Introduction
Anesthetic preconditioning (APC) refers to exposure
of the heart to a volatile anesthetic followed by its washout
(1,2). We previously demonstrated that APC
protects the heart against subsequent ischemia-reperfusion (IR)
injury (1,2). Although APC is an effective strategy
to reduce myocardial injury, its exact underlying mechanism remains
poorly understood.
The autonomic balance between the sympathetic and
parasympathetic nervous systems plays an important role in the
regulation of the cardiovascular system (3). Acetylcholine (ACh) is the main
neurotransmitter of the vagus nerve. It may mimic the effects of
myocardial ischemic preconditioning (IPC) and protect the heart
against myocardial IR injury (4).
This indicates that the activation of ACh receptors is involved in
cardioprotective signaling pathways (4-6).
There are two main types of cholinergic receptors in the heart,
namely, the muscarinic (mAChR) and the nicotinic receptors (nAChR)
(4,7,8).
Evidence has shown that through pharmacological or direct-current
electrical stimulation, both mAChRs and nAChRs can trigger
signaling pathways that protect the heart against IR injury
(9,10).
Nitric oxide (NO) is an endogenous regulatory
molecule involved in various physiological processes (11). The endogenous NO synthase (eNOS)
pathway is expressed in isolated cardiomyocytes and regulates the
negative chronotropic effects of cholinergic receptor stimulation
(12). In addition, activation of
the neuronal NOS (nNOS)/AMP-activated protein kinase (AMPK)/mTOR
pathway has been linked to the cardioprotective effects caused by
IPC (13). ACh prevents
cardiomyocyte damage by activating AMPK and inhibiting reactive
oxygen species (ROS) formation (14). Recently, it was reported that during
myocardial ischemia, vagus nerve stimulation (VNS) may activate
AMPK, leading to the phosphorylation of
calcium/calmodulin-dependent protein kinase β (CaMKKβ) (15). This suggested that the CaMKKβ/AMPK
signaling pathway is involved in VNS-mediated protective effects.
However, whether and how APC regulates cholinergic receptors to
prevent IR injury and improve cardiac function remains unclear. ACh
initiates downstream signaling by activating G-protein-coupled
mAChRs or by binding to nAChRs, which are ligand-gated ion channels
(16). Complex neural processing
occurs within the heart, not only in response to central efferent
input, but also to sensory afferent information from the myocardium
(17). Nevertheless, whether APC
exerts cardioprotection through the upregulation of cholinergic
receptors in the isolated heart remains to be determined.
Therefore, the aim of the present study was to determine the role
of cholinergic receptors in APC-induced cardioprotection against IR
injury in an isolated rat heart model. In addition, whether the NOS
and CaMKKβ/AMPK pathways are involved in the beneficial effects of
cholinergic receptor activation was also examined.
Materials and methods
Animals
The present study was approved by The Institutional
Animal Care and Use Committee of the Affiliated Suzhou Science
& Technology Town Hospital of Nanjing Medical University. Male,
8-10-week-old Sprague-Dawley rats (250±50 g) were purchased from
the Animal Center of Suzhou University and housed under a 12-h
light/dark cycle at 25˚C and 60% humidity. All rats were provided
with food and water ad libitum. All animals were treated in
accordance with the National Institutes of Health's Guidelines for
the Care and Use of Experimental Animals.
Isolated heart preparation
Preparation of isolated heart was performed as
described previously (2,18). Briefly, rats were intraperitoneally
anesthetized with 50 mg/kg pentobarbital sodium, then decapitated
when they did not respond to a noxious stimulus to the hind limb.
The heart was excised and perfused using the Langendorff method at
a perfusion pressure of 80 mmHg. A thermostatically controlled
water circulator (Lauda E100; Lauda) was used to maintain the
temperature of the perfusion and bath at 37.2±0.1˚C. Left
ventricular pressure (LVP) was measured using a volume equalizer of
a saline-filled latex balloon connected to the left atrium through
a mitral valve into the left ventricle. The heart was immersed in
aerated physiological buffer solution at 37.2˚C for 30 min of
global ischemia and then reperfused for 120 min. At the end of the
experiment, the heart was frozen and kept at -80˚C until use.
