Introduction
Lung cancer is a cause of cancer mortality in
patients. In total, >two-thirds of all lung cancer types are a
part of the subtype of non-small cell lung cancer (NSCLC) (1). In NSCLC, high expression levels of
glucose ttransporter 1 (GLUT1) and hexokinase 2 (HK2) facilitate
glucose uptake and are the initial steps of glucose metabolism
(2,3). Previous studies have revealed high
glucose metabolism as one of the hallmarks of numerous cancers, in
which glycolysis provides ATP rapidly and the pentose phosphate
pathway (PPP) generates pentose phosphates for ribonucleotide
synthesis and NADPH to meet the demand of rapid proliferation
(4-6).
Previous studies have reported that inhibition of glycolysis and
PPP influences the growth of NSCLC cells (7-9).
MicroRNAs (miRNA/miRs) are non-coding RNA molecules
of 19-25 nucleotides that negatively regulate gene expression by
interacting with 3'untranslated regions (3'UTRs) of the target
genes (10). Several miRNAs have
been reported to be associated with the progression of NSCLC,
serving as either oncogenes or tumor suppressors (11-13).
Previous studies have suggested that miR-218 expression levels are
reduced in multiple cancer types (14,15),
including NSCLC (16,17). Previous studies have also revealed
that miR-218 can regulate the metastasis and chemosensitivity of
NSCLC (18-20).
GLUT1 is indicated to be a direct target of miR-218 in bladder
cancer (21), however the function
of miR-218 in glucose metabolism in NSCLC remains unclear and the
underlying molecular mechanism is still unknown.
The present study aimed to investigate the effect of
miR-218 on the glycolysis and PPP pathways and assess the impact of
miR-218 on the expression levels of related key enzymes in NSCLC
cell lines. Furthermore, the potential mechanisms associated with
these effects were investigated.
Materials and methods
Cell culture and treatment
NCI-H23 and A549 human NSCLC cells were purchased
from Shanghai Cell Biology Institute of Chinese Academy of
Sciences. Cells were maintained in RPMI-1640 medium (cat. no.
22400089; Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (cat. no. 10437028; Gibco; Thermo Fisher Scientific, Inc.).
NCI-H23 and A549 cells (2x105 cells/well) were seeded
into 6-well plates and transiently transfected with 50 nM miR-218
mimics (cat. no. B02003) or mimics control (cat. no. B04002; both
Shanghai GenePharma Co., Ltd.) using Lipofectamine 2000 reagent
(cat. no. 11668019; Thermo Fisher Scientific, Inc.). After 48 h of
transfection the subsequent experimentations were performed.
Preparation of lentivirus and
establishment of stable cell lines
The GLUT1 gene sequence was amplified from the cDNA
of A549 cell line by PCR and the following primers:
5'-CCGTCTAGAGCCACCATGGAGCCCAGCAGCAAGAAG-3' (forward);
5'-GGCGGATCCTCACACTTGGGAATCAGCCC-3' (reverse) were inserted into
the plasmid pCDH1-CMV-MSC-EF1-Puro (System Biosciences) at the
XbaI and BamHI sites. The acquired vector was termed
‘pCDH-GLUT1’. A sequence containing the U6 promoter and a template
of inhibitor of κB (IκBα) short hairpin RNA (shRNA), shown in
Table. SI, were synthesized,
inserted in pCDH-GLUT1 (30 ng/µl) by BamHI and NotI
restriction enzymes (New England Biolabs, Ltd.), and the acquired
vector was termed ‘pCDH-GLUT1-shIκBα’. The package method of
lentivirus preparation was performed as previously described
(22). A549 cells were transfected
with lentivirus for 48 h and with 30 µg/ml puromycin (cat. no.
P8230; Beijing Solarbio Science & Technology Co., Ltd.) for 2
weeks.
Total RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from cells or tissue samples
using TRIzol® reagent (cat. no. 15596026; Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. Total RNA (1 µg) was reverse transcribed into cDNA using
the RevertAid First Strand cDNA Synthesis kit (cat. no. K1622;
Thermo Fisher Scientific, Inc.) at 42˚C, according to the
manufacturer's protocol. qPCR was subsequently performed using the
SYBR® Premix Ex Taq™ kit (cat. no. RR420L;
Takara Biotechnology Co., Ltd.). The thermocycling protocol for the
reaction was as follows: Initial denaturation at 95˚C for 10 min;
40 cycles of 95˚C for 15 sec and 60˚C for 1 min. Gene expression
was quantified using the 2-ΔΔCq method (23). The primers used in the research were
as follows: miR-218 forward, 5'-TTGCGGATGGTTCCGTCAAGCA-3'; miR-218
reverse, 5'-ATCCAGTGCAGGGTCCGAGG-3'; U6 forward,
5'-CGCTTCGGCAGCACATATAC-3'; U6 reverse,
5'-AAAATATGGAACGCTTCACGA-3'. U6 was used as a reference gene.
Measurement of glucose, lactate and
NADPH/NADP+
NCI-H23 and A549 cells were seeded into 6-well
plates (2x105 cells/well), followed by culturing till
90% confluence was reached, replaced with fresh medium and then
cultured for 6 h at 37˚C. The supernatant was collected, in which
the contents of glucose and lactate were determined by using a
glucose assay kit and a lactic acid assay kit produced by Nanjing
Jiancheng Bioengineering Institute Co., Ltd. (cat. no. F006-1-1 and
cat. no. A019-2-1). Glucose consumption was calculated by
subtracting the final concentration from the initial concentration
of the medium. The NADPH/NADP+ ratios in cellular
lysates were determined using an NADP/NADPH assay kit (cat. no.
ab65349; Abcam) at an optical density of 450 nm. All these tests
were performed according to the manufacturer's protocol.
