Introduction
Rheumatoid arthritis (RA), an autoimmune disease
caused by unclear factors, occurs more commonly among females of
30-50 years of age (1). Its
incidence in China is 0.2-0.4%, and its prevalence ratio of male to
female is 1:3 (2,3). Clinically, patients with RA experience
systemic inflammatory responses that cause an imbalance between
pro- and anti-inflammatory cytokines, which results in more
immunologic complications if the patients are not treated in time
(4). Although remarkable progress
has been made in the treatment of RA, the specific mechanism of the
disease remains unclear, and the patients suffer great direct and
indirect losses (5). Therefore, it
is necessary for clinical researchers to explore the pathogenesis
of RA and find new therapeutic schemes.
Cyclooxygenase (COX) is an important modulator of
prostaglandin synthesis and mainly exists as COX-1 and
COX-2(6). The former is expressed in
most tissues and is directly responsible for prostaglandin
production (7). The expression of
the latter is extremely low in normal tissues and cells, and is
massively produced by cells only when induced (8). According to studies, the overexpression
of COX-2 inhibits apoptosis (9), and
the expression is closely related to the conditions of patients
with RA (10). Forkhead box O3a
(FOXO3a) belongs to the forkhead family of transcription factors,
and its differential expression is closely associated with the
proliferation of helper T cells (11). There are numerous studies on FOXO3a
in tumors, however, there are few studies on FOXO3a in RA. For
example, FOXO3a controls the development and progression of tumors
by regulating related proteins (PUMA and Noxa) in lymphocytes and
neuroblastoma. The detected FOXO3a expression can be used as a
prognostic marker for patients with breast cancer (12,13). The
dysfunction of FOXO3a aggravates arthritis (14). However, the correlation of FOXO3a
with patients' disease activity remains unclear.
Therefore, the expression levels of FOXO3a and COX-2
and their correlation with the disease activity of patients with RA
were explored in this study to provide new targets for the
treatment of the disease.
Patients and methods
Clinical data
Sixty patients with RA admitted to the People's
Hospital of Guangxi Zhuang Autonomous Region (Nanning, China) from
May 2016 to May 2017 (study group) were enrolled in this study. The
patients consisted of 16 males and 44 females, with an average age
of 60.4±10.7 years. Further 30 healthy subjects undergoing physical
examinations in the hospital during the same period (control group)
were also enrolled. The healthy subjects had normal biochemical,
blood routine, immunological and microbial indicators, without
congenital immunodeficiency. The study was approved by the Medical
Ethics Committee of the People's Hospital of Guangxi Zhuang
Autonomous Region. Inclusion criteria: Patients who met the
diagnostic criteria for RA of the European League Against
Rheumatism of the American College of Rheumatology in 2009(15); patients with complete clinical data;
patients who were evaluated by DAS28 score; patients who they or
their families were informed and signed an informed consent form;
active patients who were initially diagnosed; stable patients who
had been confirmed and who were in the remissive stage after
treatment. Exclusion criteria: Patients <18 years of age;
patients with tumors; patients with other congenital
immunodeficiency diseases; patients with congenital defects of
heart, lung, or brain; patients who were unsound on their feet;
active patients who had taken hormone and immunosuppressive drugs
before treatment.
Sources of kits
COX-2 enzyme-linked immunosorbent assay (ELISA) kit
(mlbio - Shanghai Enzyme-linked Biotechnology Co., Ltd.; cat. no.
ml062904), C-reactive protein (CRP) kit (Shanghai Tellgen Life
Science Co., Ltd.; BH031), TRIzol total RNA extraction reagent
(Invitrogen; Thermo Fisher Scientific, Inc.; 15596018 and N8080234,
respectively), PrimeScript™ RT reagent kit (Takara Bio, Inc.;
RR037A). Primer sequences were designed and synthesized by Sangon
Biotech Co., Ltd. PCR instrument (Applied Biosystems; Thermo Fisher
Scientific, Inc.; ABI 7500).
Sample collection
Fasting peripheral venous blood (10 ml) was
respectively extracted from the patients of the study and the
control group, and was distributed into 4 tubes. The blood in the 3
tubes was allowed to stand for 30 min, and then, it was centrifuged
at 1,509 x g at 25˚C for 10 min to collect the supernatant for
subsequent experiments. The blood in the other tube was detected
for erythrocyte sedimentation rate (ESR).
Detection of COX-2
The collected serum was detected using ELISA.
