1. Introduction
It is well known that
[Ca2+]cyt has an essential role in numerous
important mammalian cell functions, including neurotransmitter
release, gene regulation, muscle contraction, cell proliferation,
differentiation and apoptosis (1).
Since the function of [Ca2+]cyt as the cell's
secondary messenger is based on the presence of a concentration
gradient between [Ca2+]cyt and external
Ca2+ ([Ca2+]ext) (2), normal [Ca2+]cyt
homeostasis is important. Under normal physiological conditions,
the concentration of [Ca2+]cyt is <100 nM,
which is ~10,000 times lower than [Ca2+]ext
(>1 mM) (3,4). Furthermore, the membrane potential of
~-60 mV adds to the large electrochemical gradient and this huge
concentration gradient favors the entry of Ca2+ into
cells (5). Therefore, when cells
are activated, Ca2+ is transported down this
electrochemical gradient into the cells through the specific
transmembrane Ca2+ channels, leading to increases in the
[Ca2+]cyt (5).
To restore the resting levels of
[Ca2+]cyt, Ca2+ is transported
back to the extracellular space or stored in intracellular
Ca2+ pools by Ca2+ pumps and transporters
(6,7). Furthermore,
[Ca2+]cyt exhibits differences functions in
different type of cells (8). These
are key factors that determine different specific
Ca2+-dependent cellular responses affected by complex,
spatiotemporal variations in [Ca2+]cyt
(Fig. 1). A major determinant of
these variations are different functionally distinct membrane
calcium channels and exchangers, such as the receptor-operated
calcium channels, voltage-gated channels,
Na+/Ca2+ exchangers (NCX) and calcium pumps
(9). In addition, the intracellular
stores are an important determinant for Ca2+ release
(10). To date, the ryanodine
receptor (RyR) and inositol triphosphate receptor (IP3R)
channels on the endoplasmic reticulum (ER), have been identified,
which lead to Ca2+-induced Ca2+ release
(CICR) or release of IP3, respectively, to mediate the
release of Ca2+ from intracellular stores and increasing
the [Ca2+]cyt (11).
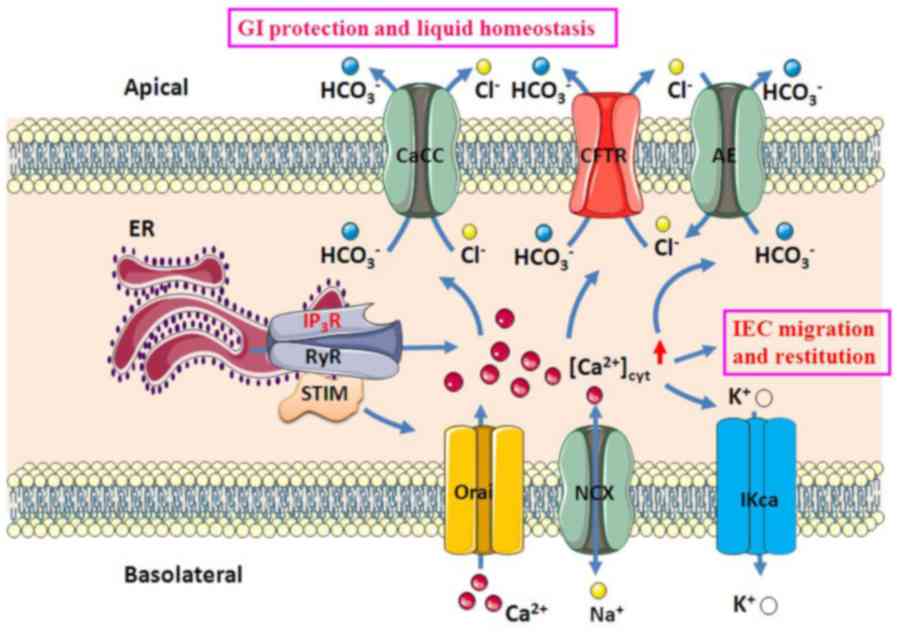 | Figure 1Ca2+-mediated GI
epithelial anion secretion. An increase in [Ca2+]cyt
resulted from intracellular Ca2+ release through IP3R or
RyR on the ER and extracellular Ca2+ entry through Orai
and NCX on the plasma membrane stimulates apical CaCC, AE and CFTR,
as well as basolateral IKCa. Ca2+ signaling activates
IEC migration and restitution and stimulates
HCO3- and Cl- secretion, which
induces epithelial protection and liquid homeostasis, respectively.
CaCC, Ca2+-activated Cl- channel; AE, anion
exchange; CFTR, cystic fibrosis transmembrane conductance
regulator; IKCa, intermediate-conductance Ca2+-activated
K+ channel; ER, endoplasmic reticulum; RyR, ryanodine
receptor; IP3R, inositol triphosphate receptor; STIM, stromal
interaction molecule; Orai, ORAI calcium release-activated calcium
modulators; NCX, Na+/Ca2+ exchangers; IEC,
intestinal epithelial cell; GI, gastrointestinal. |
In the digestive system,
[Ca2+]cyt also has critical roles in the
regulation of digestive functions (12,13).
This includes GI motility and ion transport, food digestion and
nutrient absorption (14). In the
epithelial cells of the GI tract, ion secretion and absorption of
electrolytes and fluid are two essential functions and ion
transport is also a critical physiological process in the human GI
tract (15). GI epithelium secretes
anions (Cl- and HCO3-), providing
the driving force for fluid transport to maintain fluid homeostasis
in the human body (16). GI
epithelial anion secretion is controlled by several neuro-humoral
factors, including prostaglandin E2 (PGE2), acetylcholine (ACh) and
5-hydroxytryptamine (5-HT) (17).
These factors mediate epithelial anion secretion mainly through
Ca2+, cyclic adenosine monophosphate (cAMP) and cyclic guanosine
monophosphate (cGMP) signaling pathways (17).
The physiological roles and molecular mechanisms of
cAMP- and cGMP-dependent regulation of GI epithelial anion
secretion have been extensively defined (18). Since certain adenylyl cyclase
subtypes are Ca2+-dependent and multiple interactions
between Ca2+ and cAMP signaling pathways exist in
mammalian cells, it was previously thought that Ca2+ may
mediate epithelial anion secretion indirectly through cAMP
signaling (19).
However, various lines of evidence indicate that
Ca2+ signaling is able to mediate epithelial anion
secretion in a cAMP-independent manner (19,20).
Although the critical role of Ca2+-dependent regulation
has been verified, the underlying regulatory mechanisms remain to
be fully elucidated. The current researchers have been
investigating the Ca2+-mediated regulation of anion
secretion by GI epithelium and provided solid evidence for a
regulatory role of Ca2+ signaling in a cAMP-independent
manner, as well as the underlying molecular mechanisms (19).
While the critical role of Ca2+ signaling
in other types of secretory cell (such as those of the airways and
salivary gland) is well known (21), the roles of Ca2+
signaling in GI epithelial secretion and its molecular mechanisms
have remained to be fully elucidated. Further research is required
to molecularly identify the Ca2+-activated ion
transporters in GI epithelial cells. In addition, the application
of muscarinic agonists was observed to lead to an increase in
[Ca2+]cyt in secretory cells and promote GI
anion secretion (22). However,
this phenomenon has been extensively studied in other types
secretory cells, such as the secretory cells in the avian basal
gland (21).
Overall, the Ca2+ signaling and anion
secretion mechanisms are of important clinical relevance and need
to be further elucidated. In cystic fibrosis (CF), certain calcium
agonists stimulate Ca2+-activated Cl- channel
(CaCC)-dependent and CF transmembrane conductance regulator
(CFTR)-independent secretion and agents that stimulate
Ca2+ signaling may be used therapeutically to restore
ion and fluid secretion defects (23). Conversely, in certain other types of
GI disease, such as intestinal inflammation and diarrhea,
inhibition of intracellular Ca2+ signaling may have
therapeutic potential to ameliorate excessive fluid secretion
(24,25) (Table
I). Therefore, in the present review, the current state of
knowledge regarding Ca2+ signaling in the regulation of
GI epithelial anion secretion and the associated GI disorders was
summarized.
 | Table ICa2+-mediated GI
epithelial anion secretion and the membrane ion channels
involved. |
Table I
Ca2+-mediated GI
epithelial anion secretion and the membrane ion channels
involved.
| Ion channel | Mechanism | Expression | Related
diseases | Author (refs) |
|---|
|
HCO3- | Maintenance of
intestinal barrier function. Mechanisms of mucosal protection.
Establishes and maintains optimal pH for the activity of luminal
digestive enzymes | GI epithelial
cells | Intestinal
inflammation, peptic ulcers, acute infectious diarrhea, metabolic
acidosis, CF, obstruction of the pancreatic duct, exocrine
pancreatic insufficiency, CLD, IBD | Chen et al
(26) Fei et al (36) Kuna et al (38) Gennari et al (40) Ramos et al (45) |
| Cl- | Transports the
secreted fluid from the lateral base to the apical side of
intestinal cells | Basolateral GI
epithelial cells | Acute infectious
diarrhea, metabolic acidosis, CLD, IBD | Frizzell et
al (27) Mohammad- Panah et
al (49) |
| CFTR | Contributes to the
secretion of anions and fluids in enterotoxin-induced secretory
diarrhea | Pancreas,
epithelial cells in airways, intestinal tract | CF | Goodman et
al (66) Deachapunya et
al (67) |
| CaCC | May participate in
anion secretion in the mammalian GI epithelium | Intestinal
tract | CF | Caputo et al
(76) Yang et al (17) Morris et al (79) Kunzelmann et al (83) |
| Anion/
HCO3- exchangers | Protects gastric
mucosa by combating aggressive factors | Apical membrane of
intestinal epithelial cells | Acute infectious
diarrhea, metabolic acidosis, IBD, CLD, CF | Singh et al
(92) Tang et al (59) Smith et al (94) |
| SOC and STIM/Orai
channels | Gene expression,
cell growth and organ development | Intestinal
epithelial cells | CF | Rao et al
(105) Onodera et al
(106) |
| KCa | Contributes to the
stabilization of membrane voltage and provides the driving force
for electrogenic anion transport | Intestinal
epithelium | CLD, IBD, secretory
diarrhea, CF | Julio-Kalajzić
et al (114) Dong et
al (121,127) Xie et al (13) Assaha et al (115) Wang et al (116) |
| NCX | Maintenance of
Ca2+ homeostasis in a variety of tissues. Involved in
the Ca2+-dependent anion secretion | Cardiomyocytes,
vascular cells, neurons, small intestinal epithelial cells | Intestinal
inflammation, acute infectious diarrhea, metabolic acidosis, CF,
CLD, IBD | Lee et al
(124) Seipet al (126) Dong et al (121,127) Kocks et al (110) |
2. General aspects of GI epithelial anion
secretion
GI epithelial
HCO3- secretion
GI epithelial bicarbonate
(HCO3-) is produced on the surface GI
epithelial cells and secreted to the luminal side of the
epithelium; it isinvolved in the formation of gastrointestinal
mucus with a slightly alkaline pH (12). The basolateral side of
electroneutral Na+-coupled HCO3-
cotransporter is one of the important transporters for
HCO3- absorption (12). With the help of the carbonic
anhydrase, the HCO3- is taken up and it is
also generated inside the cells (26).
