Introduction
MicroRNAs (miRNAs/miRs) are small non-coding RNAs
that may endogenously regulate gene expression by partially binding
to the 3'-untranslated region (3'UTR) of their target mRNAs
(1,2). Previous studies have reported that
miRNAs are involved in cell differentiation, development and
carcinogenesis (3,4).
Diabetic nephropathy (DN) is the chronic loss of
kidney function that frequently occurs in patients with diabetes
mellitus (5,6). Although the accurate molecular
mechanisms underlying the occurrence and progression of DN have
remained to be fully elucidated, previous studies have reported
that increased mesangial cell proliferation and abnormal apoptosis
are major pathological features of early-stage DN (7,8).
Recently, a number of studies have demonstrated that certain miRNAs
exhibit aberrant expression in the kidneys of mice with DN
(9-11).
Furthermore, miR-181-5p has been reported to be dysregulated in
diabetes mellitus (12,13). Kruppel-like factors (KLFs), a family
of DNA-binding transcriptional regulators, are involved in cellular
processes (14). KLF6 may function
as a putative tumor suppressor in various cancer types, including
prostate (15) and colorectal cancer
(16). A study indicated that
fibrotic kidneys have increased KLF6 expression (17). However, the interaction between
miR-181a-5p and KLF6, the roles of either factor on its own in DN,
or their interaction in DN-associated processes have remained
elusive.
The aim of the present study was to identify the
underlying function and molecular mechanisms of miR-181a-5p in DN.
In particular, the anti-proliferative and pro-apoptotic effects of
miR-181a-5p on high glucose treated glomerular mesangial cells
(GMCs) were assessed.
Materials and methods
Cell culture and treatment
The GMC line SV40-MES-13 (no. YS-ATCC311) was
purchased from Shanghai Yansheng Industrial Co., Ltd. The cells
were cultured in Dulbecco's modified Eagle's medium (DMEM;
containing 5.6 nM glucose; Invitrogen; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (10099-1741; Gibco;
Thermo Fisher Scientific, Inc.) in a humidified atmosphere
containing 5% CO2 at 37˚C. A total of 100 U/ml
penicillin and 100 µg/ml streptomycin (Thermo Fisher Scientific,
Inc.) were added to the medium in order to prevent bacterial
contamination. After 24 h of pre-incubation in the presence of
normal glucose levels (DMEM containing 5.6 nM glucose), further
glucose (25 nM glucose; Sigma Aldrich; Merck KGaA) was added to
provide a high-glucose (total 31.6 nM glucose) environment
(18), and the cells were incubated
for another 24 h at 37˚C in a humidified atmosphere with 5%
CO2. Following high-glucose treatment, the expression
levels of miR-181a-5p and KLF6 were measured accordingly.
Cell transfection
Following treatment, the cells were transfected with
miR-181a-5p mimics and control mimics (Shanghai Gene Pharma Co.,
Ltd.) using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). In order to determine the transfection
efficiency of miR-181a-5p, cells were divided into three groups: i)
Control group, untransfected cells; ii) negative control (NC)
group, cells were transfected with miR-181a-5p mimics NC; and iii)
mimics group, cells were transfected with miR-181a-5p mimics. The
sequences were as follows: miR-181a-5p mimics,
5'-AACAUUCAACGCUGUCGGUGAGU-3' and control mimics,
5'-UUUGUACUACACAAAAGUACUG-3'.
Plasmid construction
A KLF6-expressing vector was constructed by
inserting the KLF6 gene into the pcDNA3.1 vector. In brief, the
sequence for KLF6 was purchased from GenePharma (Shanghai
GenePharma Co., Ltd.) and inserted into the pcDNA3.1 vector
(GeneChem). In order to determine the effects of KLF6 transfection,
cells were divided into three groups: i) Control group,
untransfected cells; ii) pcDNA3.1 group, cells transfected with
pcDNA3.1; and iii) KLF6 group, cells transfected with
pcDNA3.1-KLF6. The human KLF6 sequence is forward,
5'-CTCTCAGCCTGGAAGCTTTTAGCCTAC-3' and reverse,
5'-ACAGCTCCGAGGAACTTTCTCCCA-3'.
MTT assay
In order to determine the effect of the vectors on
the amount of viable cells, the high-glucose-treated cells were
divided into four groups: i) ontrol group, untransfected cells; ii)
NC group, cells were transfected with miR-181a-5p mimics NC; iii)
mimics group, cells were transfected with miR-181a-5p mimics; and
iv) mimics + KLF6 group, cells were transfected with miR-181a-5p
mimics and pcDNA3.1-KLF6. An MTT assay (Sigma-Aldrich; Merck KGaA)
was applied to determine the cell viability. In brief, the treated
cells were seeded in a 96-well plate at a density of
1x104 cells/well. Subsequently, 20 µl MTT was added to
each well at a concentration of 5 mg/ml after 12, 24 or 48 h of
culture, followed by further incubation for 4 h at 37˚C in a
humidified atmosphere with 5% CO2. Following the removal
of all medium, dimethylsulfoxide (100 µl) was added to each well,
followed by shaking for 5 min until the formazan crystals had
dissolved completely. The absorbance was measured at 490 nM using a
microplate reader (M680-UV Spectrophotometer; Bio-Rad Laboratories,
Inc.).
Flow cytometry
An Annexin V-FITC/PI Apoptosis Detection kit (Keygen
Biotech) was used to assess the apoptosis rate. In brief, the
treated cells were seeded into a 6-well plate at a density of
4x105 cells/well and re-suspended in 500 µl binding
buffer after 24 h incubation. Subsequently, 5 µl Annexin V-FITC and
5 µl propidium iodide were added to the suspension, followed by
incubation for 20 min at room temperature in a humidified
atmosphere with 5% CO2 in the dark. Finally, cells were
analyzed using flow cytometry (Beckman Coulter, Inc.) according to
the manufacturer's protocol.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Complementary
(c)DNA was synthesized from RNA using a High Capacity cDNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) and amplified using Power SYBR Green Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) on an Applied
Biosystems Prism 7900HT sequence detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The PCR amplification was performed as
follows: A total of 40 cycles at 95˚C for 10 sec and 65˚C for 20
sec. U6 and GAPDH were used as the internal controls to normalize
the expression of miR-181a and associated proteins in different
groups using the 2-ΔΔCq method (19). Primer sequences were as follows:
miR-181a-5p forward, 5'-ACACTCCAGCTGGGAACATTCAACGCTGTCGG-3' and
reverse, 5'-TGGTGTCGTGGAGTCGA-3'; U6 forward,
5'-CTCGCTTGGGCAGCACA-3' and reverse, 5'-AACGCTTCACGAATTTGCGT-3'.
