Introduction
Steroid-induced avascular necrosis of the femoral
head (SANFH) is a serious orthopedic disease associated with the
clinical application of steroid hormones, characterized by
progressive osteonecrosis leading to structural alterations to the
femoral head, which can ultimately result in femoral head collapse
(1). According to the duration and
dosage of the steroid hormone, femoral head necrosis occurs in
5-40% of patients a few years after the onset of the disease
(2-4).
Although the mechanism underlying ischemic necrosis of the femoral
head is not completely understood (1), the mechanism underlying osteocyte
apoptosis has received increased attention (5-8).
Glucocorticoid-induced osteocyte apoptosis is a cumulative and
irreparable deficiency defect that destroys the mechanical sensory
function of the osteocyte-lacunar-tubule system, thereby initiating
a succession of events that inevitably lead to joint collapse
(9). A possible mechanism resulting
in apoptosis is that the long-term use of glucocorticoids can lead
to excessive oxidative stress, which may activate
apoptosis-associated signaling pathways when the antioxidant
capacity of the cells is exceeded (6). Another study demonstrated that
glucocorticoids are able to induce apoptosis via Dickkopf-1
(DKK-1)-mediated blocking of the Wnt/β-catenin signaling pathway, a
process that reduces osteoblast proliferation, activates apoptosis
and induces osteoclast differentiation and maturation, subsequently
disrupting bone homeostasis and leading to a series of bone
diseases, including osteoporosis and osteonecrosis (10). DKK-1 regulates myeloma bone disease
by disrupting osteoblast differentiation and function. A study
conducted by Lu et al (11)
described a DKK1 vaccine that displayed therapeutic effects against
the established disease via induction of active immunity. However,
apoptosis is mediated by the strict control of multiple genes,
which involves the activation, expression and regulation of a
series of genes. In a mouse model of steroid-induced osteonecrosis
of the femoral head, Zhang et al (12) demonstrated that aggravation of
femoral head cell apoptosis may be associated with downregulated
11β-hydroxysteroid dehydrogenase (11β-HSD)-2 expression, providing
insights into the mechanism underlying cell apoptosis during
SANFH.
The regulation of glucocorticoid levels in humans
and other animals depends predominantly on 11β-HSD, an enzyme that
catalyzes the mutual transformation of active and inert
glucocorticoids. 11β-HSD has two isozymes, 11β-HSD1 and 11β-HSD2,
which are involved in regulation of the aforementioned reaction
(13). Compared with 11β-HSD1,
11β-HSD2 has a higher affinity for its steroid substrate and relies
on NAD+ to convert active glucocorticoids into their
inactive metabolites (14,15), thereby preventing the body from
accumulating large amounts of active hormones. 11β-HSD2 is widely
distributed in tissues such as the kidney, placenta, heart and
colon (16); therefore, it has been
hypothesized that low expression levels of 11β-HSD2 are unfavorable
to the body.
Several overlapping binding sites for Sp1
transcription factor (Sp1) have been identified in multiple CpG
islands in the promoter region of 11β-HSD2(17). Sp1 is a member of the zinc-finger
transcription factor family that binds to a consistent promoter
site, including a central CpG dinucleotide (18,19). In
particular, Sp1 specifically binds to the promoter region of
11β-HSD2 and recruits the transcriptional co-activator p300, which
affects histone acetylation modification of the 11β-HSD2 promoter
region, by increasing H3K9Ac and decreasing H3K9me2, to promote the
expression of 11β-HSD2(20). Wang
et al (21) reported that
cortisol-induced aromatase is expressed in human placental
syncytiotrophoblast cells via the cAMP/Sp1 signaling pathway. A
previous study demonstrated that the tissues of the femoral head
also expressed 11β-HSD, and in a model of steroid-induced
osteonecrosis of the femoral head, the expression of 11β-HSD1 was
increased, whereas the expression of 11β-HSD2 was decreased, which
lead to further increases in the local GC concentration of the
femoral head tissue (22).
Therefore, the present study aimed to investigate the effects of
altering cAMP levels on the expression of Sp1 and 11β-HSD2 in
osteocytes and osteoblasts.
Materials and methods
Cell culture and grouping
MLO-Y4 cells were purchased from Procell Life
Science & Technology Co., Ltd and MC3T3-E1 cells were purchased
from iCell. MLO-Y4 cells can be used as representative models of
primary osteocytes (23) and
MC3T3-E1 cells are a reliable substitute in vitro model of
human osteoblasts (24). Cells were
cultured in MEM-α medium (Hyclone; GE Healthcare Life Sciences)
containing 10% FBS (Zhejiang Tianhang Biotechnology Co., Ltd.) and
1% penicillin/streptomycin at 37˚C with 5% CO2. At 80%
confluence, cells were subcultured. Trypsin-EDTA (0.25% trypsin and
0.02% EDTA; Genom Biotech Pvt., Ltd.) was added to the cells for
digestion purposes. Subsequently, the cell layer was infiltrated
and observed under a light microscope (magnification, x20) until
the cells became round and dispersed. The cell suspension was
centrifuged at 300 x g for 5 min at room temperature, the
supernatant was discarded and the cells were suspended in fresh
MEM-α medium. Cells were subsequently cultured at 37˚C with 5%
CO2. Cells in the exponential phase of growth were
seeded into 6-well plates with 2x105 cells per well and
cultured at 37˚C for 24 h. Subsequently, cells were divided into
three groups: i) The NC group (negative control group), which was
incubated with 100 µmol PBS; ii) the activator group, which was
incubated with 100 µmol forskolin (Selleck Chemicals); and iii) the
inhibitor group, which was incubated with 100 µmol SQ22536 (Selleck
Chemicals). Following incubation at 37˚C for 24 h, cells were used
for subsequent experiments.