To date, several pathways for the export
ofHCO3- into the luminal side of the GI
mucosa have been elucidated: i) The CFTR or the CaCC is able to
promote electrogenic HCO3- efflux, ii)
luminal electroneutral anion/HCO3- exchangers
have been confirmed to contribute to the transport of
HCO3- and iii) HCO3-
may betransported via the short-chain fatty acids
(SCFA)/HCO3- exchanger in the colon (27,28).
Electroneutral secretion of HCO3- is
paralleled by the activity of Na+/H+
exchanger-3(29). Furthermore, the
luminal Cl- channels, as a recycling pathway for
Cl-, are important for HCO3-
secretion and they may serve in the electrogenic secretion of
HCO3- via the luminal
Cl-/HCO3- antiporters (27). One of the Cl- channels,
CFTR, which is located on the apical side, was clearly demonstrated
to be involved in the response of HCO3-
secretion in the intestine a pancreatic duct (30). Besides the CFTR, Ca2+,
cAMP and cGMP were indicated to induce HCO3-
secretion in the small intestine (31). Current data has also demonstrated
that the CFTR is involved in the regulation of the intracellular pH
and that the expression and function of
Cl-/HCO3- exchangers and
down-regulated in adenoma were regulated by the CFTR (32). Of note, CFTR is necessary for
HCO3- secretion in numerous other epithelial
tissues as well (33). Thus, it is
likely that the CFTR contributes to anion secretion and control of
the luminal pH in the entire GI tract, but in the mammalian colon,
the SCFA-dependent HCO3- secretion is the
primary mechanism of HCO3- secretion
(34). Well-regulated
HCO3- secretion is critical for the mucosal
defense against luminal acid due to its neutralization effect in
the upper GI tract and against bacteria in the lower GI tract due
to its stimulation of mucus secretion and maintenance of the
intestinal barrier function (35).
Aside from mechanisms of mucosal protection, normal
HCO3- secretion in the small intestine is
assumed to establish and maintain an optimal pH for the activity of
luminal digestive enzymes (35).
The small intestine is in an alkaline range of pH 6.7-8.0, which is
the best pH value for the optimal activity of pancreatic enzymes
(36). The duodenum in particular
is an important organ exerting pH control for enzymatic digestion
(37).
Defective intestinal HCO3-
secretion has been indicated to be a risk factor for intestinal
inflammation and peptic ulcer diseases (38). Furthermore, intestinal
HCO3- secretion has been critically involved
in the pathophysiology of acute infectious diarrhea (39). Cholera and numerous other acute
diarrheal illnesses may increase the intestinal secretion and loss
of HCO3-, which may result in a severe
HCO3- deficit and metabolic acidosis
(40). Defective GI epithelial
HCO3- secretion has been critically
implicated in the pathogenesis of CF (30). A previous study examining the human
duodenum indicated a CFTR-dependent alkaline transport in subjects
without CF, which was absent in patients with CF (41). Furthermore, electrogenic
HCO3- secretion was detected in the colon of
mice without CF, while it was absent in mice with CF (42). The defective
HCO3- transport in CF may be crucial for the
severity of the symptoms of CF (43). Defective HCO3-
transport probably causes obstruction of the pancreatic duct and
exocrine pancreatic insufficiency (44). Impaired duodenal
HCO3- production and failure to buffer
gastric acid is responsible for an increased incidence of
epigastric pain and morphological changes in the duodenum of
patients with CF (45).
GI epithelial Cl-
secretion
In GI physiology, fluid secretion has a critical
role and is driven by active Cl- transport from the
basolateral to the apical side of enterocytes (46). The basolateral
Na+-K+-2Cl-cotransporter (NKCC1)
is one of the important transporters for Cl- secretion
(47). The rate secretion of
Cl- is regulated by the activity of NKCC1, which is
dependent on the intracellular Cl- concentration, cell
swelling and probably phosphorylation (47). It has also been confirmed that the
cAMP-activated KVLQT1/KCNE3 and Ca2+-activated
K+ channels are able to maintain Cl-
transport (48). The basolateral
Cl- is taken up by NKCC; however, its exit is primarily
via the apical CFTR. Channels such as CaCC and other Cl-
channels may also take part in apical Cl- secretion
(49). Na+ and water
follow via a paracellular route (50). These ion and fluid transports
initiated by pathogens (e.g., cholera toxin and rotavirus) involve
multiple factors, such as 5-HT, substance P, ACh and vasoactive
intestinal peptide, as well as the release of inflammatory
mediators from mast cells and neutrophils [e.g. interleukins (ILs)
and prostaglandins] (51). Ion and
fluid secretion may be activated by different mechanisms that
involve second messengers (cAMP, cGMP or Ca2+) to
activate membrane ion channels (20).
Differences between GI epithelial
secretion of Cl- and HCO3-
It is generally assumed that the GI epithelial
HCO3- and Cl- secretion have the
same regulatory mechanisms (52).
However, this notion requires to be confirmed through a systematic
comparison between them (52). As
mentioned earlier, GI epithelial Cl- and
HCO3- secretion is mainly controlled by cAMP
and Ca2+ signaling, which may interact and cross-talk to
regulate epithelial ion transport (13,27).
Previous studies have demonstrated that most well-known
secretagogues, including 5-HT, ACh, forskolin and PGE2,
stimulate intestinal HCO3- and Cl-
secretion in parallel (53-55).
However, whether epithelial HCO3- and
Cl- secretion occur in parallel and whether they are
regulated by the same or different signaling/mechanisms currently
remains elusive. Notably, it has been indicated that both
forskolin- and carbachol (CCh)-induced rat colonic Cl-
secretion was inhibited by estrogen (56) and further studies by the current
researchers revealed that estrogen stimulates duodenal bicarbonate
secretion (DBS) in humans and mice without altering basal duodenal
short-circuit current (Isc), an index primarily of
epithelial Cl- secretion (57,58).
These results demonstrated that estrogen may have different roles
in regulating intestinal HCO3- and
Cl- secretion. These findings also suggest that GI
epithelial HCO3- and Cl- secretion
may not be necessarily triggered in the same way or by identical
signaling/mechanisms. Furthermore, a previous study by the current
researchers revealed that calcium-sensing receptor (CaSR)
activation raises [Ca2+]cyt; however, it
reduces cAMP-induced exclusive duodenal HCO3-
secretion without simultaneously altering duodenal Cl-
secretion (13). Similarly, Tang
et al (59) demonstrated
that CaSR activation stimulated colonic HCO3-
secretion via SCFA/HCO3- and
Cl-/HCO3- exchangers; however, it
inhibited Cl-secretion via the cAMP/CFTR pathway. It
may, therefore, be proposed that a different regulatory mechanism
likely exists for GI epithelial HCO3- and
Cl- secretion. While cAMP may have a critical role in
CFTR-mediated Cl- secretion, Ca2+ signaling
may be critical in anion/HCO3--mediated
HCO3- secretion.
3. Ca2+ modulation of GI
epithelial anion secretion
Evidence for Ca2+-mediated
anion secretion
Although Ca2+ may mediate epithelial
anion secretion through the cAMP signaling pathway, growing lines
of evidence indicate that Ca2+ signaling is able to
mediate epithelial anion secretion in a cAMP-independent manner
(19). The evidence is as follows:
i) The increase in [Ca2+]cyt induced by
stimulation of cholinergic muscarinic type 3 receptor (M3R) were
indicated to be due to activation of basolateral K+
channels, which enhanced the driving force for luminal anion exit
(60); ii)
Ca2+/calmodulin and protein kinase C (PKC) was
demonstrated to be involved in the CCh-mediated regulation of
luminal and basolateral K+ channels (61); iii) several previous studies
suggested a contribution of Ca2+/PKC to CFTR activation
(62,63); and iv) activation of muscarinic
receptors resulted in an increase in
[Ca2+]cyt; however, it decreased cAMP levels,
which indeed triggered Ca2+-dependent duodenal
transepithelial HCO3- secretion (13). Since cAMP-mediated ion transport has
been extensively reviewed (64),
the present study focused on Ca2+-mediated GI epithelial
anion secretion and the membrane ion channels involved.
Apical CFTR
CFTR is expressed in different tissue types,
including the pancreas, epithelial cells in the airways, GI tract
and other fluid-transporting tissues (30). CF is caused by mutations in the CFTR
gene, resulting in impaired Cl- and
HCO3-transport and plasma membrane targeting
(65). CFTR is mainly located in
the luminal membrane of enterocytes and has a major role to
contribute to the secretion of anions and fluid in
enterotoxin-induced secretory diarrheas such as cholera (66). Numerous lines of solid evidence
suggest a pivotal role for CFTR in GI anion and fluid secretion
(27,30,65,66).
Numerous in vitro studies have indicated
that the application of glibenclamide and
5-nitro-2-(3-phenylpropylamino) benzoic acid further inhibited the
PGE2 and cAMP-mediated increase in Isc
and anion secretion in GI epithelial sheets and cell lines
(67,68). Mice with gene ablation of CFTR
developed intestinal obstruction (69,70).
