Introduction
Senescence is a complex process that has not yet
been fully elucidated. Senescence is associated with the
accumulation of toxic materials in cells. This accumulation leads
to a decrease in cell physiological function and ultimately results
in a variety of diseases (1). The
number of cells exhibiting senescence increases with age and these
cells exhibit an enlarged volume and flattened shape (2). Furthermore, specific markers including
senescence-associated β-galactosidase (SA-β-gal) (3), the senescence-associated secretory
phenotype (4) and an increased
level of cell cycle inhibitors, including tumor protein p53, p21
and retinoblastoma-associated protein (5), have been identified as typical of
senescent cells. The activation of these markers has been
associated with a variety of different factors, including the
presence of insulin-like growth factor 1 (IGF-1) (6,7).
IGF-1 was first discovered in 1957(8) and has since been reported as being
highly homologous with insulin (9).
IGF-1 is also known as somatomedin C and is regulated by growth
hormone and subsequently effects bodily functions (10) such as glucose metabolism and organ
homeostasis (11). IGF-1 serves an
important role in the regulation of cell growth, division and
proliferation, and its aberrant expression can cause growth defects
(11,12). A number of studies have assessed the
association between the insulin/IGF-1 signaling pathway and cell
longevity and senescence (13-15).
It has been previously reported that reduced IGF signaling leads to
increased longevity (16,17).
Cellular senescence contributes to aging and
age-associated diseases including osteoarthritis (18). The presence of a variety of
senescence markers has been observed in the chondrocytes of
osteoarthritic lesions (19) and
senescent chondrocytes (20).
Furthermore, it has been reported that IGF-1 serves a key role in
different cellular senescence types. Handayaningsih et al
(21) demonstrated that IGF-1
enhanced the senescence of rat vascular smooth muscle cells, and
mouse and human fibroblasts in vitro. However, the effect of
IGF-1 on the premature senescence of rat articular chondrocytes is
yet to be elucidated.
IGF-1 is a major regulator in a variety of tissue
types and previous research has demonstrated that IGF-1 regulated
numerous cell types through a variety of signaling pathways
(15,22). IGF-1 exerts a biological effect by
activating the PI3K and ERK/mitogen-activated protein kinase (MAPK)
pathways (23,24). Furthermore, it has been revealed
that IGF-1 stimulated proteoglycan synthesis via the PI3K pathway
in chondrocytes (25). The
activation of Akt has been indicated to be essential for
IGF-1-induced survival signaling and has been previously suggested
to be a target of IGF-1 signaling (26). Although the role of IGF-1 in the
survival of some cell types has been clarified, the association
between IGF-1 and Akt in rat articular chondrocyte senescence has
not yet, to the best of our knowledge, been fully elucidated. The
current study aimed to assess the effects of IGF-1 on rat articular
chondrocyte senescence and focused on determining the molecular
mechanisms underlying the activation of the Akt signaling
pathway.
Materials and methods
Materials
Recombinant murine IGF-1 was obtained from
PeproTech, Inc. The Akt inhibitor MK-2206 (MK) was purchased from
Selleck Chemicals. A SA-β-gal staining kit was purchased from
Beyotime Institute of Biotechnology. Anti-Akt rabbit pAb was
purchased from Wanleibio Co., Ltd. (cat. no. WL0003b). p53 (cat.
no. E1A6073), p21 (cat. no. E1A6290) and phosphorylated p-Akt
(Ser473) (cat. no. E1A0016) antibodies were obtained from EnoGene
Biotech Co., Ltd. A rabbit monoclonal antibody against GAPDH was
purchased from Abcam (cat. no. ab181602). Peroxidase-conjugated
goat anti-rabbit IgG was purchased from Origene Technologies,
Inc.
Isolation and culture of rat articular
chondrocytes
All animal experiments were approved and conducted
in accordance with the guidelines of the Ethical Committee for
Animal Experiments (Northeast Agricultural University, Harbin,
China). All rats had access to water and food ad libitum.
Animals were purchased from the Animal Experimental Center of the
Second Affiliated Hospital of the Harbin Medical University
(Harbin, China) and housed in a controlled environment (light/dark,
12/12 h; temperature 23±1˚C; relative humidity, 50-60%. All rats
were healthy upon purchase. The rats were observed for bite and
skin damage daily; rats with skin damage were not used. All efforts
were made to minimize suffering.
A total of 40 Sprague-Dawley rat pups (age, 17-21
days; weight, 30-40 g) were used for chondrocyte preparation.
Articular cartilage was aseptically isolated from the femoral
condyle, tibial plateau and femoral heads of 17-21 day old
Sprague-Dawley rat pups. Digestion was performed for 30 min at 37˚C
in a 50 ml centrifuge tube containing 0.25% (w/v) trypsin (Gibco;
Thermo Fisher Scientific, Inc.) by shaking at 85 rpm/min. Trypsin
was then removed and cartilage pieces were transferred into a new
centrifuge tube and digested for 4 h at 37˚C with 0.2% (w/v)
collagenase II (Gibco; Thermo Fisher Scientific, Inc.) by shaking
at 85 rpm/min. The digested cartilage pieces were washed with equal
quantities of DMEM/F-12 (Corning Inc.) supplemented with 10% FBS
(Biological Industries) and centrifuged at 400 x g for 7 min at
4˚C. Obtained cells were then seeded in 25 cm2 sterile
plastic culture flasks and incubated at 37˚C in a 5% CO2
incubator. The culture medium was changed every 2 days. Cells at
the third and fifth passages were used for the subsequent
experiments.
Treatment of rat articular
chondrocytes
IGF-1 stock solution was prepared at 100 µg/ml in
PBS according to manufacturer's protocol. MK was prepared as a 20
µM stock solution in DMSO. DMSO was prepared as a 20 µM stock
solution in PBS. Cells sub-cultured from primary chondrocytes at
80% confluence were trypsinized and chondrocytes from the third and
fifth passages were used in the subsequent experiments.
Chondrocytes at the third and fifth passages and at 80% confluence
were serum-starved in DMEM containing 0.05% FBS for 12 h. Cells
were divided at random into five groups, which were as follows:
Control group (DMEM alone), IGF-1 group, MK + IGF-1 group, MK
group, DMSO group. In the IGF-1 group, 10 µl IGF-1 stock solution
was added to DMEM at a final concentration of 100 ng/ml. In the MK
+ IGF-1 group, cells were pretreated with MK (5 µl) for 90 min.