KLF6 forward, 5'-CGGTGTGCTTTCGGAAGTG-3' and reverse,
5'-CGGTGTGCTTTCGGAAGTG-3'; Bcl-2 forward,
5'-TGGGATGCCTTTGTGGAACTAT-3' and reverse,
5'-AGAGACAGCCAGGAGAAATCA-3'; Bax forward,
5'-TCGTAGATCTATGGACGGGTCCGGGGAGCAGCT-3' and reverse,
5'-ATTAGCGGCCGCTCAGCCCATCTTCTTCCAGAT-3'; caspase-3 forward,
5'-TGTCATCTCGCTCTGGTACG-3' and reverse, 5'-AAATGACCCCTTCATCACCA-3';
GAPDH forward, 5'-AACTTTGGCATTGTGGAAGG-3' and reverse,
5'-GGAGACAACCTGGTCCTCAG-3'.
Western blot analysis
The treated cells were lysed in
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) containing protein inhibitor cocktail. The
concentration of proteins was measured using a BCA Protein Assay
kit (Thermo Fisher Scientific, Inc.). Proteins (10 µg/lane) were
then separated via 10% SDS-PAGE (cat. no. S1052;
Solarbio® Life Sciences) and transferred to a
polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc.).
After blocking in 5% non-fat milk with 0.1% Tris-buffered saline
containing Tween 20 (10X TBST; cat. no. T1081; Solarbio®
Life Sciences) for 1 h at room temperature, the membrane was then
incubated with primary antibodies overnight at 4˚C. The following
primary antibodies were used: Rabbit anti-KLF6 (1:1,000 dilution;
cat. no. DF13114; Affinity Biosciences), rabbit anti-Bcl-2 (1:1,000
dilution; cat. no. ab32124), rabbit anti-Wnt1 (1:1,000 dilution;
cat. no. ab15251), rabbit anti-β-catenin (1:5,000 dilution; cat.
no. ab32572), rabbit anti-Bax (1:1,000 dilution; cat. no. ab32503),
rabbit anti-caspase-3 (1:500 dilution; cat. no. ab13847) and rabbit
anti-GAPDH (1:10,000 dilution; cat. no. AB181602) (all from Abcam
unless stated otherwise). Subsequently, the secondary antibodies
IgG H&L (Cy2®; 1:1,000 dilution; cat. no. ab6940;
Abcam) were added, followed by incubation for 1 h at room
temperature. Finally, NovexTM ECL Chemiluminescent Substrate
Reagent Kit (WP2005; Thermo Fisher Scientific, Inc.) were used to
detect bound antibodies by exposure to X-ray film. All results were
normalized to the absorbance of the internal control GAPDH.
Luciferase activity assay
Following confirmation that KLF6 may be a predictive
target gene of miR-181a using TargetScan, a luciferase reporter
gene assay was performed. In brief, the target sequence purchased
from GenePharma (Shanghai GenePharma Co., Ltd.) was inserted into
the luciferase reporter plasmid pGL-3-Basic (Promega Corp.)
recombinant reporter vector containing the predictive miR-181a-5p
binding sequence. The mutant recombinant reporter vector was
designed and synthesized by GenePharma (Shanghai GenePharma Co.,
Ltd.). Cells were then cultured in 24-well plates at a density of
2x104 cells/well until they reached 80% confluence.
Subsequently, the cells were co-transfected with 0.2 µg mutant or
wild-type (WT) reporter plasmids and 10 nmol miR-181a-5p mimics or
miR-NC mimics using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). At 48 h post-transfection, the
harvested cells were analyzed using the Dual-luciferase Reporter
Assay system (Promega Corp.) according to the manufacturer's
protocol.
Statistical analysis
All of the aforementioned procedures were performed
in triplicate. Values are expressed as the mean ± standard
deviation and differences were assessed using one-way analysis of
variance, followed by the Newman-Keuls multiple-comparisons test.
SPSS software (version 17.0; SPSS Inc.) was used for the
statistical analyses and P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-181a-5p expression is
downregulated following treatment with high glucose
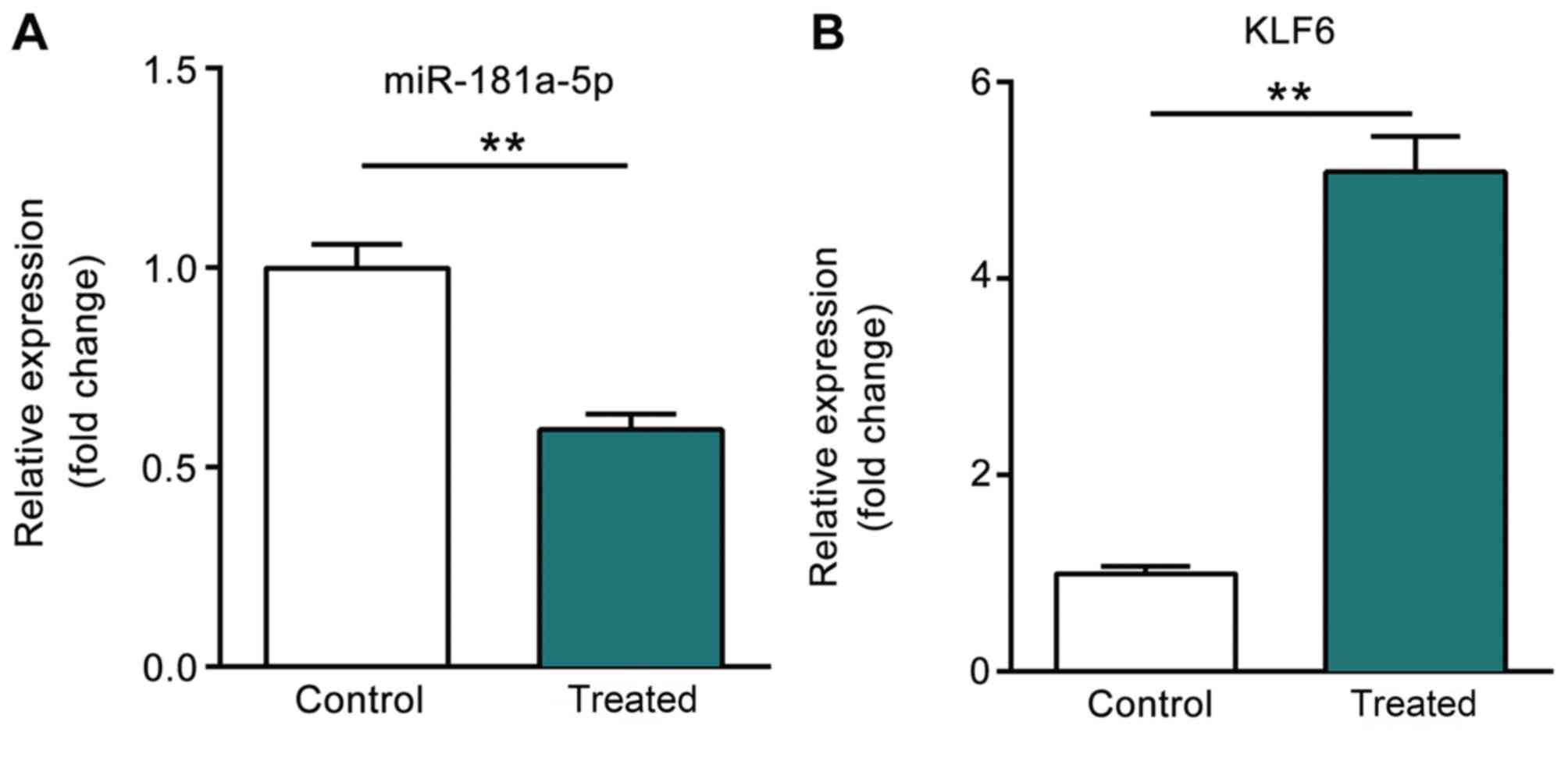
As presented in Fig.
