Introduction
Preeclampsia (PE) is a severe idiopathic obstetric
complication that occurs during pregnancy (1). PE affects ~3% of pregnant women
worldwide (2). PE has many
contributing factors and a multi-pathway pathogenesis (3), however the specific pathogenesis of
this condition is not fully understood. The main factors causing PE
include superficial implantation of the placenta, insufficient
placental perfusion, placental hypoxia and ischemia, oxidative
stress and immune imbalance (4). A
functional placenta is important for maintaining pregnancy and
fetal development (5). At the start
of a pregnancy, extravillous trophoblasts invade the decidua and
inner myometrium of the mother, a process which is required for the
progression of the pregnancy (6,7).
Transforming growth factor-β1 (TGF-β1) is a
well-known pleiotropic cytokine. TGF-β1 can induce a number of
biological functions, including embryogenesis, tissue morphogenesis
of the embryo, cell proliferation, migration, differentiation and
immune responses (8-11).
The role of TGF-β1 in different biological functions is determined
by the context in the body and interactions with other signaling
pathways (12). It has been reported
that TGF-β1 serves oncogenic roles in malignant tumors, such as in
gastrointestinal, breast and liver cancer (13,14).
TGF-β1 is also involved in the invasion of trophoblasts (15,16), as
well as placenta progenitor cell differentiation into trophoblasts
(17). The canonical pathway, in
which TGF-β1 regulates cell invasion and migration, is mediated by
SMAD (18,19). SMADs are intracellular proteins that
transduce extracellular signals from TGF-β ligands to the nucleus,
where they activate downstream gene transcription (20). SMAD2 was demonstrated to serve
critical roles in the migration and invasion of cancer cells
(21-23).
MicroRNAs (miRNAs/miRs) are small, single-stranded
non-coding RNAs that serve a variety of roles in a series of
disorders, including in malignant tumors and PE, via
post-translational modifications (24,25). A
previous study measured cell-free miRNAs in plasma from patients
with prostate cancer and healthy donors, and demonstrated that
miR-141 expression levels were high in patients with advanced stage
cancer and associated with the Gleason score value (26). Peng et al (27) reported that miR-429 acted as a tumor
suppressor gene by inhibiting astrocytoma proliferation and
invasion. Additionally, miRNAs have been indicated to serve
critical roles in the pathogenesis of PE (28). In a previous study, miR-29b
suppressed trophoblast cell invasion and promoted apoptosis in
patients with PE (29). miR-30a-3p
was overexpressed in the placenta of women with PE, and was
revealed to induce HTR-8/SVneo cell apoptosis while inhibiting the
invasive capacity of JEG-3 cells (30). The overexpression of microRNA-376c
promotes the proliferation, migration and invasion of trophoblasts,
and the growth of placental explants by inhibiting TGF-β and Nodal
signaling (31). miR-27a has also
been reported to serve oncogenic roles in a variety of malignant
tumors (32). In lung cancer,
miR-27a is significantly overexpressed and is indicated to
stimulate cancer cell proliferation and invasion by targeting SMAD2
and SMAD4(21). In addition, the
miR-27a/miR-27a complex was demonstrated to contribute to the
metastasis of osteosarcoma (32). A
previous microarray study indicated that multiple miRNAs, including
miR-27a/b, regulate the onset of PE (33). Moreover, miR-27a has been shown to be
significantly upregulated in the plasma and placenta of patients
with PE (34). However, to the best
of our knowledge, the mechanisms via which miR-27a regulates PE
have not yet been elucidated.
The present study investigated the expression levels
of miR-27a in plasma and placentas from patients with PE. To
identify the molecular mechanisms underlying miR-27a regulation of
trophoblast function, the present study investigated cell functions
after overexpressing or inhibiting miR-27a. Additionally, the
present study predicted the potential target of miR-27a using miRNA
target prediction databases. The present results may facilitate the
development of novel diagnostic and latent targeted therapies for
PE.
Materials and methods
Sample collection and cell
culture
A total of 35 pregnant women with severe PE and 20
healthy pregnant women who underwent caesarean section at the
Department of Obstetrics and Gynecology of Renmin Hospital of Wuhan
University from May 2015 to June 2016 were recruited for the
current study. The diagnostic criteria for PE followed that of the
American College of Obstetricians and Gynecologists, with either
severe hypertension (≥160 mmHg and/or 110 mmHg) plus mild
proteinuria or mild hypertension plus severe proteinuria (>2
g/24 h or >2+) (29). Other
recruitment criteria for patients in the two groups included
singleton pregnancies and no other complications, including
premature membrane rupture, cardiac or renal disease, hypertension
history and maternal infection. The clinical features of all
patients are presented in Table I.
Placenta tissues (five sites) were collected from different
placental lobules and stored at -80˚C. Peripheral blood samples (5
ml) from patients in the two groups were collected at delivery. The
current study was approved by The Ethics Committee of Renmin
Hospital of Wuhan University, and written informed consent was
obtained from all patients.
 | Table IClinicopathological characteristics
of PE and healthy pregnant women. |
Table I
Clinicopathological characteristics
of PE and healthy pregnant women.
| Clinicopathological
features | Healthy control
(n=20) | Patients with PE
(n=35) | P-value |
|---|
| Maternal age,
years | 27.3±1.9 | 28.2±2.1 | >0.05 |
| Gestational age,
weeks | 40±2.1 | 36.6±3.4 | <0.05 |
| Placental weight,
g | 462±56.3 | 425.8±50.2 | <0.05 |
| Systolic blood
pressure, mmHg | 104.5±12.4 | 157.5±15.3 | <0.05 |
| Diastolic blood
pressure, mmHg | 79.3±7.8 | 121.5±6.4 | <0.05 |
| Infant birth
weight, g | 3563.4±234.2 | 2783.4±312.2 | <0.05 |
| Sex of infants | | | >0.05 |
|
Male | 8 | 16 | |
|
Female | 12 | 19 | |
| Proteinuria, g/24
h | None | 3.51±0.98 | <0.05 |
HTR-8/SVneo cells, a human trophoblast cell line,
were purchased from the American Type Culture Collection and
cultured in RPMI 1640 medium (Sigma-Aldrich; Merck KGaA)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin (New Cell and Molecular Biotech Co., Ltd.) and 1%
streptomycin (New Cell and Molecular Biotech Co., Ltd) at 37˚C with
5% CO2.
