Introduction
Toxoplasmosis is caused by the protozoan parasite
Toxoplasma gondii, where >60% of populations are infected
with these parasites (1,2). The infection often occurs in areas of
lower altitudes, hot and humid climates (2). Our previous study demonstrated that a
low dose of the virulent type I RH strain of Toxoplasma
gondii (100 parasites) rapidly caused a lethal infection in
mice within 4 days (3). By
contrast, mortality with the avirulent type II ME49 strain of T.
gondii occurred with a higher initial dose (1x105
parasites) at 6-8 days post-infection (3). The T. gondii strain RH exhibits
high virulence in animals, but mice have been demonstrated to
survive infection with the low virulence ME49 strain of T.
gondii (4). It is hypothesised
that parasitized host cells release soluble factors into the
conditioned medium following T. gondii infection to regulate
the parasite invasion into the host cells. However, the differences
of these soluble factors in the conditioned media of host cells
parasitized with RH and ME49 remain unclear. Different soluble
factors are believed to be secreted into the conditioned medium by
host cells parasitized with various strains of T. gondii, as
these factors provide a possible selective advantage for the
parasite to rapidly invade host cells (5). A previous study has demonstrated that
the ability to develop chronicity of infection is dependent on type
1 T helper (Th1) cells (6). In
addition, overstimulation of Th1 cytokines has been associated with
the acute virulence of T. gondii (3). Therefore, soluble Th1 cytokines are
likely to constitute the factors that underlie the differences
between the conditioned media of host cells parasitized with RH and
ME49.
The present study investigated the levels of soluble
Th1 cytokines in the conditioned media of host cells parasitized
with the RH and ME49 strains of T. gondii at different time
intervals. The current study also compared the levels of soluble
factors in the conditioned media of IL-21-silenced colorectal
cancer cells and the impact of the soluble factors on the mRNA
expression level of cell proliferation markers in both the host
cells post parasitic infection and colorectal cancer cells post
silencing of the IL-21 gene. The IL-21 gene was silenced in a
colorectal cancer cell line, as it has been indicated to reduce the
proliferation of the cells, and it may also be used to study the
molecular mechanisms of host-parasite interactions that cause
chronic diseases with respect to colorectal cancer (7). The present study provided useful
information on the fundamental molecular mechanisms of the
host-parasite interactions that may aid in early diagnosis, novel
prescription drugs and cost-effective strategies for the treatment
of infectious disease-associated colorectal cancer in the
future.
Materials and methods
Preparation of conditioned media of
host cells parasitized with RH and ME49
The RH and ME49 strains of T. gondii were a
kind gift from Prof. Rahmah Noordin, INFORMM, Universiti Sains
Malaysia. The stocks were stored in liquid nitrogen. The parasites
were cultured and propagated in vitro and adjusted to a
concentration of 100 parasites/ml. The parasite numbers were
estimated using a Neubauer haemocytometer chamber (Electron
Microscopy Sciences, Inc.). Human foreskin fibroblasts (HFFs),
which were originally purchased from the American Type Culture
Collection (cat no. CRL-2522) and maintained in the laboratory,
were used as the host cells and cultured in DMEM supplemented with
10% FBS, 100 U/ml penicillin and 100 mg/ml streptomycin (all from
Thermo Fisher Scientific, Inc.). HFFs were used as the host for
parasites since they are not differentiated, which allows the
parasites to propagate rapidly (8).
The growth medium of HFFs was changed every 2-3 days, and the cells
were cultured at 37˚C in a humidified atmosphere with 5%
CO2. HFFs were seeded at a density of 1,000 cells/ml in
T-75 cell culture flasks (Thermo Fisher Scientific, Inc.). The cell
number was also estimated using a haemocytometer chamber. When HFFs
were 80% confluent, RH or ME49 (100 parasites/ml) were added to the
HFF feeder layer. The co-culture was incubated for 2-10 days. The
growth medium was removed from the co-culture at different time
intervals; every 2 days post-infection and used as the conditioned
medium. The collected conditioned media, as well as the control
media from non-parasitized HFFs (culture supernatants of HFFs
only), were passed through a 0.22-mm filter (Thermo Fisher
Scientific, Inc.) to separate the parasites, host cells and other
cell debris. The conditioned media collected at various time
intervals were used for the immunoassays of Th1 cytokines.
Immunoassay of Th1 cytokines in the
conditioned media of parasite-infected host
Immunoassays for human IL-1β (cat no. KA0356), IL-10
(cat no. KA0125), IL-12p40 (cat no. KA0178), IL-18 (cat no.
KA0561), IFN-γ (cat no. 3045) and TNF-α (cat no. P3453) were
performed using commercially available ELISA kits (Abnova).
According to the manufacturer's instructions, microtiter plates
were pre-coated with antibodies specific for the Th1 cytokines. The
collected conditioned media (~200 µl) were added to the respective
wells and allowed to react with the bound antibody for 2.5 h at
room temperature. The unbound substances were removed with a 1X PBS
washing buffer, according to the manufacturer's instructions. An
enzyme-linked antibody specific for each Th1 cytokine was added to
the wells and incubated for 1 h at room temperature. Following
another washing step, a substrate solution was added to the wells
for colour development. The colour intensity was measured using a
plate reader (Tecan Group, Ltd.) at 450 nm, and the colour
development was proportional to the amount of the Th1 cytokines
present in the samples. The cytokine level was calculated as the
ratio of the experimental value (pg/ml) relative to the value in
non-infected host cells. The statistically significant difference
relative to day 2 ratio was considered.
Incubation of HFFs with media
containing specific soluble factors
Briefly, HFFs were seeded at a density of 100
cells/ml into T-25 cell culture flasks (Thermo Fisher Scientific,
Inc.) and maintained as aforementioned. When HFFs were ~80%
confluent, the growth media were removed from the culture flasks.
The HFF feeder layer was then exposed to growth medium containing 1
ng/ml human IFN-γ research grade (cat. no.: 130-096-873), 1 ng/ml
Abnova human IL-18 recombinant protein (cat. no. P3632) or growth
medium only for 4-10 days in triplicate (day 2 was excluded to
reduce the use of cytokines and growth media). It is expected that
an alteration in HFF proliferation was observed when the HFFs were
incubated in growth media containing IFN-γ and IL-18 compared with
growth medium alone. The incubated HFFs were subsequently harvested
for analysis of mRNA expression, as described in the following
section.
Analysis of the mRNA expression levels
of cell proliferation markers in HFFs
Total RNA was extracted from HFFs incubated with
specific soluble factors for 4-10 days using a commercially
available RNeasy Mini Kit (Qiagen, Inc.) for total RNA extraction.