1A, the expression level of miR-181a-5p was significantly
downregulated following treatment with high glucose as compared
with that in the control group. However, the expression level of
KLF6, the predictive target gene of miR-181a-5p, was significantly
upregulated following treatment with high glucose (P<0.01;
Fig. 1B).
KLF6 is a target of miR-181a-5p
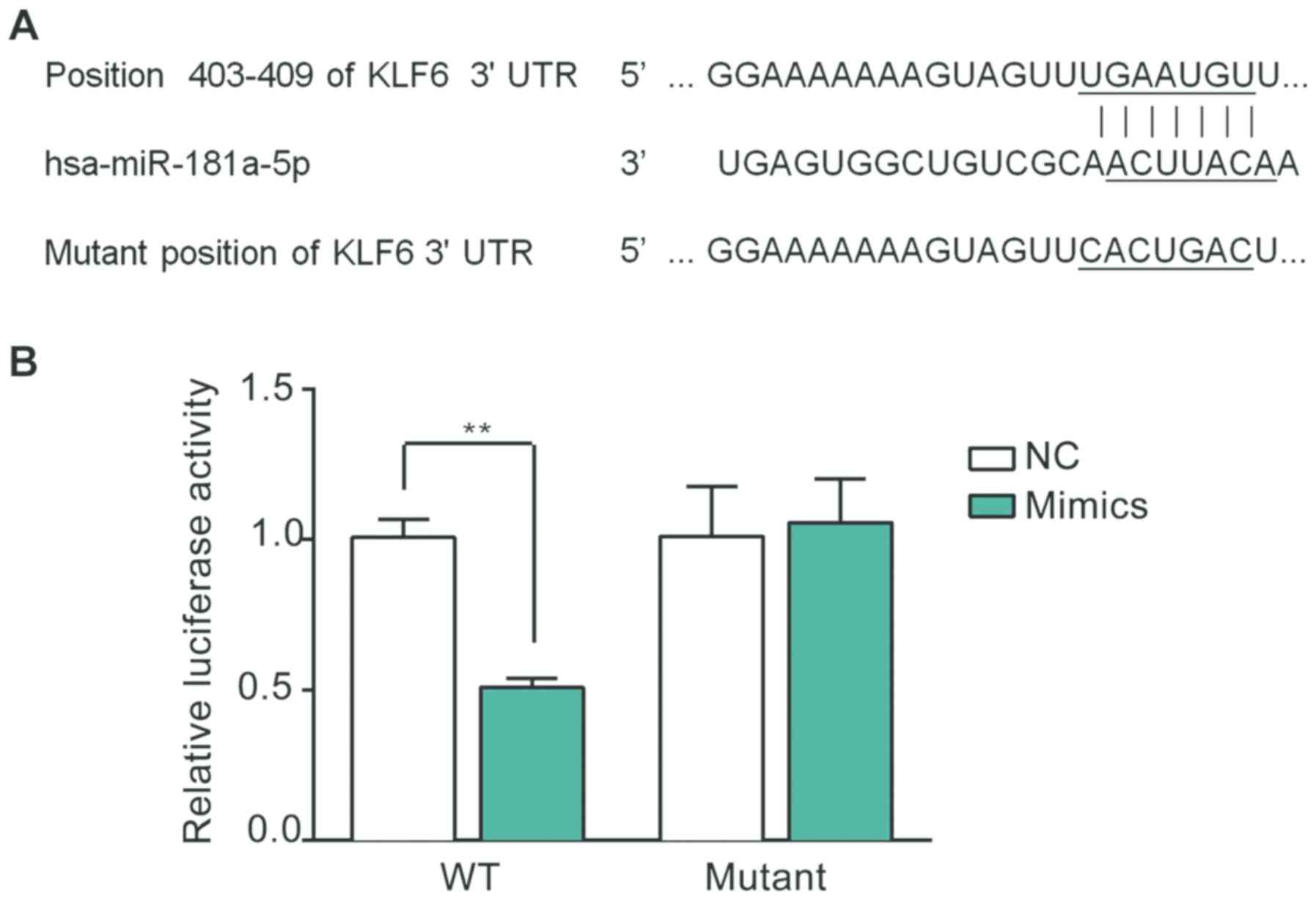
As indicated in Fig.
2A, the 3'UTR of KLF6 mRNA contains a potential binding
sequence for miR-181a-5p, which was identified using a
bioinformatics analysis. A luciferase assay was subsequently
performed using the GMCs in order to verify whether KLF6 is a
target of miR-181a-5p. As presented in Fig. 2B, the relative luciferase activity
was markedly decreased in the miR-181a-5p-transfected GMCs
following co-transfection with the WT recombinant plasmid
(P<0.01). However, there was no significant difference in
luciferase activity between the miR-181a-5p and NC groups following
co-transfection with the mutant recombinant plasmid.