The resultant characteristic ion transport impairment resulted in
defective intestinal anion and fluid secretion and increased fluid
absorption (71). In CFTR-null
mice, increased expression of alternative Cl- channels
was present and the development of mild intestinal symptoms was
observed (72). Furthermore, in
CFTR-knockout mice, cholera toxin failed to cause massive fluid
secretion through CFTR-dependent protein (72). CFTR has long been considered a
primarily cAMP-activated Cl- channel to activate GI
epithelial anion secretion (30).
The majority of studies have confirmed that CFTR also responds to
Ca2+-mobilizing secretagogues and contributes
substantially to cholinergic and purinergic responses in native
tissues (30,62).
CFTR channels may be stimulated by the G
protein-coupled receptor-mediated signal via Gq protein α subunit,
further activating Ca2+-dependent adenylyl cyclase and
tyrosine kinases, and by inhibition of protein phosphatase type 2A
(PP2A) (72). For instance, the M3R
couples strongly to Gαq. Stimulation of M3R
produces diacylglycerol and IP3 to activate PKC and
mobilize intracellular Ca2+, which in turn activates the
proline-rich tyrosine kinase 2/Src complex (62). Src stimulates CFTR activity by
phosphorylating it directly and inhibiting its dephosphorylation
through the inactivation of PP2A (62). Under basal conditions, constitutive
Ca2+ entry through store-operated Ca2+
channels partially activates adenylyl cyclase and induces tonic
CFTR activity (62).
A recent in vitro study by the current
researchers revealed that the stimulation of mouse duodenal
Isc by CCh was significantly inhibited in a
Ca2+-free solution (17). After the application of CCh, the
intracellular calcium was significantly increased; however, there
was no increase cAMP and compared to the CFTR-knockout mice.
CCh-induced Ca2+ was involved in the duodenal
Cl- and HCO3- secretion in
wild-type mice. The CCh-induced intracellular calcium signaling
also stimulated the phosphorylation of CFTR and promoted the CFTR
transport to the plasma membrane of duodenal epithelial cells.
Furthermore, CCh induced duodenal ion secretion and stimulated
PI3K/Akt signaling pathway in duodenal epithelium and all of these
effects were attenuated by selective PI3K inhibitors. Therefore, a
novel molecular mechanism of Ca2+ signaling in
CFTR-mediated ion secretion via PI3K/Akt was indicated.
Rasmussen et al (73), revealed that cigarette smoking
increased [Ca2+]cyt-induced CFTR
internalization, which was prevented by chelation of cytoplasmic
Ca2+. Furthermore, this phenomenon was inhibited by the
macrolide antibiotic bafilomycin A1, which inhibited cigarette
smoking-induced Ca2+ release and prevented CFTR
clearance from the plasma membrane, further linking cytoplasmic
Ca2+ and CFTR internalization. Patel et al
(74), also indicated that an
increase in [Ca2+]cyt induced a reduction of
cell surface CFTR expression. Therefore, CFTR appears to be the
channel that is in charge of not cAMP-activated,
Ca2+-activated Cl- and
HCO3- secretion in human GI mucosa (30).
Apical CaCC
There is currently evidence that the CaCC are a
further class of important Cl- channels that may
participate in anion secretion in the mammalian GI epithelium
(75). In luminal membranes of GI
epithelia of subjects with and without CF, CaCC are stimulated by
Ca2+ ionophores and Ca2+-mobilizing
secretagogues (76), including
acetylcholine, bradykinin, histamine, CCh and extracellular
nucleotides adenosine triphosphate (ATP) and uridine triphosphate
(UTP) (77,78). In mice with CF, the expression of
Ca2+-dependent Cl- channels was detectable in
the intestine and was age-dependent. In young mice (age, 2-3
weeks), Cl− secretion was induced by carbachol in the
small intestine (17). Furthermore,
it was indicated that in non-CF and CF mouse pup crypts, the
application of nonstructural protein 4 (NSP4) caused severe
diarrhea (79). However, compared
to the young CF mice, the NSP4-induced Cl- secretion was
largely reduced in adult CF mice. These data further support that
the expression and function of CaCC are age-dependent. Indeed, it
was also revealed that the adult CF mice (age, 6-12 weeks) did not
exhibit CFTR-dependent Cl-secretion; however, they did
have a partial CFTR-independent duodenal
HCO3- secretion in response to CCh (17). Therefore, CaCC may have an important
role in the regulation of intestinal Cl-secretion in
young CF mice and may be important for duodenal
HCO3- secretion in adult CF mice.
The Ca2+-activated TMEM16A anion channel
(or anoctamin 1) was reported to be able to conduct
HCO3- upon a significant increase in
cytosolic Ca2+ levels (80). However, the role of anoctamin 1 in
GI epithelial anion secretion remains under debate (81). More recently, a study by the current
researchers indicated that caffeine-stimulated
Ca2+-dependent duodenal anion secretion was attenuated
by niflumic acid and T16Ainh-A01, two selective CaCC blockers with
different chemical structures, suggesting that the TMEM16A anion
channel is likely one of the downstream effectors of
Ca2+ signaling (82).
It has been demonstrated that a residual
cholinergic Cl- secretion was preserved in a subset of
patients with CF with a mild phenotype (83). In T84 colonic carcinoma cells, the
role of CaCC has also been characterized and it was indicated to be
responsible for Ca2+-mediated Cl-secretion in
these cells (83). However, other
studies suggested that the integrated function of CFTR is important
for CaCC (84), as
Ca2+-dependent cholinergic Cl- secretion was
able to be completely inhibited by the deactivation of CFTR
(85). All those results suggest
that residual cholinergic Cl-secretion in CF tissues
depends on the residual function of mutant CFTR. Therefore,
although there is evidence for an alternative CaCC in the mouse
colon and human colonic carcinoma cell lines, the promotion by CaCC
is probably limited (86).
Apical
anion/HCO3-exchangers
It has been generally accepted that
HCO3- secretion from the upper GI tract is
important for the protection of normal mucosa (87). There are three
anion/HCO3- exchangers: Solute carrier family
26 member 6 [SLC26A6; also known as putative anion transporter 1
(PAT1)], DAR and SLC4A9 (also known as anion exchange protein 4);
all those channels contribute to the DBS to resist various
aggressive factors, such as the acidic gastric output (88,89).
However, at least three distinct mechanisms of
HCO3- secretion have been described in the
distal colon of rats (90,91): i) Cl-dependent: The
HCO3- secretion mediated by a brush-border
Cl-/HCO3- exchange; ii)
SCFA-dependent: HCO3- secretion as a result
of activation of SCFA/HCO3- exchange; and
iii) cAMP-induced: HCO3- secretion associated
with a CFTR (91).
As Cl-/HCO3-
exchanger was expressed on the apical membrane of the small
intestinal epithelium and likely has a role in
secretagogue-stimulated DBS (92,93),
its possible involvement in estrogen-stimulated DBS was assessed in
a study by the current researchers (94). The results suggested that estradiol
(E2) indeed stimulated murine DBS, which was attenuated
by 4,4'-diisothiocyanostilbene-2-2'-disulfonic acid, a commonly
used inhibitor of Cl-/HCO3-
exchanger. E2 was also able to increase
[Ca2+]cyt in duodenal epithelial cells
expressing estrogen receptor, whereas
1,2-Bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic
acid tetrakis (acetoxymethyl ester) (BAPTA-AM) one of an
intracellular calcium chelator inhibited the
E2-stimulated murine DBS. It was therefore suggested
that the activation of estrogen receptor stimulates the
Ca2+-dependent DBS via
Cl-/HCO3- exchanger.
Colonic bicarbonate secretion (CBS) is closely
linked to electrolyte movement and overall fluid in the colon
(59). As mentioned earlier, CaSR
activation increases [Ca2+]cyt; however, it
decreases intracellular cAMP production. Consistently, Tang et
al (59) reported that CaSR
activation inhibited cAMP-activated CBS; however, it increased the
lumen Cl-- and SCFA-dependent CBS. Consequently, upon
activation of electroneutral
Cl-/HCO3- and
SCFA/HCO3- exchangers, CaSR stimulated CBS;
by contrast, if forskolin-stimulated electrogenic CFTR-mediated
HCO3- conductance dominated, CaSR inhibited
CBS. Consistently with the results on the Ca2+-mediated
regulation of Cl-/HCO3-
exchanger-mediated DBS reported by the current researchers
(13), these results further
suggest a critical role of Ca2+ signaling in the
regulation of Cl-/HCO3- and
SCFA/HCO3- exchanger-mediated CBS (59). Therefore, modulation of CaSR
activity may provide a new therapeutic approach to correct
HCO3- deficits and metabolic acidosis, a
primary cause of morbidity and mortality in acute infectious
diarrheal illnesses (59). However,
Lamprecht et al (95)
reported on Ca2+-mediated inhibition of colonic DRA.
Both the calcium ionophore A23187 and UTP that increased
Ca2+ were able to inhibit DRA in these cells.
Basolateral store-operated channels and stromal
interaction molecule (STIM)/Orai calcium release-activated calcium
modulators. Store-operated calcium channels (SOC) are a major
pathway for calcium signaling in virtually all mammalian cells and
involved a variety of functions, including gene expression, cell
growth and organ development (96-98).
The SOC is stimulated by the diverse set of surface receptors via
depletion of the Ca2+ concentration from the ER
(99). The stromal interaction
molecule (STIM) proteins were identified as the ER Ca2+
sensors and the Orai proteins as store-operated channels and since
then, rapid progress has been made in the elucidation of the unique
mechanisms of store-operated calcium entry (SOCE) (99). The role of STIM1/Orai signaling was
previously studied mostly in nonpolarized cells, such as
lymphocytes (100,101) or nonconfluent 293 cells (102,103). However, only a few studies have
assessed the function of STIM1/Orai signaling in polarized GI
epithelia (104-106).
In the human colonic tumor cell line NCM460, STIM1 stimulated by
the emptying of intracellular Ca2+ stores after the
production of cAMP (104). In
addition, in the intestinal epithelial cell (IEC)-6 cell line from
rat intestinal crypts, STIM1/Orai was indicated to have a role in
wound healing (105). In the rat
colonic epithelium, STIM1/Orai was identified as a key component of
intracellular Ca2+ signaling involved in the regulation
of both apical and basolateral Ca2+ influx (106).
