Introduction
Lung ischemia-reperfusion injury (LIRI) is an
intricate pathological process that occurs during numerous clinical
conditions, including lung transplantation, pulmonary embolism,
resuscitation for circulatory arrest and cardiopulmonary bypass
cardiac surgery (1,2). Lung transplantation, which induces the
most severe form of LIRI, causes primary graft failure, leading to
short- and long-term morbidity and mortality (3); therefore, novel therapeutic strategies
are required to improve the clinical outcomes of patients with
LIRI.
Ischemia-reperfusion (IR) induces inflammation and
injury by rapidly activating the innate immune system (4). The mechanisms underlying LIRI involve
the release of inflammatory cytokines and an increase in their
expression levels, which results in cell damage, necrosis and
apoptosis in the lungs (5,6).
In 1988, Warltier et al (7) reported that pretreatment of cells with
isoflurane improved left ventricular systolic function following
occlusion of the left anterior descending coronary artery for 15
min. To date, the protective functions of isoflurane against IR
injury (IRI) have been confirmed by numerous studies. For example,
Kehl et al (8) reported that
low concentration isoflurane was sufficient to precondition
myocardial tissue against infarction. In addition, Lv et al
(9) indicated that pretreatment of
rats with isoflurane ameliorated IR combined with
lipopolysaccharide (LPS)-induced liver injury. Liang et al
(10) reported that isoflurane
pretreatment of rats also attenuated renal IRI by reducing
inflammation and apoptosis. Furthermore, it has been reported that
emulsified isoflurane pretreatment of rats ameliorated hepatic
IR-induced lung injury (11).
However, whether isoflurane attenuates LIRI via an
anti-inflammatory mechanism and the inhibition of apoptosis is not
completely understood; therefore, the present study aimed to
investigate the mechanisms underlying the effects of isoflurane
during LIRI.
Materials and methods
Cell culture and hypoxia-reoxygenation
(HR) model
A549 cells are derived from a human alveolar cell
carcinoma and are the most widely used in vitro model of
type 2 pulmonary alveolar epithelial cells, possessing multiple
properties of these cells (12).
A549 cells (American Type Culture Collection) were cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 4.5 g/l
glucose (Gibco; Thermo Fisher Scientific, Inc.), 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.) in
a humidified incubator with 5% CO2 at 37˚C.
A549 cells were pretreated with isoflurane (1.4%
v/v) prior to HR induction according to a previously method
(13). Briefly, cells were placed
in a sealed acrylic chamber with a circular opening in which a
rubber cannula was inserted and a mixture of 95% air and 5%
CO2 was delivered. Subsequently, isoflurane was
delivered into the chamber using a vaporizer for 60 min. Untreated
cells served as a control group. Capnography was conducted using
the Networked Multiparameter Veterinary Monitor LifeWindow 6000V
(Digicare Animal Health).
An in vitro model of LIRI was established by
HR induction. A549 cells were placed in a hypoxic chamber (0%
O2) and incubated with 95% N2 and 5%
CO2 for 25 min at room temperature. Subsequently, cells
in the hypoxic chamber (95% N2 and 5% CO2)
were incubated in a 37˚C incubator for 3 h to establish hypoxia.
A549 cells were transferred to a normoxic incubator with 5%
CO2 at 37˚C for 1 h to induce reoxygenation. Following
incubation under hypoxic conditions for 3 h, the partial percentage
of O2 in the culture media was 5% compared with 21% in
the normoxic culture media.
Cell proliferation assay
Cells (3x104 cells/well) were seeded into
96-well plates and cultured at 37˚C with 5% CO2. Cell
proliferation was determined using the Cell Counting Kit-8 (CCK-8;
Dojindo Molecular Technologies, Inc.) assay. CCK-8 solution (10
µl) was added to each well and incubated at 37˚C for 3 h.
The absorbance of each well was measured at a wavelength of 450 nm
using a spectrophotometer.
Cell apoptosis assay
Cells (1x106 cells/well) were seeded into
6-well plates and cultured at 37˚C with 5% CO2. Cells
were stained at room temperature for 10 min in the dark using the
Annexin V-FITC/PI Apoptosis Detection kit (Sigma-Aldrich; Merck
KGaA) according to the manufacturer's protocol. Early and late
apoptotic cells were analyzed using a BD FACSCalibur flow cytometer
(BD Biosciences) and CellQuest software (version 3.3; BD
Biosciences).
Detection of interleukin (IL)-8, IL-6,
superoxide dismutase (SOD) and malondialdehyde (MDA) content
IL-8 (cat. no. S8000C) and IL-6 (cat. no. S6050)
ELISA kits (R&D Systems, Inc.) were used to measure IL-8 and
IL-6 protein concentrations, according to the manufacturer's
protocols. The induction of oxidative stress was evaluated using
SOD (cat. no. A001-3) and MDA (cat. no. A003-1) assay kits (Nanjing
Jiancheng Bioengineering Institute) according to the manufacturer's
protocol.
Detection of NF-κB activity
A549 cells (1x105) were seeded into
24-well plates and transfected with the pBIIx-luc NF-κB-dependent
luciferase reporter construct (0.4 mg; GenScript Biotech
Corporation) and the Renilla luciferase vector (Promega
Corporation) using Lipofectamine® 3000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Following incubation
for 24 h at 37˚C, cells were pretreated with isoflurane and HR was
induced according to the aforementioned protocol. Subsequently,
luciferase activities were detected using a Dual-Luciferase
Reporter assay system (Promega Corporation) according to the
manufacturer's protocol. Firefly luciferase activity was normalized
to Renilla luciferase activity.
Western blotting
Western blotting was performed to measure the
protein expression levels of NF-κB phosphorylated (p)-p65, NF-κB
p65, Bax, Bcl-2 and proliferating cell nuclear antigen (PCNA).
Total protein was extracted from A549 cells using cold RIPA buffer
(Roche Diagnostics) and total protein was quantified using the
Bicinchoninic Acid Protein Assay kit (Applygen Technologies, Inc.).
