Introduction
Ultraviolet A (UVA) radiation, which penetrates deep
into the dermis and affects human dermal fibroblasts (HDFs), has an
important role in inducing photoaging (1). The histopathological changes of
photoaging are mainly manifested by the reduction of collagens and
the denaturation and accumulation of elastic fibers (1,2). UVA
not only affects the appearance of the skin, but also causes a
variety of light-associated diseases, including actinic granuloma,
squamous cell carcinoma and malignant melanoma (1,3-5).
Although the mechanism of photoaging remains to be
fully elucidated, there have been numerous advances in recent
years. For example, microRNA (miR)-377 have been reported to
promote the senescence of human dermal fibroblasts by targeting DNA
methyltransferase (6), whilst the
long noncoding RNA (lncRNA) RP11-670E13.6 delays the senescence of
UVB-irradiated dermal fibroblasts by sponging microRNA-663a
(7). These previous studies have
emphasized the essential roles of non-coding RNAs in
photoaging.
Circular (circ)RNAs, in contrast to conventional
linear RNAs, are a class of non-coding RNAs formed by reverse
splicing, without a 5' cap and a 3' polyA tail (8). Compared with other biomolecules,
circRNAs are durable and stable functional molecules that do not
get degraded (9). circRNAs bind
miRNAs with complementary sequences through base-pair binding, thus
acting like a sponge to regulate the expression of miRNA target
genes (10).
It has been previously reported that circRNAs are
involved in the development and progression of various diseases,
including heart failure, cancer and Alzheimer disease (11-13).
However, the role of circRNAs in photoaging remains to be fully
elucidated. Therefore, the present study explored the expression
profiles of circRNAs in ultraviolet A (UVA)-irradiated human dermal
fibroblasts compared with those in the non-irradiated control
group.
Materials and methods
Cell culture and UVA irradiation
The present study was approved by the Medical Ethics
Committee of the Third Affiliated Hospital of Sun Yat-sen
University (Guangzhou, China). The foreskin dermis of six healthy
children aged from 3 to 9 years (7.00±1.63 years) was used to
isolate and culture fibroblasts using procedures previously
described (14). Cells were
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 100 U/ml penicillin
(Sigma-Aldrich; Merck KGaA;), 100 U/ml streptomycin (Sigma-Aldrich;
Merck KGaA) and 10% fetal bovine serum (PAN-Biotech GmbH) in a 37˚C
humidified incubator with 5% CO2. Cells between the 4
and 6th passages were utilized for the assays. When the fibroblasts
were in the exponential growth phase and their density reached 70%,
cells were exposed to UVA irradiation, and control cells were
treated under the same conditions but without UVA exposure. The
radiation device was a UVA lamp with a wavelength of 320-400 nm
(Sigma-Aldrich; Merck KGaA), and the UVA dose was measured using a
UVX digital radiometer (Shenzhen Sunkun Technology Co., Ltd.). The
daily dose was 10 J/cm2 (15). After 14 days of UVA irradiation,
cells were harvested for subsequent experiments.
Confirmation of establishment of
UVA-irradiated HDF model of ageing
A Senescence-Associated β-Galactosidase (SA-β-Gal)
Staining kit (cat. no. C0602), purchased from Beyotime Institute of
Biotechnology, was used to detect the senescent cells using
manufacturer's protocol.
Detection of cell cycle-associated
protein expression
Protein was extracted from cells using a Protein
Extraction kit (Nanjing Keygen Biotech Co., Ltd.). The BCA Protein
Assay kit (Thermo Fisher Scientific, Inc.) was used to detect the
protein concentration. The protein (50 µg/lane) was separated by
12% SDS-PAGE and electro-transferred onto a 0.45-µm nitrocellulose
membrane (EMD Millipore) for 90 min. The membrane was blocked with
5% bovine serum albumin (NeoFROXX GmbH) and incubated overnight at
4˚C on a shaker with the following monoclonal primary antibodies:
Rabbit P21 (1:1,000 dilution; cat. no. 2947; Cell Signaling
Technology, Inc.), rabbit P16 (1:1,000 dilution; cat. no. ab51243;
Abcam) and mouse P53 (1:1,000 dilution; cat. no. 9282; Cell
Signaling Technology, Inc.). Subsequently, the membrane was washed
with TBS-T (0.05% Tween-20 in TBS; pH 7.4) and incubated with
horseradish peroxidase-conjugated secondary antibodies (1:5,000
dilution; cat. nos. 7074 and 7076; Cell Signaling Technology, Inc.)
for 1 h at room temperature and protein bands were visualized by
chemiluminescence (ECL Advanced Detection Kit; EMD Millipore).
GAPDH (1:1,000 dilution; cat. no. 2118; Cell Signaling Technology,
Inc.) was used as an internal control. The band densities were
measured using ImageJ software (version 1.50; National Institutes
of Health).
RNA extraction and quality
control
Total RNA was extracted from fibroblasts by
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol, and a NanoDrop
ND-1000 (NanoDrop; Thermo Fisher Scientific, Inc.) was used to
determine the purity and concentration of total RNA in the
samples.
circRNA microarrays and bioinformatics
analysis
Total RNAs from each sample were treated with RNase
R (cat. no. M1228-500; BioVision, Inc.) to enrich circRNAs.