Overexpression efficiency of
miR-181a-5p and KLF6
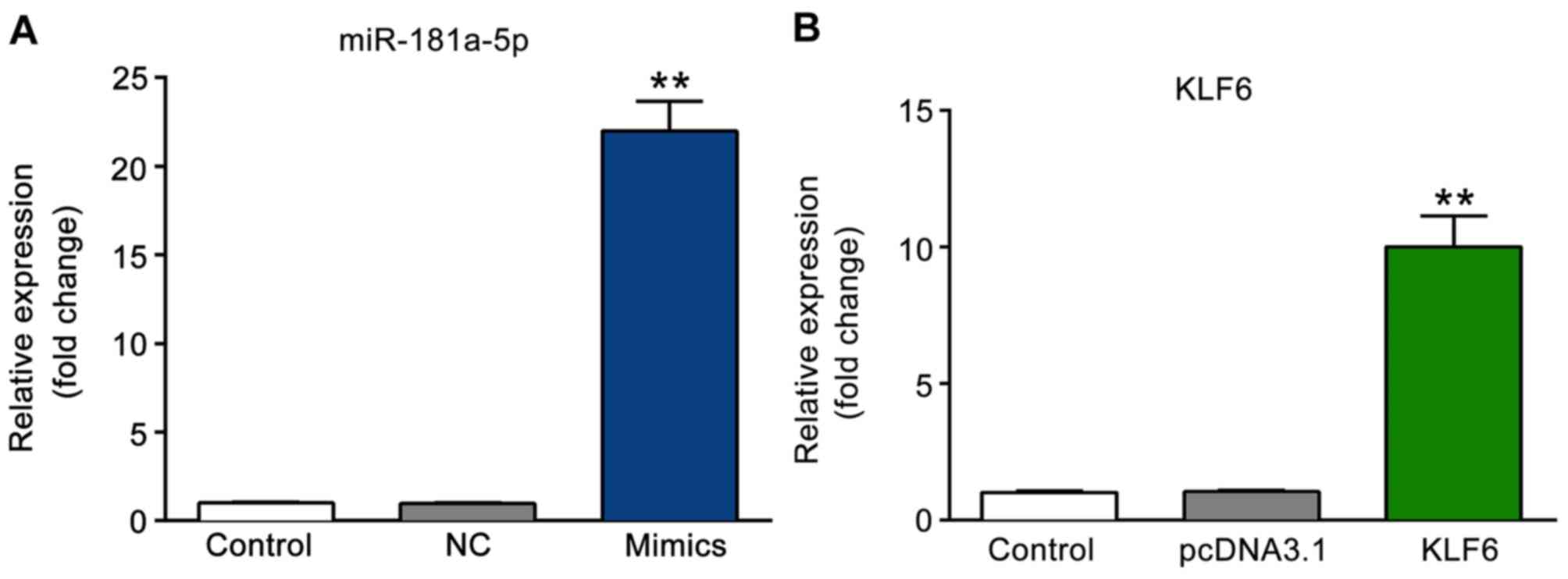
The expression levels of miR-181a-5p and KLF6 were
detected using RT-qPCR following transfection. As presented in
Fig. 3A, the expression level of
miR-181a-5p was significantly increased in the miR-181a-5p mimics
group as compared with that in the NC and control groups
(P<0.01). Furthermore, the expression level of KLF6 was markedly
increased in the KLF6 group as compared with that in the pcDNA3.1
and control groups (P<0.01; Fig.
3B).
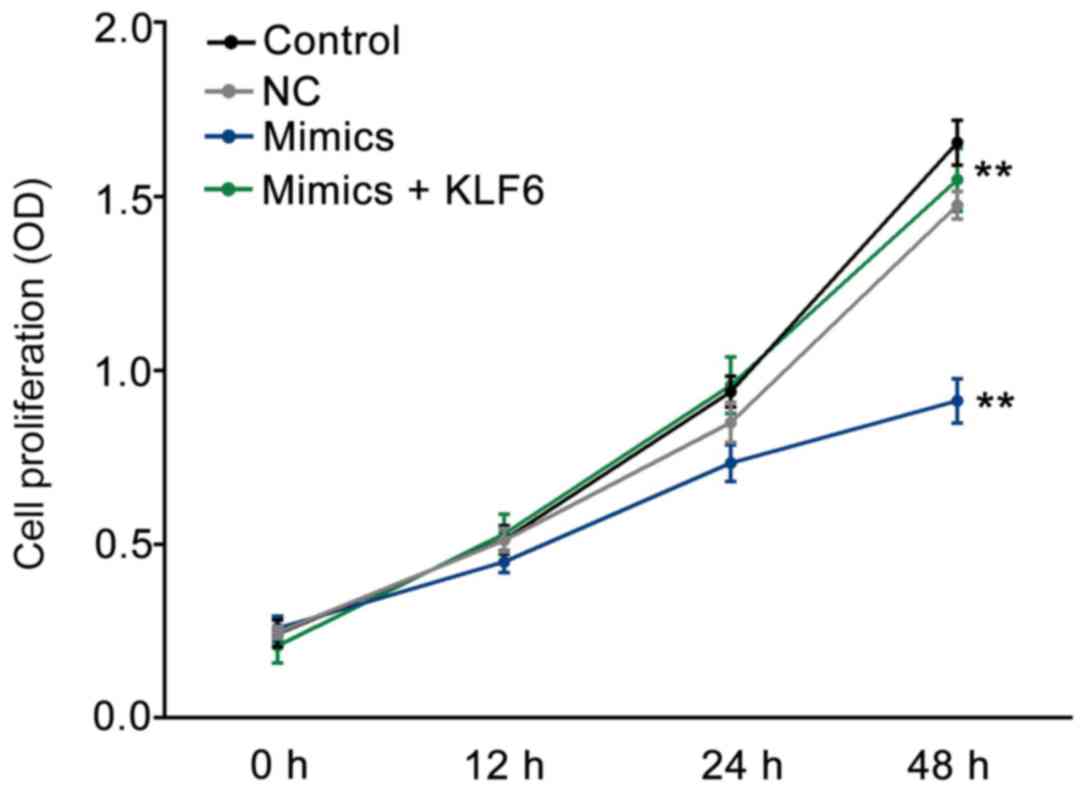
miR-181a-5p suppresses GMC
proliferation stimulated by high glucose
As presented in Fig.
4, after 48 h incubation, the proliferation of GMC cells was
significantly decreased in the mimics group as compared with that
in the NC or control groups (P<0.01); this variation was
reversed by co-transfection with pcDNA3.1-KLF6 (P<0.01).
Overexpression of KLF6 promotes human
GMC proliferation
As presented in Fig.
S1, the cell viability was prominently increased in the KLF6
group in comparison to that in the pcDNA3.1 and control groups
(P<0.01).