RNA (1.0 µg) was reverse-transcribed into cDNA at 60˚C using a
commercially available RevertAid First Strand cDNA Synthesis kit
(Thermo Fisher Scientific, Inc.), which was subsequently used for
the analysis of cell proliferation markers at the mRNA expression
level by quantitative PCR (qPCR). Primers for Ki67 and
proliferating cell nuclear antigen (PCNA) were designed using
Primer Express v2.0, and qPCR was performed using ABI 7500 Fast
Sequence Detection System (both from Applied Biosystems; Thermo
Fisher Scientific, Inc.). The primer sequences used were as
follows: Ki67 forward, 5'-CCAACTGTTGGTCTCGCGTAAG-3' and reverse,
5'-ATCTGTCCAGCTGTAGTGCCCA-3'; PCNA forward,
5'-GGCACTCAAGGACCTCATCAAC-3' and reverse,
5'-GTGAGCTGCACCAAAGAGACGT-3'; Transforming growth factor-α forward,
5'-CAGACCTTCCTACTTGGCCTGTAA-3' and reverse,
5'-GACGGAGTTCTTGACAGAGTTTTG-3'; Chemokine C-C motif ligand 5
forward, 5'-AGCCTCTCCCACAGGTACCAT-3' and reverse,
5'-GGCAGTAGCAATGAGGATGACA-3'; Epidermal growth factor forward,
5'-TGTGGTTCTCAGATTGGGCTATG-3' and reverse
5'-GATGAGGGCTTCAGCATGCT-3'; and GAPDH forward,
5'-CAAGTTCAACGGCACAGTCAAG-3' and reverse,
5'-CTCCTGGAAGATGGTGATTGGT-3'. GAPDH was used as the internal
control. A total reaction volume of 25 µl was used, which included
SYBR® Green Master Mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.), 900 nM of each primer and 5 µl cDNA (25
ng). The following thermocycling conditions were used: 95˚C for 10
min, followed by 40 cycles of 95˚C for 15 sec and 60˚C for 1 min.
Fold-changes in gene expression were calculated using the
2-ΔΔCq method (9). The
relative expression of each target gene in the samples of the host
cells incubated with growth media containing soluble factors was
normalised to that of the target gene in the samples of the host
cells incubated with growth media alone (control). The expression
level of each gene in fold-change was calculated relative to day 4
gene expression level.
Systematic review of the associations
between IL-18, IFN-γ and IL-21
The following procedure was used for the systematic
review: i) The research question ‘what is the relationship between
IL-18, IFN-γ and IL-21 in human diseases’ was used as the topic of
the analysis; ii) the systematic reference management software
EndNote 9.0.1 (Clarivate Analytics) was used to extensively search
the published studies on this topic worldwide; iii) the studies
associated with this topic were screened in the PubMed databases at
the National Library of Medicine (NLM; https://pubmed.ncbi.nlm.nih.gov/) to identify evidence
for subsequent examination; iv) the studies on the associations
between IL-18, IFN-γ and IL-21 in infectious disease and colorectal
cancer research in an endemic region were extracted to answer the
research question; v) the contents of each article were analysed,
and the evidence was synthesised to determine bias with qualitative
statistics tools, for example PCR and Immunoassays; and vi) the
conclusion was returned into a report to support the analysis.
Silencing of the IL-21 gene in
colorectal cancer cells
A mouse specific lyophilised IL-21 small interfering
RNA (siRNA) reagent (cat. no. sc-39663; Santa Cruz Biotechnology,
Inc.) was used to silence the IL-21 gene in HCT116 cells (American
Type Culture Collection). The scrambled siRNA was included in the
system. The cells were seeded in 6-well culture plates (Thermo
Fisher Scientific, Inc.) at a density of 2x105
cells/well in 2 ml antibiotic-free DMEM supplemented with 10% FBS.
The cells were incubated at 37˚C in a humidified atmosphere of 5%
CO2 until they reached 80% confluency. Solution A was
prepared by diluting 4 µl fluorescein-conjugated IL-21 siRNA duplex
with 100 µl siRNA transfection medium (cat. no. sc-36868; Santa
Cruz Biotechnology, Inc.). Solution B was prepared by diluting 6 µl
of siRNA siRNA transfection reagent (cat. no. sc-29528; Santa Cruz
Biotechnology, Inc.; as optimised in our previous study) with 100
µl siRNA transfection medium (7).
Solution A was then directly added to Solution B and mixed gently
by pipetting before mixing with 800 µl of siRNA transfection
medium. The final mixture was incubated at room temperature for
15-45 min. The cells were washed with 2 ml siRNA transfection
medium following the addition of the prepared mixture. The
transfected cells were incubated at 37˚C for ≥72 h in a
CO2 incubator. The efficacy of gene silencing compared
with that in cells transfected with scrambled siRNA was examined
using a ZEISS Axio Observer Research Inverted Fluorescence
Microscope (Carl Zeiss AG) with a magnification of x100. The
IL-21-silenced cells were subjected to analysis of cell
proliferation markers at the mRNA level, whereas the conditioned
media collected from the IL-21-silenced cells were subjected to
immunoassay of cytokines.
Analysis of cell proliferation markers
at mRNA level and cytokines in conditioned media of IL-21-silenced
cells by qPCR and ELISA
Total RNA was extracted from IL-21-silenced HCT116
cells at 1-3 days post-transfection. A total of 1.0 µg RNA was
reverse-transcribed into cDNA, which was used for the analysis of
cell proliferation marker expression at the mRNA level by qPCR. The
same primers for the detection of Ki67 and PCNA were used as
aforementioned, and the fold-changes in gene expression were
calculated relative to those of the control cells (day 1), as
aformentioned. On the other hand, the conditioned media collected
from IL-21-silenced HCT116 cells at 1-3 days post-transfection were
added to the wells and allowed to react with the bound antibodies
on the microtiter plates that were pre-coated with antibodies
specific for IL-18 and IFN-γ for 2.5 h at room temperature. The
unbound substances were removed with a wash buffer, according to
the manufacturer's instructions, and ELISA was subsequently
performed as aforementioned. The cytokine level was calculated as
the ratio of the experimental value in the silenced cells (pg/ml)
relative to the value in control cells. Statistically significant
differences were considered relative to day 1 ratio.
Statistical analysis
Data are presented as the mean ± SD. All statistical
analyses were performed with one-way ANOVA followed by Tukey's post
hoc test using GraphPad v6.01 software (GraphPad Software, Inc.).