Specific steps were as follows: 50 µl of serum and sample diluent
(1:1), respectively, were added to a 96-well plate. Blank and
standard wells were set up, and 50 µl of biotin-labeled antibodies
were added to each well. The ELISA plate was sealed, incubated at
37˚C for 1 h, and then washed. Next, streptavidin-HRP monoclonal
antibody (80 µl) was added to each well and after incubation at
37˚C for 30 min the wells were washed. Substrates A and B (50 µl
each) were respectively added to each well and incubation followed
at 37˚C for 10 min in the dark. Finally, stop solution (50 µl) was
added to each well. Optical density (OD) values at 450 nm were
detected using a microplate reader within 15 min. The experiment
was repeated 3 times to obtain the average value.
Detection of FOXO3a
TRIzol reagent was used to extract total RNA from
the collected serum. UV spectrophotometer and agarose gel
electrophoresis were used to detect its purity, concentration and
integrity. Total RNA was reverse transcribed using the PrimeScript™
RT reagent kit and cDNA was collected for subsequent experiments,
with the operation steps carried out according to the
manufacturer's instructions. The specific steps were as follows: 4
µl of 5X RT buffer, 2 µl of 10 mM dNTPs, 0.4 µl of RNasin (40
U/µl), 1 µl of M-MLV-RTase (200 U/µl), and RNase free
H2O was added to a final volume of 25 µl. The PCR
amplification system was as follows: 2 µl of cDNA, each 0.8 µl of
upstream and downstream primers, 10 µl of TB Green Premix Ex Taq II
(Tli RNaseH Plus) (2X), 0.4 µl of ROX Reference Dye or Dye II
(50X), and ddH2O was finally added to a final volume of
20 µl. The TaqMan™ reverse transcription reagent kit was used for
TB Green Premix Ex Taq II (Tli RNaseH Plus) and ROX Reference Dye
or Dye Ⅱ. The thermocycling conditions were as follows:
Pre-denaturation at 95˚C for 30 sec, denaturation at 95˚C for 5
sec, annealing and extension at 60˚C for 34 sec, for 40 cycles.
Each sample was provided with 3 identical wells, and the experiment
was carried out 3 times. GAPDH was used as an internal reference
and 2-∆Cq was used to analyze the data (16). The upstream and downstream primers of
FOXO3a were 5'-AAGCCAGCTACCTTCTCTTCCA-3' and 5'-CTGGCT
AAGTGAGTCCGAAGTGA-3', respectively, and of GAPDH were
5'-CACCCACTCCTCCACCTTTG-3' and 5'-CCACCA CCCTGTTGCTGTAG-3',
respectively.
Detection of ESR and CRP
ESR was detected by an automatic ESR analyzer (Vital
Diagnostics), and CRP was detected by a Hitachi 7600 fully
automatic biochemical analyzer (Hitachi, Ltd.) using immune
nephelometry, in strict accordance with the manufacturer's
instructions.
Observational indices
Main observational indices: The expression levels of
serum COX-2 and FOXO3a in the control and study groups were
observed. According to the DAS28 score, the patients were divided
into active and remissive patients (≥2.6 points for active stage
and <2.6 points for remissive stage), between whom the
expression levels were compared.
Secondary observational indices: Receiver operating
characteristic (ROC) curves were plotted to analyze the diagnostic
values of COX-2 and FOXO3a for disease activity. Pearson's
correlation coefficient was used to analyze the correlation of the
two markers with ESR, CRP, and DAS28 score.
Statistical analysis
SPSS 20.0 (Cabit Information Technology Co., Ltd.)
was used to statistically analyze the data. GraphPad Prism 7
(Softhead, Inc.) was used to plot figures. Count data were
expressed as percentage (%), analyzed by Chi-square test, and
represented by χ2. Kolmogorov-Smirnov test was used to
analyze the data distribution. Measurement data were expressed by
mean ± standard deviation (means ± SD). The data conforming to
normal distribution were analyzed by independent samples t-test,
and represented by t. Comparison between multiple groups was made
by one-way analysis of variance and represented by F. Pairwise
comparison between groups was analyzed by univariate LSD-t test.
Pearson's correlation coefficient was used for analyzing the
relationship between indices. ROC curves were plotted to analyze
the diagnostic values of COX-2 and FOXO3a for disease activity.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical data
There were statistically significant differences
between the study and the control group in terms of CRP and ESR
(P<0.05), but not in terms of sex, age, body mass index (BMI),
past medical history, history of smoking, history of alcoholism,
and place of residence (P>0.05) (Table I).
 | Table IBaseline data. |
Table I
Baseline data.
