Education Program (20,21), respectively. Family history of CAD
was defined as the presence of CAD or sudden cardiac death in a
first-degree relative prior to the age of 55 years for males and
prior to the age of 65 years for females.
Blood sampling and measurements of
serum S100A12
Peripheral blood samples from all patients had been
obtained upon admission prior to coronary angiography after
overnight fasting. Blood samples for measurement of S100A12 were
collected in tubes containing potassium EDTA and then centrifuged
for 20 min at 670.8 x g and 4˚C and stored at -80˚C until analysis.
S100A12 levels in serum were determined using the Human S100A12
Platinum ELISA (eBioscience) according to the manufacturer's
protocol. The overall intra- and inter-assay coefficient of
variation were 5.5 and 11.9%, respectively. All measurements were
performed in a blinded manner in duplicate.
Statistical analysis
The Kolmogorov-Smirnov test was used to assess the
distribution pattern of data. Continuous variables were expressed
as the mean ± standard deviation or median with interquartile range
and were compared by analysis of one-way ANOVA or unpaired
Student's t-test. Categorical variables were expressed as counts
and percentages and were compared by using the χ2 test.
Patients with ISR were categorized into 4 groups according to the
quartile distribution of serum S100A12 levels (µg/ml). Spearman's
correlation coefficient was determined to evaluate the association
between two continuous variables.
Two multivariate stepwise logistic regression models
were constructed to determine the association between ISR and
circulating levels of S100A12. In Model 1, age, sex, BMI,
conventional risk factors, biochemical parameters, medication,
angiographic and procedure-associated features, were included. In
model 2, the analysis was additionally adjusted for the serum
levels of S100A12. The two models were tested for their ability to
predict ISR using receiver operating characteristic (ROC) analysis,
and the areas under the ROC curve (C statistics) were compared
using MedCalc software for Windows (version 5.0; MedCalc Software)
to evaluate whether S100A12 levels have an incremental predictive
value for ISR. Net reclassification improvements (NRI) and
integrated discrimination improvements (IDI) were also calculated
to assess the improvement of the regression model with further
inclusion of the circulating S100A12 concentration. The calibration
of the model was checked using the Hosmer-Lemeshow χ2
test. All tests were 2-tailed and P<0.05 was considered to
indicate statistical significance. All analyses were performed
using SPSS version 19.0 for Windows (IBM Corp.).
Results
Clinical characteristics
A total of 516 patients were enrolled in the present
study. The mean age in the study population was 61.2±10.2 years and
361 (69.9%) subjects were males. The baseline clinical, biochemical
and angiographic characteristics of patients with and without ISR
are detailed in Table I. The
prevalence rates of hypertension was similar in the two groups.
Patients with ISR were more likely to have a family history of CAD
(P<0.001), to smoke (P<0.001) and to have hyperlipidemia
(P=0.033) and diabetes mellitus (P=0.046) compared with those
without ISR, and they had a higher BMI (P=0.033).
