Introduction
Renal fibrosis is a frequent occurrence during the
deterioration of chronic kidney diseases into end-stage renal
disease (1). It is a chronic
clinical disease that is characterized by the formation of
superfluous fibrous connective tissues, gradual reduction in
glomerular filtration and the gradual decline in renal tubular
function (2). In addition to
chronic kidney diseases, other factors can also lead to renal
fibrosis, including systemic lupus erythematosus, genetic factors,
diabetes, hypertension, drugs, hepatitis B, immune deficiency and
kidney transplantation. At present, the incidence of chronic kidney
disease is between 6.6 and 13% in China (3). Renal interstitial fibrosis (RIF) and
glomerulosclerosis are two principal features of renal fibrosis
(4). Compared with
glomerulosclerosis, RIF holds higher research significance since
renal interstitial lesions can be used as an indicator of the
severity of decline in renal function (4). The pathological features of renal
damage during RIF are mainly reflected by the observed accumulation
of cells and collagen, atrophy and dilation of renal tubules and
the loss of renal tubules and interstitial capillaries (5). Therefore, it remains urgent to
investigate the mechanism underlying the RIF process.
RIF is a complex biological process that involves a
variety of cytokines, signaling pathways and processes, including
inflammation, apoptosis and oxidative stress (6). At present, the precise molecular
mechanism underlying renal fibrosis remains poorly understood.
Renal fibrosis has been previously reported to involve a multitude
of signaling pathways, including transforming growth factor
(TGF)-β/Smad (7), apoptosis
signal-regulating kinase (8),
5'AMP-activated protein kinase/NADPH oxidase 4(9) and the Janus kinase/STAT/glycogen
synthase kinase-3β/β-catenin (10)
pathways. Regardless of the etiology, high levels of TGF-β
activation have been frequently associated with fibrosis and
disease progression (11). It has
been previously reported that the NF-κB family of transcription
factors can promote the expression of TGF-β, leading to the
activation of the TGF-β/Smad pathway to mediate downstream
physiological effects (12). Smad
proteins are intracellular effectors of TGF-β (13). Smad4 is a key regulator in the Smad
protein family that can interact with Smad7 and Smad3 to modulate
their transcriptional activities, upstream of the renal
inflammatory and fibrotic processes (14). It has also been previously
demonstrated that dysregulation of the TGF-β/Smad pathway is an
important cause of tissue fibrosis (11). TGF-β1 can upregulate the expression
of Smad2 and Smad3 during tissue fibrosis, a process that is
negatively regulated by Smad7 as part of a negative feedback loop
(15). By contrast, previous
studies have demonstrated that the long-term persistence of
inflammatory factors, such as tumor necrosis factor-α (TNF-α) and
interleukin-1β (IL-1β), in renal tissues were closely associated
with RIF, which sensitizes the kidney cells to TGF-β to aggravate
fibrosis further (16,17). Of note, macrophages are the main
type of infiltrating immune cells that have been found to mediate
this inflammatory process (18).
The kidney produces a large number of monocytes/macrophages, which
constantly infiltrates the kidney to produce pro-inflammatory
cytokines, including TNF-α and IL-1β, to induce kidney inflammation
(19). It has been previously
reported that inhibiting the release of inflammatory factors can
attenuate the progress of RIF. These previous observations
aforementioned suggest that the TGF-β/Smad pathway and inflammatory
proteins serve important roles in the development of RIF.
Naringin is a dihydroflavonoid that can be found in
the immature or near-mature dry outer pericarp of pomelo,
grapefruit and the citrus of Rutaceae (20,21). A
number of studies have previously demonstrated that naringin
exhibits several biologically active effects, including
anti-inflammation, hepato-protection, anti-apoptosis, antioxidant
in addition to inhibiting genetic toxicity (22-25).
Naringin can effectively inhibit carbon tetrachloride-induced acute
liver and kidney injury in mice by serving as an antioxidant and
scavenging free radicals (26).
Additionally, it has been previously shown that naringin can
alleviate sodium arsenite-induced liver fibrosis in rats by
suppressing TGF-β (27,28). However, the potential effects of
naringin on RIF remain poorly understand. Therefore, a rat model of
renal interstitial fibrosis and a fibrosis cell model was
established to evaluate the effects of naringin on inflammatory
proteins and fibrosis markers in kidney of rats and NRK-52E cells,
and to elucidate the mechanism governing this.
Materials and methods
Materials and reagents
Naringin was purchased from Dalian Meilun Biotech
Co., Ltd. FBS was purchased from Hyclone, Cytiva. TGF-β was
purchased from ProteinTech Group, Inc. Cell proliferation Kit I MTT
(cat. no. 11465007001) was purchased from Roche Diagnostics. DAPI,
DMEM, Tween-20 and 5% skimmed milk powder were purchased from
Beijing Solarbio Science & Technology Co., Ltd. Blood urea
nitrogen (BUN; cat. no. C013-2) and creatinine (Scr) kits (cat. no.
C011-1) were purchased from Nanjing Jiancheng Bioengineering
Institute. Bicinchoninic acid (BCA) protein concentration
determination and tissue protein extraction kits were purchased
from Beyotime Institute of Biotechnology. Anti-α-smooth muscle
actin (α-SMA; cat. no. BM0002) antibody used for immunofluorescence
was purchased from Wuhan Boster Biological Technology, Ltd.
Anti-phosphorylated (p)-Smad2/3 (cat. no. WL02305), Smad7 (cat. no.
WL02975), α-SMA (cat. no. WL02510) and NF-κB (cat. no. WL01980)
were purchased from Wanleibio Co., Ltd. Anti-collagen 1 (COL1A1;
cat. no. 14695-1-AP), TGF-β (cat. no. 21898-1-AP), Smad2 (cat. no.
12570-1-AP), Smad3 (cat. no. 25494-1-AP), Smad4 (cat. no.
51144-1-AP), cyclooxygenase (COX)-2 (cat. no. 12375-1-AP),
activator protein-1 (AP-1; cat. no. 22114-1-AP), high-mobility
group protein B1 (HMGB1; cat. no. 10829-1-AP), GAPDH (cat. no.
10494-1-AP), HRP-conjugated Affinipure Goat Anti-Rabbit IgG (H+L)
(secondary antibody, cat. no. SA00001-2), fluorescent anti body and
chemiluminescence Western Blot kit (cat. no. B500034) were
purchased from Wuhan Sanying Biotechnology (https://www.ptgcn.com/).
Experimental cells
The rat renal tubular epithelial cell line NRK-52E
was purchased from the Institute of Biochemistry and Cell Biology,
Shanghai Academy of Life Sciences, Chinese Academy of Sciences.
NRK-52E cells were incubated in DMEM containing 10% FBS under 5%
CO2 and 37˚C.
MTT assay
NRK-52E cells were seeded into 96-well plates at a
concentration of 5x104 cells/ml. Different
concentrations of naringin (0, 50, 100, 200, 400 and 800 ng/ml)
were then added to the cells. After 24 h incubation at 37˚C 50 µl
serum-free media and 50 µl MTT reagent were added into each well
(29). After incubation at 37˚C,
the MTT reagent-supplemented media was removed and 150 µl
solubilization solution was added into each well. The plates were
then shaken on an orbital shaker for 15 min at 37˚C before
absorbance at 590 nm was read for each well, which was used to
calculate the cell survival rate. The formula used was %viable
cells=Absorbancesample-Absorbanceblank)
x100/(Absorbancecontrol-Absorbanceblank).
For TGF-β treatment, NRK-52E cells at a
concentration of 1x105/ml were seeded into 96-well
plates. After 24 h of culture at 37˚C, cells in the blank group
were incubated with DMEM without FBS, whilst those in the model
group was provided with TGF-β (10 ng/ml). Cells in the treatment
group were treated with TGF-β (10 ng/ml) and naringin at different
concentrations (50, 100 and 200 ng/ml) for 24 h at 37˚C, following
which MTT assay was used to calculate the cell survival rate.
Experimental animals
A total of 36 Male Sprague-Dawley (SD) rats of
7-weeks old weighing 200-220 g were purchased from the SPF
Experimental Animal Center of Dalian Medical University (permit no.
SCXK 2013-0003; Dalian, China). Rats were housed in an animal room
under a 12-h light/dark cycle, at 20˚C and 60% relative humidity,
and were provided with food and drink ad libitum. Rats were
acclimatized for 1 week and fasted for 12 h before each experiment
(30,31). All rat experiments were conducted in
accordance with the National Institutes of Health guide for the
care and use of Laboratory animals (NIH Publications no. 85-23,
revised 1985) (32). All efforts
were made to minimize the number of animals used and their
suffering.
A total of 36 SD rats were divided into the
following groups (n=6): i) Sham group; ii) UUO group; iii) high
dose naringin administration group (80 mg/kg); iv) medium dose
naringin administration group (40 mg/kg); v) low dose naringin
administration group (20 mg/kg); and vi) single naringin
administration group (80 mg/kg).
RIF was induced by unilateral ureteral obstruction
(UUO) in rats (33,34). The operation procedure was as
follows: Rats were fasted for 12 h prior to operation but have free
access to drinking water. Rats were fixed on the operating table
following anesthesia with pentobarbital (60 mg/kg intraperitoneal
injection). After sterilization and shaving, a longitudinal
incision was made on the left side of the abdomen and the left
kidney and ureter were exposed. Rats in the UUO groups were
achieved by ligating the left ureter with 3-0 silk through a left
lateral incision. The abdomen was finally sutured in layers. The
left ureter was exposed but not ligated in the sham-operated or the
single administration groups. On day 2 after operation, rats in the
high, middle, low dose administration groups and the single
administration group were given naringin daily by intragastrical
administration for 28 consecutive days. The sham-operated and model
groups received an equivalent volume of 0.5% CMC-Na. Rats were then
fasted overnight and fixed in a supine position on the operating
table on day 28 before blood samples were collected from abdominal
aorta from rats after anesthesia with pentobarbital (60 mg/kg
intraperitoneal injection). Blood samples were placed in heparin
tubes and immediately centrifuged at 4,000 x g for 10 min at 4˚C.