Protein extraction and western
blotting
Expression levels of target proteins and GLUT1
membrane localization were examined as previously described
(24). Whole-cell proteins and
membrane proteins were extracted using RIPA lysis buffer (Beijing
Solarbio Science & Technology Co., Ltd.) and a membrane protein
extraction kit (Sangon Biotech Co., Ltd.), respectively. Protein
concentration was quantified by bicinchoninic acid protein assay
kit (cat. no. PC0020; Beijing Solarbio Science & Technology
Co., Ltd.) and proteins (40 µg per lane) were separated in 12%
polyacrylamide gel. After electrophoresis, the proteins were
transferred into PVDF membrane (cat. no. IPVH00010; EMD Millipore),
followed by blocking with 5% BSA (cat. no. A8020; Beijing Solarbio
Science & Technology Co., Ltd.) in TBST (with 0.1% Tween-20) at
room temperature for 3 h and then incubating with primary
antibodies (1:1,000) at 4˚C overnight. Next, the membranes were
incubated with horseradish peroxidase-conjugated goat anti-mouse
(cat. no. SE12; 1:2,000; Beijing Solarbio Science & Technology
Co., Ltd.) or anti-rabbit (cat. no. SE13; 1:2,000; Beijing Solarbio
Science & Technology Co., Ltd.) secondary antibodies for 1 h at
room temperature. The protein bands were visualized by BioVision
ECL western blotting substrate kit (cat. no. K820-500; BioVision,
Inc.). ImageJ software (version 1.5; National Institutes of Health)
was used to analyze the intensities of the band signals obtained.
The antibodies of GLUT1 (cat. no. ab115730), HK2 (cat. no.
ab104836), glucose-6-phosphate dehydrogenase (G6PD; cat. no.
ab993), NF-κB p65 (cat. no. ab32536), and phosphorylated (p-)NF-κB
p65 (cat. no. ab86299) were obtained from Abcam. The antibodies of
phosphofructokinase-1 (PFK1; cat. no. sc-377346), ERK1/2 (cat. no.
sc-514302), p-ERK1/2 (cat. no. sc-81492), JNK1/2 (cat. no.
sc-137019) and p-JNK1/2 (cat. no. sc-293136) were obtained from
Santa Cruz Biotechnology, Inc. The antibodies for Akt (cat. no.
9272) and p-Akt (cat. no. 4060) were produced by Cell Signaling
Technology, Inc..
Luciferase reporter assay
The 3'UTRs of GLUT1, HK2, FPK1 and G6PD were
synthesized and inserted into psiCHECK-2 plasmid (Promega
Corporation) at the restriction enzyme sites of XhoI and
NotI. Sequences are shown in Table SI. The acquired vectors were termed
as ‘psiGLUT1’, ‘psiHK2’, ‘psiFPK1’ and ‘psiG6PD’. Then cancer cells
at a density of 4x105/well were seeded into 6-well
plates and cotransfected with either 50 nm miR-218 mimics or miRNA
negative control (NC) and 2 µg above vectors, using Lipofectamine
2000 according to the manufacturer's protocol. The cells were lysed
and then assayed for luciferase activity at 48 h post-transfection
using a Dual-luciferase® reporter assay kit (Promega
Corporation). The assays were repeated independently >3 times.
Firefly luciferase was used as a reference for normalization.
Statistical analysis
All data were obtained from 3-5 independent
experiments and are presented as mean ± SD. Two-sided, unpaired
Student's t-test was used for comparing between groups. One-way
ANOVA was used to compare categorical variables. Significant
differences between experimental groups were assessed using
Bonferroni post hoc test. P<0.05 was considered to indicate a
statistically significant difference. The analysis was performed
using GraphPad Prism 5 (GraphPad Software Inc.).
Results
miR-218 reduces glucose consumption
and activities of glycolysis and PPP
To study the effect of miR-218 on glucose metabolism
in NSCLC cell lines, miR-218 mimics or NC were transfected into
NCI-H23 and A549 cells. miR-218 expression levels were
significantly increased in the two cell lines after transfected
with mimics compared with the NC group (P<0.01; Fig. 1A). Then the levels of glucose and
lactate in the supernatant, as well as the NADPH/NADP+
ratio in cellular lysates, were measured. Glucose uptake was
significantly reduced by transfection with miR-218 mimics in both
NSCLC cell lines (Fig. 1B), as well
as lactate production, which represents the activity of glycolysis
(Fig. 1C). Similarly, the ratio of
NADPH, which is a product of PPP, to NADP+ in miR-218
mimic transfection group showed a significant decrease compared
with the NC group (P<0.05; Fig.
1D). The present results indicated that miR-218 could decrease
glucose uptake and the activities of glycolysis and PPP in NSCLC
cells. In addition, the expression levels of key enzymes involved
in glucose uptake, glycolysis and PPP in the transfected NCI-H23
and A549 cells were investigated by western blotting. The present
results suggested that GLUT1, HK2, PFK1 and G6PD expression levels
were significantly decreased by miR-218 in both cell lines
(Fig. 1E).