Cell transfection
miR-27a inhibitor and mimic, and the corresponding
negative controls (Shanghai GenePharma Co., Ltd.) were transfected
into HTR-8/SVneo cells at a concentration of 2.5 µg/well using
Lipofectamine 3000® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols. The
sequences were as follows (5'-3'): miR-27a mimic,
UUCACAGUGGCUAAGUUCCGC; mimic NC, UUGUACUACACAAAAGUACUG; inhibitor,
GCGGAACUUAGCCACUGUGAA; inhibitor NC, CAGUACUUUUGUGUAGUACAA. In
addition, SMAD2 overexpression plasmid and negative control
(Shanghai GenePharma Co., Ltd.) were transfected into HTR-8/SVneo
cells with Lipofectamine® 3000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). HTR-8/SVneo cells were seeded into
six-well plates at a density of 60-70%. After adhesion, miR-27a
inhibitor (2.5 µg/well) and mimic (2.5 µg/well), or SMAD2
overexpression plasmid (2.5 µg/well) and the corresponding negative
controls (2.5 µg/well) were transfected into cells using
Lipofectamine 3000® reagent. A period of 6 h later, the
culture medium with the transfection reagent was changed to RPMI
1640 medium (Sigma-Aldrich; Merck KGaA) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin (NCM
Biotech, China) and 1% streptomycin (NCM Biotech, China). After a
period of 48 h, the transfection efficiency was detected and
confirmed. Cells were harvested for further experiments after 48 h
of transfection.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was isolated from placentas and cell lines
using a Takara RNA extraction kit (Takara Bio, Inc.) and quantified
using a spectrophotometer (BioTek, US). cDNA was synthesized from
miRNA using a SuperScript II kit (Invitrogen; Thermo Fisher
Scientific, Inc), and cDNA was reverse transcribed from mRNA using
a PrimeScript RT reagent kit with gDNA Eraser (Takara Bio, Inc.).
The RT temperature protocol is as follows: 37˚C for 15 min, 85˚C
for 5 sec and 4˚C for 10 min. PCR was performed using a SYBR Premix
Ex Taq II kit (Takara Bio, Inc.). The following thermocycling
conditions were used for the qPCR: Initial denaturation at 95˚C for
30 sec; 40 cycles of denaturation at 95˚C for 5 sec, primer
annealing and extension at 60˚C for 34 sec; and melting curve
analysis denaturation at 95˚C for 15 sec, primer annealing at 60˚C
for 1 min and denaturation at 95˚C for 15 sec. U6 and GAPDH were
used as internal controls for the quantification of miRNA and mRNA,
respectively. The following primers were used: miR-27a forward,
5'-ACAGGCTAGCGCCGCCTAAC-3' and reverse, 5'-CCTTAAGGCCCAAGATTACG-3';
SMAD2 forward, 5'-TGAGTGTGGGATTTGACCAG-3' and reverse,
5'-TGTGTTTGGAGTGGGTTTCA-3'; GAPDH forward, 5'-TCCACTGGCGTCTTCACC-3'
and reverse, 5'-GGCAGAGATGATGACCCTTTT-3'; and U6 forward,
5'-TGCGGGTGCTCGCTTCGCAGC-3' and reverse, 5'-CCAGTGCAGGGTCCGAGGT-3'.
Each assay was performed in duplicate with triplicate samples, and
expression levels were calculated using the 2-ΔΔCq
method (35).
Transwell assay
Transwell assay chambers were purchased from
Corning, Inc. Transfected HTR-8/SVneo cells were seeded into the
upper chambers at a density of 4x104 cells per well with
(for invasion assays) or without Matrigel (for migration assays)
and without FBS. RPMI 1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) with 20% FBS was added to the lower chambers. Cells were then
incubated at 37˚C with 5% CO2. After 48 h, cells in the
upper chambers were wiped away using cotton swabs, and cells in the
lower chambers were fixed in 4% paraformaldehyde at room
temperature for 15 min and stained using a 0.1% crystal violet
solution at room temperature for 10 min. The migrated or invaded
cells in five random fields were counted and photographed under a
light microscope (magnification, x200).
Western blot analysis
Total protein was extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology). Cells were
incubated with RIPA lysis buffer for 20 min on ice and centrifuged
at 4˚C for 16,099 x g for 30 min. The supernatants were removed and
the protein concentrations were determined using a BCA kit
(Beyotime Institute of Biotechnology). Then, 40 µg of denatured
protein was separated on 10% SDS-PAGE gels and transferred onto
PVDF membranes. After blocking in 5% non-fat milk at room
temperature for 1 h, the membranes were incubated with primary
antibodies at 4˚C overnight. The primary antibodies used were
monoclonal rabbit anti-Smad2 (cat. no. ab33875), anti-Bax (cat. no.
ab32503) and anti-Bcl-xl (cat. no. ab32370) (all 1:1,000; Abcam). A
GAPDH antibody (1:1,000; cat. no. ab181602; Abcam) was used as a
control for normalizing protein expression. On the second day, the
membranes were incubated with secondary antibody (goat anti-rabbit
immunoglobulin G H&L (HRP) (1:10,000; cat. no. ab97051; Abcam)
at room temperature for 1 h and washed three times with 1x
PBS-Tween-20 for 10 min. Bands were detected using a
chemiluminescence kit (Sigma-Aldrich; Merck KGaA). The band
intensities were analyzed using ImageJ 1.51j8 (National Institutes
of Health).
Cell Counting Kit-8 (CCK-8) assay
CCK-8 was performed according to the manufacturer's
protocol. Transfected HTR-8/SVneo cells were seeded in 96-well
plates at a density of 5x103 cells/well. After 48 h, 100
µl of RPMI 1640 medium and 10 µl of CCK-8 reagent (Dojindo
Molecular Technologies, Inc.) were added to each well and incubated
at 37˚C for 40 min. The relative proliferation ability was measured
at a wavelength of 450 nm.
Cell apoptosis assay
The apoptotic rate of cells was detected using an
Annexin V-FITC Apoptosis Detection kit (Becton, Dickinson and
Company) following the manufacturer's protocol. Transfected
HTR-8/SVneo cells were trypsinized and washed with cold PBS three
times. The resuspended cells were stained with 5 µl Annexin V-FITC
and 5 µl PI at room temperature for 5 min in the dark. The
apoptotic rate was analyzed using a flow cytometer (FlowJo 7.6;
Becton, Dickinson and Company).