Subsequently, referring to the Arraystar super RNA Labeling scheme
(Arraystar, Inc.), the enriched circRNAs were amplified with random
primers and transcribed into fluorescent complementary RNA (cRNA).
The labeled cRNAs were hybridized onto Arraystar Human circRNA
Arrays V2 (8x15 K; Arraystar, Inc.) and incubated for 17 h at 65˚C
in an Agilent hybridization oven (Agilent Technologies, Inc.).
After washing, the slides were scanned with the Agilent Scanner
G2505C (Agilent Technologies, Inc.). Agilent Feature Extraction
software (Version 11.0.1.1) was used to extract the data (Agilent
Technologies, Inc.). Subsequently, the R software package ‘limma’
(16) was used to perform a series
of data processing steps. After the original data was normalized,
high-quality probes were screened. The probes were marked as
present (P), marginal (M) or absent (A). In total, ≥ three of the
six samples of circRNAs were flagged in ‘P’ or ‘M’ under ‘All
Targets Value’, and these circRNAs were retained for improved
differential analysis. A statistically significant difference (fold
change >1.5) in expression of circRNAs was identified between
two groups by multiple change cut-off or volcano plot filtration.
The Arraystar proprietary miRNA target prediction software and
TargetScan Human 7.2 (http://www.targetscan.org/vert_72/) were used to
identify the potential interactions between circRNAs and their
target microRNAs. TargetScan v7.2 and miRDB v5 were used to predict
the target genes of these microRNAs. Using the overlapping data,
three circRNAs were selected that were associated with COL1A1 and
ELN for further qPCR verification. Gene Ontology (GO) annotation
and KEGG pathway analysis were performed to provide evidence for
the further functional prediction of the differentially expressed
circRNAs. These circRNAs were input into the Database for
Annotation, Visualization and Integrated Discovery (http://david.abcc.ncifcrf.gov/) and the KEGG
database (http://www.genome.jp/kegg/). The
P-values denote the significance of the GO term enrichment and the
correlation of KEGG pathway (P<0.05 was considered to indicate a
statistically significant result). The circRNA expression chip and
bioinformatics data analyses were completed by Shanghai Kangcheng
Biotechnology Co., Ltd.
RT-qPCR
Total RNA was isolated from the samples using the
TRIzol® Plus RNA Purification kit (Thermo Fisher
Scientific, Inc.), and its concentration and purity were determined
using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific,
Inc.). Complementary (c)DNA was synthesized by reverse-transcribing
2 µg total RNA using the PrimeScript™ RT Reagent kit (Takara Bio,
Inc.). cDNA was amplified according to the instructions of the TB
Green Fast qPCR Mix (Takara Bio Inc.). The volume of the PCR
amplification mixture was 20 µl, including 10 µl 2X qPCR
SYBR®-Green Master Mix, 0.5 µl forward primer (10 µM),
0.5 µl reverse primer (10 µM), 1 µl cDNA and 8 µl RNase-free
H2O. The amplification conditions were as follows:
Pre-denaturation at 95˚C for 5 min, followed by 40 cycles of
denaturation at 95˚C for 10 sec and annealing at 60˚C for 34 sec.
The relative expression was quantified using the 2-ΔΔCq
method (17), and GAPDH was used as
an internal reference gene. Sequences of the primer pairs were
listed in Table I.
 | Table IPrimer sequences of circular RNAs. |
Table I
Primer sequences of circular RNAs.
| circBase ID | Sequence (5'-3') | Size, bp |
|---|
|
hsa_circ_0006766-F |
TGTTGCCATTACAGGGGTAAGT | 146 |
|
hsa_circ_0006766-R |
CGGGAAGGAAAGTGATATTTGG | |
|
hsa_circ_0011129-F |
CTTCCGGGCCCAGGTCCTTC | 149 |
|
hsa_circ_0011129-R |
AGTGCAGGCGCCAGAAGCTGG | |
|
hsa_circ_0017502-F |
CCAGATCATGCCAGGTGACAT | 135 |
|
hsa_circ_0017502-R |
TACGGAACTGCACGCTGAAC | |
| GAPDH-F |
GACACCATGGGGAAGGTGAA | 79 |
| GAPDH-R |
AGTTAAAAGCAGCCCTGGTG | |
Statistical analysis
The results are representative of three independent
experiments and are presented as the mean ± standard error of the
mean. Statistical analysis was performed using SPSS 20.0
statistical software (IBM Corp.). The F-test was used to test the
homogeneity of variance. The independent samples Student's t-test
was used to compare the means between the chronic UVA irradiation
group and the unexposed control group. P<0.05 was considered to
indicate a statistically significant difference.
