Introduction
Systemic lupus erythematosus (SLE) is a multisystem
autoimmune disease of the connective tissue characterized by
heterogeneous manifestations, production of autoantibodies (AAbs)
and chronic inflammation of numerous organs, with a higher
prevalence among females (1). The
pathogenesis includes local deposition of anti-nuclear antibodies
(ANA) and activation of the complement system (2). Although the prognosis of patients with
SLE has improved in the past decade as a result of improvements in
diagnosis and medical technology, the mortality rates in patients
with lupus remain 2-5 times higher than in the general population
(3). Autoimmune diseases may be
divided into organ-specific and systemic autoimmune diseases. The
former most frequently affect the thyroid gland, characterized by
destruction of thyroid follicular cells and leading to
hypothyroidism. The latter mainly refer to connective tissue
diseases, including rheumatoid arthritis, SLE, myositis and
systemic sclerosis (4,5). Hypothyroidism and SLE are autoimmune
diseases and may occur together, but usually have their own
characteristic clinical manifestations. The present study reported
on a case of SLE with hypothyroidism as the initial clinical
manifestation and the literature was reviewed.
Case report
A 31-year-old female with a history of face and
bilateral leg swelling without any obvious inducement over the
preceding 6 months, accompanied by lethargy, memory loss, lack of
concentration, shortness of breath and wheezing, presented to the
department of Endocrinology, China-Japan Union Hospital of Jilin
University, Changchun, Jilin in May 2019. As the symptoms did not
significantly impact the patient's daily life, no systematic
diagnosis or treatment were determined. However, 5 and a half
months after the patient first presented, these symptoms worsened
and were accompanied by dyspnea, at which point the patient was
examined at the outpatient department of China-Japan Union Hospital
of Jilin University (Changchun, China). Assessment of thyroid
function provided the following values: Thyroid-stimulating
hormone, 100.00 mUI/l; free triiodothyronine, 0.78 pmol/l; and free
thyroxine, 1.3 pmol/l (Table I).
Cardiac ultrasonography revealed massive pericardial effusion.
During the course of the disease, there was no facial erythema,
photosensitivity, recurring oral ulcers; when the patient first
presented they were occasional morning stiffness, hair loss or
myalgia, which was apparent in the proximal extremities of the
bilateral legs. Physical examination revealed the following: Body
temperature, 36.5˚C; blood pressure, 112/70 mmHg; pulse, 72
beats/min; respiration, 16 times/min. The patient did not have any
rash; however, the patient presented with slow response, apathy,
dry skin, edema of eyelids and face and hypertrophy of lips; the
enlargement of thyroid is in the stage of first degree, thyroid
texture is tough, no tenderness, and no murmur on auscultation of
thyroid. The patient's heart rhythm was uniform, the sound of the
heart was distant, low and dull, and no murmur was heard on
auscultation of heart. The patient exhibited severe non-concave
edema of the bilateral lower limbs, normal muscle tension of limbs,
muscle strength at level 5, weakened bilateral knee reflex and
ankle reflex, and no joint swelling or motion disorder. The
patient's body weight was 80 kg. Relevant examinations were
completed on admission and results are listed in Table I. Immunoglobulin and complement were
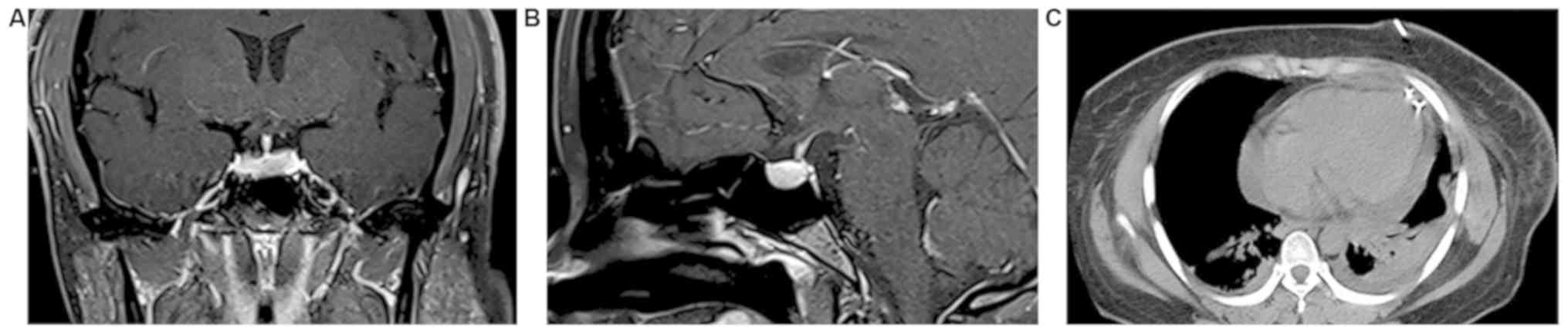
normal. A microadenoma was identified by pituitary nuclear magnetic
resonance imaging (Fig. 1A and
B). A chest CT scan revealed
pericardial effusion (Fig. 1C).