Proteins (15 µg/lane) were separated via SDS-PAGE on 10%
gels and transferred onto nitrocellulose membranes. The membranes
were incubated overnight at 4˚C with primary antibodies which were
all purchased from Cell Signaling Technology, Inc. targeted
against: Bax (cat. no. 2772; 1:1,000) and Bcl-2 (cat. no. 3498;
1:1,000), PCNA (cat. no. 13110; 1:1,000), NF-κB p-p65 (cat. no.
3033; 1:1,000), NF-κB p65 (cat. no. 8242; 1:1,000) and GAPDH (cat.
no. 5174; 1:1,000). Following primary antibody incubation, the
membranes were blocked in 5% non-fat milk at room temperature for 1
h and incubated with anti-rabbit IgG secondary antibody (cat. no.
7074, 1:10,000; Cell Signaling Technology, Inc.) at room
temperature for 2 h. Protein bands were visualized by enhanced
chemiluminescence (Thermo Fisher Scientific, Inc.) using the
Odyssey Infrared Imaging system (LI-COR Biosciences). Densitometry
was quantified by ImageJ software (version. 1.8.0; National
institutes of Health). GAPDH was used as the loading control.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from A549 cells using the
Easystep Universal RNA Extraction kit (Promega Corporation) and RNA
concentration was quantified using a NanoDrop spectrophotometer
(NanoDrop; Thermo Fisher Scientific, Inc.). Total RNA was reverse
transcribed into cDNA at 37˚C for 10 min followed by incubation at
85˚C for 5 sec using the M-MLV reverse transcriptase [50 mM
Tris-HCl (pH 8.3), 40 mM KCl, 6 mM MgCl2, 1 mM DTT, 0.5
mM [3H]dTTP, 0.1 mM poly(A), 0.1 mM
oligo(dT)12-18, 0.1 mg/ml BSA and reverse transcriptase,
Thermo Fisher Scientific, Inc.]. Subsequently, qPCR was performed
using SYBRGreen Master Mix (Promega Corporation) and the Real-Time
PCR system (Bio-Rad Laboratories, Inc.). The primer sequences were
as listed: PCNA forward, 5'-GCGTGAACCTCACCAGTATGT-3' and reverse,
5'-TCTTCGGCCCTTAGTGTAATGAT-3'; BAX forward,
5'-CACCAGCTCTGAACAGATCATGA-3' and reverse,
5'-TCAGCCCATCTTCTTCCAGATGT-3'; Bcl-2 forward,
5'-CACCCCTGGCATCTTCTCCTT-3' and reverse,
5'-AGCGTCTTCAGAGACAGCCAG-3'; IL-6 forward,
5'-AGCCACTCACCTCTTCAGAACGAA-3' and reverse,
5'-TACTCATCTGCACAGCTCTGGCTT-3'; IL-8 forward,