Following IGF-1 (100 ng/ml) stimulation, cells were further
cultured for 24 h at 37˚C. In the MK group, 5 µl MK stock solution
was added to DMEM at a final concentration of 10 nM. In the DMSO
group, an equal volume of DMSO stock solution was added to DMEM to
serve as a vehicle control. A previous study determined that there
was no toxicity at the final concentration 0.02% of DMSO (27). Cells were incubated for 24 h at 37˚C
in a 95% O2 humidified atmosphere with 5% CO2
in DMEM containing 0.05% FBS.
Toluidine blue staining
Isolated chondrocytes were cultured in six-well
plates at a density of 5.0x104 cell/well and
subsequently stained at 80% confluence using the following
procedure. Medium was removed and cells were washed with PBS. Cells
were then fixed in 4% paraformaldehyde for 60 min at 4˚C and
stained with a solution of 1% toluidine blue (Beijing Solarbio
Science & Technology Co., Ltd.) for 90 min at room temperature.
Cells were rinsed for 3 min, three times with PBS to remove the
excess dye. The staining results were observed using an inverted
light microscope (magnification, x100; Nikon Corporation).
Type II collagen immunofluorescence
staining
Isolated chondrocytes were cultured in culture
dishes at a density of 5.0x104 cell/well and stained at
80% confluence using the following procedure. For type II collagen
immunofluorescence staining, 4% paraformaldehyde was added and
cells were fixed at 37˚C for 30 min. Permeabilization was performed
using 0.1% Triton X-100/PBS for 20 min. Cells were then blocked
with 10% goat serum (Boster Biological Technology) for 30 min at
room temperature and incubated overnight at 4˚C with rabbit
polyclonal antibody against collagen type II (cat. no. ab34712;
Abcam, 1:200). Samples were washed with PBS and incubated at 37˚C
for 60 min with fluorescein-conjugated goat anti-rabbit IgG (cat.
no. ZF-0311; OriGene Technologies, Inc.; 1:100). Samples were
finally rinsed with PBS prior to nuclear staining with DAPI
(Beyotime Institute of Biotechnology) for 5 min at room
temperature. Images were analyzed using a fluorescence microscope
(magnification, x200; Nikon Corporation). Clear fluorescence was
observed under green light wavelength 543 nm, blue light wavelength
458 nm.
SA-β-gal activity assay for adherent
cultured cells
After passages two and four chondrocytes were
digested and cells were cultured at 37˚C in six-well plates at a
density of 5x104 cell/well for 20 days following the 24
h of aforementioned treatments at 37˚C in a 95% humidified
atmosphere with 5% CO2 in DMEM containing 0.05% FBS.
SA-β-gal activity assay was performed on all five experimental
groups using a SA-β-gal staining kit according to manufacturer's
protocol. Cells were fixed in fixative solution for 15 min at room
temperature. Following washing with PBS, samples were incubated
with freshly prepared SA-β-gal staining solution (1 ml/well
containing 10 µl of β-galactosidase staining solution A and B, 930
µl staining solution C and 50 µl X-gal solution) overnight at 37˚C
in the dark without CO2. Cells were rinsed with PBS for
10 min and subsequently images were captured under an inverted
light microscope. A total of 300 cells were counted in 3 random
fields of view at x100 magnification to determine the percentage of
positive blue staining cells, and quantitative analysis of SA-β-gal
positive cells was performed for each group using Microsoft Excel
97-2003.
Reverse transcription-quantitative
(RT-q)PCR analysis
After treatment for 24 h, total RNA was extracted
from 80% confluent rat articular chondrocytes using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. RNA concentration
was evaluated at 260/280 nm using a spectrophotometer. Total RNA
was reverse transcribed using PrimeScript™ RT Master Mix. According
to the manufacturer's protocol (Takara Bio, Inc.), the protocol for
reverse transcription was as follows: 37˚C for 15 min, 85˚C for 5
sec and 4˚C for 1 min. The qPCR reaction system consisted of 2 µl
cDNA, 7 µl RNase-free water, 0.5 µl each of upstream and downstream
primers at a concentration of 10 µM with 10 µl SYBR qPCR Master Mix
(Toyobo Life Science). The relative expression of mRNA was
quantified using RT-qPCR according to following protocols: 30 sec
of denaturation (95˚C), amplification consisted of 5 sec of
denaturation (95˚C) for 30 cycles, followed by 50 sec of annealing
(34˚C) and 72 sec of extension (72˚C) steps in a
LightCycler® 2.0 (Roche Diagnostics). GAPDH was used as
a housekeeping gene. Primers were designed by Sangon Biotech Co.,
Ltd. and the primer sequences are as follows: p53 forward,
5'-TCCTCTGGGCCTTCTAACAAC-3' and reverse,
3'-CACAGTCGGATATGAGCATCG-5'; p21 forward,
5'-CTCCTGAGCCTGTTTCGTGTC-3' and reverse,
3'-CTCTGAAGATGTGCCTATGGT-5'; GAPDH forward,
5'-GATGCCCCCATGTTTGTGAT-3' and reverse,
3'-GGCATGGACTGTGGTCATGAG-5'. The 2-ΔΔCq method was used
to calculate differences in relative mRNA levels against GAPDH
(28).
Western blot analysis
After treatment for 24 h, western blot analysis was
performed using standard procedures. Cells were lysed in RIPA
buffer supplemented with 1 mM PMSF. Protein concentration was
measured by the bicinchoninic acid kit (Beyotime Institute of
Biotechnology). A total of 30 µg protein was loaded into an 5-13%
SDS-PAGE gel, separated and transferred to nitrocellulose filter
membranes. Membranes were blocked with 5% nonfat dry milk for 2 h
at room temperature and subsequently incubated in 5% nonfat dry
milk with corresponding primary antibodies (29), including GAPDH (1:5,000), Akt
(1:500), p-Akt (Ser473; 1:500), p53 (1:500), p21 (1:500) overnight
at 4˚C. Membranes were washed with TBST containing Tris-buffered
saline and 0.1% Tween-20. Following washing, membranes were
incubated with peroxidase-conjugated goat anti-rabbit IgG (1:1,000)
for 1 h at 37˚C and rinsed with TBST containing Tris-buffered
saline and 0.1% Tween-20. The blots were developed using Luminata
Crescendo Western HRP substrate (EMD Millipore) and were
subsequently exposed to an Amersham Imager 600 (GE Healthcare Life
Sciences). Subsequently, bands were quantified using Image J 1.50i
software (National Institutes of Health).