Results
Verification of the skin fibroblast
model of photoaging
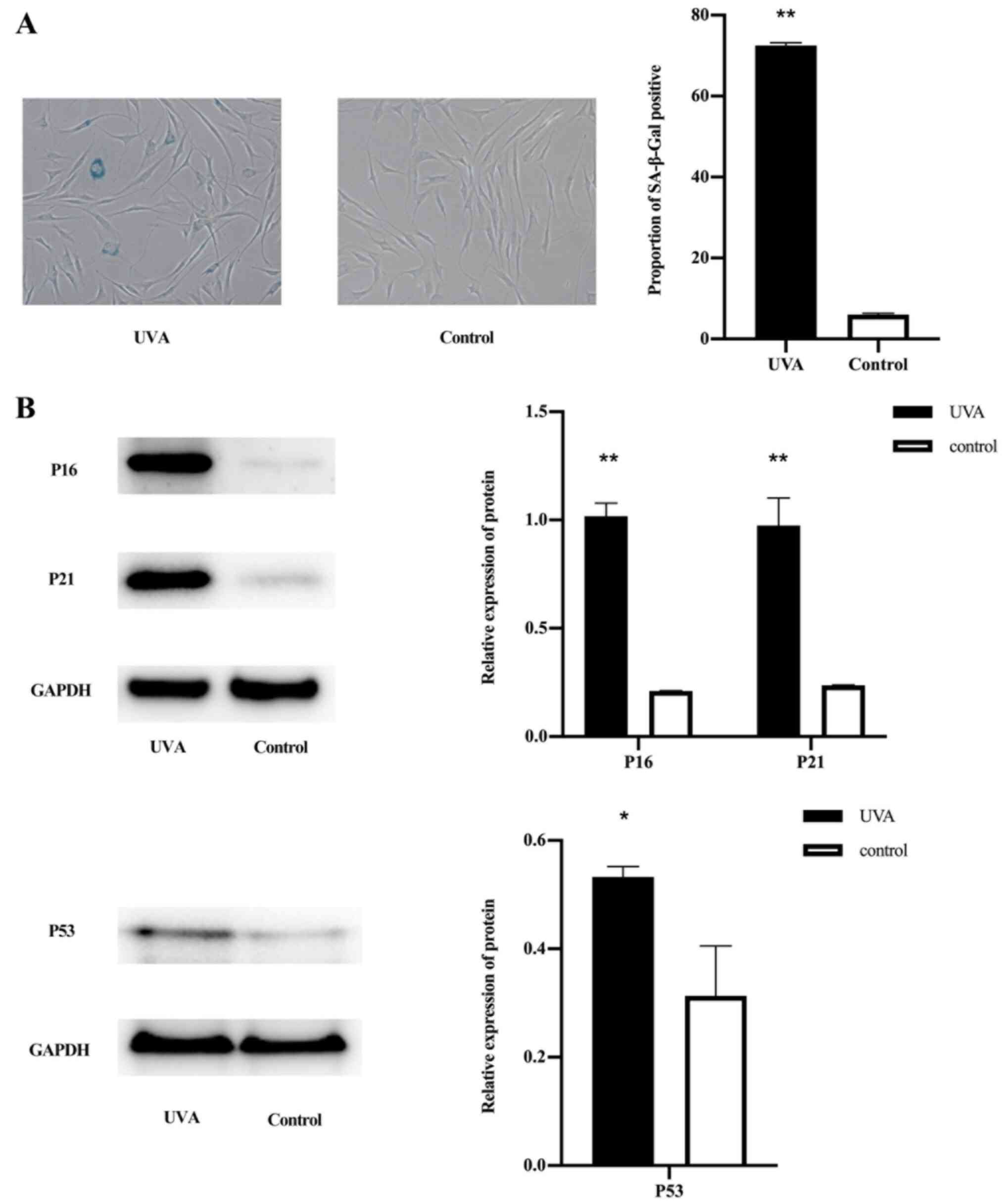
A number of changes including increased SA-β-Gal
activity and cell cycle-associated protein expression have been
reported to be induced by UVA irradiation (18,19).
Compared with that in the normal control group, the senescence
staining was increased in the UVA-treated group (Fig. 1A). Western blot analysis revealed
that the expression of p16, p21 and p53 in the UVA-treated group
was higher compared with that in the control group (Fig. 1B).