 | Table IBaseline clinical, biochemical and
angiographic characteristics of the study population. |
Table I
Baseline clinical, biochemical and
angiographic characteristics of the study population.
| Parameter | ISR(-) (n=258) | ISR(+) (n=258) | P-value |
|---|
| Age (years) | 59.1 ± 10.4 | 61.8 ± 10.1 | 0.569 |
| Sex
(male/female) | 178/80 | 183/75 | 0.631 |
| Body mass index
(kg/m2) | 26.82 ± 3.96 | 29.19 ± 3.41 | 0.001 |
| Systolic blood
pressure (mmHg) | 137.6 ± 18.2 | 139.8 ± 20.7 | 0.329 |
| Diastolic blood
pressure (mmHg) | 85.1 ± 10.1 | 84.7 ± 12.8 | 0.239 |
| Family history of
CAD | 33 (12.8) | 68 (26.4) | <0.001 |
| Cardiovascular risk
factors |
|
Hypertension | 192 (74.4) | 181 (70.2) | 0.279 |
|
Diabetes
mellitus | 70 (27.1) | 91 (35.3) | 0.046 |
|
Hyperlipidemia | 78 (29.7) | 97 (40.8) | 0.033 |
|
Smoking | 76 (27.5) | 133 (51.6) | <0.001 |
| Biochemical
measurements |
|
Total
cholesterol (mmol/l) | 4.85 ± 1.09 | 4.96 ± 1.13 | <0.005 |
|
LDL-cholesterol
(mmol/l) | 2.77 ± 0.83 | 2.91 ± 1.54 | <0.001 |
|
HDL-cholesterol
(mmol/l) | 1.25 ± 0.31 | 1.13 ± 0.28 | <0.001 |
|
Triglyceride
(mmol/l) | 1.93 ± 1.51 | 1.96 ± 1.75 | 0.326 |
|
White blood
cells (x109/l) | 7.82 ± 1.51 | 7.22 ± 2.08 | 0.369 |
|
Fibrinogen
(g/l) | 3.11 ± 1.17 | 3.22 ± 0.91 | 0.129 |
|
Fasting
glucose (mmol/l) | 5.72 ± 1.42 | 5.92 ± 1.96 | 0.251 |
|
eGFR
(ml/min/1.73 m2) | 83.74 ± 26.04 | 86.01 ± 22.06 | 0.170 |
|
hsCRP
(mg/l) | 5.33 ± 2.55 | 5.47 ± 6.02 | 0.087 |
|
S100A12
(ng/ml) | 32.61 ± 5.82 | 39.84 ± 9.15 | <0.001 |
| LVEF (%) | 64.6 ± 3.7 | 58.6 ± 5.3 | 0.005 |
| Coronary
angiography Gensini score | 62.4 ± 31.16 | 62.2 ± 36.9 | 0.129 |
| Lesion
characteristics |
|
CTO | 21 (8.1) | 55 (21.3) | <0.001 |
|
Bifurcation
lesions | 53 (20.5) | 83 (32.2) | 0.003 |
|
LM
stenosis | 16 (6.2) | 16 (6.2) | 1.0 |
| Stent
characteristics |
|
Number of
stents | 1.91 ± 1.1 | 2.1 ±1.3 | <0.001 |
|
Diameter
(mm) | 3.24 ± 0.34 | 3.18 ± 0.39 | <0.001 |
|
Length
(mm) | 34.2 ± 22.3 | 42.8 ± 27.8 | 0.008 |
| Stent type |
|
Sirolimus-eluting
stent | 169 | 176 (68.2) | 0.513 |
|
Zotarolimus-eluting
stent | 53 | 48 (18.6) | 0.579 |
|
Everolimus-eluting
stent | 20 | 16 (6.2) | 0.489 |
|
Tacrolimus-eluting
stent | 16 | 18 (7.0) | 0.814 |
| Cardiac medication
after PCI |
|
Dual
anti-platelet therapy | 253 (98.1) | 255 (98.8) | 0.476 |
|
β-blockers | 205 (79.5) | 220 (85.3) | 0.083 |
|
ACEI/ARB | 130 (50.4) | 152 (58.9) | 0.052 |
|
Calcium
antagonists | 105 (40.7) | 102 (39.5) | 0.778 |
|
Statins | 245(95) | 255 (98.8) | 0.011 |
As for the angiography results, despite the similar
degree of stenosis prior to PCI, left main coronary artery stenosis
and type of DES implanted, patients in the ISR group were observed
to more frequently have severe coronary lesions, including chronic
total occlusion and bifurcation lesions. Furthermore, patients with
ISR received smaller in diameter but longer stents and they had a
higher percentage of statin therapy after the procedure as compared
to patients without ISR.
Association between serum S100A12 and
ISR
The serum levels of S100A12 were significantly
elevated in patients in the ISR group compared with those in the
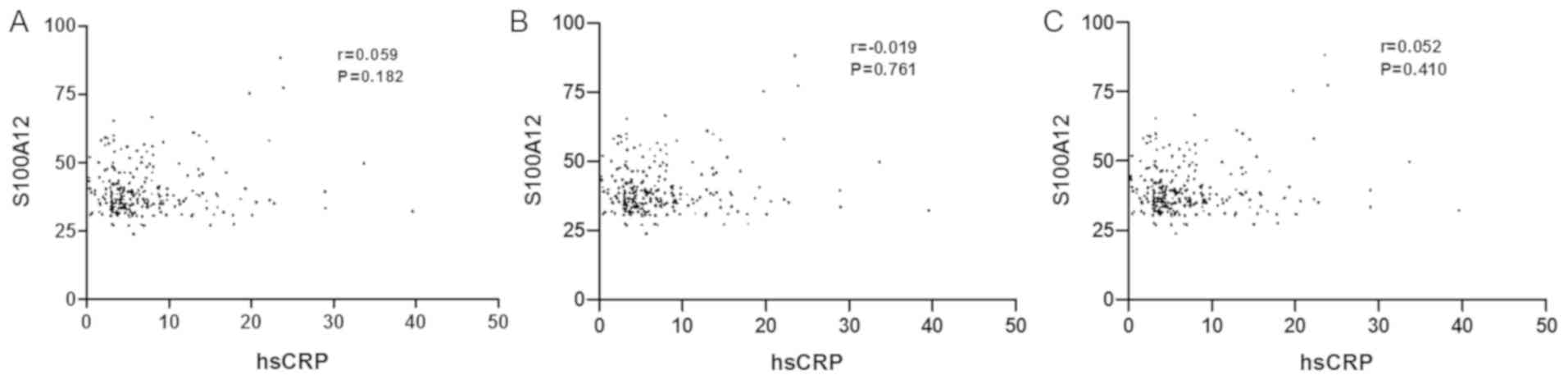
non-ISR group (P<0.001). However, no significant correlation was
observed between S100A12 and hsCRP (Fig.