| Factors | Study group
(n=60) | Control group
(n=30) | t/χ2
value | P-value |
|---|
| Sex | | | 0.433 | 0.511 |
|
Male | 16 (26.67) | 10 (33.33) | | |
|
Female | 44 (73.33) | 20 (66.67) | | |
| Age (years) | 60.4±10.7 | 58.7±8.9 | 0.750 | 0.455 |
| BMI
(kg/m2) | 22.04±1.77 | 22.59±1.80 | 1.382 | 0.171 |
| Past medical
history | | | | |
|
Hypertension | 19 (31.67) | 8 (26.67) | 0.238 | 0.626 |
|
Diabetes | 22 (36.67) | 10 (33.33) | 0.097 | 0.756 |
|
Hyperlipemia | 12 (20.00) | 5 (16.67) | 0.145 | 0.703 |
| History of
smoking | | | 0.407 | 0.524 |
|
Yes | 18 (30.00) | 11 (36.67) | | |
|
No | 42 (70.00) | 19 (63.33) | | |
| History of
alcoholism | | | 0.078 | 0.781 |
|
Yes | 5 (8.33) | 2 (6.67) | | |
|
No | 55 (91.67) | 28 (93.33) | | |
| Place of
residence | | | 0.215 | 0.643 |
|
City | 37 (61.67) | 20 (66.67) | | |
|
Countryside | 23 (38.33) | 10 (33.33) | | |
| Course of disease
(years) | 8.54±2.11 | | | |
| CRP (mg/l) | 53.84±22.06 | 5.81±2.87 | 11.842 | <0.001 |
| ESR (mm/h) | 33.15±18.22 | 9.88±4.45 | 6.876 | <0.001 |
| DAS28 score | 3.12±1.45 | | | |
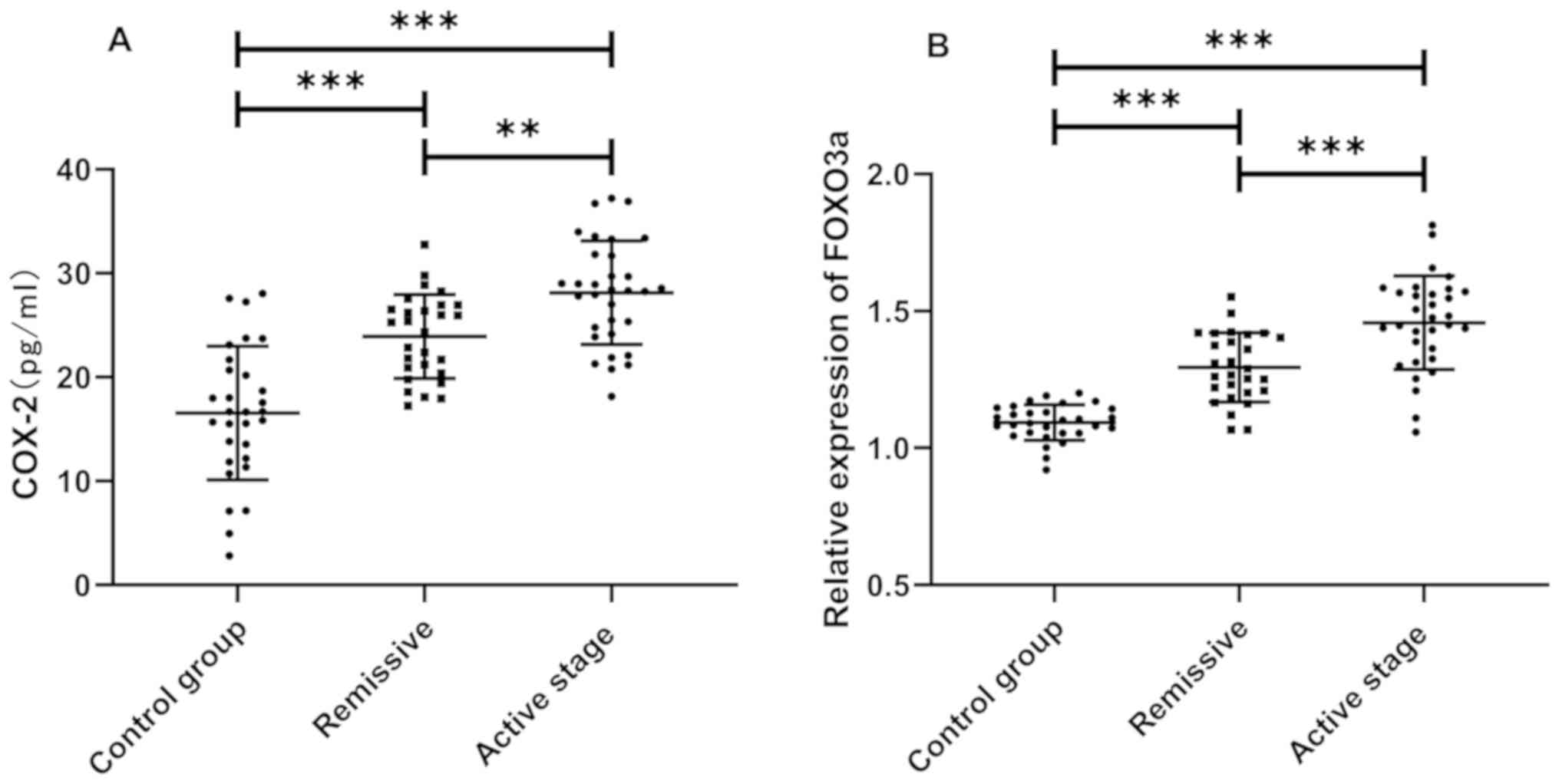
Expression levels of COX-2 and FOXO3a
in the study and the control group
According to the detection results, the expression
levels of COX-2 and FOXO3a in the study group were significantly
higher than those in the control group (P<0.05). The comparison
of COX-2 and FOXO3a expression levels between the active and
remissive patients showed that the expression levels in active
patients were significantly higher than those in remissive patients
(P<0.05) (Fig. 1).