The experiments were repeated at least twice in three technical
replicates to ensure reproducibility. P<0.05 was considered to
indicate a statistically significant difference.
Results
Th1 cytokine levels in the conditioned
media of host cells parasitized with T. gondii
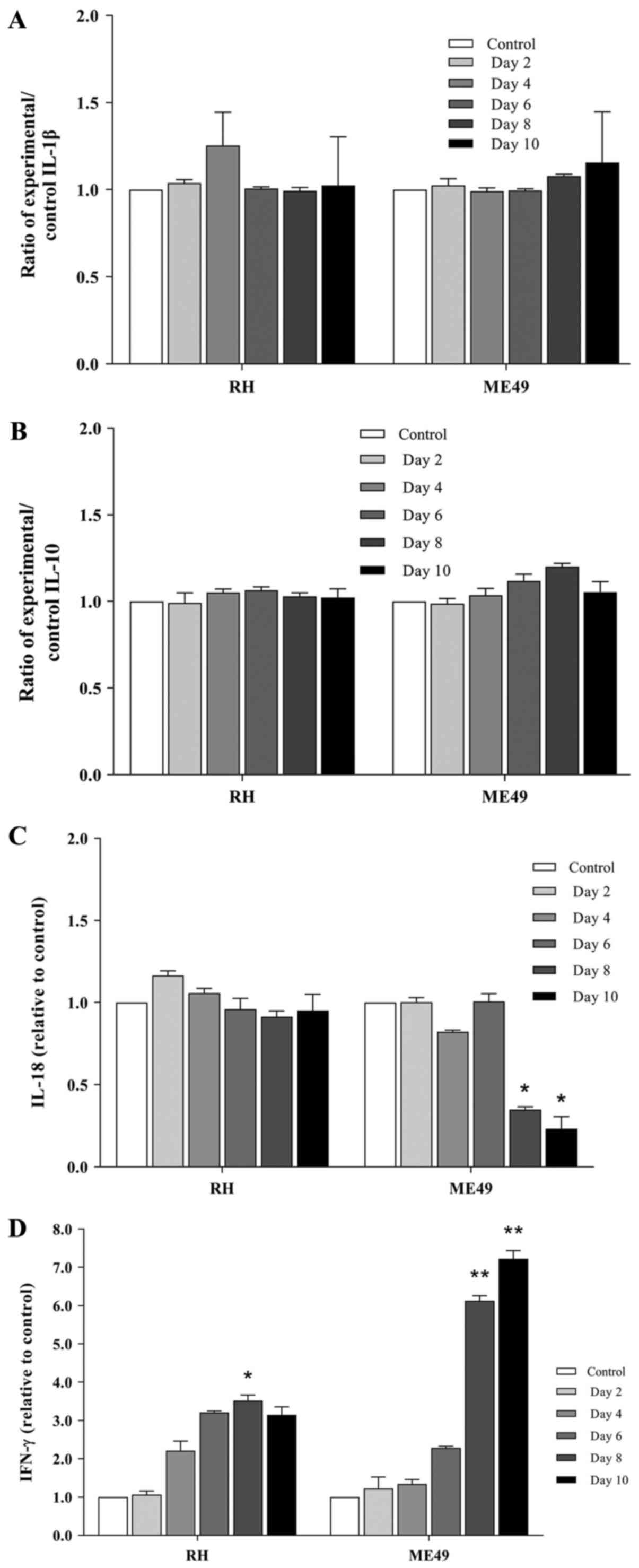
Investigation of the soluble factors IL-1β, IL-10,
IL-12p40, IL-18, IFN-γ and TNF-α in the conditioned media of HFFs
infected with strains of T. gondii at a range of time
intervals revealed that host cells infected with the RH and ME49
strains of T. gondii released various levels of soluble
factors into the conditioned media. The host cells parasitized with
RH did not exhibit notable differences in the levels of soluble
factors. IFN-γ was only significantly increased in the conditioned
media compared with the control at day 8 of incubation (3.523-fold
change; P<0.05; Fig. 1C).
However, ME49-parasitized host cells released lower levels of IL-18
at day 8 (0.349-fold change; P<0.05) and day 10 (0.234-fold
change; P<0.05) of incubation (Fig.
1D), and exhibited elevated levels of IFN-γ at day 8
(6.126-fold change; P<0.01) and day 10 (7.223-fold change;
P<0.01) of incubation (Fig. 1C)
in the conditioned media compared with those in the control cells.
The levels of other soluble factors were either not detected
(IL-12p40; TNF-α; data not shown) or exhibited no significant
differences (IL-1β, Fig. 1A; IL-10,
Fig. 1B) in the conditioned media
of the host cells parasitized with RH or ME49.