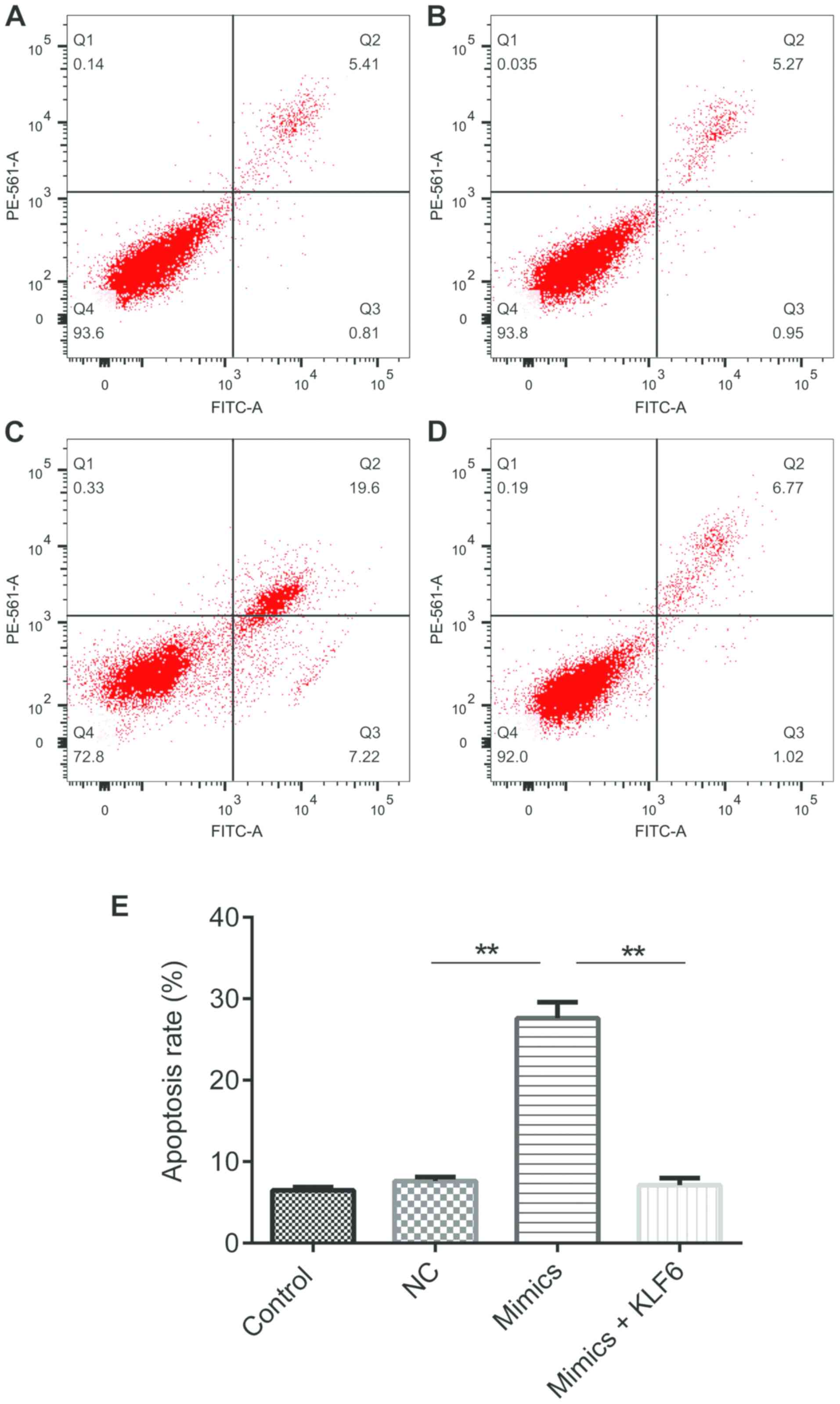
miR-181a-5p promotes GMC apoptosis
stimulated by high glucose
As presented in Fig.
5, cell apoptosis was significantly increased in the mimics
group as compared with that in the NC or control groups
(P<0.01); however, the variation was reversed by co-transfected
with pcDNA3.1-KLF6 (P<0.01).
Overexpression of KLF6 suppresses
human GMC apoptosis
As presented in Fig.
S2, cell apoptosis was prominently decreased in the KLF6 group
as compared with that in the pcDNA3.1 and control groups
(P<0.01).
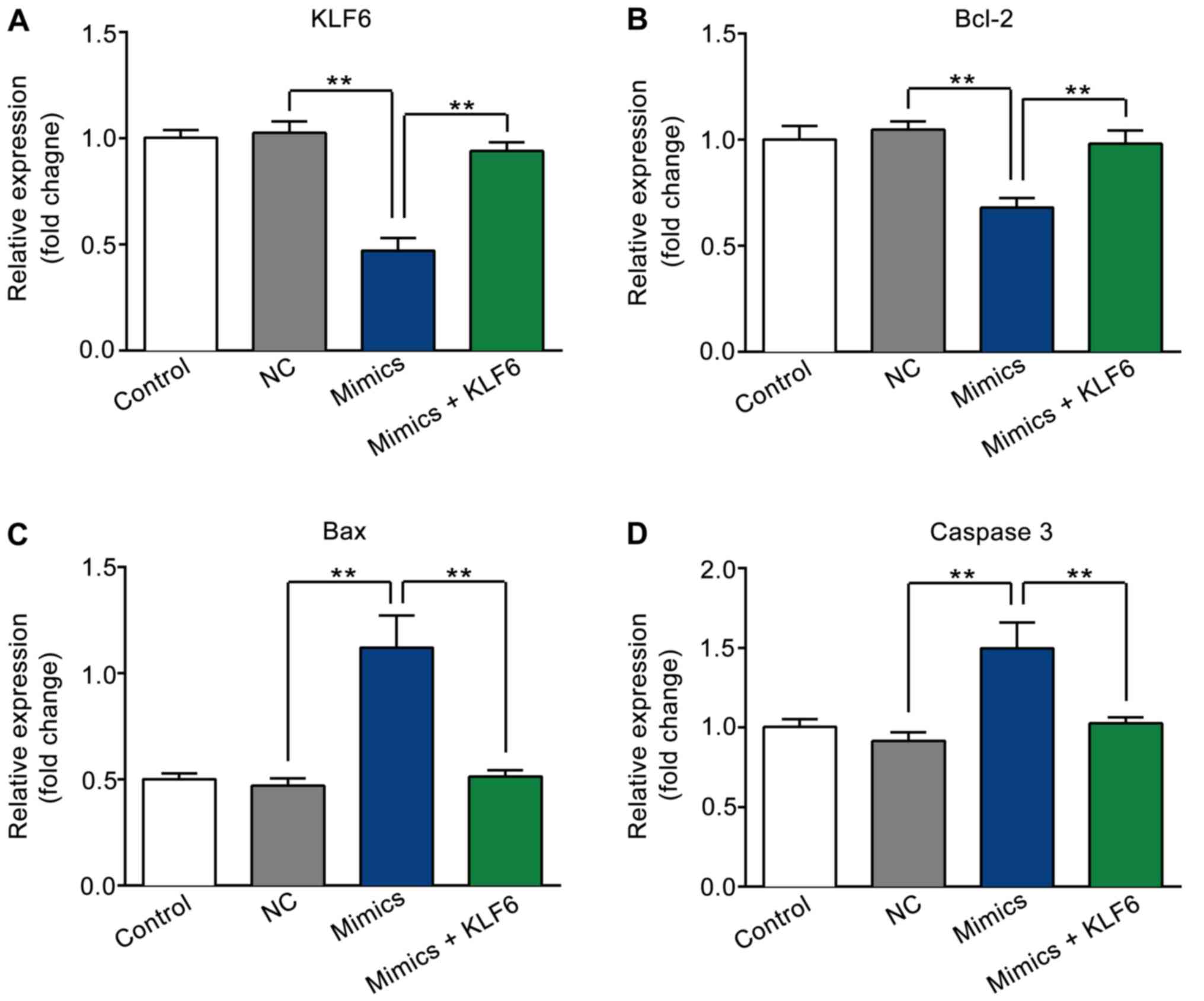
mRNA expression levels of KLF6, Bcl-2,
Bax and caspase-3 following transfection
As presented in Fig.