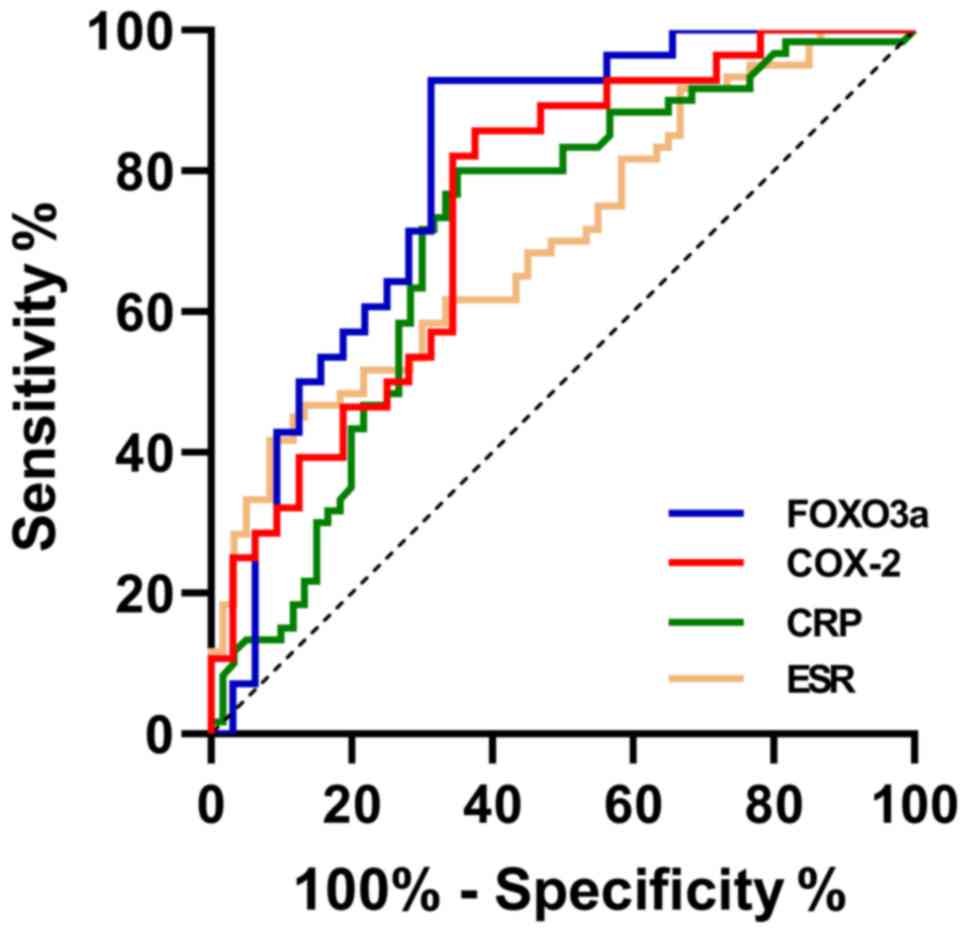
Diagnostic values of COX-2 and FOXO3a
for disease activity
According to the ROC curves, the area under the
curve (AUC) of COX-2 was 0.748. When the specificity was 62.50% and
the sensitivity was 85.71%, the best cut-off value of COX-2 was
27.671 pg/ml. The AUC of FOXO3a was 0.802. When the specificity was
68.75% and the sensitivity was 92.85%, the best cut-off value of
FOXO3a was 1.424. The AUC of CRP was 0.708. When the specificity
was 80.00% and the sensitivity was 65.00%, the best cut-off value
of CRP was 7.25 mg/l. The AUC of ESR was 0.702. When the
specificity was 86.67% and the sensitivity was 46.67%, the best
cut-off value of ESR was 14.85 mm/h (Table II and Fig. 2).
