Introduction
Dental implantation at the posterior maxilla can be
challenging due to insufficient subantral bone volume that is
primarily caused by alveolar ridge resorption and pneumatization of
the maxillary sinus (1). To ensure
sufficient bone volume, sinus floor elevation (SFE) is now widely
utilized and has yielded clinically favorable results (1). Various bone substitutes including
allograft, xenograft and alloplastic materials are used for SFE,
and their use as a scaffold for osteoconduction and vascular
ingrowth has been documented in numerous studies (2-5).
Previous studies have shown that SFE without graft material can
also provide new bone formation that would be sufficient to support
a dental implant by lifting the human maxillary sinus membrane
(hMSM) and maintaining its position (6-8).
These findings confirm the importance of secluded spaces where
blood clots can form and act as a scaffold to allow osteoconduction
and vascular ingrowth (9,10).
The residual maxillary bone, including the sinus
floor and sinus walls, provides cellular components, such that bone
and vessels can grow centripetally into grafted materials or into
the secluded spaces from the residual bone following SFE (11,12).
In addition, it is possible that the hMSM contains cells with
osteogenic potential that may act as an additional source for new
bone formation in SFE (13).
Although the hMSM does not contain osteogenic cells in the tissue,
several studies confirmed that the hMSM contains progenitor cells
with a mesenchymal lineage that can potentially differentiate into
an osteogenic lineage (14-19).
Bone morphogenetic proteins (BMPs) are
multi-functional growth factors and members of the transforming
growth factor-β (TGF-β) superfamily (20); of these, BMP-2 modulates
osteoblastic differentiation through the BMP/Smad pathway (21-23).
BMP-2 binds to the BMP receptor and activates the cytoplasmic
serine/threonine kinase of the BMP receptor (BMPR)-I. The activated
BMPRs phosphorylate BMP-specific Smad1/5/8 in the cytoplasm.
Smad1/5/8 binds Smad4, and the resultant complex is transported to
the nucleus to promote the expression of a transcription factor
with a homeodomain called Dlx5. This protein domain promotes the
expression of runt-related transcription factor 2 (RUNX2) and
Osterix, both of which are key transcription factors involved in
osteoblast differentiation (24,25).
Therefore, recombinant human BMP (rhBMP-2) among other types of
BMPs have been primarily utilized with a variety of bone graft
materials to accelerate bone regeneration (26-33).
The application of rhBMP-2 in SFE has been widely assessed in
preclinical and clinical studies in which its osteoinductive and
osteogenic capacities were confirmed (34,35);
however, with the increased use of rhBMP-2, its side effects became
apparent, and these include inflammatory complications, ectopic
bone formation, bone resorption and inflammatory swelling (36,37).
Inflammatory swelling is the most common side effect following SFE
with rhBMP-2(38). It has been
suggested that the cellular components in the hMSM and gingival
tissue are involved in the inflammatory response following rhBMP-2
treatment (36,37). However, the effect of rhBMP-2 on
hMSM-derived cells has not been investigated.
The aim of the present study was to investigate the
osteogenic differentiation potential of hMSM-derived cells and the
effect of rhBMP-2 on these cells with the aim of identifying the
cause of the inflammatory response.
Materials and methods
Subjects
hMSM samples were collected from three individuals
(1 male and 2 females, 17-33 years of age) who underwent Le Fort I
osteotomy as the orthognathic surgery between April and October
2016, with a discarded hMSM available. Informed consent was
obtained, and all samples were collected in accordance with
relevant guidelines under and ethically approved by the Ethics
Committee at the Kyung Hee University Dental Hospital (approval no.
KHD IRB 1509-1). Patients who neither had experienced nor were
diagnosed with sinus pathology, maxillary neoplasm, metabolic
diseases, genetic disease, nor had a history of previous sinus
surgery were selected. After the collection, the samples were
suspended in Dulbecco's PBS (DPBS; Corning, Inc.) containing 1%
penicillin-streptomycin (PS; Corning, Inc.). Samples that were ~1x1
cm in size were used for cell culture.
Histological analysis
Samples were fixed in 3.7% paraformaldehyde pH 7.4
(cat. no. P2031; Biosesang, Inc.) overnight at 4˚C,
dehydrated using a series of ethanol solutions of increasing
concentrations (50% ethanol, 70% ethanol and 100% ethanol), and
embedded in paraffin. Tissue sections 4 µm thick were incubated in
Mayer's hematoxylin solution (Lillie's Modification) for 5 min and
eosin Y solution (modified alcoholic) for 3 min at 25˚C
using hematoxylin and eosin (H&E) staining kit (cat. no.
ab245880; Abcam), and mouse and rabbit specific HRP/DAB IHC
detection kit (cat. no. ab236466; Abcam, the
avidin-biotin-peroxidase complex (ABC) method according to the
manufacturer's protocol. Cell markers, including STRO-1 (cat. no.
MAB1038-SP; 1:100; R&D Systems, Inc.), high mobility group
AT-hook 2 (HMGA-2; cat. no. 8179S; 1:400; Cell Signaling
Technology, Inc.), epithelial cell adhesion molecule (EpCAM; cat.
no. 2929S; 1:500; Cell Signaling Technology, Inc.) and
fibroblast-specific protein-1 (FSP-1; 13018S; 1:400; Cell Signaling
Technology, Inc.) were used as the primary antibodies.
Isolation and culture of hMSM
cells
For the isolation of hMSM cells, the samples were
rinsed with DPBS to remove erythrocytes. Tissues were cut into 1-2
mm pieces and digested with 1% type I collagenase (Gibco; Thermo
Fisher Scientific, Inc.) at 37˚C for 3 h in 60 mm petri dishes.
Enzyme activity was neutralized with the addition of DMEM
containing 10% FBS (Corning, Inc.) and 1% PS, and the samples were
centrifuged at 196 x g at 25˚C for 3 min. The pellet was
resuspended and transferred into a plate containing the culture
medium, and the cells were incubated overnight at 37˚C with 5%
CO2 to allow adherence. Subsequently, the cell cultures
were washed with DPBS to remove residual non-adherent tissues and
erythrocytes. The morphology of hMSM cells was observed daily using
an inverted phase-contrast microscope and the culture medium was
changed every two days. When the monolayer of adherent cells
reached 70-80% confluence, the cells were trypsinized
(TrypLE™Express; Gibco; Thermo Fisher Scientific, Inc.),
resuspended in growth medium and subcultured.
Immunohistochemical (IHC)
analysis
hMSM-derived cells recovered from passage 6 (P6)
were subcultured in 12-well culture plates at a density of
1x105 cells/well. Cells were fixed in 3.7%
paraformaldehyde for 20-30 min at 25˚C and blocked with antibody
diluent (GBI Labs, Inc.) overnight at 4˚C. Subsequently, cells were
incubated with anti-STRO-1 (cat. no. sc-47733; 1:100; Santa Cruz
Biotechnology, Inc.), HMGA-2 (1:400; Cell Signaling Technology,
Inc.), CD44 (cat. no. sc-7297; 1:200; Santa Cruz Biotechnology,
Inc.), CD105 (cat. no. ab169545; 1:400; Abcam), EpCAM (1:400),
FSP-1 (1:400) or CD34 (cat. no. sc-7324; 1:200; Santa Cruz
Biotechnology, Inc.) overnight at 4˚C. After incubation, the wells
were washed five times with DPBS. Each sample was incubated with
secondary antibodies (cat. no. A11001; 1:1,000; Invitrogen; Thermo
Fisher Scientific, Inc.; cat. no. A11034; 1:1,000; Invitrogen;
Thermo Fisher Scientific, Inc.) for 1 h at 25˚C. The cells were
counterstained with DAPI. Images were analyzed under x200 and 400
magnification fields using light and fluorescence microscopy
(IX71-F32PH; Olympus Corporation).