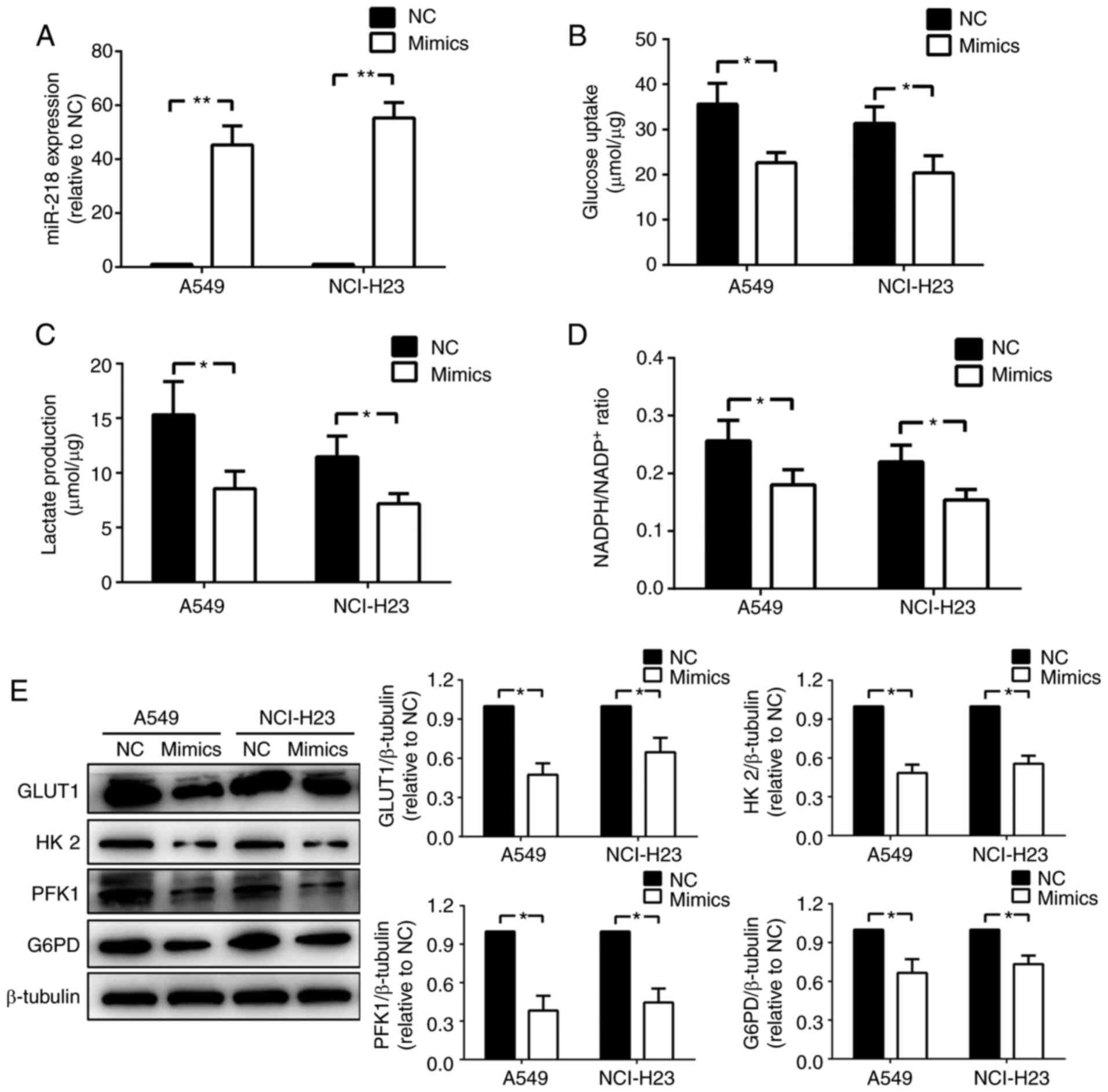 | Figure 1miR-218 reduces glucose consumption
and activities of glycolysis and pentose phosphate pathway. (A)
Expression levels of miR-218 were measured in NCI-H23 and A549
cells after transfection with NC or miR-218 mimics, n=3. Then (B)
glucose consumption, (C) lactate production and (D) ratio of NADPH
to NADP+ were investigated, n=5. (E) Western blotting
was performed to determine the protein expression levels of key
enzymes involved in glucose metabolism, n=5. *P<0.05
and **P<0.01. Data are presented as mean ± SD. NC,
negative control; miR-218, microRNA-218; GLUT1, glucose transporter
1; HK2, hexokinase 2; PFK1, phosphofructokinase-1; G6PD,
glucose-6-phosphate dehydrogenase. |
miR-218 inhibits glucose metabolism by
downregulating GLUT1
To investigate whether miR-218 regulated the
expression of GLUT1, HK2, PFK1 and G6PD directly, the 3'UTRs of
these were inserted into luciferase reporter vectors. Then the
luciferase reporter assay was used for detection after
cotransfection with reporter vector and mimics or NC in A549 cells.
The present results suggested a significant decrease in activity
only by 3'UTRs of GLUT1 (Fig. 2A).
Then A549-GLUT1, a cell line that overexpressed GLUT1 stably, was
used to explore whether there are any other mechanisms involved in
mediating glucose metabolism inhibition of miR-218 except for the
downregulation of GLUT1 directly and was identified by western
blotting (Fig. 2B). The present
results indicated that glucose uptake, lactate production and
NADPH/NADP+ were reduced after transfection with miR-218
mimics in A549-GLUT1 (Fig. 2C-E),
suggesting the possibility of other mechanisms.