Although the critical role of Ca2+
signaling in GI epithelial anion secretion is well-known (13), the mechanisms by which
[Ca2+]cyt homeostasis in GI epithelial cells
is controlled remain to be fully elucidated (11). Under normal physiological
conditions, in non-excitable epithelial cells, the occurrence of
Ca2+ entry mainly depends on the SOC (99). In non-excitable cells, agonists
induce Ca2+ signaling mostly depending on the
intracellular Ca2+ release mainly from the ER and
Ca2+ influx from the extracellular medium (107). IP3-sensitive and
ryanodine-sensitive Ca2+ stores have been identified
within the ER (108,109). The former is activated by the
binding of IP3 to IP3R, while the latter is
activated by the binding of ryanodine to RyR to induce ER
Ca2+ release into the cytosol (110).
The IP3R-mediated Ca2+ influx
pathway was reported to have a role in the regulation of GI
epithelial anion secretion (109,111). Similarly, a study by the current
researchers indicated that muscarinic receptors were activated
after the application of CCh and induced mouse intestinal
Cl- secretion, which was significantly inhibited by
selective SOC blockers added to the serosal side of duodenal
tissues in a Ca2+-free serosal solution (82). Furthermore, the study revealed that
calcium release-activated calcium/Orai channels may represent the
molecular candidate of SOC involved in the CCh-induced increase of
intracellular Ca2+ in GI epithelium. As the underlying
mechanisms of RyR-mediated ER Ca2+ release as an
important component of SOCE to contribute to GI epithelial anion
secretion had remained elusive, the role of RyR/Ca2+
storage was also further investigated by Dong et al
(82). The results suggested that
caffeine, a selective RyR activator, markedly increased mouse
intestinal Cl- and HCO3-
secretion. However, this process was suppressed by
Ca2+-free serosal solutions and selective blockers of
SOC/Ca2+ and knockdown of the protein expression of
Orai1 channels also inhibited the Cl- and
HCO3- secretion on the serosal side of
duodenal tissue. Furthermore, the caffeine-induced anion secretion
was inhibited by ER Ca2+ chelator and RyR blockers
(82). In addition, the protein
expression of STIM1 and Orai1 was detected. In IEC cells, the
caffeine-induced SOCE was attenuated by selective SOC inhibitor
(82).
It was therefore concluded that the
RyR/Orai1/Ca2+ signaling on the basolateral side has a
critical role in the regulation of GI epithelial anion secretion
(67). Lefkimmiatis et al
(112) indicated that in a newly
identified type of SOC termed ‘store-operated cAMP signaling’
(SOcAMPS), the luminal ER Ca2+ sensor STIM1 does not
depend on changes in [Ca2+]cyt. The
decreasing free Ca2+ concentration within the ER lumen
induces a rise in intracellular cAMP. Therefore, they proposed the
SOcAMPS, in which the content of internal Ca2+ stores is
directly connected to cAMP signaling through a process that
involves STIM1. Subsequently, Nichols et al (113) determined that in T84 colonic
cells, the Isc, cAMP and PKA activity was increased
under Ca2+-free conditions after treatment with
Ca2+-releasing agonist CCh and Ca2+ ionophore
and suppressed by pre-treatment with BAPTA-AM. Furthermore, the
effects of ER Ca2+ store depletion on cAMP/PKA activity
were attenuated by Ca2+ entering from the extracellular
space, indicating that the production of cAMP decreased after
Ca2+ influx. They proposed that a discrete component of
the ‘Ca2+-dependent’ secretory activity in the colon is
derived from cAMP generated through SOcAMPS. These studies further
support the notion that Ca2+ and cAMP signaling may
independently trigger epithelial ion transport.
Basolateral Ca2+-activated
K+ channels (Kca)
In GI epithelial cells, K+ channels are
important in the intestinal epithelium, contribute to the
stabilization of membrane voltage and provide the driving force for
electrogenic anion transport (114). The concept that cholinergic agents
promote the intestinal Cl- secretion via the activation
of membrane K+ conductance and maintain the cellular
Cl- transport has been widely accepted (115). Certain cholinergic agents, such as
CCh, activate muscarinic receptors or acetylcholine raises the
[Ca2+]cyt, which activates KCa
conductance and secondarily stimulates Cl- secretion via
the apical CFTR (116). Therefore,
basolateral K+ channels hyperpolarize apical membrane
potential and increase the electrical driving force for anion
(Cl- and HCO3-) secretion to
maintain electroneutrality.
To date, three different subtypes of KCa
channels expressed on colonic surface and crypt cells have been
identified: Large-conductance KCa channels,
intermediate-conductance KCa channels (IKCa)
and small-conductance KCa channels (117,118). Among them, IKCa
channels have an important role in epithelial Cl-
secretion. A selective blocker of IKCa channels,
clotrimazole, inhibited the Cl- secretion in intact
colonic epithelium and human colonic T84 cells (119). In addition, it has been
demonstrated that activation of CFTR alone is insufficient to evoke
transepithelial Cl- secretion and that basolateral
membrane K+ channels are also necessary components of
the secretory response (30).
Therefore, basolateral membrane KCa channels represent
an important potential therapeutic target to increase
Cl- secretion in patients with CF.
While the expression and function of KCa
channels and their role in the regulation of duodenal epithelial
ion transport and DBS in the duodenal epithelium have remained
elusive, it is well known that [Ca2+]cyt has
an important role in epithelial ion transport (2,11);
however, the underlying mechanisms of
[Ca2+]cyt to induce duodenal
HCO3- secretion, or indeed other ion
transport systems, had not been explored in detail. Therefore, the
functionality of Kca and their role in the regulation of
duodenal mucosal ion transport were explored. A previous review by
the current researchers provided evidence that IKCa or
intermediate conductance calcium-activated potassium channel
protein 4/SK4 channels are located on the basolateral side of
duodenal epithelial cells and are involved in the regulation of
Ca2+-mediated duodenal Cl- and
HCO3- secretion (120). Furthermore, it was indicated that
clotrimazole, a selective blocker of basolateral IKCa,
was able to inhibit Ca2+-mediated duodenal
Cl- and HCO3- secretion,
suggesting its potential utility as an anti-diarrheal drug for the
treatment of secretory diarrhea (13,121).
NCX
The plasma membrane NCX is an important membrane
transporter and has a critical role in the maintenance of
Ca2+ homeostasis in a variety of tissue types (122).
NCX is a bidirectional plasma membrane transporter
and in each cycle, three Na+ for one Ca2+ are
transported in the opposite direction and this process depends on
electrochemical gradients (123).
The expression and function of NCX have been demonstrated in
cardiomyocytes, vascular cells and neurons (124). They are able to function in a
forward mode to excrete intracellular Ca2+ and in
reverse mode to induce extracellular Ca2+ entry and
various associated signal transduction pathways (124). NCX was previously reported to be
expressed in small intestinal epithelial cells and to function in
the forward mode that is involved in the absorption of
Ca2+ into the bloodstream. However, NCX also has a role
in GI epithelial anion secretion (125).
Seip et al (126), demonstrated an interaction between
SOC and NCX in the rat colon, where the influx of Na+
across SOC serves to reduce the driving force for Ca2+
extrusion via the NCX and thereby maintains the increase in
[Ca2+]cyt during the induction of rat colonic
anion secretion. Consistently, Kocks et al (110) reported a cross-talk between the
depletion of intracellular Ca2+ stores and NCX, which
may maintain a long-lasting increase in
[Ca2+]cyt to amplify
Ca2+-dependent colonic Cl- secretion. While
it was demonstrated that muscarinic receptor induced the activation
of [Ca2+]cyt increases, which regulates anion
secretion, the underlying mechanisms of Ca2+ remained
largely elusive. A previous study by the current researchers
determined whether NCX has a role in the regulation of duodenal
mucosal anion secretion by controlling Ca2+ homeostasis
(127). The results indicated that
activation of muscarinic receptors stimulated NCX activity in a
reverse mode to increase [Ca2+]cyt in
epithelial cells, leading to Ca2+-dependent
HCO3- and Cl- secretion (127). In conclusion, NCX has an important
role in Ca2+-dependent anion secretion by controlling
Ca2+ homeostasis in GI epithelial cells.
4. Associated GI diseases
Ulcers
Ulcers refer to mucosal injury reaching the
submucosa in the GI tract (128).
Peptic ulcers may develop in the stomach or proximal duodenum and
at the margin of a gastroenterostomy, Meckel's diverticulum or the
esophagus (128). Helicobacter
pylori (H. pylori) infection, non-steroidal
anti-inflammatory drugs and stress cause a large proportion of
peptic ulcers (129). Since
patients with ulcers usually have hyperchlorhydria, proton-pump
inhibitors are used to inhibit gastric acid secretion, besides
eradication of H. pylori infection with antibiotics
(130). It is well established
that GI epithelial HCO3- secretion is
critical for defending the vulnerable epithelium against various
aggressive factors (87). The mucus
secreted on the surface of GI mucosa and the bicarbonate ions
secreted by the GI epithelium form a mucous bicarbonate barrier
(87). When H+ in
gastric acid diffuses to the stomach wall, it is neutralized by
HCO3- secreted by epithelial cells (87). In this way, the surface of the
gastric mucosa remains in a neutral or partially alkaline state,
preventing gastric acid and pepsin from attacking the mucosa
(131). The esophagus also
requires HCO3- secretion to protect the
epithelial surface from acid reflux (132). Furthermore, normal mucus release
from GI epithelium requires concurrent HCO3-
secretion, which is essential for the release of mucin molecules
and their proper expansion on the surface of epithelium as well
(133). As a matter of fact, DBS,
as an important protector, has been confirmed in patients with
duodenal ulcer whose acid-stimulated DBS is only 41% of that of
healthy subjects (94). The defect
in intestinal HCO3- secretion has further
been indicated to be a risk factor for peptic ulcer diseases
(134).
Additionally, normal colonic
HCO3- secretion is critical for the mucosal
defense against bacteria in the lower GI tract (87). The luminal pH was indicated to be
acidic in the colon of patients with ulcerative colitis (UC), which
may be caused at least in part by disturbances in the ion transport
in the inflamed colon (135).
