Introduction
Radix Astragali, popularly known as Huangqi
in Chinese or Milkvetch in English, mainly contains astragaloside
IV, Astragalus polysaccharide (APS) and Astragalus
flavonoids (1). Scientific
investigations have revealed that APS has various biological
activities, such as immunomodulatory, antioxidant, antitumor,
anti-inflammatory, anti-atherosclerotic, hematopoietic and
neuroprotective properties (2,3).
Previous studies have also shown that APS significantly increased
the phagocytotic activity of macrophages and promoted lymphocyte
proliferation (4,5). RAW264.7 mouse macrophages, popularly
used in the study of immune mechanisms, occupy a unique niche in
the immune system. They can kill pathogens directly by phagocytosis
and indirectly via the secretion of pro-inflammatory factors
(6). RAW264.7 mouse macrophages are
responsible for functions such as antigen processing and
presentation to antigen-specific T cells (7). Following activation, RAW264.7 cells
can induce the expression of accessory and co-stimulatory molecules
that promote sustained stimulatory interactions with T cells and
the generation of adaptive immunity (8-10).
CD40, CD80 and CD86 are known as co-stimulatory
molecules that are required for T cell activation (11). It was demonstrated that the
differential expression of co-stimulatory molecules on
antigen-presenting cells plays an essential role in directing the
T-cell response to proinflammatory or regulatory effector functions
(12). CD40 interacts with CD40L,
which is known to play key roles in the in vitro and in
vivo activation and differentiation of B cells (13). In vivo results indicated that
aberrant expression of co-stimulatory molecules is sufficient for
the initiation of an autoimmune response (14). Therefore, CD40, CD80 and CD86 are
responsible for functions such as antigen processing and
presentation to antigen-specific T cells. Thus, the increase in the
expression of the surface molecules CD40, CD80 and CD86 indicates
enhanced immune response. It was previously demonstrated that
RAW264.7 cells play an important role as an interface between
innate and adaptive immunity (15).
Therefore, it was deemed necessary to investigate the effects of
APS on CD40, CD80 and CD86 expression in RAW264.7 cells.
NF-κB is a ubiquitous transcription factor of
inflammatory genes. The MAPK signaling pathway is an important
regulator of numerous extracellular stimuli (16). In recent years, it was reported that
the activation of the NF-κB/MAPK signaling pathway plays a key role
in resistance to pathogens or mechanical injury (17). A number of experimental and clinical
studies have investigated the effects of APS in the clinical
setting. APS regulates humoral and cellular immunity via the
Toll-like receptor 4 signaling pathway, affecting the function of T
cells and thus may be used as an adjuvant for vaccines (18). The activation of NF-κB/Rel may be
involved in the ability of APS to induce cytokine production by
macrophages and RAW264.7 macrophages, leading to the enhancement of
immune function (19,20). However, the exact molecular
mechanism through which this induction affects immune function
remains to be elucidated. The aim of the present study was to study
the effects of APS on the activation of the NF-κB/MAPK signaling
pathway.
Materials and methods
Reagents
APS (purity >98%; cat. no. B20562-20mg) was
purchased from Shanghai Yuanye Bio-Technology Co., Ltd. DMEM with
high glucose was obtained from HyClone (Cytiva). FBS was purchased
from Clark Bioscience. Cell Counting Kit-8 (CCK-8) was purchased
from Dojindo Molecular Technologies, Inc. Cell cycle and mouse
TNF-α (cat. no. KGEMC102a), mouse IL-6 (cat. no. KGEMC004-1) and
mouse IL-1β (cat. no. KGEMC001b) ELISA kits were obtained from
Nanjing KeyGen Biotech Co., Ltd. Nitric oxide (NO) assay kit (cat.
no. S0021) was obtained from Beyotime Institute of Biotechnology.
Biozol RNA extraction reagent, Biomiga First-Strand cDNA Synthesis
kit and quantitative PCR (qPCR) kit were obtained from Biomiga,
Inc. The primary antibodies used in this study included
anti-β-actin (cat. no. AF0003; 1:500), anti-p38 (cat. no. AM065;
1:500), anti-phosphorylated (p)-p38 (cat. no. AM063; 1:500),
anti-p65 (cat. no. AF0246; 1:500), anti-p-p65 (cat. no. AN371;
1:500), anti-ERK (cat. no. AM076; 1:500), anti-p-ERK (cat. no.
AP328; 1:500), anti-JNK (cat. no. AJ516; 1:500) and anti-p-JNK
(cat. no. AJ518; 1:500), and HRP-conjugated anti-mouse and
anti-rabbit secondary antibodies (cat. nos. A0216 and A0208; 1:500)
were purchased from Beyotime Institute of Biotechnology. The
pacific blue anti-mouse CD40 (cat. no. 124626; 1:500),
phycoerythrin anti-mouse CD80 (cat. no. 104708; 1:400) and FITC
anti-mouse CD86 (cat. no. 105006; 1:500) antibodies and the
corresponding isotype controls (cat. no. 405404; 1:1000) were
purchased from BioLegend, Inc.
Cell culture and treatment
RAW264.7 macrophages were obtained from Jilin
University (Jilin, China) and cultured in DMEM with high glucose
containing 10% FBS and 1% antibiotics (100 U/ml penicillin and 100
µg/ml streptomycin) as previously described (19,20).
The cells were cultured at 37˚C and 5% CO2 and the
culture medium was replaced every 48 h. The cells were pretreated
with or without 5 µmol/l pyrrolidine dithiocarbamate (PDTC; NF-κB
inhibitor; cat. no. S1809; Beyotime Biotechnology, China) 1 h
before treatment with 0, 25, 50 or 100 µg/ml APS.
Evaluating the optimal concentration
of APS and RAW264.7 cell viability by CCK-8 assay
Cells were adjusted to a volume of 1x105
cells/ml and 100 µl of the cultured cells were selected, plated in
a 96-well plate and incubated at 37˚C and 5% CO2 for 24
h. Subsequently, 10 µl CCK-8 solution was added to each well and
the cells were incubated for an additional 4 h. The optical density
was measured at a wavelength of 450 nm using a plate reader
(Multiskan MK3; Thermo Fisher Scientific, Inc.).
Evaluation of IL-6, TNF-α, IL-β and NO
secretion by ELISA and mRNA expression levels by reverse
transcription (RT)-qPCR analysis
RAW264.7 cells (1x105 cells/ml) were
seeded in 6-well plates, incubated overnight and then exposed to
APS (0, 25, 50 and 100 µg/ml) and PDTC (5 µmol/l ) for 24 h as
previously described (19,20). Cell supernatants were collected by
centrifugation at 3,500 x g (4˚C) for 10 min. The amount of IL-6,
TNF-α, IL-1β and NO secretion in the culture supernatants were
determined in duplicate using respective ELISA kits according to
the manufacturer's instructions.