circRNA expression profiles in
UVA-irradiated human dermal fibroblasts
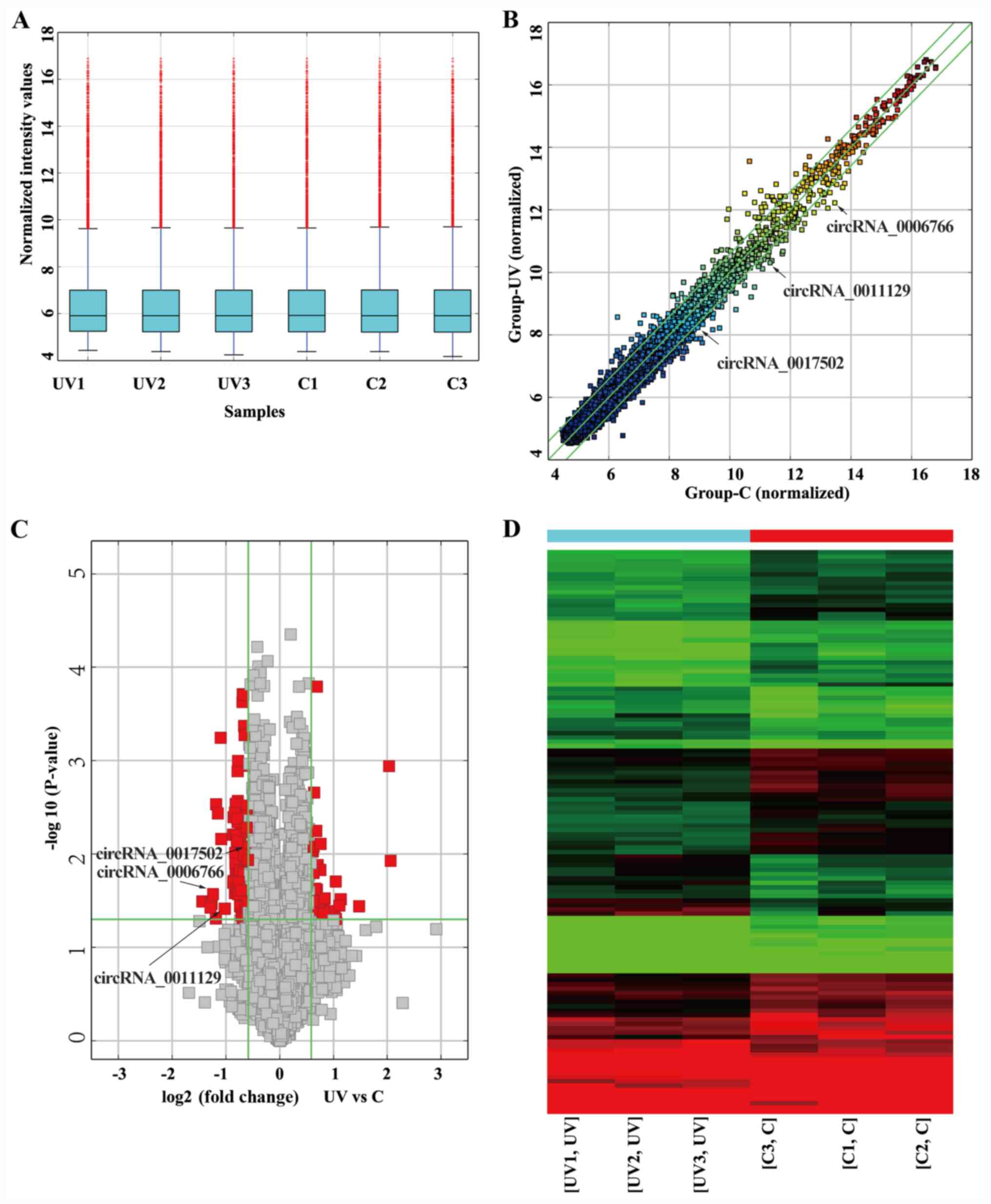
A circRNA microarray was used to obtain the
expression profiles of circRNAs in UVA-irradiated human dermal
fibroblasts. The distribution of circRNA expression profiles
exhibited no differences between the UVA-treated group and the
non-irradiated control group (Fig.
2A). Differentially expressed circRNAs between two compared
groups were identified through Fold Change filtering (Fig. 2B) or Volcano Plot filtering
(Fig. 2C). In Fig. 2D, hierarchical clustering was
utilized to show circRNA expression profiling among the all
samples. The data showed that there was a distinctly
distinguishable circRNAs expression profile between the UVA-treated
group and non-irradiated group. Compared with the non-irradiated
control group, 128 circRNAs were determined to be differentially
expressed after UVA exposure based on the criteria FC>1.5 and
P<0.05, including 39 upregulated and 89 downregulated circRNAs.
The top 20 aberrant circRNAs are listed in Table II.
 | Table IIcircRNAs differentially expressed
following ultraviolet A treatment. |
Table II
circRNAs differentially expressed
following ultraviolet A treatment.
| A, Upregulated
circRNAs |
|---|
| circRNA | FC (abs) | Gene symbol | P-value |
|---|
|
hsa_circRNA_103065 | 4.1715 | MYBL2 | 0.0118 |
|
hsa_circRNA_100445 | 4.0847 | TATDN3 | 0.0011 |
|
hsa_circRNA_103361 | 2.7804 | SMARCC1 | 0.0362 |
|
hsa_circRNA_000401 | 2.1920 | BRD9 | 0.0305 |
|
hsa_circRNA_048148 | 2.1574 | CNN2 | 0.0356 |
|
hsa_circRNA_104484 | 2.0623 | ZC3HC1 | 0.0494 |
|
hsa_circRNA_404870 | 2.0559 | METTL15 | 0.0197 |
|
hsa_circRNA_005411 | 2.0248 | EXTL3 | 0.0407 |
|
hsa_circRNA_101093 | 1.7798 | NUP107 | 0.0299 |
|
hsa_circRNA_092418 | 1.7606 | B2M | 0.0434 |