6A-D, the expression levels of KLF6 and Bcl-2 were markedly
decreased, while Bax and caspase-3 levels were increased in the
mimics group as compared with those in the NC and control groups
(P<0.01). However, the variation induced by transfection with
miR-181a-5p mimics was reversed by co-transfection with
pcDNA3.1-KLF6 (P<0.01).
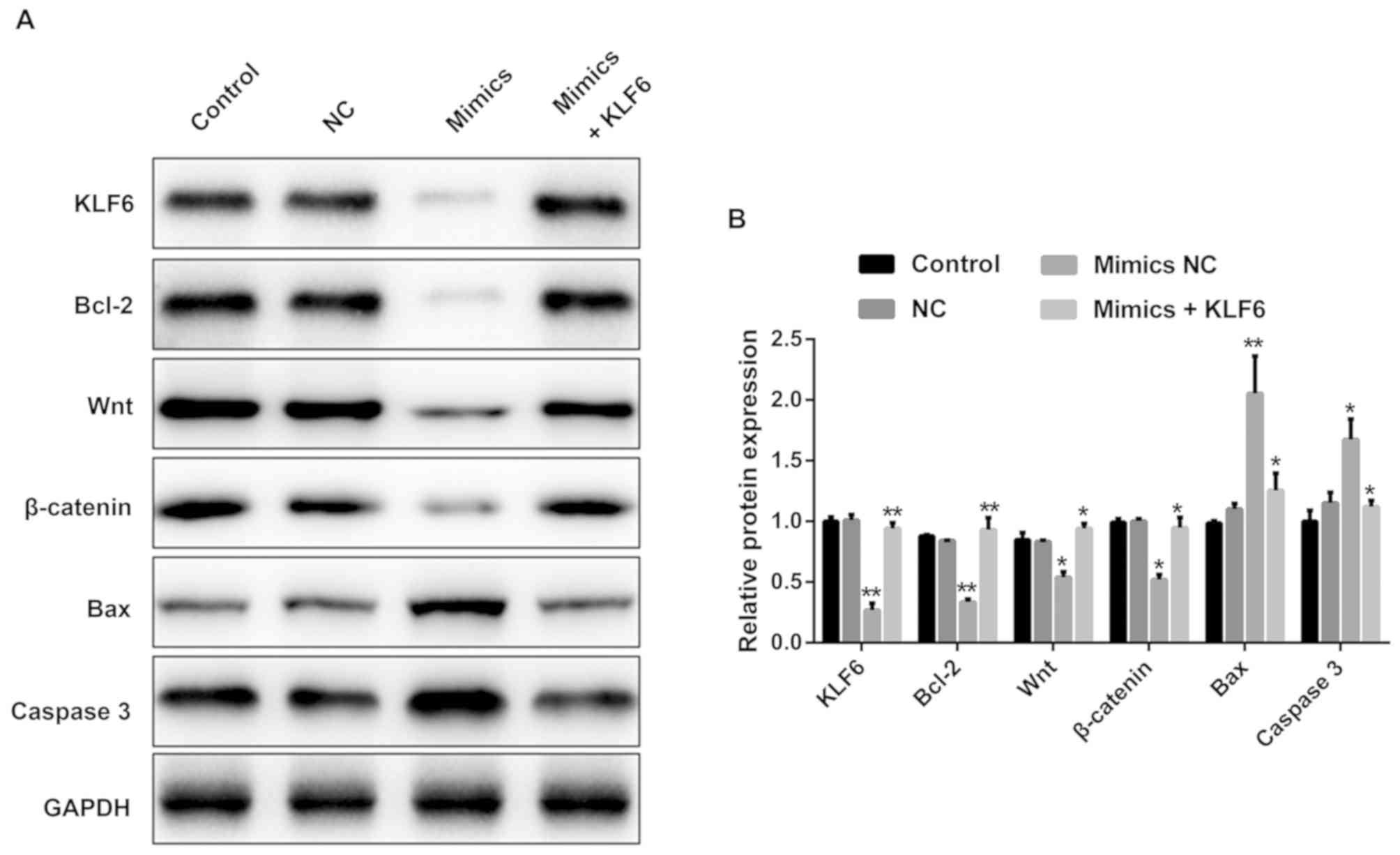
miR-181a-5p may regulate GMC
proliferation and apoptosis via the Wnt/β-catenin signaling
pathway
As presented in Fig.
7A and 7B, the protein
expression levels of KLF6, Bcl-2, Wnt, β-catenin were significantly
decreased, while Bax and caspase-3 were increased in the mimics
group as compared with those in the NC and control groups. However,
the variation was reversed by co-transfection with
pcDNA3.1-KLF6.
KLF6 may regulate human GMC
proliferation and apoptosis via the Wnt/β-catenin signaling
pathway
As presented in Fig.
S3, the protein expression levels of KLF6, Bcl-2, Wnt and
β-catenin were prominently increased, while Bax and caspase-3 were
decreased in the KLF6 group as compared with those in the pcDNA3.1
and control groups.
Discussion
miR-181a-5p belongs to the miR-181a family, which
has been reported to participate in malignant transformation
(20-22)
and various diseases (23-25).