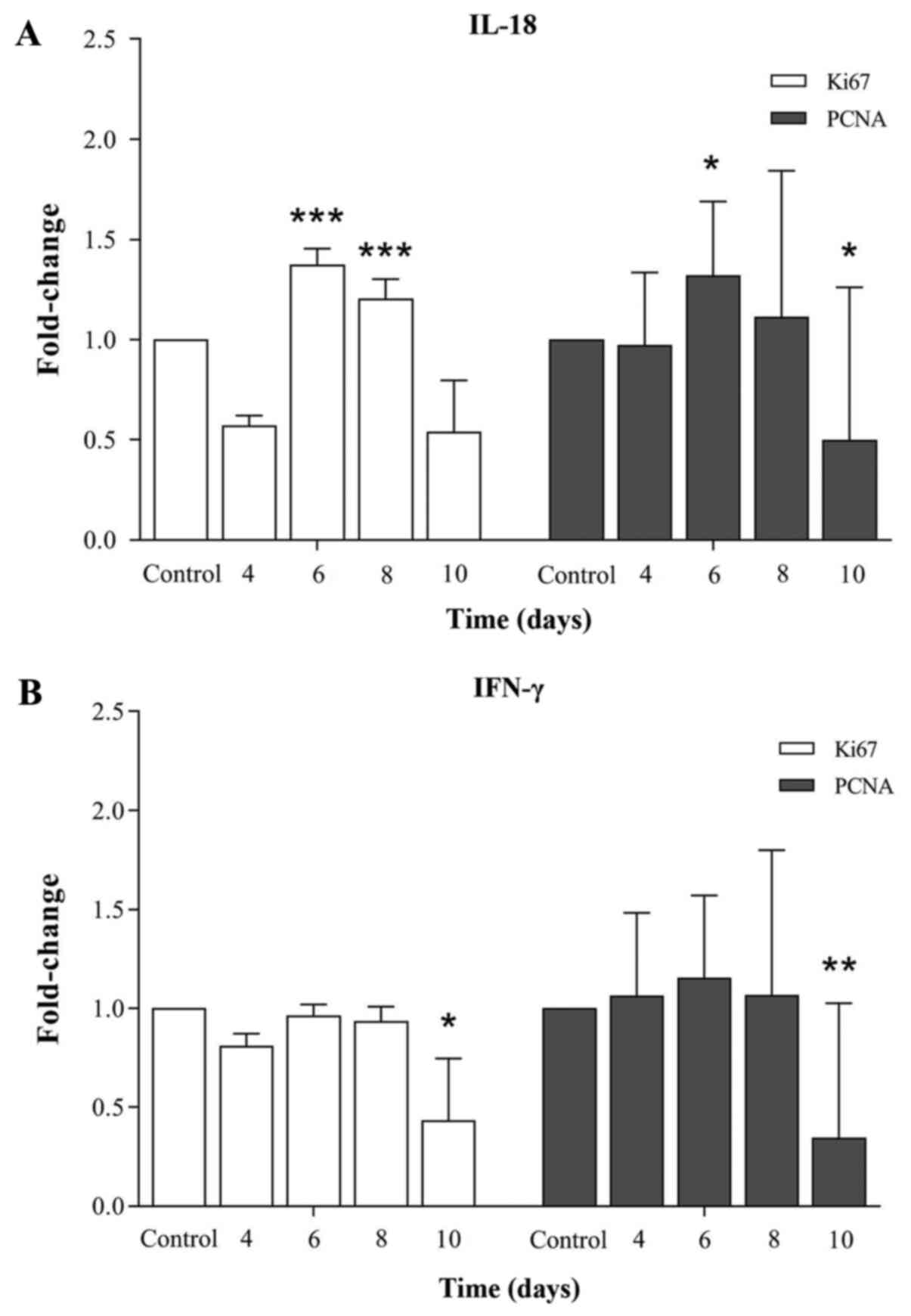
mRNA expression levels of cell
proliferation markers in IFN-γ- and IL-18-incubated HFFs
Determination of the effects of IL-18 and IFN-γ on
the mRNA expression levels of cell proliferation markers at
different time intervals using reverse transcription (RT)-qPCR
demonstrated that incubation of HFFs with growth media containing
IL-18 resulted in a time-dependent bell-shaped curve in the mRNA
expression levels of Ki67 and PCNA compared with the controls (day
4 gene expression level; Fig. 2A),
whereas incubation with medium containing IFN-γ inhibited the mRNA
expression of cell proliferation markers compared with the controls
(day 4 gene expression level; Fig.
2B). In the growth media containing IL-18, the mRNA expression
of Ki67 was increased at day 6 (1.376-fold change; P<0.001) and
day 8 (1.204-fold change; P<0.001) of incubation, and it
decreased to the initial level of mRNA expression at day 10
(0.539-fold change) of incubation. A similar level of mRNA
expression was also revealed for PCNA in the IL-18-incubated host
cells, where the mRNA expression of PCNA was increased to
1.321-fold change (P<0.05) and 1.114-fold change at days 6 and 8
of incubation, respectively, compared with the controls. The mRNA
expression level of PCNA reached a 0.500-fold change (P<0.05) at
day 10 of incubation. This pattern of alteration (bell-shaped
curve) was not observed in the mRNA expression levels of the cell
proliferation markers when the cells were incubated with growth
media containing IFN-γ for different time intervals. The mRNA
expression level of Ki67 and PCNA decreased to 0.434-fold
(P<0.05) and 0.346-fold (P<0.01), respectively, at day 10 of
incubation in the IFN-γ-incubated host cells compared with the
controls. These results suggested that IL-18 was likely required
for the host cell proliferation, whereas IFN-γ inhibited the host
cell proliferation.
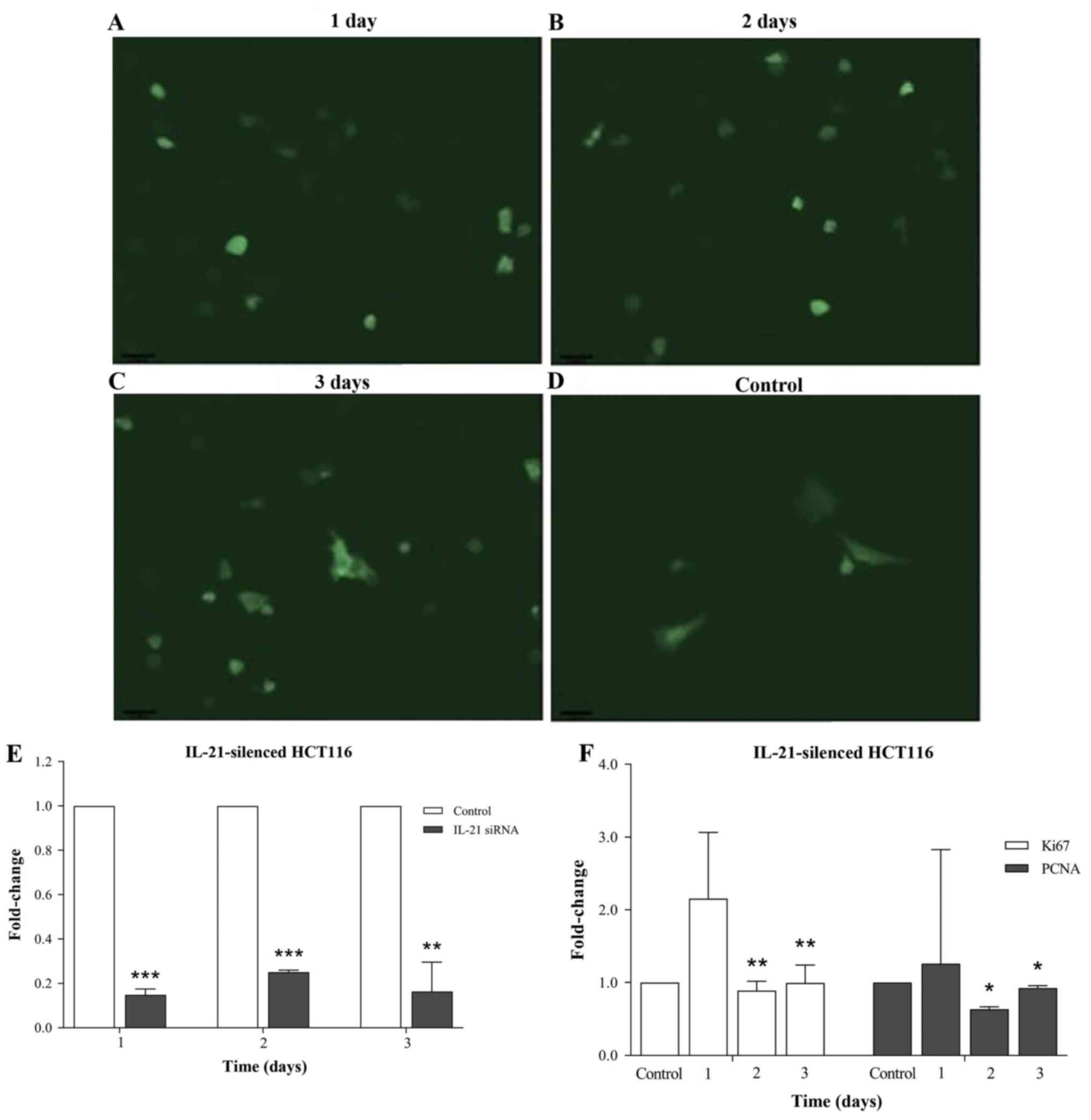
mRNA expression of cell proliferation
markers in IL-21-silenced HCT116 cells
The images of IL-21-silenced HCT116 cells captured
under an inverted fluorescence microscope are presented in Fig. 3A, and IL-21 mRNA expression
differences in cells transfected with IL-21 siRNA and control