Cardiac ultrasonography revealed massive pericardial effusion. A
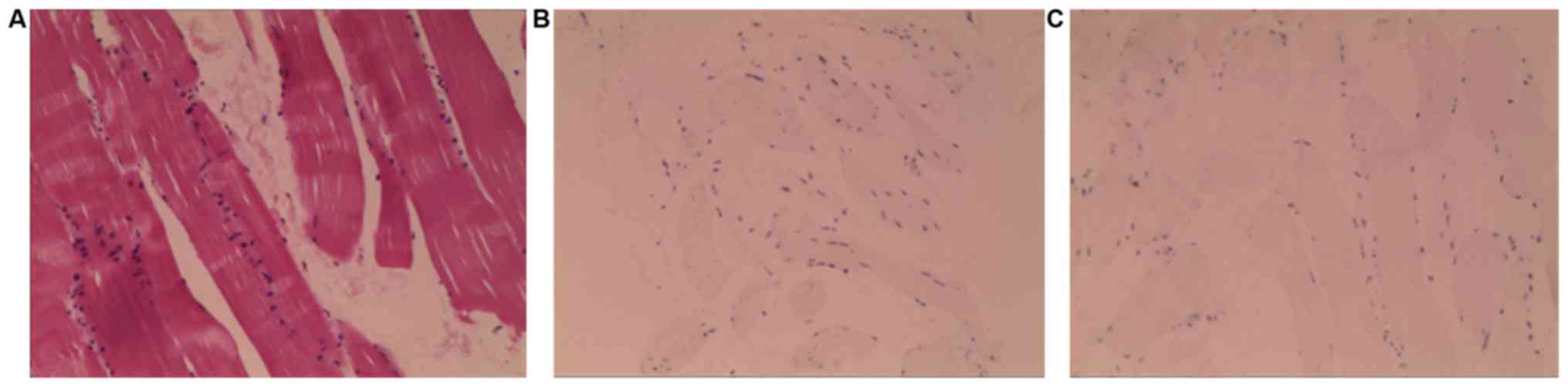
right deltoid muscle biopsy was performed and indicated that the
majority of the muscle fiber tissues were atrophied and
degenerated, and lymphocytes were occasionally observed among the
muscle fibers (Fig. 2A). Myositis
was not excluded based on morphology. Lymphocyte
immunohistochemistry was as follows: Lymphocytes were negative for
CD4 and CD8 (Fig. 2B and C). The patient was treated with initiated
thyroid hormone replacement therapy with oral levothyroxine (25
µg/day). Pericardiocentesis was performed under CT guidance, and
~1,000 ml of pericardial fluid was removed via an anterior approach
with placement of a catheter. The characteristics of the
pericardial fluid were as follows: The rivalta experiment was
positive, which is a qualitative determination test of serous mucin
used to distinguish the exudate from the leaking fluid and the
result of the exudate should be positive (6); leucocyte count, 7.1x107/l;
glucose, 5.0 mmol/l; protein, 58.2 g/l; chloride, 106.8 mmol/l;
mononuclear leucocytes, 82%; polynuclear leucocytes, 18%; adenosine
deaminase, 11.7 U/l; and lactate dehydrogenase, 466.19 IU/l. After
pericardiocentesis, the dyspnea symptoms gradually alleviated. The
dose of thyroxine was gradually adjusted during hospitalization.
After 18 days, the patient was discharged on oral thyroxine (87.5
µg/day). The patient was followed up at the outpatient clinic 3
weeks and 3 months after being discharged (Table I).
 | Table IVital signs and laboratory results at
hospitalization and follow-ups. |
Table I
Vital signs and laboratory results at
hospitalization and follow-ups.
