Introduction
Mild traumatic brain injury (mTBI) is a growing
public health concern globally, representing 70-90% of all TBIs
(1). Traumatic brain injury
resulting from impact-acceleration forces leads to diffuse axonal
injury (DAI), which is considered to be a key feature of pathology
following mTBI. mTBI is difficult to detect on conventional
computed tomography (CT) or magnetic resonance imaging (MRI)
(2). While most patients with mTBI
symptoms recover in 7-10 days, 10-40% of patients may suffer
persistent symptoms, with long-term cognitive deficits and white
matter changes (3). Furthermore,
repeated mTBI may cause an increased incidence of chronic traumatic
encephalopathy (4). However, it is
difficult to predict the prognosis of neural functional recovery
after DAI at the early stage, and clinically relevant experimental
models of DAI still require improvement (5). Consequently, we simulated the DAI
occurrence mechanism with reference to Namjoshi et al
(6) and Li et al (7) injury device and constructed an
in-house rat model of DAI, which enabled simultaneous and
instantaneous extra-large rotational acceleration and linear
acceleration at the head of the rat, thus providing a useful tool
for DAI study (8).
The diffusion of water molecules is closely related
to changes to the myelin sheath, axolemma, and ultra-structure
inside the axon after axonal injury. Diffusion tensor imaging (DTI)
can detect the diffusion changes in water molecules and the
coherence of fibrous structures sensitively in patients with DAI
(9). DTI can assess the random
movement of protons of water molecules in terms of the apparent
diffusion coefficient (ADC) and orientational dependence
(fractional anisotropy, FA). The corpus callosum is a common site
of high tension from impact, with frequent abnormalities identified
by DTI (10,11). Axonal injury has been characterized
histologically using β-amyloid precursor protein (β-APP)
immunohistochemistry (IHC), which normally traverses the length of
the axon, and accumulates in response to injury (12). The aims of this study were to
determine the diagnostic accuracy of DTI in a rat model of DAI with
direct comparison to histopathologically-confirmed axonal injury,
and to identify any correlations with neural functional
recovery.
Materials and methods
DAI model establishment
All studies were approved by the Animal Ethics
Committee of Chongqing Medical University (approval no. 20170301).
One hundred adult male Sprague-Dawley rats, aged 2-3 months and
weighing 300-400 g, were used in this study. The animals were kept
in cages at room temperature of 22-25˚C, air humidity of 40%, air
pressure of 101.325 kPa and 12 h light/dark cycles. All animals had
free access to common rat feed and water. The rats were randomly
allocated to two groups (control, n=25; experimental, n=75). The
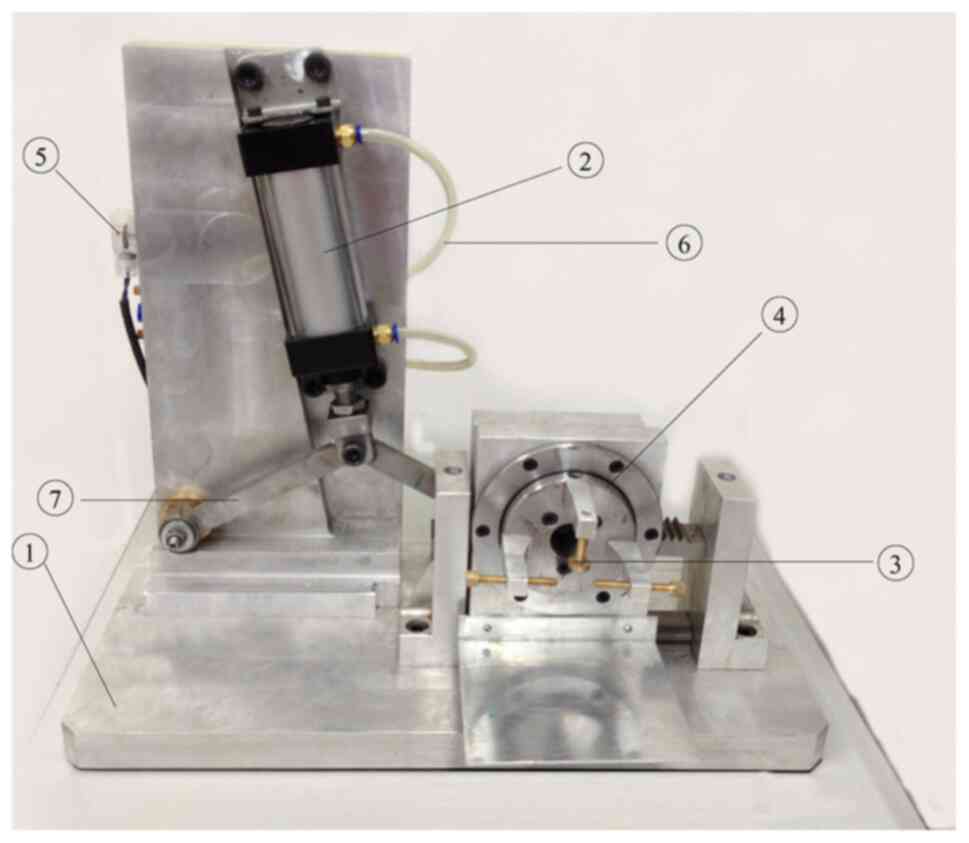
self-made injury device was used to establish the DAI rat model at
different injury levels. The device comprises a pneumatic
conversion device driven by a high air flow rate. The air pressure
of the power cylinder reaches the preset air pressure (0.8 kPa)
through the charging of the electric air charging pump. The
pneumatic conversion system is started by pushing the gear slider
to make a linear acceleration movement to the right in an instant.