Luciferase reporter assay
The wild-type 3'untranslated region (UTR) of SMAD2
and a mutated (MUT) 3'UTR containing the predicted binding sites of
miR-27a were cloned into the pGL3 vector (Promega Corporation). The
binding sites were predicted using TargetScan (http://www.targetscan.org/vert_72/) and miRanda
(http://www.microrna.org/). The reporters
containing mutant (MUT) or wild-type (WT) SMAD2 were transfected
into trophoblast cell lines along with a miR-27a mimic, inhibitor
or negative control with Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.). After 48 h,
luciferase activity was detected using a
Dual-Luciferase® Reporter Assay System kit (Promega
Corporation). Renilla luciferase activity was used as a
normalization control.
Statistical analysis
SPSS 16.0 software (SPSS, Inc.) was used to perform
statistical analyses. Data are presented as the mean ± standard
deviation, and experiments were repeated ≥3 times. Independent
Student's t-test was performed to compare the differences of
measurement data between two groups and χ2-test was
performed to compare the differences of categorical data. In
addition, a one-way ANOVA followed by Tukey test was performed to
compare the differences among multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-27a is significantly upregulated
in PE placentas and serum
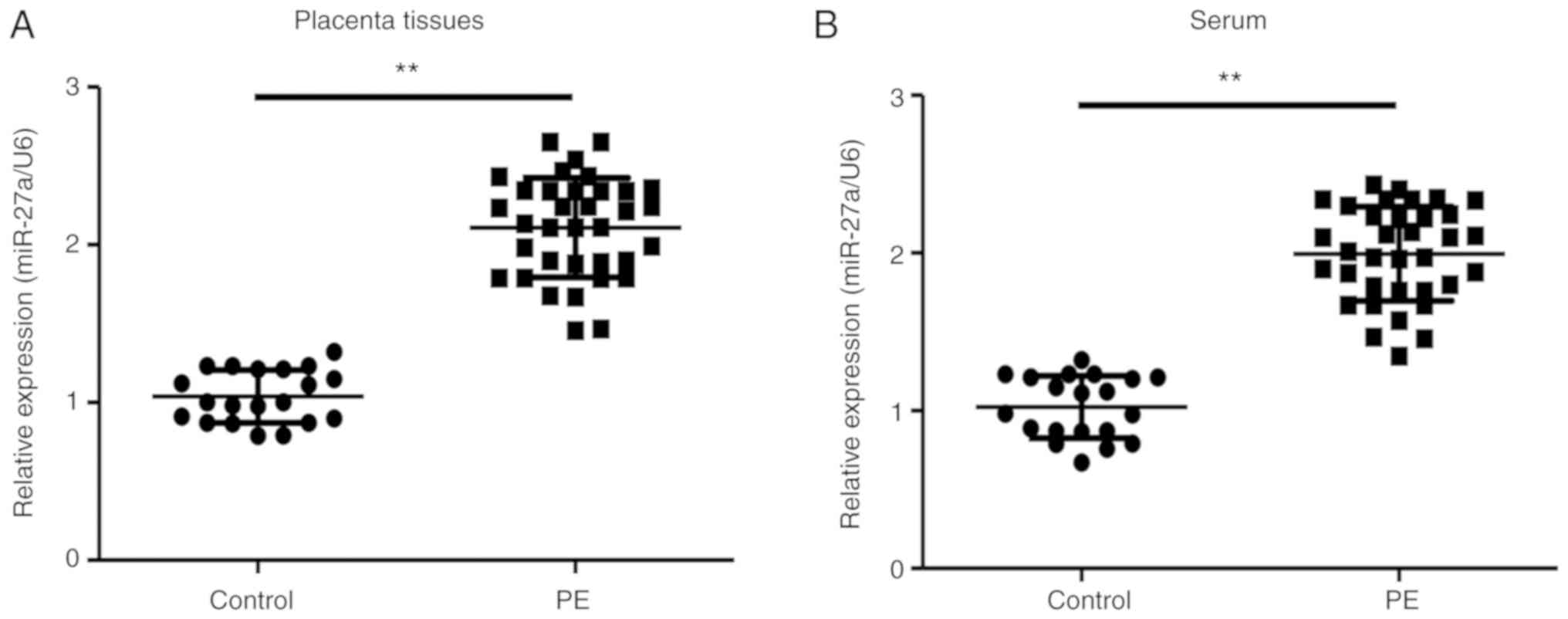
To investigate the potential roles of miR-27a in PE,
PE and healthy control clinical samples were collected and the
expression levels of miR-27a was detected using RT-qPCR. The
present results indicated that miR-27a expression levels were
significantly increased in the placenta and serum from pregnant
women with PE compared with healthy pregnant women (Fig. 1). Therefore, miR-27a may serve a
critical role in PE pathogenesis.