Total RNA was isolated from RAW264.7 cells using
TRIzol® reagent and the RNA was reverse transcribed into
cDNA (1 h at 37˚C) with a First Strand cDNA Synthesis kit. The
relative mRNA expression levels were detected using a Biomiga SYBR
qPCR mix kit on an ABI 7500 Real-Time PCR system (Applied
Biosystems, Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used for the qPCR: Initial
denaturation for 5 min at 94˚C; followed by 30 cycles of 30 sec at
94˚C, 40 sec at 55˚C and 2 min at 72˚C; and a final extension for 7
min at 72˚C. Analysis of relative gene expression data was
performed using the 2-∆∆Cq method
(21). The primer sequences of
IL-1β, IL-6, TNF-α, inducible nitric oxide synthase (iNOS) and
GAPDH that were used for RT-qPCR are designed by Primer 5.0
software according to the sequences in Genebank and are presented
in Table I.
 | Table IPrimer sequences used in reverse
transcription-quantitative PCR analysis. |
Table I
Primer sequences used in reverse
transcription-quantitative PCR analysis.
| Gene name | Product size
(bp) | Gene ID | Primer sequence
(5'-3') |
|---|
| IL-1β | 165 | 16176 | Forward:
GCCACCTTTTGACAGTGATGAG |
| | | | Reverse:
AGTGATACTGCCTGCCTGAAG |
| IL-6 | 201 | 16193 | Forward:
CAACGATGATGCACTTGCAGA |
| | | | Reverse:
TCTCTCTGAAGGACTCTGGCT |
| TNF-α | 161 | 21926 | Forward:
ACCTGGCCTCTCTACCTTGT |
| | | | Reverse:
CCCGTAGGGCGATTACAGTC |
| iNOS | 156 | 18125 | Forward:
AGGGACTGAGCTGTTAGAGACA |
| | | | Reverse:
AAGAGAAACTTCCAGGGGCAAG |
| GAPDH | 232 | 14433 | Forward:
GGTGAAGGTCGGTGTGAACG |
| | | | Reverse:
CCCGTAGGGCGATTACAGTC |
Evaluation of CD40, CD80 and CD86
expression
Cells were collected following treatment with PDTC
and different concentrations (0-100 µg/ml) of APS for 24 h. The
cell surfaces were blocked with 5% goat serum (cat. no. C0256;
Beyotime Institute of Biotechnology) at 4˚C for 15 min and washed
twice with PBS (pH 7.2). Subsequently, the cells were incubated
with monoclonal antibodies against CD40, CD80, CD86 and the
corresponding fluorescent markers for 30 min at 4˚C. After the
cells were washed twice with PBS and resuspended in PBS, they were
subjected to flow cytometry on a FACS platform and CellQuest
software Version 3.0 (Becton, Dickinson & Company).
Flow cytometry analysis of cell cycle
regulation
RAW264.7 cells (1x106 cells/ml) were
seeded in 6-well plates and treated with various concentrations
(0-100 µg/ml) of APS in the presence or absence of PDTC for 24 h.
Subsequently, the cells were collected, washed once with cold PBS,
fixed in 75% cold ethanol overnight at 4˚C and washed twice with
cold PBS. The fixed cells were resuspended in 100 µl RNase (cat.
no. ST577; Beyotime Institute of Biotechnology) at 37˚C and
incubated with 400 µl PI at 4˚C in a dark room for 30 min. Cell
cycle progression was analyzed using flow cytometry on a FACS
platform and Cell Quest software Version 3.0 (Becton, Dickinson
& Company).
Evaluation of p65 expression by
electrophoretic mobility shift assay (EMSA)
Briefly, nuclear protein from RAW264.7 cells was
extracted with a nuclear protein extraction kit (cat. no. C500009;
Sangon Biotech Co., Ltd.) according to the manufacture's
instruction. After the cell medium was aspirated, 10 ml pre-cold 1X
PBS was added to remove the culture solution. The cells were
scraped with a cell scraper. The cells and culture medium were
transferred to a centrifuge tube and centrifuged at 800 x g for 10
min at 4˚C. The supernatant was discarded and 10 ml pre-cold 1X PBS
was added to wash twice via centrifuging at 800 x g (3,000 rpm) for
5 min at 4˚C. 0.3 ml pre-cold hypotonic buffer (include 5 µl
phosphatase inhibitor, 10 µl PMSF and 1 µl DTT in 1 m of pre-cold
hypotonic buffer) was added to centrifugate tube and then
transferred to new tubes. The tube was flicked with fingers to
suspend the precipitate, bathed on ice for 10 min, shaken for 10
sec. The suspension was centrifuged at 800 x g (3,000 rpm) at 4˚C
for 5 min. The supernatant was discarded immediately. 0.4 ml
pre-cold hypotonic buffer added to wash the pellet with shaking for
30 sec. Then, the tubes were centrifuged at 4˚C, 2,500 x g (5,000
rpm) for 5 min. The supernatant was discarded and the precipitate
was saved. 0.2 ml lysis buffer (contain 5 µl phosphatase inhibitor,
10 µl PMSF and 1 µl DTT in 1 ml pre-cold lysis buffer)) was added
to the precipitate to suspend the precipitate. Then the suspension
was bathed in ice for 20 min and centrifuged at 20,000 x g for 10
min at 4˚C. The precipitate was discarded and the supernatant is
the nuclear protein extract. The nuclear protein extract was stored
at -80˚C for EMSA assay. The protein concentration was measured
using a Bio-Rad protein assay reagent (Bio-Rad Laboratories, Inc.).
p65 probes were labeled with biotin for 30 min at 37˚C. Equal
amounts (4 µg) of nuclear protein from each sample were included in
the binding reaction for 20 min at room temperature using a
Lightshift EMSA Optimization and Control kit (Pierce; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Protein-DNA complexes were separated using non-denaturing 6.5%
polyacrylamide Tris-borate-EDTA electrophoresis gels and
transferred to nylon membranes. Biotin-labelled probes were
detected using a chemiluminescence solution (Pierce; Thermo Fisher
Scientific, Inc.). Labelled bands were measured using a G-BOX Chemi
XR5 gel imager (Syngene Europe).
Evaluation of NF-κB/MAPK signaling
pathway by western blotting
RAW264.7 cell proteins were extracted with RIPA
lysis buffer (cat. no. C500007; Sangon Biotech Co., Ltd.)
containing protease (cat. no. BL104A; BioSharp Life Sciences) and
phosphatase inhibitor (cat. no. BL612A; BioSharp Life Sciences).
Protein (40 µg/lane) was electrophoresed on a 12% acrylamide
resolving gel and 5% stacking gel, then transferred to a PVDF
membrane (EMD Millipore) for 30 min at 120 V. The membrane was
blocked with 5% BSA (Biosharp Life Sciences) for 4 h at 26˚C and
incubated with the appropriate diluted primary antibody at 4˚C for
16 h. Following five washes with TBS containing 0.05% Tween-20
(TBS-T), the membrane was incubated with HRP-conjugated anti-mouse
or anti-rabbit secondary antibodies (cat. nos. A0216 and A0208;
1:500). The membrane was washed in TBS-T thrice, visualized by
chemiluminescence (cat. no. 32109; Thermo Fisher Scientific, Inc.)
and the results were analyzed using Quantity One Software version
4.6.9 (Bio-Rad Laboratories, Inc.).