With the action of the slider, the gears rotate and move around its
center at the same time via the meshing action of the gears and
racks to produce the compound motion of instantaneous super linear
acceleration and angular acceleration. The start switch of the
pneumatic transfer system is triggered continuously, which can
realize multiple round-trip rotation and acceleration. According to
the number of repeated injuries delivered, the rats were divided
into the mild injury group A, the moderate injury group B, and the
severe injury group C, with 25 rats in each group (please refer to
the schematic diagram shown in Fig.
1).
All the rats of the experimental group were
anesthetized via an intraperitoneal injection of chloral hydrate
(300 mg/kg). The head of the rat in the experimental group was
fixed in the rat connector. The actuating cylinder was inflated to
0.8 kPa. The rats in group A, B, and C received repeated axial
linear plus sagittal rotational acceleration impact for 1, 2, and 3
times, respectively, through the swinging lever and rotating part.
The injured rats were monitored post-injury until they awakened and
could move normally. The wake-up time and recovery time interval of
neurophysiological reflexes (corneal reflex, hindlimb claw reflex,
and righting reflex) in the injured groups were observed.
Methods of rat euthanasia
Humane endpoints were established following the
University of Pennsylvania's Institutional Animal Care and Use
Committee (IACUC) guideline in order to minimize pain or distress
to experimental animals. Cervical vertebra dislocation was used for
euthanasia, as rapid brain tissue collection is required for
neurophysiological studies. The rats were anesthetized via an
intraperitoneal injection of chloral hydrate (300 mg/kg) prior to
euthanasia by cervical vertebra dislocation. Cardiorespiratory
arrest was used to verify rat death.
Magnetic resonance imaging
At 6, 24, 72 h, and 1, 2 weeks post-injury, to
prevent the influence on the DTI scanning result caused by limb
exercise, unstable breath, and heart rate, 10 of the 25 rats in
each group (control group and experimental group A, B, C) received
intraperitoneal anesthetization with chloral hydrate (300 mg/kg).
An MRI scanner (Philips Achieva 3.0T) and an 8-channel
knee-phased-array coil were used for MRI.
Turbo spin-echo T2-weighted image parameters were as
follows: Repetition time (TR)=5,000 msec; echo time (TE)=100 msec;
slice thickness=2.0 mm; field of view=74x74x42 mm3; and
voxel size=0.3x0.3x2.0 mm3. DTI data were acquired using
a spin echo planar sequence with TR/TE=2907 msec/63 msec; b0=0
sec/mm2; b1=1,000 sec/mm2; direction
number=25, slice thickness=2.0 mm; number of signal averages
(NSA)=5; and field of view (FOV)=74x74x42 mm.