Assessment of cellular viability
Cells in the exponential phase of growth were
digested and collected. A cell suspension (1x105
cells/ml) was prepared and 100 µl/well cell suspension was added to
a plate. Cells were cultured at 37˚C for 24 h to allow the cells to
adhere. Subsequently, 10 µl medium containing forskolin or SQ22536
(0, 5, 10, 50, 100 and 200 µmol) was added to each well. For the
negative control group, 10 µl medium containing solvent was added
to each well. The blank group consisted of wells containing 10 µl
cell-free medium only. Following incubation at 37˚C for 24 h, 10 µl
CCK-8 solution (Shanghai Rebiosci Biotech Co., Ltd.) was added to
each well at 37˚C for 2 h. The absorbance of each well was measured
at a wavelength of 450 nm using an ELISA reader (Diatek Healthcare
Pvt. Ltd.).
Quantitative determination of cAMP
levels in each group by ELISA
Cells were centrifuged at 2,000 x g at 4˚C for 10
min to remove insoluble cell debris. Subsequently, the supernatant
was separated and analyzed using the mouse cAMP ELISA kit [cat. no.
ELK8116; Elk (Wuhan) Biotechnology Co. Ltd.], according to the
manufacturer's protocol.
RNA extraction and RT-qPCR
Cells were washed with pre-cooled PBS. Total RNA was
extracted using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.). Subsequently, total RNA was reverse transcribed
into cDNA using the PrimeScript™ RT reagent kit with genomic (g)DNA
Eraser (Clontech Laboratories, Inc.) according to the manufacturers
protocol. To remove gDNA, the reaction was performed on ice.
Subsequently, qPCR was performed on a StepOne™ Real-Time PCR
machine (Thermo Fisher Scientific, Inc.) using the SYBR®
Premix Ex Taq™ kit (Clontech Laboratories, Inc.) with three
replicate wells per sample. The following thermocycling conditions
were used for qPCR: Incubation at 95˚C for 1 min; followed by 40
cycles of 95˚C for 15 sec, 58˚C for 20 sec and 72˚C for 45 sec. The
primers used for qPCR were synthesized by Wuhan GeneCreate
Biological Engineering Co., Ltd. and are presented in Table I. Following qPCR, agarose gel (2.0%)
electrophoresis of the PCR products was performed to observe band
intensity. mRNA expression levels were quantified using the
2-∆∆Cq method and normalized to
the internal reference gene GAPDH (25). RT-qPCR was performed in
triplicate.
 | Table IPrimer sequences used for reverse
transcription-quantitative PCR. |
Table I
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Sequence (5'-3') | PCR product size,
bp |
|---|
| GAPDH | F:
TGAAGGGTGGAGCCAAAAG | 227 |
| | R:
AGTCTTCTGGGTGGCAGTGAT | |
| Sp1 | F:
AACCTCAGTGCATTGGGTACTTC | 164 |
| | R:
CTATTCTCTCCTTCTCCACCTGC | |
| 11βHSD2 | F:
TGCTTCAAGACAGATGCAGTGAC | 175 |
| | R:
CAACTGGGCTAAGGTCAGGC | |
Protein extraction and western
blotting
Total protein was extracted from cells using RIPA
protein lysate (Wuhan Aspen Biotechnology Co., Ltd.) and quantified
using a bicinchoninic acid protein concentration assay kit (Wuhan
Aspen Biotechnology Co., Ltd.). Subsequently, the protein
expression levels of 11β-HSD2 and Sp1 were detected following a
standard western blotting protocol. Proteins (40 µg) were added to
an appropriate amount of protein loading buffer (5X) and incubated
at 95-100˚C in a boiling water bath for 5 min. Subsequently,
proteins were separated via 8% SDS-PAGE and transferred onto
methanol pre-activated PVDF membranes (EMD Millipore). The
membranes were blocked with 5% non-fat milk at room temperature for
1 h. Subsequently, the membranes were incubated overnight with the
following primary antibodies: Anti-Sp1 (cat. no. ab227383; 1:5000;
Abcam), anti-11β-HSD2 (cat. no. ab80317; 1:1,000; Abcam) and
anti-GAPDH (cat. no. ab37168; 1:10,000; Abcam). After washing three
times with TBS containing the 0.05% of Tween-20 (TBST), the
membranes were incubated with the corresponding horseradish
peroxidase-conjugated secondary antibody (cat. no. AS1107;
1:10,000; Wuhan Aspen Biotechnology Co., Ltd.) for 30 min. Protein
bands were visualized using the ECL system (Thermo Fisher
Scientific, Inc.).The optical density of the target bands was
assessed using the AlphaEaseFC™ 4.0 software processing system
(ProteinSimple) with GAPDH as the loading control. Western blotting
was performed in triplicate.
Statistical analysis
All experiments were performed at least three times.
Data are presented as the mean ± SEM. One-way ANOVA followed by
Tukey's post hoc test was used to analyze the cell viability data.
One-way ANOVA followed by the Student-Newman-Keuls post hoc test
was used to analyze the ELISA, RT-qPCR and western blotting data.
Statistical analyses were performed using GraphPad Prism software
(version 5; GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of forskolin and SQ22536 on
MLO-Y4/MC3T3-E1 cell activity
The effects of forskolin and SQ22536 on MLO-Y4 and
MC3T3-E1 cell viability were assessed using the Cell Counting Kit-8
assay (Fig. 1). Compared with the 0
µmol forskolin group, cell viability was increased in the 10 µmol
forskolin group following incubation for 24 h (P>0.05). Cell
viability in the 50, 100 and 200 µmol forskolin groups was
significantly increased compared with the 0 µmol forskolin group
(P<0.05). However, the 100 µmol forskolin group displayed the
most significant increase in cell viability compared with the 0
µmol forskolin group; therefore, 100 µmol forskolin was selected
for subsequent experiments (Fig.
1A). Furthermore, compared with the 0 µmol SQ22536 group, cell
viability was significantly decreased in the 100 and 200 µmol
SQ22536 groups (P<0.001), but there was no significant
difference between the two groups (P>0.05); therefore, 100 µmol
SQ22536 was selected for subsequent experiments (Fig. 1B).