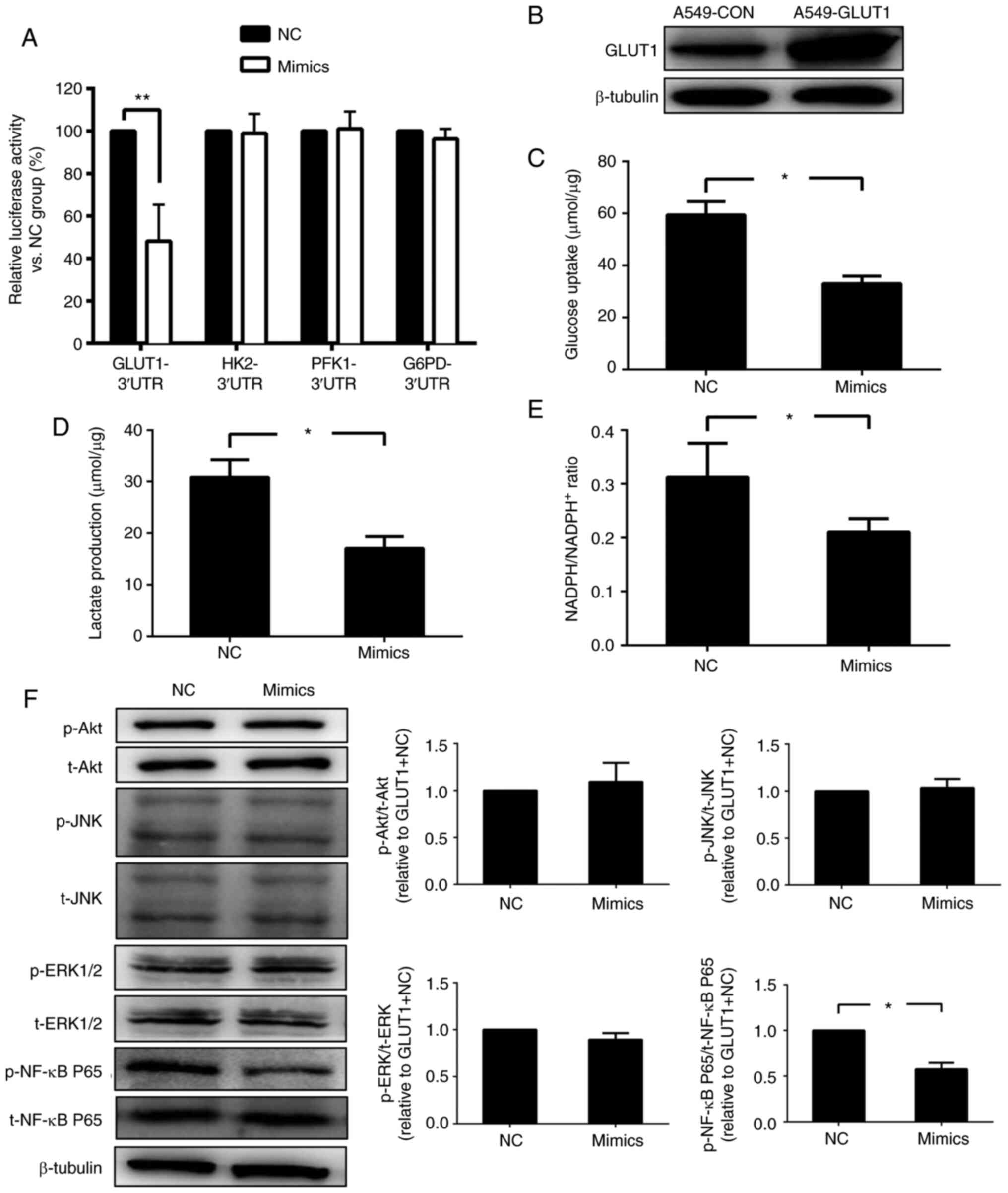 | Figure 2miR-218 inhibits glucose metabolism
via downregulation of GLUT1. (A) A luciferase reporter assay was
conducted to investigate whether key enzymes involved in glucose
metabolism are direct targets of miR-218, n=5. (B) A549-GLUT1, a
stable cell line that overexpressed GLUT1 was constructed and
identified by western blotting. (C) Glucose consumption, (D)
lactate production and (E) the ratio of NADPH to NADP+
were measured after transfection with NC or miR-218 mimics, n=5.
(F) Expression levels of several factors related to cell
proliferation and survival were detected in A549-GLUT1 cells in
both NC and mimics groups, n=5. *P<0.05 and
**P<0.01. Data are presented as mean ± SD. NC,
negative control; miR-218, microRNA-218; GLUT1, glucose
ttransporter 1; HK2, hexokinase 2; PFK1, phosphofructokinase-1;
G6PD, glucose-6-phosphate dehydrogenase; p-, phosphorylated; t-,
total; UTR, untranslated region. |
To explore the signaling pathway involved in glucose
metabolism inhibition of miR-218, phosphorylation levels of Akt,
ERK1/2, JNK1/2 and NF-κB p65 were detected in A549-GLUT1
transfected with mimic or NC. The present results indicated that
the expression level of p-NF-κB p65, but not the other three
proteins, was significantly decreased after transfection with the
mimic (Fig. 2F). Therefore, the
present results suggested that miR-218 may regulate glucose
metabolism by modulating the activity of NF-κB.
miR-218 inhibits glucose metabolism
via the NF-κB signaling pathway
To investigate whether the NF-κB pathway mediated
glucose metabolism inhibition induced by miR-218, an inhibitor of
NF-κB IκBα was stably knocked down by shRNA in A549-GLUT1 cells
(Fig. 3A). The acquired cell line
was termed ‘A549-GLUT1-shIκBα’. After transfection with miR-218
mimics, the A549-GLUT1 cells showed reduced glucose uptake, lactate
production and NADPH/NADP+ ratio, and vice versa in the
A549-GLUT1-shIκBα (Fig. 3B-D).