| B, Downregulated
circRNAs |
| circRNA | FC (abs) | Gene symbol | P-value |
|
hsa_circRNA_101621 | 2.7131449 | CEMIP | 0.032211923 |
|
hsa_circRNA_007624 | 2.4404224 | BCAR3 | 0.037482416 |
|
hsa_circRNA_004585 | 2.4282438 | CEMIP | 0.032890625 |
|
hsa_circRNA_000675 | 2.4117771 | NDUFA10 | 0.034232964 |
|
hsa_circRNA_006766 | 2.3634626 | CCL24 | 0.026828638 |
|
hsa_circRNA_100191 | 2.2776152 | PTPRF | 0.048836243 |
|
hsa_circRNA_004594 | 2.2729704 | MLLT1 | 0.002923375 |
|
hsa_circRNA_102817 | 2.219963 | SAP130 | 0.003660652 |
|
hsa_circRNA_062539 | 2.1416076 | BCR | 0.00056699 |
|
hsa_circRNA_104647 | 2.1249749 | ZFHX4 | 0.006894599 |
GO and KEGG pathway analyses
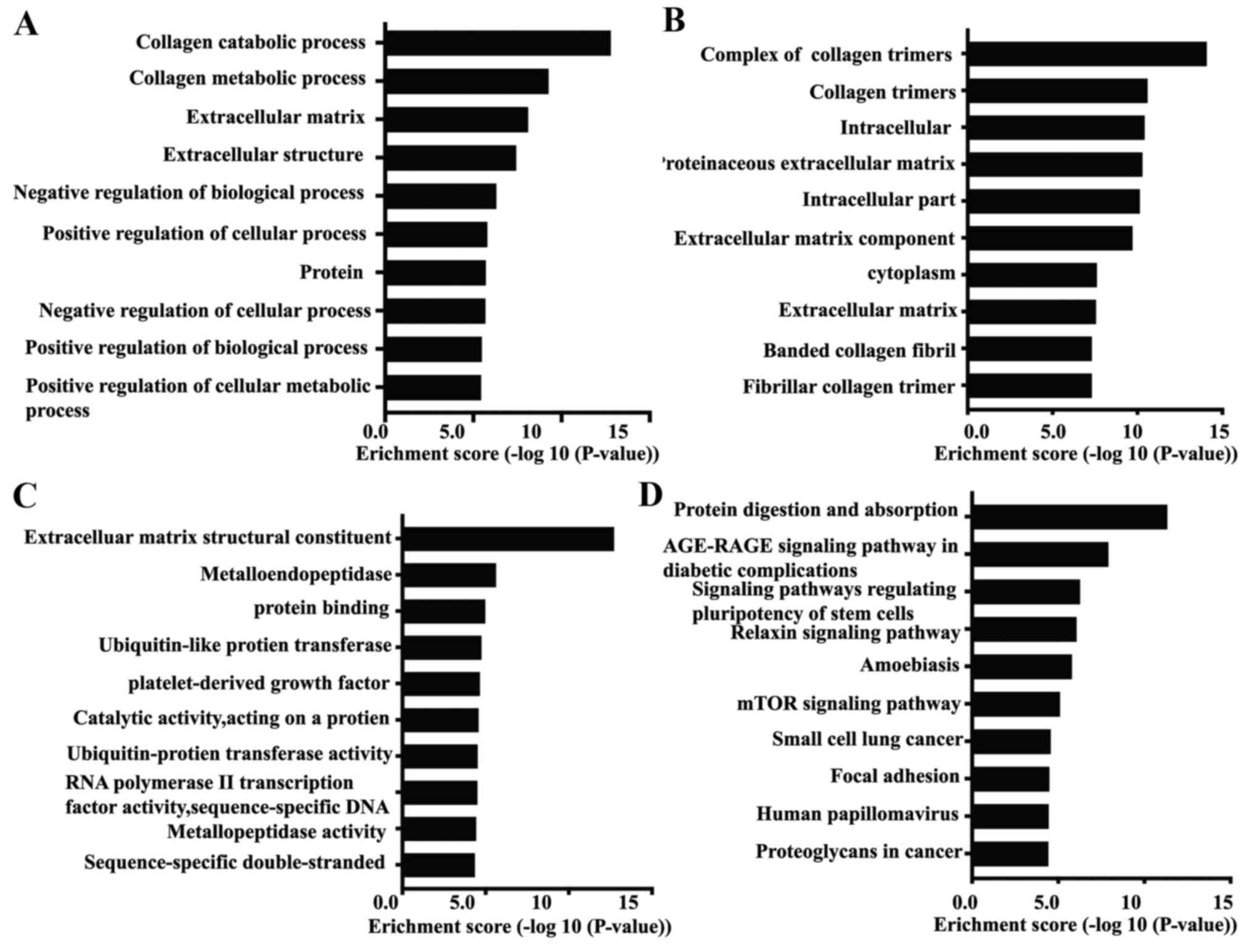
To determine the roles of circRNAs in the physiology
and pathology of fibroblasts after UVA irradiation, GO and KEGG
pathway analyses of mRNAs transcribed from parental genes of the
differentially expressed circRNAs were performed. The results
demonstrated that the most significantly enriched relevant GO terms
in the biological process category were ‘collagen catabolic
process’ and ‘collagen metabolic process’, as well as
‘extracellular matrix’ (ECM; Fig.
3A). In the cellular component category, the parental genes of
the differentially expressed circRNA were identified to be
accumulated in the ‘complex of collagen trimers’ (Fig. 3B). In the molecular function
category, the most significantly enriched terms were ‘extracellular
matrix structural construction’, ‘metalloendopeptidase’ and
‘protein binding’ (Fig. 3C). KEGG
pathway analysis revealed the top 10 pathways that may be involved
in skin photoaging (Fig. 3D). Among
these, the ‘Age-RAGE signaling pathway in diabetic complications’
had been reported to be involved in skin photoaging (20,21).