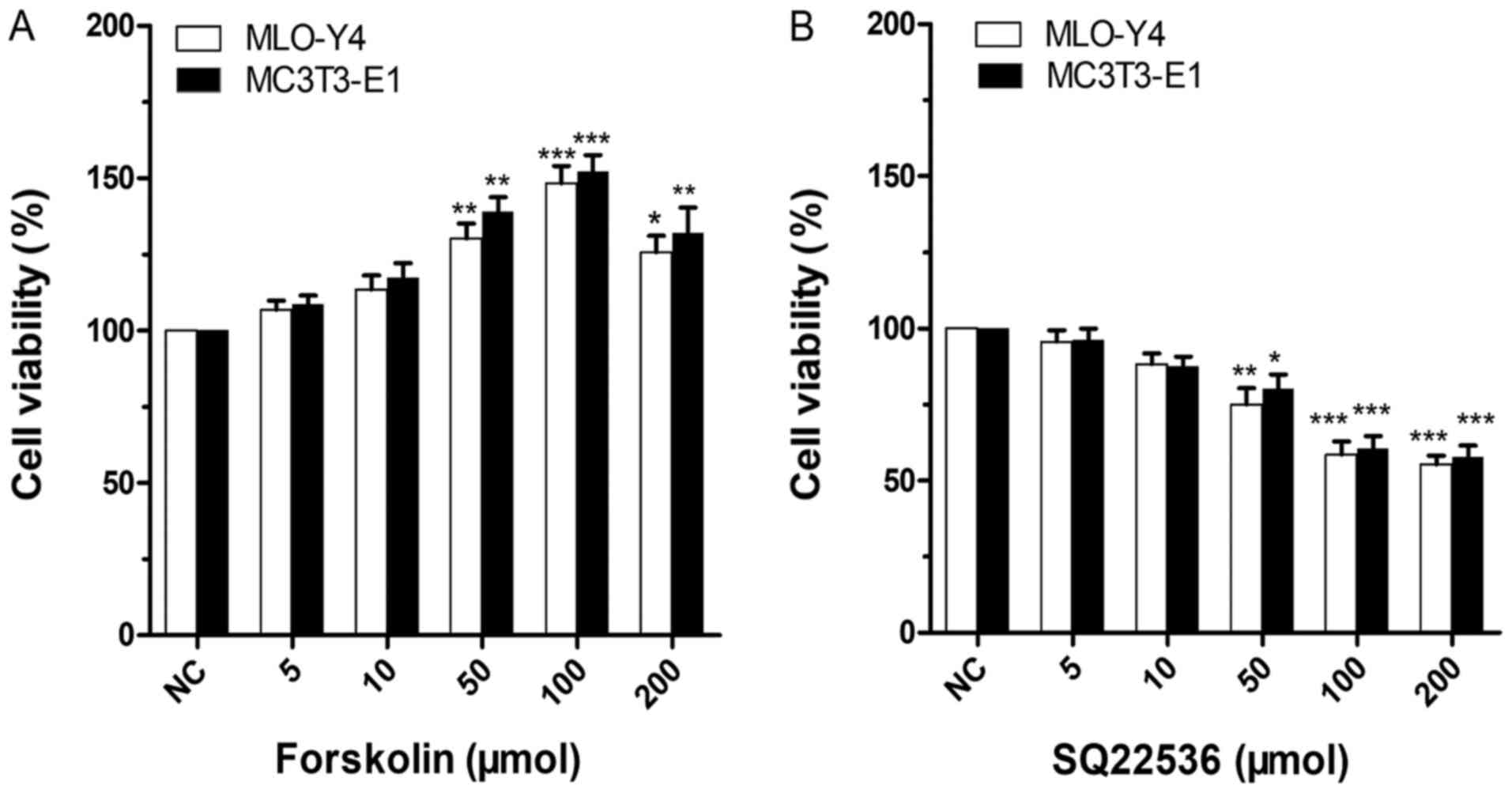 | Figure 1Effects of forskolin and SQ22536 on
MLO-Y4 and MC3T3-E1 cell viability. The NC group was treated with 0
µmol forskolin or SQ22536. (A) Effect of forskolin on MLO-Y4 and
MC3T3-E1 cell viability. Compared with the NC group, the 100 µmol
forskolin group exhibited the most significant increase in MLO-Y4
cell viability (P<0.001), followed by the 50 µmol forskolin
group (P<0.01). Moreover, 200 µmol forskolin significantly
increased MLO-Y4 cell viability compared with the NC group
(P<0.05). In MC3T3-E1 cells, compared with the NC group, the 50
and 200 µmol forskolin groups exhibited significantly enhanced cell
viability (P<0.01). The 100 µmol forskolin group also
significantly increased MC3T3-E1 cell viability compared with the
NC group (P<0.001). (B) Effect of SQ22536 on MLO-Y4 and MC3T3-E1
cell viability. In MLO-Y4 and MC3T3-E1 cells, the 100 and 200 µmol
SQ22536 groups displayed significantly decreased cell viability
compared with the NC group (P<0.001). Moreover, compared with
the NC group, MLO-Y4 cell viability was significantly decreased in
the 50 µmol SQ22536 group (P<0.01). Similarly, MC3T3-E1 cell
viability was significantly decreased in the 50 µmol SQ22536 group