5'-ATGACTTCCAAGCTGGCCGTGGCT-3' and reverse,
5'-TCTCAGCCCTCTTCAAAAACTTCT-3'; RELA forward,
5'-CCCACGAGCTTGTAGGAAAGG-3' and reverse,
5'-GGATTCCCAGGTTCTGGAAAC-3'; IκBa forward,
5'-ACCTGGTGTCACTCCTGTTGA-3' and reverse, 5'-CTGCTGCTGTATCCGGGTG-3';
IκB1, forward, 5'-GATATCGCCCTGATCTTGCT-3' and reverse,
5'-AGGTTGGCTCCTGACATCAC-5'; and GAPDH forward,
5'-TTGGTATCGTGGAAGGAC-3' and reverse, 5'-TGTCATCATATTTGGCAGGTT-3'.
The thermocycling conditions were as listed: 94˚C for 30 sec;
followed by 40 cycles of 94˚C for 5 sec, 60˚C for 15 sec and 72˚C
for 10 sec). mRNA expression levels were quantified using the
2-ΔΔCq method (14) and
normalized to the internal reference gene GAPDH.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analyses were performed using SPSS software (version
14.0; SPSS, Inc.). Comparisons among groups were analyzed by
one-way ANOVA followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Isoflurane pretreatment reverses
HR-induced reductions in cell proliferation
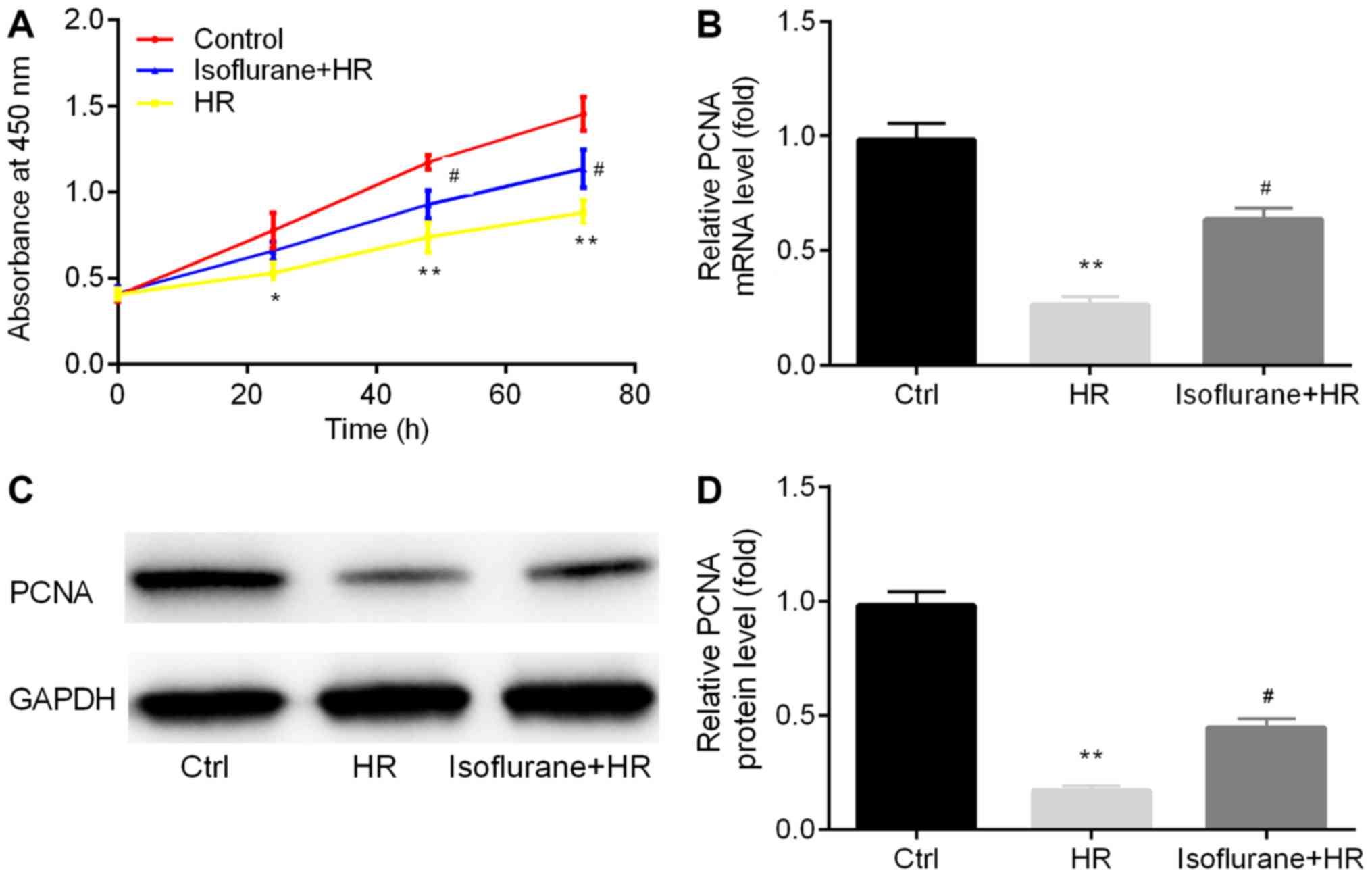
The effects of isoflurane on cell proliferation were
assessed. Cell proliferation in the HR group was significantly
decreased compared with the control group, whereas pretreatment
with isoflurane reversed the effects of HR on cell proliferation
(Fig. 1A).
In addition, the expression levels of the cell
proliferation-associated molecule, PCNA, were assessed in
the different groups by RT-qPCR and western blotting. The mRNA
expression levels of PCNA were significantly decreased in
the HR group compared with the control group (Fig. 1B). Conversely, pretreatment with
isoflurane increased PCNA mRNA expression in the HR group
(Fig. 1B). PCNA protein levels
displayed a similar pattern to PCNA mRNA levels (Fig. 1C and D).
Isoflurane pretreatment inhibits
HR-induced cell apoptosis
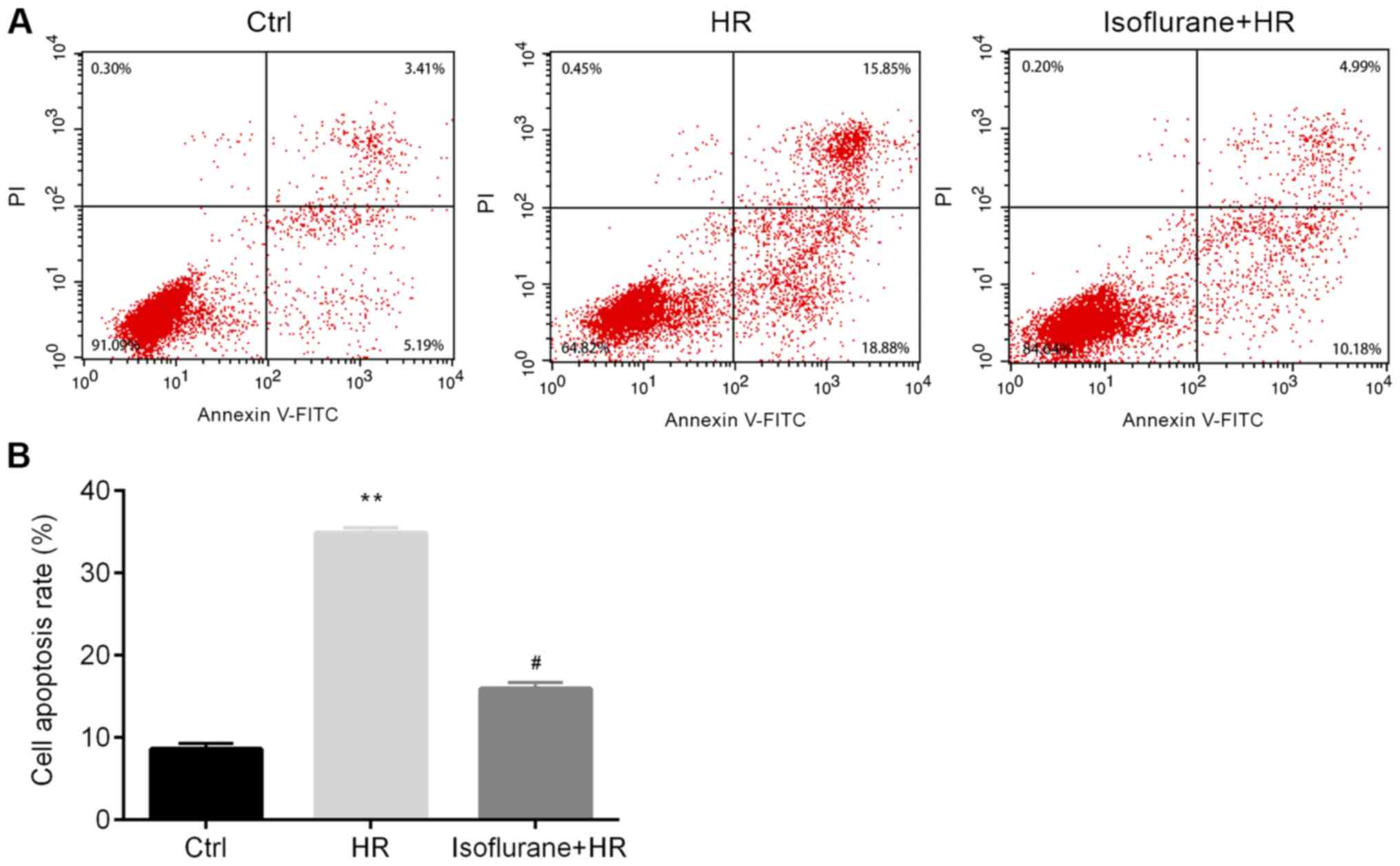
Subsequently, the effects of isoflurane on cell
apoptosis were investigated. The rate of apoptosis was
significantly increased in the HR group compared with the control
group, and pretreatment with isoflurane significantly reversed
HR-induced cell apoptosis (Fig.