Flow cytometry analysis
hMSM-derived cells obtained at passage (P)2, P4, P6
and P8 were analyzed by flow cytometry to assess the expression of
various markers. Cultures with a density of 1x106
cells/ml were fixed in 3.7% paraformaldehyde for 20 min at 25˚C and
then blocked with antibody diluent overnight at 4˚C. The cells were
then labeled with monoclonal antibodies against STRO-1, EpCAM,
HMGA2 and FSP-1 overnight at 4˚C and washed five times with DPBS.
The secondary antibodies coupled with FITC were added, and the
cells were incubated for 1 h at 25˚C in the dark. After labeling,
cells were washed and resuspended in DPBS, and analyzed using a
LSRFortessa™ X-20 flow cytometer (BD Biosciences).
Alizarin Red staining
hMSM-derived cells from P6 were cultured in 6-well
plates (3x105 cells/well) with non-osteogenic medium
(DMEM containing 10% FBS and 1% PS), osteogenic medium [DMEM
containing 10% FBS, 1% PS, 5 µM β-glycerophosphate (Sigma-Aldrich;
Merck KGaA), 0.1 mM ascorbic acid (Sigma-Aldrich; Merck KGaA) and
0.1 µM dexamethasone (Sigma-Aldrich; Merck KGaA)] or osteogenic
medium supplemented with rhBMP-2 (10 ng/ml; PeproTech Inc.). After
0, 7 and 14 days of culture, the cells were fixed with 3.7%
paraformaldehyde (cat. no. P2031; Biosesang, Inc.) for 30 min at
25˚C and rinsed with DPBS. The cells were subsequently
stained with 2% Alizarin Red solution (cat. no. 6B7131;
Sigma-Aldrich; Merck KGaA) for 20 min at 25˚C, and
rinsed five times with DPBS to remove non-specific stained
cells.
Reverse transcription-quantitative
(RT-q)PCR for osteogenic activity and inflammatory reaction
To investigate the expression of genes associated
with osteogenic differentiation, hMSM-derived cells from P6 were
cultured (3x105 cells/well) in 6-well plates for 14
days. The cells were cultured in the osteogenic medium with or
without 10 ng/ml rhBMP-2. The markers used for RT-qPCR are
presented in Table I, including the
sequences of the primers used and the expected amplicon size. To
investigate the expression of genes relevant to the inflammatory
response caused by rhBMP-2, MSM-derived cells and gingival
fibroblast cells (P4) were cultured (5x105 cells/well)
for 0, 24, 48 and 72 h. The control group was cultured in DMEM
containing 10% FBS and 1% PS. The experimental groups were cultured
in DMEM containing 10% FBS and 1% PS with 10 ng/ml rhBMP-2. Total
RNA was isolated from control and experimental cultures at defined
time intervals using a Ribospin™ RNA isolation kit (GeneAll,
Biotechnology, Co. Ltd.) according to the manufacturer's protocol.
RNA was collected in RNAse free water, and its total quantity and
quality were measured spectrophotometrically (Nanodrop 2000/2000c
spectrophotometer; Thermo Fisher Scientific, Inc.). First strand
cDNA was synthesized from total RNA using AccuPower®
CycleScript RT PreMIX(dT20) (Bioneer Corporation) according to the
manufacturer's protocol. After cDNA synthesis, qPCR was performed
using 1 µg cDNA mixed with 10 µl SYBR-Green using TOPreal™ qPCR 2x
PreMIX (Enzynomics, Co., Ltd.), with 5 µM each of the forward and
reverse primers. The PCR thermocycling conditions were: Initial
denaturation at 95˚C for 15 min; followed by 40 cycles of
denaturation at 95˚C for 15 sec, annealing at primer melting
temperature (Tm) for 10 sec and extension at 72˚C for 30 sec.
Expression levels of the target genes were quantified after
normalization to the levels of β-actin using the 2-ΔΔCq
method (39).
 | Table ISequences of the primers used and the
expected amplicon size. |
Table I
Sequences of the primers used and the
expected amplicon size.
| Gene | Sequence,
5'-3' | Size, bp |
|---|
| β-actin | | 110 |
|
Forward |
GTCAGGCAGCTCGTGCTCT | |
|
Reverse |
TCGTGCGTGACATTAAGGAG | |
| RUNX2 | | 189 |
|
Forward |
GTAGCTACTTGGGGAGGATT | |
|
Reverse |
AGATGGGACTGTGGTTACTG | |
| ALP | | 102 |
|
Forward |
TCCATGTTGAGATGAGCTG | |
|
Reverse |
ACACACAGTGAACCGCAACT | |
| Osteocalcin | | 143 |
|
Forward |
CGCCTGGGTCTCTTCACTAC | |
|
Reverse |
CTCACACTCCTCGCCCTATT | |
| Type I
collagen | | 105 |
|
Forward |
ATGACAATCTGCTCCCAAC | |
|
Reverse |
CAATGCTGTTCTTGCAGTGG | |
| NF-κB | | 158 |
|
Forward |
AGATGTGGTGGAGGATTTGC | |
|
Reverse |
TGGGGTGGTCAAGAAGTAGTG | |
| TNF-α | | 116 |
|
Forward |
CAAGGATGTCATTGGTGACG | |
|
Reverse |
CCTTGGTCTGCTTCTTCTCC | |
| IL-1β | | 133 |
|
Forward |
TCCAGGGACAGGATATGGAG | |
|
Reverse |
TCTTTCAACACGCAGGACAG | |
| IL-6 | | 179 |
|
Forward |
AGGCACTGGCAGAAAACAAC | |
|
Reverse |
AGCTCTGGCTTGTTCCTCAC | |
Statistical analysis
Results are expressed as individual data or as the
mean ± the standard error of the mean of at least three repeats.
Statistical analysis was performed using a Wilcoxon signed ranks
test and a Mann-Whitney U test in SPSS version 15.0 (SPSS, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Histological findings
H&E staining allowed visualization of the
epithelial lining, lamina propria and periosteal-like lining
(Fig. 1A-C). The hMSM sections were
also stained using the ABC method, which revealed a number of cells
positive for the mesenchymal stem/stromal cell (MSC) marker
(STRO-1), fibroblast marker (FSP-1) and epithelial cell marker
(EpCAM) (Fig. 1D-F).