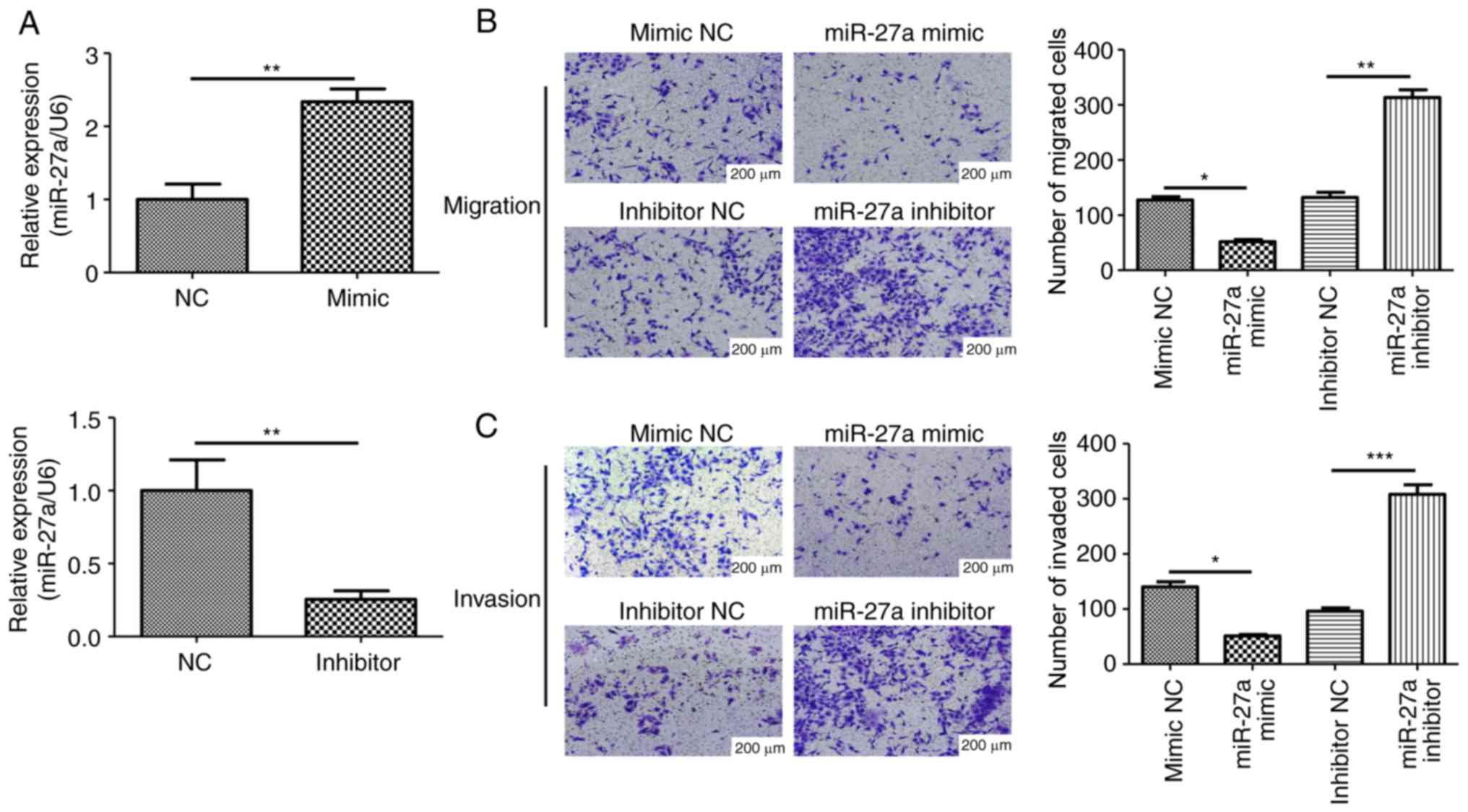
Overexpression of miR-27a suppresses
the migration and invasion of human trophoblasts
To further investigate the role of miR-27a in PE
development, miR-27a mimic, inhibitor or negative controls were
transfected into trophoblasts. The transfection efficiency was
verified using RT-qPCR, with the mimic and inhibitor increasing and
decreasing the expression level of miR-27a, respectively, compared
with the corresponding controls (Fig.
2A). The present study also examined the migratory and invasive
abilities of HTR-8/SVneo cells after miR-27a mimic or inhibitor
transfection. The migratory and invasive abilities were
significantly inhibited after miR-27a overexpression, while miR-27a
inhibition improved the migratory and invasive abilities of
HTR-8/SVneo cells (Fig. 2B and
C). Collectively, the present
results suggested that the ectopic expression of miR-27a impaired
the migratory and invasive abilities of human trophoblasts, and may
contribute to the pathogenesis of PE.
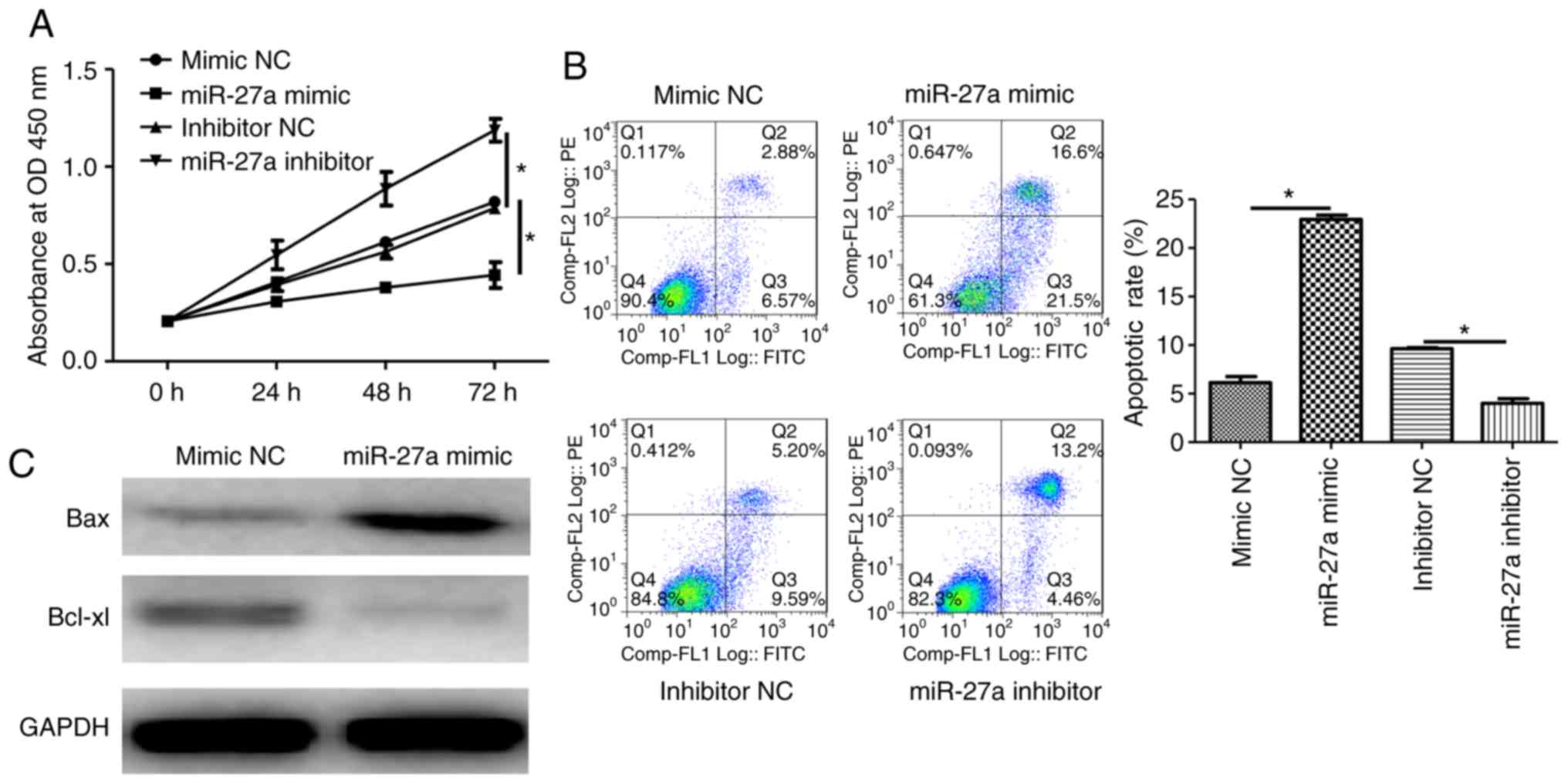
Overexpression of miR-27a impairs cell
proliferation and promotes apoptosis in human trophoblasts
To investigate the effects of miR-27a expression
level on the proliferation of HTR-8/SVneo cells, CCK-8 and cell
apoptosis assays were performed after miR-27a overexpression or
inhibition. Additionally, apoptotic-related proteins were analyzed
using western blot analysis. The results demonstrated that the
overexpression of miR-27a impaired proliferation and promoted
apoptosis in human trophoblasts (Fig.