Statistical analysis
All values are expressed as the mean ± SD with three
or five replications in one treatment and analyzed with the SPSS
software package 17.0 (SPSS Inc.). The differences among
experimental groups were analyzed using one-way ANOVA with post hoc
multiple comparison of means using the Duncan's multiple range
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of APS on cell
proliferation
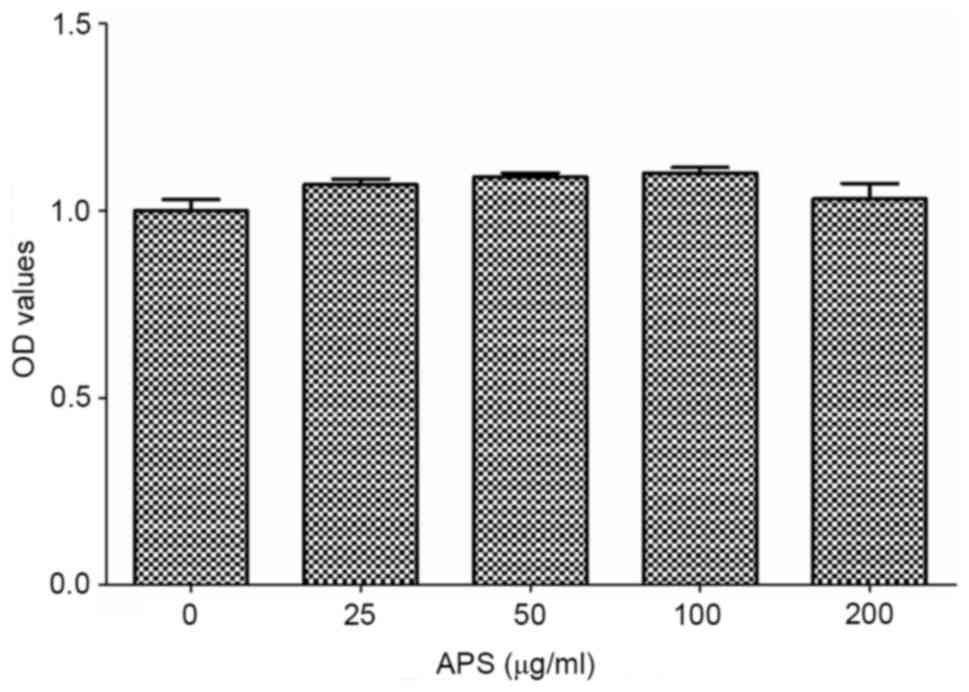
As shown in Fig. 1,
RAW264.7 cells were treated with different concentrations of APS
(0-200 µg/ml). At the concentration range of 0-100 µg/ml, the cell
viability gradually increased with the increase in APS
concentration and reaching the maximum at 100 µg/ml. When the
concentration of APS was 100-200 µg/ml, the cell viability
decreased gradually upon increased APS concentration. Therefore,
100 µg/ml APS was determined as the optimum concentration for
subsequent experiments.
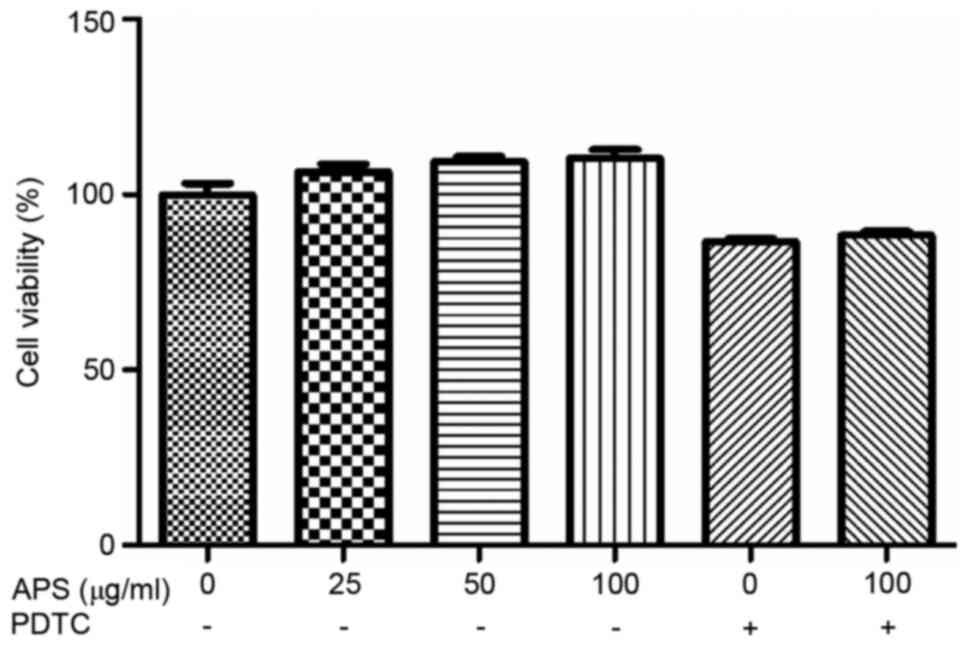
As shown in Fig. 2,
compared with 0 µg/ml APS treatment group, 25-100 µg/ml APS could
increase the cell viability to a certain extent. However, following
the addition of PDTC, the cell viability was suppressed. When APS
was used at 100 µg/ml with PDTC, PDTC exerted no suppressive
effects on cell viability.
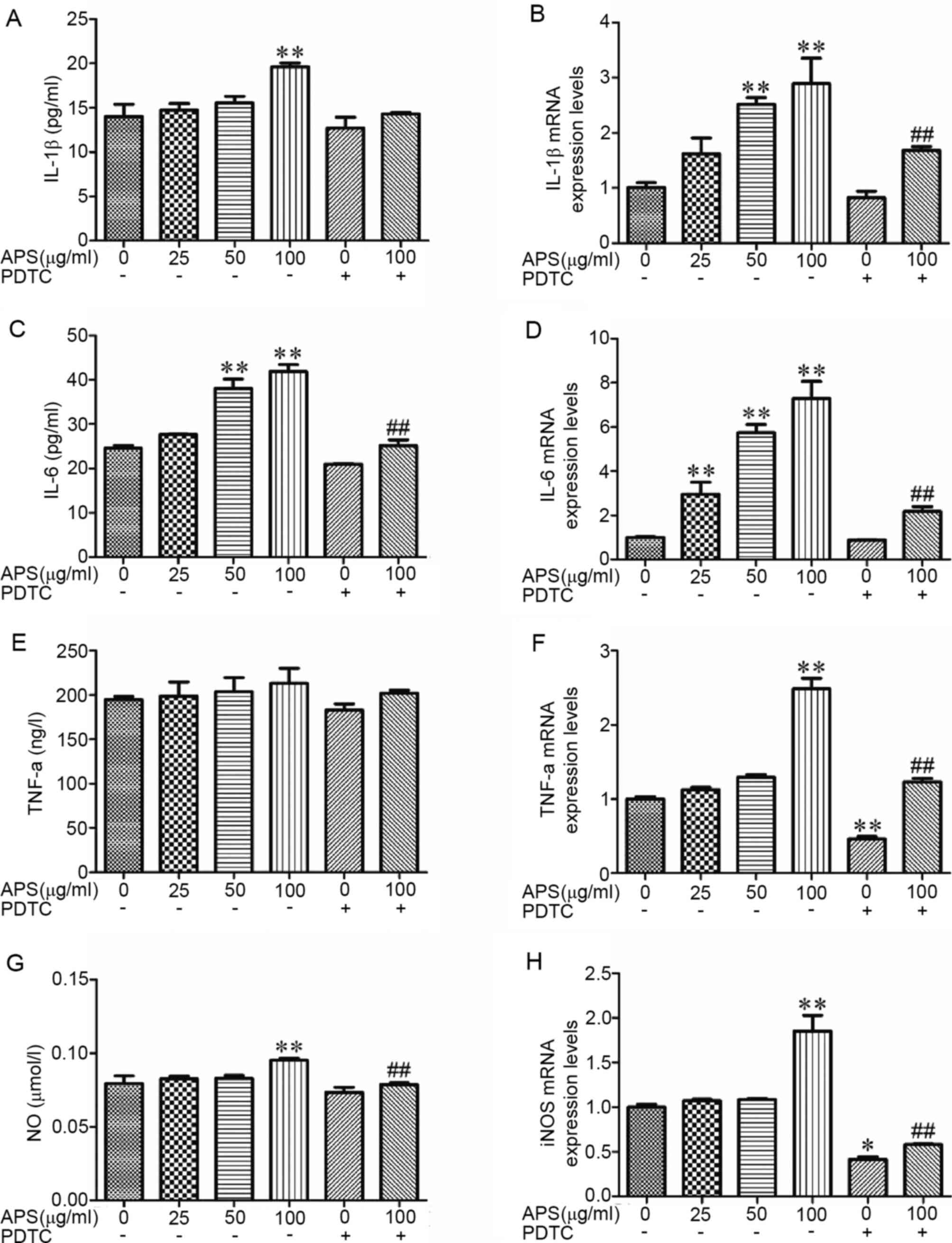
APS promotes cytokine secretion from
RAW264.7 cells
RAW264.7 cell treatment with APS resulted in a
marked increase in the concentrations of IL-1β, TNF-α and NO in a
dose-dependent manner (Fig. 3A,
C and G). The gene expression levels were
evaluated by RT-qPCR. The results demonstrated that following APS
stimulation, the mRNA expression levels of pro-inflammatory factors
and chemokines were increased (Fig.