(untransfected cells) were demonstrated using RT-qPCR (Fig. 3B). As the cells transfected with
scrambled siRNA did not exhibit any significant differences
compared with non-transfected cells in our previous study (7,10),
non-transfected cells were used as control. Evaluation of the mRNA
expression level of the cell proliferation markers Ki67 and PCNA
using RT-qPCR demonstrated that the mRNA expression levels of Ki67
and PCNA were decreased in the IL-21-silenced HCT116 cells compared
with those in the controls. The reduction in the mRNA levels of
both markers was observed as early as day 1 following IL-21
silencing. The mRNA expression levels of Ki67 were significantly
downregulated at days 2 (0.891-fold change; P<0.01) and 3
(0.993-fold change; P<0.01), and those of PCNA were also
significantly downregulated at days 2 (0.636-fold change;
P<0.05) and 3 (0.924-fold change; P<0.05) following silencing
of IL-21 in HCT116 compared to day 1 fold change (Fig. 3C). The mRNA expression level of
other cell proliferation markers, such as transforming growth
factor-α, chemokine C-C motif ligand 5 and epidermal growth
factor, were not significantly altered in the IL-21-silenced
HCT116 cells (data not shown).
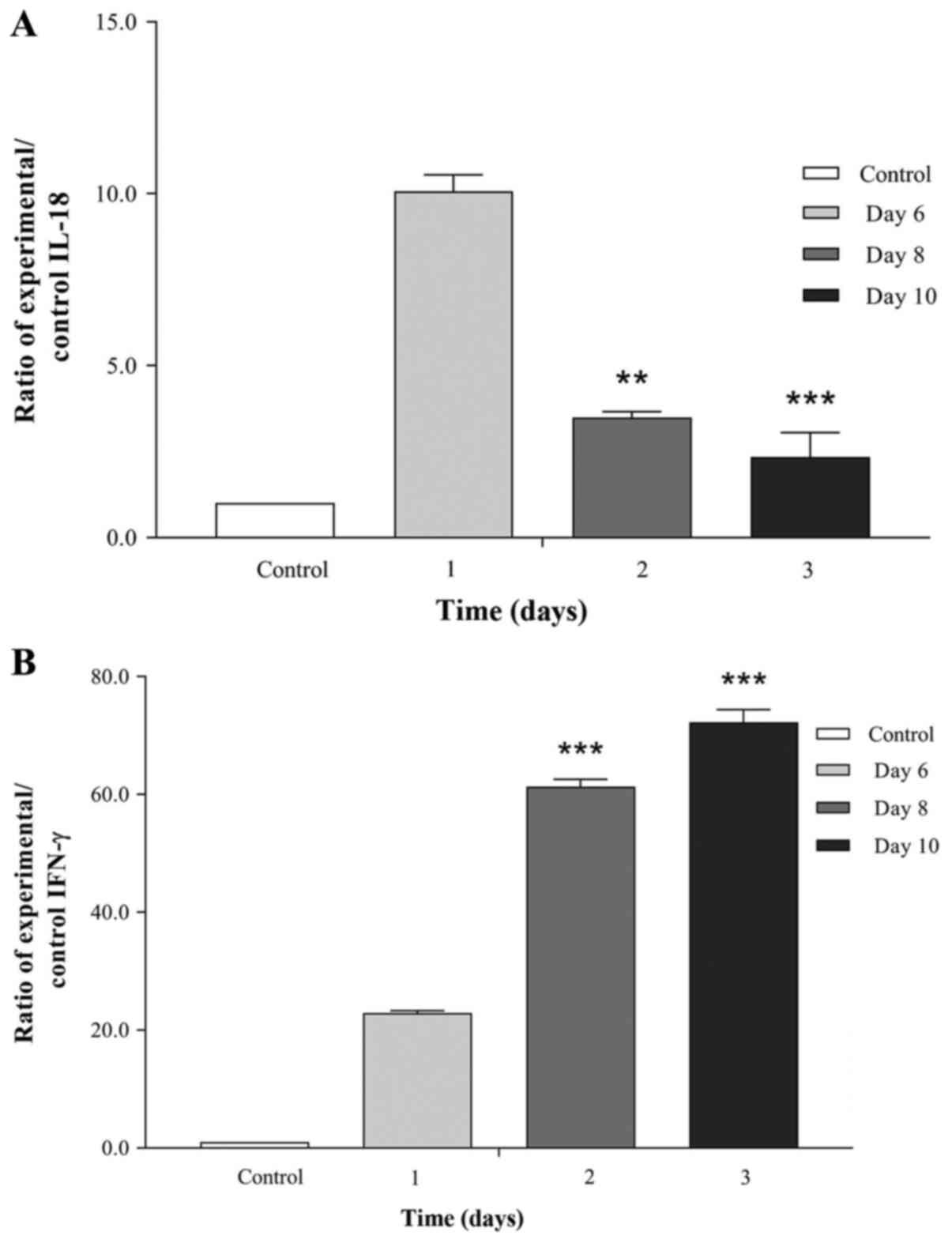
IL-18 and IFN-γ levels in the
conditioned media of IL-21-silenced HCT116 cells
The levels of the soluble factors IL-18 and IFN-γ in
the conditioned media of IL-21-silenced HCT116 cells were analysed,
and the results demonstrated that IL-21 silencing resulted in
similar alterations of IL-18 and IFN-γ levels in the conditioned
media of parasitized host cells. A reduction in IL-18 levels was
observed on days 2 (3.459-fold change; P<0.01) and 3 (2.347-fold
change; P<0.001) following IL-21 silencing compared with day 1
(10.070-fold change). By contrast, increased IFN-γ levels were
observed on days 2 (61.260-fold change; P<0.001) and 3
(72.293-fold change; P<0.001) following IL-21 silencing compared
with day 1 (Fig. 4A and B). Therefore, these results indicated that
IL-18 and IFN-γ may be the soluble factors associated with the
alterations in the levels of proliferation markers in
IL-21-silenced HCT116 and parasite-infected host cells.