3A and B). The pro-apoptotic
protein Bax was upregulated, while the anti-apoptotic protein
Bcl-xl was downregulated following miR-27a mimic transfection
(Fig. 3C). Therefore, miR-27a
deficiency may stimulate cell proliferation and suppress apoptosis
in trophoblasts in PE.
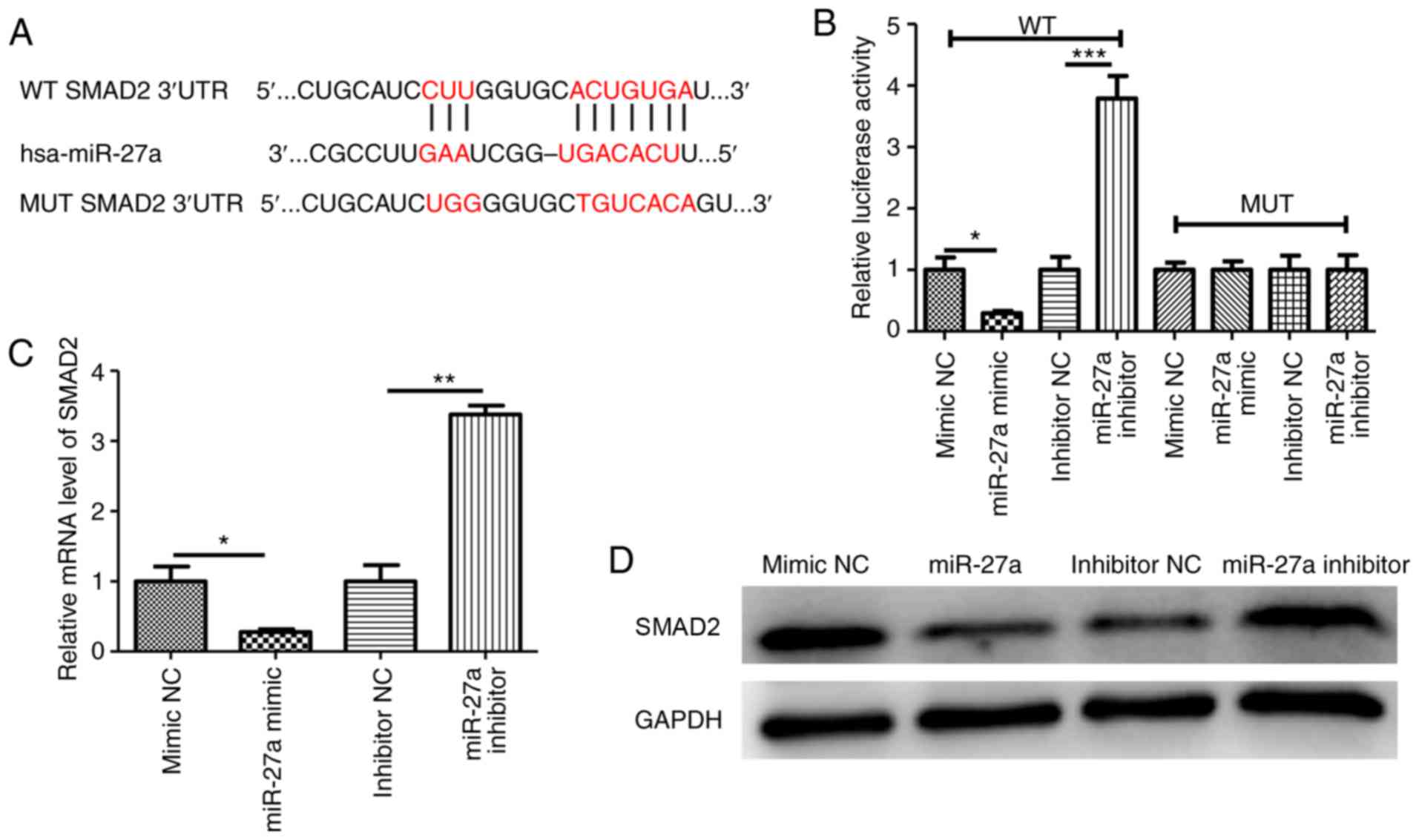
miR-27a targets SMAD2
To further elucidate the mechanisms by which miR-27a
overexpression regulates the migration and invasion of
trophoblasts, a number of databases (miRanda and TargetScan) were
used to predict the possible targets of miR-27a. The bioinformatic
prediction results indicated that SMAD2 was a target of miR-27a,
with a binding site in the 3'UTR (Fig.
4A). To investigate whether miR-27a regulated the invasion of
HTR-8/SVneo cells by targeting SMAD2, WT and MUT luciferase
reporters containing the 3'UTR of SMAD2 were constructed. After
co-transfection with miR-27a mimic or inhibitor, the luciferase
activity was measured. The results suggested that in the WT group,
miR-27a overexpression decreased the luciferase activity of cells
compared with the miR-27a negative control, while miR-27a
downregulation increased luciferase activity (Fig. 4B). However, no changes were
identified in the MUT 3'UTR groups compared with their
corresponding controls (Fig. 4B).
Additionally, the mRNA and protein levels of SMAD2 were
significantly decreased in the miR-27a-overexpressing cells, but
increased in the miR-27a-deficient cells (Fig. 4C and D).