Post-image processing
Post-image processing was acquired at the Extended
MR WorkSpace workstation (version no. 2.6.3.5; Philips Medical
Systems Mr, Inc.) with the production of an FA map, an ADC map and
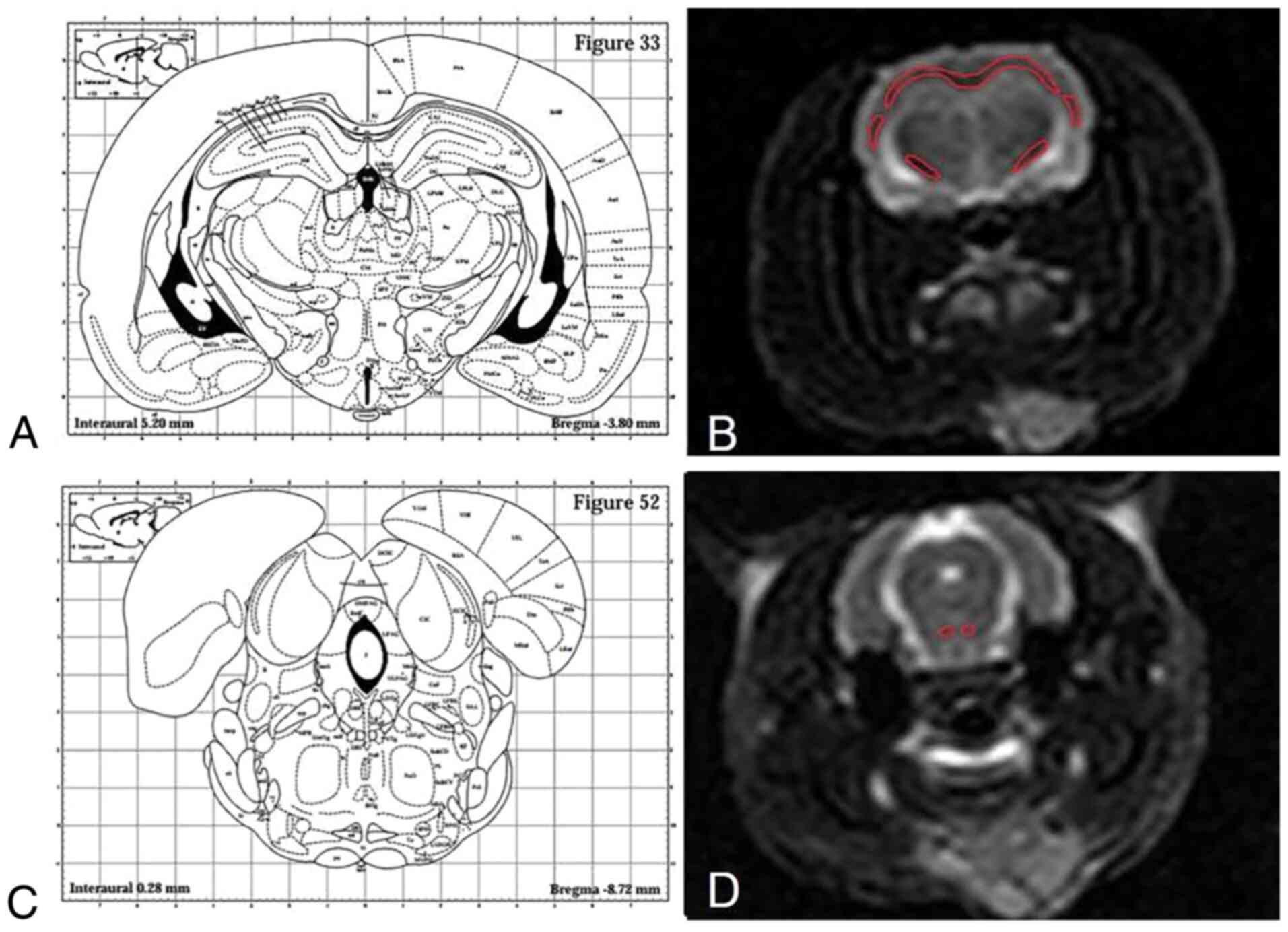
a 3D tensor tractography (3D-DTT) map. Since the corpus callosum
and external capsule of the rat have a continuous white board
layer, the dividing line between the two was unified and defined as
the following, according to brain stereotaxic atlas: Inferior
margin ligature of cingulate cortex (bregma + 1.60-0.92 mm),
inferior margin ligature of fimbria hippocampi (bregma-0.92-2.80
mm) and inferior margin ligature of hippocampi (bregma-2.80-5.30
mm) (13). The dividing line for
the corpus callosum and external capsule was the transverse
ligature of the inferior margin of hippocampi, based on which the
external capsule was divided into the left part and the right part
(Fig. 2).
One radiologist with 8 years of neural imaging
experience, who was blinded to the experimental grouping, carefully
delineated the regions of interest (ROIs) of the corpus callosum,
left and right internal capsule, external capsule, and the
pyramidal tract, to acquire the FA and ADC values on the DTI image.