Experimental protocols
The present experimental protocols were similar to
our previous study (2). Each
experiment lasted 220 min and a total of 60 rats were used. After
30 min of perfusion, when functional parameters reached equilibrium
(steady state); the hearts were randomly divided into five groups
(n=12 hearts in each group): i) Untreated sham group: Continuous
perfusion for 190 min without ischemia or drug administration; ii)
IR group, after an additional 40 min of perfusion, hearts received
30 min of global ischemia and 120 min of reperfusion; iii) APC
group, 3.5% sevoflurane (Abbott Pharmaceutical Co. Ltd.) was
administered for 15 min, then washed out for 15 min priorto
ischemia; iv) an atropine (ATR; mAChR antagonist; 100 nM;
Sigma-Aldrich; Merck KGaA) group, used based on its affinity to
mAChR (KD=0.36 nM) and administered 10 min before APC
and v) an hexamethonium (HEM; nAChR antagonist; Sigma-Aldrich;
Merck KGaA) group, 50 µM was used to achieve specificity at nAChRs
within the cardiac ganglia.
Sevoflurane was bubbled into the perfusate using an
agent-specific vaporizer placed in the O2-CO2
gas mixture line. Samples of coronary perfusate were collected from
a port in the aortic cannula to measure sevoflurane concentration
by gas chromatography. Inflow sevoflurane concentration was
0.64±0.02 mM, which is equivalent to 3.34±0.22% atmosphere and a
minimal alveolar concentration of 1.5±0.4% (1). The ATR and HEM groups were used to
evaluate the effects of cholinergic receptors. ATR and HEM doses
were selected according to a previous study (17).
Measurement of hemodynamic
function
Hemodynamic parameters were monitored throughout the
experiment. After 30 min of ischemia, hemodynamic function was
assessed by determining the left ventricular developed pressure
(LVDP), left ventricular end-diastolic pressure (LVEDP) and the
maximal and minimal derivatives of LVP (+dP/dt and -dP/dt). These
parameters represent the major indices of myocardial contractility
and relaxation. In particular, LVEDP is not only a marker of
diastolic function but also a good predictor of cardiac
mortality.
Myocardial high-energy phosphate
analysis
At the end of reperfusion, the heart was frozen
using aluminum forceps pre-cooled in liquid nitrogen to measure
myocardial ATP and creatine phosphate (CP) levels, as described
previously (19). Cardiac CP is
converted to ATP by the enzymatic reaction of creatine kinase
(20). Briefly, frozen ventricles
were ground and mixed with 0.3 M HClO4 and 0.25 mM EDTA
under liquid nitrogen cooling. The extract was centrifuged at 8,000
x g for 15 min at 4˚C, and the resulting supernatant was sampled
using high-pressure liquid chromatography to measure myocardial ATP
and CP.
Determination of NOS activity and NO
levels
At the end of the experiment, the heart was removed
and homogenized in a 0.9% ice-cold saline solution, then
centrifuged at 600 x g for 10 min at 4˚C. NOS activity and NO
levels were measured using a diagnostic assay kit (Nanjing
Jiancheng Bioengineering Institute). The absorbance at a wavelength
of 530 nm was measured using a DU-640 spectrophotometer (Beckman
Coulter, Inc.), and normalized to the control according to the
manufacturer's instructions (11).
Western blot analysis
Western blot analysis was performed as described
previously (2). Hearts were
homogenized using RIPA lysis buffer (cat. no. 20-188; EMD
Millipore) and a complete mammalian proteinase inhibitor cocktail
(cat. no. PI101; Roche Diagnostics GmbH) and then centrifuged at
13,200 x g for 20 min at 4˚C. A BCA assay kit (cat. no. P0010;
Beyotime Institute of Biotechnology) was used to determine the
protein concentration. After denaturation, 20 µg of each sample was
dissolved in Laemmli sample buffer (cat. no. S3401; Sigma-Aldrich;
Merck KGaA) and separated using SDS-PAGE on a 10% gel. The samples
were then transferred to a nitrocellulose membrane, which was
blocked with 5% skim milk in PBS for 1 h at room temperature, The
membranes were subsequently incubated with primary antibody at 4˚C
overnight, and then incubated with HRP-conjugated goat anti-rabbit
secondary antibody (1:10,000; cat. no. ab6721; Abcam) or
HRP-conjugated goat anti-mouse secondary antibody (1:10,000; cat.
no. sc-2031; Santa Cruz Biotechnology, Inc.) at room temperature
for 1 h. Protein bands were visualized using a SuperSignal West
Pico kit (cat. no. 34577; Pierce; Thermo Fisher Scientific, Inc.).