3B, D, F and H).
Following treatment with APS at 50 µg/ml, IL-6 expression was
significantly increased (Fig. 3C)
compared the 0 µg/ml APS treatment group (P<0.01). At 100 µg/ml
APS treatment, the production of IL-1β, IL-6 and NO were
significantly increased (Fig. 3A,
C and G) compared with the 0 µg/ml APS treatment
group (P<0.01), whereas PDTC was able to inhibit the increase of
IL-1β, IL-6 and NO (Fig. 3A,
C, E and G;
P>0.05). Compared with the 0 µg/ml APS treatment group, the
production of TNF-α have an increased trend with the increased
concentration of APS, but there was no significance (Fig. 3E; P>0.05). Compared with 100
µg/ml APS, the levels of IL-6 and NO in the APS 100 µg/ml + PDTC
treatment group were significantly decreased (Fig. 3C and G; P<0.01). Compared with the 0 µg/ml
APS treatment group, IL-6 mRNA expression was significantly
increased (Fig. 3D; P<0.01) in
the APS 25 µg/ml treatment group (P<0.01) and the IL-1β and IL-6
mRNA expression levels were significantly higher (Fig. 3B and D; P<0.01) in the APS 50 µg/ml group
(P<0.01). The expression of IL-1β, IL-6, TNF-α and iNOS mRNA in
the APS 100 µg/ml group were significantly increased (Fig. 3B, D,
F and H; P<0.01) compared with 0 µg/ml APS
treatment group (P<0.01). These results demonstrated that PDTC
inhibited the effects of APS.
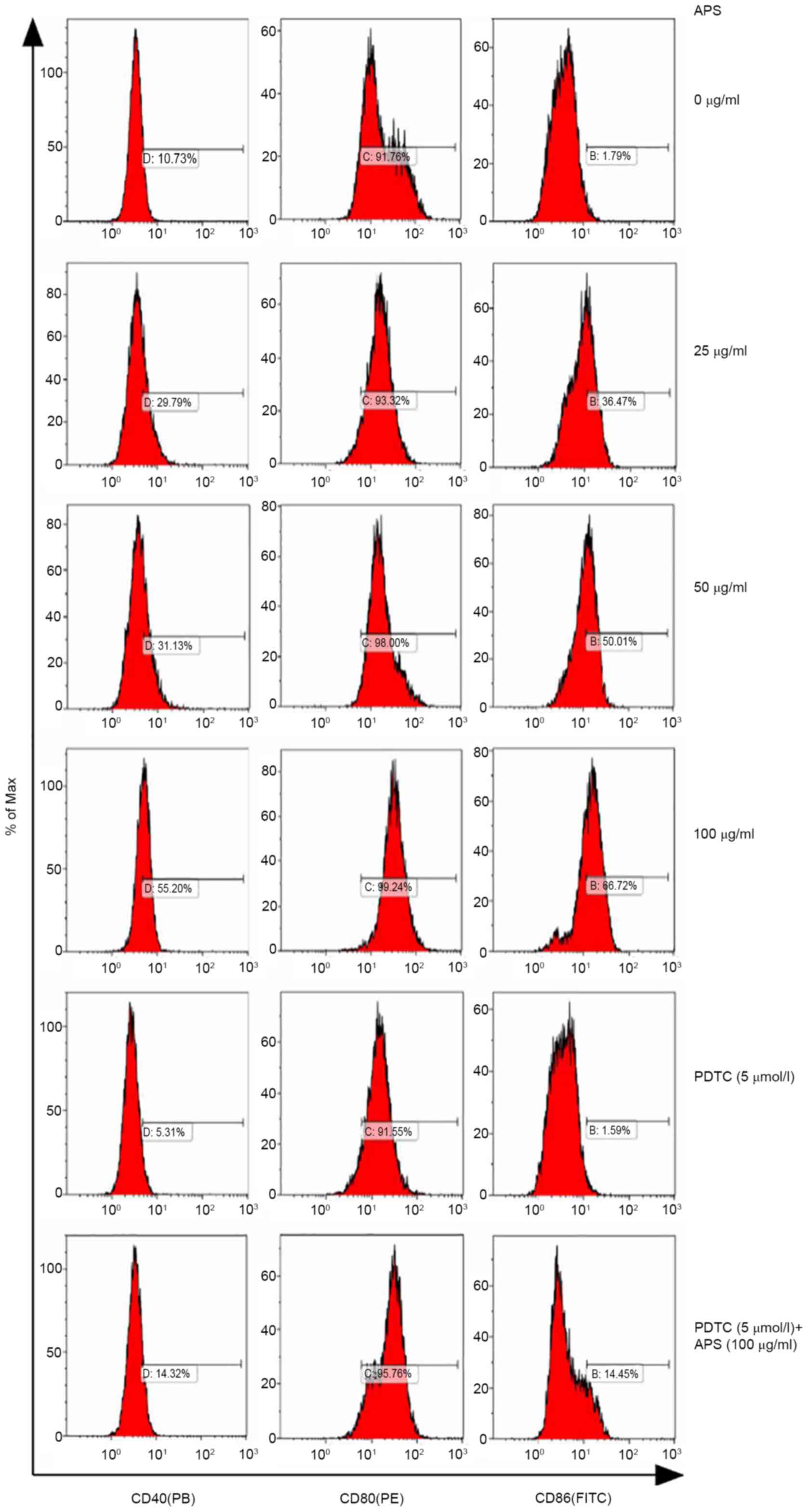
APS upregulates the expression of
surface molecules on RAW264.7 cells
The expression of CD40, CD80 and CD86 on RAW264.7
cells treated with APS were measured by flow cytometry (Fig. 4). Compared with the 0 µg/ml APS
treatment group, the expression levels of CD40 increased gradually
with increasing concentrations of APS. Following treatment with 100
µg/ml APS, CD40 expression reached 55.20%; however, following the
addition of PDTC, the expression levels of CD40 decreased. Compared
with 100 µg/ml APS, CD40 expression levels decreased in the 100
µg/ml APS + PDTC group. APS exerted no effects on CD80 expression.
CD86 secretion increased from 36.47 to 66.72% with 25-100 µg/ml
APS, while PDTC inhibited APS-induced upregulation on CD86
expression.