Therefore, it appears important to recover normal GI epithelial
HCO3- secretion in patients with peptic
ulcers and inflamed colon to prevent their recurrence. It is of
growing interest to discover novel drugs to stimulate sufficient GI
epithelial HCO3- secretion for mucosal
protection as a potential adjuvant therapy for ulcer diseases or
prevention of their recurrence.
CF
Epithelial HCO3- secretion is
impaired in the GI tract of patients with CF, suggesting a pivotal
role of the CFTR in mediating epithelial
HCO3- secretion (64). Patients with CF usually have an
epithelial HCO3- deficit. As discussed
earlier, while the CFTR is mainly triggered by the cAMP/PKA
pathway, most of the channels involved in GI epithelial anion
secretion, including CaCC, anion exchangers, KCa and
even CFTR, may be generally triggered by Ca2+ signaling
(136). For instance, the CaCC is
stimulated by Ca2+ ionophores and
Ca2+-mobilizing secretagogues in luminal membranes of GI
epithelia from subjects with or without CF (136), including ACh, CCh, histamine,
bradykinin, ATP and UTP (77,78).
Furthermore, a previous study by the current researchers
demonstrated that adult CF mice exhibited a partial
CFTR-independent duodenal HCO3- secretion in
response to CCh, although they did not display CFTR-dependent
Cl-secretion (58). More
recently, a study by the current researchers demonstrated that
caffeine stimulated Ca2+-dependent duodenal anion
secretion, which was able to be attenuated by selective CaCC
blockers, suggesting that the CaCC is one of the downstream
effectors of Ca2+ signaling (82). Therefore, after the cAMP-activated
CFTR is impaired in CF, targeting the Ca2+-mediated
pathway may be a potential adjuvant for CF therapy. Calcium ions
have a critical role in the normal functioning of the
gastrointestinal system (137).
Certain calcium channel blockers were used to affect all of the
organs of the gastrointestinal tract and may have therapeutic
efficacy against esophageal spasm, mesenteric vascular
insufficiency, irritable bowel syndrome, dyskinesis of the
Sphincter of Oddi and insulinoma (137); however, this requires further
intensive investigation.
Inflammatory bowel disease (IBD)
IBD, including Crohn's disease and UC, is a group
of chronic inflammatory disorders of the GI tract. Diarrhea is the
most highly prevalent and debilitating symptom of IBD (138). The pathogenesis of IBD is
multifactorial and involves variations in patients' genome, immune
response, the intestinal microbiome and environmental factors to
result in an excessive and abnormal host immune response (139). However, the change of expression
and/or function of epithelial ion channels and transporters may
result in electrolyte retention and water accumulation in the
intestinal lumen, leading to diarrhea in IBD (139). IBD is a chronic inflammatory
disorder with high complex endogenous inflammatory meditators,
including IL-1β, tumor necrosis factor-α, interferon-γ, IL-6,
monochloramine and nitric oxide (140). They may act on intestinal
epithelial ion transport and smooth muscle (141). Furthermore, the colon of patients
with UC has an acidic luminal pH, which impairs the ion transport
in the inflamed colon (142).
Consistently, the expression of
Cl-/HCO3- exchanger SLC26A3 (DRA)
was reported to be markedly decreased in the inflamed colon
(143). The expression of DRA was
also indicated to be absent exclusively in UC patients, indicating
inadequate membrane trafficking events (144,145). Furthermore, in a recent
genome-wide association study, a single-nucleotide polymorphism in
the SLC26A3 gene was identified as a risk factor for UC development
(146). A strong reduction in
Cl- absorption was identified in parallel with a low
expression of DRA in UC colonic crypts (147). Therefore, decreased DRA expression
may lead to a deficient Cl- absorption in UC, which
emphasizes the important role of DRA in UC-associated diarrhea
(148).
Congenital chloride diarrhea
(CLD)
It is well established that DRA and PAT-1 are the
two major transporters involved in apical
Cl-/HCO3- exchange in the GI tract
(88,90). As mentioned above, loss of the
expression and function of DRA may induce diarrheal disorders
(143). However, mutations in the
DRA gene that encode Cl-/HCO3-
exchange cause a rare diarrheal disorder named CLD, which is
associated with a high stool concentration of Cl-,
metabolic alkalosis and physiologic evidence of an absence of
Cl-/HCO3- exchange in the colon
and ileum (149). Therefore, the
characteristics of patients with CLD include voluminous diarrhea,
massive loss of Cl- via the stool and metabolic
alkalosis. Furthermore, the pH of the ileocolonic lumen in patients
with IBD has been reported to be reduced due to limited
HCO3- secretion (146). Since DRA serves as the major
luminal intestinal Cl-/HCO3-
exchanger responsible for bulk intestinal Cl- absorption
and HCO3- secretion, DRA deficiency is one of
the important factors in the pathogenesis CLD and IBD (147). Therefore, based on the reported
Ca2+-mediated inhibition of colonic DRA, it may be
speculated that inhibition of intracellular Ca2+
signaling may have therapeutic potential to improve excessive fluid
secretion, thereby providing a novel research direction for the
treatment of CLD and IBD.
5. Conclusion
GI epithelial anion and fluid transport have
critical roles in maintaining normal physiological functions in the
GI tract. Defective GI epithelial anion secretion has been
critically implicated in the pathophysiology of ulcer diseases, CF,
intestinal inflammation, diarrhea/constipation and even metabolic
acidosis. Similarly, [Ca2+]cyt also has a
critical role in the regulation of digestive functions. GI
epithelial anion secretion is known to be controlled by several
neuro-humoral factors, including PGE2, ACh and 5-HT.
These factors mediate epithelial anion secretion mainly through
Ca2+, cAMP and cGMP signaling pathways. Although
multiple interactions exist between Ca2+ signaling and
the cAMP pathway to trigger GI epithelial anion secretion, growing
lines of evidence indicated that Ca2+ signaling may
mediate epithelial anion secretion in a cAMP-independent manner.
Ca2+ signaling modulates GI epithelial anion secretion
through acting on CFTR, CaCC,
Cl-/HCO3- exchanger, SOC,
Kca and NCX. It was previously assumed that those
channels and transporters involved in GI epithelial secretion of
Cl- and HCO3- are identical;
however, emerging evidence suggests they are different. While cAMP
may be a critical factor in CFTR-mediated Cl- secretion,
Ca2+ signaling may have a critical role in
Cl-/HCO3--mediated
HCO3- secretion. Elucidation of the precise
regulatory mechanisms of Ca2+-mediated GI epithelial
Cl- and HCO3- secretion will
markedly enhance the current knowledge of ion and fluid transport
in the GI tract. Further investigation on the differences between
GI epithelial secretion of Cl- and
HCO3- may provide novel potential drug
targets to protect the upper GI tract against ulcer diseases and
promote epithelial HCO3- secretion.
Acknowledgements
The authors thank Professor Biguang Tuo (Department
of Gastroenterology, Affiliated Hospital to Zunyi Medical
University, Zunyi, China) for his assistance with the grammar,
spelling and formatting of the manuscript.
Funding
The current study was supported by research grants
of the National Natural Science Foundation of China (no. 81660412
to RX and no. 81970541 to JYX).
Availability of data and materials
Not applicable.
Authors' contributions
WS, YH, JD, XY, JL, QD, QL and LL conceived the
current review article. JX and RX were responsible for the
collection and assembly of the articles/published data for
inclusion and interpretation in this review. All authors were
involved in the writing of the manuscript. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Berridge MJ, Lipp P and Bootman MD: The
versatility and universality of calcium signalling. Nat Rev Mol
Cell Biol. 1:11–21. 2000.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Clapham DE: Calcium signaling. Cell.
131:1047–1058. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kristián T and Siesjö BK: Calcium in
ischemic cell death. Stroke. 29:705–718. 1998.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Berridge MJ, Bootman MD and Roderick HL:
Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev
Mol Cell Biol. 4:517–529. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dong Z, Saikumar P, Weinberg JM and
Venkatachalam MA: Calcium in cell injury and death. Annu Rev
Pathol. 1:405–434. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Romac JM, Shahid RA, Swain SM, Vigna SR
and Liddle RA: Piezo1 is a mechanically activated ion channel and
mediates pressure induced pancreatitis. Nat Commun.
9(1715)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Criddle DN, McLaughlin E, Murphy JA,
Petersen OH and Sutton R: The pancreas misled: Signals to
pancreatitis. Pancreatology. 7:436–446. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee PJ and Papachristou GI: New insights
into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 16:479–496.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Karlstad J, Sun Y and Singh BB: Ca(2+)
signaling: An outlook on the characterization of Ca(2+) channels
and their importance in cellular functions. Adv Exp Med Biol.
740:143–157. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kinjo TG and Schnetkamp PPM: Ca2+
chemistry, storage and transport in biologic systems: An overview.
Mol Biol Intell Unit, pp1-11, 1970.
|
|
11
|
Foskett JK, White C, Cheung KH and Mak DO:
Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev.
87:593–658. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
He J, Yang X, Guo Y, Zhang F, Wan H, Sun
X, Tuo B and Dong H: Ca2+ signaling in
HCO3- secretion and protection of upper GI
tract. Oncotarget. 8:102681–102689. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xie R, Dong X, Wong C, Vallon V, Tang B,
Sun J, Yang S and Dong H: Molecular mechanisms of calcium-sensing
receptor-mediated calcium signaling in the modulation of epithelial
ion transport and bicarbonate secretion. J Biol Chem.