To increase the accuracy of the measured values, T2-weighted image
(T2WI) images were first merged with the corresponding parameter
maps, and then used to demonstrate the margins of the freehand
ROIs.
IHC
At 6, 24 and 72 h, and 1 and 2 weeks post-injury, 3
rat brains from each group from the remaining 15 rat brains were
extracted and fixed in 4% paraformaldehyde for 48 h at room
temperature. Following this, the brains were cryosectioned at 5 µm
for β-APP IHC analysis, according to previously published protocol
(14). The IHC data were quantified
independently by two investigators from the corresponding locations
matching to the MRI data for correlation analysis via the threshold
of the normal staining intensity as determined by the control
staining. IHC quantification by each investigator was averaged to
represent the percentage of positive β-APP staining in the images
(magnification, x400).
Morris water maze test
Morris water maze tests were performed at 2 weeks
post-injury to test learning and memory deficits in the rats. The
Morris water maze was comprised of a video monitoring system, round
water pool and safety platform. The safety platform was at the 3rd
quadrant. The water temperature was 23-26˚C. The video monitoring
system was used to film and record the incubation period (time
required to locate the platform) and track by which the rat locates
the safety platform. The rats were trained for 8 days. The first
day was used to get the rats accustomed to the water environment
without a safety platform. During the following 6 days, the water
level was raised by 1 cm to test each rat for 4 times to locate the
platform, with each test lasting 60 sec. If the rat failed to
locate the platform, the incubation period was recorded as 60 sec.
The test on day 7 was conducted with the removal of the safety
platform and testing for 60 sec. The first 3 days comprised the
training period and days 4-7 were used to measure the incubation
period while the rats were trying to locate the safety platform.
These were tests of the positioning and navigation ability. On day
8, the safety platform was removed and the times that the rats
succeeded in crossing the safety platform within 60 sec were
recorded. Furthermore, the percentage of searching time for the
safety platform quadrant was recorded, which was a test of space
exploring ability.
Statistical analysis
Statistical analysis was conducted by using SPSS for
Windows software (version no. 20.0; IBM, Corp.). Data are presented
as mean ± standard deviation. One-way ANOVA followed by
Bonferroni's correction was used for multiple comparisons. The
correlation between two variables was assessed by Pearson
correlation analysis. Enumeration data are presented as a
percentage and compared using the χ2 test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Behavioral observation of rats in the
acute stage of injury
After injury, the rats developed limb convulsions,
incontinence, and apnea in varying degrees. Corneal reflex, hind
paw reflex, and righting reflex disappeared in all three injury
groups. The average recovery time of corneal reflex, hind paw
reflex, and righting reflex, and the wake-up time were
significantly different between group A and group B, and between
group B and group C (P<0.05; Table
I). After waking, the rats showed decreased activity, slow
reaction, and unsteady walking.
 | Table IBehavioral observation of rats of each
group (n=25, mean ± SD). |
Table I
Behavioral observation of rats of each
group (n=25, mean ± SD).
| Recovery time
(min) | Group A | Group B | Group C | P-value |
|---|
| Corneal reflex | 28.50±5.72 |
39.50±4.79a |
44.80±2.35a,b | <0.05 |
| Hind paw reflex | 42.80±4.50 |
52.80±8.27a |
69.80±11.08a,b | <0.05 |
| Righting reflex | 58.10±7.14 |
80.80±11.16a |
106.20±16.23a,b | <0.05 |
| Wake-up time | 56.84±8.43 |
76.54±10.85a |
101.57±15.56a,b | <0.05 |
DTI image and diffusion parameter
analysis
There were no obvious changes in the signal
intensity or gray gradient in the T2WI, FA map, and ADC map of the
corpus callosum, bilateral internal capsule, external capsule, and
pyramidal tract of each group.