(P<0.05). *P<0.05, **P<0.01,
***P<0.001 vs. NC (0 µmol). NC, negative control. |
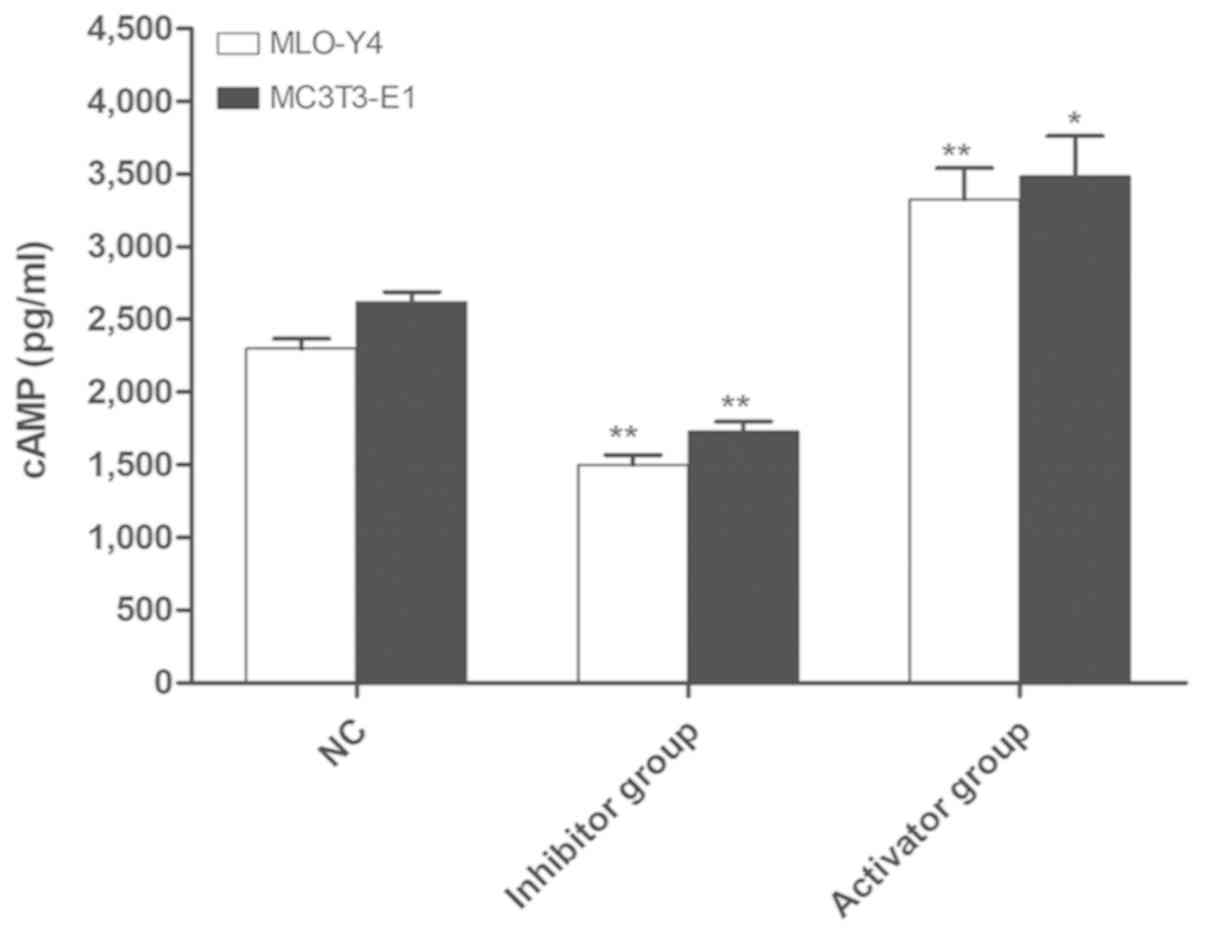
Detection of cAMP expression in MLO-Y4
and MC3T3-E1 cells by ELISA
Subsequently, whether forskolin and SQ22536 had a
significant effect on the expression of cAMP in MLO-Y4 and MC3T3-E1
cells was investigated. Compared with the NC group, the content of
cAMP in the activator group was significantly increased
(P<0.05), whereas the content of cAMP was significantly
decreased in the inhibitor group (P<0.01). The results suggested
that the adenylate cyclase activator and inhibitor significantly
upregulated and downregulated the content of cAMP, respectively, in
mouse osteoblast-like and bone-like cells (Fig. 2).
Sp1 and 11β-HSD2 expression levels in
MLO-Y4 and MC3T3-E1 cells
The effect of upregulation and downregulation of
cAMP content on the expression levels of Sp1 and 11β-HSD2 were
detected by RT-qPCR (Fig. 3). The
expression levels of Sp1 and 11β-HSD2 were significantly increased
in the activator group compared with the NC group (P<0.05). By
contrast, the expression levels of Sp1 and 11β-HSD2 in the
inhibitor group were significantly decreased compared with the NC
group (P<0.05).