Band density was quantified using UN-SCAN-IT software (v.7.0; Silk
Scientific, Inc.). The primary antibodies were specific for eNOS
(cat. no. sc-376751; 1:1,000; Santa Cruz Biotechnology, Inc.), nNOS
(cat. no. sc-5302; 1:1,000; Santa Cruz Biotechnology, Inc.); AMPK
(cat. no. 2352; 1:1,000; Cell Signaling Technology, Inc.);
phosphorylated (p)-AMPK (Thr172; cat. no. 5884; 1:1,000; Cell
signaling Technology, Inc.); p-CaMKK2 (Ser511; cat. no. AF4487;
1:1,000; Affinity Biosciences) and CAMKK2 (polyclonal antibody;
cat. no. DF4793; 1:1,000; Affinity Biosciences); as well as the
housekeeping protein GAPDH (cat. no. AG019; 1:1,000; Shanghai
Biyuntian Biotechnology Co., Ltd.).
Statistical analysis
Data are presented as the mean ± SD. Each experiment
was repeated at least three times. SPSS 19.0 (IBM Corp.) was used
to conduct statistical analyses. One-way ANOVA was used to compare
the differences among five groups, followed by Tukey's post-hoc
test to determine the differences between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Cardiac performance
The LVDP significantly decreased following IR