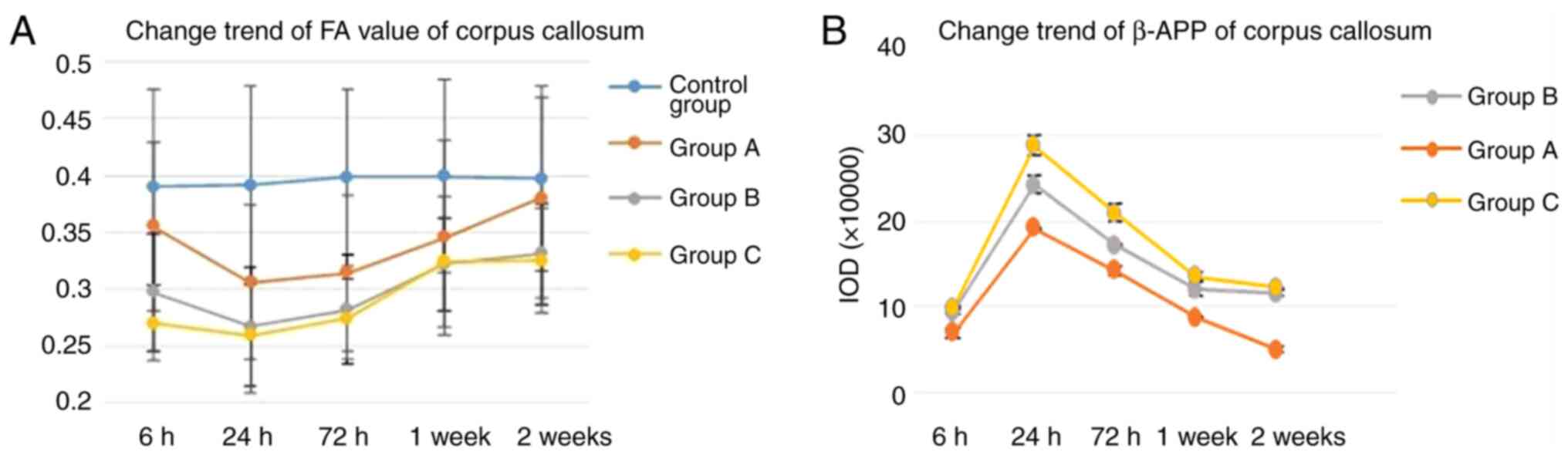
Quantitative analysis of diffusion parameters showed
that FA values of the corpus callosum in each injury group
decreased from 6 h post-injury, reached the lowest level at 24 h
post-injury (P<0.05), and then started to recover. FA values of
the corpus callosum in the mild injury group A recovered to normal
level at 1 and 2 weeks post-injury, but did not recover in group B
and C (Fig. 3A). FA and ADC values
of the bilateral external capsule, internal capsule, and pyramidal
tract in each injury group showed no significant differences with
those of the control group (Table
SI).
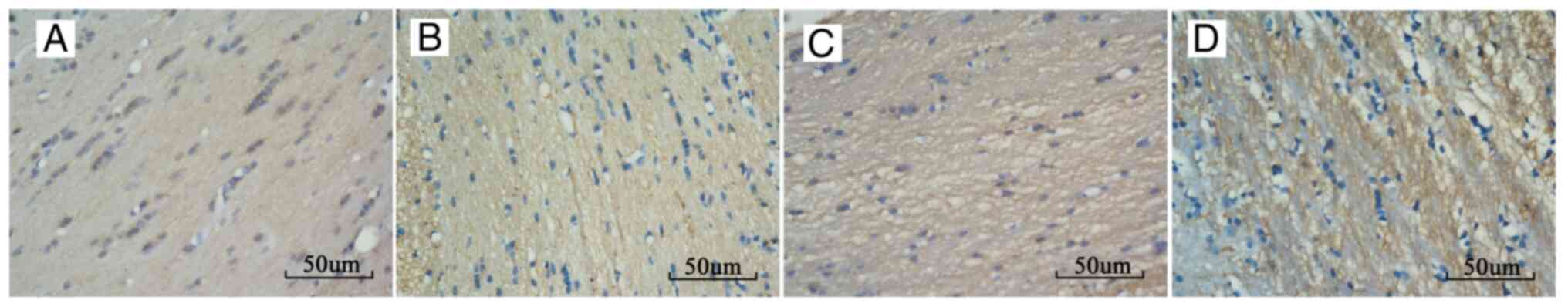
IHC results
IHC staining in the corpus callosum of the injury
groups demonstrated changes consistent with white matter damage
(Fig. 4). The percentage of
positive β-APP staining in the corpus callosum of group A, B, and C
at each time point post-injury increased compared with that of the
control group, and there was a significant difference between each
injury group. The percentage of positive β-APP staining increased
from 6 h post-injury, reached the highest level at 24 h
post-injury, and then started to recover. The rats in group A
recovered normal β-APP levels at 2 weeks post-injury (Fig. 3B and Table II).
 | Table IIThe percentage of positive β-APP
staining of each group (n=3, mean ± SD). |
Table II
The percentage of positive β-APP
staining of each group (n=3, mean ± SD).