The levels of BUN and Scr were calculated according to the
manufacturer's protocol. After sacrificing the rats, the left
kidney was quickly excised, decapsulated and immediately placed
into oxygenated buffer at 4˚C and then split into two halves. One
part of the kidney tissue was stored at -20˚C until further
biochemical testing whereas the other part was fixed for 1 week in
10% formaldehyde solution at 25˚C for histopathological
examination.
Histopathological examination
According to the routine method of histopathology,
kidney samples fixed in 10% formaldehyde solution (pH 7.2) were
embedded in paraffin to make wax blocks. Using a rotatory
microtome, 4-µm thick kidney tissue sections were prepared for
histopathological examination. Histopathological analysis was
conducted under an light microscope (magnification, x400) after
hematoxylin and eosin staining (H&E), Masson's trichrome
staining and Sirius red staining (all, Wuhan Servicebio Technology
Co., Ltd.). The protocol for H&E staining is briefly described
as follows: Slices were stained with hematoxylin for 10 min at 25˚C
and then dehydrated in 85 and 95% ethanol for 4 min, before being
stained with eosin for 4-5 min at 25˚C and dehydrated again using
three cylinders of 100% anhydrous ethanol. Slices were washed with
n-butanol and xylene before sealing with neutral gum. The protocol
for Masson's trichrome staining is briefly described as follows:
Slices were immersed in Masson solution A overnight at 25˚C before
incubation in Masson solution A at a 65˚C for 30 min. The slices
were then immersed in mixed dye solution for 1 min, differentiated
with 1% hydrochloric acid alcohol for ~1 min and incubated in
Masson solution D for 6 min at 25˚C. Incubation in Masson solution
E for 1 min and Masson solution F for 8-15 sec at 25˚C then ensued,
before the slices were sealed after dehydration with anhydrous
ethanol. The protocol for Sirius red staining is briefly described
as follows: Slices were immersed in Sirius red solution for 8 min
at 25˚C and dehydrated by anhydrous ethanol. Slices were washed
with xylene and sealed with neutral gum.
Immunofluorescence analysis α-SMA
NRK-52E cells were collected and seeded into
six-well plates at 1x105 cells/ml. After 24 h at 37˚C,
the blank group was replaced with DMEM without FBS, the model group
was treated with TGF-β (10 ng/ml), whilst the other groups were
treated with TGF-β (10 ng/ml) and naringin (50, 100 and 200 ng/ml)
for 24 h at 37˚C. Cells were rinsed three times with PBS, fixed at
room temperature in 10% formaldehyde for 20 min and incubated with
0.2% Triton X-100 for 10 min. The cells were then treated with the
immunofluorescence blocking solution for 1 h at 25˚C and incubated
overnight with the anti-α-SMA antibody (1:50) at 4˚C.
Rhodamine-conjugated fluorescent secondary antibody IgG h + L
(1:70) was subsequently added and incubated at 37˚C for 1 h. Cells
were washed three times with PBS before DAPI (10 g/ml) was added
and incubated at 37˚C for 10 min. Cells were washed and imaged at
x400 magnification using an inverted fluorescence microscope.
For tissue sections, they were first washed in
xylenes, followed by 2x10 min and then washed in 100% ethanol for 5
min, washed in 95% ethanol for 5 min, washed in 90% ethanol for 5
min, washed in 80% ethanol for 5 min, washed in 70% ethanol for 5
min and washed in water for 5 min. Antigens were retrieved and the
sections were sealed using immunofluorescent blocking solution at
25˚C. After PBS cleaning, the sections were incubated with
anti-α-SMA antibody (1:70) overnight at 4˚C before being washed
three times with PBS and incubated with fluorescein-conjugated
secondary antibodies (1:70) at 37˚C for 1 h. DAPI (10 g/ml) was
then added and incubated at 37˚C for 10 min. The sections were
finally imaged at x400 magnification using a fluorescence
microscope.
Reverse transcription-quantitative
PCR
Total RNA was extracted from renal and NRK-52E cells
using RNAiso Plus® Reagent Kit according to
manufacturer's protocol (Takara Biotechnology Co., Ltd.) and then
reverse-transcribed into cDNA using PrimeScript™ RT
Reagent kit with DNA Eraser (Takara Biotechnology Co., Ltd.). The
cDNA was amplified using SYBR® Premix Ex Taq™ kit
(Takara Biotechnology Co., Ltd.). Primer sequences are shown in
Table I. The thermocycling
conditions of RT PCR were as follows: Initial denaturation at 95˚C
for 30 sec, followed by 40 cycles of 95˚C for 5 sec and 60˚C for 30
sec, dissociation at 95˚C for 15 sec and 60˚C for 60 sec and 95˚C
for 15 sec. qPCR was subsequently performed using SYBR-Green PCR
Master Mix in an ABI prism 7500 Sequence Detection System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions of qPCR were as follows: Initial denaturation at 95˚C
for 5 min, followed by 40 cycles of 95˚C for 5 sec and 60˚C for 30
sec, dissociation at 95˚C for 15 sec and 60˚C for 60 sec and 95˚C
for 15 sec. The 2-ΔΔCq method was used to calculate the
fold change for each gene relative to that of GAPDH (35).
 | Table IPrimers used for reverse
transcription-quantitative PCR. |
Table I
Primers used for reverse
transcription-quantitative PCR.
| Gene | Forward primer
(5'-3') | Reverse primer
(5'-3') |
|---|
| Rat-GAPDH |
GAAAGACAACCAGGCCATCAG |
TCATGAATGCATCCTTTTTTGC |
| Rat-IL-1β |
CCCTGAACTCAACTGTGAAATAGCA |
CCCAAGTCAAGGGCTTGGAA |
| Rat-IL-6 |
ATTGTATGAACAGCGATGATGCAC |
CCAGGTAGAAACGGAACTCCAGA |
| Rat-TNF-α |
TCAGTTCCATGGCCCAGAC |
GTTGTCTTTGAGATCCATGCCATT |
| Rat-α-SMA |
AGCCAGTCGCCATCAGGAAC |
GGGAGCATCATCACCAGCAA |
| Rat-COL1A1 |
GACATGTTCAGCTTTGTGGACCC |
AGGGACCCTTAGGCCATTGTGTA |
| Rat-COL3A1 |
TTTGGCACAGCAGTCCAATGTA |
GACAGATCCCGAGTCGCAGA |
Protein isolation and western blotting
assay
NRK-52E cells and kidney tissues were utilized to
extract proteins by homogenization in RIPA Buffer (cat. no. R0278;
Sigma-Aldrich; Merck KGaA) buffer containing PMSF (Beyotime
Institute of Biotechnology). Protein concentration was determined
using the BCA kit. Protein samples (30 µg) were separated by 10%
SDS-PAGE and transferred onto PVDF membranes (Immobilon-P; EMD
Millipore). Anti-Smad2/3, Smad7, α-SMA, NF-κB, TGF-β, Smad2, Smad3,
Smad4, COX-2, AP-1 and HMGB1 antibodies (all, 1:2,000) were
incubated overnight at 4˚C. GAPDH (1:3,000) were incubated for 2 h
at 4˚C as the secondary antibody. The protein bands were visualized
using a chemiluminescence Western Blot kit and identified using the
ChemiDoc™ XRS and Imaging system (Bio-Rad Laboratories,
Inc.). Quantification of protein expression was performed using the
Image Lab™ Software (version 4.0.1 build 6; Bio-Rad
Laboratories, Inc.).
Statistical analysis
The experimental data were presented as the mean ±
SD (n=6 for rats; n=6 for cells). To test for statistically
significant differences among multiple treatments for a given
parameter, One-way ANOVA followed by Tukey's multiple comparisons
test was performed using the GraphPad Prism version 5.00 software
(GraphPad Software, Inc.).
Results
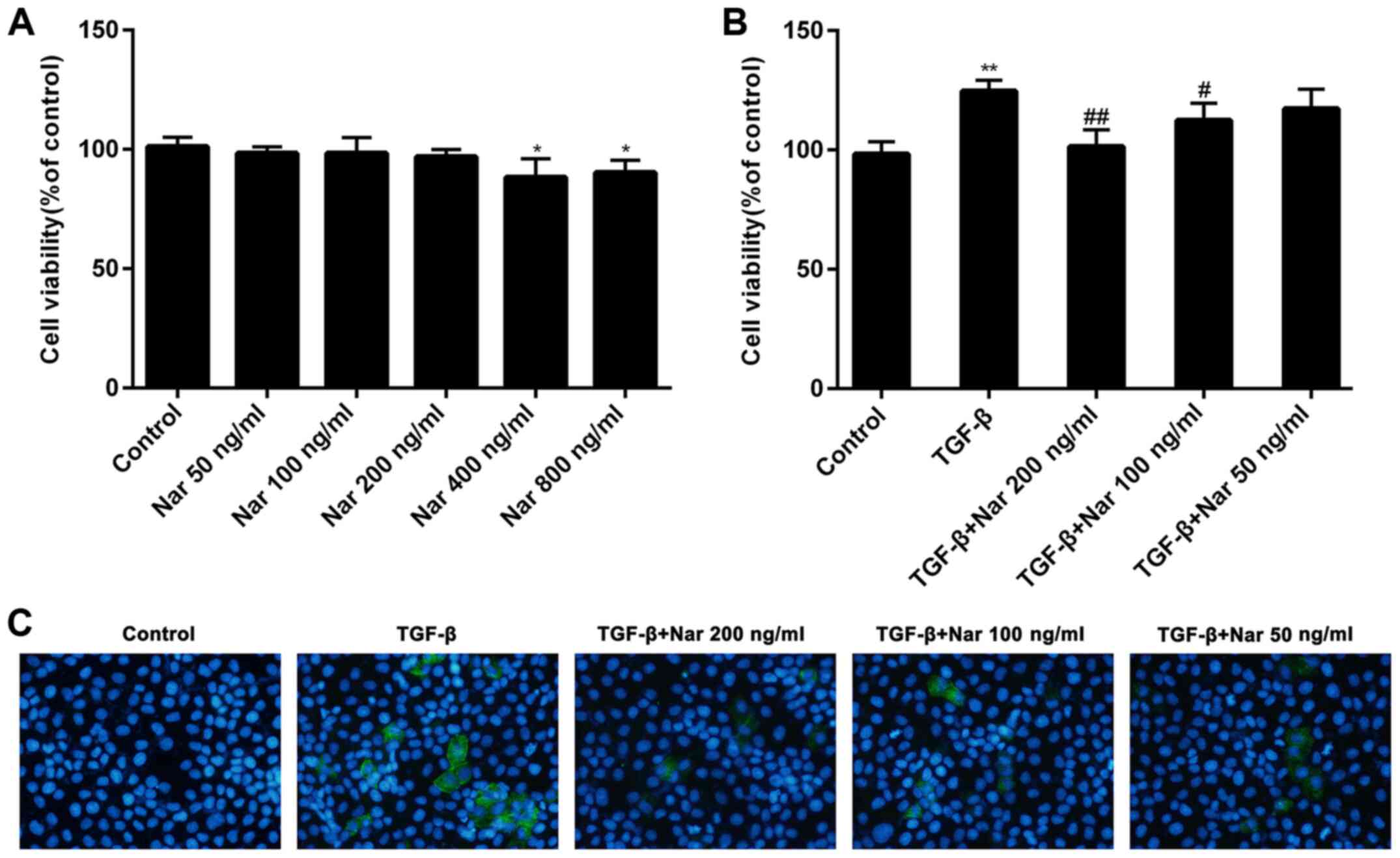
Naringin reduces NRK-52E cell
viability induced by TGF-β
To investigate the toxicity of naringin on NRK-52E
cells, ascending concentrations of naringin (0, 50, 100, 200, 400
and 800 ng/ml) were incubated with NRK-52E cells for 24 h. Naringin
exerted little or no toxicity on NRK-52E cells at concentrations of
<200 ng/ml (Fig. 1A). It was
also found that naringin (100 and 200 ng/ml) could significantly
reduce the viability of NRK-52E cells induced by TGF-β (Fig. 1B). These results suggest that
naringin can reverse the potentiating effects of TGF-β on NRK-52E
cell viability. Naringin at concentrations of 50, 100 and 200 ng/ml
was therefore chosen for subsequent experiments.