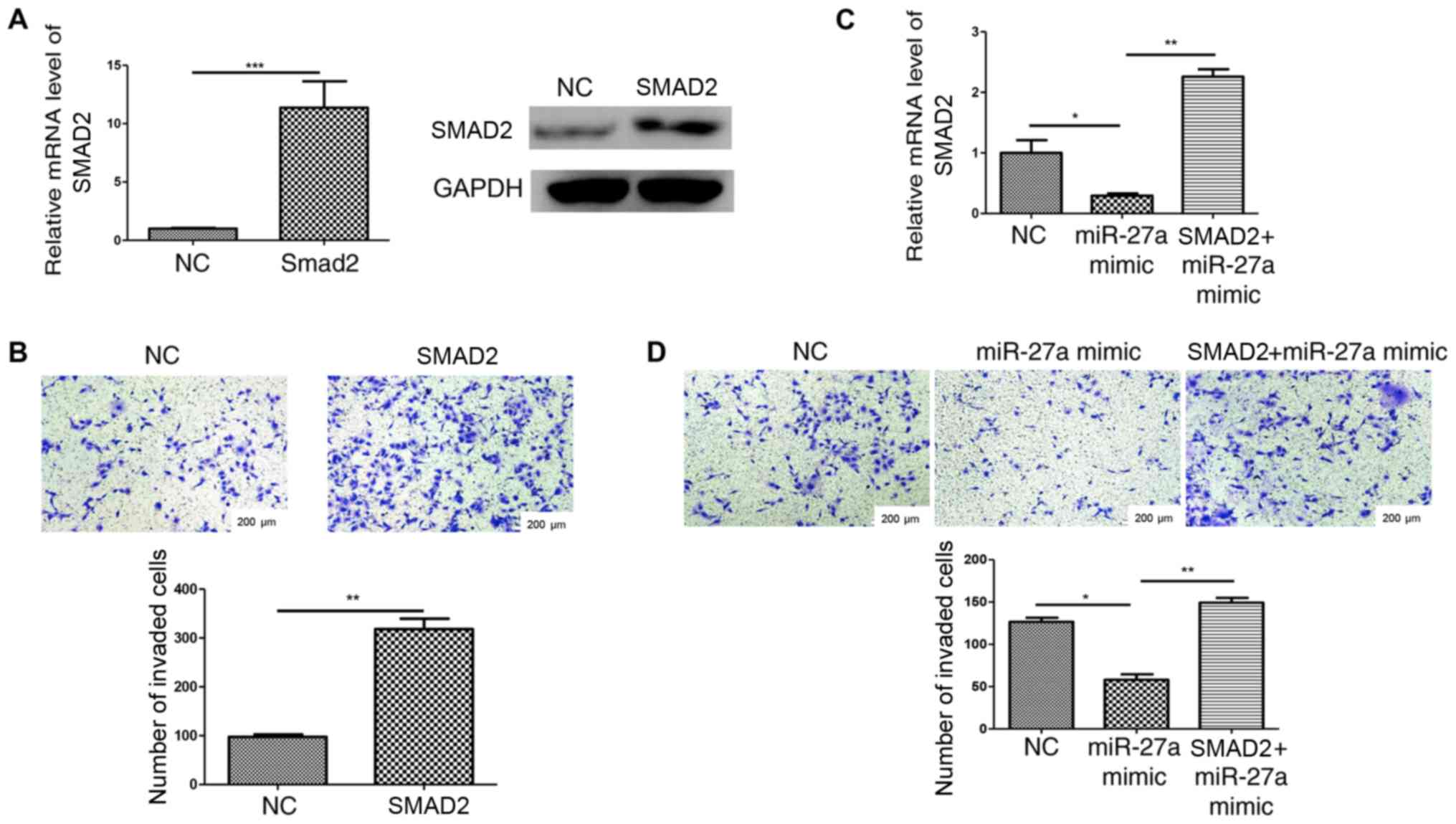
Overexpression of SMAD2 stimulates the
invasion of HTR-8/SVneo cells and reverses the miR-27a
upregulation-mediated inhibition of cell invasion
Cell invasion was detected following transfection
with a SMAD2 overexpression plasmid or negative control (Fig. 5A). The results revealed that
HTR-8/SVneo cell invasion was increased by SMAD2 overexpression
compared with the negative control group (Fig. 5B). To further demonstrate that
miR-27a suppressed HTR-8/SVneo cell invasion by targeting SMAD2,
the SMAD2 overexpression plasmid was transfected into
miR-27a-overexpressing cells and confirmed using RT-qPCR (Fig. 5C). Transwell assay results indicated
that cell invasion inhibition that was caused by miR-27a
overexpression was reversed by SMAD2 overexpression (Fig. 5D). Collectively, the present results
suggested that miR-27a may regulate the invasion of human
trophoblasts, at least partially by targeting SMAD2.
Discussion
The present results suggested that miR-27a
expression level was significantly higher in placenta and blood
samples from pregnant women with PE compared with healthy pregnant
women. The overexpression of miR-27a impaired the proliferative
ability and induced apoptosis of human trophoblasts. In addition,
the migratory and invasive capacities of trophoblasts were
significantly reduced after miR-27a overexpression. To investigate
the underlying mechanisms governing this, bioinformatics analysis
was performed to identify potential targets of miR-27a, and SMAD2
was predicted as a direct target of miR-27a. Luciferase reporter
assays, RT-qPCR, western blot analysis and rescue experiments were
used to demonstrate that miR-27a regulated HTR-8/SVneo invasion, at
least in part, by targeting SMAD2. Therefore, miR-27a may be a
promising therapeutic target for PE.
miRNAs have been shown to serve critical roles in a
variety of biological and pathological pathways (24). It has been reported that abnormal
expression of miRNAs is involved in pregnancy-associated disorders,
including PE (36,37). miR-136 is highly expressed in
decidua-derived mesenchymal stem cells from patients with PE and
impairs capillary formation at the maternal-fetal interface by
suppressing vascular endothelial growth factor (38). Another previous study indicated that
increased miR-34a expression levels promoted trophoblast apoptosis
via targeting BCL-2 in PE (39).
miR-27a has been studied in number of disease types (36). It has been previously demonstrated
that miR-27a and miR-27b regulate the expression of PTEN-induced
kinase 1, which is critical for the selective autophagic clearance
of damaged mitochondria in Parkinson's disease (40). In maternal diabetes, oxidative stress
upregulates miR-27a, which in turn suppresses nuclear factor
erythroid 2-related factor 2, and this feedback results in diabetic
embryopathy (41). In lung cancer,
miR-27a functions as an oncogene contributing to cancer cell
proliferation and invasion by targeting SMAD2 and SMAD4(21). Similar studies have indicated that
miR-27a is involved in obesity, gastric cancer chemoresistance and
osteosarcoma (32,42,43).