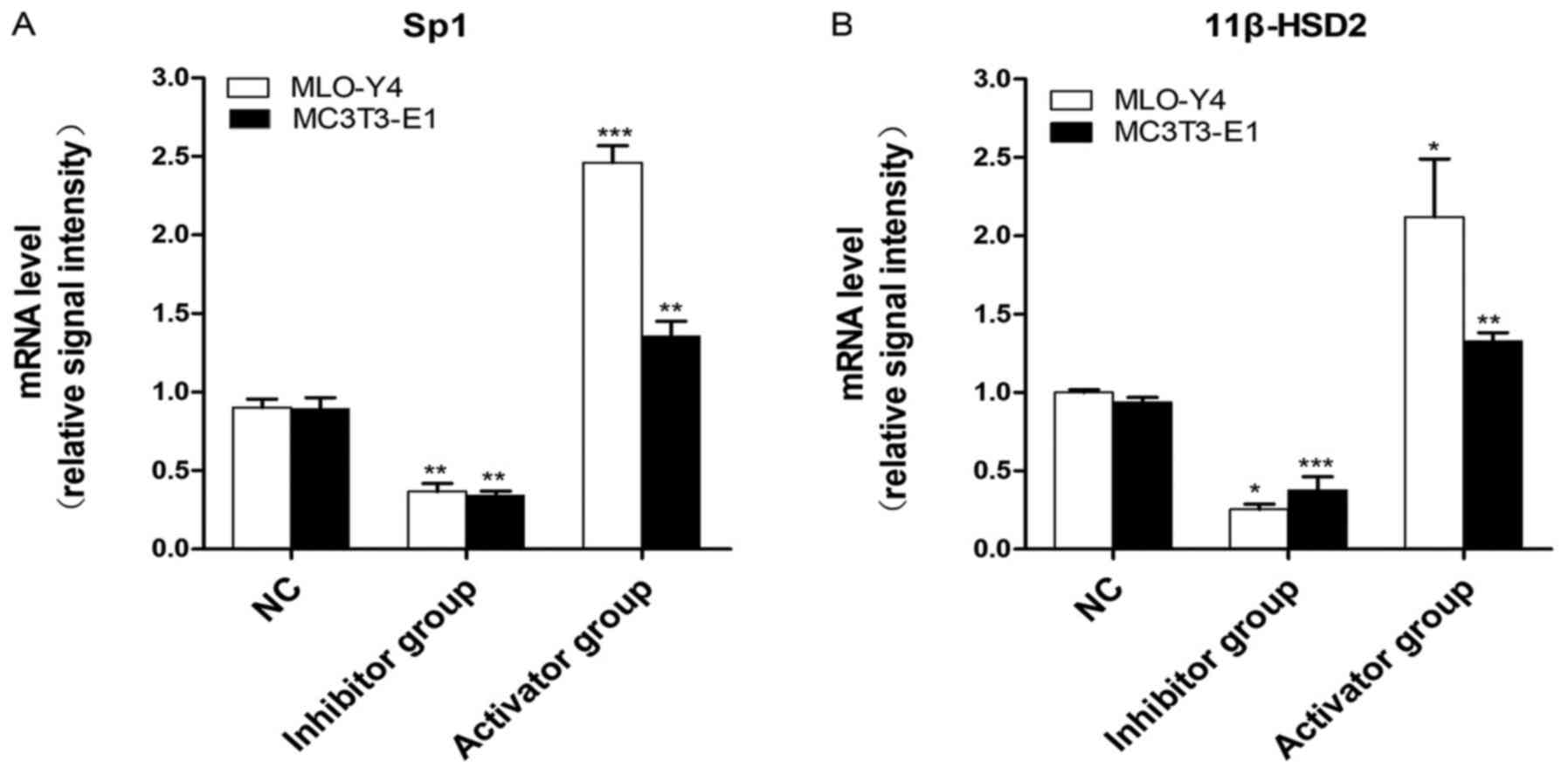 | Figure 3Sp1 and 11β-HSD2 expression levels in
MLO-Y4 and MC3T3-E1 cells. (A) Sp1 mRNA expression levels in MLO-Y4
and MC3T3-E1 cells. Compared with the NC group, the expression of
Sp1 was significantly reduced in the inhibitor group (P<0.01).
By contrast, the expression of Sp1 was significantly increased in
the activator group compared with the NC group in MLO-Y4
(P<0.001) and MC3T3-E1 cells (P<0.01). (B) 11β-HSD2 mRNA
expression levels in MLO-Y4/MC3T3-E1 cells. In MLO-Y4 cells,
compared with the NC group, the expression of 11β-HSD2 in the
inhibitor and activator groups was decreased and increased,
respectively (P<0.05). Compared with the NC group, the
expression of 11β-HSD2 in MC3T3-E1 cells was significantly
decreased in the inhibitor group (P<0.001), and significantly
increased in the activator group (P<0.01).
*P<0.05, **P<0.01,
***P<0.001 vs. NC. Sp1, Sp1 transcription factor;
11β-HSD2, 11β-hydroxysteroid dehydrogenase-2; NC, negative
control. |
Protein expression levels of Sp1 and
11β-HSD2
Western blotting was performed to detect whether
alterations to cAMP content had an effect on the expression levels
of Sp1 and 11β-HSD-2 (Fig. 4). The
expression levels of Sp1 and 11β-HSD2 in the activator group were
significantly increased compared with the NC group (P<0.05).
Moreover, the expression levels of Sp1 and 11β-HSD2 were
significantly decreased in the inhibitor group compared with the NC
group (P<0.05).
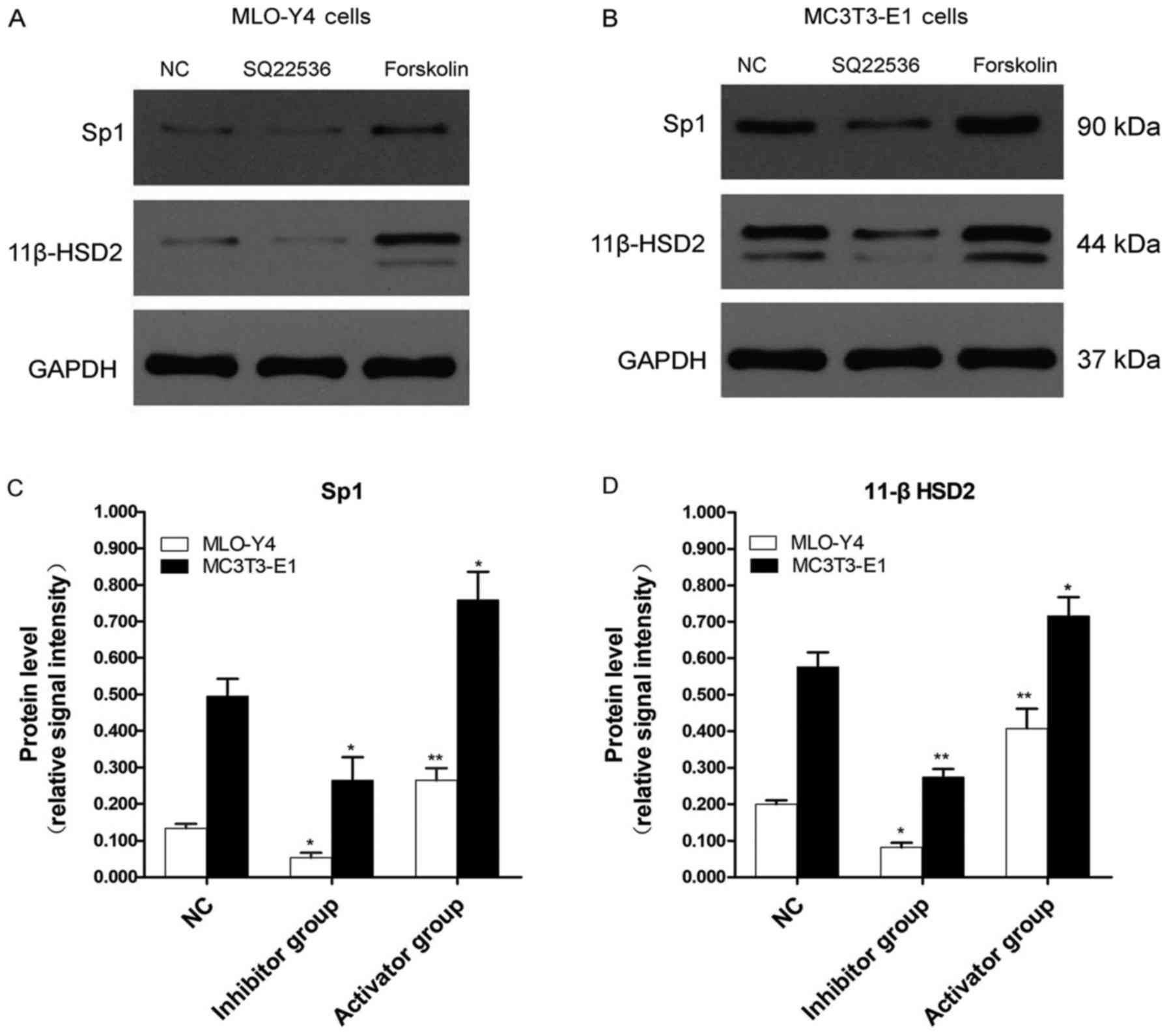 | Figure 4Western blotting was performed to
measure the relative protein expression of Sp1 and 11β-HSD2 in
MLO-Y4 and MC3T3-E1 cells. Protein expression levels were
determined by western blotting in (A) MLO-Y4 and (B) MC3T3-E1
cells, and (C) Sp1 and (D) 11β-HSD2 expression levels were
quantified. In MLO-Y4 cells, compared with the NC group, Sp1
protein expression was significantly decreased (P<0.05) and
increased (P<0.01) in the inhibitor and activator groups,
respectively. In MC3T3 cells, Sp1 protein expression in the
inhibitor and activator groups was significantly decreased
(P<0.05) and increased (P<0.05) compared with the NC group,
respectively. Similarly, in MLO-Y4 cells, compared with the NC
group, 11β-HSD2 expression was significantly decreased (P<0.05)
and increased (P<0.01) in the inhibitor and activator groups,
respectively. Furthermore, in MC3T3-E1 cells, 11β-HSD2 expression
was significantly decreased (P<0.01) and increased (P<0.05)
in the inhibitor and activator groups compared with the NC group,
respectively. *P<0.05, **P<0.01 vs. NC.
Sp1, Sp1 transcription factor; 11β-HSD2, 11β-hydroxysteroid
dehydrogenase-2; NC, negative control. |
Discussion
The results of the present study supported the
hypothesis that, compared with the negative control group, the
relative expression of Sp1 and 11β-HSD2 in the activator group was
significantly increased. In contrast, the relative expression of
Sp1 and 11β-HSD2 in the inhibitor group was significantly decreased
compared with the negative control group. Specifically, the data