Similarly, HK-2, PFK1 and G6PD expression levels were decreased by
miR-218 in A549-GLUT1 cells, but this effect was diminished in
A549-GLUT1-shIκBα cells (Fig. 3E).
Moreover, this was in accordance with results reported in a
previous study (24), in which the
plasma membrane translocation of GLUT1 was reduced in mimic
transfected A549-GLUT1 cells. No difference was identified between
A549-GLUT1-shIκBα transfected with NC or mimics (Fig. 3F). The present results indicated
that glucose metabolism inhibition of miR-218 may be reversed by
the activation of the NF-κB signaling pathway.
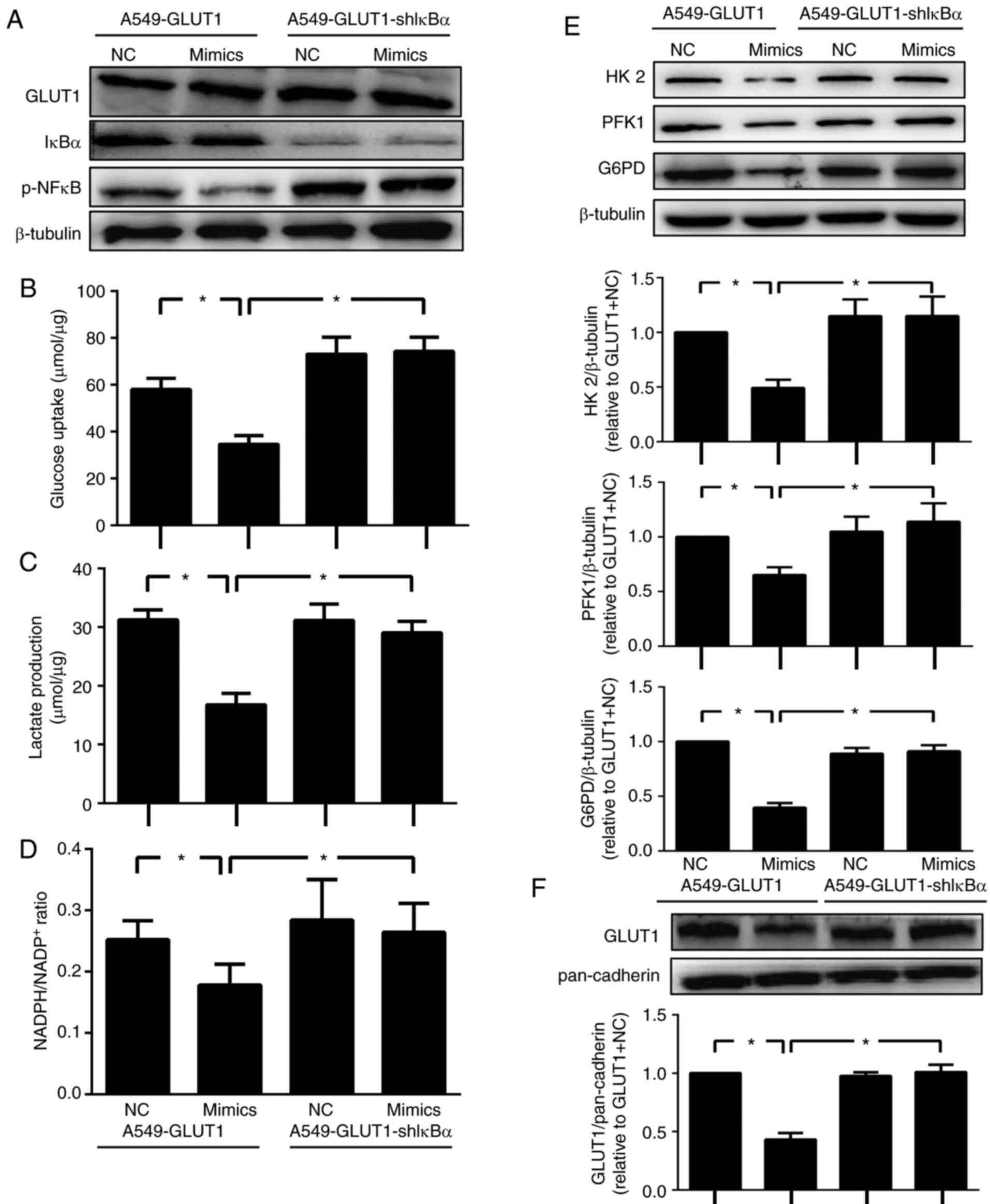 | Figure 3miR-218 inhibits glucose metabolism
via the NF-κB signaling pathway. (A) A549-GLUT1-shIκBα, a stable
GLUT1 overexpressing and IκBα silencing cell line was constructed
and identified by western blotting. (B) Glucose consumption, (C)
lactate production and (D) the ratio of NADPH to NADP+
were examined in A549-GLUT1 and A549-GLUT1-shIκBα cells after
transfection with NC or miR-218 mimics, n=5. (E) Western blotting
was performed to determine the protein expression levels of key
enzymes involved in glucose metabolism and (F) the location of
GLUT1 on cytomembrane, n=5. *P<0.05. Data are
presented as mean ± SD. NC, negative control; miR-218,
microRNA-218; shRNA; short hairpin RNA; shIκBα, short hairpin IκBα
RNA; GLUT1, glucose ttransporter 1; p-, phosphorylated; HK2,
hexokinase 2; PFK1, phosphofructokinase-1; G6PD,
glucose-6-phosphate dehydrogenase; IκBα, inhibitor of κB. |
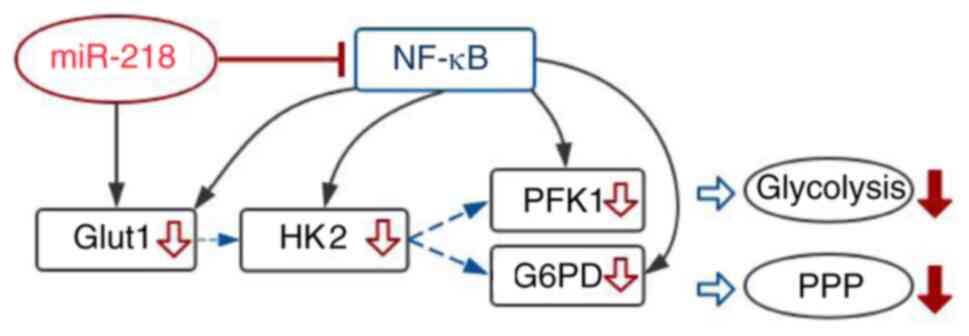
The proposed mechanisms of miR-218 regulation of
glucose metabolism are shown in Fig.
4. miR-218 reduced the expression levels of GLUT1 directly, but
also affected the membrane translocation of GLUT1 and expression
levels of key enzymes in glycolysis and PPP pathways indirectly by
inhibiting the NF-κB signaling pathway.
Discussion
Cancer cells have aberrant expression of miRNAs that
are involved in modulating numerous cell signaling pathways
(25). Previous studies have shown
that miR-218 expression level is significantly reduced in NSCLC
(16,17). Recently, miR-218 has been reported
as a tumor suppressor in lung cancer cells by regulating the
interleukin-6/STAT3 pathway (26).
The present study investigated the effect of miR-218 on glucose
metabolism and the possible underlying molecular mechanisms in
NSCLC cells.
The present results suggested that miR-218 inhibited
glucose uptake, glycolysis and PPP. Several rate-limiting enzymes
such as GLUT1, HK2, PFK1 and G6PD play crucial roles during these
glucose metabolism processes (27).
Previous studies have shown that these enzymes are regulated
directly or indirectly by miRNAs (28-32).