Inhibitory effects of naringin on cell
fibrosis induced by TGF-β
To investigate the effect of naringin on cell
fibrosis induced by TGF-β, the expression of fibrotic markers α-SMA
was detected by immunofluorescence. Compared with cells treated
with TGF-β alone, the expression levels of α-SMA protein in NRK-52E
cells treated with naringin (50, 100 and 200 ng/ml) was found to be
markedly reduced (Fig. 1C). These
results indicated that naringin could effectively inhibit cell
fibrosis induced by TGF-β.
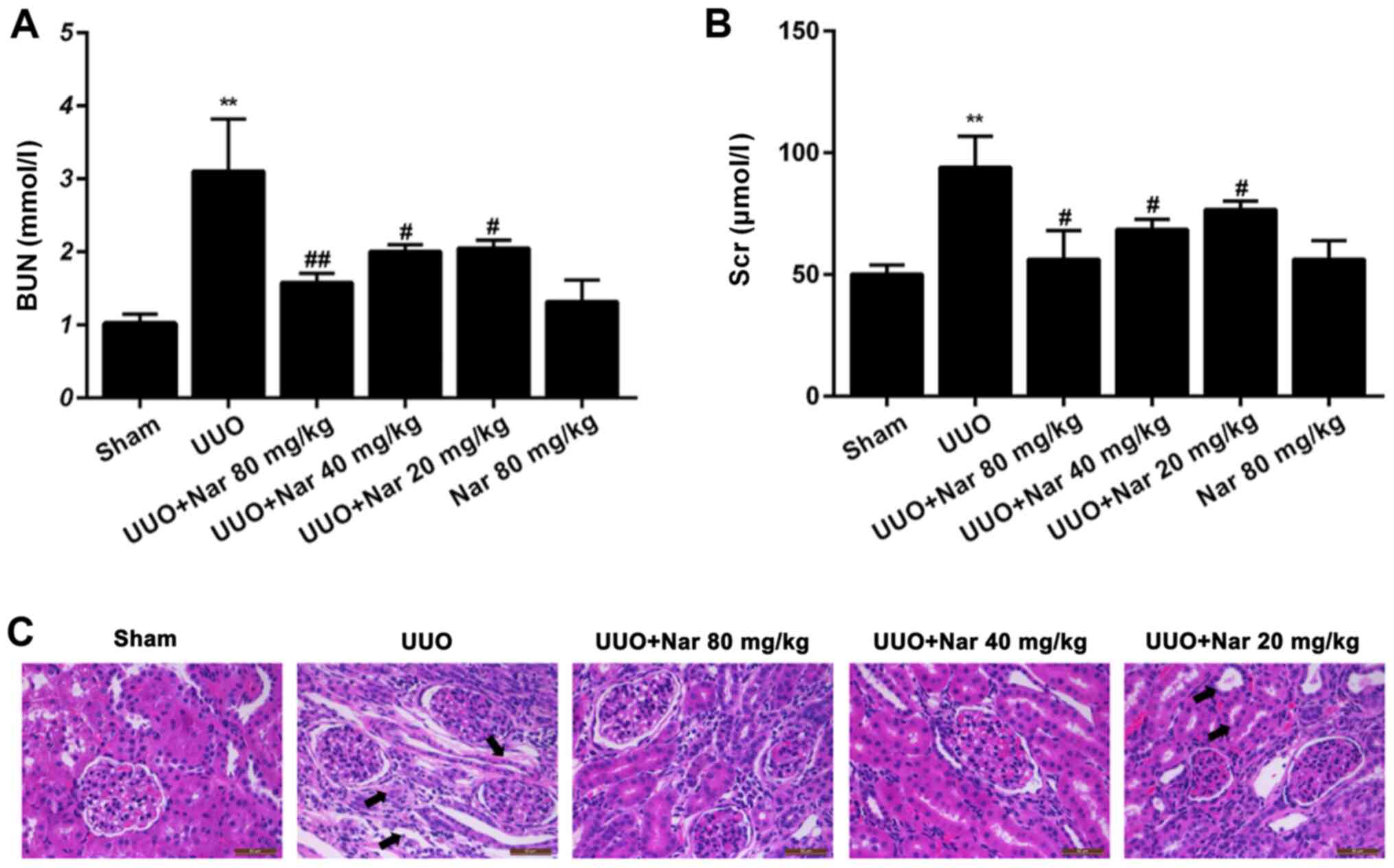
Effect of naringin on renal function
in rats with RIF induced by UUO
To investigate the effect of naringin on renal
function in rats following UUO, blood samples were collected for
BUN and Scr analysis. Compared with those in the control group, BUN
and Scr levels were demonstrated to be significantly increased in
the UUO group (Fig. 2A and B). Compared with those in the UUO group,
BUN levels were demonstrated to be significantly reduced,
specifically by 49.1, 35.4 and 33.9% in the high, middle and low
dose groups, respectively (Fig.
2A). Similarly. Scr levels were also significantly decreased,
by 40.1, 27.2 and 18.5%, respectively, compared with those in the
UUO groups (Fig. 2B). These
findings suggested that the levels of BUN and Scr can be
significantly reduced after naringin treatment in UUO rats. There
was no significant difference in the levels of BUN and Scr between
those in the single naringin administration and sham groups,
suggesting that naringin 80 mg/kg exerted no adverse effects on the
renal function of rats.
Effect of naringin on renal pathology
in rats following UUO-induced RIF
To further evaluate the histological damage in the
kidney tissues, the histological sections of the kidneys of rats in
each group were analyzed by H&E staining. Compared with those
in the sham group, some renal tubules in the UUO group were
demonstrated to be dilated, atrophied and necrotized, where a large
number of monocytes and lymphocytes infiltrated (Fig. 2C). Compared with tissues in the UUO
group, the expansion or atrophy of the renal tubules treated with
naringin (20, 40 and 80 mg/kg) were improved, where the degree of
vacuolar degeneration was reduced (Fig.
2C). These results suggest that naringin can effectively
improve the histopathological changes in the kidney after UUO.