| Time points | Control group | Group A | Group B | Group C |
|---|
| 6 h | 4.31±0.19 |
6.92±0.58a |
9.34±0.25a,b |
9.86±0.17a,b |
| 24 h | 4.54±0.17 |
19.18±0.11a |
24.29±1.00a,b |
28.70±1.21a-c |
| 72 h | 4.44±0.38 |
14.16±1.53a |
17.18±0.15a,b |
20.92±0.98a-c |
| 1 week | 4.50±0.26 |
8.65±0.10a |
12.03±0.74a,b |
13.50±0.45a-c |
| 2 week | 4.68±0.31 | 4.97±0.34 |
11.52±0.33a,b |
12.24±0.02a-c |
Correlation between β-APP and FA
values of the corpus callosum
The correlations between the β-APP and FA values of
the corpus callosum in group A, B, and C at 24 h post-injury were
analyzed. The results showed an obvious negative correlation, with
an r value of -0.723.
Result of the Morris water maze
test
Test of positioning and navigation
ability
From the test of positioning and navigation ability,
it was found that in the 6-day training period, the incubation
period (time required to find the platform) of the rats in each
group decreased as the training time increased. The incubation
period of group B and C was longer than that of the control group
(P<0.05), whereas the difference between group A and the control
group was not statistically significant (P>0.05). The
differences among groups A, B, and C showed statistical
significance (P<0.05; Table
SII).
Test of space exploration ability
In the test of space exploration ability, the
platform searching ability of groups B and C was poorer compared
with that of the control group (P<0.05), whereas the difference
between group A and the control group was not statistically
significant (P>0.05). The difference among group A, B, and C
showed statistical significance (P<0.05). The times of crossing
safety platform of the control group and group A, B, C were
6.60±0.89, 6.20±1.09, 3.33±0.89, and 1.67±0.82, respectively
(Table SIII).
Trajectory chart analysis
During the training period, the rats in the control
group firstly sailed along the pool wall and then along the
diagonal line of the pool. Their motion track was mostly a straight
line. The injured rats spent longer time to sail along the pool
wall. In addition, the track that the rats used to look for the
platform was mostly curved (Fig.
S1).
Correlation analysis for FA, β-APP and
learning and memory deficits of the rats during the recovery
stage
Correlations among the FA, β-APP staining of the
corpus callosum at 24 h post injury and the results of crossing the
safety platform at 2 weeks post injury in group A, B, and C were
analyzed. The result showed an obvious positive correlation with an
r value of 0.881 between the FA and the results of crossing the
safety platform (Fig. S2A), and
obvious negative correlation with an r value of -0.931 between the
β-APP staining and the results of crossing the safety platform
(Fig. S2B).
Discussion
Diffuse axonal injury (DAI) resulting from
impact-acceleration forces, including linear, rotational, or some
combination, is a common cause of substantial neurological
impairment. The pathologies following DAI including primary axonal
damage and secondary degeneration, remain insufficiently
characterized (15). Compared with
widely used experimental models of mild traumatic brain injury
(mTBI), such us fluid percussion (FP) and controlled cortical
impact (CCI) systems, and closed head injury (CHI) models, various
other models have been developed to increase rotational
acceleration, which are more clinically relevant to human head
movement following impact-related Mtbi (5,6,14). We
simulated the DAI occurrence mechanism with reference to Namjoshi
et al (6) and Li et
al (7) injury devices and
constructed an in-house rat model of DAI, which enabled
simultaneous and instantaneous extra-large sagittal rotational
acceleration and axial linear acceleration at the head of the rat,
with direct comparison to histopathologically-confirmed axonal
injury, as assessed by β-amyloid precursor protein (β-APP), which
normally traverses the length of the axon and accumulates in
response to injury (12). We
demonstrated previously that this model integrates
histopathological and behavioral characteristics of DAI, without
focal injuries, such as skull fracture, hemorrhage, and surgery
(8,16).
In the present study, the percentage of positive
β-APP staining in the corpus callosum of group A, B, and C at each
time point post-injury increased compared with that in the control
group, and there was a significant difference between each injury
group, which demonstrated that the extent of DAI that incurred
using our in-house rat model was directly proportional to the
severity of injury.