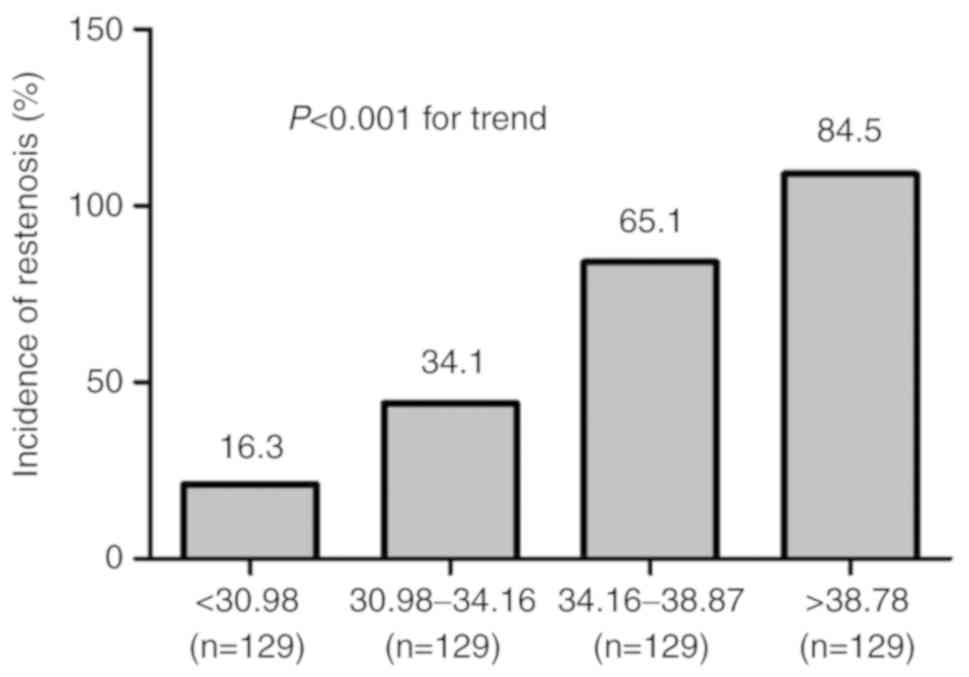
1). The patients were further categorized into four groups
according to the quartile distribution of S100A12. The incidence of
ISR increased gradually across the quartiles of S100A12
(P<0.001; Fig. 2). This
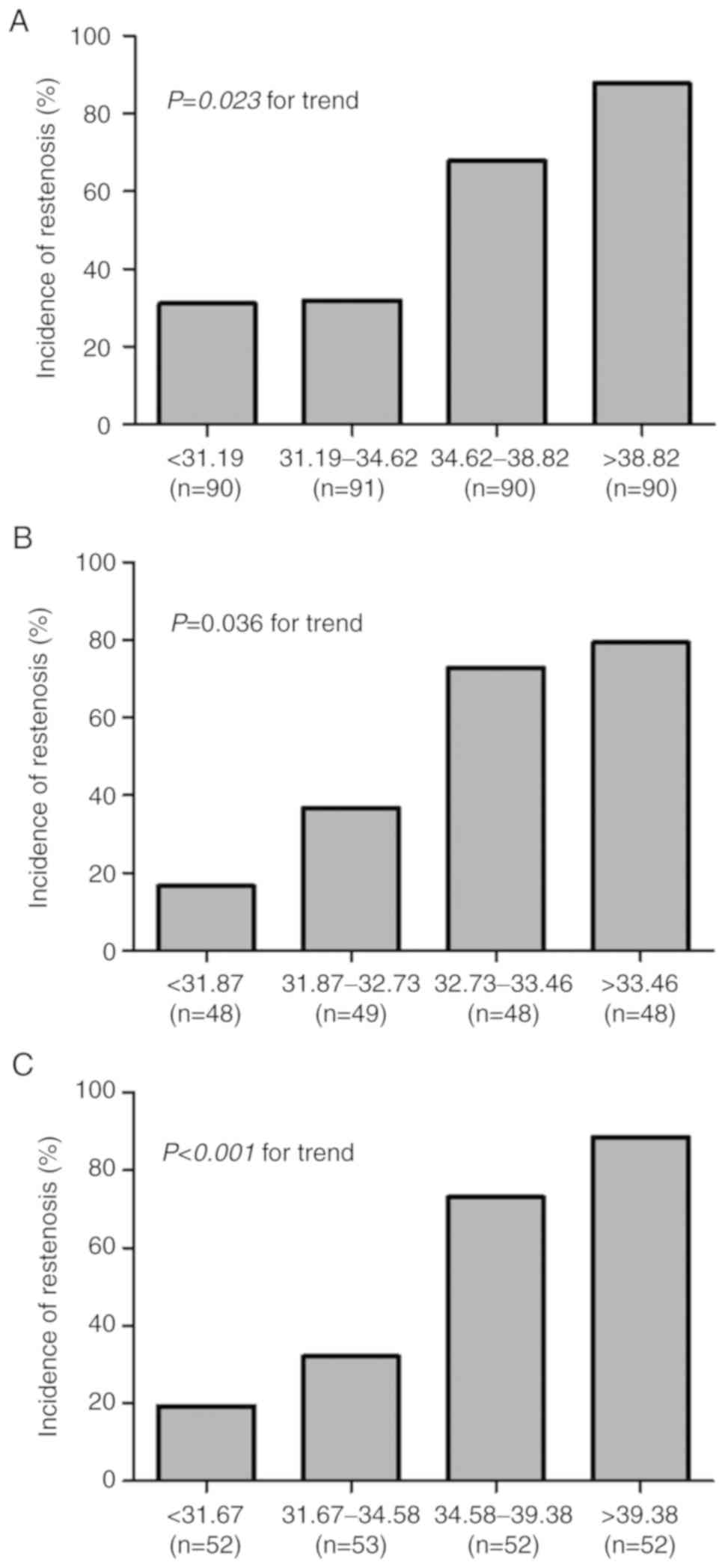
association remained significant in subgroups of males (P=0.023),
older patients (age, >65 years; P=0.036) and smokers
(P<0.001; Fig. 3).
In the multivariate logistic regression analysis
(Table II), after adjusting for
conventional cardiovascular risk factors, laboratory parameters,
medication after the procedure, angiographic and procedural
features, it was determined that smoking, LVEF, bifurcation lesion
and stent diameter were independent risk factors for ISR (Model 1).
When S100A12 was further included in model 2, in addition to the
factors in model 1, they all (including S100A12) remained
independently associated with ISR.
 | Table IIMultivariate logistic regression
analysis for the risk of in-stent restenosis. |
Table II
Multivariate logistic regression
analysis for the risk of in-stent restenosis.