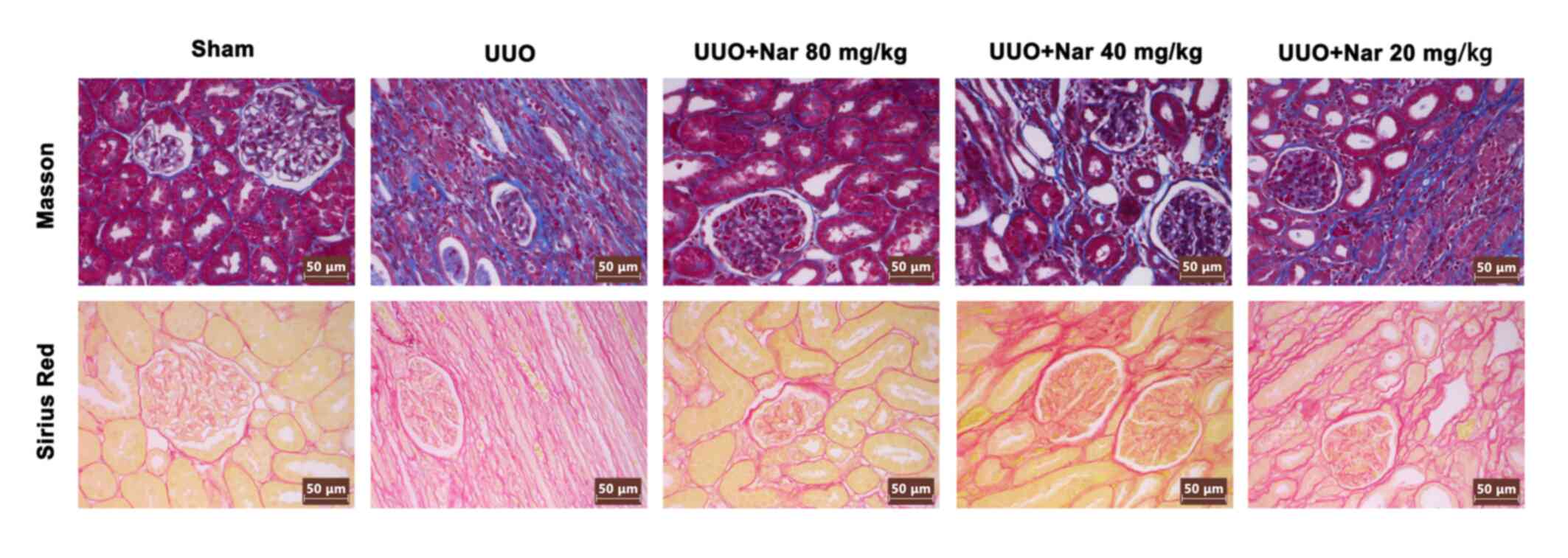
Naringin inhibits RIF induced by
UUO
To investigate the effects of naringin on RIF
following UUO, Masson's trichrome and Sirius red staining were used
to evaluate the degree of RIF in rats in each group. Compared with
those in the sham group, the proliferation and deposition of
collagen fibrous connective tissues in the kidney tissues of rats
in the UUO group were markedly increased. By contrast, compared
with tissues from the UUO group, the extent of collagen fibrous
connective tissue deposition and proliferation in the kidneys of
rats treated with naringin (20, 40 and 80 mg/kg) were visibly
reduced (Fig. 3). The content of
blue and red fibers were all shown to be reduced after treatment
with naringin, with the magnitude of reduction the highest in the
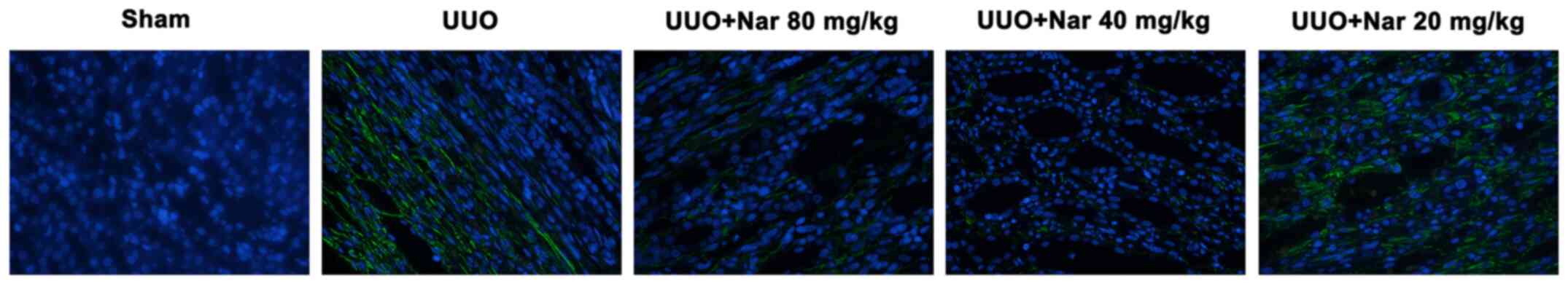
high dose group. In addition, results of immunofluorescence
analysis showed that naringin administration could reduce the
expression of α-SMA in the kidney tissues of UUO rats (Fig. 4). These results indicated that
naringin can notably reduce the degree of RIF induced by UUO.
Effect of naringin on the expression
of fibrotic markers
To examine the effect of naringin on the expression
of fibrotic markers in NRK-52E cells and rat kidneys, the
expression of TGF-β, α-SMA and COL1A1 was next measured. The
protein expression levels of TGF-β, α-SMA and COL1A1 in the NRK-52E
fibrosis cell model induced by TGF-β and kidney tissues of rats
following UUO were found to be significantly higher compared with
those in the control group (NRK-52E) and sham group (rat models).
In addition, the mRNA expression levels of α-SMA, COL1A1 and COL3A1
were revealed to be significantly reduced in NRK-52E cells and rats
in the UUO group treated with naringin (Fig. 5A and C). Compared with those in the control
NRK-52E cell and UUO rat groups, naringin treatment significantly
reduced the expression of TGF-β, α-SMA and COL1A1 in both
TGF-β-treated NRK-52E cell models and kidney tissues of rat models
following UUO, respectively (Fig.
6). These results suggest that the degree of fibrosis in both
the fibrotic cell and UUO rat models was improved following
treatment with naringin.
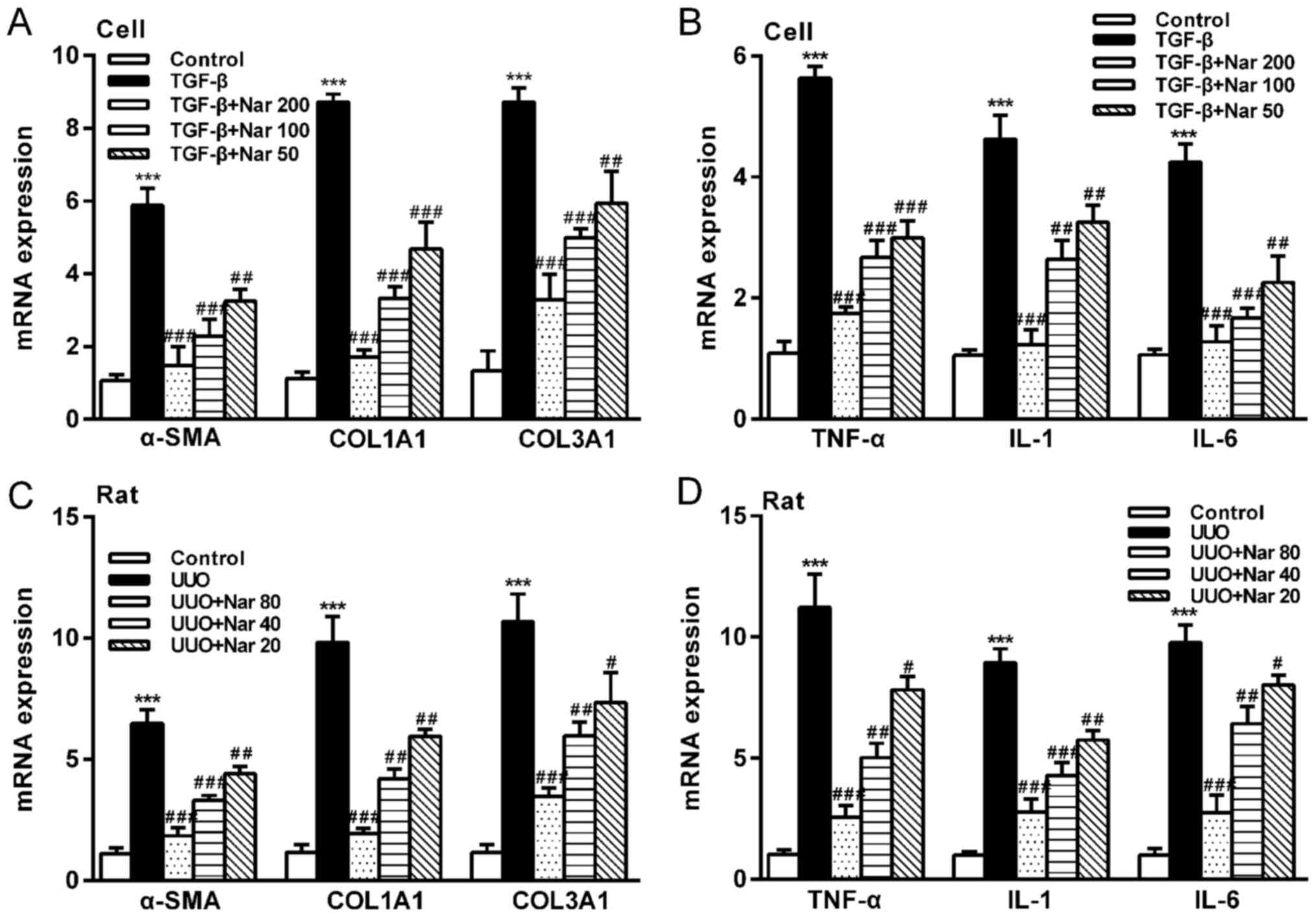 | Figure 5Effect of naringin on the mRNA
expression of fibrotic markers and inflammatory factors. (A) Effect
of naringin on the gene expression of α-SMA, COL1A1 and COL3A1 in
NRK-52E cells. (B) Effect of naringin on the gene expression of
TNF-α, IL-1 and IL-6 in NRK-52E cells. (C) Effect of naringin on
gene expression of α-SMA, COL1A1 and COL3A1 in rat kidney tissues.