2).
Isoflurane pretreatment reverses
HR-induced increases in the Bax/Bcl-2 ratio
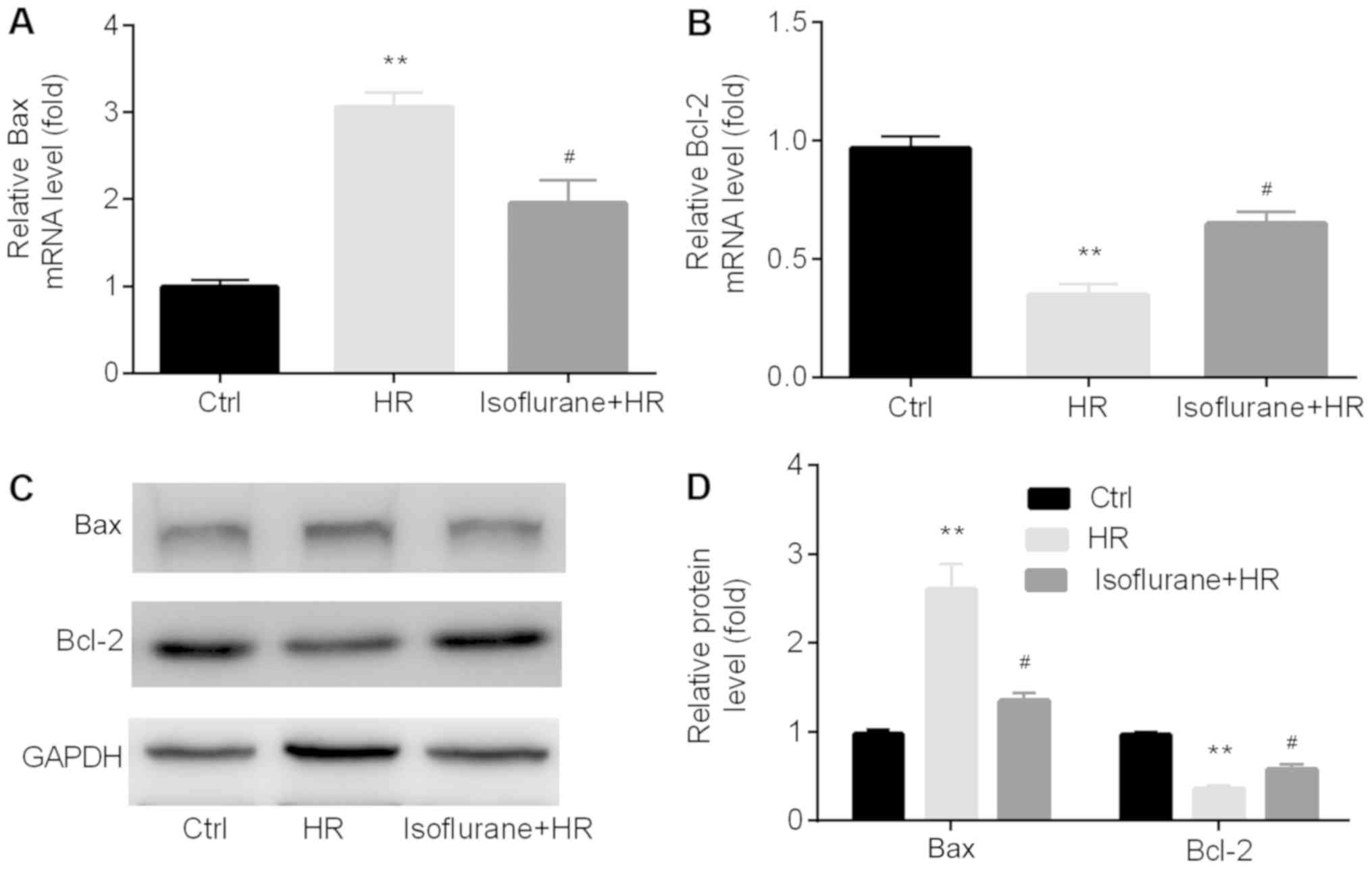
Furthermore, the expression levels of
apoptosis-related proteins Bax and Bcl-2 were examined in the
different groups by RT-qPCR and western blotting. The mRNA
expression levels of Bax were significantly higher in the HR
group compared with the control group, whereas the opposite effect
was observed for Bcl-2. Pretreatment with isoflurane significantly
reversed the HR-induced effects on apoptosis-related genes
(Fig. 3A and B). The western blotting results indicated
that the protein expression levels of Bax and Bcl-2 displayed a
similar trend to the mRNA expression levels (Fig. 3C and D).