The present results indicated that the expression levels of these
enzymes were decrease by miR-218 in NSCLC cell lines. Subsequently,
to identify whether the four rate-limiting enzymes were the direct
targets of miR-218, a luciferase reporter assay was performed. The
present results suggested that GLUT1 was directly modulated by
miR-218, but the other enzymes were not. The miR-218 mimics had
suppressive effects on glucose uptake, glycolysis and PPP. The
present results suggested the presence of an indirect mechanism
during the expression of rate-limiting enzymes mediated by miR-218.
Previous studies have indicated that Akt, JNK, ERK and NF-κB
signaling pathways play important roles in glucose metabolism
regulation (33-40).
Therefore, in the present study the phosphorylation of these
proteins was detected in A549-GLUT1 cells after transfection with
miR-218 mimics. The present results suggested there were no
significant differences in the phosphorylation levels of Akt, JNK
and ERK, however p-NF-κB p65 was reduced by miR-218 mimics. The
present results indicated that NF-κB may be responsible for the
role of miR-218 in glucose metabolism. To investigate this effect,
changes on glucose metabolism in A549-GLUT1-shIκBα cells,
A549-shIκBα cells and A549-GLUT1 cells were studied. The present
results suggested that glucose uptake, lactate production and
NADPH/NADP+ ratio in A549-GLUT1-shIκBα cells and A549-shIκBα cells
had no significant differences (data not shown). In addition, the
present results showed that reduction of IκBα, whose absence
results in the activation of NF-κB (41), inhibited the curb of miR-218 on
glucose metabolism. The decreasing effects of miR-218 on membrane
translocation of GLUT1 and expression levels of HK2, PFK1 and G6PD
were all reversed by the activation of NF-κB.
In the present study the hypothesis was investigated
in vitro, however metabolism in long-term cultivated cell
lines may not fully reflect the situation in growing tumors, thus
in vivo experiments are required to further investigate the
effect of miR-218.
In the present study glycolysis and PPP were
affected by miR-218 in NSCLC cell lines, thus similar effects based
on the present results are expected in vivo. Although the
present results suggested miR-218 inhibited glucose metabolism by
inactivating the NF-κB signaling pathway in NSCLC cells, the
details of the underlying regulatory mechanism still remain
unclear. Therefore, the targets of miR-218 in NF-κB pathway should
be investigated in future studies. The reasons for miR-218
expression level change in NSCLC, especially whether it was
affected due to low glucose metabolism caused by itself, should be
focused on.