APS promotes cell proliferation by
inducing cell cycle progression in RAW264.7 cells
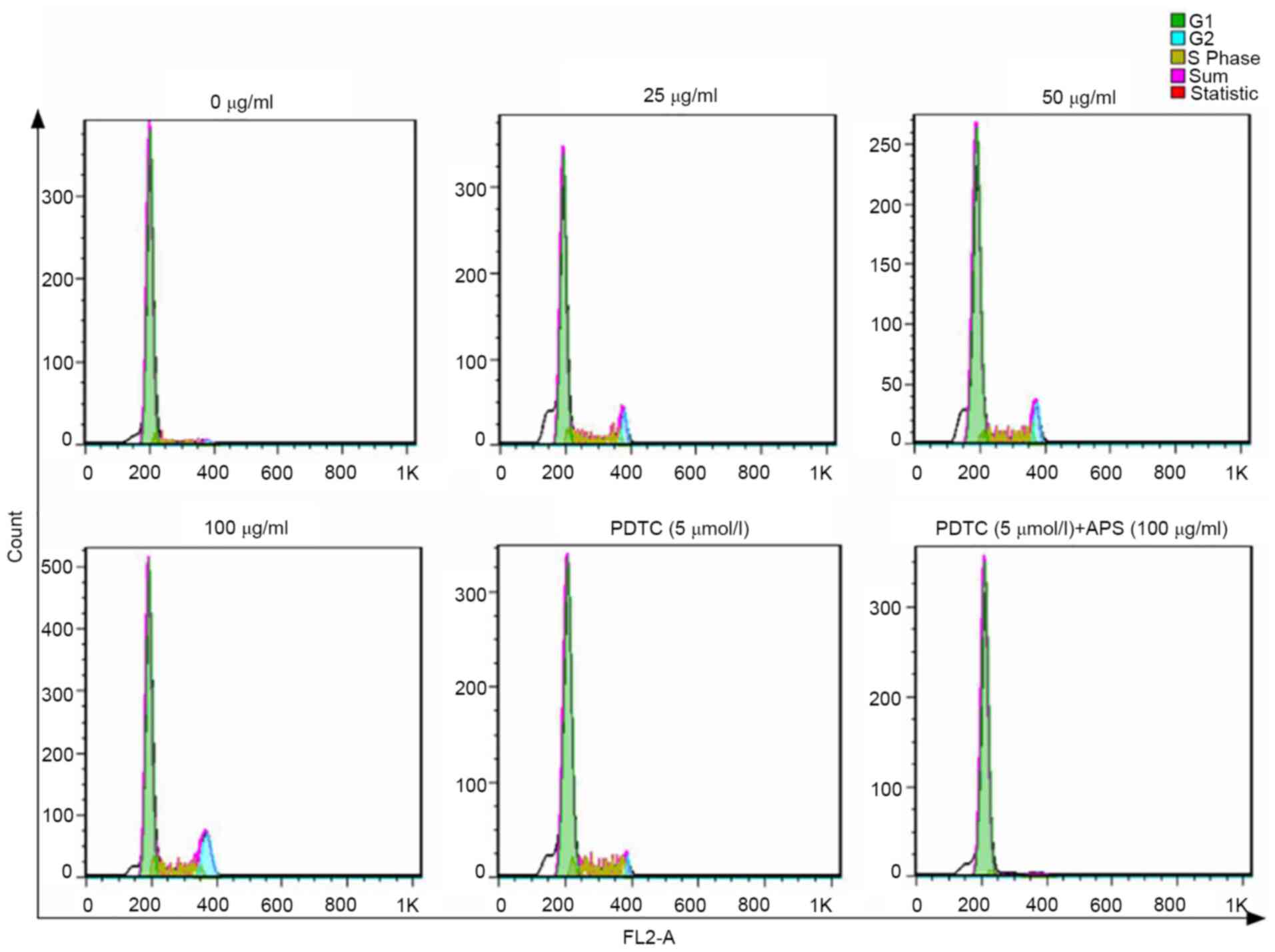
As shown in Fig. 5
and Table II, APS increased the
ratio of G2/M cells in a dose-dependent manner, where the effects
of 100 µg/ml APS was the most prominent. The addition of PDTC
lowered the ratio of G2/M cells. The number of G2/M phase cells was
slightly higher compared with the 0 µg/ml APS treatment group when
both APS and PDTC were administered simultaneously. Therefore, the
results indicated that APS promoted cell proliferation.
 | Table IICell cycle data. |
Table II
Cell cycle data.
| | APS (µg/ml) | | |
|---|
| Items | 0 | 25 | 50 | 100 | PDTC (5
µmol/l) | PDTC (5 µmol/l) +
100 µg/ml APS |
|---|
| RMS | 1.66 | 2.57 | 2.17 | 2.32 | 2.16 | 1.46 |
| Freq.G1 | 86.20 | 66.44 | 80.31 | 66.57 | 75.84 | 96.54 |
| Freq.S | 5.48 | 14.02 | 12.37 | 15.70 | 14.70 | 3.41 |
| Freq.G2 | 2.45 | 7.01 | 8.40 | 15.65 | 2.26 | 2.68 |
| G1 mean | 201.93 | 194.46 | 190.73 | 192.66 | 208.15 | 210.00 |
| G2 mean | 383.19 | 379.63 | 373.81 | 368.87 | 387.07 | 376.00 |
| G1 cv | 6.42 | 6.65 | 8.54 | 6.76 | 7.96 | 7.19 |
| G2 cv | 6.24 | 2.98 | 3.35 | 5.90 | 1.74 | 11.44 |
| Freq.sub-G1 | 5.62 | 14.38 | 4.46 | 3.34 | 9.25 | 2.91 |
| Freq.sub-G2 | -0.75 | -0.46 | -0.13 | -1.19 | -0.05 | -0.82 |
APS promotes p65 expression in
RAW264.7 cells
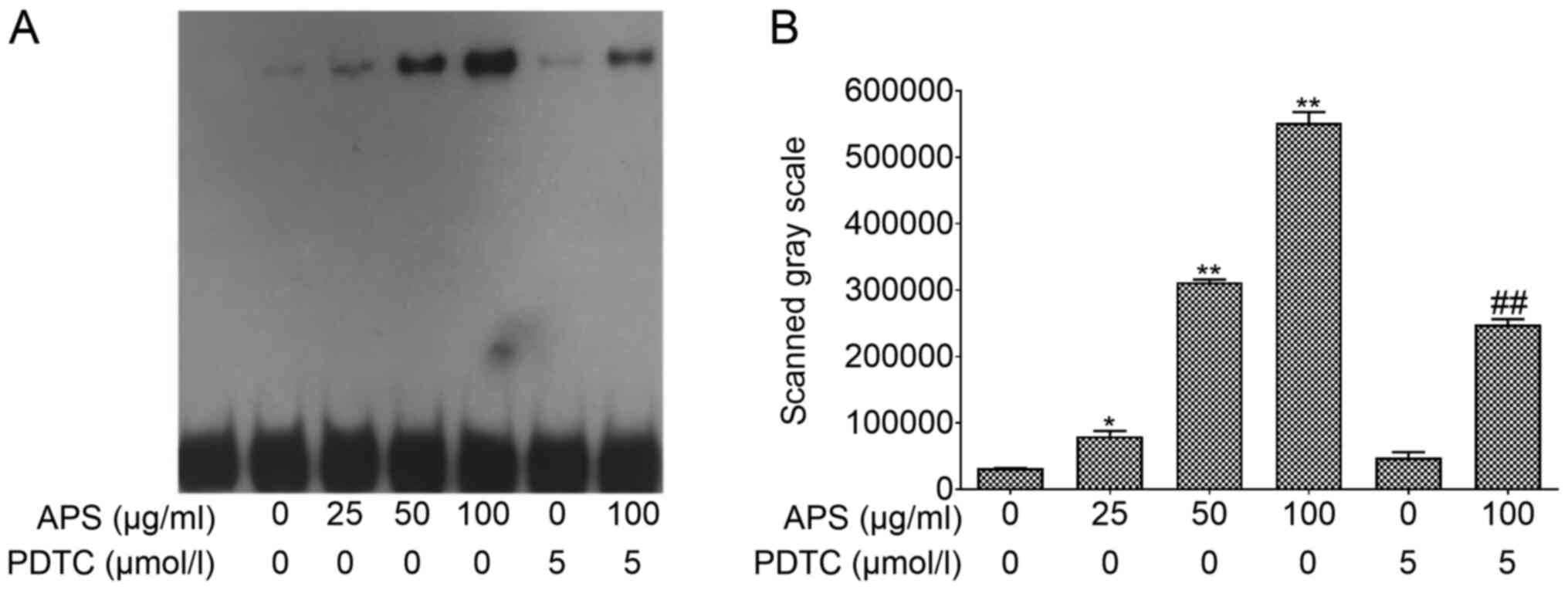
EMSA was performed using nuclear protein extracts to
assess p65 expression in the nucleus (Fig. 6). APS treatment resulted in an
increase of p65 expression in a dose-dependent manner. However,
this effect was inhibited by PDTC. Compared with the 0 µg/ml APS
treatment group, the expression levels of p65 in the nucleus
significantly increased in the 25 µg/ml (P<0.05), 50 µg/ml
(P<0.01) and 100 µg/ml APS (P<0.01) groups. Compared with 100
µg/ml APS, p65 expression of p65 significantly decreased in the 100
µg/ml APS + PDTC group (P<0.01). These results suggested that
PDTC can inhibit the expression of p65 in the nucleus, opposing the
effects of APS treatment.