Isoflurane pretreatment reverses
HR-induced increases in MDA concentration and decreases in SOD
activity
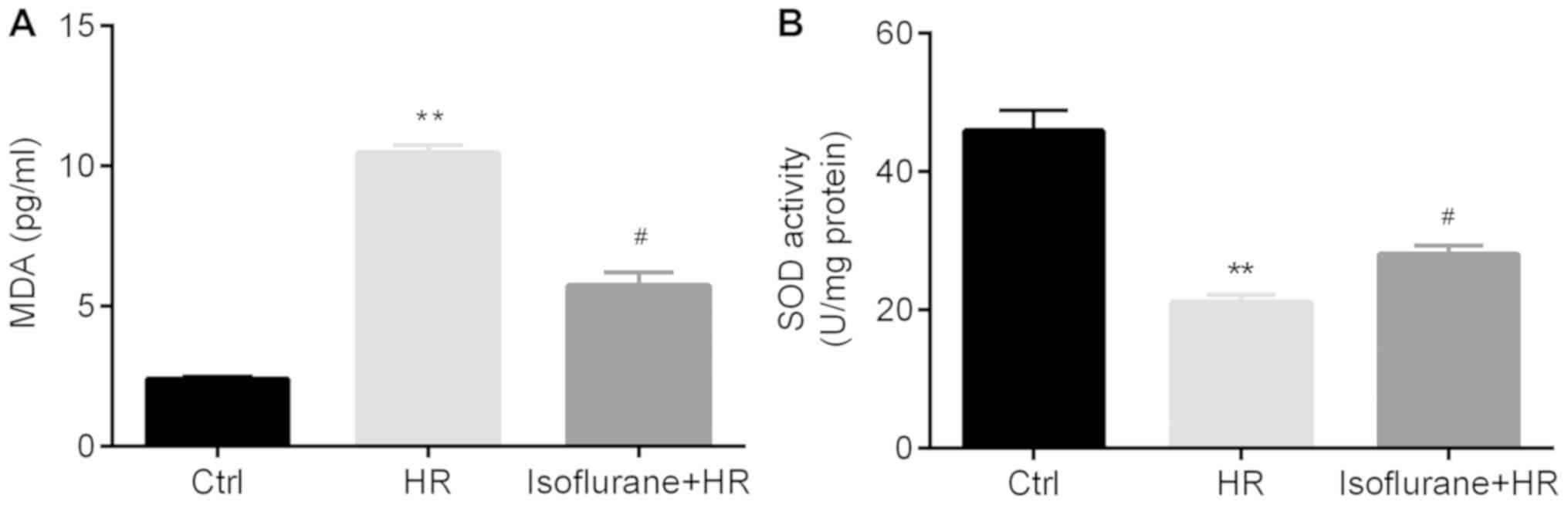
Subsequently, the effects of isoflurane on reactive
oxygen species (ROS)-associated markers, including SOD activity and
MDA levels, were investigated. Significantly higher MDA
concentrations and lower SOD activity levels were observed in the
HR group compared with the control group; however, pretreatment
with isoflurane significantly reversed these effects (Fig. 4A and B).
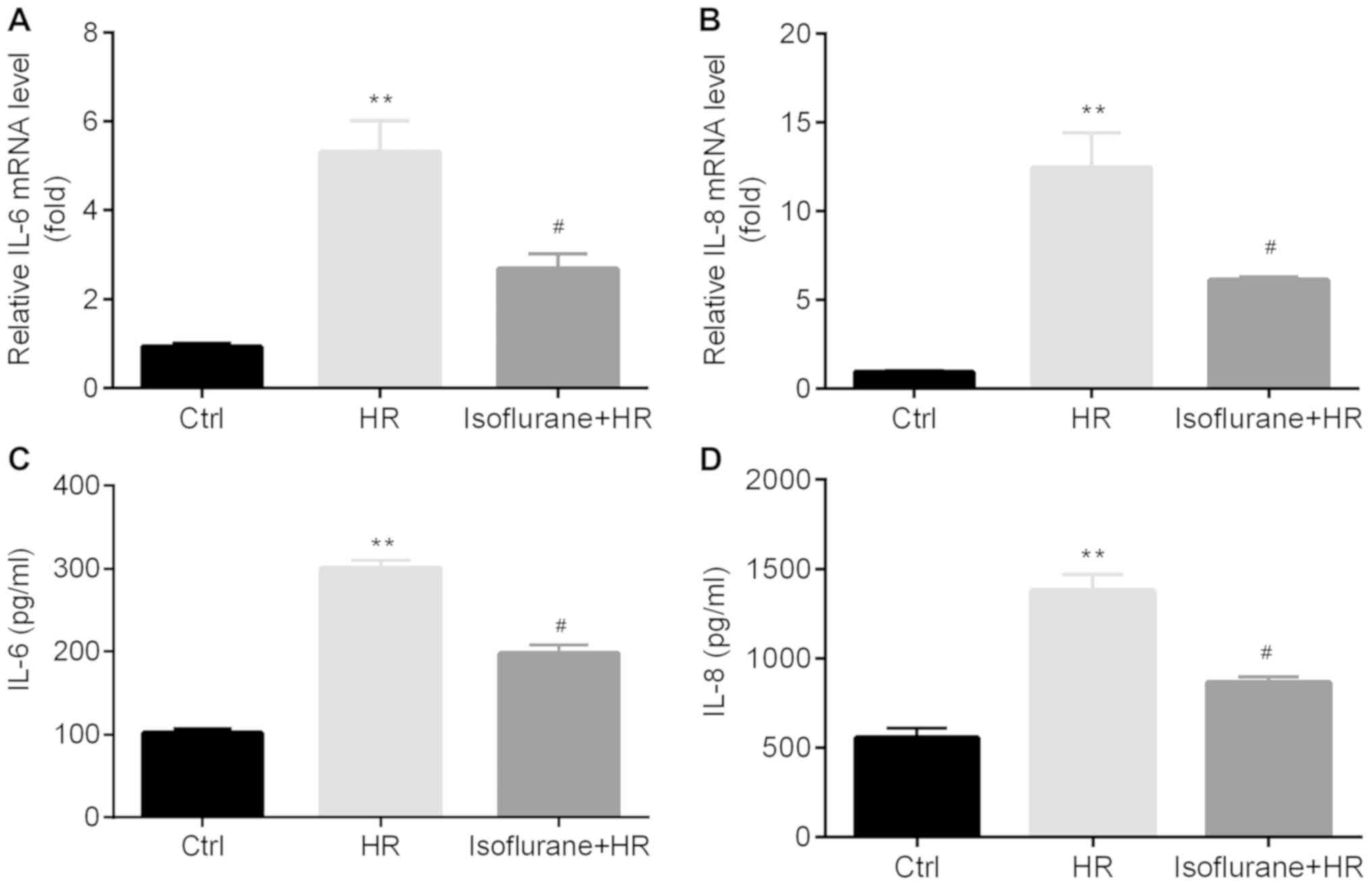
Isoflurane pretreatment reduces
HR-induced inflammatory cytokine release
The effect of isoflurane on the production and
release of inflammatory cytokines, including IL-8 and IL-6, was
examined in A549 cells and the cell culture medium, respectively.