Verification of differentially
expressed circRNAs by RT-qPCR
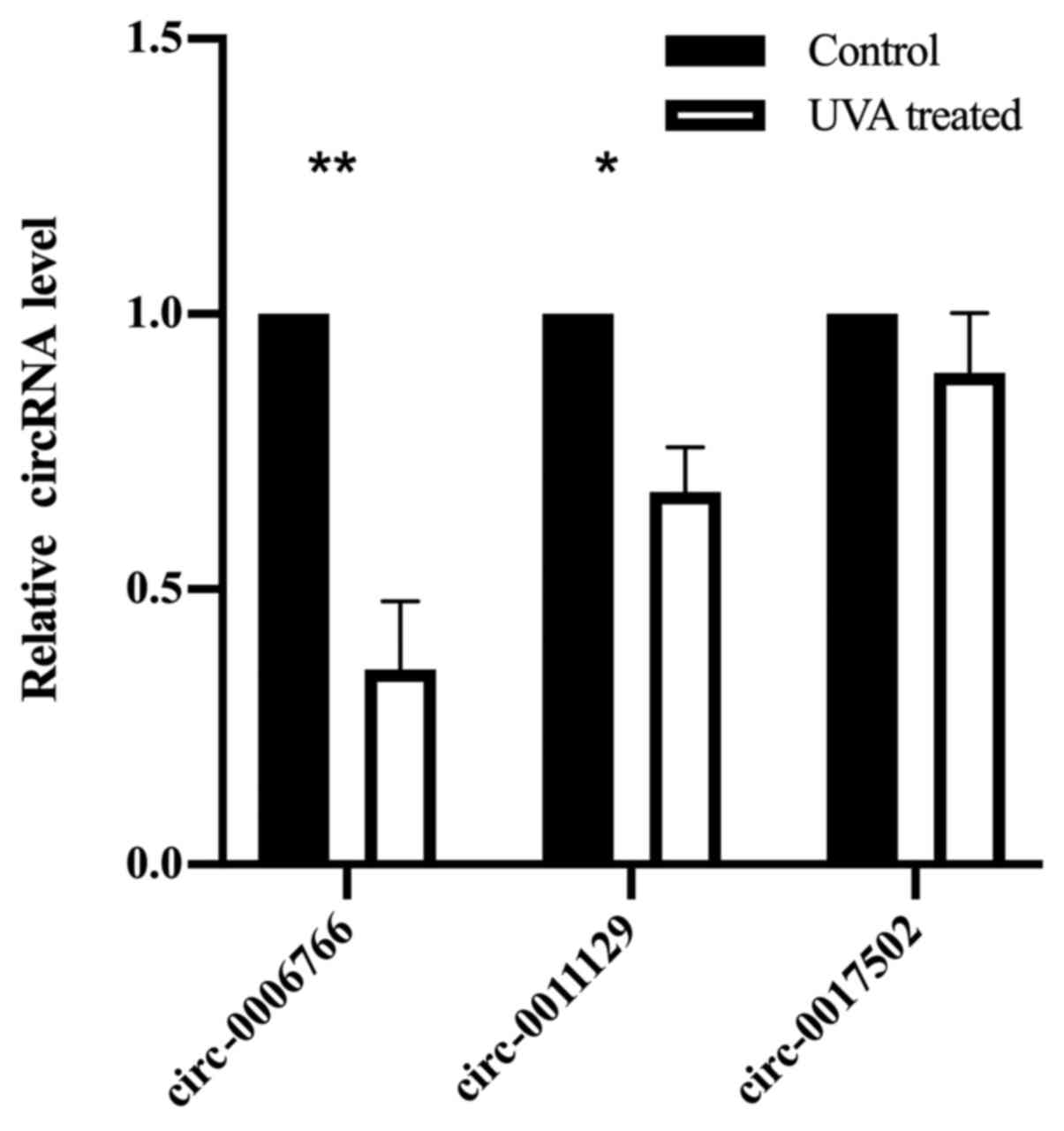
The main pathological changes of photoaging are the
accumulation of abnormal elastin and a severe loss of collagen
fibers in dermis (1,2). To verify the data of the circRNA
microarray, three circRNAs that were predicted to be associated
with collagen type I (COL1A1) and elastin (ELN) were selected to be
confirmed by RT-qPCR analysis. There were no significant
differences in the expression levels of circ-0017502, but there
were significant downregulations in the expression levels of
circ-0006766 and circ-0011129 in the UVA-irradiated group compared
with those in the control group (Fig.
4). The expression pattern was similar to that obtained from
the circRNA microarray.
Discussion
Accumulating evidence indicates UVA to be a major
contributing factor in photoaging, but its mechanisms remain to be
fully elucidated (1). Furthermore,
UVA is associated with a variety of light-associated diseases,
including skin cancer (4).
Therefore, developing new markers for early diagnosis and the
design of preventive strategies would be of benefit. Non-coding
RNAs have been widely assessed in studies on gene function and
disease treatment. For example, MRX34, a microRNA (miRNA) mimic,
has reached phase 1 studies in patients with advanced solid tumors,
including hepatocellular carcinoma, pancreatic cancer and
cholangiocarcinoma (22). miR-146a
may serve as a promising therapeutic target for dengue (23). Wheatley et al (24) previously developed a DNA vaccine
vector co-expressing an miRNA designed in silico miRNA to
inhibit PERK with a HIV-1 envelope. In the field of skin
photoaging, non-coding RNA has also received increasing attention.