APS activates the NF-κB/MAPK signaling
pathway in RAW264.7 cells
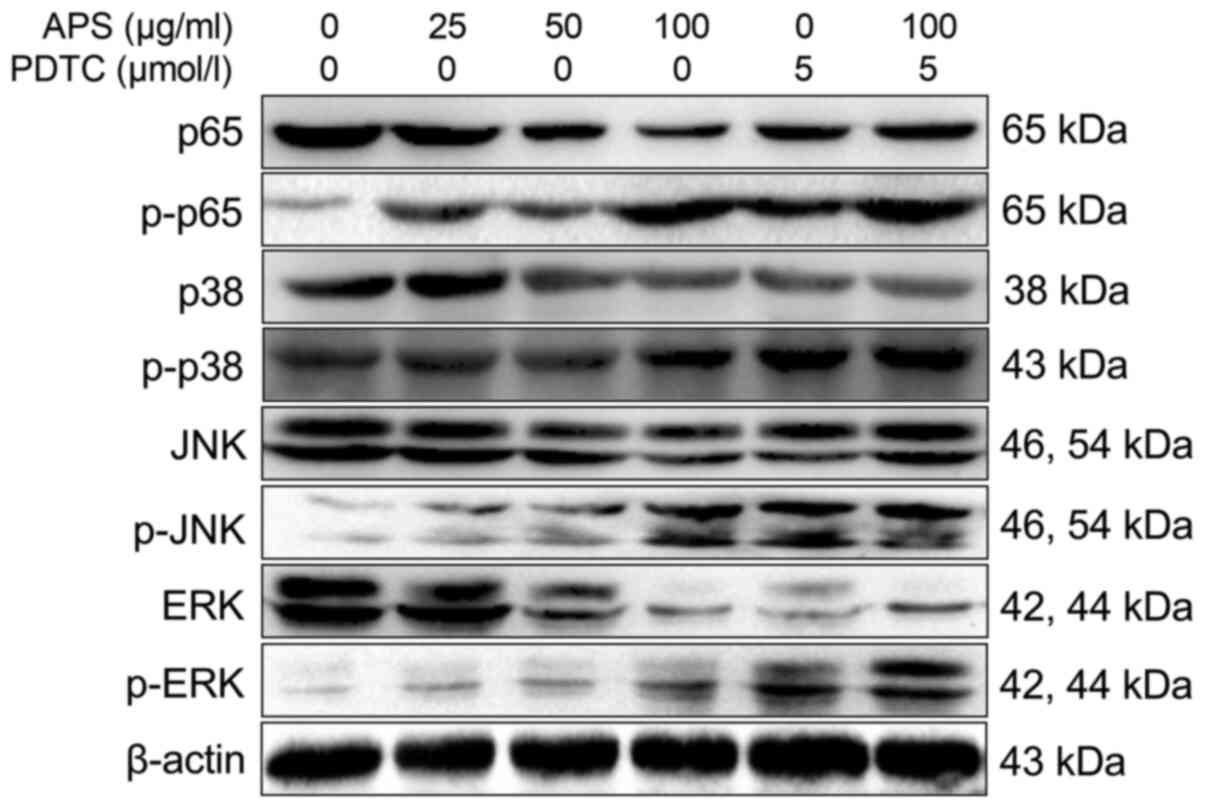
Western blot analysis results are shown in Fig. 7. The levels of p-p65 in RAW264.7
cells increased significantly with increasing concentrations of
APS. Compared with the 0 µg/ml APS treatment group, the levels of
NF-κB p65, p-p38, p-JNK and p-ERK were significantly increased in
the 25-100 µg/ml APS groups. Compared with 100 µg/ml APS, the
levels of p-p38 and p-JNK were decreased in the 100 µg/ml APS +
PDTC group.
Discussion
APS is the main bioactive ingredient of
Astragalus membranaceus and is widely studied for their
immunopotentiating components on murine B-cell proliferation
stimulation (22,23). Besides, previous studies reported
that APS can promote cell proliferation within a certain
concentration range (24). To
investigate the immune regulation of APS, CCK-8 assay was taken. In
the present study, the result showed that different concentrations
of APS increased the proliferation of cells to a certain extent
which indicated that APS promoted the differentiation of RAW264.7.
In addition, it is reported that APS was not associated with toxic
effects in 3T3-L1 adipocytes and ob/ob diabetic mice (25). Our results further confirm that APS
play a vital role in RAW264.7 differentiation without cytotoxicity.
Meanwhile, APS was shown to potentiate immune response of murine
macrophages via increasing the production of IL-1β and TNF-α
(23). It was previously reported
that pachymaran polysaccharides enhance cellular immune function by
increasing the secretion of NO and TNF-α in macrophages (26). Pleurotus nebrodensis polysaccharide
can activate RAW264.7 cells and increase the expression of IL-6,
TNF-α, IFN-γ, NO and iNOS, thereby exerting an immune-enhancing
effect (7). In a study of mice, APS
significantly suppressed coxsackievirus B3-induced expression of
IL-1β, IL-6, TNF-α, INF-γ and MCP-1 in the heart (27). In addition, APS can modulate
cytokine-induced immune dysfunction, mainly by downregulation of
the expression of IL-1β, IL-2, IL-6, TNF-α and IFN-γ in non-obese
diabetic mice T-cell subsets (28).
However, it is not clear whether APS can induce the production of
cytokines in RAW264.7 in vitro. The results of the present
study demonstrated that APS promoted the secretion of IL-1β, IL-6
and TNF-α and increased the content of NO in RAW264.7 cells, which
indicated that APS can enhance the immune function of RAW264.7
cells by directly promoting the production of cytokines. It is well
known that the production of cytokines is regulated on
transcriptional, post-transcriptional and translational level
(29). Furthermore, it was
demonstrated that polysaccharides can exert immunopotentiation
effects by increasing the levels of cytokines and NO and enhancing
gene expression (30). In the
present study, the levels of IL-1β, IL-6, TNF-α and NO were
measured. The results demonstrated that the expression of IL-1β,
IL-6, TNF-α and iNOS was increased after treated with APS and was
decreased after inhibitor PDTC treatment. These results suggest
that APS can promote the expression of cytokines and the production
of NO, exerting potent immunomodulatory effects.