| | Model 1 | Model 2 |
|---|
| Factor | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Smoking | 7.853
(2.597-23.743) | 0.001 | 4.952
(1.893-12.954) | <0.001 |
| LVEF | 0.765
(0.674-0.868) | <0.001 | 0.739
(0.644-0.848) | <0.001 |
| Bifurcation
lesion | 2.021
(1.018-5.332) | 0.021 | 1.912
(1.236-6.398) | 0.046 |
| Stent diameter | 0.48
(0.11-0.818) | <0.001 | 0.201
(0.102-0.763) | <0.001 |
| S100A12 | NA | NA | 1.228
(1.152-1.309) | <0.001 |
In the study population, addition of S100A12 to the
model enhanced the predictive value for ISR with an increase of the
C statistic of 0.027 (95% CI, 0.011-0.043, P=0.026). At the same
time, the values of NRI and IDI achieved by inclusion of S100A12
were also significantly improved (6.1% for NRI, P=0.003; 5.3% for
IDI, P<0.0001, respectively; Table
III).
 | Table IIIIncremental predictive value of S100
calcium-binding protein A12 level for in-stent restenosis
calculated by AUC difference, NDR and IDI. |
Table III
Incremental predictive value of S100
calcium-binding protein A12 level for in-stent restenosis
calculated by AUC difference, NDR and IDI.