289:34642–34653. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Abdulnour-Nakhoul S, Nakhoul HN, Kalliny
MI, Gyftopoulos A, Rabon E, Doetjes R, Brown K and Nakhoul NL: Ion
transport mechanisms linked to bicarbonate secretion in the
esophageal submucosal glands. Am J Physiol Regul Integr Comp
Physiol. 301:R83–R96. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kiela PR and Ghishan FK: Physiology of
intestinal absorption and secretion. Best Pract Res Clin
Gastroenterol. 30:145–159. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bachmann O and Seidler U: News from the
end of the gut-how the highly segmental pattern of colonic
HCO3- transport relates to absorptive
function and mucosal integrity. Biol Pharm Bull. 34:794–802.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang X, Wen G, Tuo B, Zhang F, Wan H, He
J, Yang S and Dong H: Molecular mechanisms of calcium signaling in
the modulation of small intestinal ion transports and bicarbonate
secretion. Oncotarget. 9:3727–3740. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tuo B, Wen G, Zhang Y, Liu X, Wang X, Liu
X and Dong H: Involvement of phosphatidylinositol 3-kinase in cAMP-
and cGMP-induced duodenal epithelial CFTR activation in mice. Am J
Physiol Cell Physiol. 297:C503–C515. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ahuja M, Jha A, Maléth J, Park S and
Muallem S: cAMP and Ca²+ signaling in secretory
epithelia: Crosstalk and synergism. Cell Calcium. 55:385–393.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lee RJ and Foskett JK: cAMP-activated Ca2+
signaling is required for CFTR-mediated serous cell fluid secretion
in porcine and human airways. J Clin Invest. 120:3137–3148.
2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kallenberg LA: Calcium signalling in
secretory cells. Arch Physiol Biochem. 108:385–390. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lee MG, Ohana E, Park HW, Yang D and
Muallem S: Molecular mechanism of pancreatic and salivary gland
fluid and HCO3 secretion. Physiol Rev. 92:39–74. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ambudkar IS: Ca²+ signaling and
regulation of fluid secretion in salivary gland acinar cells. Cell
Calcium. 55:297–305. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Linan-Rico A, Ochoa-Cortes F, Beyder A,
Soghomonyan S, Zuleta-Alarcon A, Coppola V and Christofi FL:
Mechanosensory signaling in enterochromaffin cells and 5-HT
release: Potential implications for gut inflammation. Front
Neurosci. 10(564)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Thiagarajah JR, Donowitz M and Verkman AS:
Secretory diarrhoea: Mechanisms and emerging therapies. Nat Rev
Gastroenterol Hepatol. 12:446–457. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen M, Praetorius J, Zheng W, Xiao F,
Riederer B, Singh AK, Stieger N, Wang J, Shull GE, Aalkjaer C and
Seidler U: The electroneutral
Na+:HCO3⁻ cotransporter NBCn1 is a
major pHi regulator in murine duodenum. J Physiol. 590:3317–3333.
2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Frizzell RA and Hanrahan JW: Physiology of
epithelial chloride and fluid secretion. Cold Spring Harb Perspect
Med. 2(a009563)2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Quinton PM: Role of epithelial
HCO3- transport in mucin secretion: Lessons
from cystic fibrosis. Am J Physiol Cell Physiol. 299:C1222–C1233.
2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Furukawa O, Bi LC, Guth PH, Engel E,
Hirokawa M and Kaunitz JD: NHE3 inhibition activates duodenal
bicarbonate secretion in the rat. Am J Physiol Gastrointest Liver
Physiol. 286:G102–G109. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Saint-Criq V and Gray MA: Role of CFTR in
epithelial physiology. Cell Mol Life Sci. 74:93–115.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang N, Garcia MA and Quinton PM: Normal
mucus formation requires cAMP-dependent HCO3- secretion and
Ca2+-mediated mucin exocytosis. J Physiol. 591:4581–4593.
2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chávez JC, Hernández-González EO,
Wertheimer E, Visconti PE, Darszon A and Treviño CL: Participation
of the Cl-/HCO(3)-exchangers SLC26A3 and SLC26A6, the Cl- channel
CFTR, and the regulatory factor SLC9A3R1 in mouse sperm
capacitation. Biol Reprod. 86:1–14. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hug MJ, Tamada T and Bridges RJ: CFTR and
bicarbonate secretion by [correction of to] epithelial cells. News
Physiol Sci. 18:38–42. 2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Binder HJ, Rajendran V, Sadasivan V and
Geibel JP: Bicarbonate secretion: A neglected aspect of colonic ion
transport. J Clin Gastroenterol. 39 (4 Suppl 2):S53–S58.
2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Feldman M: Gastric bicarbonate secretion
in humans. Effect of pentagastrin, bethanechol, and
11,16,16-trimethyl prostaglandin E2. J Clin Invest. 72:295–303.
1983.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fei G, Fang X, Wang GD, Liu S, Wang XY,
Xia Y and Wood JD: Neurogenic mucosal bicarbonate secretion in
guinea pig duodenum. Br J Pharmacol. 168:880–890. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rune SJ: pH in the human duodenum. Its
physiological and pathophysiological significance. Digestion.
8:261–268. 1973.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kuna L, Jakab J, Smolic R, Raguz-Lucic N,
Vcev A and Smolic M: Peptic ulcer disease: A brief review of
conventional therapy and herbal treatment options. J Clin Med.
8(179)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Field M: Intestinal ion transport and the
pathophysiology of diarrhea. J Clin Invest. 111:931–943.
2003.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gennari FJ and Weise WJ: Acid-base
disturbances in gastrointestinal disease. Clin J Am Soc Nephrol.
3:1861–1868. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Pratha VS, Hogan DL, Martensson BA,
Bernard J, Zhou R and Isenberg JI: Identification of transport
abnormalities in duodenal mucosa and duodenal enterocytes from
patients with cystic fibrosis. Gastroenterology. 118:1051–1060.
2000.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Xiao F, Li J, Singh AK, Riederer B, Wang
J, Sultan A, Park H, Lee MG, Lamprecht G, Scholte BJ, et al: Rescue
of epithelial HCO3- secretion in murine intestine by apical
membrane expression of the cystic fibrosis transmembrane
conductance regulator mutant F508del. J Physiol. 590:5317–5334.
2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ehre C, Ridley C and Thornton DJ: Cystic
fibrosis: An inherited disease affecting mucin-producing organs.
Int J Biochem Cell Biol. 52:136–145. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wilschanski M and Novak I: The cystic
fibrosis of exocrine pancreas. Cold Spring Harb Perspect Med.
3(a009746)2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ramos AF, de Fuccio MB, Moretzsohn LD,
Barbosa AJ, Passos Mdo C, Carvalho RS and Coelho LG: Cystic
fibrosis, gastroduodenal inflammation, duodenal ulcer, and H.
pylori infection: The ‘cystic fibrosis paradox’ revisited. J
Cyst Fibros. 12:377–383. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kuwahara A: Involvement of the gut
chemosensory system in the regulation of colonic anion secretion.
Biomed Res Int. 2015(403919)2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Markadieu N and Delpire E: Physiology and
pathophysiology of SLC12A1/2 transporters. Pflugers Arch.
466:91–105. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Flores CA, Melvin JE, Figueroa CD and
Sepúlveda FV: Abolition of Ca2+-mediated intestinal anion secretion
and increased stool dehydration in mice lacking the intermediate
conductance Ca2+-dependent K+ channel Kcnn4. J Physiol.
583:705–717. 2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Mohammad-Panah R, Ackerley C, Rommens J,
Choudhury M, Wang Y and Bear CE: The chloride channel ClC-4
co-localizes with cystic fibrosis transmembrane conductance
regulator and may mediate chloride flux across the apical membrane
of intestinal epithelia. J Biol Chem. 277:566–574. 2002.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Argenzio RA, Whipp SC and Glock RD:
Pathophysiology of swine dysentery: Colonic transport and
permeability studies. J Infect Dis. 142:676–684. 1980.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lakhan SE and Kirchgessner A:
Neuroinflammation in inflammatory bowel disease. J
Neuroinflammation. 7(37)2010.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Park HW and Lee MG: Transepithelial
bicarbonate secretion: Lessons from the pancreas. Cold Spring Harb
Perspect Med. 2(a009571)2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kaji I, Akiba Y, Said H, Narimatsu K and
Kaunitz JD: Luminal 5-HT stimulates colonic bicarbonate secretion
in rats. Br J Pharmacol. 172:4655–4670. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Sugamoto S, Kawauch S, Furukawa O, Mimaki
TH and Takeuchi K: Role of endogenous nitric oxide and
prostaglandin in duodenal bicarbonate response induced by mucosal
acidification in rats. Dig Dis Sci. 46:1208–1216. 2001.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Devor DC, Singh AK, Lambert LC, DeLuca A,
Frizzell RA and Bridges RJ: Bicarbonate and chloride secretion in
Calu-3 human airway epithelial cells. J Gen Physiol. 113:743–760.
1999.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Condliffe SB, Doolan CM and Harvey BJ:
17beta-oestradiol acutely regulates Cl- secretion in rat distal
colonic epithelium. J Physiol. 530:47–54. 2001.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Tuo B, Wen G, Wei J, Liu X, Wang X, Zhang
Y, Wu H, Dong X, Chow JY, Vallon V and Dong H: Estrogen regulation
of duodenal bicarbonate secretion and sex-specific protection of
human duodenum. Gastroenterology. 141:854–863. 2011.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Yang X, Guo Y, He J, Zhang F, Sun X, Yang
S and Dong H: Estrogen and estrogen receptors in the modulation of
gastrointestinal epithelial secretion. Oncotarget. 8:97683–97692.
2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Tang L, Peng M, Liu L, Chang W, Binder HJ
and Cheng SX: Calcium-sensing receptor stimulates Cl(-)- and
SCFA-dependent but inhibits cAMP-dependent HCO3(-) secretion in
colon. Am J Physiol Gastrointest Liver Physiol. 308:G874–G883.
2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Nathanson NM: Synthesis, trafficking, and
localization of muscarinic acetylcholine receptors. Pharmacol Ther.
119:33–43. 2008.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Gustafsson JK, Lindén SK, Alwan AH,
Scholte BJ, Hansson GC and Sjövall H: Carbachol-induced colonic
mucus formation requires transport via NKCC1, K+
channels and CFTR. Pflugers Arch. 467:1403–1415. 2015.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Billet A and Hanrahan JW: The secret life
of CFTR as a calcium-activated chloride channel. J Physiol.