indicated that upregulating or downregulating intracellular cAMP
levels in MLO-Y4 and MC3T3-E1 cells increased or decreased the
expression levels of Sp1 and 11β-HSD2, respectively. Therefore, the
results suggested that in osteoblasts and osteocytes, cAMP mediated
the expression of the transcription stimulator Sp1 and the
glucocorticoid-regulated key enzyme 11β-HSD2, which is closely
related to SANFH-associated apoptosis.
Increasing evidence has suggested that activation of
the cAMP signaling pathway serves an important role in hormone
metabolism. Cabrera-Sharp et al (26) reported that cortisol-cortisone
metabolism in boar testis and caput epididymis could be regulated
by the cAMP-protein kinase A (PKA) signaling pathway via their
selective effects on the reductase activity of 11β-HSD. Prenatal
ethanol exposure reduced placental 11β-HSD2 expression, which was
controlled via the mechanism underlying ethanol-induced histone
modification alterations to the 11β-HSD2 promoter via the cAMP/PKA
signaling pathway (27). However,
biological analysis of the promoter sequence of the 11β-HSD2 gene
indicated that the 11β-HSD2 promoter did not contain a
cAMP-response element; therefore, the effect of cAMP on the
expression of 11β-HSD2 was potentially indirectly mediated via
other transcription factors (28).
Sp1 is a sequence-specific DNA-binding protein that regulates the
transcription of cellular and viral genes containing GC-rich
promoters (29). A previous study
suggested that the expression of 11β-HSD2 is highly tissue-specific
in vivo (16). 11β-HSD2 is
highly expressed in target organs containing mineralocorticoid
receptors that are involved in mediating steroid resistance, and
the tissue-specific profile of 11β-HSD2 expression has been
attributed to differential methylation of the CpG island-rich
11β-HSD2 promoter (16,30). Sp1 domain B is a high affinity
DNA-binding structure that includes a PKA-compliant phosphorylation
site at Thr366(31). The cAMP/PKA
signaling pathway regulates the transactivation of Sp1 and
DNA-binding activity, and PKA stimulates transcription of the
Sp1-dependent promoter in intact cells and activates the
DNA-binding activity of Sp1 in vitro (32). In a study investigating the mechanism
by which the placental glucocorticoid barrier is established, it
was reported that human chorionic gonadotropin altered histone
modification and increased the expression of Sp1 via activation of
the cAMP/PKA signaling pathway, which activated 11β-HSD2
transcription (33). A further study
demonstrated that 11β-HSD2 expression was influenced by increased
Sp1 expression following activation of the cAMP signaling pathway,
rather than the differential methylation of the 11β-HSD2 promoter
(20). The interaction between Sp1
and PKA is similar to the complex formed between the PKA catalytic
subunit and NF-κB (34). Another
previous study reported that Sp1 is the substrate of DNA-dependent
protein kinase in vitro, and is phosphorylated at multiple
sites in HeLa nuclear extract; however, the study did not determine
the degree of DNA binding, or Sp1-dependent transcription or
specificity (35).