The mRNA expression levels and protein concentrations of IL-8 and
IL-6 in the HR group were significantly increased compared with the
control group; however, pretreatment with isoflurane significantly
reduced the expression and production of IL-8 and IL-6 compared
with the HR group (Fig. 5).
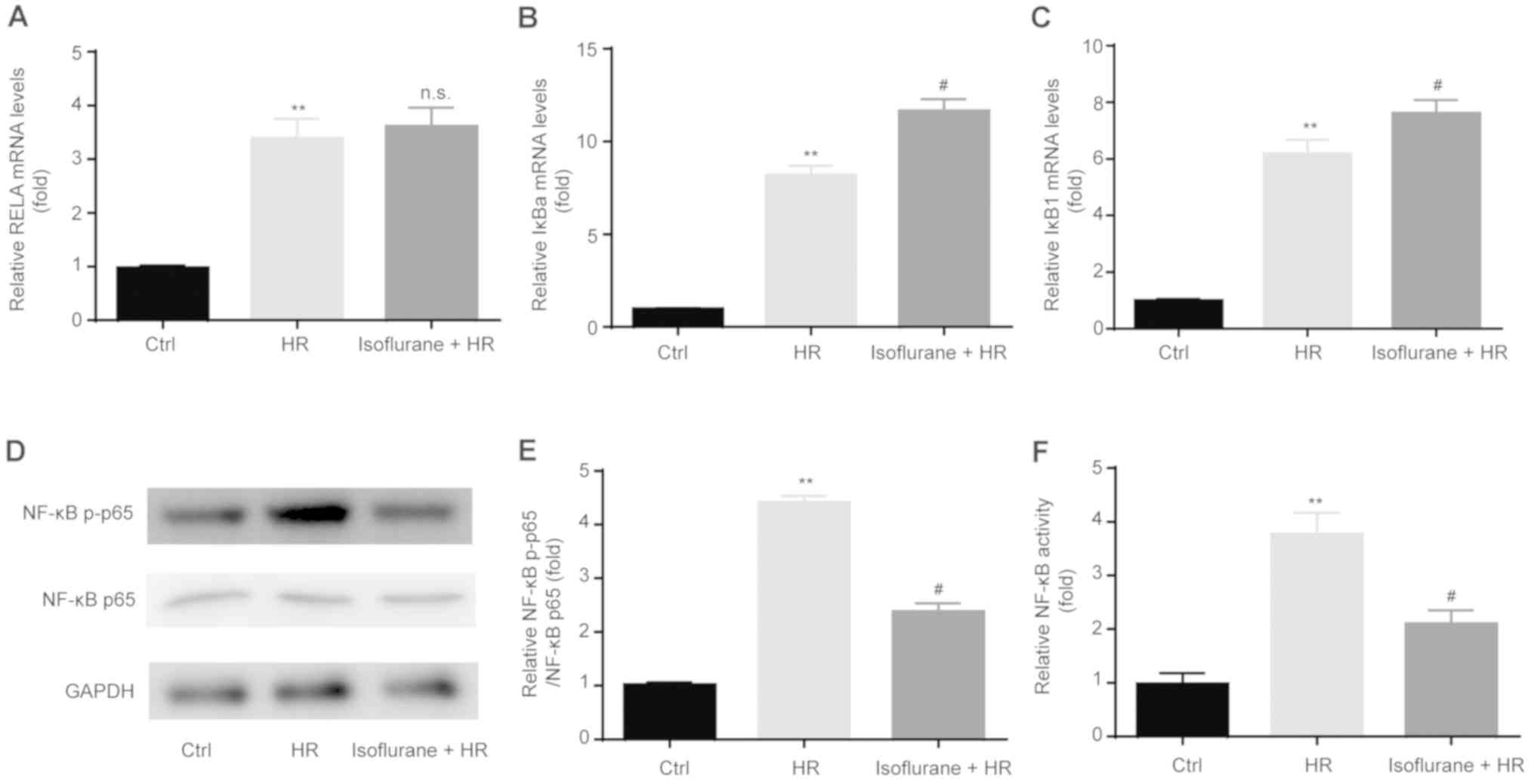
Isoflurane pretreatment suppresses
HR-induced NF-κB activation
To investigate the effect of isoflurane on the
expression of NF-κB-associated genes, RT-qPCR was performed.
Increased levels of NF-κB inhibitor α (IκBα) and IκB1
were observed in the HR group compared with the control group.
Pretreatment with isoflurane upregulated the mRNA expression levels
of IκBa and IκB1, whereas these effects were not
observed for RELA proto-oncogene, NF-κB subunit (RELA;
Fig. 6A-C).