| Parameter | Initial visit | Follow-up at 3
weeks | Follow-up at 3
months | Reference
range |
|---|
| Body weight
(kg) | 80 | 73.5 | 71.8 | - |
| Blood pressure
(mmHg) | 106/63 | 100/70 | 110/80 | - |
| Heart rate
(beats/min) | 78 | 75 | 86 | - |
| TSH (mIU/l) | 100.00 | 30.79 | 11.32 | 0.37-4.94 |
| FT3 (pmol/l) | 0.40 | 4.42 | 4.68 | 3.10-6.80 |
| FT4 (pmol/l) | 0.90 | 11.30 | 9.20 | 12.00-22.00 |
| Tg (ng/ml) | 14.21 | 9.65 | 0.04 | 0.10-23.00 |
| Anti-Tg (IU/l) | 297.80 | - | 313.80 | 0.00-35.00 |
| TPO (IU/l) | 160.40 | 130.50 | 131.70 | 0.00-34.00 |
| WBC
(x109/l) | 3.17 | 5.76 | 4.98 | 4.00-10.00 |
| HB (g/l) | 73.00 | 97.40 | 112.00 | 110.00-150.00 |
| PLT
(x109/l) | 328.00 | 328.00 | 333.00 | 100.00-300.00 |
| Coomb's test | (-) | (-) | (-) | (-) |
| TG (mmol/l) | 2.09 | 1.89 | 1.00 | <1.70 |
| TC (mmol/l) | 6.91 | 4.26 | 4.35 | 3.00-5.70 |
| LDL-C (mmol/l) | 4.18 | 2.71 | 2.67 | <4.13 |
| ESR (mm) | 18.00 | 30.00 | 12 | 0.00-5.00 |
| CRP (mg/l) | 6.26 | 13.43 | 9.86 | 0.00-5.00 |
| CK (IU/l) | 1339.76 | 149.71 | 93.00 | 26.00-140.00 |
| CK-MB (U/l) | 37.20 | 11.23 | 16.20 | 0.00-16.00 |
| LDH (U/l) | 1645.01 | 254.32 | 216.00 | 313.00-618.00 |
| PRL (mIU/l) | 1280.90 | - | 794.9 | 108.80-557.10 |
| ANA | 1:100 (+) | 1:100 (+) | 1:100 (+) | (-) |
| ANUA | (+) | (+) | (+) | (-) |
| Anti-nRNP
Antibodies | (+) | (-) | (+) | (-) |
| Anti-Sm
antibodies | (+) | (+) | (+) | (-) |
| Pericardial
effusion | Deepest, 37 mm | Deepest, 13 mm | (-) | (-) |
Discussion
A systematic literature search of the PubMed and
CNKI databases was performed to identify studies published from
1987 to the present day containing information on the prevalence of
hypothyroidism in SLE patients (Table
II). The majority of the studies identified reported a high
incidence of hypothyroidism in patients with SLE; the prevalence of
clinical hypothyroidism reported in the literature ranged from 3.0
to 21.4% (7-25).
The prevalence was higher in females compared with that in males,
with the incidence rate estimated to be 9-fold higher in females
(26). Hypothyroidism in patients
with SLE was associated with the presence of abnormal AAbs,
hypoechoic pattern of the thyroid and small thyroid volume,
suggesting that autoimmunity has an important role in the
pathogenesis of hypothyroidism in SLE (9). Studies have indicated that arthritis
and skin damage are more common in patients with SLE with
hypothyroidism, while fewer neuropsychiatric symptoms and
hematological abnormalities were observed (15). The association between SLE and
thyroid dysfunction has been established since 1961(27). Most of the previous studies reported
a higher prevalence of SLE in patients with hypothyroidism
(4-26).
However, there were no reported cases of SLE with the only or the
initial symptom of hypothyroidism, the present study reported on a
case to highlight the diversity of the disease. SLE is a
multifactorial autoimmune disease with a complex pathogenesis and
clinical manifestations (28). The
present case and previous studies suggest that hypothyroidism is
more likely to occur in patients with SLE in whom there is a higher
incidence of anti-thyroid antibodies (7-26);
the reason for this remains elusive. The pathogenesis of
hypothyroidism in patients with SLE may be due to the following
factors: i) Autoimmunity has an important role in hypothyroidism
associated with SLE (29). Systemic
and organ-specific autoimmune diseases are associated with each
other. SLE is a prototype of autoimmune diseases characterized by
its multi-organ involvement. Previous reports indicated that the
presence of positivity for anti-thyroglobulin antibodies (A-TG) and
anti-thyroid peroxidase antibodies (TPOAb) in patients with SLE
were significantly higher than those in the control group of
healthy individuals (8-24).
Patients with SLE have a variety of AAbs, including anti-Smith
(anti-Sm) and anti-nucleosome antibodies (30), which tend to cause autoimmune
inflammation. Lymphocytic infiltration of the thyroid gland
destroys thyroid follicles and induces apoptosis of thyroid tissue
cells, and A-TG and TPOAb may result from polyclonal B-cell
activation (31). ii) An early
animal study indicated that the incidence of autoimmune thyroid
diseases in a murine lupus model, MRL-lpr/lpr mice, was
significantly increased, suggesting that the two diseases may have
similar immune deficiency or immune regulatory mechanisms (32). Later studies suggested that two human
leukocyte antigens (HLA), HLA-DRW3 and HLA-B8, and the interactions
among autoimmune cytokines have important roles in the occurrence
of hypothyroidism in SLE (5,33-37).