Data analysis
All data were statistically analyzed using GraphPad
Prism software (version 7.0; GraphPad Software, Inc.). Results are
presented as the mean ± standard deviation. Significant differences
were analyzed by one-way ANOVA followed by Bonferroni's post hoc
test. All data are representative of three independent experiments.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Identification of chondrocytes
Toluidine blue staining and type II collagen
immunofluorescence staining were used for the identification of
chondrocytes. In the present study, the strong toluidine blue
staining of cytoplasm and membranes confirmed that cultured
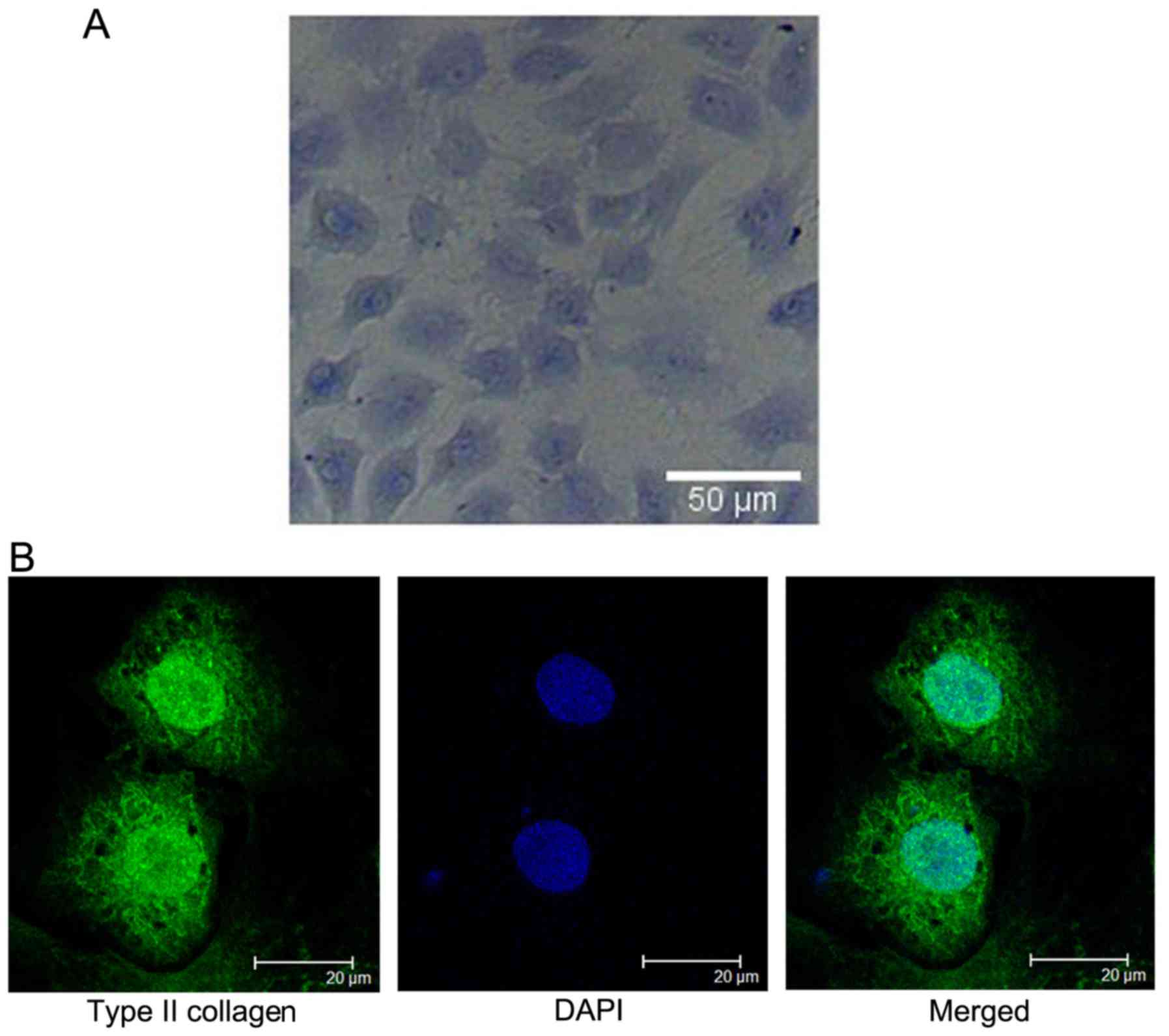
chondrocytes synthesized and secreted proteoglycan (Fig. 1A).
An evaluation of collagen type II expression in rat
articular chondrocytes was performed in vitro. The results
suggest that type II collagen was secreted by chondrocytes.
Chondrocyte nuclei were stained blue with DAPI. The cultured
chondrocytes maintained the characteristic of a phenotype
expressing type II collagen (Fig.
1B).