NF-κB activity was determined following HR and
isoflurane pretreatment by western blotting and the dual-luciferase
reporter assay. The HR group displayed significantly increased p-p65
expression levels compared with the control group, which were
significantly decreased by isoflurane pretreatment (Fig. 6D and E). Furthermore, pretreatment with
isoflurane significantly suppressed the enhanced NF-κB activation
in the HR group (Fig. 6F).
Discussion
LIRI is the second most common cause of respiratory
insufficiency (15,16). Although the current treatment
strategies used for lung protection are effective, they may not be
sufficient to prevent LIRI (17);
therefore, identifying a novel strategy to protect against LIRI is
required.
Apoptosis is the process of programmed cell death
and is associated with the pathogenesis of LIRI. It has been
reported that the inhibition of apoptosis may ameliorate LIRI
(18). The present study suggested
that LIRI induced an increased rate of apoptosis compared with the
control group, which was significantly reversed by pretreatment
with isoflurane. Furthermore, the expression levels of
apoptosis-related markers, including Bcl-2 and Bax, were
investigated. The Bcl-2 family can be divided into three subgroups
that modulate cell apoptosis: Anti-apoptotic Bcl-2, proapoptotic
Bax and the BH3-only subfamily (19,20).
The results indicated that LIRI induced significantly higher Bax
expression and reduced Bcl-2 expression compared with the control
group, and pretreatment with isoflurane significantly reversed
LIRI-induced effects.
In a previous study, isoflurane pretreatment reduced
injury to normal lung cells in Sprague-Dawley rats by regulating
tumor necrosis factor-α, intercellular adhesion molecular-1 and
NF-κB (11). In the present study,
isoflurane pretreatment reversed LIRI-induced reductions in cell
proliferation. In addition, the expression of PCNA, a cell
proliferation-associated marker (21), was significantly reduced in the LIRI
group compared with the control group, which was reversed by
isoflurane pretreatment.
During LIRI, the imbalance between the demand and
supply of pulmonary oxygen leads to oxidative stress (2), which leads to an excessive
accumulation of ROS (16,22). MDA is the final product of
peroxidation, and SOD is an antioxidant enzyme that protects the
epithelium/endothelium in the lung from oxidant injury and
inflammation (23,24). The results of the present study
demonstrated that LIRI increased MDA levels and decreased SOD
activity, and that these effects were reversed by isoflurane
pretreatment.
Moreover, the infiltrating ability of inflammatory
cells in the lungs during LIRI is considered a crucial source of
ROS production (16,25). LIRI is associated with the
expression of IL-6 and IL-8 in small airway epithelial cells
(26). Isoflurane decreased
LPS-induced production of proinflammatory cytokines in rats,
including IL-6(27). A similar
protective role was identified in a rat model of renal IRI
(28). The present study indicated
that the expression levels of IL-8 and IL-6 in the LIRI group were
significantly higher compared with the control group, which was
reversed by isoflurane pretreatment.
NF-κB is an important transcription factor that is
involved in inflammation and LIRI; when activated, NF-κB promotes
the expression of various inflammatory molecules, including
cytokines, chemokines and adhesion molecules (29,30),
contributing to lung injury. Sevoflurane pretreatment of the heart
tissue decreased NF-κB activation and IR-induced production of
inflammatory mediators, thus attenuating myocardial IRI (31). Emulsified isoflurane pretreatment of
A549 cells displayed a similar effect on NF-κB activation. In the
present study, higher mRNA expression levels of IκBa,
IκB1 and RELA (an NF-κB subunit) were observed in the
LIRI group compared with the control group. Isoflurane pretreatment
significantly increased the expression levels of IκBa and
IκB1, whereas this effect was not observed for RELA
expression. In addition, significantly increased levels of p-p65
NF-κB were observed in the LIRI group compared with the control
group; however, isoflurane pretreatment decreased LIRI-induced
effects on p-p65. Furthermore, the dual-luciferase reporter assay
suggested that isoflurane pretreatment inactivated NF-κB
hyperactivation in the LIRI group.
Collectively, the results indicated that isoflurane
suppressed LIRI by inhibiting the activation of NF-κB and the
induction of cell apoptosis, suggesting that isoflurane may serve
as a therapeutic agent for LIRI.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NL and XL carried out the experiments and analyzed
the data. NL prepared the manuscript. All authors read and approved
the final manuscript for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ovechkin AV, Lominadze D, Sedoris KC,
Robinson TW, Tyagi SC and Roberts AM: Lung ischemia-reperfusion
injury: Implications of oxidative stress and platelet-arteriolar
wall interactions. Arch Physiol Biochem. 113:1–12. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Den Hengst WA, Gielis JF, Lin JY, Van
Schil PE, De Windt LJ and Moens AL: Lung ischemia-reperfusion
injury: A molecular and clinical view on a complex
pathophysiological process. Am J Physiol Heart Circ Physiol.
299:H1283–H1299. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Porteous MK, Diamond JM and Christie JD:
Primary graft dysfunction: Lessons learned about the first 72 h
after lung transplantation. Curr Opin Organ Transplant. 20:506–514.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kreisel D and Goldstein DR: Innate
immunity and organ transplantation: Focus on lung transplantation.
Transpl Int. 26:2–10. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ng CS, Wan S, Arifi AA and Yim AP:
Inflammatory response to pulmonary ischemia-reperfusion injury.
Surg Today. 36:205–214. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Welborn MB III, Douglas WG, Abouhamze Z,
Auffenburg T, Abouhamze AS, Baumhofer J, Seeger JM, Pruitt JH,
Edwards PD, Chizzonite R, et al: Visceral ischemia-reperfusion
injury promotes tumor necrosis factor (TNF) and interleukin-1
(IL-1) dependent organ injury in the mouse. Shock. 6:171–176.
1996.PubMed/NCBI
|
|
7
|
Warltier DC, Al Wathiqui MH, Kampine JP
and Schmeling WT: Recovery of contractile function of stunned
myocardium in chronically instrumented dogs is enhanced by
halothane or isoflurane. Anesthesiology. 69:552–565.