CD40, CD80 and CD86 are antigen-presenting cells
that play a costimulatory role in immune response regulation
(31). Previous studies indicate
that CD40, CD80 and CD86 are indispensable for immune responses and
inducing the expression of cytokines (32). In the present study, APS increased
the secretion of CD40 and CD80 in RAW264.7 cells. There is no
significant changes in the secretion of CD80. On the basis of these
findings, CD40 and CD80, not CD 86, are included in the immune
regulation stimulated with APS. It is reported that G1 period and S
period are preparing for the presynthesis of RNA and synthesis of
DNA, respectively (31). The
regulation of the cell cycle is necessary to cell proliferation
(33). It was found that APS
exhibited an anti-proliferative effect on cancer and displayed
differentiation induction on erythroid (34). In the present study, the G2/M of
RAW264.7 cells was gradually increased with the increased APS and
was lowed with the inhibitor PDTC treatment. Combined with the
viability results, APS promoted the proliferation of RAW264.7
cells.
APS regulates immune function through a variety of
extracellular and intracellular signaling pathways, of which the
most important is the NF-κB signaling pathway (35-39).
NF-κB plays a key role in the regulation of immune and inflammatory
responses through phosphorylation, DNA binding, dimerization and
nuclear translocation methods (39,40).
p65 is key in the activation of the NF-κB family of transcription
factors and translocates from the cytoplasm to the nucleus after
stimulation (41,42). In the present study, p65 nuclear
translocation increased in RAW264.7 cells treated with APS as
determined by EMSA. The results demonstrated that different
concentrations of APS could promote p65 nuclear translocation,
particularly at high concentration, whereas PDTC could inhibit the
function of APS. It is reported that p38, JNK and ERK MAPK
signaling pathway is upstream of IL-1β, IL-6 and TNF-α (43,44).
As one of the main components of Radix Astragali,
Astragaloside IV was found to increase the phosphorylation of p65,
p38, ERK and JNK (31). Limited
studies report whether APS affects the protein expression of p65
and MAPK. To further investigate the roles of APS in the MAPK and
NF-κB pathways, western blotting was used. In the present study,
western blotting results revealed that the levels of p-p65, p-p38,
p-JNK and p-ERK were significantly increased following treatment
with APS. The changes of p65 and MAPK were reversed by the
inhibitor PDTC. These results suggested that APS can enhance immune
function via the NF-κB p65/MAPK signaling pathway and PDTC can
suppress the effects of APS. Overall, the activation of NF-κB
p65/MAPK signaling pathway provides a molecular explanation for the
well-known immune regulation with APS.
In conclusion, the results of the present study
demonstrated that APS affected the expression of inflammatory
cytokines and associated genes, promoting the secretion of
co-stimulatory molecules on the surface of RAW264.7 cells and
promoted cell proliferation to enhance immune function partly via
the NF-κB/MAPK signaling pathway. However, the present study did
not perform in vivo studies, which will further clarify the
immunoregulatory effects of APS. However, the present study
provided a foundation for further investigation of the immune
function enhancement of APS.
Acknowledgements
Not applicable.
Funding
This research was funded by the National Key
Research and Development Program of China (grant no.
2016YFD0501009) and the Project of Modern Agricultural Industry and
Technology System of Anhui Province (grant no. AHCYJSTX-07).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SF and YL performed the experiments, collected the
results and wrote the manuscript. HD, LL, CP, YH, FZ, WL and TM
contributed to data analysis and manuscript revision. JL, XW, YL
and JW conceived the study and contributed to reviewing/editing the
manuscript. All authors read, approved the final manuscript and
agreed to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu J, Zhao ZZ and Chen HB: Review of
Astragali radix. Chin Herb Med. 2:90–105. 2011.
|
|
2
|
Zhao LH, Ma ZX, Zhu J, Yu XH and Weng DP:
Characterization of polysaccharide from Astragalus radix as
the macrophage stimulator. Cell Immunol. 271:329–334.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang X, Li Y, Yang X and Yao J:
Astragalus polysaccharide reduces inflammatory response by
decreasing permeability of LPS-infected Caco2 cells. Int J Biol
Macromol. 61:347–352. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kong X, Hu Y, Rui R, Wang D and Li X:
Effects of Chinese herbal medicinal ingredients on peripheral
lymphocyte proliferation and serum antibody titer after vaccination
in chicken. Int Immunopharmacol. 4:975–982. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xu HD, You CG, Zhang RL, Gao P and Wang
ZR: Effects of Astragalus polysaccharides and astragalosides
on the phagocytosis of Mycobacterium tuberculosis by macrophages. J
Int Med Res. 35:84–90. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Han J, Yue WP, Guang-Zhi HE and Tian WY:
Effects of polysaccharide of eight traditional chinese medicine on
cytokine releasing from mouse macrophages. Curr Immunol.
31:495–498. 2011.
|
|
7
|
Sun H, Zhang J, Chen F, Chen X, Zhou Z and
Wang H: Activation of RAW264.7 macrophages by the polysaccharide
from the roots of Actinidia eriantha and its molecular mechanisms.
Carbohydr Polym. 121:388–402. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Choi YH, Jin GY, Li GZ and Yan GH:
Cornuside suppresses lipopolysaccharide-induced inflammatory
mediators by inhibiting nuclear factor-kappa B activation in RAW
264.7 macrophages. Biol Pharm Bull. 34:959–966. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dunzendorfer S, Lee HK, Soldau K and
Tobias PS: TLR4 is the signaling but not the lipopolysaccharide
uptake receptor. J Immunol. 173:1166–1170. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hsu BY, Kuo YC and Chen BH: Polysaccharide
Isolated from Zizyphus jujuba (Hóng Zǎo) Inhibits
Interleukin-2 Production in Jurkat T Cells. J Tradit Complement
Med. 4:132–135. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Van Gool SW, Vandenberghe P, de Boer M and
Ceuppens JL: CD80, CD86 and CD40 provide accessory signals in a
multiple-step T-cell activation model. Immunol Rev. 153:47–83.
1996.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chang TT, Kuchroo VK and Sharpe AH: Role
of the B7-CD28/CTLA-4 pathway in autoimmune disease. Curr Dir
Autoimmun. 5:113–130. 2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yellin MJ, Brett J, Baum D, Matsushima A,
Szabolcs M, Stern D and Chess L: Functional interactions of T cells
with endothelial cells: The role of CD40L-CD40-mediated signals. J
Exp Med. 182:1857–1864. 1995.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Harlan DM, Hengartner H, Huang ML, Kang
YH, Abe R, Moreadith RW, Pircher H, Gray GS, Ohashi PS and Freeman
GJ: Mice expressing both B7-1 and viral glycoprotein on pancreatic
beta cells along with glycoprotein-specific transgenic T cells
develop diabetes due to a breakdown of T-lymphocyte
unresponsiveness. Proc Natl Acad Sci USA. 91:3137–3141.
1994.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Khatua S and Acharya K: Alkali treated
antioxidative crude polysaccharide from Russula alatoreticula
potentiates murine macrophages by tunning TLR/NF-κB pathway. Sci
Rep. 9(1713)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jia XJ, Li X, Wang F, Liu HQ, Zhang DJ and
Chen Y: Berbamine Exerts Anti-inflammatory effects via inhibition
of NF-κB and MAPK signaling pathways. Cell Physiol Biochem.