Effects of IGF-1 on SA-β-gal
activity
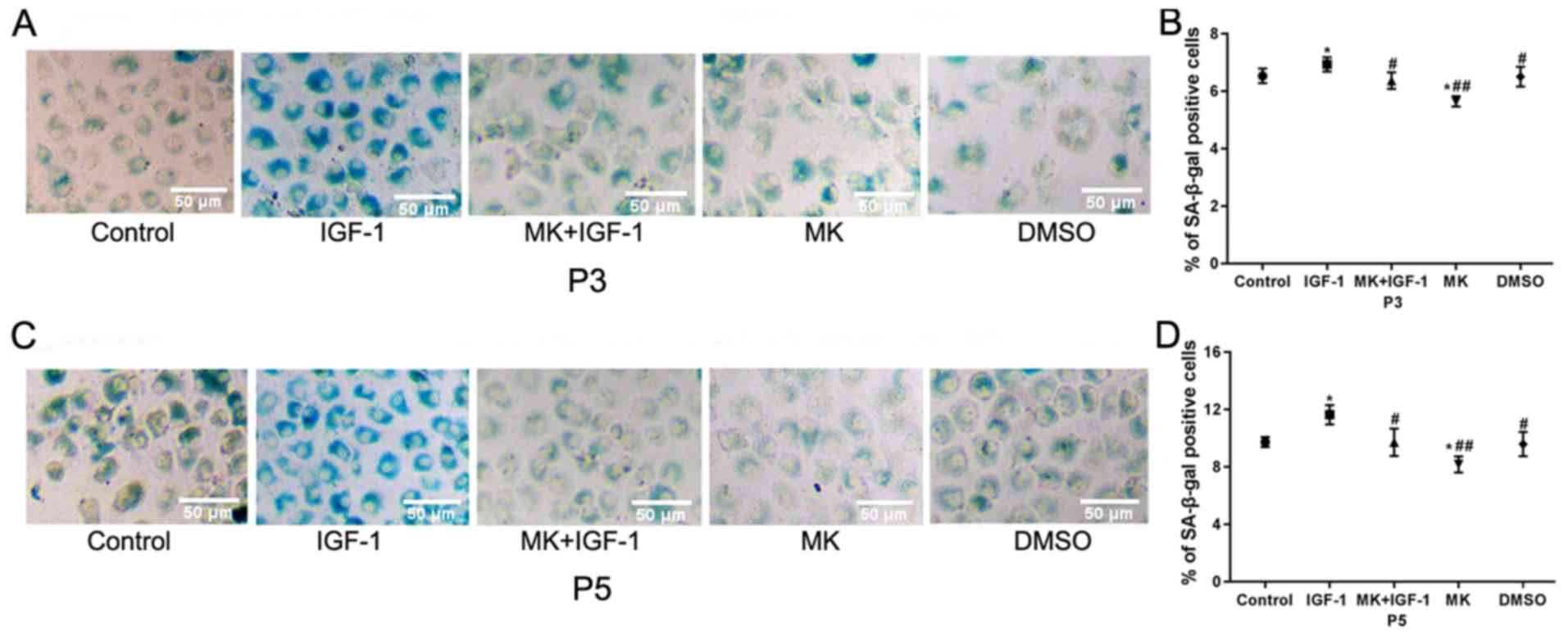
Cellular senescence was confirmed using SA-β-gal
staining, a biomarker of cellular senescence (3). The staining results revealed a
blue-stained cytoplasm (Fig. 2A and
C). The SA-β-gal-positive cells in
the IGF-1 group which was significantly higher compared with the
control group (6.94±0.25% vs. 6.54±0.26%; P<0.05; Fig. 2B). When cells were pretreated with
MK for 90 min prior to IGF-1 treatment, cells were
SA-β-gal-positive and this was a significant reduction compared
with the IGF-1 group (6.17±0.29% vs. 6.94±0.25%; P<0.05). No
significant differences were observed in the DMSO group compared
with the control group (6.51±0.45% vs. 6.54±0.26%; P>0.05).
Similar trends were also observed in the fifth passage chondrocytes
(Fig. 2D).
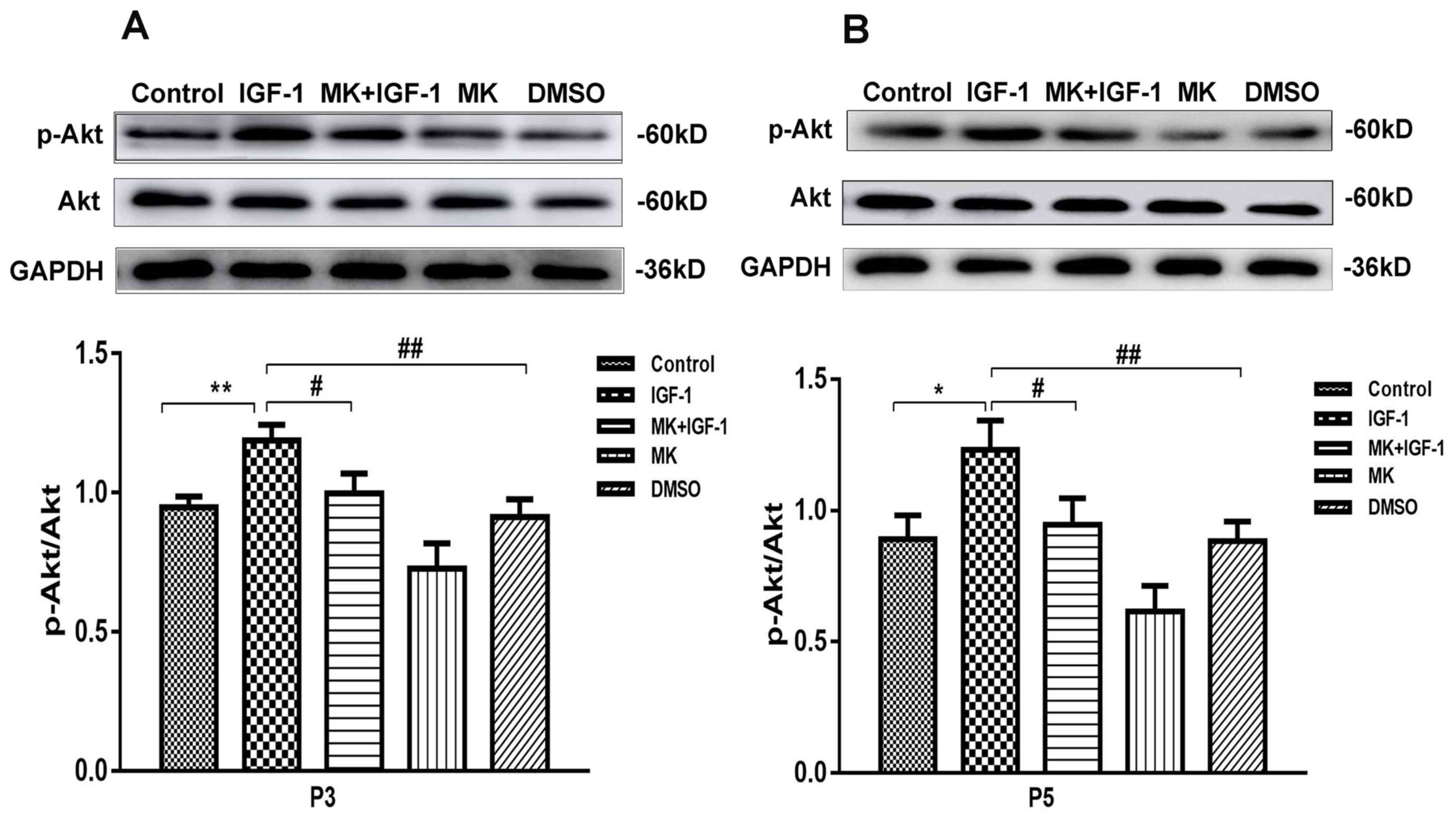
IGF-1 effects on the Akt signaling
pathway
In order to elucidate the role of IGF-1 in the
regulation of cellular senescence in rat articular chondrocytes,
the effect of IGF-1 on cellular senescence in the third and fifth
passages was assessed. The involvement of the Akt signaling pathway
in the induction of cellular senescence by IGF-1 was also assessed.