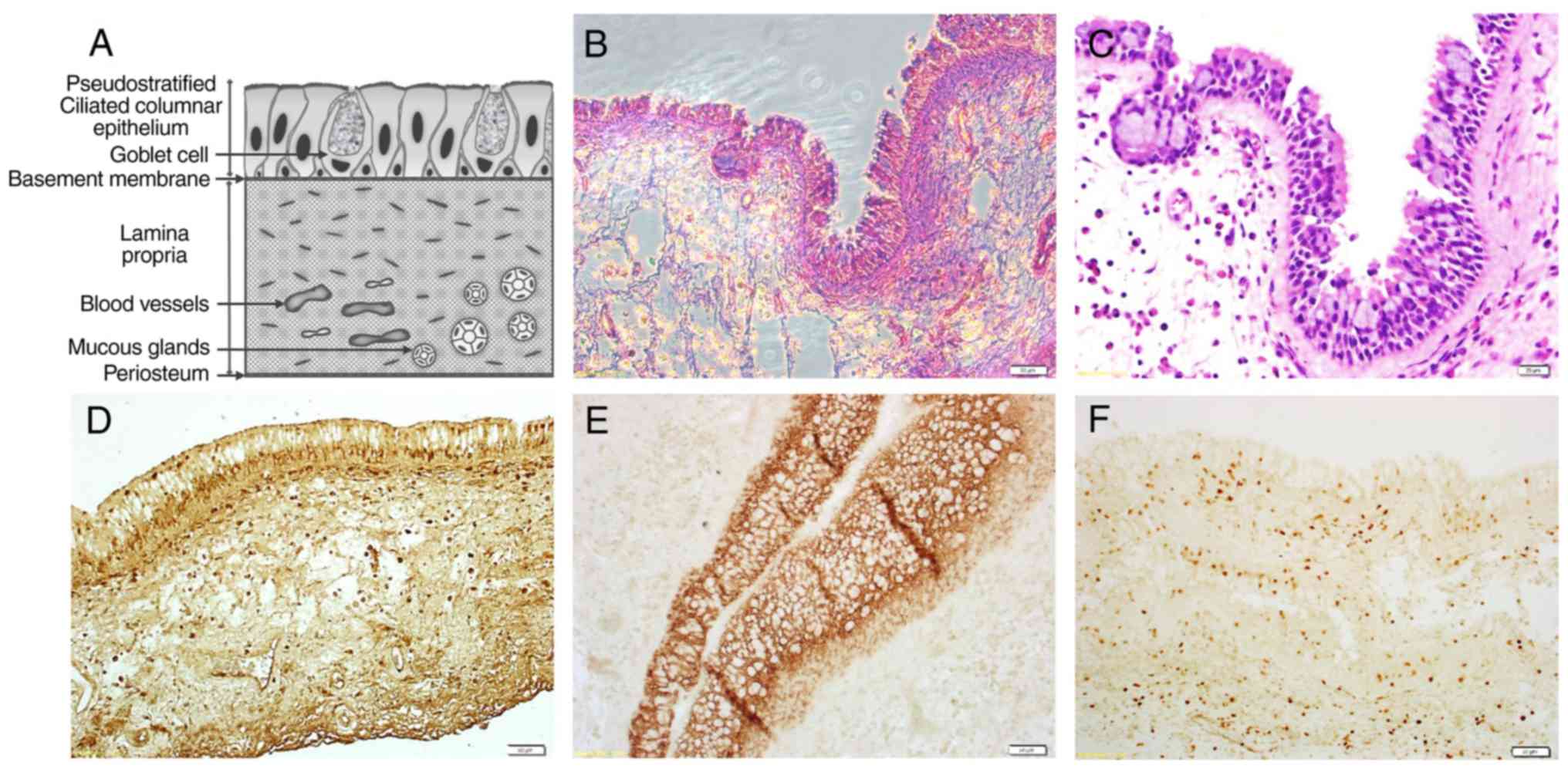 | Figure 1Tissue section of the hMSM. A general
view of a stained hMSM section, showing the epithelial lining,
vascularized lamina propria and deepest periosteal-like lining. (A)
An illustration of an hMSM section. (B and C) Hematoxylin &
eosin staining. Scale bar, 50 and 20 µm, respectively.
Avidin-biotin-peroxidase complex staining for (D) STRO-1, (E)
EpCAM, and (F) FSP-1, respectively. Scale bar, 50 µm. hMSM, human
maxillary sinus membrane; EpCAM, epithelial cell adhesion
molecule. |
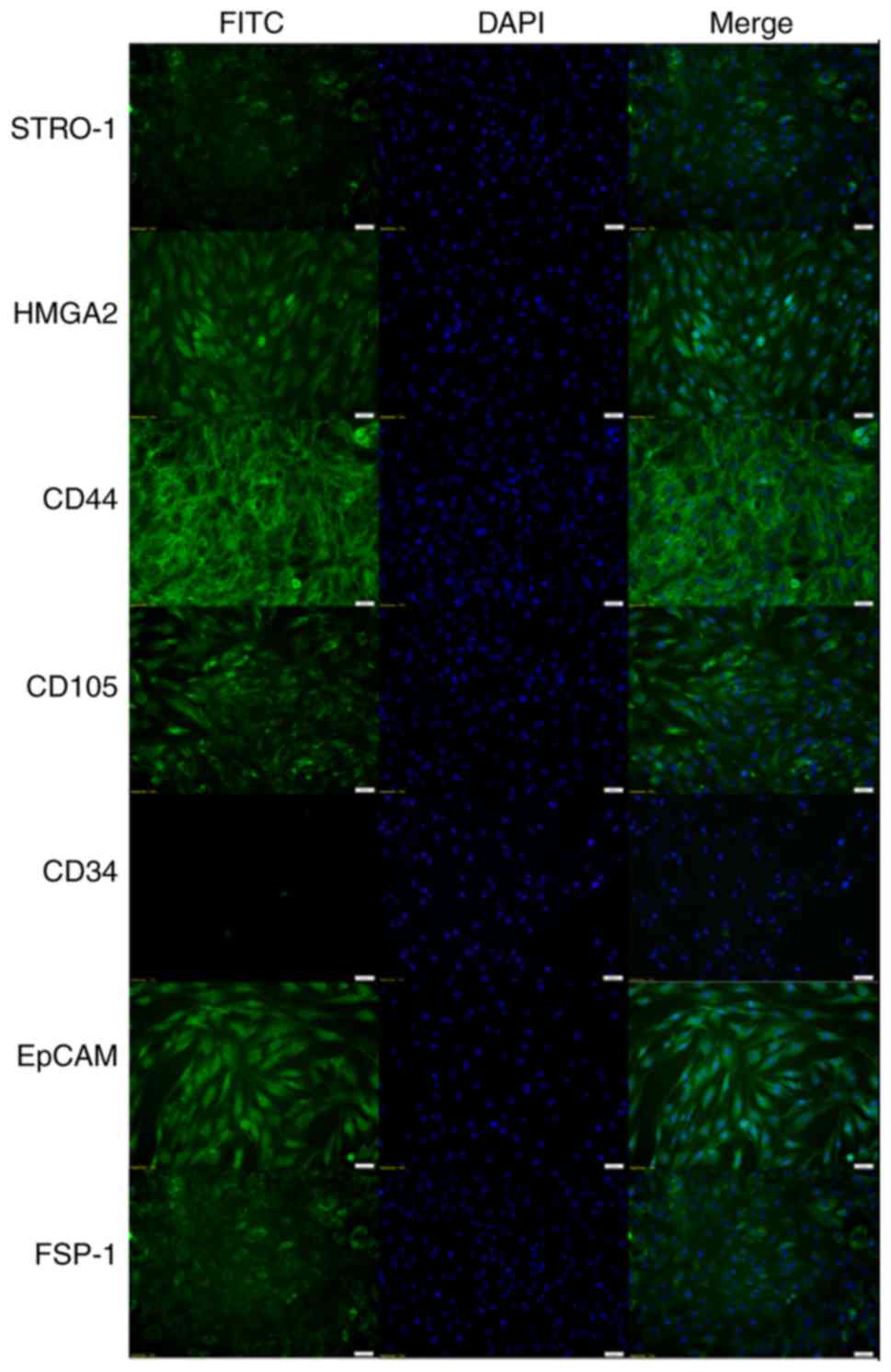
Morphological and IHC findings
Adherent hMSM-derived cells were heterogeneous,
consisting of epithelial-like cells with a polygonal shape and
fibroblast-like cells with a bipolar elongated shape. As the number
of cell passages increased, the number of fibroblast-like cells
also increased. The hMSM-derived cells expressed MSC markers such
as STRO-1, HMGA2, CD44 and CD105 but they did not express the
hematopoietic marker CD34. The epithelial cell marker EpCAM and
fibroblast marker FSP-1 were also expressed (Fig. 2).