compared with the sham group (Fig.
1A). Moreover, the LVDP significantly increased in the APC
group compared with the IR group. By contrast, APC-induced
improvement of LVDP was significantly inhibited by ATR or HEM
treatment (P<0.05). Hearts subjected to IR displayed a
significant increase in LVDP (Fig.
1B). However, a significant reduction in LVEDP was observed
after APC treatment. The effect of APC treatment was inhibited by
ATR or HEM treatment. Cardiac contractility (+dP/dt) and relaxation
(-dP/dt) (Fig. 1C and D) were
reduced during ischemia in all groups. Following reperfusion,
contractility increased but still remained lower than that recorded
before ischemia throughout reperfusion in each group. APC
significantly improved contractile recovery in IR hearts, which was
also abolished by ATR or HEM treatment.
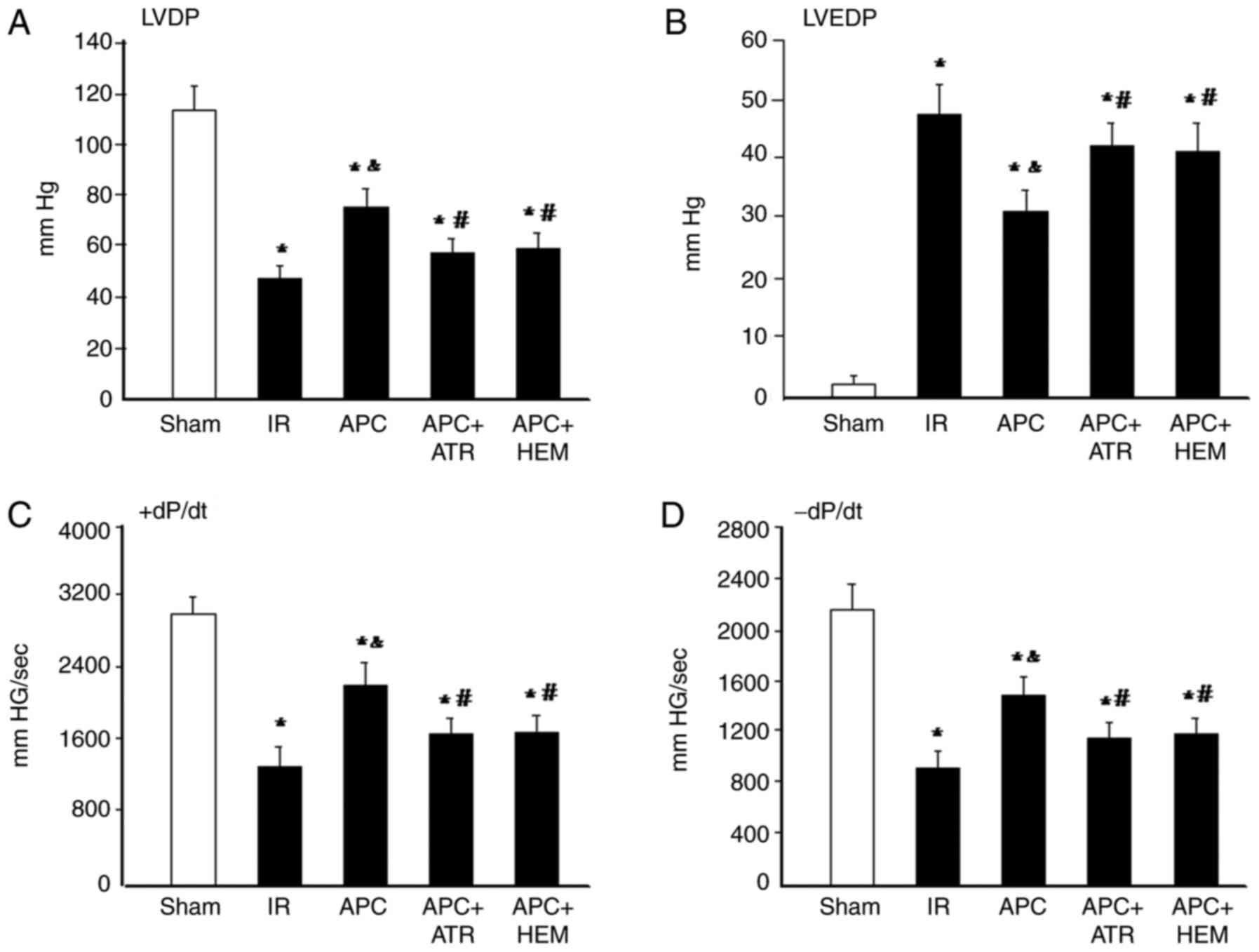 | Figure 1LVDP, LVEDP, +dP/dt and -dP/dt after
IR. (A) LVDP, (B) LVEDP, (C) +dP/dt and (D) -dP/dt after 30 min
ischemia and 120 min reperfusion. Values are presented as the mean
± standard deviation. *P<0.05 vs. Sham group;
&P<0.05 vs. IR group; #P<0.05 vs.