As presented in Fig. 3, in passages
three and five, Akt was phosphorylated in chondrocytes with
senescence induced upon IGF-1 stimulation compared with the control
and DMSO groups. Treatment with MK, an Akt inhibitor, significantly
decreased the IGF-1-induced activation of Akt and led to a decrease
in phosphorylated Akt levels.
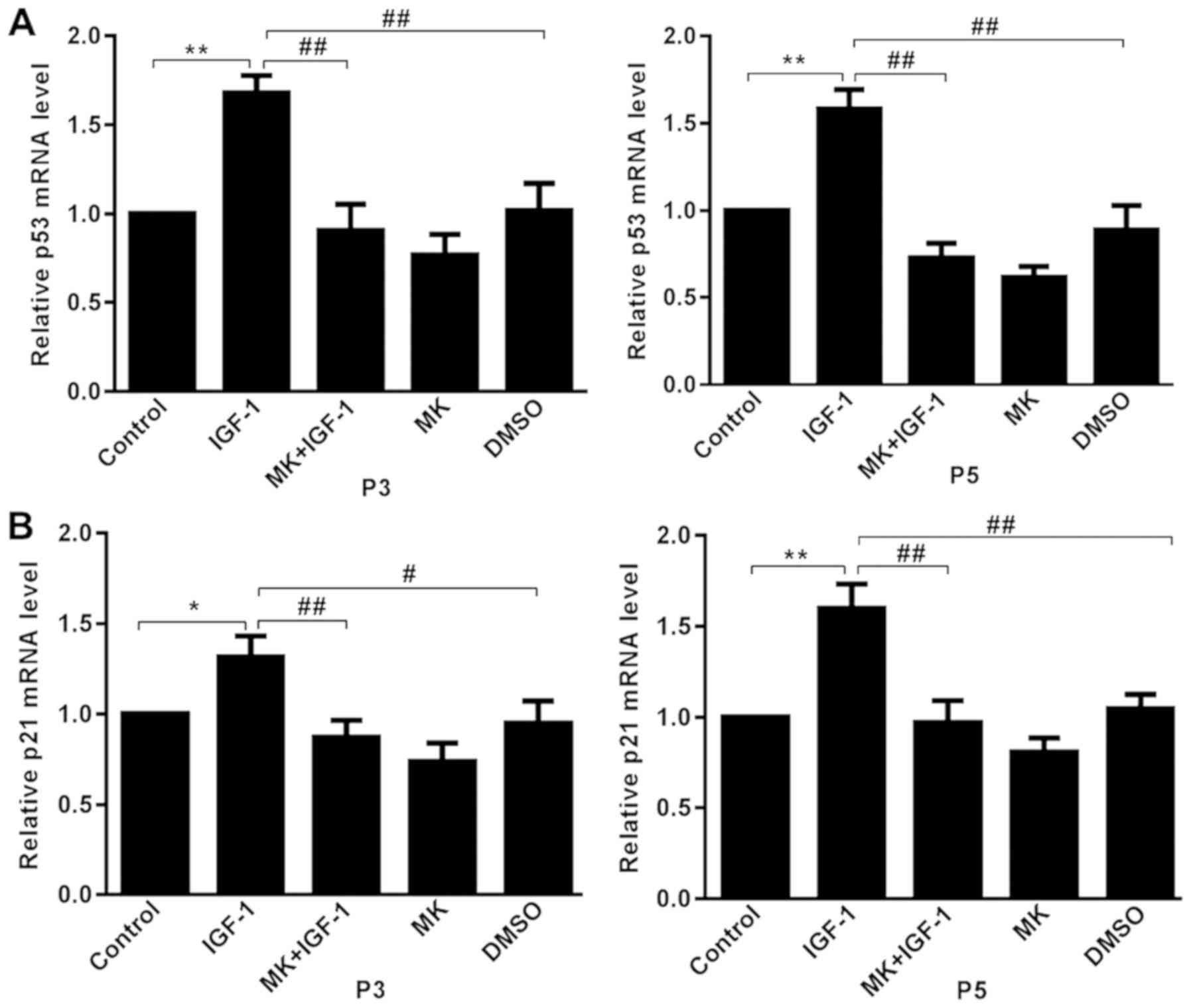
Effects of IGF-1 on p53 and p21
expression
The expression aging markers p53 and p21 were
detected to further confirm the IGF-1-induced cellular senescence
via the Akt signaling pathway (30). Relative levels of p53 and p21 mRNA
were determined using RT-qPCR. Treatment with 100 ng/ml IGF-1
induced an increase in p53 mRNA in rat articular chondrocytes when
compared with the control (15),
and this effect was significantly decreased by pretreatment with MK
(Fig. 4A). Subsequently, mRNA
expression of p21 was assessed, a target gene of p53(31). The mRNA expression of p21 was
significantly higher in the IGF-1 group compared with the control
group and was significantly decreased by pretreatment with MK
(Fig. 4B). Chondrocytes at passages
third and fifth exhibited similar trends.