41:2307–2318. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhou L, Liu Z, Wang Z, Yu S, Long T, Zhou
X and Bao Y: Astragalus polysaccharides exerts
immunomodulatory effects via TLR4-mediated MyD88-dependent
signaling pathway in vitro and in vivo. Sci Rep.
7(44822)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Du X, Chen X, Zhao B, Lv Y, Zhang H, Liu
H, Chen Z, Chen Y and Zeng X: Astragalus polysaccharides
enhance the humoral and cellular immune responses of hepatitis B
surface antigen vaccination through inhibiting the expression of
transforming growth factor β and the frequency of regulatory T
cells. FEMS Immunol Med Microbiol. 63:228–235. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang S: Effects of water soluble alfalfa
and Astragalus polysaccharide on proliferation of lymphocyte
in broilers. Chin J Anim Nutr. 22:670–674. 2010.
|
|
20
|
Li Y, Hao N, Zou SP, Meng TT, Tao HQ, Ming
PF, Li MM, Ding HY, Li JC, Feng SB, et al: Immune regulation of
RAW264. 7 cells in vitro by flavonoids from Astragalus
complanatus via activating the NF-κB signalling pathway. J
Immunol Res. 2018:1–9. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li XT, Zhang YK, Kuang HX, Jin FX, Liu DW,
Gao MB, Liu Z and Xin XJ: Mitochondrial protection and anti-aging
activity of Astragalus polysaccharides and their potential
mechanism. Int J Mol Sci. 13:1747–1761. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shao BM, Xu W, Dai H, Tu P, Li Z and Gao
XM: A study on the immune receptors for polysaccharides from the
roots of Astragalus membranaceus, a Chinese medicinal herb.
Biochem Biophys Res Commun. 320:1103–1111. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang NW, Li JF, Hu YX, Cheng GL, Zhu XY,
Liu FQ, Zhang YJ, Liu ZJ and Xu JQ: Effects of Astragalus
polysaccharide on the immune response to foot-and-mouth disease
vaccine in mice. Carbohydr Polym. 82:680–686. 2010.
|
|
25
|
Ma L, Huang L, Pei H, Liu Z, Xie C, Lei L,
Chen X, Ye H, Peng A and Chen L: Pharmacological effects of the
water fraction of key components in the traditional Chinese
prescription Mai Tong Fang on 3T3-L1 adipocytes and ob/ob diabetic
mice. Molecules. 19:14687–14698. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chung HS, Shin CH, Lee EJ, Hong SH and Kim
HM: Production of nitric oxide and tumor necrosis factor-alpha by
Smilacis rhizoma in mouse peritoneal macrophages. Comp Biochem
Physiol C Toxicol Pharmacol. 135:197–203. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu T, Zhang M, Niu H, Liu J, Ruilian M,
Wang Y, Xiao Y, Xiao Z, Sun J, Dong Y, et al: Astragalus
polysaccharide from Astragalus Melittin ameliorates
inflammation via suppressing the activation of TLR-4/NF-κB p65
signal pathway and protects mice from CVB3-induced virus
myocarditis. Int J Biol Macromol. 126:179–186. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wei C, Li YM, Yu MH and Shi XM:
Immunoloregulation effects of Astragalus polysaccharides on
T helper lymphocyte subgroups in nonobese diabetic mice. China J
Mod Med. 17:28–35. 2007.(In Chinese).
|
|
29
|
Stoecklin G, Mayo T and Anderson P:
ARE-mRNA degradation requires the 5'-3' decay pathway. EMBO Rep.
7:72–77. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Luo T, Qin J, Liu M, Luo J, Ding F, Wang M
and Zheng L: Astragalus polysaccharide attenuates
lipopolysaccharide-induced inflammatory responses in microglial
cells: Regulation of protein kinase B and nuclear factor-κB
signaling. Inflamm Res. 64:205–212. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li Y, Meng T, Hao N, Tao H, Zou S, Li M,
Ming P, Ding H, Dong J, Feng S, et al: Immune regulation mechanism
of Astragaloside IV on RAW264.7 cells through activating the
NF-κB/MAPK signaling pathway. Int Immunopharmacol. 49:38–49.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li J, Wang F, Wang G, Sun Y, Cai J, Liu X,
Zhang J, Lu X, Li Y, Chen M, et al: Combination epidermal growth
factor receptor variant III peptide-pulsed dendritic cell vaccine
with miR-326 results in enhanced killing on EGFRvIII-positive
cells. Oncotarget. 8:26256–26268. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang Z, Deng X, Xiong R, Xiong S, Liu J,
Cao X, Lei X, Chen Y, Zheng X and Tang G: Design, synthesis and
biological evaluation of 3',4',5'-trimethoxy flavonoid
benzimidazole derivatives as potential anti-tumor agents.
MedChemComm. 9:305–315. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zong A, Cao H and Wang F: Anticancer
polysaccharides from natural resources: A review of recent
research. Carbohydr Polym. 90:1395–1410. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jiang CL, Tang C, Qiang Y, Suo HY and Li
L: Immunoregulation effect of Astragalus polysaccharides.
Shipin Kexue. 94:90–99. 2013.
|
|
36
|
Wang Z, Liu Z, Zhou L, Long T, Zhou X and
Bao Y: Immunomodulatory effect of APS and PSP is mediated by
Ca2+-cAMP and TLR4/NF-κB signaling pathway in
macrophage. Int J Biol Macromol. 94:283–289. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen JL, Zhang YQ, Yuan Y, Wu SR and Ming
J: Progress in research on immune-regulatory effects of plant
polysaccharides on macrophages through NF-κB signaling pathway.
Food Sc. 36:288–294. 2015.
|
|
38
|
Chen JL, Zhang YQ, Yuan Y, Wu SR and Ming
J: Research progress on the immune-regulatory effects for
macrophages of plant polysaccharides by NF-κB signaling pathway.
Klin Oczna. 46(1441)2015.
|
|
39
|
Lamparter CL, Philbrook NA and Winn LM:
Valproic acid increases NF-κB transcriptional activation despite
decreasing DNA binding ability in P19 cells, which may play a role
in VPA-initiated teratogenesis. Reprod Toxicol. 74:32–39.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Panday A, Inda ME, Bagam P, Sahoo MK,
Osorio D and Batra S: Transcription factor NF-κB: An update on
intervention strategies. Arch Immunol Ther Ex. 64:1–21.
2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhang Q, Lenardo MJ and Baltimore D: 30
years of NF-κB: A blossoming of relevance to human pathobiology.
Cell. 168:37–57. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhang JZ, Liu N, Sun C, Sun DQ and Wang
YJ: Polysaccharides from polygonatum sibiricum delar. ex redoute
induce an immune response in the RAW264.7 cell line via an
NF-κB/MAPK pathway. RSC Advances. 9:17988–17994. 2019.
|
|
43
|
Cho JW, Lee KS and Kim CW: Curcumin
attenuates the expression of IL-1β, IL-6, and TNF-α as well as
cyclin E in TNF-α-treated HaCaT cells; NF-kappaB and MAPKs as
potential upstream targets. Int J Mol Med. 19:469–474.
2007.PubMed/NCBI
|
|
44
|
Fisher WG, Yang PC, Medikonduri RK and
Jafri MS: NFAT and NFkappaB activation in T lymphocytes: A model of
differential activation of gene expression. Ann Biomed Eng.
34:1712–1728. 2006.PubMed/NCBI View Article : Google Scholar
|