The two HLAs are highly expressed in patients with SLE and
hypothyroidism and may promote the formation of AAbs. These two
autoimmune diseases are mediated by T cells and B cells and the
mechanism for autoimmune destruction probably involves cellular
immunity and humoral immunity (38),
causing lymphocytic infiltration of the thyroid gland by B cells
and cytotoxic T cells; the function of T suppressor cells (Ts) is
low, T helper cells (Th) and B cells are hyperactive and the Ts/Th
ratio decreases, causing the production of a large number of AAbs
(39), and destruction of the
thyroid gland. In addition, T cell-mediated antibodies inhibit the
function of thyroid cells, leading to insufficient release of
thyroxine, which causes hypothyroidism. iii) A previous study
suggested that gene mutations have an important role in SLE with
hypothyroidism (40). It examined
the R620W polymorphism of the protein tyrosine phosphatase
non-receptor type 22 gene, which encodes for a protein present on T
cells, and discovered that individuals with this mutation were more
likely than individuals without or with other gene mutations to
develop concurrent SLE and hypothyroidism. iv) According to a study
by Hijmans et al (27),
organ-specific antigens are able to evoke auto-antibody production.
The auto-antibodies are mistakenly directed to attack healthy
tissue. A degree of overlap of auto-antibodies appears to exist
between SLE and thyroid autoimmune disease, either thyroid-specific
antibodies or antibodies typical for systemic lupus. The prevalence
of anti-TPO and A-TG is higher in SLE patients (4), but there is disagreement regarding
which antibody is responsible for thyroid disease.
 | Table IIPrevalence of hypothyroidism in
patients with SLE in previously published studies. |
Table II
Prevalence of hypothyroidism in
patients with SLE in previously published studies.
| | Patients with
SLE | Controls | | |
|---|
| Author, year | Cases (n) | Hypothyroidism
(%) | Cases (n) | Hypothyroidism
(%) | P-value | (Refs.) |
|---|
| Pyne and Isenberg,
2002 | 300 | 5.70 | - | - | NR | (7) |
| Antonelli et
al, 2010 | 201 | 5.90 | 402 | 0 | <0.01 | (8) |
| Chan et al,
2001 | 69 | 4.30 | 0 | 0 | NR | (9) |
| Park et al,
1995 | 63 | 9.50 | - | - | NR | (10) |
| Boey et al,
1993 | 129 | 3.90 | - | - | NR | (11) |
| Miller et
al, 1987 | 332 | 6.60 | - | - | NR | (12) |
| Vianna et
al, 1991 | 100 | 3.00 | 100 | 0 | <0.05 | (13) |
| Tsai et al,
1993 | 45 | 4.40 | - | - | <0.05 | (14) |
| Mader et al,
2007 | 77 | 11.60 | 52 | 1.9 | 0.048 | (15) |
| Appenzeller et
al, 2009 | 524 | 5.30 | 50 | 2 | >0.05 | (16) |
| Kumar et al,
2012 | 100 | 14.00 | 100 | 8 | NR | (17) |
| Stagnaro-Green
et al, 2011 | 63 | 11.00 | 0 | 0 | NR | (18) |
| Gao et al,
2011 | 1,006 | 1.69 | - | - | <0.01 | (19) |
| Ong and Choy,
2016 | 189 | 3.70 | - | - | NR | (20) |
| Watad et al,
2016 | 5,018 | 15.58 | 25,090 | 5.75 | <0.001 | (21) |
| Franco et
al, 2015 | 376 | 12.00 | - | - | NR | (22) |
| Song et al,
2014 | 220 | 57.80 | 160 | - | <0.05 | (23) |
| Domingues et
al, 2017 | 79 | 21.5 | 159 | 6.9 | 0.02 | (24) |
Hypothyroidism is an organ-specific autoimmune
disease. It is a systemic hypometabolic syndrome caused by thyroid
hormone deficiency or resistance due to various reasons, and its
clinical manifestations include intolerance of cold, fatigue,
lethargy, memory impairment, female menstrual disorders and
infertility (41). Typical patients
may have blank facial expressions, slow response, hoarse voice,
hearing impairment, pale complexion, facial and/or eyelid edema,
thick lips and enlarged tongue, frequently with tooth marks, dry
and rough skin, peeling skin, decreased temperature, and sparse and
dry hair. In a few cases, pretibial myxoedema occurs, and
pericardial effusion and heart failure may occur when the heart is
involved. In severe cases, myxoedema coma may occur (42).
SLE is a systemic non-specific autoimmune disease
and clinical manifestations include weakness, fever, weight loss,
photosensitivity, hair loss, oral ulcers, erythema, skin rash,
joint pain, muscle aches and Raynaud's phenomenon. SLE may cause
damage to numerous organs through the immune system, including the
thyroid, joints, skin, blood vessels, heart, lungs, liver, kidney
and nervous system (25,43). Lupus and thyroid disorders may cause
fatigue, focal edema, weakness, myalgias, arthralgias and a variety
of other non-specific complaints. According to the classification
and diagnostic criteria for SLE formulated by the American College
of Rheumatology (ACR) in 2019(44).