Discussion
The present study on Th1 immune responses following
parasitic infection of host cells revealed that IL-18 and IFN-γ
likely represented the growth factors that differentiated a
possible selective advantage and disadvantage for RH and ME49
strains of T. gondii to invade into surrounding host cells.
These factors may modify the proliferation of the host cells, which
was observed as a reduction in Ki67 and PCNA mRNA expression levels
in the IL-18- and IFN-γ-stimulated host cells at the end of the
experiments. These soluble factors may also allow the parasites,
such as ME49, to slowly invade into a higher number of surrounding
host cells, resulting in chronic diseases. Similarly, reduction in
the mRNA expression level of the cell proliferation markers Ki67
and PCNA was also observed in the IL-21-silenced HCT116 cells in
the present study, which indicated that IL-21 may regulate the
proliferation of diseased cells. Different levels of IL-18 and
IFN-γ were also detected in the conditioned media of IL-21-silenced
HCT116 cells. By studying HFFs (host cells) and HCT116 cells
(diseased cells), the present study aimed to elucidate the
fundamental molecular mechanisms of the host-parasite interactions
that subsequently cause chronic diseases, such as colorectal
cancer, and examine whether these two cell types released the same
soluble factors in the conditioned media. The collective
information may be useful for future treatment of infectious
disease-associated colorectal cancer, and IL-21, IL-18 and IFN-γ
may be used to develop tools for early diagnosis, novel
prescription drugs and cost-effective strategies for the treatment
of these diseases.
IL-18 and IFN-γ are Th1-type cytokines that are
produced by the immune system in response to parasitic infection
(11-13).
Only host cells infected with ME49 were indicated to release
reduced levels of IL-18 and elevated levels of IFN-γ in the
conditioned media compared with the controls in the present study.
It may be hypothesised that the ME49-parasitized host cells also
secrete IL-21 into the conditioned media. IL-21 is a member of the
common c chain family of cytokines, which includes IL-2, IL-4, IL-7
and IL-15 that has been reported to be involved in T-cell
proliferation and homeostasis (14). A previous study has demonstrated
that IL-21r−/− mice infected with T.
gondii survived for ≥100 days post-infection; however, these
mice displayed a defect in serum IgG production (15). IL-21 has been also associated with
the differentiation of IL-10-producing CD4+ T cells,
which have been reported to limit immune-mediated pathology during
toxoplasmosis (16,17). IL-21-deficient mice chronically
infected with T. gondii have been demonstrated to exhibit
high numbers of parasites in the brain, which is associated with a
decrease in parasite-specific antibody production and a marked
reduction in the numbers of CD4+ and CD8+
effector T cells in the brain, resulting in diminished IFN-γ
production (18). However,
reductions in IFN-γ levels were not observed in the present study
in the conditioned media of host cells parasitized with RH and
ME49. By contrast, the levels of IFN-γ were elevated in the
conditioned media of host cells parasitized with ME49 for ³8 days,
which suggested that the levels of IFN-γ production may be
tissue-specific, and the alterations in the cytokine levels may
provide a potential selective advantage for ME49 to cause chronic
infection. However, this phenomenon was not observed in the type I
parasitic infection with the RH strain.
A previous study has demonstrated that IL-18 and
IL-21 in different combinations enhance IFN-γ production in human
NK and T cells (19). The results
of the present study revealed that infection with ME49 reduced the
levels of IL-18, but increased those of IFN-γ in the conditioned
media compared with those in the control cells. The overview of the
association between IL-18 and IFN-g with IL-21 is summarised in
Table I, whereby a low expression
level of IL-21 is hypothesised at early chronic parasitic infection
when both IL-18 and IFN-γ are maintained at moderate levels. When
the infection time is prolonged, IL-21 continues to be induced,
while IL-18 and IFN-γ are decreased and maintained at a high level,
respectively. IL-18 is required for IFN-γ gene activation in both
bacterial and viral infections (20-23).
Therefore, a low level of IL-18 in the conditioned media of
parasite-infected host cells may be insufficient to induce high
levels of IFN-γ. The presence of other factors in the conditioned
media, e.g. IL-21, likely contributes to high levels of IFN-γ and
to the development of cancerous cells. This phenomenon may also
explain the relationship between parasitic infection and colorectal
cancer. IL-21 was the focus of the present study, as a high level
of IL-21 expression has been detected in colorectal cancer cells,
such as HT29 and HCT116(7). In our
previous study, a high level of IL-21 was also detected in the
serum samples of patients diagnosed with colorectal cancer with a
history of parasitic infections (24). IL-18 or IL-21 alone have been
demonstrated to represent weak inducers of IFN-γ production, but
the combination of IL-18 and IL-21 has been reported to induce
notable activation of IFN-γ gene expression (19). Therefore, it was hypothesised that
IL-21 is another soluble factor that may be present in the
conditioned media, and it was examined in the present study whether
IL-21 silencing resulted in the reduction of cell proliferation
that may be associated with the levels of IL-18 and IFN-γ in the
conditioned media.
 | Table ISummary of the associations between
IL-18 and IFN-γ with IL-21 observed in the present and previous
studies. |
Table I
Summary of the associations between
IL-18 and IFN-γ with IL-21 observed in the present and previous
studies.
| | Cytokine | |
|---|
| Day | IL-18a | IFN-γa | IL-21b | Comment |
|---|
| 2 | + | + | - | Early chronic
parasitic infection |
| 4 | + | + | - | Early chronic
parasitic infection |
| 6 | + | ++ | + | IFN-γ and cancerous
factor levels start to be induced in host cells with chronic
parasitic infection |
| 8 | - | +++ | ++ | IFN-γ and cancerous
factor levels continue to be induced when IL-18 is decreased in the
conditioned media of host cells with chronic parasite
infection |
| 10 | - | +++ | +++ | IFN-γ is maintained
at a high level, and the cancerous factor continues to be induced
when IL-18 is decreased in the conditioned media of host cells with
chronic parasite infection |
In the present study, IL-18 and IFN-γ were
demonstrated to alter the mRNA expression of the cell proliferation
markers Ki67 and PCNA in IL-18- and IFN-γ-stimulated host cells,
which was also observed in IL-21-silenced HCT116 cells. IL-18 is a
uniquely pleiotropic member of the IL-1 family, and it is
synthesised as a 24 kDa precursor protein and cleaved into an 18
kDa mature form by caspase-1(25).