(D) Effect of naringin on the gene expression of TNF-α, IL-1 and
IL-6 in rat kidney tissues. Data is presented as the mean ± SD
(n=6). ***P<0.001 vs. Control; #P<0.05,
##P<0.01 and ###P<0.001 vs. UUO or
TGF-β. Nar, naringin; UUO, unilateral ureteral obstruction; COL,
collagen; TNF-α, tumor necrosis factor-α; α-SMA, α-smooth muscle
actin; IL, interleukin. |
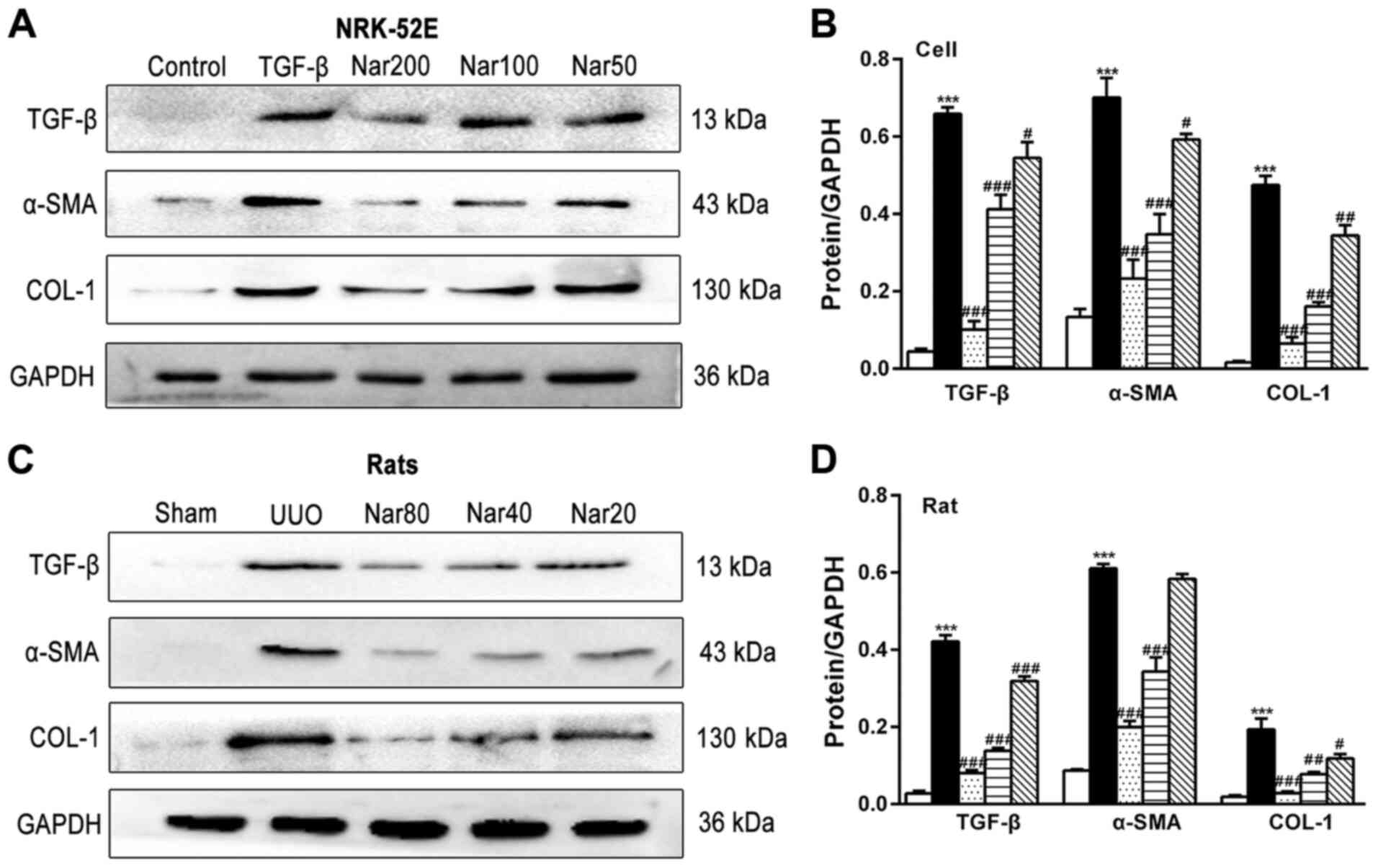 | Figure 6Effect of naringin on the protein
expression of fibrotic markers in NRK-52E cells and rat kidneys.
(A) Effect of naringin on TGF-β, α-SMA and COL1A1 protein
expression in NRK-52E cells. (B) Quantitative analysis of the
protein expression of TGF-β, α-SMA and COL1A1 in NRK-52E cells. (C)
Effect of naringin on TGF-β, α-SMA and COL1A1 in rat kidneys. (D)
Quantitative analysis of the protein expression of TGF-β, α-SMA and
COL1A1 in rat kidneys. Data is presented as mean ± SD (n=6).
***P<0.001 vs. Control; #P<0.05,
##P<0.01, ###P<0.001 vs. UUO or TGF-β.
Nar, naringin; UUO, unilateral ureteral obstruction; COL, collagen;
TNF-α, tumor necrosis factor-α; α-SMA, α-smooth muscle actin; IL,
interleukin; TGF-β, transforming growth factor-β. |
Effect of naringin on the expression
of components of the TGF-β/Smad pathway
To investigate the mechanism of the inhibitory
effects of naringin on TGF-β-induced RIF, the effect of naringin on
the expression of Smad, downstream regulators of TGF-β was
subsequently investigated (Fig. 7).
Compared with those in the control cell group and the sham group,
the degree of Smad2/3 phosphorylation and Smad4 expression in the
TGF-β-treated cell group and kidney tissues of rats in the UUO
group were found to be significantly increased (Fig 7). Compared with those in the
TGF-β-treated cell group or rats in the UUO group, the
phosphorylation of Smad2/3 and Smad4 expression in TGF-β-treated
NRK-52E cells and rats in the UUO group were significantly reduced
following treatment with naringin in both models (Fig. 7). By contrast, the expression of
Smad7 exhibited opposite trends in both in vitro and in
vivo models. These observations suggest that naringin treatment
can alleviate fibrosis by regulating the expression of Smad
proteins.
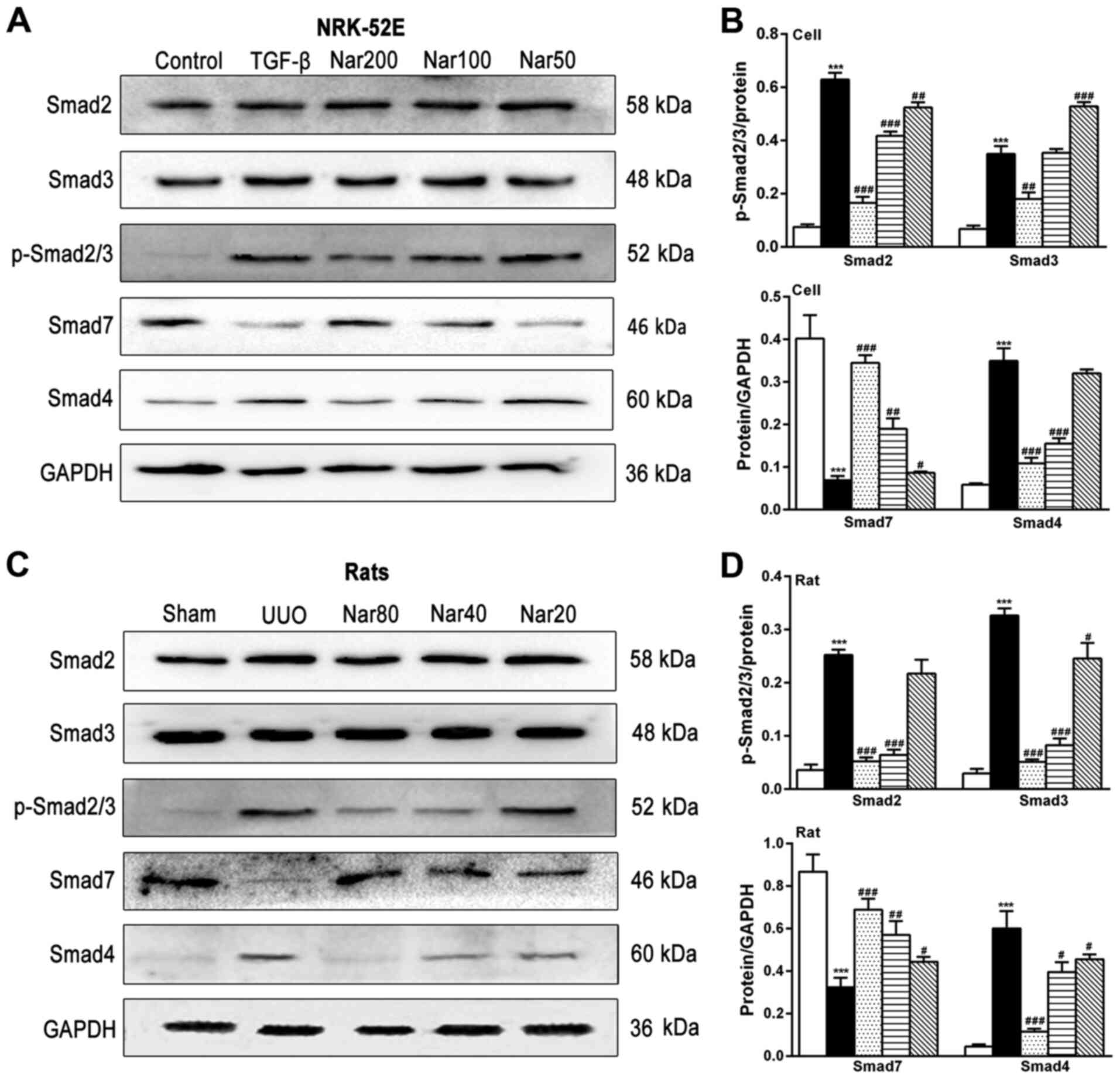 | Figure 7Effect of naringin on the expression
of key components of the TGF-β/Smad pathway. (A) Effect of naringin
on the protein levels of Smad2, Smad3, p-Smad2/3, Smad7 and Smad4
in NRK-52E cells. (B) Quantitative analysis of the protein levels
of Smad2, Smad3, p-Smad2/3, Smad7 and Smad4 in NRK-52E cells. (C)
Effect of naringin on the protein levels of Smad2, Smad3,
p-Smad2/3, Smad7 and Smad4 in rat kidneys. (D) Quantitative
analysis of the protein levels of Smad2, Smad3, p-Smad2/3, Smad7
and Smad4 in rat kidneys. Data is presented as mean ± SD (n=6).
***P<0.001 vs. Control; #P<0.05,
##P<0.01 and ###P<0.001 vs. UUO or
TGF-β. Nar, naringin; UUO, unilateral ureteral obstruction; COL,
collagen; TNF-α, tumor necrosis factor-α; α-SMA, α-smooth muscle
actin; IL, interleukin; TGF-β, transforming growth factor-β. |
Effect of naringin on the expression
of inflammatory proteins
Since renal fibrosis is frequently accompanied with
inflammation (36), the effect of
naringin on the expression of inflammatory proteins was next
investigated. Naringin treatment significantly reduced the
expression of inflammatory factors TNF-α, IL-1β and IL-6 in the
kidney tissues of UUO rats and TGF-β-treated NRK-52E cells
(Fig. 5B and D). In addition, the expression levels of
HMGB1, AP-1, NF-κB and COX-2 proteins in TGF-β-treated NRK-52E
cells and kidney tissues of UUO rats were found to be significantly
higher compared with those in the control cell group and sham
group, respectively (Fig. 8).
Naringin treatment significantly reversed the increased expression
of HMGB1, AP-1, NF-κB and COX-2 in both the TGF-β-treated NRK-52E
cell model and kidney tissues of UUO rats (Fig. 8). In summary, these results
suggested that naringin may also reduce the degree of fibrosis by
suppressing the expression of inflammatory proteins.