For instance, downregulation of miR-34c-5p may inhibit the p53
pathway by increasing the level of the downstream target E2F
transcription factor 3 and ultimately impair cell senescence
(25). However, miRNAs and lncRNAs
have a linear structure, due to which their stability is
insufficient to withstand treatment with RNase (the 3' exonuclease
of hydrolyzable linear RNAs) (26),
limiting their clinical application. Compared with linear
non-coding RNAs such as miRNAs and lncRNAs, circRNAs have the
advantage of being more stable (9,27).
The role of circRNAs in photoaging remains to be
fully elucidated. Previous studies by our group have indicated that
circCOL3A1-859267, which targets miR-29c, is able to regulate the
expression of type I collagen in fibroblasts (19,28).
In the present study, the expression profile of circRNAs in
UVA-irradiated fibroblasts was determined, and 128 differentially
expressed circRNAs (FC>1.5 and P<0.05) were identified,
including 39 upregulated and 89 downregulated circRNAs. GO and KEGG
analyses indicated that the aberrant circRNAs were implicated in
ECM organization and metabolism. Subsequently, RT-qPCR was used to
verify three differentially expressed circRNAs and the results were
similar to those of the circRNA microarray. Of note, the
differentially expressed circRNAs identified in the present study
require further assessment by bioinformatics analyses and in
vivo and in vitro experiments. For instance, the
aberrant accumulation of ELN and the extensive loss of collagen
fibers in dermis are the fundamental pathological changes of skin
photoaging (29). Through the
Arraystar proprietary miRNA target prediction software and
TargetScan, the present study identified that the miRNAs that
circ-0011129 may sponge, including hsa-miR-484, hsa-miR-3619-5p and
hsa-miR-6732-5p, share binding sites with photoaging-related
proteins, such as collagen type I α1 (COL1A1), COL3A1 or
elastin.
Zhang et al (30) previously revealed that miR-6732-5p
was one of seven significantly upregulated miRNAs in atypical
meningioma patients that are resistant to radiotherapy, where the
differentially expressed miRNAs were enriched mostly in the
transforming growth factor-β (TGF-β) signaling pathway. In
addition, Quan et al (31)
revealed that UV irradiation impaired the TGF-β/Smad pathway in
human dermal fibroblasts, thereby downregulating the expression of
TGF-β target genes such as type I procollagen. Type I collagen is
one of the main components of the dermal extracellular matrix.
Extensive degradation of type I collagen is a fundamental
pathological change of skin photoaging (1). Therefore, circ-0011129 is hypothesized
to regulate the expression of type I collagen by targeting the
miR-6732-5p and TGF-β/Smad pathway.
Several limitations of the present study should be
acknowledged. The effect of ultraviolet A (UVA) on fibroblasts was
explored but not ultraviolet B (UVB). Although both UVA and UVB are
involved in skin photoaging, UVA penetrates deeper than UVB and
induces more profound alterations in the dermal connective tissue
(31,32). Therefore, for fibroblasts in the
dermis, UVA has a greater impact. In addition, regulatory
mechanisms of the differentially expressed circRNAs on photoaging
remain to be fully elucidated, which serve to be the next step in
this research.
In conclusion, the present study provided a unique
circRNA profile in UVA-irradiated fibroblasts. These altered
circRNAs may provide an avenue to elucidate the mechanisms of skin
photoaging, as well as facilitate the development of small
molecules to prevent and treat photoaging and associated skin
diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81673047).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL proposed the experimental concept and designed
the study. ML and YZ established the UVA irradiation model and were
major contributors in writing the manuscript. ML, QL, YL, QX and YL
performed the microarray data analysis and performed critical data
analyses. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Informed written consent of patients' parents was
obtained prior to tissue resection. The present study and all
procedures were approved by the Medical Ethics Committee of the
Third Affiliated Hospital of Sun Yat-sen University [no.
(2016)2-63].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gilchrest BA: Photoaging. J Invest
Dermatol. 133:E2–E6. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Weihermann AC, Lorencini M, Brohem CA and
de Carvalho CM: Elastin structure and its involvement in skin
photoaging. Int J Cosmet Sci. 39:241–247. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schneider SL and Lim HW: Review of
environmental effects of oxybenzone and other sunscreen active
ingredients. J Am Acad Dermatol. 80:266–271. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Burke KE: Mechanisms of aging and
development-a new understanding of environmental damage to the skin
and prevention with topical antioxidants. Mech Ageing Dev.