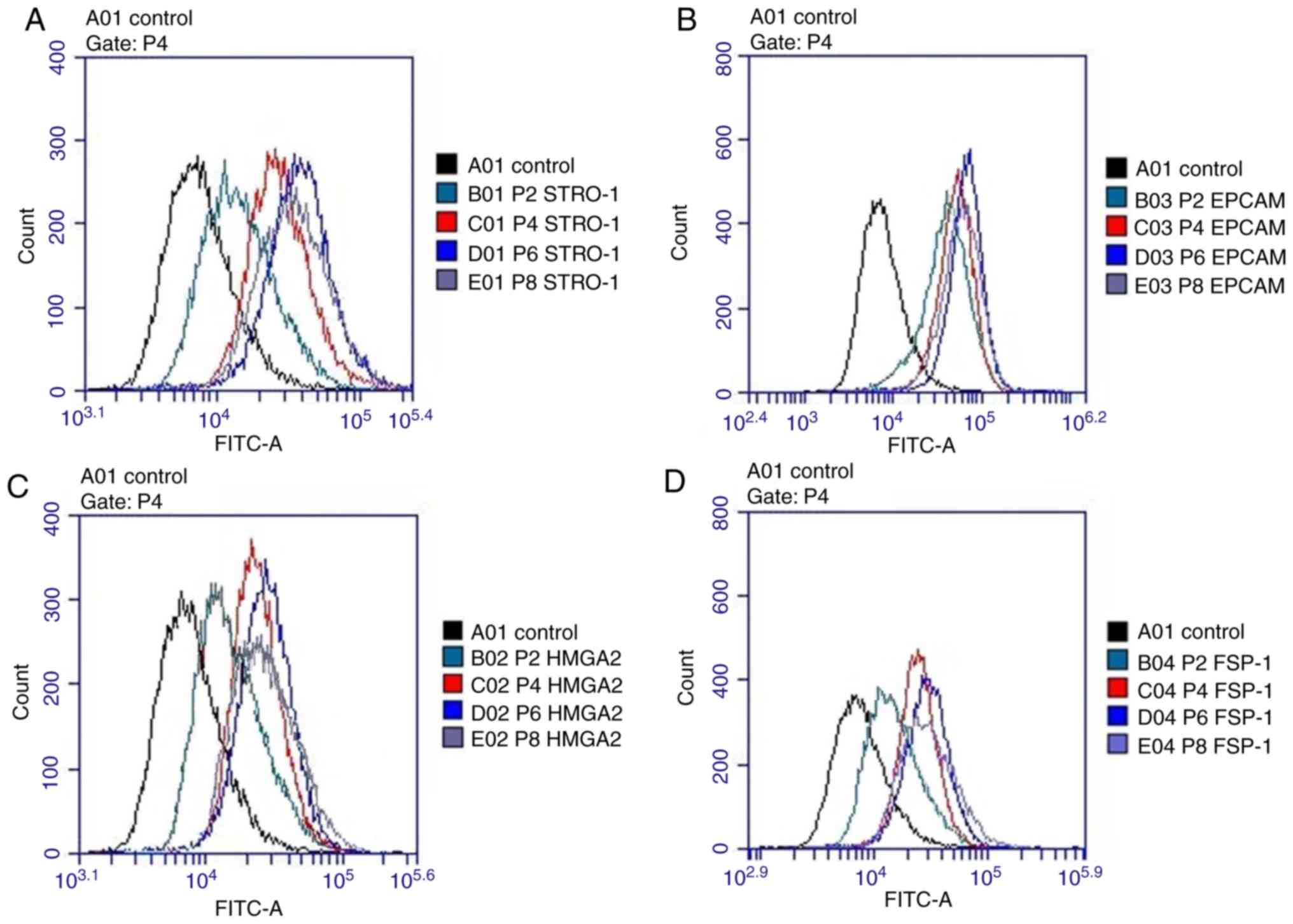
Flow cytometry
The hMSM-derived cells expressed STRO-1, HMGA2,
EpCAM and FSP-1 markers in all examined passages (P2, P4, P6 and
P8). The signals for these markers increased between P2 to P6, but
decreased at P8. Cells positive for the MSC markers (STRO-1 and
HMGA2) peaked at P6 (Fig. 3).
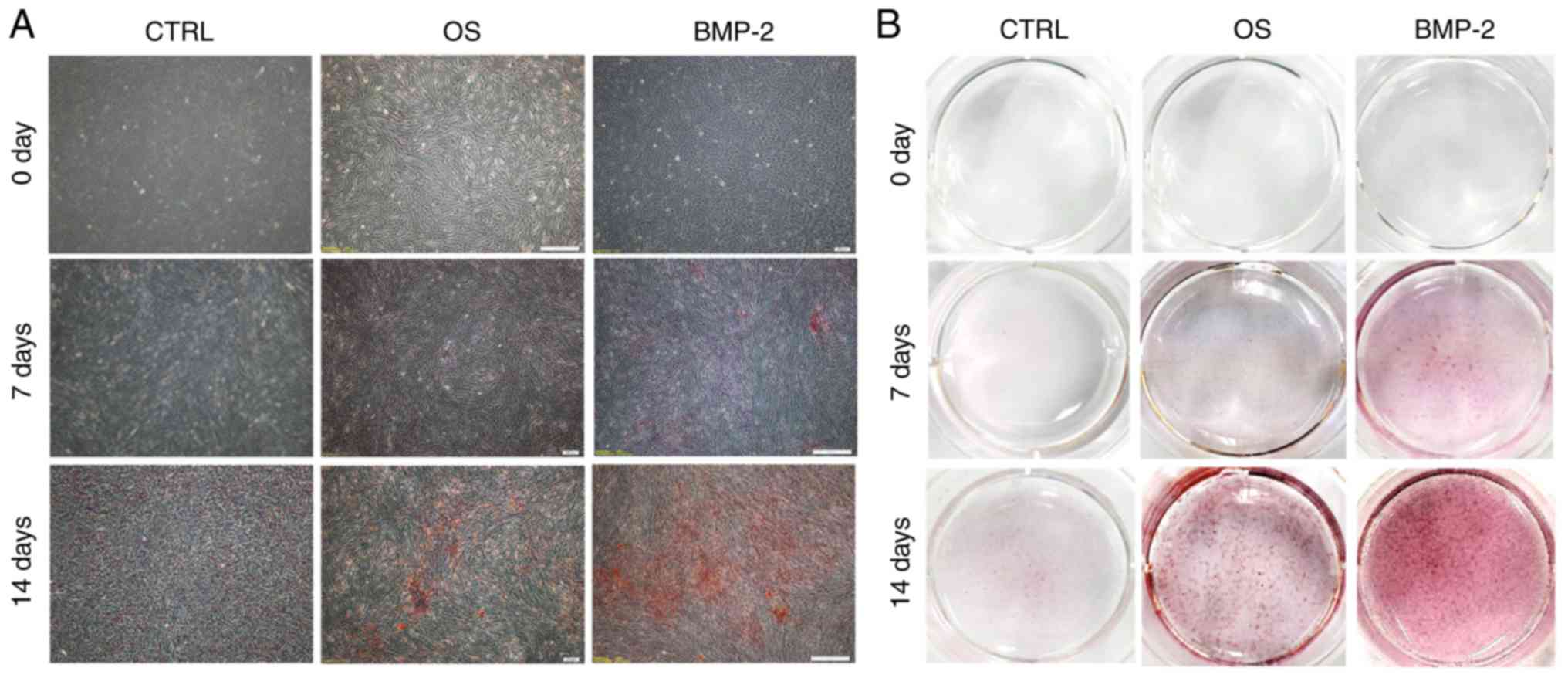
Osteogenic activity
Calcium nodules stained with Alizarin Red S became
more evident as the culture period increased, and were most
numerous in the rhBMP-2 group (Fig.