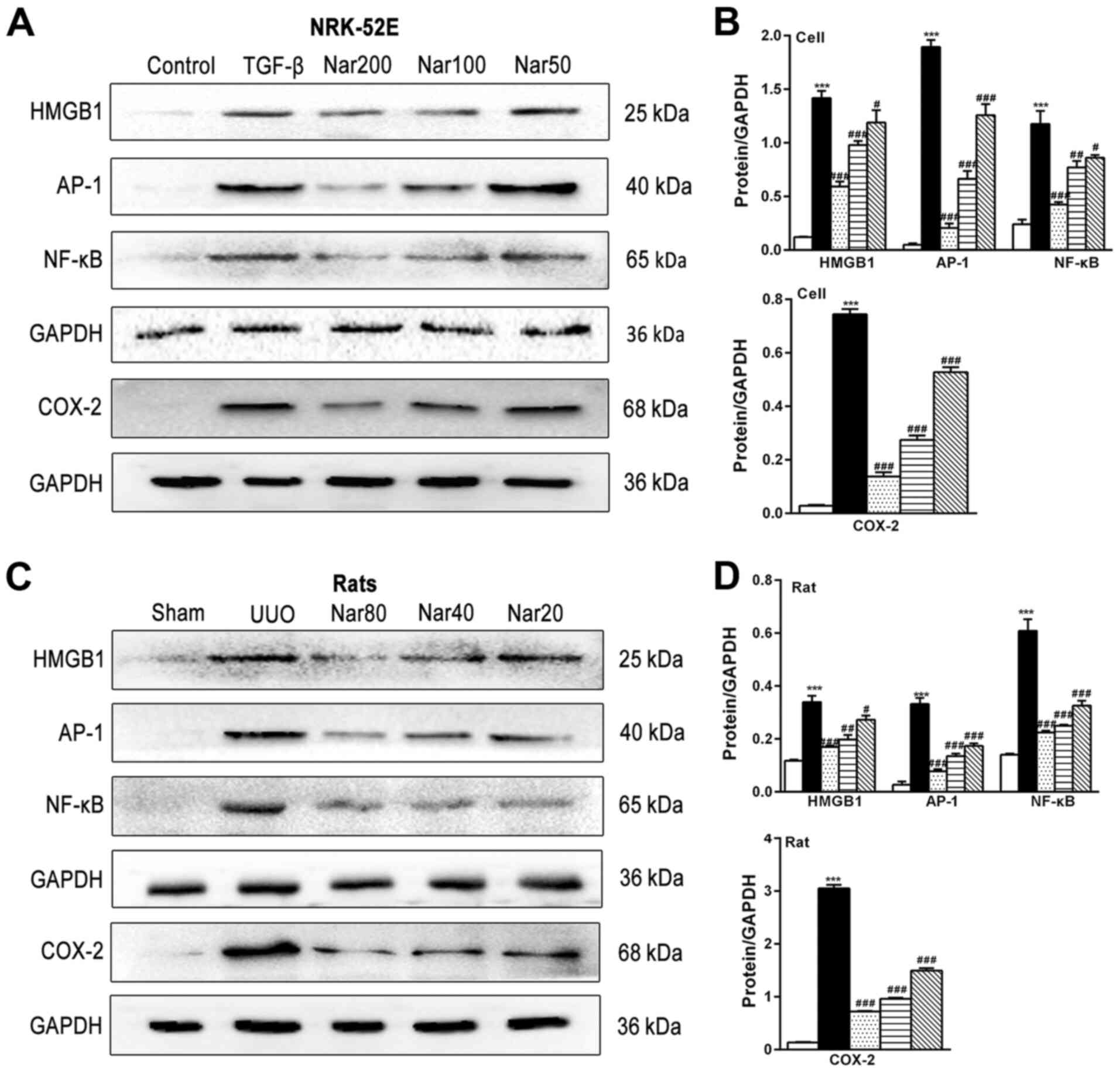 | Figure 8Effects of naringin on the expression
of HMGB1, AP-1, NF-κB and COX-2 in NRK-52E cells and rat kidneys.
(A) Effect of naringin on HMGB1, AP-1, NF-κB and COX-2 in NRK-52E
cells. (B) Quantitative analysis of the protein expression of
HMGB1, AP-1, NF-κB and COX-2 in NRK-52E cells. (C) Effect of
naringin on HMGB1, AP-1, NF-κB and COX-2 in rat kidneys. (D)
Quantitative analysis of the protein expression of HMGB1, AP-1,
NF-κB and COX-2 in rat kidneys. Data is presented as mean ± SD
(n=6). ***P<0.001 vs. Control; #P<0.05,
##P<0.01, ###P<0.001 vs. UUO or TGF-β.
Nar, naringin; UUO, unilateral ureteral obstruction; HMGB-1,
high-mobility group protein B1; COX-2, cyclooxygenase-2; AP-1,
activator protein-1; TGF-β, transforming growth factor-β. |
Discussion
Renal fibrosis is a frequent pathological outcome
during the latter stages chronic kidney disease (37). RIF is a pathological manifestation
of renal fibrosis that is regularly observed and is closely
associated with reductions in renal function in patients with
chronic kidney disease (38).
Therefore, prevention and intervention strategies of RIF would be
of great benefit for patients with chronic kidney diseases.
Although considerable amount of information has been obtained
regarding the underlying mechanism of renal fibrosis over the past
decade, effective therapeutic methods for the prevention and
treatment of RIF remain elusive. Naringin is a type of
dihydroflavonoids with previously reported pharmacological effects,
including antioxidant, hypolipidemic, antimicrobial,
anti-inflammatory and anti-fibrotic effects in the liver (39). The present study found that naringin
also exhibited anti-fibrotic effects in the rat kidney, possibly by
regulating the TGF-β/Smad signaling pathway to inhibit the
expression of inflammatory factors.
In the present study, the rat model of RIF was used
to investigate the potential effects of naringin. There are
currently three main methods used for establishing renal fibrosis
in animals: i) Surgical methods, including UUO (40), 5/6 nephrectomy (41) and ischemia-reperfusion injury model
(42); ii) drug or toxic induction
methods, including treatment with adenine, aristolochic acid and
cyclosporine, which induce renal fibrosis after long-term
administration (43); and iii)
compound models, including folate-Phd14 gene
knockout-induced fibrosis (44) and
transgenic-KIM-1REC-tg mouse models (45). Due to potential drug interactions,
drug or toxic induction methods were not used in the present study.
UUO, 5/6 nephrectomy and ischemia-reperfusion models are widely
applied for renal fibrosis surgery. The establishment of 5/6
nephrectomy models requires two separate operations followed by
obligatory regular monitoring for ≥5 weeks (41). This procedure is highly complex,
where the time of model establishment is substantially longer
compared with UUO. Ischemia-reperfusion model mainly simulates the
changes in renal function and renal fibrosis after renal
transplantation that can be used to reflect glomerulosclerosis and
RIF (42). UUO model is used to
mimic RIF in a manner that is characterized by rapid pathological
changes in 1-2 weeks, reduced animal mortality rates, involves
operation procedures that are less complex and good reproducibility
(40,46). Following successful model
establishment, pathological manifestations include collagen
deposition in the renal interstitium, infiltration of inflammatory
cells and dilatation or atrophy of renal tubules (47). In addition, serological indicators
BUN were increased 1.5-fold whilst Scr increased by 2-fold
(48-50).
Therefore, for the present study the UUO rat model was chosen as
the animal model. According to H&E staining, compared with
those in the sham group, kidney tissues of rats in the UUO group
exhibited a large number of infiltrating inflammatory cells,
nuclear deformation and renal tubule dilatation or atrophy.
Masson's trichrome and Sirius red staining revealed large
quantities of collagen deposition in the kidneys of the UUO group
of rats. The serum BUN levels of rats in the UUO group was found to
be three times higher compared with those in the sham group, whilst
serum Scr levels was found to be 1.6 times higher compared with
those in the sham group.
The effects of naringin treatment on RIF in rats
were subsequently investigated. In vivo results showed that
naringin administration (20, 40 and 80 mg/kg) reduced the levels of
BUN and Scr in the serum samples of UUO rats, improved the dilation
or atrophy of renal tubules and reduced the extent of vacuolar
degeneration and inflammatory cell infiltration in the kidneys of
UUO rats. These observations suggest that naringin can improve
renal function and pathological changes in UUO rats. Additionally,
naringin reduced collagen fibrous connective tissue proliferation
and the deposition of collagen fibers in the kidneys of UUO rats.
To evaluate the effect of naringin on RIF in rats further, the
expression of fibrosis indicators α-SMA, COL1A1 and COL3A1 was
measured. α-SMA expression is an important marker of fibroblasts
that also serve a key role in the migratory behavior of fibroblasts
(51), whilst COL1A1 and COL3A1 are
key components of the skin connective tissue (52). Aberrant expression of COL1A1 and
COL3A1 are primary causes of pathological changes in the dermal
connective tissue and fibroplasia (53). In the present study, naringin
significantly reduced the expression of these three fibrotic
markers aforementioned in the kidneys of UUO rats, suggesting that
naringin can preserve renal function and inhibit RIF in rats.
TGF-β is a well-studied fibroblast-promoting factor
and a powerful anti-inflammatory cytokine that regulates renal
inflammation (54-56).
It has been previously reported to modulate cell growth,
differentiation and proliferation. Overexpression of TGF-β can
cause renal fibrosis (57). Results
from the present study demonstrated that TGF-β expression in the
rat kidney was significantly increased by UUO induction, which was
reversed by naringin treatment. This observation suggest that
naringin may downregulate the expression of TGF-β in kidney
tissues, thus inhibiting RIF. There are ≥9 different isoforms of
proteins in the Smad superfamily, which serve as the main
intracellular signal transducers of TGF-β (58). Smad2 and Smad3 are
receptor-activated Smads that can be activated by TGF-β (59). Following activation, they form
complexes with other Smad proteins such as Smad4 to regulate cell
cycle progression, cell proliferation, differentiation, adhesion,
metastasis, apoptosis and collagen expression (15). By contrast, Smad7 is an inhibitory
Smad, which can block receptor-activated Smad phosphorylation.