| | AUC | P-value | NRI | P-vlaue | IDI | P-value |
|---|
| Model 1 | 0.916 | <0.0001 | Reference | Reference | Reference | Reference |
| Model 2 | 0.943 | <0.0001 | 6.1% | 0.003 | 5.3% | <0.0001 |
| AUCModel
2- AUCModel 1 | 0.027 | 0.026 | | | | |
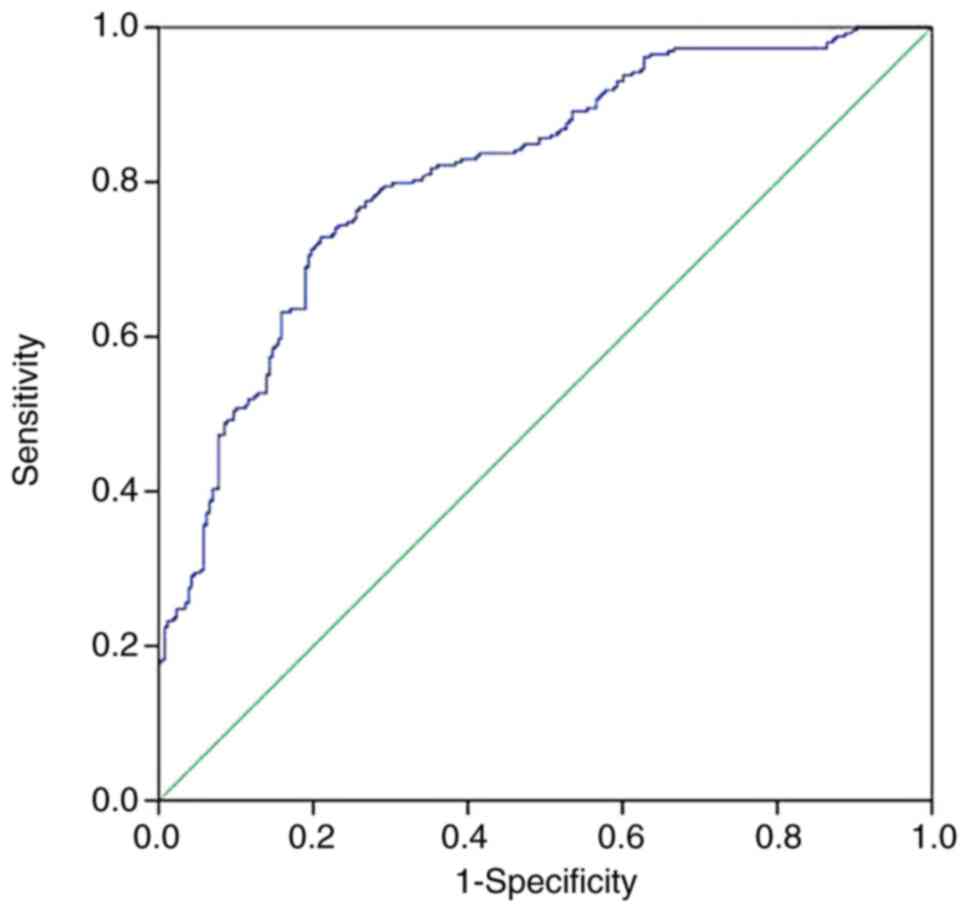
ROC analysis revealed that when a cut-off for the
serum levels of S100A12 of 34.75 ng/ml was used, it was possible to
predict ISR with 72.8% sensitivity and 79.1% specificity, and the
area under the ROC curve was 0.796 (95% CI: 0.757-0.834;
P<0.001; Fig. 4).
Discussion
The major results of the present study are as
follows: i) Serum levels of S100A12 were significantly elevated in
patients with ISR compared with those in patients without ISR. ii)
Multivariate logistic regression analysis further revealed that
S100A12 was an independent risk factor for IRS, beyond the
predictive capacity of traditional risk factors, biochemical
measurements, angiographic and procedural characteristics. iii)
Baseline levels of S100A12 provide incremental predictive value for
ISR in patients undergoing coronary DES implantation.
PCI with DES has revolutionized the treatment of
patients with CAD over the past decade; however, its benefits are
overshadowed by the occurrence of in-stent restenosis (1,2).
Vascular inflammation has a pathogenetic role in the development of
ISR after deployment of DES (3,5). It is
generally accepted that the vascular inflammatory status prior to
the intervention and the severity of the inflammatory reaction
after the procedure are determinants of ISR (22). In parallel, due to the involvement of
the inflammatory process that follows DES implantation, the use of
non-invasive biomarkers for the identification of patients at an
increased risk of ISR appears suitable.
Due to its pro-inflammatory features, S100A12 has
been indicated to predict outcomes in different subsets of
patients, including those with chronic heart failure, acute
coronary syndrome (ACS) and stable coronary artery disease (SCAD),
as well as in the general population (11,16,23,24).