 | Table IIROC parameters. |
Table II
ROC parameters.
| Indicators | AUC | 95% CI | Specificity
(%) | Sensitivity
(%) | Youden index
(%) | Cut-off |
|---|
| COX-2 | 0.748 | 0.624-0.871 | 62.50 | 85.71 | 48.21 | <27.671
pg/ml |
| FOXO3a | 0.802 | 0.689-0.916 | 68.75 | 92.85 | 61.61 | <1.424 |
| CRP | 0.708 | 0.613-0.803 | 80.00 | 65.00 | 45.00 | <7.25 mg/l |
| ESR | 0.702 | 0.609-0.794 | 86.67 | 46.67 | 33.33 | <14.85 mm/h |
Correlation of COX-2 and FOXO3a with
ESR, CRP, and DAS28 score
According to Pearson's correlation coefficient, the
expression levels of COX-2 and FOXO3a were positively correlated
with ESR, CRP, and DAS28 score of active and remissive patients
(both P<0.05) (Tables III and
IV, and Fig. 3).
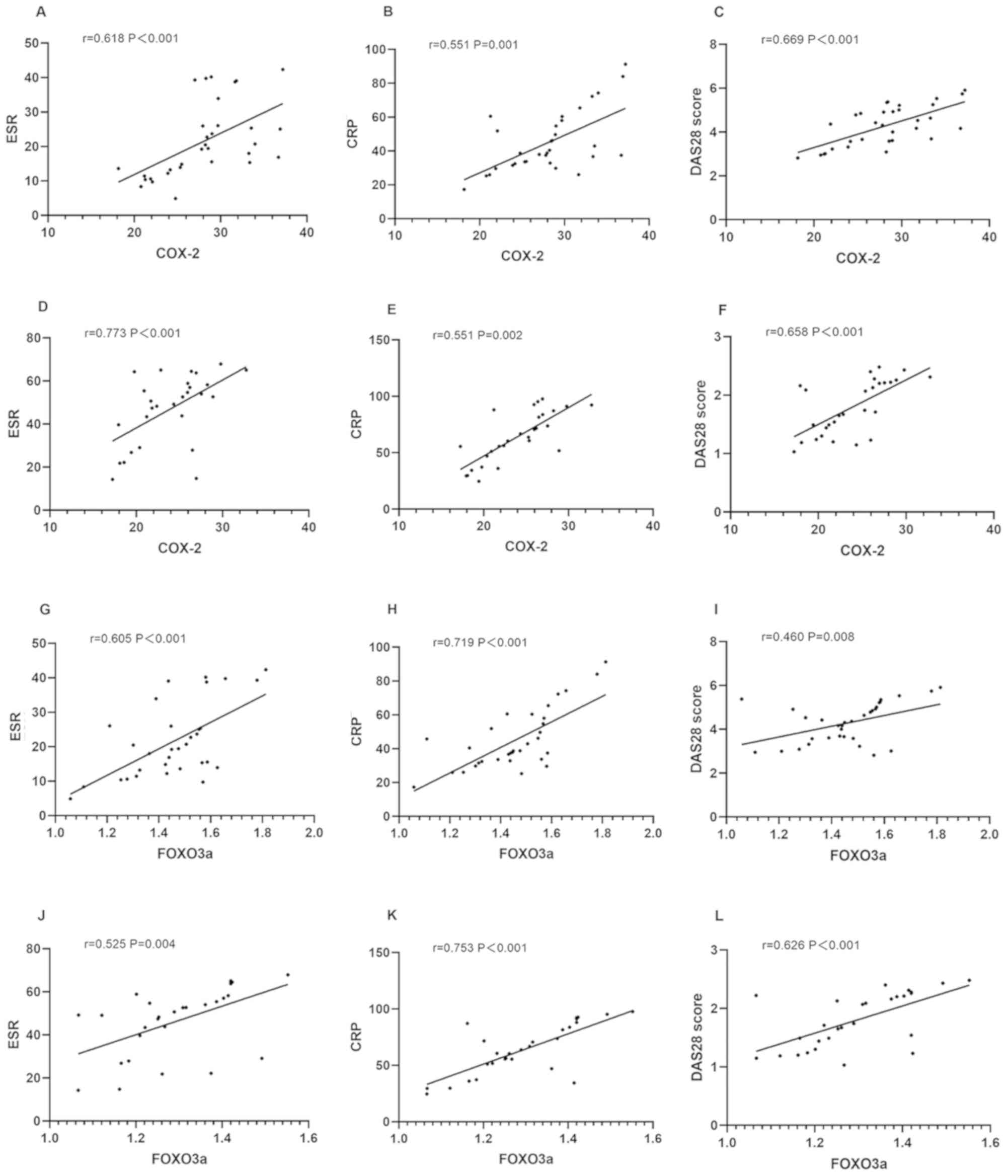 | Figure 3Correlation of COX-2 and FOXO3a with
ESR, CRP, and DAS28 score. The correlation of COX-2 with (A) ESR,
(B) CRP, and (C) DAS28 score of active patients is presented. The
correlation of COX-2 with (D) ESR, (E) CRP, and (F) DAS28 score of
remissive patients is presented. The correlation of FOXO3a with (G)
ESR, (H) CRP, and (I) DAS28 score of active patients is presented.