High glucose concentrations have been demonstrated to contribute to
the uncontrolled proliferation of mesangial cells (26,27) and
high glucose was reported to be the principal cause of renal damage
in diabetes (28), indicating that
uncontrolled proliferation stimulated by high glucose in mesangial
cells may contribute to the progression of DN. The results of the
present study demonstrated that miR-181a-5p was highly expressed,
while KLF6 exhibited low expression in GMCs following treatment
with high glucose. Furthermore, overexpression of miR-181a-5p was
indicated to inhibit the proliferation but promote apoptosis of
GMCs stimulated by high glucose. Collectively, these results
indicated that miR-181a-5p may have a pivotal in the genesis and
progression of DN and may serve as a diagnostic marker.
Bcl-2 is a member of the Bcl-2 family, which is able
to regulate cell apoptosis and functions as an anti-apoptotic
protein (29,30). Bax is a pro-apoptotic protein, which
is activated in response to apoptotic stimuli (31). Caspase-3 is a member of the family of
cysteine proteases, originally identified by their role in
apoptosis, functioning as a pro-apoptotic protein in various
cellular processes (32). KLF6 has
been reported to be a regulator for the progression of DN,
functioning as a potential pathogenic factor in DN (33). The Wnt/β-catenin signaling pathway,
controlled by miRNAs, exerts its function to maintain proper
cell-cell junctions and tissue homeostasis (34). Inactivation or inhibition of the
Wnt/β-catenin signaling pathway has been demonstrated to promote
apoptosis and suppress cell proliferation in various cancer types
(35,36). Of note, a previous study reported
that blocking the Wnt/β-catenin signaling pathway may attenuate the
proliferation of GMCs induced by high glucose levels (37). Consistent with previous studies, the
results of the present study suggested that miR-181a-5p may be able
to inhibit GMC proliferation but increase apoptosis by
downregulating KLF6 and Bcl-2 expression and upregulating Bax and
caspase-3 expression via the Wnt/β-catenin signaling pathway. To
the best of our knowledge, the present study was the first to
demonstrate that miR-181a-5p could inhibit GMC proliferation while
it promoted apoptosis by inhibition of KLF6 through Wnt/β-catenin
signaling pathway. However, there are some limitations in our
study. In the future, we will conduct further experiments
concerning the effect of high glucose on GMC proliferation and
apoptosis. Meanwhile, more targets and underlying molecular
mechanism remain to be further elucidated.
Xu et al (38)
reported that miR-181a-5p expression was downregulated in rats with
DN and may prevent fibrosis in HK-2 cells by targeting early growth
response 1. In the present study, miR-181a-5p overexpression was
indicated to inhibit GMC proliferation but increase apoptosis by
targeting KLF6 under high-glucose conditions. It was confirmed that
KLF6 was a target gene of miR-181a-5p for the first time.
In conclusion, the present study indicated that
miR-181a-5p was downregulated in GMCs following treatment with high
glucose. Overexpression of miR-181a-5p inhibited GMC proliferation
but promoted apoptosis at least partially through targeting KLF6
via the Wnt/β-catenin signaling pathway. Overall, the results of
the present study suggest that miR-181a-5p may have a crucial role
in the occurrence and development of DN and may be a valuable
diagnostic marker and therapeutic target for DN.
Supplementary Material
Overexpression of KLF6 promotes human
glomerular mesangial cell proliferation. The cell proliferation in
the different groups is presented. **P<0.01, KLF6 vs.
pcDNA3.1 group. KLF6, kruppel-like factor 6; OD, optical
density.
Overexpression of KLF6 suppresses
human glomerular mesangial cell apoptosis. (A-C) Representative
flow cytometry dot plots for (A) Control group, (B) pcDNA3.1 group
and (C) KLF6 group. (D) The corresponding results for A-C are
presented. **P<0.01, KLF6 vs. pcDNA3.1 group. KLF6,
kruppel-like factor 6; Q, quadrant.
KLF6 may regulate human glomerular
mesangial cell proliferation and apoptosis via the Wnt/β-catenin
signaling pathway. The protein expression levels of the associated
factors in different groups were assessed by western blot analysis.
KLF6, kruppel-like factor 6.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL prepared the manuscript, performed some
experiments and revised the manuscript for important content. WX
performed the statistical analysis and helped with the study
design, critical discussion and manuscript revision. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nav Rev Genet. 5:522–531.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu X, Li M, Peng Y, Hu X, Xu J, Zhu S, Yu
Z and Han S: miR-30c regulates proliferation, apoptosis and
differentiation via the Shh signaling pathway in P19 cells. Exp Mol
Med. 48(e248)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vidal AF, Cruz AM, Magalhães L, Pereira
AL, Anaissi AK, Alves NC, Albuquerque PJ, Burbano RM, Demachki S
and Ribeiro-dos-Santos Â: hsa-miR-29c and hsa-miR-135b differential
expression as potential biomarker of gastric carcinogenesis. World
J Gastroenterol. 22:2060–2070. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bansal N, Dhaliwal R and Weinstock RS:
Management of diabetics in the elderly. Med Clin North Am.
99:351–377. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nam S, Chesla C, Stotts NA, Kroon L and
Janson SL: Barriers to diabetes management: Patient and provider
factors. Diabetes Res Clin Pract. 93:1–9. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Abboud HE: Mesangial cell biology. Exp
Cell Res. 318:979–985. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Danesh FR, Sadeghi MM, Amro N, Philips C,
Zeng L, Lin S, Sahai A and Kanwar YS: 3-Hydroxy-3-methylglutaryl
CoA reductase inhibitors prevent high glucose-induced proliferation
of mesangial cells via modulation of Rho GTPase/p21 signaling
pathway: Implications for diabetic nephropathy. Proc Natl Acad Sci
USA. 99:8301–8305. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu ZM, Zheng HY, Chen LH, Li YL, Wang Q,
Liao CF and Li XW: Low expression of miR-203 promoted diabetic
nephropathy via increasing TLR4. Eur Rev Med Pharmacol Sci.
22:5627–5634. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhao D, Jia J and Shao H: iR-30e targets
GLIPR-2 to modulate diabetic nephropathy: In vitro and in vivo
experiments. J Mol Endocrinol. 59:181–190. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang J, Duan L, Gao Y, Zhou S, Liu Y, Wei
S, An S, Liu J, Tian L and Wang S: Angiotensin II receptor blocker
valsartan ameliorates cardiac fibrosis partly by inhibiting miR-21
expression in diabetic nephropathy mice. Mol Med Endorcrinol.