Furthermore, the predictive value of S100A12 for ISR after DES has
remained elusive. Of note, as an important supplement to previous
studies, the present study demonstrated the independent and
incremental predictive value of the circulating S100A12
concentration for ISR following deployment of DES.
Intimal and medial injury during PCI provokes a
perivascular inflammatory response (22,25). The
vascular inflammatory status prior to intervention and the severity
of inflammatory reaction after the procedure appear to be the
determinants of ISR. The pro-inflammatory and atherogenic
properties of local S100A12 expression have been well elucidated.
Consistent with the results of basic studies, S100A12 levels have
been demonstrated to be a reliable and independent marker of
increased cardiovascular risk in different subgroups of patients,
including those with recent ACS and SCAD, as well as in healthy
individuals (11,18,26).
S100A12 concentrations have also been reported to be associated
with the extent of atherosclerosis in coronary arteries and the
presence of complex lesion morphology as detected by coronary
angiogram (24,27). The levels of S100A12 were reported to
be elevated in ACS patients as compared with those in patients with
SCAD and healthy controls, and to have a favorable prognostic value
for ACS (23), suggesting a
significant link between plaque vulnerability and S100A12. All of
this suggests that the circulating levels of S100A12 may be capable
of reflecting the inflammatory status in the vascular wall,
considering the consistency between the local expression and the
circulation level. Furthermore, patients with more extensive
vascular inflammation after PCI may be more vulnerable to recurrent
lumen narrowing; thus, the higher pre-procedural S100A12 values are
bound to aggravate the extent and susceptibility of the
inflammation in response to vascular injury, including hypertrophic
vascular remodeling and excessive neointima formation, which are
hallmarks of ISR.
One of the possible mechanisms for the involvement
of S100A12 in ISR after PCI is its affinity to bind to MOK protein
kinase and TLR4. The interaction of S100A12 with MOK protein kinase
or TLR4 triggers activation of NF-κB, which results in the
production of pro-inflammatory cytokines, including TNF-α and IL-1β
(8,28,29). All
of these cytokines are known to have a pathogenetic role in the
development of atherosclerosis and restenosis after PCI (30). The chemotactic effect of S100A12 may
be another mechanism underlying the association of S100A12 with
ISR. S100A12 was reported to enhance the expression of adhesion
molecules, including intercellular adhesion molecule-1 and vascular
cell adhesion molecule-1, contributing to monocyte recruitment as
well as migration (8,31). This is important, as
monocytes/macrophages are directly involved in ISR by secretion of
pro-inflammatory cytokines (32).
Furthermore, the pivotal role of augmented reactive oxygen species
(ROS) in the pathogenesis of ISR has been well established
(33). Tardif et al (34) indicated that ROS were involved in the
secretion of S100A12 by neutrophils. It may be speculated that
ROS-mediated S100A12 production, as observed in the serum of
patients with ISR, also provides a possible mechanistic explanation
for the strong positive link between elevated levels of S100A12 and
increased risk for ISR.
The present study has several limitations that
should be addressed. First, the observations of the present
cross-sectional study should be further evaluated in large-scale
studies prior to establishing a causal association between S100A12
and ISR following DES implantation. The present study was a
single-center and non-randomized study and the sample size was
relatively small. This may limit the interpretation and application
of the present results to a certain extent. In addition, the
inclusion of patients may have been biased due to symptom-driven
coronary angiography at follow-up, which may limit the reliability
of the results.
In conclusion, the present study was the first, to
the best of our knowledge, to indicate that the elevated level of
S100A12 at baseline is independently associated with an increased
risk for restenosis after DES-based PCI. The present results
support a prognostic utility of S100A12 in ISR. Although the
utility of serum S100A12 as a single surrogate biomarker for
predicting ISR is limited, it may be useful as part of a
multi-marker panel for improved identification of patients with
increased risk of ISR after DES implantation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Development Plans of Traditional Chinese Medicine in
Shandong Province (grant no. 2019-0304), the National Nature
Science Foundation of China (grant no. 81600222 to YC), the Taishan
Young Scholar Program of Shandong Province (grant no. tsqn201812142
to YC) the Shandong Provincial Nature Science Foundation of China
(grant no. ZR2016HM22 to LC) and the Clinical Medical Science and
Technology Innovation Development Plan Project of Jinan in China
(grant no. 201704106 to LC).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LMC designed the study. YHL and YQC reviewed the
medical records and collected the data. HRB, HL, PCY and LMC
performed data analysis. YHL and YQC and wrote the manuscript. LMC
and YQC was responsible for manuscript revisions and all the
authors reviewed the manuscript.