However, the roles of miR-27a in PE pathogenesis have not yet been
elucidated. In the present study, miR-27a was highly expressed in
PE placenta and serum, and contributed to the migration and
invasion of trophoblasts, suggesting that miR-27a, at least in
part, is involved in PE progression.
SMAD2 belongs to the SMAD family (44). SMAD proteins are signal transducers
and transcriptional modulators that mediate multiple signaling
pathways (44). Similar to other
SMADs, SMAD2 serves a role in the transmission of extracellular
signals from TGF-β ligands into the cell nucleus and mediates cell
proliferation, apoptosis and differentiation (45). The TGF-β-SMAD2/3 signaling pathway is
involved in epithelial-mesenchymal transition in several malignant
tumors, including hepatocellular carcinoma (46), breast cancer (47) and colorectal cancer (48). In addition, SMAD2 also contributes to
PE initiation (49). TGF-β1
suppresses trophoblast invasion by increasing the expression level
of connective tissue growth factor mediated by SMAD2/3(49). Given the significance of miR-27a and
SMAD2 in cell invasion, the present study investigated the
relationship between them and identified that the anti-invasion
function of miR-27a in human trophoblasts in PE partially targets
SMAD2.
In conclusion, the present results indicated that
miR-27a promoted apoptosis and inhibited proliferation, migration
and invasion in human trophoblasts. However, the present study has
some limitations, such as the association between the miR-27a
expression level and blood pressure or placenta weight was not
investigated. Additionally, primary trophoblasts and other human
trophoblast cell lines should be included in future studies.
Moreover, sample size should be increased in future studies to
obtain further evidence. However, the present results indicated
that miR-27a may be a promising novel therapeutic target for
PE.
Acknowledgements
Not applicable.
Funding
This study was supported by Medical and health
research projects of Yichang (grant no. A19-301-28).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WFZ performed experiments and drafted the
manuscript. AHC and HJY analyzed data and reviewed the manuscript.
LH designed the study and reviewed the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by The Ethics Committee of
Renmin Hospital of Wuhan University. Written informed consents were
collected from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ghulmiyyah L and Sibai B: Maternal
mortality from preeclampsia/eclampsia. Semin Perinatol. 36:56–59.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mol BWJ, Roberts CT, Thangaratinam S,
Magee LA, de Groot CJM and Hofmeyr GJ: Pre-eclampsia. Lancet.
387:999–1011. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tenorio MB, Ferreira RC, Moura FA, Bueno
NB, de Oliveira ACM and Goulart MOF: Cross-talk between oxidative
stress and inflammation in preeclampsia. Oxid Med Cell Longev.
2019(8238727)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sircar M, Thadhani R and Karumanchi SA:
Pathogenesis of preeclampsia. Curr Opin Nephrol Hypertens.
24:131–138. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bhorat I: Pre-eclampsia and the foetus: A
cardiovascular perspective. Cardiovasc J Afr. 29:387–393.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Staun-Ram E and Shalev E: Human
trophoblast function during the implantation process. Reprod Biol
Endocrinol. 3(56)2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rebahi H, Elizabeth Still M, Faouzi Y and
Rhassane El Adib A: Risk factors for eclampsia in pregnant women
with preeclampsia and positive neurosensory signs. Turk J Obstet
Gynecol. 15:227–234. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xu T, Ni MM, Xing-LI Li XF, Meng XM, Huang
C and Li J: NLRC5 regulates TGF-β1-induced proliferation and
activation of hepatic stellate cells during hepatic fibrosis. Int J
Biochem Cell Biol. 70:92–104. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Miyazono K, Suzuki H and Imamura T:
Regulation of TGF-beta signaling and its roles in progression of
tumors. Cancer Sci. 94:230–234. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kawamoto K, Pahuja A, Hering BJ and
Bansal-Pakala P: Transforming growth factor beta 1 (TGF-beta1) and
rapamycin synergize to effectively suppress human T cell responses
via upregulation of FoxP3+ Tregs. Transpl Immunol. 23:28–33.
2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Serban AI, Stanca L, Geicu OI, Munteanu MC
and Dinischiotu A: RAGE and TGF-β1 cross-talk regulate
extracellular matrix turnover and cytokine synthesis in AGEs
exposed fibroblast cells. PLoS One. 11(e0152376)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Seoane J and Gomis RR: TGF-β family
signaling in tumor suppression and cancer progression. Cold Spring
Harb Perspect Biol. 9(a022277)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Katz LH, Likhter M, Jogunoori W, Belkin M,
Ohshiro K and Mishra L: TGF-β signaling in liver and
gastrointestinal cancers. Cancer Lett. 379:166–172. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang L, Zhou F, Garcia de Vinuesa A, de
Kruijf EM, Mesker WE, Hui L, Drabsch Y, Li Y, Bauer A, Rousseau A,
et al: TRAF4 promotes TGF-β receptor signaling and drives breast
cancer metastasis. Mol Cell. 51:559–572. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lash GE, Otun HA, Innes BA, Bulmer JN,
Searle RF and Robson SC: Inhibition of trophoblast cell invasion by
TGFB1, 2, and 3 is associated with a decrease in active proteases.
Biol Reprod. 73:374–381. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhao MR, Qiu W, Li YX, Zhang ZB, Li D and
Wang YL: Dual effect of transforming growth factor beta1 on cell
adhesion and invasion in human placenta trophoblast cells.
Reproduction. 132:333–341. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Albers RE, Selesniemi K, Natale DRC and
Brown TL: TGF-β induces Smad2 phosphorylation, ARE induction, and
trophoblast differentiation. Int J Stem Cells. 11:111–120.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Attisano L and Wrana JL: Signal
transduction by the TGF-beta superfamily. Science. 296:1646–1647.
2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huang Z, Li S, Fan W and Ma Q:
Transforming growth factor β 1 promotes invasion of human JEG-3
trophoblast cells via TGF-β/Smad3 signaling pathway. Oncotarget.
8:33560–33570. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang J, Zhang X, Xie F, Zhang Z, van Dam
H, Zhang L and Zhou F: The regulation of TGF-β/SMAD signaling by
protein deubiquitination. Protein Cell. 5:503–517. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chae DK, Ban E, Yoo YS, Kim EE, Baik JH
and Song EJ: MIR-27a regulates the TGF-β signaling pathway by
targeting SMAD2 and SMAD4 in lung cancer. Mol Carcinog.