The diagnostic criteria for SLE are positive ANA as an entry
criterion, weighted criteria in seven clinical domains
(constitutional, haematological, neuropsychiatric, mucocutaneous,
serosal, musculoskeletal and renal) and three immunological domains
[anti-phospholipid antibodies, low complements, anti-Sm and
anti-double-stranded (ds)DNA as SLE-specific antibodies] and a
classification threshold score of ≥10. However, early clinical
manifestations of SLE are atypical, and therefore, laboratory tests
are necessary. The detection of autoantibodies has become an
important and reliable basis for the diagnosis of SLE, as patients
with SLE present with a variety of AAbs (45). A previous study revealed that
positive detection of anti-nuclear antibodies has significance in
the diagnosis of SLE (46). Among
the 15 different IgG antibodies, anti-Sm, anti-dsDNA and
anti-nucleosome antibodies are specific antibodies for SLE. Among
them, the anti-Sm antibody occurs only in SLE, has high specificity
and is considered to be a marker antibody for SLE. Anti-nucleosome
antibodies appear in the early stage of SLE and contribute to the
early diagnosis of SLE in combination with anti-nuclear antibodies.
Anti-ribonucleoprotein (nRNP) antibodies may be expressed in a
variety of autoimmune diseases without specificity (47). Zeng and Wu (48) analyzed AAbs in 150 patients with SLE
and indicated that 5.33% of patients were positive for a single
antibody, while the remaining 94.67% were positive for ≥2
antibodies at the same time. The patient in the present case report
had no obvious facial erythema, photosensitivity or recurrent oral
ulcers, but did exhibit morning stiffness, hair loss and
pericardial effusion, and laboratory results were positive for
anti-nRNP and anti-Sm antibodies accompanied by changes in blood
images. These symptoms were consistent with positive ANA as an
entry criterion, leucopenia (white blood cell count
<4.0x109/l), pericardial effusion and positivity for
anti-Sm antibody, which correspond to the diagnostic criteria for
SLE formulated by the ACR (44).
Therefore, the diagnosis of SLE was confirmed. According to the
international SLE disease activity index scoring standard (49), the patient of the present study was
identified as having a score of 14 points. Despite the definitive
diagnosis of SLE, the patient had mild symptoms, clinical stability
and no apparent organ damage. Considering the low activity and
severity of the disease, as well as the potential of risk
associated with treatment, SLE-associated treatment was not started
at this point. In the present case, it was not clear whether
hypothyroidism was a manifestation of SLE or a co-existing disease.
The majority of patients present with SLE first and develop thyroid
dysfunction at a later stage. Thyroid diseases may be the result of
AAbs produced as a consequence of SLE. In the present case,
hypothyroidism was the initial clinical manifestation and was
apparently not linked to SLE in the course of the disease, and no
similar manifestation was present in the patient's medical history.
A possible reason for the initial occurrence of hypothyroidism and
subsequent manifestation of SLE is that the two autoimmune diseases
have a similar pathogenesis. Another potential reason for the
unusual presentation in the present case is that the patient may
have had low-severity SLE, which did not cause any specific
clinical symptoms and was not detected. Hypothyroidism, caused by
the destruction of thyroid follicular cells by autoimmune
inflammation, may be the initial manifestation.
The association between pericardial effusion and
hypothyroidism was first reported in the early 20th century
(50), and has been reported
successively since then (51-53).
Pericardial effusion is a complication and indicator of the stage
of SLE (54). Severe hypothyroidism
and active SLE are known to lead to the development of pericardial
effusion. In the present case, pericardial effusion may have been
caused by hypothyroidism alone or in combination with SLE.
Hypothyroidism was the first clinical manifestation in the current
case and the patient had normal blood sedimentation, no history of
tuberculosis contact, normal tumor markers and multiple negative
examinations of pericardial effusion-shedding cells. Therefore,
tuberculous pericardial effusion and neoplastic pericardial
effusion were excluded according to the differential diagnosis
(55,56) combined with the patient's symptoms.
Due to technical limitations, anti-nuclear antibody testing of
pericardial effusion was not possible; however, this indicator may
have been helpful in determining the etiology.
The clinical symptoms of hypothyroidism myopathy,
muscle weakness and elevation of serum enzymes are not specific and
are similar to polymyositis, defined as hypothyroidism polymyositis
syndrome (57). The difference
between the two diseases is mainly based on muscle biopsies
indicating normal or slight non-specific changes in hypothyroidism
polymyositis syndrome. The major feature of polymyositis is
lymphocyte infiltration, with the characteristic immunopathology of
CD8+T/major histocompatibility complex-I injury
(58). Combined with the results of
muscle biopsy in the present case, myositis was excluded. Prolactin
was increased in the patient of the present study, although there
were no symptoms of lactation or menoxenia. However, pituitary MRI
revealed the presence of a pituitary microadenoma. At present, MRI
is the preferred imaging method for pituitary lesions. Pituitary
tumors and hypothyroidism-induced pituitary hyperplasia have
certain characteristic manifestations, but both may present on MRI
as pituitary enlargement, spherical or nodular. Primary
hypothyroidism may also lead to pituitary feedback neoplasia, which
is challenging to identify, and in the present case, it may not be
excluded that the increase of pituitary hormone secretion was due
to hypothyroidism or a pituitary tumor. Therefore, no specific
treatment was provided and the patient was instructed to present
for regular review to observe whether prolactin normalized under
the control of primary hypothyroidism (59). After 3 months of thyroid hormone
replacement therapy, the patient was followed up at the outpatient
clinic and pituitary MRI revealed that the size of pituitary
microadenoma decreased from 6.4 mm at the first presentation to 1.8
mm, and prolactin levels were significantly decreased from 1,280.90
to 794.9 mIU/l.