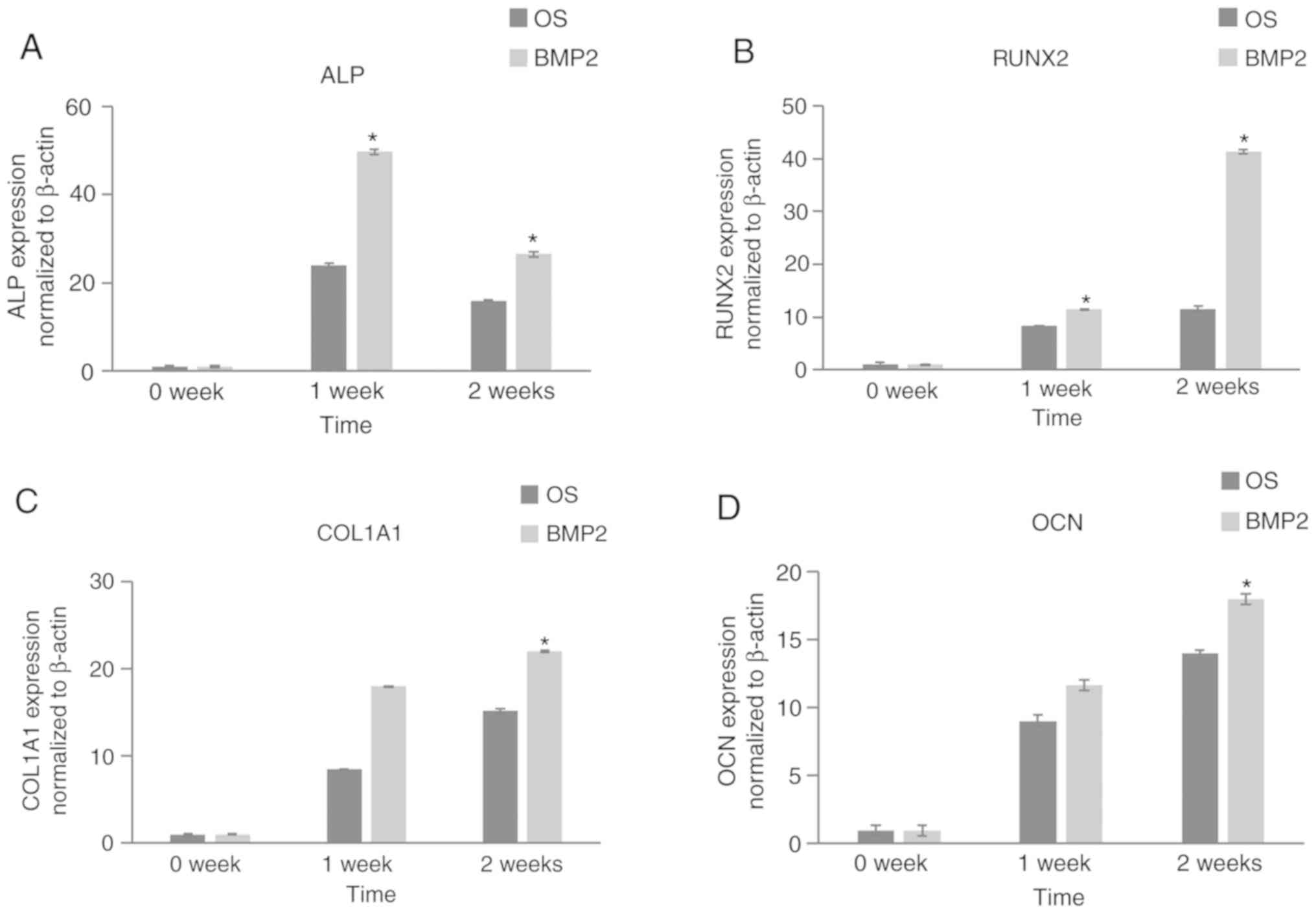
4A and B). RT-qPCR was
performed to analyze the gene expression of the osteogenic markers
including alkaline phosphatase (ALP), RUNX2, type 1 collagen and
osteocalcin following osteogenic differentiation. The expression of
the osteogenic genes was significantly higher in the rhBMP-2 groups
compared with the control group (Fig.
5). Expression of all these markers were upregulated after 7
and 14 days of culture. Apart from ALP, the expression of the
markers in the rhBMP-2 group was significantly higher after 14 days
compared with after 7 days. ALP expression in the rhBMP-2 group was
higher (up to 24-fold) upon measurement after 7 days of culture
compared with day 0, and subsequently significantly decreased in
both control and rhBMP-2 groups.
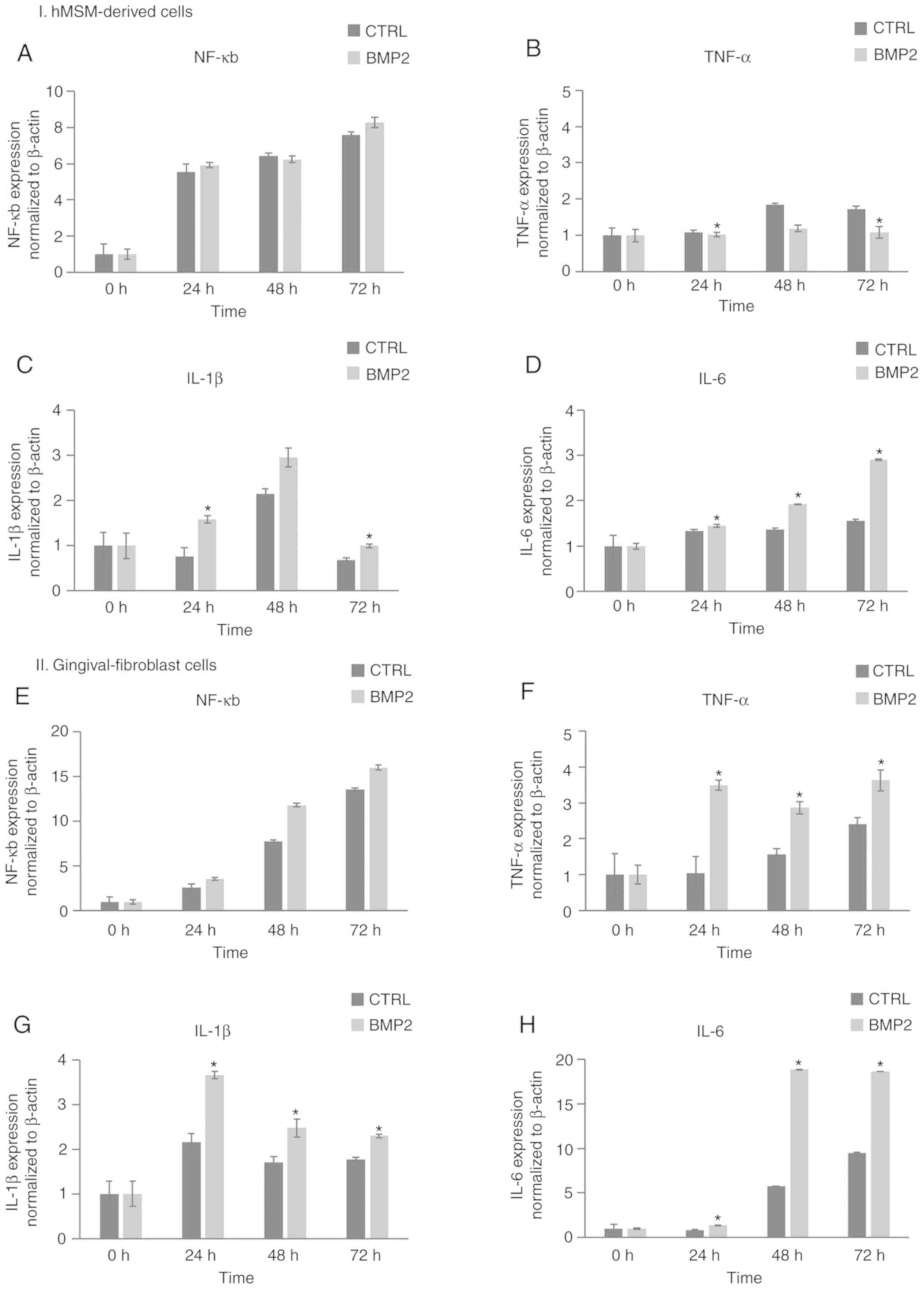
Inflammatory reaction to rhBMP-2
As shown in Fig. 6,
the gene expression of nuclear factor-κB (NF-κB) in the
hMSM-derived cell and gingival fibroblast groups gradually
increased over time, with an 8.2-fold and 15.9-fold increase in
expression after 72 h of incubation, respectively. However, the
gene expression of NF-κB in the hMSM-derived cells did not differ
significantly compared with the control group. Tumor necrosis
factor (TNF)-α in the hMSM-derived cell group treated with rhBMP-2
was significantly lower compared with the control group; however,
in the gingival fibroblast group, it was significantly higher
compared with the control group. Interleukin (IL)-1β expression
peaked at 48 h with a 2.9-fold increase in expression compared with
the control in the hMSM-derived cells, and at 24 h with a 3.8-fold
increase in gingival fibroblasts. The expression of IL-6 gene
expression peaked at 72 h, with a 3-fold increase in the
hMSM-derived cells, and an 18-fold increase in gingival fibroblast
cells. The expression of IL-1β and IL-6 in both groups was
significantly higher compared with the control group.
Discussion
SFE has become a standard procedure to increase
subantral bone volume at the atrophic posterior maxilla (39-41).
Autogenous bone, allograft, xenograft and alloplastic materials
have been used as bone graft materials (2). Since xenograft and alloplastic
materials are only osteoconductive, osteogenic and angiogenic cells
and growth factors from the residual maxillary bone serve a crucial
role in osteogenesis (42). In
addition, the presence of human (h)MSCs is also an important factor
for SFE, as hMSCs can differentiate into osteogenic cells (43). Therefore, if the hMSM contains
hMSCs, osteogenesis is expected to occur following SFE, which is
far from the sinus floor and walls. BMP-2 modulates osteoblastic
differentiation through the BMP/Smad pathway by binding to the BMP
receptor (21-24).
Accordingly, the present study was designed to verify if the hMSM
contains a type of MSCs that exhibits the potential to
differentiate into osteogenic cells, and if BMP-2 could enhance the
osteogenic differentiation of the hMSM derived cells. In addition,
the role of BMP-2 in eliciting an inflammatory response was
investigated according to cellular composition and tissue type, as
it is possible that the use of BMP-2 may increase postoperative
complications following SFE (44).
The isolated hMSM-derived cells showed
characteristics of epithelial-like cells and fibroblast-like cells
morphologically, indicating that the cells were heterogeneous and
may contain hMSCs (43-45).
The presence of hMSCs was confirmed by STRO1, CD44, CD105 and
HMGA2-positive cells (46). As
STRO1-positive progenitors are considered to be derived from a
perivascular niche, the MSCs could have arisen from developing
blood vessels that are abundant in the hMSM tissue (15,47,48).
In addition, the presence of fibroblasts and epithelial cells were