The level of IL-18 was reduced in the conditioned media of host
cells parasitized with ME49 for 8-10 days in the present study.
Stimulation of the host cells with IL-18 induced an optimal level
of cell proliferation markers dependent on the time following
stimulation (bell shaped curve), which was reflected in the mRNA
expression levels of Ki67 and PCNA. However, prolonged incubation
of the host cells with IL-18 also reduced cell proliferation marker
expression. These results were consistent with those of a previous
study indicating that IL-18 exerted both cancer-promoting and
cancer-suppressing functions (26).
IL-18 has been revealed to promote the proliferation and invasion
of pancreatic cancer cells, and higher IL-18 levels in pancreatic
cancer tissues were associated with a shorter overall survival
(OS), increased invasion and metastasis, compared with patients
with lower IL-18 levels (26).
However, in the same study, higher plasma levels of IL-18 were
associated with longer OS. IL-18 has been demonstrated to exhibit
antitumourtumour activity in preclinical models and increase the
serum concentrations of IFN-γ, granulocyte macrophage
colony-stimulating factor and soluble Fas ligand (27). By contrast, the role of IFN-γ was
demonstrated to be more direct and straightforward in the present
study. IFN-γ was induced in the conditioned media of the host cells
parasitized with ME49, and stimulation of the host with IFN-γ
reduced cell proliferation markers. These results suggested that
IFN-γ may reduce cell proliferation markers, which was also
observed in IL-21-silenced HCT116 cells in the present study. A
previous study reported that intravesical instillations of 0.7 mg
IFN-γ produced a significant cytostatic effect on superficial
bladder cancer cells, which was evidenced by the decrease in growth
fractions, measured using antigens of PCNA and Ki67(28). IFN-γ has been also demonstrated to
sustain the expression of PCNA and the G1/S regulator
retinoblastoma proteins, including cyclin D1, cyclin E and cdk2,
and maintain low p27 levels (29).
However, the effects of prolonged IFN-γ stimulation on Ki67 and
PCNA expression were only observed at day 10 in the current study.
A more direct method, such as flow cytometry or western blotting,
to evaluate the cell cycle-associated proteins should be utilised
in future research to produce a stronger evidence for this
hypothesis. The results of the analysis of the mRNA expression
levels of cell proliferation markers in IL-21-silenced HCT116 cells
indicated that IL-21 likely regulated the proliferation of HCT116
cells. The IL-21-silenced HCT116 cells may be used as a model to
ensure whether the modification of diseased cell proliferation
released the soluble factors as the aforementioned investigation
outlines.
A previous study has revealed that IL-18-deficient
mice were highly resistant to chronic T. muris infection,
and in vivo treatment of normal mice with recombinant IL-18
suppressed IL-4 and IL-13 secretion. However, the treatment did not
affect the level of IFN-γ in the mice (6). The present in vitro study did
not observe an association between IL-18 and ME49 infection
resistance. However, the levels of IL-18 and IFN-γ exhibited
opposing trends during ME49 infection for 10 days, which supported
the hypothesis that IL-18 does not function as an IFN-γ-inducing
cytokine during chronic infections, but serves other roles, such as
direct regulation of Th2 cytokines (30). Another study has demonstrated that
CD8+ T cells and IFN-γ were required for the host
immunity to RH and ME49(31).
However, these differences may reflect stage-specific (tachyzoite
vs. bradyzoite) or strain-specific (RH vs. ME49) requirements for
the host immunity, which remain unclear, primarily since the
majority of toxoplasma strains have been indicated to be virulent
during secondary infections (32).
Additional studies should be conducted in the future to provide
stronger evidence for the validation of the hypothesis of the
present study. To the best of our knowledge, Schistosomiasis
attracts little attention and support worldwide owing to the
geographical barriers and certain political issues (33). The disease may also be considered as
unimportant as it primarily occurs in individuals living in poor,
rural communities and in endemic regions (33). Moreover, current studies focus
mainly on pandemic issues rather than neglected diseases.
In conclusion, the results of the present study may
elucidate the fundamental molecular mechanisms of host-parasite
interactions that cause chronic diseases. The results may also
provide useful information for future studies on groups of genes
that regulate Th cell responses during colorectal cancer and
parasitic infection.
Acknowledgements
The authors would like to thank Professor Rahmah
Noordin of Institute for Research in Molecular Medicine (INFORMM),
Universiti Sains Malaysia for providing the RH and ME49 strains of
T. gondii for the present study.
Funding
The present study was funded by the Universiti Sains
Malaysia Short-term Grant Scheme (grant no. 304/CIPPM/6311018) and
Fundamental Research Grant Scheme Fasa 1/2017 (grant no.
203/CIPPM/6711599).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PG, BYK and BY made substantial contributions to the
design of the present study. CYO and AEA participated in all
experiments under technical support provided by BYK. BYK
interpreted the results, drafted and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferra B, Holec-Gąsior L and Grąźlewska W:
Toxoplasma gondii recombinant antigens in the serodiagnosis
of toxoplasmosis in domestic and farm animals. Animals (Basel).
10(1245)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Galeh TM, Sarvi S, Montazeri M, Moosazadeh
M, Nakhaei M, Shariatzadeh SA and Daryani A: Global status of
Toxoplasma gondii seroprevalence in rodents: A systematic
review and meta-analysis. Front Vet Sci. 7(461)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mordue DG, Monroy F, La-Regina M,
Dinarello CA and Sibley LD: Acute toxoplasmosis leads to lethal
overproduction of Th1 cytokines. J Immunol. 167:4574–4584.
2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gavrilescu LC and Denkers EY: IFN-gamma
overproduction and high level apoptosis are associated with high
but not low virulence Toxoplasma gondii infection. J
Immunol. 167:902–909. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hakimi MA, Olias P and Sibley LD:
Toxoplasma effectors targeting host signaling and transcription.