APC group. LVDP, left ventricular developed pressure; LVEDP, left
ventricular end-diastolic pressure; ±dP/dt, the maximal and minimal
derivatives of LVDP; IR, ischemia-reperfusion; APC, anesthetic
preconditioning; ATR, atropine; HEM, hexamethonium. |
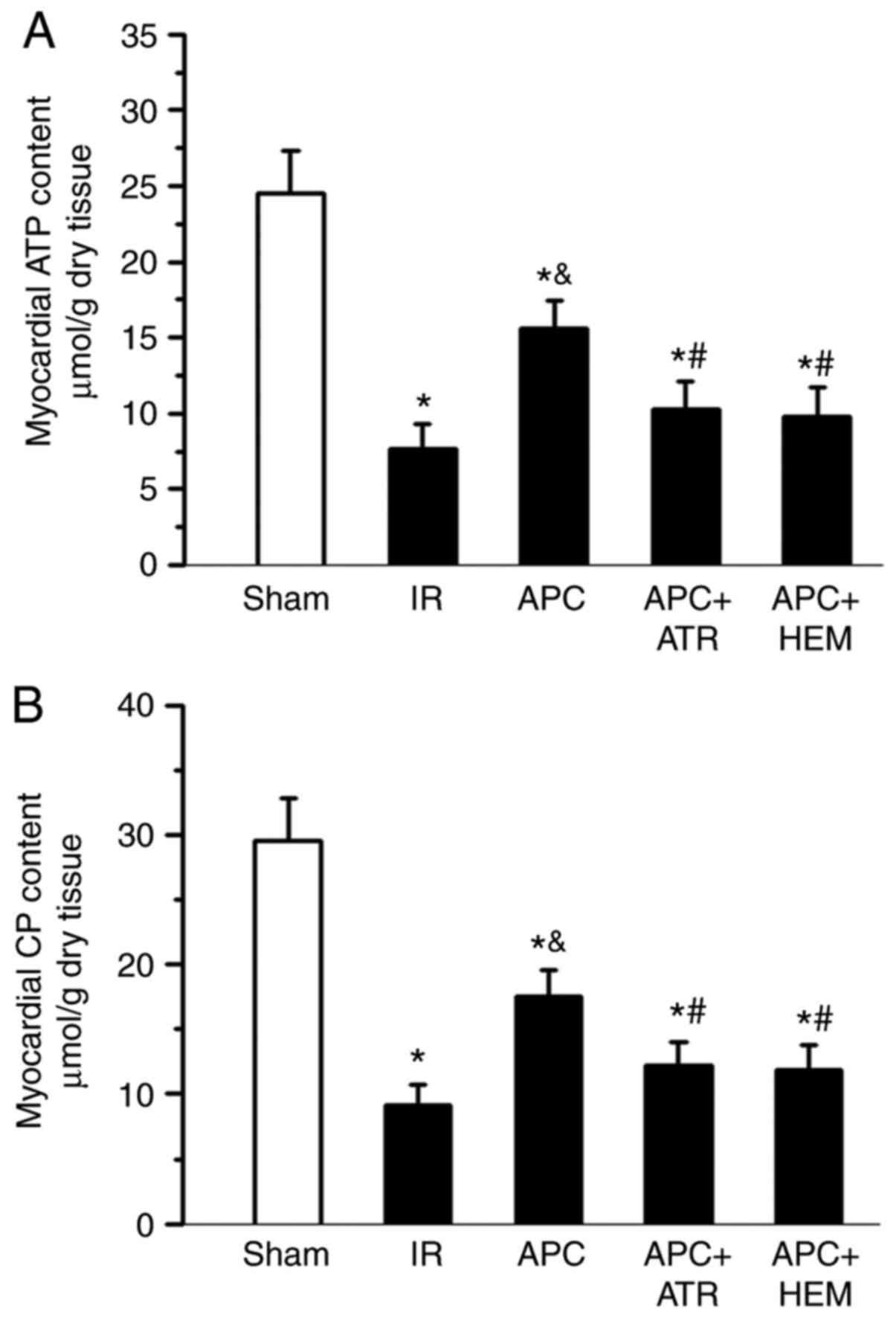
ATP and CP content
At the end of reperfusion, the ATP and CP content
were determined in trans-mural sections obtained from the left
ventricular free wall (Fig. 2). In
the IR groups, the ATP content was significantly reduced (7.7±1.6
µmol/g) compared with the sham group (24.5±2.8 µmol/g; Fig. 2A). In addition, the CP content was
also significantly decreased in the IR group compared with the sham
group (9.2±1.5 vs. 29.6±3.3 µmol/g; Fig. 2B). However, the levels of ATP and CP
were better preserved in the APC-treated group (15.6±1.8 and
17.5±2.1 µmol/g) compared with the IR group. Both the mAChR
antagonist ATR and nAChR antagonist HEM abolished the preserving
effects of APC.
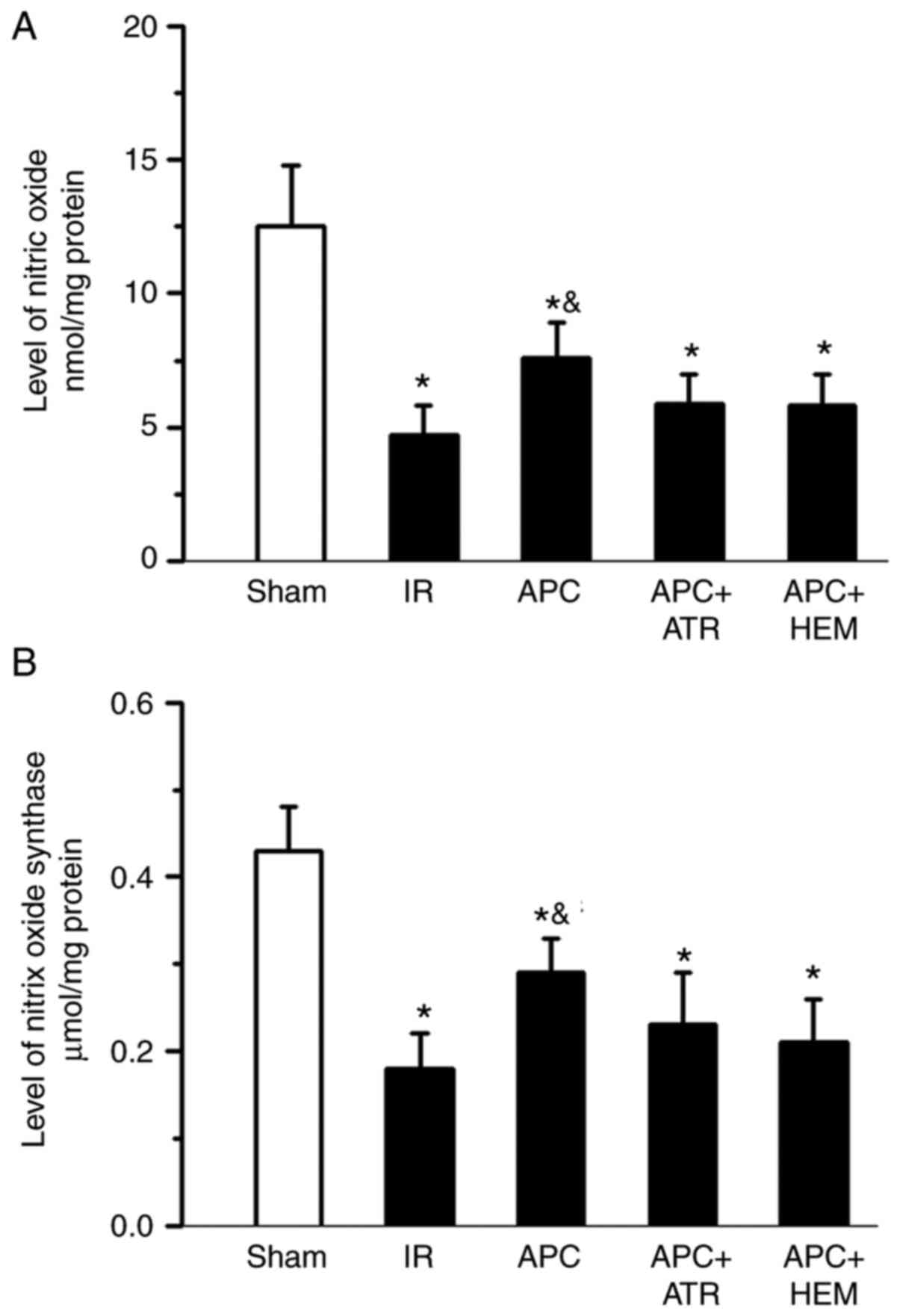
Effects of APC on NOS activity and NO
content
Following IR, both NOS activity and NO levels were
significantly decreased in the IR group compared with the sham
group (Fig. 3). Moreover, NOS
activity and NO levels significantly increased in the APC group
compared with the IR group (Fig. 3A
and B). However, ATR and HEM
treatment abolished the APC-induced increase in NOS activity and NO
levels. These results indicated that both NOS activity and NO
levels were decreased following IR injury, but increased after APC
treatment. However, the effects of APC were reversed by ATR and HEM
administration.
Effects of APC on eNOS and nNOS
phosphorylation
Western blotting bands of eNOS, p-eNOS, nNOS and
phosphorylated nNOS (p-nNOS) protein in Fig. 4A. The percentile ratio of
p-eNOS/total eNOS and p-nNOS/total nNOS are displayed in Fig. 4B and C. There was a significant increase in eNOS
(Fig. 4B) and nNOS (Fig. 4C) phosphorylation in the IR group
compared with the sham group. Treatment with APC further increased
the phosphorylation of eNOS and nNOS (APC group vs. IR group).
However, this effect was significantly attenuated following ATR and
HEM administration (APC+ATR group or APC+HEM group vs. APC
group).