Smad7 can inhibit the expression of Smad2, Smad3 and Smad4, thereby
suppressing and the formation of fibrosis (60). Therefore, the effect of naringin on
the TGF-β/Smad signaling pathway was assessed in the present study.
Application of TGF-β for inducing cell proliferation in
vitro is a well-reported method of inducing fibrosis (61,62).
Therefore, the present study evaluated the effect of naringin on
cell fibrosis following treatment with TGF-β. Naringin could reduce
the phosphorylation of Smad2/3 and Smad4 expression whilst
increasing the expression of Smad7 in TGF-β-treated NRK-52E cells
and kidneys of UUO rats. These findings suggest that naringin can
inhibit RIF by regulating the expression of Smad proteins.
In addition to the TGF-β/Smad signaling pathway,
Wnt, MAPK and connective tissue growth factor (CTGF) signaling
pathways have also been documented to be involved in mediating
renal fibrosis and inflammation (63-65).
The Wnt signaling pathway is a highly conserved signaling pathway
in cells that has been reported to crosstalk with the TGF-β pathway
(66). The MAPK pathway consists of
a large family of protein kinases, including ERK, JNK/ SAPK, p38
and ERK5/MAPK, and phosphorylated NF-κB after activation of MAPK
protein (67). TGF-β forms a
positive feedback loop with the MAPK pathway via mitogen-activated
protein kinase 7, which is an upstream p38/JNK activator. JNK
signaling can enhance the tubular production of thrombospondin-1
which, in turn, activates the latent form of TGF-β (68,69).
In addition, it has been revealed that TGF-β can induce the
expression of extracellular matrix (ECM) proteins in mesenchymal
cells to stimulate the production of protease inhibitors and
prevent the enzymatic decomposition of ECM (18). CTGF is induced by TGF-β, which
triggers the synthesis of ECM proteins (70). Of note, Smad has been previously
demonstrated to lie at the center of an intracellular signaling
crosstalk network of Wnt, MAPK and CTGF pathways, to serve an
important regulatory role in a number of biological processes
(71). Therefore, it could be
hypothesized that the anti-fibrotic effects of naringin could be
attributed to these three pathways aforementioned.
The occurrence and development of renal fibrosis are
not separate processes and involves inflammation. Following the
inhibition of AP-1 and NF-κB, the expression of inflammatory
cytokines can be inhibited. Inflammatory factors can lead to the
increase of stromal cells and intercellular stroma, thus increasing
the accumulation of extracellular matrix such as COL1A1 and COL3A1.
This is consistent with findings in the present study that the
expression of COL1A1 and COL3A1 is increased in fibrotic model
cells and kidneys of UUO rats, which was confirmed by the results
of Masson and Sirius red staining. These observations showed that
naringin can antagonize fibrosis by inhibiting the expression of
inflammatory factors.
In conclusion, results from the present study
demonstrated that naringin exerted anti-fibrotic effects in the
kidney both in vivo and in vitro, possibly through
the TGF-β/Smads signaling pathway whilst suppressing the expression
of inflammatory factors. This provides further evidence for the
application of naringin as a therapeutic agent for RIF.
Acknowledgements
Not applicable.
Funding
The present study was supported by Liaoning
Province's Education Department Program (grant no. L201631),
Natural Science Foundation of Liaoning Province (Grant nos.
201602223 and 2019-ZD-0652) and Dalian Medical Science Research
Project (grant no. 1912006).
Availability of data and materials
All data generated or analysed during the present
study are included in this published article.
Authors' contributions
RCW, GLW, SLY and DSD designed the work; RCW, GLW,
TTD, YTL and ZCC collected data; RCW, GLW, SLY and DSD contributed
to analysis and interpretation of data. All authors read and
approved the final version of the manuscript..
Ethics approval and consent to
participate
Ethical approval for the study was granted from the
Ethics Committee of Dalian Medical University (Dalian, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lawson J, Elliott J, Wheeler-Jones C, Syme
H and Jepson R: Renal fibrosis in feline chronic kidney disease:
Known mediators and mechanisms of injury. Vet J. 203:18–26.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kanasaki K, Taduri G and Koya D: Diabetic
nephropathy: The role of inflammation in fibroblast activation and
kidney fibrosis. Front Endocrinol (Lausanne). 4(7)2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hill NR, Fatoba ST, Oke JL, Hirst JA,
O'Callaghan CA, Lasserson DS and Hobbs FD: Global prevalence of
chronic kidney disease-A systematic review and meta-analysis. PLoS
One. 11(e0158765)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gewin LS: Renal fibrosis: Primacy of the
proximal tubule. Matrix Biol. 68-69:248–262. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen PS, Li YP and Ni HF: Morphology and
evaluation of renal fibrosis. Adv Exp Med Biol. 1165:17–36.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wilson PC, Kashgarian M and Moeckel G:
Interstitial inflammation and interstitial fibrosis and tubular
atrophy predict renal survival in lupus nephritis. Clin kidney J.
11:207–218. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhao D and Luan Z: Oleanolic acid
attenuates renal fibrosis through TGF-β/Smad pathway in a rat model
of unilateral ureteral obstruction. Evid Based Complement Alternat
Med. 2020(2085303)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Deng T, Wei Z, Gael A, Deng X, Liu Y, Lai
J, Hang L, Yan Q, Fu Q and Li Z: Higenamine improves cardiac and
renal fibrosis in rats with cardiorenal syndrome via ASK1 signaling
pathway. J Cardiovasc Pharmacol. 75:535–544. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li W, Cheng F, Songyang YY, Yang SY, Wei J
and Ruan Y: CTRP1 attenuates UUO-induced renal fibrosis via
AMPK/NOX4 pathway in mice. Curr Med Sci. 40:48–54. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu Y, Feng Q, Miao J, Wu Q, Zhou S, Shen
W, Feng Y, Hou FF, Liu Y and Zhou L: C-X-C motif chemokine receptor
4 aggravates renal fibrosis through activating
JAK/STAT/GSK3β/β-catenin pathway. J Cell Mol Med. 24:3837–3855.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Meng XM, Nikolic-Paterson DJ and Lan HY:
TGF-β: The master regulator of fibrosis. Nat Rev Nephrol.
12:325–338. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tieri P, Termanini A, Bellavista E,
Salvioli S, Capri M and Franceschi C: Charting the NF-κB pathway
interactome map. PloS One. 7(e32678)2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Meng XM, Huang XR, Xiao J, Chung AC, Qin
W, Chen HY and Lan HY: Disruption of Smad4 impairs TGF-beta/Smad3
and Smad7 transcriptional regulation during renal inflammation and
fibrosis in vivo and in vitro. Kidney Int. 81:266–279.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hu HH, Chen DQ, Wang YN, Feng YL, Cao G,
Vaziri ND and Zhao YY: New insights into TGF-β/Smad signaling in
tissue fibrosis. Chem Biol Interact. 292:76–83. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Campbell MT, Hile KL, Zhang H, Asanuma H,
Vanderbrink BA, Rink RR and Meldrum KK: Toll-like receptor 4: A
novel signaling pathway during renal fibrogenesis. J Surg Res.
168:e61–69. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pulskens WP, Rampanelli E, Teske GJ,
Butter LM, Claessen N, Luirink IK, van der Poll T, Florquin S and
Leemans JC: TLR4 promotes fibrosis but attenuates tubular damage in
progressive renal injury. J Am Soc Nephrol. 21:1299–1308.
2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jang HR and Rabb H: Immune cells in
experimental acute kidney injury. Nat Rev Nephrol. 11:88–101.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yao L, Li J, Li L, Li X, Zhang R, Zhang Y
and Mao X: Coreopsis tinctoria Nutt ameliorates high
glucose-induced renal fibrosis and inflammation via the
TGF-β1/SMADS/AMPK/NF-κB pathways. BMC Complement Altern Med.
19(14)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zeng X, Su W, Zheng Y, He Y, He Y, Rao H,
Peng W and Yao H: Pharmacokinetics, tissue distribution,
metabolism, and excretion of naringin in aged rats. Front
Pharmacol. 10(34)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen R, Qi QL, Wang MT and Li QY:
Therapeutic potential of naringin: an overview. Pharm Biol.
54:3203–3210. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Adil M, Kandhare AD, Ghosh P, Venkata S,
Raygude KS and Bodhankar SL: Ameliorative effect of naringin in
acetaminophen-induced hepatic and renal toxicity in laboratory
rats: Role of FXR and KIM-1. Ren Fail. 38:1007–1020.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
El-Desoky AH, Abdel-Rahman RF, Ahmed OK,
El-Beltagi HS and Hattori M: Anti-inflammatory and antioxidant
activities of naringin isolated from Carissa carandas L.: In
vitro and in vivo evidence. Phytomedicine. 42:126–134.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Caglayan C, Temel Y, Kandemir FM, Yildirim
S and Kucukler S: Naringin protects against
cyclophosphamide-induced hepatotoxicity and nephrotoxicity through
modulation of oxidative stress, inflammation, apoptosis, autophagy,
and DNA damage. Environ Sci Pollut Res Int. 25:20968–20984.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ahmed S, Khan H, Aschner M, Hasan MM and
Hassan STS: Therapeutic potential of naringin in neurological
disorders. Food Chem Toxicol. 132(110646)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dong D, Xu L, Yin L, Qi Y and Peng J:
Naringin prevents carbon tetrachloride-induced acute liver injury
in mice. J Functional Foods. 12:179–191. 2015.
|
|
27
|
Adil M, Kandhare AD, Visnagri A and
Bodhankar SL: Naringin ameliorates sodium arsenite-induced renal
and hepatic toxicity in rats: decisive role of KIM-1, Caspase-3,
TGF-β, and TNF-α. Ren Fail. 37:1396–1407. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Adil M, Kandhare AD, Ghosh P and Bodhankar
SL: Sodium arsenite-induced myocardial bruise in rats: Ameliorative
effect of naringin via TGF-β/Smad and Nrf/HO pathways. Chem Biol
Int. 253:66–77. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen F, Zhang N, Ma X, Huang T, Shao Y, Wu
C and Wang Q: Naringin alleviates diabetic kidney disease through
inhibiting oxidative stress and inflammatory reaction. PLoS One.