172:123–130. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ma DL and Vano-Galvan S: Actinic
Granuloma. N Engl J Med. 376(475)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xie HF, Liu YZ, Du R, Wang B, Chen MT,
Zhang YY, Deng ZL and Li J: MiR-377 induces senescence in human
skin fibroblasts by targeting DNA methyltransferase 1. Cell Death
Dis. 8(e2663)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li M, Li L, Zhang X, Zhao H, Wei M, Zhai
W, Wang B and Yan Y: LncRNA RP11-670E13.6, interacted with hnRNPH,
delays cellular senescence by sponging microRNA-663a in UVB damaged
dermal fibroblasts. Aging (Albany NY). 11:5992–6013.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kristensen LS, Andersen MS, Stagsted LVW,
Ebbesen KK, Hansen TB and Kjems J: The biogenesis, biology and
characterization of circular RNAs. Nat Rev Genet. 20:675–691.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Guo JU, Agarwal V, Guo H and Bartel DP:
Expanded identification and characterization of mammalian circular
RNAs. Genome Biol. 15(409)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang K, Long B, Liu F, Wang JX, Liu CY,
Zhao B, Zhou LY, Sun T, Wang M, Yu T, et al: A circular RNA
protects the heart from pathological hypertrophy and heart failure
by targeting miR-223. Eur Heart J. 7:2602–2611. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen J, Li Y, Zheng Q, Bao C, He J, Chen
B, Lyu D, Zheng B, Xu Y, Long Z, et al: Circular RNA profile
identifies circPVT1 as a proliferative factor and prognostic marker
in gastric cancer. Cancer Lett. 388:208–219. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dube U, Del-Aguila JL, Li Z, Budde JP,
Jiang S, Hsu S, Ibanez L, Fernandez MV, Farias F, Norton J, et al:
An atlas of cortical circular RNA expression in Alzheimer disease
brains demonstrates clinical and pathological associations. Nat
Neurosci. 22:1903–1912. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen FG, Zhang WJ, Bi D, Liu W, Wei X,
Chen FF, Zhu L, Cui L and Cao Y: Clonal analysis of nestin(-)
vimentin(+) multipotent fibroblasts isolated from human dermis. J
Cell Sci. 120:2875–2883. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lamore SD and Wondrak GT: UVA causes dual
inactivation of cathepsin B and L underlying lysosomal dysfunction
in human dermal fibroblasts. J Photchem Photobiol B. 123:1–12.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43(e47)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak K and Schmittgen T: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Park YM and Park SN: Inhibitory effect of
lupeol on MMPs expression using aged fibroblast through repeated
UVA Irradiation. Photochem Photobiol. 95:587–594. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Peng Y, Song X, Zheng Y, Cheng H and Lai
W: circCOL3A1-859267 regulates type I collagen expression by
sponging miR-29c in human dermal fibroblasts. Eur J Dermatol.
28:613–620. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bouchie A: First microRNA mimic enters
clinic. Nat Biotechnol. 31(577)2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Farrar MD: Advanced glycation end products
in skin ageing and photoageing: What are the implications for
epidermal function? Exp Dermatol. 25:947–948. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Beg MS, Brenner AJ, Sachdev J, Borad M,
Kang YK, Stoudemire J, Smith S, Bader AG, Kim S and Hong DS: Phase
I study of MRX34, a liposomal miR-34a mimic, administered twice
weekly in patients with advanced solid tumors. Invest New Drugs.
35:180–188. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wong RR, Abd-Aziz N, Affendi S and Poh CL:
Role of microRNAs in antiviral responses to dengue infection. J
Biomed Sci. 27(4)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wheatley AK, Kramski M, Alexander MR, Toe
JG, Center RJ and Purcell DF: Co-expression of miRNA targeting the
expression of PERK, but not PKR, enhances cellular immunity from an
HIV-1 Env DNA vaccine. PLoS One. 6(e18225)2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhou BR, Guo XF, Zhang JA, Xu Y, Li W, Wu
D, Yin ZQ, Permatasari F and Luo D: Elevated miR-34c-5p mediates
dermal fibroblast senescence by ultraviolet irradiation. Int J Biol
Sci. 9:743–752. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ivanov A, Memczak S, Wyler E, Torti F,
Porath HT, Orejuela MR, Piechotta M, Levanon EY, Landthaler M,
Dieterich C, et al: Analysis of intron sequences reveals hallmarks
of circular RNA biogenesis in animals. Cell Rep. 10:170–177.
2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
You X, Vlatkovic Babic A, Will T, Epstein
Tushev G, Akbalik G, Wang M, Glock C, Quedenau C, et al: Neural
circular RNAs are derived from synaptic genes and regulated by
development and plasticity. Nat Neurosci. 18:603–910.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Peng Y, Song X, Zheng Y, Wang X and Lai W:
Circular RNA profiling reveals that circCOL3A1-859267 regulate type
I collagen expression in photoaged human dermal fibroblasts.
Biochem Biophys Res Commun. 486:277–284. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xu D, Li D, Zhao Z, Wu J and Zhao M:
Regulation by walnut protein hydrolysate on the components and
structural degradation of photoaged skin in SD rats. Food Funct.
10:6792–6802. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang X, Zhang G, Huang H, Li H, Lin S and
Wang Y: Differentially expressed MicroRNAs in radioresistant and
radiosensitive atypical meningioma: A clinical study in chinese
patients. Front Oncol. 10(501)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Quan T, He T, Kang S, Voorhees JJ and
Fisher GJ: Solar ultraviolet irradiation reduces collagen in
photoaged human skin by blocking transforming growth factor-beta
type II receptor/Smad signaling. Am J Pathol. 165:741–751.
2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Battie C, Jitsukawa S, Bernerd F, Del Bino
S, Marionnet C and Verschoore M: New insights in photoaging, UVA
induced damage and skin types. Exp Dermatol. 23 (Suppl 1):7–12.
2014.PubMed/NCBI View Article : Google Scholar
|