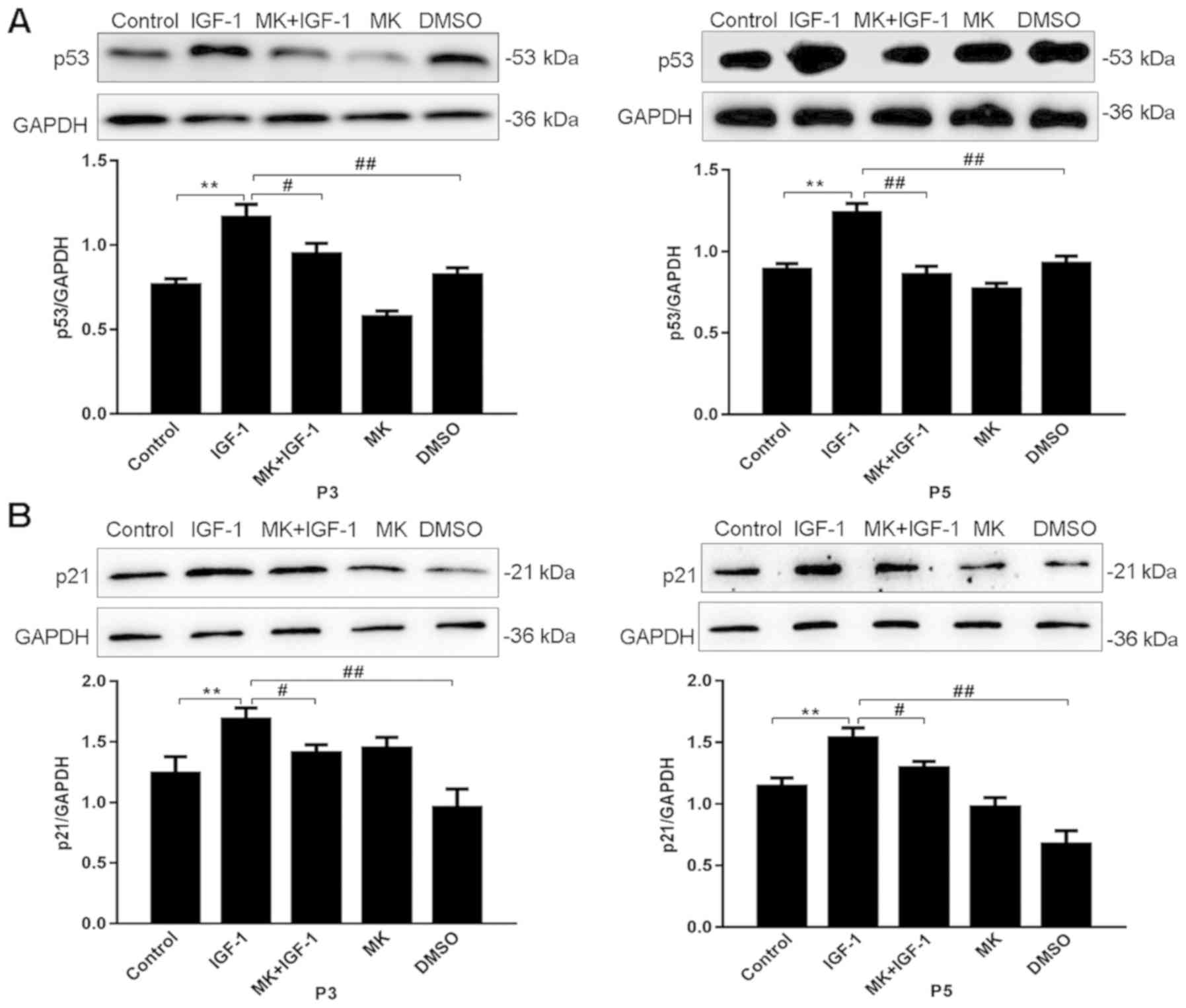
p53 and p21 protein expression was subsequently
detected using western blot analysis (Fig. 5A and B). In passages three and five, when
compared with the control group, p53 and p21 protein expression was
significantly increased in the presence of IGF-1 (P<0.01).
Following incubation with MK+IGF-1, p53 and p21 protein expression
levels were significantly reduced compared with the IGF-1 group
(P<0.05).
Discussion
Several studies assessing cellular senescence have
previously demonstrated that IGF-1 induced cellular senescence in
multiple cell types (21,22). The results of the present study
demonstrated a similar process occurring in rat articular
chondrocytes. The results revealed that IGF-1 promoted cellular
senescence, increased SA-β-gal staining and increased
senescence-associated p53 and p21 expression. SA-β-gal activity is
a typical biomarker of cellular senescence. SA-β-gal has been
revealed to be expressed in senescent cells, but not in
pre-senescent or quiescent fibroblasts and not in early passage
terminally differentiated keratinocytes (3). A variety of other cell types have also
been reported to express SA-β-gal. For example, in a previous
study, human prostatic epithelial cells exhibited extensive
expression of SA-β-gal during prolonged culture (32). The activity of SA-β-gal has also
been revealed to increase in senescent human endothelial cells
(33).
A previous study demonstrated that chondrocyte
senescence serves an essential role in the cartilage degeneration
that occurs with aging and also increases with subculture-induced
dedifferentiation of chondrocytes (34). A previous study reported that
chondrocyte viability was high in early passages and reduced in
later passages. Chondrocytes appeared fibroblast-like after the
seventh passage (35). As the
passage number increased, cell growth rate and viability decreased.
The percentage of SA-β-gal positive cells was 26 and 52% in third
and fifth passage chondrocytes, respectively (35). Although the proliferative activity
of chondrocytes within early passages is high, cells in late
passages no longer exhibit high proliferative activity (36). Therefore, third and fifth passage
cells were used in the present study. To synchronize cells and
avoid serum interference with drugs, cells were serum-starved for
12 h in DMEM containing 0.05% FBS. IGF-1 was used to induce cells
in serum-free or low-concentration serum DMEM, a method which has
been previously revealed to exhibit no effect on cell growth
(15,21,22,37).
In the current study, the results of SA-β-gal staining revealed an
upregulation of positive cells upon IGF-1 treatment in the third
and fifth passage chondrocytes. The number of positive cells in the
fifth generation chondrocytes was higher compared with the third
generation. These results indicated that senescent cells increased
over time. The results are in agreement with those reported by
Handayaningsih et al (21)
who demonstrated that IGF-1 significantly enhanced cell
senescence.
In the present study, other aging markers were
additionally assessed, including p53 and p21 expression. Tumor
suppressor protein p53 is one of the major biochemical mediators of
senescence in human cells which induces p21 expression (30,31).
P53 and p21 are directly associated with cellular senescence and
have been used to identify senescent cells (38). p53 is associated with lifespan
regulation and is a critical meditor of the senescence response to
a variety of stimuli (39,40). It has been previously reported that
p53 is activated and accumulates in senescent fibroblasts (39). A recent study has demonstrated that
senescence-associated p53 level increased in aged mouse
cardiomyocytes (40). p21 is a
downstream target of p53 and is directly regulated by p53 (38,41).
p21 expression has been revealed to increase in human fibroblasts
upon replicative senescence (42,43).