DAI is usually irregular, without hemorrhage, and is
difficult to detect on conventional CT or MRI, and mainly relies on
patient self-reporting for clinical diagnosis (17). Diffusion tensor imaging (DTI) can
detect the diffusion changes of water molecules and the coherence
of fibrous structures in patients with DAI, which provides
important information in vivo (9). The fractional anisotropy (FA) value
reflects the anisotropy of water molecule diffusion, and is
sensitive to axonal integrity. The apparent diffusion coefficient
(ADC) reflects the water molecule diffusion characteristics,
influenced by cellular brain edema (CBE) (18). The results of the present study
showed that the FA value could reflect varying axonal injury states
over time, and was obviously correlated with the change in β-APP
levels in the corpus callosum. The more severe the axonal injury
was, the higher β-APP value and the lower FA value would be. As an
in vivo radiological parameter, the FA value might replace
β-APP, the early molecular marker of DAI injury, to reflect the
degree of early axonal injury.
Cognitive and behavioral deficits caused by DAI may
persist for months to years (19).
Axonal injury of the brain centerline and neural pathway are
closely related to learning and memory deficits (20). Kraus et al (3) and Lipton et al (21) reported that DTI might determine the
relationship between cognitive deficits and TBI. However, it is
difficult to predict the prognosis of neural functional recovery
after DAI at the early stage. We detected significant differences
in learning and memory deficits between the injury groups, which
demonstrated that the more severe the injury the rats suffered, the
worse were their learning and memory deficits. Furthermore, an
obvious correlation between the FA value of the corpus callosum at
the early stage and learning and memory deficits of the rats at the
recovery stage were found, and both the FA value and the learning
and memory deficits of the rats in the mild injury group could
recover to normal levels after 2 weeks of follow up. This suggested
that DTI could predict the recovery of the rat's learning and
memory deficits at an early stage post-injury.
The present study had some limitations. First, the
magnetic field intensity of the 3T MRI machine is relatively low,
making it difficult to obtain a clear image of small animals.
Additionally, if different pathology components coexist in one
image voxel, because of the partial volume effects, DTI measurement
would be affected, making accurate estimation of the diffusion
parameters difficult (22). Further
evaluation of axonal injury, demyelination, axonal loss, and
inflammation with improved parametric diffusion methods will be of
interest to determine the overall water molecule diffusion features
of the white matter after DAI.
In conclusion, DTI provided complementary insight
into the underlying pathologies reflecting varying injury states
over time, and could be used to predict the neural functional
recovery at the early stage post-injury in a DAI rat model. We
propose DTI as a sensitive quantification method for early-stage
diagnosis and prognostic analysis of DAI.
Supplementary Material
Figure S1. Trajectory chart analysis
of the Morris water maze test. (A) Control group; (B) group A; (C)
group B; (D) group C.
Figure S2. (A) Correlation analysis
for FA and learning and memory deficits of the rats during the
recovery stage. (B) Correlation analysis for β‑APP and learning and
memory deficits of the rats during the recovery stage. FA,
fractional anisotropy; β‑APP, β‑amyloid precursor protein.
Table SI. Diffusion parameters of the
region of interests of each group at different post.injury time
points (n=10).
Table SII. The incubation period (time
in sec required to find the platform) of the rats of each group
(n=6).
Table SIII. Times of crossing the
safety platform and the percentage of searching time for the safety
platform quadrant of the rats in each group (n=6).
Acknowledgements
The authors would like to thank Dr Xianyong Tang
(College of Mechanical Engineering, Chongqing University), and Dr
Jin Zhu (Department of Pathology, Children's Hospital of Chongqing
Medical University), for the experimental guidance of
immunohistochemistry.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL and LH conceived and designed the study. YZ and
LL performed the experiments. YZ, LL and LH wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The current study were approved by the Animal Ethics
Committee of Chongqing Medical University (approval no.
20170301).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cassidy J, Carroll L, Peloso P, Borg J,
von Holst H, Holm L, Kraus J and Coronado V: WHO Collaborating
Centre Task Force on Mild Traumatic Brain Injury: Incidence, risk
factors and prevention of mild traumatic brain injury: Results of
the WHO collaborating Centre task force on mild traumatic brain
injury. J Rehabil Med. 36 (Suppl 43):S28–S60. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Smith DH, Meaney DF and Shull WH: Diffuse
axonal injury in head trauma. J Head Trauma Rehabil. 18:307–316.
2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kraus MF, Susmaras T, Caughlin BP, Walker
CJ, Sweeney JA and Little DM: White matter integrity and cognition
in chronic traumatic brain injury: A diffusion tensor imaging
study. Brain. 130:2508–2519. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
McKee AC, Stern RA, Nowinski CJ, Stein TD,
Alvarez VE, Daneshvar DH, Lee HS, Wojtowicz SM, Hall G, Baugh CM,
et al: The spectrum of disease in chronic traumatic encephalopathy.