The correlation of FOXO3a with (J) ESR, (K) CRP, and (L) DAS28
score of remissive patients is presented. COX, cyclooxygenase;
FOXO3a, forkhead box O3a; ESR, erythrocyte sedimentation rate; CRP,
C-reactive protein. |
 | Table IIICorrelation of COX-2 with ESR, CRP,
and DAS28 score. |
Table III
Correlation of COX-2 with ESR, CRP,
and DAS28 score.
| | Active patients
(n=28) | Remissive patients
(n=32) |
|---|
| Factors | r value | P-value | r value | P-value |
|---|
| ESR | 0.618 | <0.001 | 0.773 | <0.001 |
| CRP | 0.551 | 0.001 | 0.551 | 0.002 |
| DAS28 | 0.669 | <0.001 | 0.658 | <0.001 |
 | Table IVCorrelation of FOXO3a with ESR, CRP,
and DAS28 score. |
Table IV
Correlation of FOXO3a with ESR, CRP,
and DAS28 score.
| | Active patients
(n=28) | Remissive patients
(n=32) |
|---|
| Factors | r value | P-value | r value | P-value |
|---|
| ESR | 0.605 | <0.001 | 0.525 | 0.004 |
| CRP | 0.719 | <0.001 | 0.753 | <0.001 |
| DAS28 | 0.460 | 0.008 | 0.626 | <0.001 |
Discussion
RA is the most common joint disease in clinical
practice. Systemic inflammatory responses stimulate the
proliferation of synovial cells, cause the formation of invasive
pannus, and damage the cartilage and bone tissue, thereby leading
to the disease (17). Statistics
have shown that the disability rate of RA in China is high, and the
disease seriously affects the patients' daily life and work
(18). The disease is currently
treated with anti-inflammatory agents and analgesics, both of which
only relieve patients' conditions, but cannot cure the disease
(19). Therefore, it is essential to
find new therapeutic targets to improve the conditions.
In this study, the expression of COX-2 in the study
group was significantly higher than that in the control group. A
large number of studies have shown that COX-2 is highly expressed
in patients with RA (20,21), which is consistent with our findings.
Therefore, COX-2 inhibitors have been widely used in clinical
practice, especially in the treatment of RA (22,23). As
a subfamily member of forkhead transcription factors, FOXO3a
mediates apoptosis, cell proliferation, cell cycle progression, DNA
damage, and tumorigenesis (24).
However, there are few studies on FOXO3a in RA. In this study, the
expression of FOXO3a in the study group was found to be
significantly higher than that in the control group, indicating
that FOXO3a is differentially expressed in patients with RA and
healthy subjects. In a study by Turrel-Davin et al (25), the expression of serum FOXO3a in
patients with RA, as detected by microarray chips, was
significantly higher than that in healthy subjects, which is
consistent with our findings.
According to different disease activities, the
patients were divided into active and remissive patients. The
expression levels of COX-2 and FOXO3a in remissive patients were
significantly lower than those in active patients. According to
Turrel-Davin et al (25), the
overexpression of FOXO3a promotes the proliferation and survival of
neutrophils and synovial T cells in the peripheral blood of
patients with RA. Therefore, the conditions of active patients were
more severe than those of remissive patients, possibly because the
promotion of the overexpression aggravates the patients'
conditions. However, it is still unclear whether COX-2 and FOXO3a
can be used as markers to distinguish active patients from
remissive patients. According to the ROC curves, the AUCs of COX-2
and FOXO3a were 0.748 and 0.802, respectively, slightly higher than
those of ESR and CRP, indicating that COX-2 and FOXO3a have good
diagnostic values for active and remissive patients.