1988.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kehl F, Krolikowski JG, Mraovic B, Pagel
PS, Warltier DC and Kersten JR: Is isoflurane-induced
preconditioning dose related? Anesthesiology. 96:675–680.
2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lv X, Li Q, Huang SD, Wu FX, Yang LQ and
Yu WF: Isoflurane pretreatment reduced liver injury induced by
ischemia/reperfusion combined with lipopolysaccharide in rats.
Zhongguo Wei Zhong Bing Ji Jiu Yi Xue (Chinese). 20:271–274.
2008.PubMed/NCBI
|
|
10
|
Liang Y, Li Z, Mo N, Li M, Zhuang Z, Wang
J, Wang Y and Guo X: Isoflurane preconditioning ameliorates renal
ischemia-reperfusion injury through antiinflammatory and
antiapoptotic actions in rats. Biol Pharm Bull. 37:1599–1605.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lv X, Wang ZM, Huang SD, Song SH, Wu FX
and Yu WF: Emulsified isoflurane preconditioning reduces lung
injury induced by hepatic ischemia/reperfusion in rats. Int J Med
Sci. 8:353–361. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lieber M, Smith B, Szakal A, Nelson-Rees W
and Todaro G: A continuous tumor-cell line from a human lung
carcinoma with properties of type II alveolar epithelial cells. Int
J Cancer. 17:62–70. 1976.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yue T, Roth Z'graggen B, Blumenthal S,
Neff SB, Reyes L, Booy C, Steurer M, Spahn DR, Neff TA, Schmid ER
and Beck-Schimmer B: Postconditioning with a volatile anaesthetic
in alveolar epithelial cells in vitro. Eur Respir J. 31:118–125.
2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Apostolakis E, Filos KS, Koletsis E and
Dougenis D: Lung dysfunction following cardiopulmonary bypass. J
Card Surg. 25:47–55. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Weyker PD, Webb CA, Kiamanesh D and Flynn
BC: Lung ischemia reperfusion injury: A bench-to-beside review.
Semin Cardiothorac Vasc Anesth. 17:28–43. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Apostolakis EE, Koletsis EN, Baikoussis
NG, Siminelakis SN and Papadopoulos GS: Strategies to prevent
intraoperative lung injury during cardiopulmonary bypass. J
Cardiothorac Surg. 5(1)2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tang PS, Mura M, Seth R and Liu M: Acute
lung injury and cell death: How many ways can cells die? Am J
Physiol Lung Cell Mol Physiol. 294:L632–L641. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lindsten T, Ross AJ, King A, Zong WX,
Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K,
et al: The combined functions of proapoptotic Bcl-2 family members
bak and bax are essential for normal development of multiple
tissues. Mol Cell. 6:1389–1399. 2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Oda E, Ohki R, Murasawa H, Nemoto J,
Shibue T, Yamashita T, Tokino T, Taniguchi T and Tanaka N: Noxa, a
BH3-only member of the Bcl-2 family and candidate mediator of
p53-induced apoptosis. Science. 288:1053–1058. 2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Goodlad RA: Quantification of epithelial
cell proliferation, cell dynamics, and cell kinetics in vivo. Wiley
Interdiscip Rev Dev Biol 6: 2017.
|
|
22
|
De Perrot M, Liu M, Wadderll TK and
Kashavjee S: Ischemia reperfusion induced lung injury. Am J Respir
Crit Care Med. 167:490–511. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Till GO, Hatherill JR, Tourtellotte WW,
Lutz MJ and Ward PA: Lipid peroxidation and acute lung injury after
thermal trauma to skin. Evidence of a role for hydroxyl radical. Am
J Pathol. 119:376–384. 1985.PubMed/NCBI
|
|
24
|
Baradaran Rahimi V, Rakhshandeh H, Raucci
F, Buono B, Shirazinia R, Samzadeh Kermani A, Maione F, Mascolo N
and Askari VR: Anti-inflammatory and anti-oxidant activity of
portulaca oleracea extract on lps-induced rat lung injury.
Molecules. 4: pii(E139)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Forman HJ and Thomas MJ: Oxidant
production and bactericidal activity of phagocytes. Annu Rev
Physiol. 48:669–680. 1986.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Watanabe K, Iwahara C, Nakayama H,
Iwabuchi K, Matsukawa T, Yokoyama K, Yamaguchi K, Kamiyama Y and
Inada E: Sevoflurane suppresses tumour necrosis factor-a-induced
inflammatory responses in small airway epithelial cells after
anoxia/reoxygenation. Br J Anaesth. 110:637–645. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li QF, Zhu YS, Jiang H, Xu H and Sun Y:
Isoflurane preconditioning ame-liorates endotoxin-induced acute
lung injury and mortality in rats. Anesth Analg. 109:1591–1597.
2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hashiguchi H, Morooka H, Miyoshi H,
Matsumoto M, Koji T and Sumikaw K: Isoflurane protects renal
function against ischemia and reperfusion through inhibition of
protein kinases, JNK and ERK. Anesth Analg. 101:1584–1589.
2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yoshidome H, Kato A, Edwards MJ and
Lentsch AB: Interleukin-10 inhibits pulmonary NF-kappaB activation
and lung injury induced by hepatic ischemia-reperfusion. Am J
Physiol. 277:919–923. 1999.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Colletti LM, Cortis A, Lukacs N, Kunkel
SL, Green M and Strieter RM: Tumor necrosis factor up-regulates
intercellular adhesion molecule 1, which is important in the
neutrophil-dependent lung and liver injury associated with hepatic
ischemia and reperfusion in the rat. Shock. 10:182–191.
1998.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhong C, Zhou Y and Liu H: Nuclear factor
kappaB and anesthetic preconditioning during myocardial
ischemia-reperfusion. Anesthesiology. 100:540–545. 2004.PubMed/NCBI View Article : Google Scholar
|