also confirmed by the presence of the fibroblast marker FSP-1 and
the epithelial marker EpCAM (49,50).
Interestingly, the number of cells exhibiting the morphological
characteristics of mesenchymal progenitor cells and STRO1-positive
cells increased with the increasing number of passages.
The hMSM-derived cells contained a cell population
capable of differentiating into osteogenic cells. Calcified nodules
were observed after 14 days of incubation in the osteogenic medium,
and mineralization was enhanced with rhBMP-2 supplementation. The
gene expression of osteogenic markers including ALP, RUNX2, Type I
collagen and osteocalcin, were also significantly upregulated in
the presence of rhBMP-2 compared with those in the control group.
These results suggest that the use of rhBMP-2 in SFE may induce and
facilitate osteogenesis initiated by the hMSM-derived MSCs.
Several studies have suggested that the hMSM
contains a population of multipotent stem cells that may contribute
to osteogenesis. A study by Graziano et al (13) in which mesenchymal progenitors in
the hMSM were isolated and characterized, showed that they
possessed the intrinsic capacity to regenerate maxillary bone
volume. Another study also reported that the hMSCs isolated from
the hMSM were capable of generating bone-like tissue (15), in agreement with the results of the
present study.
Currently, rhBMP-2 is widely used for bone
regeneration as an osteoinductive adjuvant; however, concerns were
raised, as postoperative complications associated with rhBMP-2 have
been reported (36-38,51,52).
In 2008, the United States Food and Drug Administration issued a
warning regarding the use of rhBMP-2, due to the risk of cervical
spine swelling and death (36,37),
and there is a study describing local reactions, infections, wound
complications and graft failures as common adverse events of BMP-2
use (38). Local reactions, such as
edema, erythema and pain were the most frequently reported events,
and this suggests that inflammatory reactions may increase with the
use of rhBMP-2. According to the side effects profile of BMP-2
reviewed by Nguyen et al (53), both in vitro and in
vivo preclinical studies show that BMP-2 induces inflammation,
as evidenced by increased levels of the inflammatory cytokines
IL-1β, IL-6, IL-10, IL-17, IL-18 and TNF-α (37,53,54).
In the present study, it was demonstrated that the
mRNA expression levels of IL-1β and IL-6 were significantly
upregulated by rhBMP-2 in both groups. However, expression of
TNF-α, which regulates immune cells and induces inflammation, was
significantly downregulated, and NF-κB expression was not
significantly different compared with the control in the
hMSM-derived cells. On the contrary, the expression of these
inflammatory markers were upregulated in the gingival fibroblast
group. This result suggests that the hMSCs may serve a role in the
decreased inflammatory response.
In agreement with this result, several studies have
shown that MSCs modulate allogeneic immune cell responses, and that
MSCs serve as guardians against excessive inflammatory responses
(55-59).
Aggarwal and Pittenger (55)
demonstrated that hMSCs interact with a variety of immune cells to
inhibit or limit the inflammatory response, and promote
anti-inflammatory pathways; however, it is difficult to conclude if
hMSCs serve a role in reducing inflammation, as the hMSM consists
of various cell types and their inflammatory response is not
balanced with the result.
Further studies are required to identify the
mechanism and the role of hMSCs in the rhBMP-2 induced inflammatory
response. The expression of the markers and BMP-2 receptor are
required to verify these results, and various concentrations of
rhBMP-2 should be examined, as rhBMP-2 initiates a dose-dependent
inflammatory response (60).