56:1992–1998. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Marmol I, Sanchez-de-Diego C, Pradilla
Dieste A, Cerrada E and Rodriguez Yoldi MJ: Colorectal carcinoma: A
general overview and future perspectives in colorectal cancer. Int
J Mol Sci. 18(E197)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tang YN, Ding WQ, Guo XJ, Yuan XW, Wang DM
and Song JG: Epigenetic regulation of Smad2 and Smad3 by profilin-2
promotes lung cancer growth and metastasis. Nat Commun.
6(8230)2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Guo L, Liu Y, Guo Y, Yang Y and Chen B:
MicroRNA-423-5p inhibits the progression of trophoblast cells via
targeting IGF2BP1. Placenta. 74:1–8. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Osipov ID, Zaporozhchenko IA, Bondar AA,
Zaripov MM, Voytsitskiy VE, Vlassov VV, Laktionov PP and Morozkin
ES: Cell-free miRNA-141 and miRNA-205 as prostate cancer
biomarkers. Adv Exp Med Biol. 924:9–12. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Peng G, Liao Y and Shen C: miRNA-429
inhibits astrocytoma proliferation and invasion by targeting BMI1.
Pathol Oncol Res. 23:369–376. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Vaiman D: Genes, epigenetics and miRNA
regulation in the placenta. Placenta. 52:127–133. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li P, Guo W, Du L, Zhao J, Wang Y, Liu L,
Hu Y and Hou Y: microRNA-29b contributes to pre-eclampsia through
its effects on apoptosis, invasion and angiogenesis of trophoblast
cells. Clin Sci (Lond). 124:27–40. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Niu ZR, Han T, Sun XL, Luan LX, Gou WL and
Zhu XM: MicroRNA-30a-3p is overexpressed in the placentas of
patients with preeclampsia and affects trophoblast invasion and
apoptosis by its effects on IGF-1. Am J Obstet Gynecol.
218:249.e1–249.e12. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fu G, Ye G, Nadeem L, Ji L, Manchanda T,
Wang Y, Zhao Y, Qiao J, Wang YL, Lye S, et al: MicroRNA-376c
impairs transforming growth factor-β and nodal signaling to promote
trophoblast cell proliferation and invasion. Hypertension.
61:864–872. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Salah Z, Arafeh R, Maximov V, Galasso M,
Khawaled S, Abou-Sharieha S, Volinia S, Jones KB, Croce CM and
Aqeilan RI: miR-27a and miR-27a* contribute to metastatic
properties of osteosarcoma cells. Oncotarget. 6:4920–4935.
2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Song J, Li Y and An RF: Identification of
early-onset preeclampsia-related genes and MicroRNAs by
bioinformatics approaches. Reprod Sci. 22:954–963. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yang S, Li H, Ge Q, Guo L and Chen F:
Deregulated microRNA species in the plasma and placenta of patients
with preeclampsia. Mol Med Rep. 12:527–534. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jairajpuri DS and Almawi WY: MicroRNA
expression pattern in pre-eclampsia (Review). Mol Med Rep.
13:2351–2358. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pineles BL, Romero R, Montenegro D, Tarca
AL, Han YM, Kim YM, Draghici S, Espinoza J, Kusanovic JP, Mittal P,
et al: Distinct subsets of microRNAs are expressed differentially
in the human placentas of patients with preeclampsia. Am J Obstet
Gynecol. 196:261.e1–6. 2007.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ji L, Zhang L, Li Y, Guo L, Cao N, Bai Z,
Song Y, Xu Z, Zhang J, Liu C and Ma X: MiR-136 contributes to
pre-eclampsia through its effects on apoptosis and angiogenesis of
mesenchymal stem cells. Placenta. 50:102–109. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Guo M, Zhao X, Yuan X and Li P: Elevated
microRNA-34a contributes to trophoblast cell apoptosis in
preeclampsia by targeting BCL-2. J Hum Hypertens. 31:815–820.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kim J, Fiesel FC, Belmonte KC, Hudec R,
Wang WX, Kim C, Nelson PT, Springer W and Kim J: miR-27a and
miR-27b regulate autophagic clearance of damaged mitochondria by
targeting PTEN-induced putative kinase 1 (PINK1). Mol Neurodegener.
11(55)2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhao Y, Dong D, Reece EA, Wang AR and Yang
P: Oxidative stress-induced miR-27a targets the redox gene nuclear
factor erythroid 2-related factor 2 in diabetic embryopathy. Am J
Obstet Gynecol. 218:136.e1–136.e10. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yao F, Yu Y, Feng L, Li J, Zhang M, Lan X,
Yan X, Liu Y, Guan F, Zhang M and Chen L: Adipogenic miR-27a in
adipose tissue upregulates macrophage activation via inhibiting
PPARgamma of insulin resistance induced by high-fat diet-associated
obesity. Exp Cell Res. 355:105–112. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Danza K, Silvestris N, Simone G, Signorile
M, Saragoni L, Brunetti O, Monti M, Mazzotta A, De Summa S, Mangia
A and Tommasi S: Role of miR-27a, miR-181a and miR-20b in gastric
cancer hypoxia-induced chemoresistance. Cancer Biol Ther.
17:400–406. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Khalil H, Kanisicak O, Prasad V, Correll
RN, Fu X, Schips T, Vagnozzi RJ, Liu R, Huynh T, Lee SJ, et al:
Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac
fibrosis. J Clin Invest. 127:3770–3783. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Fu H, He Y, Qi L, Chen L, Luo Y, Chen L,
Li Y, Zhang N and Guo H: cPLA2α activates PI3K/AKT and inhibits
Smad2/3 during epithelial-mesenchymal transition of hepatocellular
carcinoma cells. Cancer Lett. 403:260–270. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lu Y, Wang L, Li H, Li Y, Ruan Y, Lin D,
Yang M, Jin X, Guo Y, Zhang X and Quan C: SMAD2 inactivation
inhibits CLDN6 methylation to suppress migration and invasion of
breast cancer cells. Int J Mol Sci. 18(E1863)2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Fleming NI, Jorissen RN, Mouradov D,
Christie M, Sakthianandeswaren A, Palmieri M, Day F, Li S, Tsui C,
Lipton L, et al: SMAD2, SMAD3 and SMAD4 mutations in colorectal
cancer. Cancer Res. 73:725–735. 2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Cheng JC, Chang HM and Leung PCK: TGF-β1
inhibits human trophoblast cell invasion by upregulating connective
tissue growth factor expression. Endocrinology. 158:3620–3628.
2017.PubMed/NCBI View Article : Google Scholar
|