In the present study, p53 and p21 mRNA expression was significantly
higher in the IGF-1 group compared with control cells. Furthermore,
it has been described that IGF-1 treatment performed on IMR90 or
MEF cells led to the appearance of cells with premature cellular
senescence characteristics, including the upregulation of p53 and
p21 proteins (22). These data are
consistent with the results obtained in the present study
demonstrating that p53 and p21 protein expression was enhanced upon
IGF-1 treatment. It may be concluded that IGF-1 induced cellular
senescence in rat articular chondrocytes.
The associated signaling pathways were assessed to
determine the mechanisms underlying cellular senescence induced by
IGF-1. Two main pathways have been previously revealed to be
associated with IGF-1 activity, including the PI3K/Akt and MAPK/ERK
pathways (23,24). Various studies have suggested that
senescence was mediated by activating the Akt signaling pathway
(44), and IGF-1 stimulation lead
to the activation of the PI3K/Akt pathway (45). Therefore, the current study assessed
the ability of IGF-1 to activate Akt and aimed to determine whether
the Akt pathway was associated with rat articular chondrocyte
senescence. The Akt phosphorylation in the IGF-1 group which was
significantly higher compared with the control group, which
demonstrated that Akt was activated by IGF-1. Additionally, the
chemical inhibition of Akt by MK led to the significant inhibition
of Akt phosphorylation induced by IGF-1 activation. Overall, the
results of the present study indicated that the inhibition of the
Akt signaling pathway reversed IGF-1 induction. Further experiments
should aim to clarify the involvement of other signaling pathways
in rat articular chondrocyte senescence.
In conclusion, the results of the present study
demonstrated that IGF-1 induced senescence in rat articular
chondrocytes and activated the PI3K/Akt pathway.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National Key
R&D Program of China (grant no. 2017YFD0502202).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ, LB and JX conceived and designed the study. LH,
ZZ, LC XM and NM were responsible for the collection and analysis
of the data. LZ, SZ and JX interpreted the data and drafted the
manuscript. LZ and JX revised the manuscript critically for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The current study was approved by The Ethical
Committee for Animal Experiments (Northeast Agricultural
University, Harbin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Childs BG, Durik M, Baker DJ and van
Deursen JM: Cellular senescence in aging and age-related disease:
From mechanisms to therapy. Nat Med. 21:1424–1435. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Muñoz-Espín D and Serrano M: Cellular
senescence: From physiology to pathology. Nat Rev Mol Cell Biol.
15:482–496. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Dimri GP, Lee X, Basile G, Acosta M, Scott
G, Roskelley C, Medrano EE, Linskens M, Rubelj I and Pereira-Smith
O: A biomarker that identifies senescent human cells in culture and
in aging skin in vivo. Proc Natl Acad Sci USA. 92:9363–9367.
1995.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tchkonia T, Zhu Y, van Deursen J, Campisi
J and Kirkland JL: Cellular senescence and the senescent secretory
phenotype: Therapeutic opportunities. J Clin Invest. 123:966–972.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Hall BM, Balan V, Gleiberman AS, Strom E,
Krasnov P, Virtuoso LP, Rydkina E, Vujcic S, Balan K, Gitlin I, et
al: Aging of mice is associated with p16(Ink4a)- and
β-galactosidase-positive macrophage accumulation that can be
induced in young mice by senescent cells. Aging (Albany NY).
8:1294–1315. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kenyon C: The first long-lived mutants:
Discovery of the insulin/IGF-1 pathway for ageing. Philos Trans R
Soc Lond B Biol Sci. 366:9–16. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lee C, Wan J, Miyazaki B, Fang Y,
Guevara-Aguirre J, Yen K, Longo V, Bartke A and Cohen P: IGF-I
regulates the age-dependent signaling peptide humanin. Aging Cell.
13:958–961. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Salmon WD Jr and Daughaday WH: A
hormonally controlled serum factor which stimulates sulfate
incorporation by cartilage in vitro. 1956. J Lab Clin Med.
116:408–419. 1990.PubMed/NCBI
|
|
9
|
Rinderknecht E and Humbel RE: The amino
acid sequence of human insulin-like growth factor I and its
structural homology with proinsulin. J Biol Chem. 253:2769–2776.
1978.PubMed/NCBI
|
|
10
|
Daughaday WH, Hall K, Raben MS, Salmon WD
Jr, van den Brande JL and van Wyk JJ: Somatomedin: Proposed
designation for sulphation factor. Nature. 235(107)1972.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Powell-Braxton L, Hollingshead P,
Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N and Stewart
TA: IGF-I is required for normal embryonic growth in mice. Genes
Dev. 7:2609–2617. 1993.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Costales J and Kolevzon A: The therapeutic
potential of insulin-like growth factor-1 in central nervous system
disorders. Neurosci Biobehav Rev. 63:207–122. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Castilla-Cortazar I, Guerra L, Puche JE,
Muñoz U, Barhoum R, Escudero E and Lavandera JL: An experimental
model of partial insulin-like growth factor-1 deficiency in mice. J
Physiol Biochem. 70:129–139. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
De Ita JR, Castilla-Cortázar I, Aguirre
GA, Sánchez-Yago C, Santos-Ruiz MO, Guerra-Menéndez L, Martín-Estal
I, García-Magariño M, Lara-Díaz VJ, Puche JE and Muñoz U: Altered
liver expression of genes involved in lipid and glucose metabolism
in mice with partial IGF-1 deficiency: An experimental approach to
metabolic syndrome. J Transl Med. 13(326)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nishizawa H, Iguchi G, Fukuoka H,
Takahashi M, Suda K, Bando H, Matsumoto R, Yoshida K, Odake Y,
Ogawa W and Takahashi Y: IGF-I induces senescence of hepatic
stellate cells and limits fibrosis in a p53-dependent manner. Sci
Rep. 6(34605)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xu J, Gontier G, Chaker Z, Lacube P,
Dupont J and Holzenberger M: Longevity effect of IGF-1R(+/-)
mutation depends on genetic background-specific receptor
activation. Aging Cell. 13:19–28. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Di Bona D, Accardi G, Virruso C, Candore G
and Caruso C: Association of Klotho polymorphisms with healthy
aging: A systematic review and meta-analysis. Rejuvenation Res.