Ethics approval and consent to
participate
The protocol was approved by the institutional
review board of Shandong Provincial Hospital (Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
References
|
1
|
Bague N, Nasr B, Chaillou P, Costargent A,
Gouailler-Vulcain F and Goueffic Y: The role for DCBs in the
treatment of ISR. J Cardiovasc Surg (Torino). 57:578–585.
2016.PubMed/NCBI
|
|
2
|
Kang IS, Shehata I, Shin DH, Kim JS, Kim
BK, Ko YG, Choi D, Jang Y and Hong MK: Comparison between
drug-coated balloon angioplasty and second-generation drug-eluting
stent placement for the treatment of in-stent restenosis after
drug-eluting stent implantation. Heart Vessels. 31:1405–1411.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Baumann F, Willenberg T, Do DD, Keo HH,
Baumgartner I and Diehm N: Endovascular revascularization of
below-the-knee arteries: Prospective short-term angiographic and
clinical follow-up. J Vasc Interv Radiol. 22:1665–1673.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
He YH, Wang XQ, Zhang J, Liu ZH, Pan WQ,
Shen Y, Zhu ZB, Wang LJ, Yan XX, Yang K, et al: Association of
serum HMGB2 levels with in-stent restenosis: HMGB2 promotes
neointimal hyperplasia in mice with femoral artery injury and
proliferation and migration of VSMCs. Arterioscler Thromb Vasc
Biol. 37:717–729. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Montone RA, Sabato V, Sgueglia GA and
Niccoli G: Inflammatory mechanisms of adverse reactions to
drug-eluting stents. Curr Vasc Pharmacol. 11:392–398.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang Z, Zhang T, Sun L, Li R, Wei Y, Fan
X, Yuan Z, Liu J and Chen T: Pioglitazone attenuates drug-eluting
stent-induced proinflammatory state in patients by blocking
ubiquitination of PPAR. PPAR Res. 2016(7407153)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Foell D, Wittkowski H, Vogl T and Roth J:
S100 proteins expressed in phagocytes: A novel group of
damage-associated molecular pattern molecules. J Leukoc Biol.
81:28–37. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Farokhzadian J, Mangolian Shahrbabaki P
and Bagheri V: S100A12-CD36 axis: A novel player in the
pathogenesis of atherosclerosis? Cytokine.
122(154104)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bagheri V: S100A12: Friend or foe in
pulmonary tuberculosis? Cytokine. 92:80–82. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Han EC, Cho SB, Ahn KJ, Oh SH, Kim J, Kim
DS, Lee KH and Bang D: Expression of pro-inflammatory protein
S100A12 (EN-RAGE) in behcet's disease and its association with
disease activity: A pilot study. Ann Dermatol. 23:313–320.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Oesterle A and Bowman MA: S100A12 and the
S100/calgranulins: Emerging biomarkers for atherosclerosis and
possibly therapeutic targets. Arterioscler Thromb Vasc Boil.
35:2496–2507. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Leach ST, Yang Z, Messina I, Song C, Geczy
CL, Cunningham AM and Day AS: Serum and mucosal S100 proteins,
calprotectin (S100A8/S100A9) and S100A12, are elevated at diagnosis
in children with inflammatory bowel disease. Scand J Gastroenterol.
42:1321–1331. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yan WX, Armishaw C, Goyette J, Yang Z, Cai
H, Alewood P and Geczy CL: Mast cell and monocyte recruitment by
S100A12 and its hinge domain. J Biol Chem. 283:13035–13043.
2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hofmann Bowman MA, Gawdzik J, Bukhari U,
Husain AN, Toth PT, Kim G, Earley J and McNally EM: S100A12 in
vascular smooth muscle accelerates vascular calcification in
apolipoprotein E-null mice by activating an osteogenic gene
regulatory program. Arterioscler Thromb Vasc Biol. 31:337–344.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Burke AP, Kolodgie FD, Zieske A, Fowler
DR, Weber DK, Varghese PJ, Farb A and Virmani R: Morphologic
findings of coronary atherosclerotic plaques in diabetics: A
postmortem study. Arterioscler Thromb Vasc Boil. 24:1266–1271.
2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mahajan N, Malik N, Bahl A and Dhawan V:
Receptor for advanced glycation end products (RAGE) and its
inflammatory ligand EN-RAGE in non-diabetic subjects with
pre-mature coronary artery disease. Atherosclerosis. 207:597–602.
2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ligthart S, Sedaghat S, Ikram MA, Hofman
A, Franco OH and Dehghan A: EN-RAGE: A novel inflammatory marker
for incident coronary heart disease. Arterioscler Thromb Vasc Biol.