In conclusion, the prevalence of hypothyroidism in
SLE is high, whereas studies regarding hypothyroidism as the first
or the only symptom of SLE are rare. Although the clinical
manifestations of SLE are diverse, the current case lacked
characteristic manifestations of SLE and hypothyroidism was the
major manifestation. Therefore, attention should be paid to
screening for SLE while diagnosing hypothyroidism and the
importance of thyroid dysfunction should also be recognized in the
treatment of SLE. This may help identify diseases at an earlier
stage.
The limitation of the present study is its
retrospection; it was only possible to review the literature and
analyze previous studies to provide relevant information.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
RL and QW analyzed and interpreted the patient data
regarding the SLE and hypothyroidism diseases, and revised the
manuscript. BFX and XJZ contributed to the conception and design of
the study and performed the data analyses and wrote the manuscript.
ZWL and YYG performed the analysis with constructive discussions,
carried out the study and collected important background
information and reviewed the references. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Ethics committee of China-Japan Union Hospital of
Jilin University (approval no. 2020032602).
Patient consent for publication
The patient provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aringer M and Schneider M: Systemic lupus
erythematosus. Dtsch Med Wochenschr. 141:537–543. 2016.(In German).
PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ekinci RMK, Balcı S, Celik G, Dogruel D,
Altıntas DU and Yilmaz M: Symptomatic multifocal avascular necrosis
in an adolescent with neuropsychiatric systemic lupus
erythematosus. Reumatologia. 57:182–187. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bharath G, Kumar P, Makkar N, Singla P,
Soneja M, Biswas A and Wig N: Mortality in systemic lupus
erythematosus at a teaching hospital in India: A 5-year
retrospective study. J Family Med Prim Care. 8:2511–2515.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Blich M, Rozin A and Edoute Y: Systemic
lupus erythematosus and thyroid disease. Isr Med Assoc J.
6:218–220. 2004.PubMed/NCBI
|
|
5
|
Luo W, Mao P, Zhang L and Yang Z:
Association between systemic lupus erythematosus and thyroid
dysfunction: A meta-analysis. Lupus. 27:2120–2128. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen X: The significance of rivalta
experiment in the diagnosis of ascites. Chinese Community Doctors.
15(253)2013.(In Chinese).
|
|
7
|
Pyne D and Isenberg DA: Autoimmune thyroid
disease in systemic lupus erythematosus. Ann Rheum Dis. 61:70–72.
2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Antonelli A, Fallahi P, Mosca M, Ferrari
SM, Ruffilli I, Corti A, Panicucci E, Neri R and Bombardieri S:
Prevalence of thyroid dysfunctions in systemic lupus erythematosus.
Metabolism. 59:896–900. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chan AT, Al-Saffar Z and Bucknall RC:
Thyroid disease in systemic lupus erythematosus and rheumatoid
arthritis. Rheumatology (Oxford). 40:353–354. 2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Park DJ, Cho CS, Lee SH, Park SH and Kim
HY: Thyroid disorders in Korean patients with systemic lupus
erythematosus. Scand J Rheumatol. 24:13–17. 1995.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Boey ML, Fong PH, Lee JS, Ng WY and Thai
AC: Autoimmune thyroid disorders in SLE in Singapore. Lupus.
2:51–54. 1993.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Miller FW, Moore GF, Weintraub BD and
Steinberg AD: Prevalence of thyroid disease and abnormal thyroid
function test results in patients with systemic lupus
erythematosus. Arthritis Rheum. 30:1124–1131. 1987.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vianna JL, Haga HJ, Asherson RA, Swana G
and Hughes GR: A prospective evaluation of antithyroid antibody
prevalence in 100 patients with systemic lupus erythematosus. J
Rheumatol. 18:1193–1195. 1991.PubMed/NCBI
|
|
14
|
Tsai RT, Chang TC, Wang CR, Chuang CY and
Chen CY: Thyroid disorders in Chinese patients with systemic lupus
erythematosus. Rheumatol Int. 13:9–13. 1993.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mader R, Mishail S, Adawi M, Lavi I and
Luboshitzky R: Thyroid dysfunction in patients with systemic lupus
erythematosus (SLE): Relation to disease activity. Clin Rheumatol.