Finally, the correlation of COX-2 and FOXO3a with
ESR, CRP, and DAS28 score of active and remissive patients were
analyzed. DAS28 score, the accuracy of which has been confirmed in
a number of tests, is an internationally recognized standard for
evaluating the conditions of patients with RA through laboratory
parameters and swollen joint count (26). CRP is not the best specific
indicator, however, is an important laboratory parameter for RA
activity, because its increase directly reflects inflammation
(27). ESR is an important index for
the clinical observation of RA. Rouleaux formation is easy to occur
in erythrocytes due to the massive production of inflammatory
cytokines in patients, thereby accelerating ESR (28). According to Pearson's correlation
coefficient, COX-2 and FOXO3a were shown to be positively
correlated the ESR, CRP, and DAS28 score of active and remissive
patients, which suggests that COX-2 and FOXO3a may relieve the
patients' conditions.
The present study proves that COX-2 and FOXO3a are
highly expressed in patients with RA, and that the two markers can
be used as potential indicators for distinguishing different
disease activities. However, there are still limitations. The
expression levels of COX-2 and FOXO3a in the synovial tissue of the
patients were not detected. The relationship between FOXO3a and
COX-2 was not fully explored in this clinical study. The mechanism
of action of FOXO3a in RA is also unclear. The expression levels of
the two markers in the patients' serum were detected, however, the
patients were not followed up. Therefore, further study is still
required.
In conclusion, the expression levels of COX-2 and
FOXO3a in patients with RA are upregulated, so the two markers may
be involved in the development and progression of the disease. The
expression levels of COX-2 and FOXO3a are related to the disease
activity of RA, and thus, they can be used as diagnostic
indicators.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BW wrote the manuscript. BW and XH performed PCR and
ELISA. JL analyzed and interpreted the patient data, and assisted
with statistical analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Medical Ethics
Committee of the People's Hospital of Guangxi Zhuang Autonomous
Region (Nanning, China). Patients who participated in this research
signed an informed consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singh JA, Saag KG, Bridges SL Jr, Akl EA,
Bannuru RR, Sullivan MC, Vaysbrot E, McNaughton C, Osani M,
Shmerling RH, et al: 2015 American College of Rheumatology
Guideline for the Treatment of Rheumatoid Arthritis. Arthritis
Rheumatol. 68:1–26. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jin S, Li M, Fang Y, Li Q, Liu J, Duan X,
Liu Y, Wu R, Shi X, Wang Y, et al: CREDIT Co-authors: Chinese
Registry of rheumatoid arthritis (CREDIT): II prevalence and risk
factors of major comorbidities in Chinese patients with rheumatoid
arthritis. Arthritis Res Ther. 19(251)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
de Achaval S and Suarez-Almazor ME:
Treatment adherence to disease-modifying antirheumatic drugs in
patients with rheumatoid arthritis and systemic lupus
erythematosus. Int J Clin Rheumatol. 5:313–326. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Malmström V, Catrina AI and Klareskog L:
The immunopathogenesis of seropositive rheumatoid arthritis: From
triggering to targeting. Nat Rev Immunol. 17:60–75. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Catrina AI, Joshua V, Klareskog L and
Malmström V: Mechanisms involved in triggering rheumatoid
arthritis. Immunol Rev. 269:162–174. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zelenay S, van der Veen AG, Böttcher JP,
Snelgrove KJ, Rogers N, Acton SE, Chakravarty P, Girotti MR, Marais
R, Quezada SA, et al: Cyclooxygenase-dependent tumor growth through
evasion of immunity. Cell. 162:1257–1270. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Depboylu C, Weihe E and Eiden LE: COX1 and
COX2 expression in non-neuronal cellular compartments of the rhesus
macaque brain during lentiviral infection. Neurobiol Dis.
42:108–115. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yang CM, Chen YW, Chi PL, Lin CC and Hsiao
LD: Resveratrol inhibits BK-induced COX-2 transcription by
suppressing acetylation of AP-1 and NF-κB in human rheumatoid
arthritis synovial fibroblasts. Biochem Pharmacol. 132:77–91.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Walther U, Emmrich K, Ramer R, Mittag N
and Hinz B: Lovastatin lactone elicits human lung cancer cell
apoptosis via a COX-2/PPARγ-dependent pathway. Oncotarget.
7:10345–10362. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lowin T, Apitz M, Anders S and Straub RH:
Anti-inflammatory effects of N-acylethanolamines in rheumatoid
arthritis synovial cells are mediated by TRPV1 and TRPA1 in a COX-2
dependent manner. Arthritis Res Ther. 17(321)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tang YL, Huang LB, Lin WH, Wang LN, Tian
Y, Shi D, Wang J, Qin G, Li A, Liang YN, et al: Butein inhibits
cell proliferation and induces cell cycle arrest in acute
lymphoblastic leukemia via FOXO3a/p27kip1 pathway. Oncotarget.