34:2695–2699. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Saito T, Hojo Y, Ogoyama Y, Hirose M,
Ikemoto T, Katsuki T, Shimada K and Kario K: S100A12 as a marker to
predict cardiovascular events in patients with chronic coronary
artery disease. Circ J. 76:2647–2652. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Williams B, Mancia G, Spiering W, Rosei
EA, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak
A, et al: 2018 ESC/ESH guidelines for the management of arterial
hypertension. Kardiol Pol. 77:71–159. 2019.PubMed/NCBI View Article : Google Scholar : (In Polish).
|
|
20
|
Introduction: Standards of medical care in
diabetes-2019. Diabetes Care. 42 (Suppl 1):S1–S2. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
National Cholesterol Education Program
(NCEP) Expert Panel on Detection Evaluation and Treatment of High
Blood Cholesterol in Adults (Adult Treatment Panel III). Third
Report of the National cholesterol education program (NCEP) expert
panel on detection, evaluation, and treatment of high blood
cholesterol in adults (Adult Treatment Panel III) final report.
Circulation. 106:3143–3421. 2002.PubMed/NCBI
|
|
22
|
Schillinger M, Exner M, Mlekusch W,
Rumpold H, Ahmadi R, Sabeti S, Haumer M, Wagner O and Minar E:
Vascular inflammation and percutaneous transluminal angioplasty of
the femoropopliteal artery: Association with restenosis. Radiology.
225:21–26. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Buyukterzi Z, Can U, Alpaydin S, Guzelant
A, Karaarslan S, Kocyigit D and Gurses KM: Enhanced S100A9 and
S100A12 expression in acute coronary syndrome. Biomark Med.
11:229–237. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhao P, Wu M, Yu H, Huang Y, Wang Y, Wang
W and Yin W: Serum S100A12 levels are correlated with the presence
and severity of coronary artery disease in patients with type 2
diabetes mellitus. J Investig Med. 61:861–866. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Schillinger M, Exner M, Mlekusch W, Haumer
M, Rumpold H, Ahmadi R, Sabeti S, Wagner O and Minar E:
Endovascular revascularization below the knee: 6-month results and
predictive value of C-reactive protein level. Radiology.
227:419–425. 2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rosenberg S, Elashoff MR, Beineke P,
Daniels SE, Wingrove JA, Tingley WG, Sager PT, Sehnert AJ, Yau M,
Kraus WE, et al: Multicenter validation of the diagnostic accuracy
of a blood-based gene expression test for assessing obstructive
coronary artery disease in nondiabetic patients. Ann Intern Med.
153:425–434. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu J, Ren YG, Zhang LH, Tong YW and Kang
L: Serum S100A12 concentrations are correlated with angiographic
coronary lesion complexity in patients with coronary artery
disease. Scand J Clin Lab Investig. 74:149–154. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li H and Sun B: Toll-like receptor 4 in
atherosclerosis. J Cell Mol Med. 11:88–95. 2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hofmann MA, Drury S, Fu C, Qu W, Taguchi
A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, et al: RAGE
mediates a novel proinflammatory axis: A central cell surface
receptor for S100/calgranulin polypeptides. Cell. 97:889–901.
1999.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rahel BM, Visseren FL, Suttorp MJ, Plokker
TH, Kelder JC, de Jongh BM, Bouter KP and Diepersloot RJ:
Preprocedural serum levels of acute-phase reactants and prognosis
after percutaneous coronary intervention. Cardiovasc Res.
60:136–140. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang Z, Tao T, Raftery MJ, Youssef P, Di
Girolamo N and Geczy CL: Proinflammatory properties of the human
S100 protein S100A12. J Leukoc Biol. 69:986–994. 2001.PubMed/NCBI
|
|
32
|
Medina I, Cougoule C, Drechsler M,
Bermudez B, Koenen RR, Sluimer J, Wolfs I, Döring Y, Herias V,
Gijbels M, et al: Hck/Fgr kinase deficiency reduces plaque growth
and stability by blunting monocyte recruitment and intraplaque
motility. Circulation. 132:490–501. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gallo G, Pierelli G, Forte M, Coluccia R,
Volpe M and Rubattu S: Role of oxidative stress in the process of
vascular remodeling following coronary revascularization. Int J
Cardiol. 268:27–33. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tardif MR, Chapeton-Montes JA, Posvandzic
A, Page N, Gilbert C and Tessier PA: Secretion of S100A8, S100A9,
and S100A12 by neutrophils involves reactive oxygen species and
potassium efflux. J Immunol Res. 2015(296149)2015.PubMed/NCBI View Article : Google Scholar
|