26:1891–1894. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Appenzeller S, Pallone AT, Natalin RA and
Costallat LT: Prevalence of thyroid dysfunction in systemic lupus
erythematosus. J Clin Rheumato. 15:117–119. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kumar K, Kole AK, Karmakar PS and Ghosh A:
The spectrum of thyroid disorders in systemic lupus erythematosus.
Rheumatol Int. 32:73–78. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Stagnaro-Green A, Akhter E, Yim C, Davies
TF, Magder L and Petri M: Thyroid disease in pregnant women with
systemic lupus erythematosus: Increased preterm delivery. Lupus.
20:690–699. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gao H, Li C, Mu R, Guo YQ, Liu T, Chen S,
Su Y and Li ZG: Subclinical hypothyroidism and its association with
lupus nephritis: A case control study in a large cohort of Chinese
systemic lupus erythematosus patients. Lupus. 20:1035–1041.
2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ong SG and Choy CH: Autoimmune thyroid
disease in a cohort of Malaysian SLE patients: Frequency, clinical
and immunological associations. Lupus. 25:67–74. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Watad A, Mahroum N, Whitby A, Gertel S,
Comaneshter D, Cohen AD and Amital H: Hypothyroidism among SLE
patients: Case-control study. Autoimmun Rev. 15:484–486.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Franco JS, Amaya-Amaya J, Molano-González
N, Caro-Moreno J, Rodríguez-Jiménez M, Acosta-Ampudia Y, Mantilla
RD, Rojas-Villarraga A and Anaya JM: Autoimmune thyroid disease in
Colombian patients with systemic lupus erythematosus. Clin
Endocrinol (Oxf). 83:943–950. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Song Q, Mao YJ, Li J and Guo XH:
Correlation between systemic lupus erythematosus and autoimmune
thyroid diseases. Chin J General Practice. 13:742–744. 2014.(In
Chinese).
|
|
24
|
Domingues SL, Gonçalves FT, Jorge MLMP,
Limongi JE, Ranza R and Jorge PT: High prevalence of hypothyroidism
in systemic lupus erythematosus patients without an increase in
circulating anti-thyroid antibodies. Endocr Pract. 23:1304–1310.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rahman A and Isenberg DA: Systemic lupus
erythematosus. N Engl J Med. 358:929–939. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pan Q, Chen X, Liao S, Chen X, Zhao C, Xu
YZ and Liu HF: Updated advances of linking psychosocial factors and
sex hormones with systemic lupus erythematosus susceptibility and
development. Peer J. 7(e7179)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hijmans W, Doniach D, Roitt IM and
Holborow EJ: Serological overlap between lupus erythematosus,
rheumatoid arthritis, and thyroid auto-immune disease. Br Med J.
2:909–914. 1961.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rivas-Larrauri F and Yamazaki-Nakashimada
MA: Systemic lupus erythematosus: Is it one disease? Reumatol Clin.
12:274–281. 2016.(In Spanish). PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu YC, Lin WY, Tsai MC and Fu LS:
Systemic lupus erythematosus and thyroid disease-experience in a
single medical center in Taiwan. J Microbiol Immunol Infect.
52:480–486. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lisnevskaia L, Murphy G and Isenberg D:
Systemic lupus erythematosus. Lancet. 384:1878–1888.
2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Posselt RT, Coelho VN and Skare TL:
Hashimoto thyroiditis, anti-thyroid antibodies and systemic lupus
erythematosus. Int J Rheum Dis. 21:186–193. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Green LM, LaBue M, Lazarus JP and Colburn
KK: Characterization of autoimmune thyroiditis in MRL-lpr/lpr mice.
Lupus. 4:187–196. 1995.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Apostolidis SA, Lieberman LA, Kis-Toth K,
Crispín JC and Tsokos GC: The dysregulation of cytokine networks in
systemic lupus erythematosus. J Interferon Cytokine Res.
31:769–779. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zlotnik A and Yoshie O: Chemokines: A new
classification system and their role in immunity. Immunity.
12:121–127. 2000.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ahmadpoor P, Dalili N and Rostami M: An
update on pathogenesis of systemic lupus erythematosus. Iran J
Kidney Dis. 8:171–184. 2014.PubMed/NCBI
|
|
36
|
Tsokos GC: Systemic lupus erythematosus. N
Engl J Med. 365:2110–2121. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kawashima A, Tanigawa K, Akama T,
Yoshihara A, Ishii N and Suzuki K: Innate immune activation and
thyroid autoimmunity. J Clin Endocrinol Metab. 96:3661–3671.