591:5273–5278. 2013.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Jia Y, Mathews CJ and Hanrahan JW:
Phosphorylation by protein kinase C is required for acute
activation of cystic fibrosis transmembrane conductance regulator
by protein kinase A. J Biol Chem. 272:4978–4984. 1997.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Kiela PR and Ghishan FK: Ion transport in
the intestine. Curr Opin Gastroenterol. 25:87–91. 2009.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Shah VS, Ernst S, Tang XX, Karp PH, Parker
CP, Ostedgaard LS and Welsh MJ: Relationships among CFTR
expression, HCO3- secretion, and host defense may inform gene- and
cell-based cystic fibrosis therapies. Proc Natl Acad Sci USA.
113:5382–5387. 2016.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Goodman BE and Percy WH: CFTR in cystic
fibrosis and cholera: From membrane transport to clinical practice.
Adv Physiol Educ. 29:75–82. 2005.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Deachapunya C and O'Grady SM: Regulation
of chloride secretion across porcine endometrial epithelial cells
by prostaglandin E2. J Physiol. 508:31–47. 1998.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Hoffmann EK, Lambert IH and Pedersen SF:
Physiology of cell volume regulation in vertebrates. Physiol Rev.
89:193–277. 2009.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Borowitz D and Gelfond D: Intestinal
complications of cystic fibrosis. Curr Opin Pulm Med. 19:676–680.
2013.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Kelly T and Buxbaum J: Gastrointestinal
manifestations of cystic fibrosis. Dig Dis Sci. 60:1903–1913.
2015.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Lavelle GM, White MM, Browne N, McElvaney
NG and Reeves EP: Animal models of cystic fibrosis pathology:
Phenotypic parallels and divergences. Biomed Res Int.
2016(5258727)2016.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Li C, Dandridge KS, Di A, Marrs KL, Harris
EL, Roy K, Jackson JS, Makarova NV, Fujiwara Y, Farrar PL, et al:
Lysophosphatidic acid inhibits cholera toxin-induced secretory
diarrhea through CFTR-dependent protein interactions. J Exp Med.
202:975–986. 2005.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Rasmussen JE, Sheridan JT, Polk W, Davies
CM and Tarran R: Cigarette smoke-induced Ca2+ release leads to
cystic fibrosis transmembrane conductance regulator (CFTR)
dysfunction. J Biol Chem. 289:7671–7681. 2014.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Patel W, Moore PJ, Sassano MF,
Lopes-Pacheco M, Aleksandrov AA, Amaral MD, Tarran R and Gray MA:
Increases in cytosolic Ca2+ induce dynamin- and
calcineurin-dependent internalisation of CFTR. Cell Mol Life Sci.
76:977–994. 2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
He J, Yang X, Guo Y, Zhang F, Wan H, Sun
X, Tuo B and Dong H: Ca2+ signaling in HCO3-
secretion and protection of upper GI tract. Oncotarget.
8:102681–102689. 2017.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Caputo A, Caci E, Ferrera L, Pedemonte N,
Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O and
Galietta LJ: TMEM16A, a membrane protein associated with
calcium-dependent chloride channel activity. Science. 322:590–594.
2008.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Zimmermann H: Extracellular ATP and other
nucleotides-ubiquitous triggers of intercellular messenger release.
Purinergic Signal. 12:25–57. 2016.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Beech DJ: Inhibitory effects of histamine
and bradykinin on calcium current in smooth muscle cells isolated
from guinea-pig ileum. J Physiol. 463:565–583. 1993.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Morris AP, Scott JK, Ball JM, Zeng CQ,
O'Neal WK and Estes MK: NSP4 elicits age-dependent diarrhea and
Ca(2+)mediated I(-) influx into intestinal crypts of CF mice. Am J
Physiol. 277:G431–G444. 1999.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Yu K, Zhu J, Qu Z, Cui YY and Hartzell HC:
Activation of the Ano1 (TMEM16A) chloride channel by calcium is not
mediated by calmodulin. J Gen Physiol. 143:253–267. 2014.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Kunzelmann K, Ousingsawat J, Cabrita I,
Doušová T, Bähr A, Janda M, Schreiber R and Benedetto R: TMEM16A in
cystic fibrosis: Activating or inhibiting? Front Pharmacol.
10(3)2019.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Zhang F, Wan H, Yang X, He J, Lu C, Yang
S, Tuo B and Dong H: Molecular mechanisms of caffeine-mediated
intestinal epithelial ion transports. Br J Pharmacol.
176:1700–1716. 2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Kunzelmann K and Mall M: Electrolyte
transport in the mammalian colon: mechanisms and implications for
disease. Physiol Rev. 82:245–289. 2002.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Berg J, Yang H and Jan LY: Ca2+-activated
Cl- channels at a glance. J Cell Sci. 125:1367–1371.
2012.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Zsembery A, Strazzabosco M and Graf J:
Ca2+-activated Cl- channels can substitute for CFTR in stimulation
of pancreatic duct bicarbonate secretion. FASEB J. 14:2345–2356.
2000.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Berkes J, Viswanathan VK, Savkovic SD and
Hecht G: Intestinal epithelial responses to enteric pathogens:
Effects on the tight junction barrier, ion transport, and
inflammation. Gut. 52:439–451. 2003.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Flemström G and Isenberg JI:
Gastroduodenal mucosal alkaline secretion and mucosal protection.
News Physiol Sci. 16:23–28. 2001.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Simpson JE, Schweinfest CW, Shull GE,
Gawenis LR, Walker NM, Boyle KT, Soleimani M and Clarke LL: PAT-1
(Slc26a6) is the predominant apical membrane Cl-/HCO3- exchanger in
the upper villous epithelium of the murine duodenum. Am J Physiol
Gastrointest Liver Physiol. 292:G1079–G1088. 2007.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Xiao F, Yu Q, Li J, Johansson ME, Singh
AK, Xia W, Riederer B, Engelhardt R, Montrose M, Soleimani M, et
al: Slc26a3 deficiency is associated with loss of colonic HCO3 (-)
secretion, absence of a firm mucus layer and barrier impairment in
mice. Acta Physiol (Oxf). 211:161–175. 2014.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Vidyasagar S, Barmeyer C, Geibel J, Binder
HJ and Rajendran VM: Role of short-chain fatty acids in colonic
HCO(3) secretion. Am J Physiol Gastrointest Liver Physiol.
288:G1217–G1226. 2005.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Vidyasagar S, Rajendran VM and Binder HJ:
Three distinct mechanisms of HCO3- secretion in rat distal colon.
Am J Physiol Cell Physiol. 287:C612–C621. 2004.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Singh AK, Riederer B, Chen M, Xiao F,
Krabbenhöft A, Engelhardt R, Nylander O, Soleimani M and Seidler U:
The switch of intestinal Slc26 exchangers from anion absorptive to
HCOFormula secretory mode is dependent on CFTR anion channel
function. Am J Physiol Cell Physiol. 298:C1057–C1065.
2010.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Singh AK, Liu Y, Riederer B, Engelhardt R,
Thakur BK, Soleimani M and Seidler U: Molecular transport machinery
involved in orchestrating luminal acid-induced duodenal bicarbonate
secretion in vivo. J Physiol. 591:5377–5391. 2013.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Smith A, Contreras C, Ko KH, Chow J, Dong
X, Tuo B, Zhang HH, Chen DB and Dong H: Gender-specific protection
of estrogen against gastric acid-induced duodenal injury:
Stimulation of duodenal mucosal bicarbonate secretion.
Endocrinology. 149:4554–4566. 2008.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Lamprecht G, Hsieh CJ, Lissner S, Nold L,
Heil A, Gaco V, Schäfer J, Turner JR and Gregor M: Intestinal anion
exchanger down-regulated in adenoma (DRA) is inhibited by
intracellular calcium. J Biol Chem. 284:19744–19753.
2009.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Feske S, Giltnane J, Dolmetsch R, Staudt
LM and Rao A: Gene regulation mediated by calcium signals in T
lymphocytes. Nat Immunol. 2:316–324. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
97
|
Rosenberg SS and Spitzer NC: Calcium
signaling in neuronal development. Cold Spring Harb Perspect Biol.
3(a004259)2011.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Stiber J, Hawkins A, Zhang ZS, Wang S,
Burch J, Graham V, Ward CC, Seth M, Finch E, Malouf N, et al: STIM1
signalling controls store-operated calcium entry required for
development and contractile function in skeletal muscle. Nat Cell
Biol. 10:688–697. 2008.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Prakriya M and Lewis RS: Store-operated
calcium channels. Physiol Rev. 95:1383–1436. 2015.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Baba Y, Hayashi K, Fujii Y, Mizushima A,
Watarai H, Wakamori M, Numaga T, Mori Y, Iino M, Hikida M and
Kurosaki T: Coupling of STIM1 to store-operated Ca2+ entry through
its constitutive and inducible movement in the endoplasmic
reticulum. Proc Natl Acad Sci USA. 103:16704–16709. 2006.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Barr VA, Bernot KM, Srikanth S, Gwack Y,
Balagopalan L, Regan CK, Helman DJ, Sommers CL, Oh-Hora M, Rao A
and Samelson LE: Dynamic movement of the calcium sensor STIM1 and
the calcium channel Orai1 in activated T-cells: Puncta and distal
caps. Mol Biol Cell. 19:2802–2817. 2008.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Smyth JT, Lemonnier L, Vazquez G, Bird GS
and Putney JW Jr: Dissociation of regulated trafficking of TRPC3
channels to the plasma membrane from their activation by
phospholipase C. J Biol Chem. 281:11712–11720. 2006.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Mercer JC, Dehaven WI, Smyth JT, Wedel B,
Boyles RR, Bird GS and Putney JW Jr: Large store-operated calcium
selective currents due to co-expression of Orai1 or Orai2 with the
intracellular calcium sensor, Stim1. J Biol Chem. 281:24979–24990.
2006.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Lefkimmiatis K, Moyer MP, Curci S and
Hofer AM: ‘cAMP sponge’: A buffer for cyclic adenosine 3',
5'-monophosphate. PLoS One. 4(e7649)2009.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Rao JN, Rathor N, Zou T, Liu L, Xiao L, Yu
TX, Cui YH and Wang JY: STIM1 translocation to the plasma membrane
enhances intestinal epithelial restitution by inducing
TRPC1-mediated Ca2+ signaling after wounding. Am J
Physiol Cell Physiol. 299:C579–C588. 2010.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Onodera K, Pouokam E and Diener M:
STIM1-regulated Ca2+ influx across the apical and the basolateral
membrane in colonic epithelium. J Membr Biol. 246:271–285.