Brain. 136:43–64. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xiong Y, Mahmood A and Chopp M: Animal
models of traumatic brain injury. Nat Rev Neurosci. 14:128–142.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Namjoshi DR, Cheng WH, McInnes KA, Martens
KM, Carr M, Wilkinson A, Fan J, Robert J, Hayat A, Cripton PA and
Wellington CL: Merging pathology with biomechanics using chimera
(closed-head impact model of engineered rotational acceleration): A
novel, Surgery-free model of traumatic brain injury. Mol
Neurodegen. 9(55)2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li XY, Dai GH, Feng DF, Li J and Gu L:
Experimental device for inducing diffuse brain injury in small
animals. BME Clin Med. 13:489–493. 2009.
|
|
8
|
Xiang L, Zhang YT, Liang P, Wei H, Peng LL
and Li LS: Study on the establishment of rat model of different
degree of diffuse axonal injury. Chongqing Med. 45:2881–2884.
2016.
|
|
9
|
Mayer AR, Ling J, Mannell MV, Gasparovic
C, Phillips JP, Doezema D, Reichard R and Yeo RA: A prospective
diffusion tensor imaging study in mild traumatic brain injury.
Neurology. 74:643–650. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
McAllister TW, Ford JC, Ji S, Beckwith JG,
Flashman LA, Paulsen K and Greenwald RM: Maximum principal strain
and strain rate associated with concussion diagnosis correlates
with changes in corpus callosum white matter indices. Ann Biomed
Eng. 40:127–140. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Eierud C, Craddock RC, Fletcher S, Aulakh
M, King-Casas B, Kuehl D and LaConte SM: Neuroimaging after mild
traumatic brain injury: Review and meta-analysis. Neuroimage Clin.
4:283–294. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bramlett HM, Kraydieh S, Green EJ and
Dietrich WD: Temporal and regional patterns of axonal damage
following traumatic brain injury: A beta-amyloid precursor protein
immunocyto-chemical study in rats. J Neuropathol Exp Neurol.
56:1132–1141. 1997.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Paxinos G and Watson C: The Rat Brain in
Stereotaxic Coordinates. Elsevier Publishing, Amsterdam, pp82-100,
1997.
|
|
14
|
Kilbourne M, Kuehn R, Tosun C, Caridi J,
Keledjian K, Bochicchio G, Scalea T, Gerzanich V and Simard JM:
Novel model of frontal impact closed head injury in the rat. J
Neurotrauma. 26:2233–2243. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fehily B and Fitzgerald M: Repeated mild
traumatic brain injury: Potential mechanisms of damage. Cell
Transplant. 26:1131–1155. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xiang L, Zhang YT, Liang P, Wei H, Peng LL
and Li LS: Value of β-APP and NF-L as markers in evaluation of rat
diffuse axonal injury. J Third Military Med Univ. 37:2255–2260.
2015.
|
|
17
|
Browne KD, Chen XH, Meaney DF and Smith
DH: Mild traumatic brain injury and diffuse axonal injury in swine.
J Neurotrauma. 28:1747–1755. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gasparetto EL, Rueda Lopes FC and
Domingues RC and Domingues RC: Diffusion imaging in traumatic brain
injury. Neuroimaging Clin N Am. 21:115–125. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
McCrory P, Meeuwisse WH, Aubry M, Cantu B,
Dvorák J, Echemendia RJ, Engebretsen L, Johnston K, Kutcher JS,
Raftery M, et al: Consensus statement on concussion in sport: The
4th International Conference on Concussion in Sport held in Zurich,
November 2012. Br J Sports Med. 47:250–258. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ham TE and Sharp DJ: How can investigation
of network function inform rehabilitation after traumatic brain
injury. Curr Opin Neurol. 25:662–669. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lipton ML, Gellella E, Lo C, Gold T,
Ardekani BA, Shifteh K, Bello JA and Branch CA: Multifocal white
matter ultrastructural abnormalities in mild traumatic brain injury
with cognitive disability: A voxel-wise analysis of diffusion
tensor imaging. J Neurotrauma. 25:1335–1342. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang Y, Wang Q, Haldar JP, Yeh FC, Xie M,
Sun P, Tu TW, Trinkaus K, Klein RS, Cross AH and Song SK:
Quantification of increased cellularity during inflammatory
demyelination. Brain. 134:3590–601. 2011.PubMed/NCBI View Article : Google Scholar
|