However, the present study showed that hMSM contributes to the
osteogenic process through hMSCs, and that the use of rhBMP-2 in
SFE increases the inflammatory response resulting in more acute
postoperative complications than without the use of rhBMP-2 in
conventional SFE. In addition, the severity of the inflammatory
response may differ by region depending on the cellular composition
of the tissue affected.
In conclusion, the present study confirmed that hMSM
contains hMSCs that are capable of differentiating into osteogenic
cells. Supplementation of rhBMP-2 enhances osteogenic
differentiation. In addition, rhBMP-2 induced an inflammatory
response, and the response was smaller in the hMSM-derived cells
and larger in the gingival fibroblasts. The use of rhBMP-2 in SFE
may increase the inflammatory response and the gingival tissue may
be responsible for the increased response and postoperative
complications. Extra precautions are required for the clinical use
of rhBMP-2.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the Korea
Health Technology R&D project through the Korea Health Industry
Development Institute, funded by the Ministry of Health &
Welfare, Republic of Korea (grant no. HI14C0175).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC and JJ wrote the manuscript. JC performed the
experiments. JJ analyzed the data and revised the manuscript. J-HL
and S-HO interpreted the results. Y-DK conceived and designed the
study.
Ethics approval and consent to
participate
Written informed consent was obtained from all
participants, and all samples were collected in accordance with
relevant guidelines and approved by the Ethics Committee at the
Kyung Hee University Dental Hospital (approval no. KHD IRB
1509-1).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Raghoebar GM, Onclin P, Boven GC, Vissink
A and Meijer HJA: Long-term effectiveness of maxillary sinus floor
augmentation: A systematic review and meta-analysis. J Clin
Periodontol. 46 (Suppl 21):S307–S318. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Stumbras A, Krukis MM, Januzis G and
Juodzbalys G: Regenerative bone potential after sinus floor
elevation using various bone graft materials: A systematic review.
Quintessence Int. 50:548–558. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Helder MN, van Esterik FAS, Kwehandjaja
MD, Ten Bruggenkate CM, Klein-Nulend J and Schulten E: Evaluation
of a new biphasic calcium phosphate for maxillary sinus floor
elevation: Micro-CT and histomorphometrical analyses. Clin Oral
Implants Res. 29:488–498. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Farré-Guasch E, Prins HJ, Overman JR, Ten
Bruggenkate CM, Schulten EA, Helder MN and Klein-Nulend J: Human
maxillary sinus floor elevation as a model for bone regeneration
enabling the application of one-step surgical procedures. Tissue
Eng Part B Rev. 19:69–82. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Solar P, Geyerhofer U, Traxler H, Windisch
A, Ulm C and Watzek G: Blood supply to the maxillary sinus relevant
to sinus floor elevation procedures. Clin Oral Implants Res.
10:34–44. 1999.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Riben C and Thor A: Follow-up of the sinus
membrane elevation technique for maxillary sinus implants without
the use of graft material. Clin Implant Dent Relat Res. 18:895–905.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sohn DS, Moon JW, Lee WH, Kim SS, Kim CW,
Kim KT and Moon YS: Comparison of new bone formation in the
maxillary sinus with and without bone grafts: Immunochemical rabbit
study. Int J Oral Maxillofac Implants. 26:1033–1042.
2011.PubMed/NCBI
|
|
8
|
Parra M, Olate S and Cantin M: Clinical
and biological analysis in graftless maxillary sinus lift. J Korean
Assoc Oral Maxillofac Surg. 43:214–220. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Stefanski S, Svensson B and Thor A:
Implant survival following sinus membrane elevation without
grafting and immediate implant installation with a one-stage
technique: An up-to-40-month evaluation. Clin Oral Implants Res.
28:1354–1359. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cricchio G, Sennerby L and Lundgren S:
Sinus bone formation and implant survival after sinus membrane
elevation and implant placement: A 1- to 6-year follow-up study.
Clin Oral Implants Res. 22:1200–1212. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cordaro L, Bosshardt DD, Palattella P, Rao
W, Serino G and Chiapasco M: Maxillary sinus grafting with Bio-Oss
or Straumann Bone Ceramic: Histomorphometric results from a
randomized controlled multicenter clinical trial. Clin Oral
Implants Res. 19:796–803. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Avera SP, Stampley WA and McAllister BS:
Histologic and clinical observations of resorbable and
nonresorbable barrier membranes used in maxillary sinus graft
containment. Int J Oral Maxillofac Implants. 12:88–94.
1997.PubMed/NCBI
|
|
13
|
Graziano A, Benedetti L, Massei G, Cusella
de Angelis MG, Ferrarotti F and Aimetti M: Bone production by human
maxillary sinus mucosa cells. J Cell Physiol. 227:3278–3281.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gruber R, Kandler B, Fuerst G, Fischer MB
and Watzek G: Porcine sinus mucosa holds cells that respond to bone
morphogenetic protein (BMP)-6 and BMP-7 with increased osteogenic
differentiation in vitro. Clin Oral Implants Res. 15:575–580.
2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Guo J, Weng J, Rong Q, Zhang X, Zhu S,
Huang D, Li X and Chen S: Investigation of multipotent postnatal
stem cells from human maxillary sinus membrane. Sci Rep.
5(11660)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Srouji S, Kizhner T, Ben David D,
Riminucci M, Bianco P and Livne E: The Schneiderian membrane
contains osteoprogenitor cells: In vivo and in vitro study. Calcif
Tissue Int. 84:138–145. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kim SW, Lee IK, Yun KI, Kim CH and Park
JU: Adult stem cells derived from human maxillary sinus membrane
and their osteogenic differentiation. Int J Oral Maxillofac
Implants. 24:991–998. 2009.PubMed/NCBI
|
|
18
|
Berberi A, Al-Nemer F, Hamade E, Noujeim
Z, Badran B and Zibara K: Mesenchymal stem cells with osteogenic
potential in human maxillary sinus membrane: An in vitro study.
Clin Oral Investig. 21:1599–1609. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cho KS, Park HY, Roh HJ, Bravo DT, Hwang
PH and Nayak JV: Human ethmoid sinus mucosa: A promising novel
tissue source of mesenchymal progenitor cells. Stem Cell Res Ther.
5(15)2014.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Lin GH, Lim G, Chan HL, Giannobile WV and
Wang HL: Recombinant human bone morphogenetic protein 2 outcomes
for maxillary sinus floor augmentation: A systematic review and
meta-analysis. Clin Oral Implants Res. 27:1349–1359.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yamaguchi A, Komori T and Suda T:
Regulation of osteoblast differentiation mediated by bone
morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev.
21:393–411. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Itoh S, Itoh F, Goumans MJ and Ten Dijke
P: Signaling of transforming growth factor-beta family members
through Smad proteins. Eur J Biochem. 267:6954–6967.
2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Miyazono K: Positive and negative
regulation of TGF-beta signaling. J Cell Sci. 113:1101–1109.
2000.PubMed/NCBI
|
|
24
|
Lee MH, Kim YJ, Kim HJ, Park HD, Kang AR,
Kyung HM, Sung JH, Wozney JM, Kim HJ and Ryoo HM: BMP-2-induced
Runx2 expression is mediated by Dlx5, and TGF-beta 1 opposes the
BMP-2-induced osteoblast differentiation by suppression of Dlx5
expression. J Biol Chem. 278:34387–34394. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lee MH, Kwon TG, Park HS, Wozney JM and
Ryoo HM: BMP-2-induced Osterix expression is mediated by Dlx5 but
is independent of Runx2. Biochem Biophys Res Commun. 309:689–694.