A previous study confirmed the expression of
11β-HSD2 in necrotic tissue of the femoral head, which was
increased in a model of steroid-induced femoral head necrosis,
whereas the expression of 11β-HSD2 was decreased (22). Furthermore, it was reported that when
the transfected recombinant adenovirus Adb-11β-HSD2 entered into
MLO-Y4 and MC3T3-E1 cells, the expression of 11β-HSD2 was
upregulated, and the number of apoptotic cells and the relative
expression of apoptotic proteins decreased, reflecting a decrease
in apoptosis (12). However, the
regulatory mechanism underlying 11β-HSD2 has not been previously
reported.
In the present study, forskolin and SQ22536 were
used as a cAMP activator and inhibitor, respectively. ELISA was
performed to identify alterations to intracellular cAMP content,
and RT-qPCR and western blotting were performed to measure the
relative expression levels of Sp1 and 11β-HSD2. Compared with the
negative control group, the expression levels of Sp1 and 11β-HSD2
were significantly increased in the activator group, and the
opposite results were observed in the inhibitor group. The results
preliminarily suggested that the expression of Sp1 and 11β-HSD2 was
regulated by cAMP in osteocytes and osteoblasts. However, the
mechanism underlying Sp1-mediated regulation of 11β-HSD2 was not
identified. Sp1 regulates gene expression via two main mechanisms.
One mechanism involves recruitment of basic transcriptional device
proteins and direct interaction with certain proteins in
transcriptional complexes, which activates gene transcription by
recruiting or promoting the assembly of the basic transcription
device (36). The other mechanism
involves alterations to chromosome modification. Sp1 can recruit
histone acetylase and deacetylase to regulate the acetylation state
of the histone at the gene promoter, thereby activating or
inhibiting gene expression (37).
However, Sp1-mediated regulation of 11β-HSD2 expression requires
further investigation.
Apoptosis is an important mechanism associated with
steroid-induced femoral head necrosis; furthermore, osteocyte and
osteoblast apoptosis result in reduced bone formation, as well as
inadequate bone remodeling and repair (38). The aim of the present study was to
identify a potential mechanism underlying 11β-HSD2 involvement in
steroid-induced bone necrosis at the cellular level. However,
combinations of Sp1 and 11β-HSD2 promoter regions,
post-modification alterations and interactions in vivo have
not yet been studied; therefore, future studies are required.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Health and
Family Planning Commission of Hubei Province (grant no.
WJ2018H0002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZP made substantial contributions to the conception
and design of the current study and gave final approval of the
version to be published. DL analyzed and interpreted data, and
drafted the manuscript. DL, YW, ZH and FC performed the
experiments. ZP supervised the project. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang A, Ren M, Song Y, Wang X, Wang Q,
Yang Q, Liu H, Du Z, Zhang G and Wang J: MicroRNA expression
profiling of bone marrow mesenchymal stem cells in steroid-induced
osteonecrosis of the femoral head associated with osteogenesis. Med
Sci Monit. 24:1813–1825. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Weinstein RS: Glucocorticoid-induced
osteonecrosis. Endocrine. 41:183–190. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xu J, Gong H, Lu S, Deasey MJ and Cui Q:
Animal models of steroid-induced osteonecrosis of the femoral
head-a comprehensive research review up to 2018. Int Orthop.
42:1729–1737. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Luo P, Gao F, Han J, Sun W and Li Z: The
role of autophagy in steroid necrosis of the femoral head: A
comprehensive research review. Int Orthop. 42:1747–1753.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Deng S, Zhou JL, Fang HS, Nie ZG, Chen S
and Peng H: Sesamin protects the femoral head from osteonecrosis by
inhibiting ROS-induced osteoblast apoptosis in rat model. Front
Physiol. 9(1787)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Youm Y-S, Lee S-Y and Lee S-H: Apoptosis
in the osteonecrosis of the femoral head. Clin Orthop Surg.
2:250–255. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sato AY, Tu X, McAndrews KA, Plotkin LI
and Bellido T: Prevention of glucocorticoid induced-apoptosis of
osteoblasts and osteocytes by protecting against endoplasmic
reticulum (ER) stress in vitro and in vivo in female mice. Bone.
73:60–68. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mutijima E, De Maertelaer V, Deprez M,
Malaise M and Hauzeur JP: The apoptosis of osteoblasts and
osteocytes in femoral head osteonecrosis: Its specificity and its
distribution. Clin Rheumatol. 33:1791–1795. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Weinstein RS, Nicholas RW and Manolagas
SC: Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis
of the hip. J Clin Endocrinol Metab. 85:2907–2912. 2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Komori T: Glucocorticoid Signaling and
Bone Biology. Horm Metab Res. 48:755–763. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lu C, Meng S, Jin Y, Zhang W, Li Z, Wang
F, Wang-Johanning F, Wei Y, Liu H, Tu H, et al: A novel
multi-epitope vaccine from MMSA-1 and DKK1 for multiple myeloma
immunotherapy. Br J Haematol. 178:413–426. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang H, Zhou F, Pan Z, Bu X, Wang Y and
Chen F: 11β-hydroxysteroid dehydrogenases-2 decreases the apoptosis
of MC3T3/MLO-Y4 cells induced by glucocorticoids. Biochem Biophys
Res Commun. 490:1399–1406. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhou HY, Chen XX, Lin H, Fei AL and Ge RS:
11β-hydroxysteroid dehydrogenase types 1 and 2 in postnatal
development of rat testis: Gene expression, localization and
regulation by luteinizing hormone and androgens. Asian J Androl.