In conclusion, the present results suggested miR-218
may inhibit glucose uptake, glycolysis and PPP in NSCLC cells by
reducing the expression or membrane translocation of rate-limiting
enzymes involved in glucose metabolism, in which NF-κB played a
critical role.
Supplementary Material
shRNA sequences.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key
Scientific Research Project of Colleges and Universities in Henan
Province (grant no. 16A320045) and the Program of Medical
Technology Plan in Henan Province (grant no. 2018020790).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY and QC designed the study. WT, XY and YS
performed the experiments. JZ and HW analyzed the data. LW and DL
contributed to constructing the engineering A549 cell lines and
writing the original draft. WT and QC revised and edited the
manuscript. All authors read and approved the final version of the
manuscript for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Janssen-Heijnen ML, van Erning FN, De
Ruysscher DK, Coebergh JW and Groen HJ: Variation in causes of
death in patients with non-small cell lung cancer according to
stage and time since diagnosis. Ann Oncol. 26:902–907.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Giatromanolaki A, Sivridis E, Arelaki S
and Koukourakis MI: Expression of enzymes related to glucose
metabolism in non-small cell lung cancer and prognosis. Exp Lung
Res. 43:167–174. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Patra KC, Wang Q, Bhaskar PT, Miller L,
Wang Z, Wheaton W, Chandel N, Laakso M, Muller WJ, Allen EL, et al:
Hexokinase 2 is required for tumor initiation and maintenance and
its systemic deletion is therapeutic in mouse models of cancer.
Cancer Cell. 24:213–228. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Patra KC and Hay N: The pentose phosphate
pathway and cancer. Trends Biochem Sci. 39:347–354. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Riganti C, Gazzano E, Polimeni M, Aldieri
E and Ghigo D: The pentose phosphate pathway: An antioxidant
defense and a crossroad in tumor cell fate. Free Radic Biol Med.
53:421–436. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pavlova NN and Thompson CB: The emerging
hallmarks of cancer metabolism. Cell Metab. 23:27–47.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li F, Han X, Li F, Wang R, Wang H, Gao Y,
Wang X, Fang Z, Zhang W, Yao S, et al: LKB1 Inactivation elicits a
redox imbalance to modulate non-small cell lung cancer plasticity
and therapeutic response. Cancer Cell. 27:698–711. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zeng C, Wu Q, Wang J, Yao B, Ma L, Yang Z,
Li J and Liu B: NOX4 supports glycolysis and promotes glutamine
metabolism in non-small cell lung cancer cells. Free Radic Biol
Med. 101:236–248. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhou L, Li M, Yu X, Gao F and Li W:
Repression of hexokinases II-mediated glycolysis contributes to
piperlongumine-induced tumor suppression in non-small cell lung
cancer cells. Int J Biol Sci. 15:826–837. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kitamura K, Ohata M, Narata M, Iida M,
Ohmori K, Irako M, Nakamura S, Natori H and Sezai Y: Surgical
treatment of bilateral spontaneous pneumothorax. Nihon Kyobu Geka
Gakkai Zasshi. 37:117–122. 1989.PubMed/NCBI(In Japanese).
|
|
12
|
Zhang JG, Guo JF, Liu DL, Liu Q and Wang
JJ: MicroRNA-101 exerts tumor-suppressive functions in non-small
cell lung cancer through directly targeting enhancer of zeste
homolog 2. J Thorac Oncol. 6:671–678. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang RT, Xu M, Xu CX, Song ZG and Jin H:
Decreased expression of miR216a contributes to non-small-cell lung
cancer progression. Clin Cancer Res. 20:4705–4716. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261.
2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Martinez I, Gardiner AS, Board KF, Monzon
FA, Edwards RP and Khan SA: Human papillomavirus type 16 reduces
the expression of microRNA-218 in cervical carcinoma cells.
Oncogene. 27:2575–2582. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Davidson MR, Larsen JE, Yang IA, Hayward
NK, Clarke BE, Duhig EE, Passmore LH, Bowman RV and Fong KM:
MicroRNA-218 is deleted and downregulated in lung squamous cell
carcinoma. PLoS One. 5(e12560)2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tatarano S, Chiyomaru T, Kawakami K,
Enokida H, Yoshino H, Hidaka H, Yamasaki T, Kawahara K, Nishiyama
K, Seki N and Nakagawa M: miR-218 on the genomic loss region of
chromosome 4p15.31 functions as a tumor suppressor in bladder
cancer. Int J Oncol. 39:13–21. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zeng XJ, Wu YH, Luo M, Cong PG and Yu H:
Inhibition of pulmonary carcinoma proliferation or metastasis of
miR-218 via down-regulating CDCP1 expression. Eur Rev Med Pharmacol
Sci. 21:1502–1508. 2017.PubMed/NCBI
|
|
19
|
Chiu KL, Kuo TT, Kuok QY, Lin YS, Hua CH,
Lin CY, Su PY, Lai LC and Sher YP: ADAM9 enhances CDCP1 protein
expression by suppressing miR-218 for lung tumor metastasis. Sci
Rep. 5(16426)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zarogoulidis P, Petanidis S, Kioseoglou E,
Domvri K, Anestakis D and Zarogoulidis K: miR-205 and miR-218
expression is associated with carboplatin chemoresistance and
regulation of apoptosis via Mcl-1 and Survivin in lung cancer
cells. Cell Signal. 27:1576–1588. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li P, Yang X, Cheng Y, Zhang X, Yang C,
Deng X, Li P, Tao J, Yang H, Wei J, et al: MicroRNA-218 increases
the sensitivity of bladder cancer to cisplatin by targeting Glut1.