17:212–216. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lotz M and Loeser RF: Effects of aging on
articular cartilage homeostasis. Bone. 51:241–248. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Price JS, Waters JG, Darrah C, Pennington
C, Edwards DR, Donell ST and Clark IM: The role of chondrocyte
senescence in osteoarthritis. Aging Cell. 1:57–65. 2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
McCulloch K, Litherland GJ and Rai TS:
Cellular senescence in osteoarthritis pathology. Aging Cell.
16:210–218. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Handayaningsih AE, Takahashi M, Fukuoka H,
Iguchi G, Nishizawa H, Yamamoto M, Suda K and Takahashi Y: IGF-I
enhances cellular senescence via the reactive oxygen species-p53
pathway. Biochem Biophys Res Commun. 425:478–484. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tran D, Bergholz J, Zhang HB, He HB, Wang
Y, Zhang YJ, Li QT, Kirkland JL and Xiao ZX: Insulin-like growth
factor-1 regulates the SIRT1-p53 pathway in cellular senescence.
Aging Cell. 13:669–678. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang M, Zhou Q, Liang QQ, Li CG, Holz JD,
Tang D, Sheu TJ, Li TF, Shi Q and Wang YJ: IGF-1 regulation of type
II collagen and MMP-13 expression in rat endplate chondrocytes via
distinct signaling pathways. Osteoarthritis Cartilage. 17:100–106.
2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ock S, Lee WS, Ahn J, Kim HM, Kang H, Kim
HS, Jo D, Abel ED, Lee TJ and Kim J: Deletion of IGF-1 receptors in
cardiomyocytes attenuates cardiac aging in male mice.
Endocrinology. 157:336–345. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Starkman BG, Cravero JD, Delcarlo M and
Loeser RF: IGF-I stimulation of proteoglycan synthesis by
chondrocytes requires activation of the PI 3-kinase pathway but not
ERK MAPK. Biochem J. 389:723–729. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kulik G and Weber MJ: Akt-dependent and
-independent survival signaling pathways utilized by insulin-like
growth factor I. Mol Cell Biol. 18:6711–6718. 1998.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Panganiban RAM and Day RM: Inhibition of
IGF-1R prevents ionizing radiation-induced primary endothelial cell
senescence. PLoS One. 8(e78589)2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xu XX, Zhang XH, Diao Y and Huang YX:
Achyranthes bidentate saponins protect rat articular chondrocytes
against interleukin-1β induced inflammation and apoptosis in vitro.
Kaohsiung J Med Sci. 33:62–68. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jiang CS, Liu G, Luckhardt T, Antony V,
Zhou Y, Carter AB, Thannickal VJ and Liu RM: Serpine 1 induces
alveolar type II cell senescence through activating p53-p21-Rb
pathway in fibrotic lung disease. Aging Cell. 16:1114–1124.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
el-Deiry WS, Tokino T, Velculescu VE, Levy
DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW and
Vogelstein B: WAF1, a potential mediator of p53 tumor suppression.
Cell. 75:817–825. 1993.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Choi J, Shendrik I, Peacocke M, Peehl D,
Buttyan R, Ikeguchi EF, Katz AE and Benson MC: Expression of
senescence-associated beta-galactosidase in enlarged prostates from
men with benign prostatic hyperplasia. Urology. 56:160–166.
2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kurz DJ, Decary S, Hong Y and Erusalimsky
JD: Senescence-associated (beta)-galactosidase reflects an increase
in lysosomal mass during replicative ageing of human endothelial
cells. J Cell Sci. 113:3613–3622. 2000.PubMed/NCBI
|
|
34
|
Loeser RF: Aging and osteoarthritis: The
role of chondrocyte senescence and aging changes in the cartilage
matrix. Osteoarthritis Cartilage. 17:971–979. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Liu ZM, Shen PC, Lu CC, Chou SH and Tien
YC: Characterization of the proliferating layer chondrocytes of
growth plate for cartilage regeneration. Tissue Eng Part A.
25:364–378. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kang SW, Yoo SP and Kim BS: Effect of
chondrocyte passage number on histological aspects of
tissue-engineered cartilage. Biomed Mater Eng. 5:269–276.
2007.PubMed/NCBI
|
|
37
|
Handayaningsih AE, Iguchi G, Fukuoka H,
Nishizawa H, Takahashi M, Yamamoto M, Herningtyas EH, Okimura Y,
Kaji H, Chihara K, et al: Reactive oxygen species play an essential
role in IGF-I signaling and IGF-I-induced myocyte hypertrophy in
C2C12 myocytes. Endocrinology. 152:912–921. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Itahana K, Dimri G and Campisi J:
Regulation of cellular senescence by p53. Eur J Biochem.
268:2784–2791. 2001.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Atadja P, Wong H, Garkavtsev I, Veillette
C and Riabowol K: Increased activity of p53 in senescing
fibroblasts. Proc Natl Acad Sci USA. 92:8348–8352. 1995.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang Z, Rong X, Luo B, Qin S, Lu L, Zhang
X, Sun Y, Hu Q and Zhang C: A natural model of mouse cardiac
myocyte senescence. J Cardiovasc Transl Res. 9:456–458.
2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jackson JG and Pereira-Smith OM: p53 is
preferentially recruited to the promoters of growth arrest genes
p21 and GADD45 during replicative senescence of normal human
fibroblasts. Cancer Res. 66:8356–8360. 2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Herbig U, Jobling WA, Chen BP, Chen DJ and
Sedivy JM: Telomere shortening triggers senescence of human cells
through a pathway involving ATM, p53, and p21(CIP1), but not
p16(INK4a). Mol Cell. 14:501–513. 2004.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Dimri GP, Testori A, Acosta M and Campisi
J: Replicative senescence, aging and growth-regulatory
transcription factors. Biol Signals. 5:154–162. 1996.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chen ZB, Trotman LC, Shaffer D, Lin HK,
Dotan Z, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al:
Crucial role of p53-dependent cellular senescence in suppression of
Pten-deficient tumorigenesis. Nature. 436:725–730. 2005.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Blume-Jensen P and Hunter T: Oncogenic
kinase signalling. Nature. 411:355–365. 2001.PubMed/NCBI View Article : Google Scholar
|