Clin Microbiol Rev. 30:615–645. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Helmby H, Takeda K, Akira S and Grencisa
RK: Interleukin (Il)-18 promotes the development of chronic
gastrointestinal helminth infection by downregulating IL-13. J Exp
Med. 194:355–364. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Abdalkareem EA, Ong CY, Lim BH and Khoo
BY: Neutralising FGF4 protein in conditioned medium of
IL-21-silenced HCT116 cells restores the invasiveness of the
colorectal cancer cells. Cytotechnology. 70:1363–1374.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Khan A and Grigg ME: Toxoplasma
gondii: Laboratory maintenance and growth. Curr Protoc
Microbiol. 44:20C.1.1–20C.1.17. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Eshtiyag A, Lim BH and Khoo BY: Silencing
of IL21 in HT29 and HCT116 cells to determine its role in the
proliferation of colorectal cancer associated with Schistosoma
mansoni infection. Australian J Basic Applied Sci. 9:39–44.
2015.
|
|
11
|
Eberl M, Beck E, Coulson PS, Okamura H,
Wilson RA and Mountford AP: IL-18 potentiates the adjuvant
properties of IL-12 in the induction of a strong Th1 type immune
response against a recombinant antigen. Vaccine. 18:2002–2008.
2000.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chang CY, Lee J, Kim EY, Park HJ, Kwon CH,
Joh JW and Kim SJ: Intratumoral delivery of IL-18 naked DNA induces
T-cell activation and Th1 response in a mouse hepatic cancer model.
BMC Cancer. 7(87)2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Srinivasan A, Salazar-Gonzalez RM, Jarcho
M, Sandau MM, Lefrancois L and McSorley SJ: Innate immune
activation of CD4 T cells in salmonella-infected mice is dependent
on IL-18. J Immunol. 178:6342–6349. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Spolski R and Leonard WJ: Interleukin-21:
Basic biology and implications for cancer and autoimmunity. Annu
Rev Immunol. 26:57–79. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ozaki K, Spolski R, Feng CG, Qi CF, Cheng
J, Sher A, Morse HC III, Liu C, Schwartzberg PL and Leonard WJ: A
critical role for IL-21 in regulating immunoglobulin production.
Science. 298:1630–1634. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pot C, Jin H, Awasthi A, Liu SM, Lai CY,
Madan R, Sharpe AH, Karp CL, Miaw SC, Ho IC and Kuchroo VK: Cutting
edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21,
and the costimulatory receptor ICOS that coordinately act together
to promote differentiation of IL-10-producing Tr1 cells. J Immunol.
183:797–801. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Spolski R, Kim HP, Zhu W, Levy DE and
Leonard WJ: IL-21 mediates suppressive effects via its induction of
IL-10. J Immunol. 182:2859–2867. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Stumhofer JS, Silver JS and Hunter CA:
IL-21 is required for optimal antibody production and T cell
responses during chronic Toxoplasma gondii infection. PLoS
One. 8(e62889)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Strengell M, Matikainen S, Sirén J,
Lehtonen A, Foster D, Julkunen I and Sareneva T: IL-21 in synergy
with IL-15 or IL-18 enhances IFN-gamma production in human NK and T
cells. J Immunol. 170:5464–5469. 2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sareneva T, Matikainen S, Kurimoto M and
Julkunen I: Influenza A virus-induced IFN-alpha and IL-18
synergistically enhance IFN-gamma gene expression in human T cells.
J Immunol. 160:6032–6038. 1998.PubMed/NCBI
|
|
21
|
Nakanishi K, Yoshimoto T, Tsutsui H and
Okamura H: Interleukin-18 regulates both Th1 and Th2 responses. Ann
Rev Immunol. 19:423–474. 2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pirhonen J, Sareneva T, Kurimoto M,
Julkunen I and Matikainen S: Virus infection activates IL-1 beta
and IL-18 production in human macrophages by a caspase-1-dependent
pathway. J Immunol. 162:7322–7329. 1999.PubMed/NCBI
|
|
23
|
Pien GC, Satoskar AR, Takeda K, Akira S
and Biron CA: Cutting edge: Selective IL-18 requirements for
induction of compartmental IFN-gamma responses during viral
infection. J Immunol. 165:4787–4791. 2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Abdalkareem EA, Tan GC, Abdalla HS, Lim BH
and Khoo BY: Identification of specific proteins in colorectal
cancer patients with Schistosoma mansoni infection as a
possible biomarker for the treatment of this infection. Asian Pac J
Trop Dis. 4 (Suppl):S720–S724. 2014.
|
|
25
|
Kuppala MB, Syed SB, Bandaru S, Varre S,
Akka J and Mundulru HP: Immunotherapeutic approach for better
management of cancer-role of IL-18. Asian Pac J Cancer Prev.
13:5353–5361. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guo X, Zheng L, Jiang J, Zhao Y, Wang X,
Shen M, Zhu F, Tian R, Shi C, Xu M, et al: Blocking NF-κB is
essential for the immunotherapeutic effect of recombinant IL-18 in
pancreatic cancer. Clin Cancer Res. 22:5939–5950. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wigginton JM, Lee JK, Wiltrout TA, Alvord
WG, Hixon JA, Subleski J, Back TC and Wiltrout RH: Synergistic
engagement of an ineffective endogenous anti-tumor immune response
and induction of IFN-gamma and Fas-ligand-dependent tumor
eradication by combined administration of IL-18 and IL-2. J
Immunol. 169:4467–4474. 2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Stavropoulos NE, Ioachim E, Pappa L,
Hastazeris K and Agnantis NJ: Antiproliferative activity of
interferon gamma in superficial bladder cancer. Anticancer Res.
19:4529–4533. 1999.PubMed/NCBI
|
|
29
|
Chew LJ, King WC, Kennedy A and Gallo V:
Interferon-gamma inhibits cell cycle exit in differentiating
oligodendrocyte progenitor cells. Glia. 52:127–143. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yasuda K, Nakanishi K and Tsutsui H:
Interleukin-18 in health and disease. Int J Mol Sci.
20(649)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gigley JP, Bhadra R and Khan IA: CD8 T
cells and Toxoplasma gondii: A new paradigm. J Parasitol
Res. 2011(243796)2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jensen KDC, Camejo A, Melo MB, Cordeiro C,
Julien L, Grotenbreg GM, Frickel EM, Ploegh HL, Young L and Saeij
JP: Toxoplasma gondii superinfection and virulence during
secondary infection correlate with the exact ROP5/ROP18 allelic
combination. mBio. 6(e02280)2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Abdalkareem EA and Yin KB: A current
perspective of schistosomiasis in association with
colorectal carcinogenesis. Open Infect Dis J. 11:7–12. 2019.
|