16:811–816. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li X, Mao B, Dong Y, Li Y, Zhan M, Bai Y,
Lian Q, Ge RS and Ye L: Effects of ziram on rat and human
11β-hydroxysteroid dehydrogenase isoforms. Chem Res Toxicol.
29:398–405. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chapman K, Holmes M and Seckl J:
11β-hydroxysteroid dehydrogenases: Intracellular gate-keepers of
tissue glucocorticoid action. Physiol Rev. 93:1139–1206.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Draper N and Stewart PM:
11β-hydroxysteroid dehydrogenase and the pre-receptor regulation of
corticosteroid hormone action. J Endocrinol. 186:251–271.
2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nawrocki AR, Goldring CE, Kostadinova RM,
Frey FJ and Frey BM: In vivo footprinting of the human
11β-hydroxysteroid dehydrogenase type 2 promoter: Evidence for
cell-specific regulation by Sp1 and Sp3. J Biol Chem.
277:14647–14656. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Black AR, Black JD and Azizkhan-Clifford
J: Sp1 and krüppel-like factor family of transcription factors in
cell growth regulation and cancer. J Cell Physiol. 188:143–160.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Kasaai B, Gaumond MH and Moffatt P:
Regulation of the bone-restricted IFITM-like (Bril) gene
transcription by Sp and Gli family members and CpG methylation. J
Biol Chem. 288:13278–13294. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li JN, Ge YC, Yang Z, Guo CM, Duan T,
Myatt L, Guan H, Yang K and Sun K: The Sp1 transcription factor is
crucial for the expression of 11beta-hydroxysteroid dehydrogenase
type 2 in human placental trophoblasts. J Clin Endocrinol Metab.
96:E899–E907. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang W, Li J, Ge Y, Li W, Shu Q, Guan H,
Yang K, Myatt L and Sun K: Cortisol induces aromatase expression in
human placental syncytiotrophoblasts through the cAMP/Sp1 pathway.
Endocrinology. 153:2012–2022. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang L, Luo DK and Pan ZY: Expression of
11β-HSD in steroid-induced avascular necrosis of the femoral head.
Mol Med Rep. 7:1482–1486. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sun T, Yan Z, Cai J, Shao X, Wang D, Ding
Y, Feng Y, Yang J, Luo E, Feng X, et al: Effects of
mechanical vibration on cell morphology, proliferation, apoptosis,
and cytokine expression/secretion in osteocyte-like MLO-Y4 cells
exposed to high glucose. Cell Biol Int: Aug 26, 2019 (Epub ahead of
print).
|
|
24
|
Czekanska EM, Stoddart MJ, Richards RG and
Hayes JS: In search of an osteoblast cell model for in vitro
research. Eur Cell Mater. 24:1–17. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cabrera-Sharp V, Mirczuk SM, Shervill E,
Michael AE and Fowkes RC: Regulation of glucocorticoid metabolism
in the boar testis and caput epididymidis by the gonadotrophin-cAMP
signalling pathway. Cell Tissue Res. 352:751–760. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yu L, Zhou J, Zhang G, Huang W, Pei L, Lv
F, Zhang Y, Zhang W and Wang H: cAMP/PKA/EGR1 signaling mediates
the molecular mechanism of ethanol-induced inhibition of placental
11β-HSD2 expression. Toxicol Appl Pharmacol. 352:77–86.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pizzolo F, Friso S, Morandini F,
Antoniazzi F, Zaltron C, Udali S, Gandini A, Cavarzere P, Salvagno
G, Giorgetti A, et al: Apparent mineralocorticoid excess by a novel
mutation and epigenetic modulation by HSD11B2 promoter methylation.
J Clin Endocrinol Metab. 100:E1234–E1241. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fan F, Shen W, Wu S, Chen N, Tong X, Wang
F and Zhang Q: Sp1 participates in the cadmium-induced imbalance of
the placental glucocorticoid barrier by suppressing 11β-HSD2
expression. Environ Pollut. 261:1–12. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Alikhani-Koopaei R, Fouladkou F, Frey FJ
and Frey BM: Epigenetic regulation of 11 β-hydroxysteroid
dehydrogenase type 2 expression. J Clin Invest. 114:1146–1157.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Chu S: Transcriptional regulation by
post-transcriptional modification - role of phosphorylation in Sp1
transcriptional activity. Gene. 508:1–8. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rohlff C, Ahmad S, Borellini F, Lei J and
Glazer RI: Modulation of transcription factor Sp1 by cAMP-dependent
protein kinase. J Biol Chem. 272:21137–21141. 1997.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhu P, Wang W, Zuo R and Sun K: Mechanisms
for establishment of the placental glucocorticoid barrier, a guard
for life. Cell Mol Life Sci. 76:13–26. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhong H, SuYang H, Erdjument-Bromage H,
Tempst P and Ghosh S: The transcriptional activity of NF-kappaB is
regulated by the IkappaB-associated PKAc subunit through a cyclic
AMP-independent mechanism. Cell. 89:413–424. 1997.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jackson SP, MacDonald JJ, Lees-Miller S
and Tjian R: GC box binding induces phosphorylation of Sp1 by a
DNA-dependent protein kinase. Cell. 63:155–165. 1990.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Taatjes DJ and Tjian R: Structure and
function of CRSP/Med2; a promoter-selective transcriptional
coactivator complex. Mol Cell. 14:675–683. 2004.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hung JJ, Wang YT and Chang WC: Sp1
deacetylation induced by phorbol ester recruits p300 to activate
12(S)-lipoxygenase gene transcription. Mol Cell Biol. 26:1770–1785.
2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kothapalli R, Aya-ay JP, Bian H, Garces A
and Kim HK: Ischaemic injury to femoral head induces apoptotic and
oncotic cell death. Pathology. 39:241–246. 2007.PubMed/NCBI View Article : Google Scholar
|