2013.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Smyth JT, DeHaven WI, Bird GS and Putney
JW Jr: Role of the microtubule cytoskeleton in the function of the
store-operated Ca2+ channel activator STIM1. J Cell Sci.
120:3762–3771. 2007.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Seo MD, Enomoto M, Ishiyama N, Stathopulos
PB and Ikura M: Structural insights into endoplasmic reticulum
stored calcium regulation by inositol 1,4,5-trisphosphate and
ryanodine receptors. Biochim Biophys Acta. 1853:1980–1991.
2015.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Putney JW Jr: Capacitative calcium entry
revisited. Cell Calcium. 11:611–624. 1990.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Kocks S, Schultheiss G and Diener M:
Ryanodine receptors and the mediation of Ca2+-dependent anion
secretion across rat colon. Pflugers Arch. 445:390–397.
2002.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Prole DL and Taylor CW: Inositol
1,4,5-trisphosphate receptors and their protein partners as
signalling hubs. J Physiol. 594:2849–2866. 2016.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Lefkimmiatis K, Srikanthan M, Maiellaro I,
Moyer MP, Curci S and Hofer AM: Store-operated cyclic AMP
signalling mediated by STIM1. Nat Cell Biol. 11:433–442.
2009.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Nichols JM, Maiellaro I, Abi-Jaoude J,
Curci S and Hofer AM: ‘Store-operated’ cAMP signaling contributes
to Ca2+-activated Cl- secretion in T84 colonic cells. Am J Physiol
Gastrointest Liver Physiol. 309:G670–G679. 2015.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Julio-Kalajzić F, Villanueva S, Burgos J,
Ojeda M, Cid LP, Jentsch TJ and Sepúlveda FV: K2P TASK-2
and KCNQ1-KCNE3 K+ channels are major players
contributing to intestinal anion and fluid secretion. J Physiol.
596:393–407. 2018.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Assaha DVM, Ueda A, Saneoka H, Al-Yahyai R
and Yaish MW: The role of Na+ and K+
transporters in salt stress adaptation in glycophytes. Front
Physiol. 8(509)2017.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Wang J, Haanes KA and Novak I: Purinergic
regulation of CFTR and Ca(2+)-activated Cl(-) channels and K(+)
channels in human pancreatic duct epithelium. Am J Physiol Cell
Physiol. 304:C673–C684. 2013.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Joiner WJ, Basavappa S, Vidyasagar S,
Nehrke K, Krishnan S, Binder HJ, Boulpaep EL and Rajendran VM:
Active K+ secretion through multiple KCa-type channels and
regulation by IKCa channels in rat proximal colon. Am J Physiol
Gastrointest Liver Physiol. 285:G185–G196. 2003.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Thompson-Vest N, Shimizu Y, Hunne B and
Furness JB: The distribution of intermediate-conductance,
calcium-activated, potassium (IK) channels in epithelial cells. J
Anat. 208:219–229. 2006.PubMed/NCBI View Article : Google Scholar
|
|
119
|
McNamara B, Winter DC, Cuffe JE,
O'Sullivan GC and Harvey BJ: Basolateral K+ channel involvement in
forskolin-activated chloride secretion in human colon. J Physiol.
519:251–260. 1999.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Du C, Chen S, Wan H, Chen L, Li L, Guo H,
Tuo B and Dong H: Different functional roles for K+
channel subtypes in regulating small intestinal glucose and ion
transport. Biol Open. 8(bio042200)2019.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Dong H, Smith A, Hovaida M and Chow JY:
Role of Ca2+-activated K+ channels in duodenal mucosal ion
transport and bicarbonate secretion. Am J Physiol Gastrointest
Liver Physiol. 291:G1120–G1128. 2006.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Ottolia M and Philipson KD: NCX1:
Mechanism of transport. Adv Exp Med Biol. 961:49–54.
2013.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Brini M and Carafoli E: The plasma
membrane Ca2+ ATPase and the plasma membrane sodium calcium
exchanger cooperate in the regulation of cell calcium. Cold Spring
Harb Perspect Biol. 3(a004168)2011.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Lee SY and Kim JH: Mechanisms underlying
presynaptic Ca2+ transient and vesicular glutamate release at a CNS
nerve terminal during in vitro ischaemia. J Physiol. 593:2793–2806.
2015.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Liao QS, Du Q, Lou J, Xu JY and Xie R:
Roles of Na+/Ca2+ exchanger 1 in digestive
system physiology and pathophysiology. World J Gastroenterol.
25:287–299. 2019.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Seip G, Schultheiss G, Kocks SL and Diener
M: Interaction between store-operated non-selective cation channels
and the Na(+)-Ca(2+) exchanger during secretion in the rat colon.
Exp Physiol. 86:461–468. 2001.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Dong H, Sellers ZM, Smith A, Chow JY and
Barrett KE: Na(+)/Ca(2+) exchange regulates Ca(2+)-dependent
duodenal mucosal ion transport and HCO(3)(-) secretion in mice. Am
J Physiol Gastrointest Liver Physiol. 288:G457–G465.
2005.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Narayanan M, Reddy KM and Marsicano E:
Peptic ulcer disease and Helicobacter pylori infection. Mo
Med. 115:219–224. 2018.PubMed/NCBI
|
|
129
|
Iijima K, Kanno T, Koike T and Shimosegawa
T: Helicobacter pylori-negative, non-steroidal
anti-inflammatory drug: Negative idiopathic ulcers in Asia. World J
Gastroenterol. 20:706–713. 2014.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Goderska K, Agudo Pena S and Alarcon T:
Helicobacter pylori treatment: Antibiotics or probiotics.
Appl Microbiol Biotechnol. 102:1–7. 2018.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Phan J, Benhammou JN and Pisegna JR:
Gastric hypersecretory states: Investigation and management. Curr
Treat Options Gastroenterol. 13:386–397. 2015.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Mejia A and Kraft WK: Acid peptic
diseases: Pharmacological approach to treatment. Expert Rev Clin
Pharmacol. 2:295–314. 2009.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Garcia MA, Yang N and Quinton PM: Normal
mouse intestinal mucus release requires cystic fibrosis
transmembrane regulator-dependent bicarbonate secretion. J Clin
Invest. 119:2613–2622. 2009.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Allen A and Flemström G: Gastroduodenal
mucus bicarbonate barrier: Protection against acid and pepsin. Am J
Physiol Cell Physiol. 288:C1–C19. 2005.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Barkas F, Liberopoulos E, Kei A and Elisaf
M: Electrolyte and acid-base disorders in inflammatory bowel
disease. Ann Gastroenterol. 26:23–28. 2013.PubMed/NCBI
|
|
136
|
Hwang SJ, Basma N, Sanders KM and Ward SM:
Effects of new-generation inhibitors of the calcium-activated
chloride channel anoctamin 1 on slow waves in the gastrointestinal
tract. Br J Pharmacol. 173:1339–1349. 2016.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Findling R, Frishman W, Javed MT, Heffer S
and Brandt L: Calcium channel blockers and the gastrointestinal
tract. Am J Ther. 3:383–408. 1996.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Zhang YZ and Li YY: Inflammatory bowel
disease: Pathogenesis. World J Gastroenterol. 20:91–99.
2014.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Tanoue T, Umesaki Y and Honda K: Immune
responses to gut microbiota-commensals and pathogens. Gut Microbes.
1:224–233. 2010.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Ghishan FK and Kiela PR: Epithelial
transport in inflammatory bowel diseases. Inflamm Bowel Dis.
20:1099–1109. 2014.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Lau KS, Nakashima O, Aalund GR, Hogarth L,
Ujiie K, Yuen J and Star RA: TNF-alpha and IFN-gamma induce
expression of nitric oxide synthase in cultured rat medullary
interstitial cells. Am J Physiol. 269:F212–F217. 1995.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Das S, Jayaratne R and Barrett KE: The
role of ion transporters in the pathophysiology of infectious
diarrhea. Cell Mol Gastroenterol Hepatol. 6:33–45. 2018.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Manoharan P, Coon S, Baseler W, Sundaram
S, Kekuda R and Sundaram U: Prostaglandins, not the leukotrienes,
regulate Cl(-)/HCO(3)(-) exchange (DRA, SLC26A3) in villus cells in
the chronically inflamed rabbit ileum. Biochim Biophys Acta.
1828:179–186. 2013.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Yang D, Shcheynikov N, Zeng W, Ohana E, So
I, Ando H, Mizutani A, Mikoshiba K and Muallem S: IRBIT coordinates
epithelial fluid and HCO3- secretion by stimulating the
transporters pNBC1 and CFTR in the murine pancreatic duct. J Clin
Invest. 119:193–202. 2009.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Lohi H, Kujala M, Kerkelä E,
Saarialho-Kere U, Kestilä M and Kere J: Mapping of five new
putative anion transporter genes in human and characterization of
SLC26A6, a candidate gene for pancreatic anion exchanger. Genomics.
70:102–112. 2000.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Anbazhagan AN, Priyamvada S, Alrefai WA
and Dudeja PK: Pathophysiology of IBD associated diarrhea. Tissue
Barriers. 6(e1463897)2018.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Priyamvada S, Gomes R, Gill RK, Saksena S,
Alrefai WA and Dudeja PK: Mechanisms underlying dysregulation of
electrolyte absorption in inflammatory bowel disease-associated
diarrhea. Inflamm Bowel Dis. 21:2926–2935. 2015.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Priyamvada S, Anbazhagan AN, Gujral T,
Borthakur A, Saksena S, Gill RK, Alrefai WA and Dudeja PK:
All-trans-retinoic acid increases SLC26A3 DRA (Down-regulated in
Adenoma) expression in intestinal epithelial cells via HNF-1β. J
Biol Chem. 290:15066–15077. 2015.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Seidler U and Nikolovska K: Slc26 Family
of anion transporters in the gastrointestinal tract: Expression,
function, regulation, and role in disease. Compr Physiol.
9:839–872. 2019.PubMed/NCBI View Article : Google Scholar
|