2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nakashima M and Reddi AH: The application
of bone morphogenetic proteins to dental tissue engineering. Nat
Biotechnol. 21:1025–1032. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Bowler D and Dym H: Bone morphogenic
protein: Application in implant dentistry. Dent Clin North Am.
59:493–503. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Srouji S, Ben-David D, Lotan R, Riminucci
M, Livne E and Bianco P: The innate osteogenic potential of the
maxillary sinus (Schneiderian) membrane: An ectopic tissue
transplant model simulating sinus lifting. Int J Oral Maxillofac
Surg. 39:793–801. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sohn DS and Moon YS: Histomorphometric
study of rabbit's maxillary sinus augmentation with various graft
materials. Anat Cell Biol. 51 (Suppl 1):S1–S12. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Valentin-Opran A, Wozney J, Csimma C,
Lilly L and Riedel GE: Clinical evaluation of recombinant human
bone morphogenetic protein-2. Clin Orthop Relat Res 110-120,
2002.
|
|
31
|
Haugen HJ, Lyngstadaas SP, Rossi F and
Perale G: Bone grafts: Which is the ideal biomaterial? J Clin
Periodontol. 46 (Suppl 21):S92–S102. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jung RE, Glauser R, Scharer P, Hammerle
CH, Sailer HF and Weber FE: Effect of rhBMP-2 on guided bone
regeneration in humans. Clin Oral Implants Res. 14:556–568.
2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Freitas RM, Spin-Neto R, Marcantonio
Junior E, Pereira LA, Wikesjö UM and Susin C: Alveolar ridge and
maxillary sinus augmentation using rhBMP-2: A systematic review.
Clin Implant Dent Relat Res. 17 (Suppl 1):e192–e201.
2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Boyne PJ, Lilly LC, Marx RE, Moy PK,
Nevins M, Spagnoli DB and Triplett RG: De novo bone induction by
recombinant human bone morphogenetic protein-2 (rhBMP-2) in
maxillary sinus floor augmentation. J Oral Maxillofac Surg.
63:1693–1707. 2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Torrecillas-Martinez L, Monje A, Pikos MA,
Ortega-Oller I, Suarez F, Galindo-Moreno P and Wang HL: Effect of
rhBMP-2 upon maxillary sinus augmentation: A comprehensive review.
Implant Dent. 22:232–237. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
James AW, LaChaud G, Shen J, Asatrian G,
Nguyen V, Zhang X, Ting K and Soo C: A review of the clinical side
effects of bone morphogenetic protein-2. Tissue Eng Part B Rev.
22:284–297. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lee KB, Taghavi CE, Murray SS, Song KJ,
Keorochana G and Wang JC: BMP induced inflammation: A comparison of
rhBMP-7 and rhBMP-2. J Orthop Res. 30:1985–1994. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Woo EJ: Adverse events reported after the
use of recombinant human bone morphogenetic protein 2. J Oral
Maxillofac Surg. 70:765–767. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pjetursson BE, Tan WC, Zwahlen M and Lang
NP: A systematic review of the success of sinus floor elevation and
survival of implants inserted in combination with sinus floor
elevation. J Clin Periodontol. 35 (Suppl 8):S216–S240.
2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mohan N, Wolf J and Dym H: Maxillary sinus
augmentation. Dent Clin North Am. 59:375–388. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tawil G, Barbeck M, Unger R, Tawil P and
Witte F: Sinus floor elevation using the lateral approach and
window repositioning and a xenogeneic bone substitute as a grafting
material: A histologic, histomorphometric, and radiographic
analysis. Int J Oral Maxillofac Implants. 33:1089–1096.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Baksh D, Song L and Tuan RS: Adult
mesenchymal stem cells: Characterization, differentiation, and
application in cell and gene therapy. J Cell Mol Med. 8:301–316.
2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kelly MP, Vaughn OL and Anderson PA:
Systematic review and meta-analysis of recombinant human bone
morphogenetic protein-2 in localized alveolar ridge and maxillary
sinus augmentation. J Oral Maxillofac Surg. 74:928–939.
2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Väänänen HK: Mesenchymal stem cells. Ann
Med. 37:469–479. 2005.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Dennis JE, Carbillet JP, Caplan AI and
Charbord P: The STRO-1+ marrow cell population is multipotential.
Cells Tissues Organs. 170:73–82. 2002.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Shi S and Gronthos S: Perivascular niche
of postnatal mesenchymal stem cells in human bone marrow and dental
pulp. J Bone Miner Res. 18:696–704. 2003.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chaudhury H, Raborn E, Goldie LC and
Hirschi KK: Stem cell-derived vascular endothelial cells and their
potential application in regenerative medicine. Cells Tissues
Organs. 195:41–47. 2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Litvinov SV, Velders MP, Bakker HA,
Fleuren GJ and Warnaar SO: Ep-CAM: A human epithelial antigen is a
homophilic cell-cell adhesion molecule. J Cell Biol. 125:437–446.
1994.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Strutz F, Okada H, Lo CW, Danoff T, Carone
RL, Tomaszewski JE and Neilson EG: Identification and
characterization of a fibroblast marker: FSP1. J Cell Biol.
130:393–405. 1995.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Draenert FG, Nonnenmacher AL, Kammerer PW,
Goldschmitt J and Wagner W: BMP-2 and bFGF release and in vitro
effect on human osteoblasts after adsorption to bone grafts and
biomaterials. Clin Oral Implants Res. 24:750–757. 2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kao DW, Kubota A, Nevins M and Fiorellini
JP: The negative effect of combining rhBMP-2 and Bio-Oss on bone
formation for maxillary sinus augmentation. Int J Periodontics
Restorative Dent. 32:61–67. 2012.PubMed/NCBI
|
|
53
|
Nguyen V, Meyers CA, Yan N, Agarwal S,
Levi B and James AW: BMP-2-induced bone formation and neural
inflammation. J Orthop. 14:252–256. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Huang RL, Yuan Y, Tu J, Zou GM and Li Q:
Opposing TNF-α/IL-1β- and BMP-2-activated MAPK signaling pathways
converge on Runx2 to regulate BMP-2-induced osteoblastic
differentiation. Cell Death Dis. 5(e1187)2014.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Aggarwal S and Pittenger MF: Human
mesenchymal stem cells modulate allogeneic immune cell responses.
Blood. 105:1815–1822. 2005.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Sotiropoulou PA and Papamichail M: Immune
properties of mesenchymal stem cells. Methods in molecular biology.
407:225–243. 2007.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Gonzalez-Rey E, Gonzalez MA, Varela N,
O'Valle F, Hernandez-Cortes P, Rico L, Büscher D and Delgado M:
Human adipose-derived mesenchymal stem cells reduce inflammatory
and T cell responses and induce regulatory T cells in vitro in
rheumatoid arthritis. Ann Rheum Dis. 69:241–248. 2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Prockop DJ and Oh JY: Mesenchymal
stem/stromal cells (MSCs): Role as guardians of inflammation. Mol
Ther. 20:14–20. 2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Castro-Manrreza ME: Participation of
mesenchymal stem cells in the regulation of immune response and
cancer development. Bol Med Hosp Infant Mex. 73:380–387.
2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zara JN, Siu RK, Zhang X, Shen J, Ngo R,
Lee M, Li W, Chiang M, Chung J, Kwak J, et al: High doses of bone
morphogenetic protein 2 induce structurally abnormal bone and
inflammation in vivo. Tissue Eng Part A. 17:1389–1399.
2011.PubMed/NCBI View Article : Google Scholar
|