Cell Physiol Biochem. 41:921–832. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yuan X, Tian W, Hua Y, Hu L, Yang J, Xie
J, Hu J and Wang F: Rhein enhances the cytotoxicity of effector
lymphocytes in colon cancer under hypoxic conditions. Exp Ther Med.
16:5350–5358. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jiang J, Geng G, Yu X, Liu H, Gao J, An H,
Cai C, Li N, Shen D, Wu X, et al: Repurposing the anti-malarial
drug dihydroartemisinin suppresses metastasis of non-small-cell
lung cancer via inhibiting NF-κB/GLUT1 axis. Oncotarget.
7:87271–87283. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yang Y, Ding L, Hu Q, Xia J, Sun J, Wang
X, Xiong H, Gurbani D, Li L, Liu Y and Liu A: MicroRNA-218
functions as a tumor suppressor in lung cancer by targeting
IL-6/STAT3 and negatively correlates with poor prognosis. Mol
Cancer. 16(141)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Abbaszadeh Z, Çeşmeli S and Biray Avcı Ç:
Crucial players in glycolysis: Cancer progress. Gene.
726(144158)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yan Y, Yan F and Huang Q: miR-200c
inhibited the proliferation of oral squamous cell carcinoma cells
by targeting Akt pathway and its downstream Glut1. Arch Oral Biol.
96:52–57. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Santasusagna S, Moreno I, Navarro A, Muñoz
C, Martinez F, Hernández R, Castellano JJ and Monzo M: miR-328
mediates a metabolic shift in colon cancer cells by targeting
SLC2A1/GLUT1. Clin Transl Oncol. 20:1161–1167. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhou Y, Zheng X, Lu J, Chen W, Li X and
Zhao L: Ginsenoside 20(S)-Rg3 inhibits the warburg effect via
modulating DNMT3A/ miR-532-3p/HK2 pathway in ovarian cancer cells.
Cell Physiol Biochem. 45:2548–2559. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang Y, Ishak Gabra MB, Hanse EA, Lowman
XH, Tran TQ, Li H, Milman N, Liu J, Reid MA, Locasale JW, et al:
miR-135 suppresses glycolysis and promotes pancreatic cancer cell
adaptation to metabolic stress by targeting phosphofructokinase-1.
Nat Commun. 10(809)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
He C and Yang J, Ding J, Li S, Wu H, Xiong
Y, Zhou F, Jiang Y, Teng L and Yang J: Downregulation of
glucose-6-phosphate dehydrogenase by microRNA-1 inhibits the growth
of pituitary tumor cells. Oncol Rep. 40:3533–3542. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yang J, Kou J, Lim JE, Lalonde R and
Fukuchi KI: Intracranial delivery of interleukin-17A via
adeno-associated virus fails to induce physical and learning
disabilities and neuroinflammation in mice but improves glucose
metabolism through AKT signaling pathway. Brain Behav Immun.
53:84–95. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Song DH, Getty-Kaushik L, Tseng E, Simon
J, Corkey BE and Wolfe MM: Glucose-dependent insulinotropic
polypeptide enhances adipocyte development and glucose uptake in
part through Akt activation. Gastroenterology. 133:1796–1805.
2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Belgardt BF, Mauer J, Wunderlich FT, Ernst
MB, Pal M, Spohn G, Brönneke HS, Brodesser S, Hampel B, Schauss AC
and Brüning JC: Hypothalamic and pituitary c-Jun N-terminal kinase
1 signaling coordinately regulates glucose metabolism. Proc Natl
Acad Sci USA. 107:6028–6033. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xu G, Wang Z, Li Y, Li Z, Tang H, Zhao J,
Xiang X, Ding L, Ma L, Yuan F, et al: Ghrelin contributes to
derangements of glucose metabolism induced by rapamycin in mice.
Diabetologia. 55:1813–1823. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang W, Thompson BJ, Hietakangas V and
Cohen SM: MAPK/ERK signaling regulates insulin sensitivity to
control glucose metabolism in Drosophila. PLoS Genet.
7(e1002429)2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li Q, Li Y, Liang L1, Li J, Luo D, Liu Q,
Cai S and Li X: Klotho negatively regulated aerobic glycolysis in
colorectal cancer via ERK/HIF1α axis. Cell Commun Signal.
16(26)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mauro C, Leow SC, Anso E, Rocha S,
Thotakura AK, Tornatore L, Moretti M, De Smaele E, Beg AA,
Tergaonkar V, et al: NF-κB controls energy homeostasis and
metabolic adaptation by upregulating mitochondrial respiration. Nat
Cell Biol. 13:1272–1279. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
40
|
Kawauchi K, Araki K, Tobiume K and Tanaka
N: p53 regulates glucose metabolism through an IKK-NF-kappaB
pathway and inhibits cell transformation. Nat Cell Biol.
10:611–618. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
41
|
Gilmore TD: Introduction to NF-kappaB:
players, pathways, perspectives. Oncogene. 25:6680–6684.
2006.PubMed/NCBI View Article : Google Scholar
|