472:149–158. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
He YQ, Ding YL, Liang BY, Lin JJ, Kim TY,
Yu HB, Hang HW and Wang K: A systematic study of dysregulated
microRNA in type 2 diabetes mellitus. Int J Mol Sci. 18:
pii(456)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Assmann TS, Recamonde-Mendoza M, De Souza
BM and Crispim D: MicroRNA expression profiles and type 1 diabetes
mellitus: Systematic review and bioinformatic analysis. Endocr
Connect. 6:773–790. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tetreault MP, Yang Y and Katz JP:
Krüppel-like factors in cancer. Nat Rev Cancer. 13:701–713.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Huang X, Li X and Guo B: KLF6 induces
apoptosis in prostate cancer cells through up-regulation of ATF3. J
Biol Chem. 283:29795–29801. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wahab AH, Kassem AM, Matter S, EI Deen AF,
Helmy AS, Ismaeil MM and Zakaria MS: Role of KLF6 tumor suppressor
gene mutations in the development of colorectal carcinoma in an
Egyptian population. Hepatogastroenterology. 57:1405–1410.
2010.PubMed/NCBI
|
|
17
|
Rane MJ, Zhao Y and Cai L: Krüppel-like
factors (KLFs) in renal physiology and disease. EBioMedicine.
40:743–750. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu S, Lv YY, Zhao J, Wang JP, Zhao X and
Wang S: Inhibitory effects of Shenkang injection and its main
component emodin on the proliferation of high glucose-induced renal
mesangial cells through cell cycle regulation and induction of
apoptosis. Mol Med Rep. 14:3381–3388. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li Y, Kuscu C, Banach A, Zhang Q,
Pulkoski-Gross A, Kim D, Liu J, Roth E, Li E, Shroyer KR, et al:
MiR-181a-5p inhibits cancer cell migration and angiogenesis via
downregulation of matrix metalloproteinase-14. Cancer Res.
75:2674–2685. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Taylor MA, Sossey-Alaoui K, Thompson CL,
Danielpour D and Schiemann WP: TGF-β upregulates miR-181a
expression to promote breast cancer metastasis. J Clin Invest.
123:150–163. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Wei Y, Tao X, Xu H, Chen Y, Zhu L, Tang G,
Li M, Jiang A, Shuai S, Ma J, et al: Role of miR-181a-5p and
endoplasmic reticulum stress in the regulation of myogenic
differentiation. Gene. 592:60–70. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li S, Yang J, Xia Y, Fan Q and Yang KP:
Long noncoding RNA NEAT1 promotes proliferation and invasion via
targeting miR-181a-5p in non-small cell lung cancer. Oncol Res.
26:289–296. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yu C, Sun J, Leng X and Yang J: Long
noncoding RNA SNHG6 functions as a competing endogenous RNA by
sponging miR-181a-5p to regulate E2F5 expression in colorectal
cancer. Cancer Manag Res. 11:611–624. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mi Y, Zhang D, Jiang W, Weng J, Zhou C,
Huang K, Tang H, Yu Y, Liu X, Cui W, et al: miR-181a-5p promotes
the progression of gastric cancer via RASSF6-mediated MAPK
signaling activation. Cancer Lett. 389:11–22. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang L, Pang S, Deng B, Qian L, Chen J,
Zou J, Zheng J, Yang L, Zhang C, Chen X, et al: High glucose
induces renal mesangial cell proliferation and fibronectin
expression through JNK/NF-κB/NADPH oxidase/ROS pathway, which is
inhibited by resveratrol. Int J Biochem Cell Biol. 44:629–638.
2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sodhi CP, Phadke SA, Batlle D and Sahai A:
Hypoxia and high glucose cause exaggerated mesangial cell growth
and collagen synthesis: Role of osteopontin. Am J Physiol Renal
Physiol. 280:F667–F674. 2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Suzaki Y, Yoshizumi M, Kagami S, Nishiyama
A, Ozawa Y, Kyaw M, Izawa Y, Kanematsu Y, Tsuchiya K and Tamaki T:
BMK1 is activated in glomeruli of diabetic rats and in mesangial
cells by high glucose conditions. Kidney Int. 65:1749–1760.
2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Singh L, Pushker N, Saini N, Sen S, Sharma
A, Bakhshi S, Chawla B and Kashyap S: Expression of pro-apoptotic
Bax and anti-apoptotic Bcl-2 proteins in human retinoblastoma. Clin
Exp Ophthalmol. 43:259–267. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H,
Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO,
Giles CB, et al: Identification of a novel senolytic agent,
navitoclax, targeting the Bcl-2 family of anti-apoptotic factors.
Aging Cell. 15:428–435. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ghibelli L and Diederich M: Multistep and
multitask Bax activation. Mitochondrion. 10:604–613.
2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Caruso Bavisotto C, Nikolic D, Marino
Gammazza A, Barone R, Lo Cascio F, Mocciaro E, Zummo G, Conway de
Macario E, Macario AJ, Cappello F, et al: The dissociation of the
Hsp60/pro-Caspase-3 complex by bis (pyridyl) oxadiazole copper
complex (CubipyOXA) leads to cell death in NCI-H292 cancer cells. J
Inorg Biochem. 170:8–16. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Qi W, Holian J, Tan CY, Kelly DJ, Chen XM
and Pollock CA: The roles of Kruppel-like factor 6 and peroxisome
proliferator-activated receptor-γ in the regulation of macrophage
inflammatory protein-3α at early onset of diabetics. Int J Biochem
Cell Biol. 43:383–392. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ghahhari NM and Babashah S: Interplay
between microRNAs and Wnt/β-catenin signaling pathway regulates
epithelial-mesenchymal transition in cancer. Eur J Cancer.
51:1638–1649. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mao J, Fan S, Ma W, Fan P, Wang B, Zhang
J, Wang H, Tang B, Zhang Q, Yu X, et al: Roles of Wnt/β-catenin
signaling in the gastric cancer stem cells proliferation and
salinomycin treatment. Cell Death Des. 5(e1039)2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gao L, Chen B, Li J, Yang F, Cen X, Liao Z
and Long X: Wnt/β-catenin signaling pathway inhibits the
proliferation and apoptosis of U87 glioma cells via different
mechanisms. PLoS One. 12(e0181346)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lan XQ, Wen HX, Aslam R, Marashi Shoshtari
SS, Mishara A, Kumar V, Wang HC, Wu GS, Luo HR, Malhotra A and
Singhal PC: Nicotine enhances mesangial cell proliferation and
fibronectin production in high glucose milieu via activation of
Wnt/β-catenin pathway. Bioscience Rep. 38:
pii(BSR20180100)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xu P, Guan MP, Bi JG, Wang D, Zheng ZJ and
Xue YM: High glucose down-regulates microRNA-181a-5p to increase
pro-fibrotic gene expression by targeting early growth response
factor 1 in HK-2 cells. Cell Signal. 31:96–104. 2017.PubMed/NCBI View Article : Google Scholar
|