2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Scharer CD, Blalock EL, Mi T, Barwick BG,
Jenks SA, Deguchi T, Cashman KS, Neary BE, Patterson DG, Hicks SL,
et al: Epigenetic programming underpins B cell dysfunction in human
SLE. Nat Immunol. 20:1071–1082. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ferrari SM, Elia G, Virili C, Centanni M,
Antonelli A and Fallahi P: Systemic lupus erythematosus and thyroid
autoimmunity. Front Endocrinol (Lausanne). 8(138)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wu H, Cantor RM, Graham DS, Lingren CM,
Farwell L, Jager PL, Bottini N, Grossman JM, Wallace DJ, Hahn BH,
et al: Association analysis of the R620W polymorphism of protein
tyrosine phosphatase PTPN22 in systemic lupus erythematosus
families: Increased T allele frequency in systemic lupus
erythematosus patients with autoimmune thyroid disease. Arthritis
Rheum. 52:2396–2402. 2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Antonelli A, Ferrari SM, Corrado A, Di
Domenicantonio A and Fallahi P: Autoimmune thyroid disorders.
Autoimmun Rev. 14:174–180. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Biondi B and Cooper DS: Thyroid hormone
therapy for hypothyroidism. Endocrine. 66:18–26. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Arbuckle MR, McClain MT, Rubertone MV,
Scofield RH, Dennis GJ, James JA and Harley JB: Development of
autoantibodies before the clinical onset of systemic lupus
erythematosus. N Engl J Med. 349:1526–1533. 2003.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Aringer M, Costenbader K, Daikh D, Brinks
R, Mosca M, Ramsey-Goldman R, Smolen JS, Wofsy D, Boumpas DT, Kamen
DL, et al: 2019 European league against Rheumatism/American College
of rheumatology classification criteria for systemic lupus
erythematosus. Arthritis Rheumatol. 71:1400–1412. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Petri M, Orbai AM, Alarcón GS, Gordon C,
Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O,
et al: Derivation and validation of the systemic lupus
international collaborating clinics classification criteria for
systemic lupus erythematosus. Arthritis Rheum. 64:2677–2686.
2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Budde P, Zucht HD, Vordenbäumen S, Goehler
H, Fischer-Betz R, Gamer M, Marquart K, Rengers P, Richter J,
Lueking A, et al: Multiparametric detection of autoantibodies in
systemic lupus erythematosus. Lupus. 25:812–822. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Tan EM: Autoantibodies, autoimmune
disease, and the birth of immune diagnostics. J Clin Invest.
122:3835–3836. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
48
|
Zeng YK and Wu J: Diagnostic value of
combined detection of anti-nuclear antibody, anti-nuclear antibody
spectrum and anti-double-stranded DNA antibody in systemic lupus
erythematosus. Med Clin Res. 2081–2083. 2014.
|
|
49
|
Zai Y, Zhong NS and Xie Y: Internal
medicine [M]. Version 7. Beijing: People's medical publishing house
86, 2008.
|
|
50
|
Gordon AH: Some clinical aspects of
hypothyroidism. Can Med Assoc J. 20:7–10. 1929.PubMed/NCBI
|
|
51
|
Wang JL, Hsieh MJ, Lee CH, Chen CC, Hsieh
IC, Lin JD, Lin FC and Hung KC: Hypothyroid cardiac tamponade:
Clinical features, electrocardiography, pericardial fluid and
management. Am J Med Sci. 340:276–281. 2010.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Martinez-Soto T, Deal C, Stephure D,
Trussell R, Boutin C, Djemli A and Ho J: Pericardial effusion in
severe hypothyroidism in children. J Pediatr Endocrinol Metab.
23:1165–1168. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Agarwal A, Chowdhury N, Mathur A, Sharma S
and Agarwal A: Pericardial effusion with cardiac tamponade as a
form of presentation of primary hypothyroidism. J Assoc Physicians
India. 64:98–100. 2016.PubMed/NCBI
|
|
54
|
Mohamed AAA, Hammam N, El Zohri MH and
Gheita TA: Cardiac manifestations in systemic lupus erythematosus:
Clinical correlates of subclinical echocardiographic features.
Biomed Res Int. 2019(e2437105)2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Abdallah R and Atar S: Etiology and
characteristics of large symptomatic pericardial effusion in a
community hospital in the contemporary era. QJM. 107:363–368.
2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ozturk E, Tanidir IC, Saygi M, Ergul Y,
Guzeltas A and Odemis E: Evaluation of non-surgical causes of
cardiac tamponade in children at a cardiac surgery center. Pediatr
Int. 56:13–18. 2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Madariaga MG: Polymyositis-like syndrome
in hypothyroidism: Review of cases reported over the past
twenty-five years. Thyroid. 12:331–336. 2002.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Scott KR, Simmons Z and Boyer PJ:
Hypothyroid myopathy with a strikingly elevated serum creatine
kinase level. Muscle Nerve. 26:141–144. 2002.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Nachawi N and Bodnar TW: Pituitary
‘pseudotumor’: An under-recognised complication of undertreated
primary hypothyroidism. BMJ Case Rep.
2018(bcr-2018-225472)2018.PubMed/NCBI View Article : Google Scholar
|