7:18651–18664. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Obexer P, Geiger K, Ambros PF, Meister B
and Ausserlechner MJ: FKHRL1-mediated expression of Noxa and Bim
induces apoptosis via the mitochondria in neuroblastoma cells. Cell
Death Differ. 14:534–547. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jiang Y, Zou L, Lu WQ, Zhang Y and Shen
AG: Foxo3a expression is a prognostic marker in breast cancer. PLoS
One. 8(e70746)2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Viatte S, Lee JC, Fu B, Espéli M, Lunt M,
De Wolf JN, Wheeler L, Reynolds JA, Castelino M, Symmons DP, et al:
Association between genetic variation in FOXO3 and reductions in
inflammation and disease activity in inflammatory polyarthritis.
Arthritis Rheumatol. 68:2629–2636. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Aletaha D, Neogi T, Silman AJ, Funovits J,
Felson DT, Bingham CO III, Birnbaum NS, Burmester GR, Bykerk VP,
Cohen MD, et al: 2010 Rheumatoid arthritis classification criteria:
An American College of Rheumatology/European League Against
Rheumatism collaborative initiative. Arthritis Rheum. 62:2569–2581.
2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Narayan N, Owen DR, Mandhair H, Smyth E,
Carlucci F, Saleem A, Gunn RN, Rabiner EA, Wells L, Dakin SG, et
al: Translocator protein as an imaging marker of macrophage and
stromal activation in rheumatoid arthritis pannus. J Nucl Med.
59:1125–1132. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gong G and Mao J: Health-related quality
of life among Chinese patients with rheumatoid arthritis: The
predictive roles of fatigue, functional disability, self-efficacy,
and social support. Nurs Res. 65:55–67. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Smith SR, Deshpande BR, Collins JE, Katz
JN and Losina E: Comparative pain reduction of oral non-steroidal
anti-inflammatory drugs and opioids for knee osteoarthritis:
Systematic analytic review. Osteoarthritis Cartilage. 24:962–972.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fan HW, Liu GY, Zhao CF, Li XF and Yang
XY: Differential expression of COX-2 in osteoarthritis and
rheumatoid arthritis. Genet Mol Res. 14:12872–12879.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Peng A, Lu X, Huang J, He M, Xu J, Huang H
and Chen Q: Rheumatoid arthritis synovial fibroblasts promote
TREM-1 expression in monocytes via COX-2/PGE 2 pathway. Arthritis
Res Ther. 21(169)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Roubille C, Richer V, Starnino T, McCourt
C, McFarlane A, Fleming P, Siu S, Kraft J, Lynde C, Pope J, et al:
The effects of tumour necrosis factor inhibitors, methotrexate,
non-steroidal anti-inflammatory drugs and corticosteroids on
cardiovascular events in rheumatoid arthritis, psoriasis and
psoriatic arthritis: A systematic review and meta-analysis. Ann
Rheum Dis. 74:480–489. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nissen SE, Yeomans ND, Solomon DH, Lüscher
TF, Libby P, Husni ME, Graham DY, Borer JS, Wisniewski LM, Wolski
KE, et al: PRECISION Trial Investigators: Cardiovascular safety of
celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med.
375:2519–2529. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Codogno P and Morel E: FOXO3a provides a
quickstep from autophagy inhibition to apoptosis in cancer therapy.
Dev Cell. 44:537–539. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Turrel-Davin F, Tournadre A, Pachot A,
Arnaud B, Cazalis MA, Mougin B and Miossec P: FoxO3a involved in
neutrophil and T cell survival is overexpressed in rheumatoid blood
and msynovial tissue. Ann Rheum Dis. 69:755–760. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yoshida K, Matsui K, Nakano H, Oshikawa H,
Utsunomiya M, Kobayashi T, Kimura M, Deshpande GA and Kishimoto M:
Response to ‘Pain persists in DAS28 rheumatoid arthritis remission
but not in ACR/EULAR remission: A longitudinal observational
study’. Arthritis Res Ther. 13(405)2011.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Sheehy C, Evans V, Hasthorpe H and
Mukhtyar C: Revising DAS28 scores for remission in rheumatoid
arthritis. Clin Rheumatol. 33:269–272. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shimada K, Komiya A, Yokogawa N, Nishino
J, Sugii S and Tohma S: Impact of the size and number of swollen
joints on serum C-reactive protein level and erythrocyte
sedimentation rate in rheumatoid arthritis: A cross-sectional study
in Japan. Clin Rheumatol. 36:427–431. 2017.PubMed/NCBI View Article : Google Scholar
|