10(e0143868)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang H, Gao M, Li J, Sun J, Wu R, Han D,
Tan J, Wang J, Wang B, Zhang L and Dong Y: MMP-9-positive
neutrophils are essential for establishing profibrotic
microenvironment in the obstructed kidney of UUO mice. Acta Physiol
(Oxf). 227(e13317)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang ZH, He JQ, Zhao YY, Chen HC and Tan
NH: Asiatic acid prevents renal fibrosis in UUO rats via promoting
the production of 15d-PGJ2, an endogenous ligand of PPAR-γ. Acta
Pharmacol Sin. 41:373–382. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Health NIo: Guide for the care and use of
laboratory animals. 1985.
|
|
33
|
Hosseinian S, Rad AK, Bideskan AE,
Soukhtanloo M, Sadeghnia H, Shafei MN, Motejadded F, Mohebbati R,
Shahraki S and Beheshti F: Thymoquinone ameliorates renal damage in
unilateral ureteral obstruction in rats. Pharmacol Rep. 69:648–657.
2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu H, Li W, He Q, Xue J, Wang J, Xiong C,
Pu X and Nie Z: Mass spectrometry imaging of kidney tissue sections
of rat subjected to unilateral ureteral obstruction. Sci Rep.
7(41954)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lan HY: Diverse roles of TGF-β/Smads in
renal fibrosis and inflammation. Int J Biol Sci. 7:1056–1067.
2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Boor P and Floege J: Chronic kidney
disease growth factors in renal fibrosis. Clin Exp Pharmacol
Physiol. 38:441–450. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Nangaku M: Chronic hypoxia and
tubulointerstitial injury: A final common pathway to end-stage
renal failure. J Am Soc Nephrol. 17:17–25. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Salehi B, Fokou PVT, Sharifi-Rad M, Zucca
P, Pezzani R, Martins N and Sharifi-Rad J: The therapeutic
potential of naringenin: A review of clinical trials.
Pharmaceuticals (Basel) 12: 2019.
|
|
40
|
Zhang M, Guo Y, Fu H, Hu S, Pan J, Wang Y,
Cheng J, Song J, Yu Q, Zhang S, et al: Chop deficiency prevents
UUO-induced renal fibrosis by attenuating fibrotic signals
originated from Hmgb1/TLR4/NFκB/IL-1β signaling. Cell Death Dis.
6(e1847)2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gong W, Mao S, Yu J, Song J, Jia Z, Huang
S and Zhang A: NLRP3 deletion protects against renal fibrosis and
attenuates mitochondrial abnormality in mouse with 5/6 nephrectomy.
Am J Physiol Renal Physiol. 310:F1081–1088. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen W, Yan Y, Song C, Ding Y and Du T:
Microvesicles derived from human Wharton's Jelly mesenchymal stem
cells ameliorate ischemia-reperfusion-induced renal fibrosis by
releasing from G2/M cell cycle arrest. Biochem J. 474:4207–4218.
2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Nogueira A, Pires MJ and Oliveira PA:
Pathophysiological mechanisms of renal fibrosis: A review of animal
models and therapeutic strategies. In Vivo. 31:1–22.
2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xiao Z, Li CW, Shan J, Luo L, Feng L, Lu
J, Li SF, Long D and Li YP: Interventions to improve chronic
cyclosporine A nephrotoxicity through inhibiting renal cell
apoptosis: A systematic review. Chin Med J (Engl). 126:3767–3774.
2013.PubMed/NCBI
|
|
45
|
Humphreys BD, Xu F, Sabbisetti V, Grgic I,
Movahedi Naini S, Wang N, Chen G, Xiao S, Patel D, Henderson JM, et
al: Chronic epithelial kidney injury molecule-1 expression causes
murine kidney fibrosis. J Clin Invest. 123:4023–4035.
2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lan HY, Mu W, Tomita N, Huang XR, Li JH,
Zhu HJ, Morishita R and Johnson RJ: Inhibition of renal fibrosis by
gene transfer of inducible Smad7 using ultrasound-microbubble
system in rat UUO model. J Am Soc Nephrol. 14:1535–1548.
2003.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ishii A, Sakai Y and Nakamura A: Molecular
pathological evaluation of clusterin in a rat model of unilateral
ureteral obstruction as a possible biomarker of nephrotoxicity.
Toxicol Pathol. 35:376–382. 2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Xin BM, Wang XX, Jin W, Yan HM, Cui B,
Zhang XW, Hua F, Yang HZ and Hu ZW: Activation of Toll-like
receptor 9 attenuates unilateral ureteral obstruction-induced renal
fibrosis. Acta pharmacologica Sinica. 31:1583–1592. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wongmekiat O, Leelarungrayub D and
Thamprasert K: Alpha-lipoic acid attenuates renal injury in rats
with obstructive nephropathy. BioMed Res Int.
2013(138719)2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lucarelli G, Mancini V, Galleggiante V,
Rutigliano M, Vavallo A, Battaglia M and Ditonno P: Emerging
urinary markers of renal injury in obstructive nephropathy. BioMed
Res Int. 2014(303298)2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Shinde AV, Humeres C and Frangogiannis NG:
The role of α-smooth muscle actin in fibroblast-mediated matrix
contraction and remodeling. Biochim Biophys Acta Mol Basis Dis.
1863:298–309. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wang Q, Peng Z, Xiao S, Geng S, Yuan J and
Li Z: RNAi-mediated inhibition of COL1A1 and COL3A1 in human skin
fibroblasts. Exp Dermatol. 16:611–617. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Artlett CM, Sassi-Gaha S, Rieger JL,
Boesteanu AC, Feghali-Bostwick CA and Katsikis PD: The inflammasome
activating caspase 1 mediates fibrosis and myofibroblast
differentiation in systemic sclerosis. Arthritis Rheum.
63:3563–3574. 2011.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zeglinski MR, Hnatowich M, Jassal DS and
Dixon IM: SnoN as a novel negative regulator of TGF-β/Smad
signaling: A target for tailoring organ fibrosis. Am J Physiol
Heart Circ Physiol. 308:H75–H82. 2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Gu YY, Liu XS, Huang XR, Yu XQ and Lan HY:
Diverse role of TGF-β in kidney disease. Front Cell Dev Biol.
8(123)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhang X, Huang H, Zhang G, Li D, Wang H
and Jiang W: Raltegravir attenuates experimental pulmonary fibrosis
in vitro and in vivo. Front Pharmacol. 10(903)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
ten Dijke P and Hill CS: New insights into
TGF-β-Smad signalling. Trends Biochem Sci. 29:265–273.
2004.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Miyazawa K, Shinozaki M, Hara T, Furuya T
and Miyazono K: Two major Smad pathways in TGF-beta superfamily
signalling. Genes Cells. 7:1191–1204. 2002.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Biernacka A, Dobaczewski M and
Frangogiannis NG: TGF-β signaling in fibrosis. Growth Factors.
29:196–202. 2011.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Yan X, Liao H, Cheng M, Shi X, Lin X, Feng
XH and Chen YG: Smad7 protein interacts with receptor-regulated
smads (R-Smads) to inhibit transforming growth factor-β
(TGF-β)/smad signaling. J Biol Chem. 291:382–392. 2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Masola V, Carraro A, Granata S, Signorini
L, Bellin G, Violi P, Lupo A, Tedeschi U, Onisto M, Gambaro G and
Zaza G: In vitro effects of interleukin (IL)-1 beta inhibition on
the epithelial-to-mesenchymal transition (EMT) of renal tubular and
hepatic stellate cells. J Transl Med. 17(12)2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Liu JH, He L, Zou ZM, Ding ZC, Zhang X,
Wang H, Zhou P, Xie L, Xing S and Yi CZ: A novel inhibitor of
homodimerization targeting MyD88 ameliorates renal interstitial
fibrosis by counteracting TGF-β1-induced EMT in vivo and in vitro.
Kidney Blood Press Res. 43:1677–1687. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Edeling M, Ragi G, Huang S, Pavenstadt H
and Susztak K: Developmental signalling pathways in renal fibrosis:
The roles of Notch, Wnt and Hedgehog. Nature Rev Nephrol.
12:426–439. 2016.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Wang D, Warner GM, Yin P, Knudsen BE,
Cheng J, Butters KA, Lien KR, Gray CE, Garovic VD, Lerman LO, et
al: Inhibition of p38 MAPK attenuates renal atrophy and fibrosis in
a murine renal artery stenosis model. Am J Physiol Renal Physiol.
304:F938–F947. 2013.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Ito Y, Aten J, Bende RJ, Oemar BS,
Rabelink TJ, Weening JJ and Goldschmeding R: Expression of
connective tissue growth factor in human renal fibrosis. Kidney
Int. 53:853–861. 1998.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Caraci F, Gili E, Calafiore M, Failla M,
Rosa CL, Crimi N, Sortino MA, Nicoletti F, Copani A and Vancheri C:
TGF-beta1 targets the GSK-3beta/beta-catenin pathway via ERK
activation in the transition of human lung fibroblasts into
myofibroblasts. Pharmacol Res. 57:274–282. 2008.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Qi W, Twigg S, Chen X, Polhill TS,
Poronnik P, Gilbert RE and Pollock CA: Integrated actions of
transforming growth factor-beta1 and connective tissue growth
factor in renal fibrosis. Am J Physiol Renal Physiol.
288:F800–F809. 2005.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Naito T, Masaki T, Nikolic-Paterson DJ,
Tanji C, Yorioka N and Kohno N: Angiotensin II induces
thrombospondin-1 production in human mesangial cells via p38 MAPK
and JNK: A mechanism for activation of latent TGF-beta1. Am J
Physiol Renal Physiol. 286:F278–F287. 2004.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Gao Y, Jiang W, Dong C, Li C, Fu X, Min L,
Tian J, Jin H and Shen J: Anti-inflammatory effects of sophocarpine
in LPS-induced RAW 264.7 cells via NF-κB and MAPKs signaling
pathways. Toxicol In Vitro. 26:1–6. 2012.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Ihn H: Pathogenesis of fibrosis: Role of
TGF-beta and CTGF. Curr Opin Rheumatol. 14:681–685. 2002.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Luo K: Signaling cross talk between
TGF-beta/Smad and other signaling pathways. Cold Spring Harb
Perspect Biol. 9(a022137)2017.PubMed/NCBI View Article : Google Scholar
|