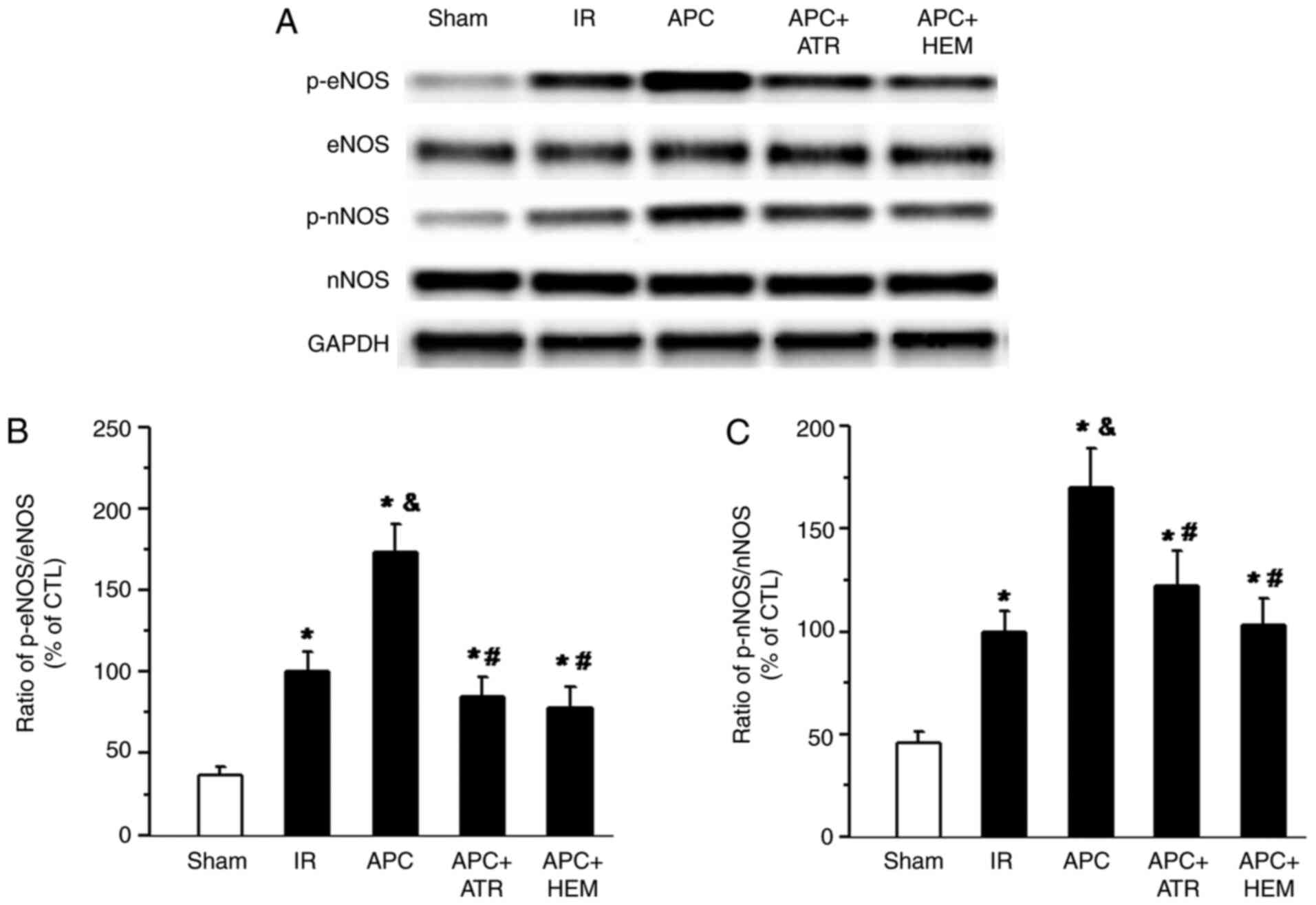 | Figure 4Western blot analysis of eNOS, nNOS,
p-eNOS and p-nNOS levels in the homogenates of myocardial tissue
from rat hearts. (A) Western blot bands of eNOS, p-eNOS, nNOS and
p-nNOS. The ratio of (B) p-eNOS/total eNOS and (C) p-nNOS/total
nNOS. APC-induced increases of the phosphorylation of eNOS and nNOS
were reduced by the muscarinic acetylcholine receptor antagonist
ATR (100 nM) and nicotinic acetylcholine receptor antagonist HEM
(50 µM). The data are presented as the mean ± SD. n=5 hearts/group.
*P<0.05 vs. Sham group; &P<0.05 vs.
IR group; #P<0.05 vs. APC group. eNOS, endogenous
nitric oxide synthase; nNOS, neuronal NOS; p, phosphorylated; IR,
ischemia-reperfusion; APC, anesthetic preconditioning; ATR,
atropine; HEM, hexamethonium; CTL, control. |
Effects of SPC on AMPK and CaMKKβ
phosphorylation
Western blotting bands of AMPK and p-AMPK(Thr172)
protein and the ratio of p-AMPK(Thr172)/total AMPK are presented in
Fig. 5A. Western blotting bands of
CAMKKβ and p-CAMKKβ (Ser511) protein and the ratio of p-CAMKKβ
(Ser511)/total CAMKKβ are displayed in Fig. 5B. There was a significant decrease
in AMPK (Fig. 5A) and CAMKKβ
(Fig. 5B) phosphorylation in the IR
group compared with the sham group. Treatment with APC
significantly increased the phosphorylation of AMPK and CAMKKβ (APC
group vs. IR group). Following ATR and HEM administration, this
effect was significantly reduced (APC+ATR group or APC+HEM group,
vs. APC group).
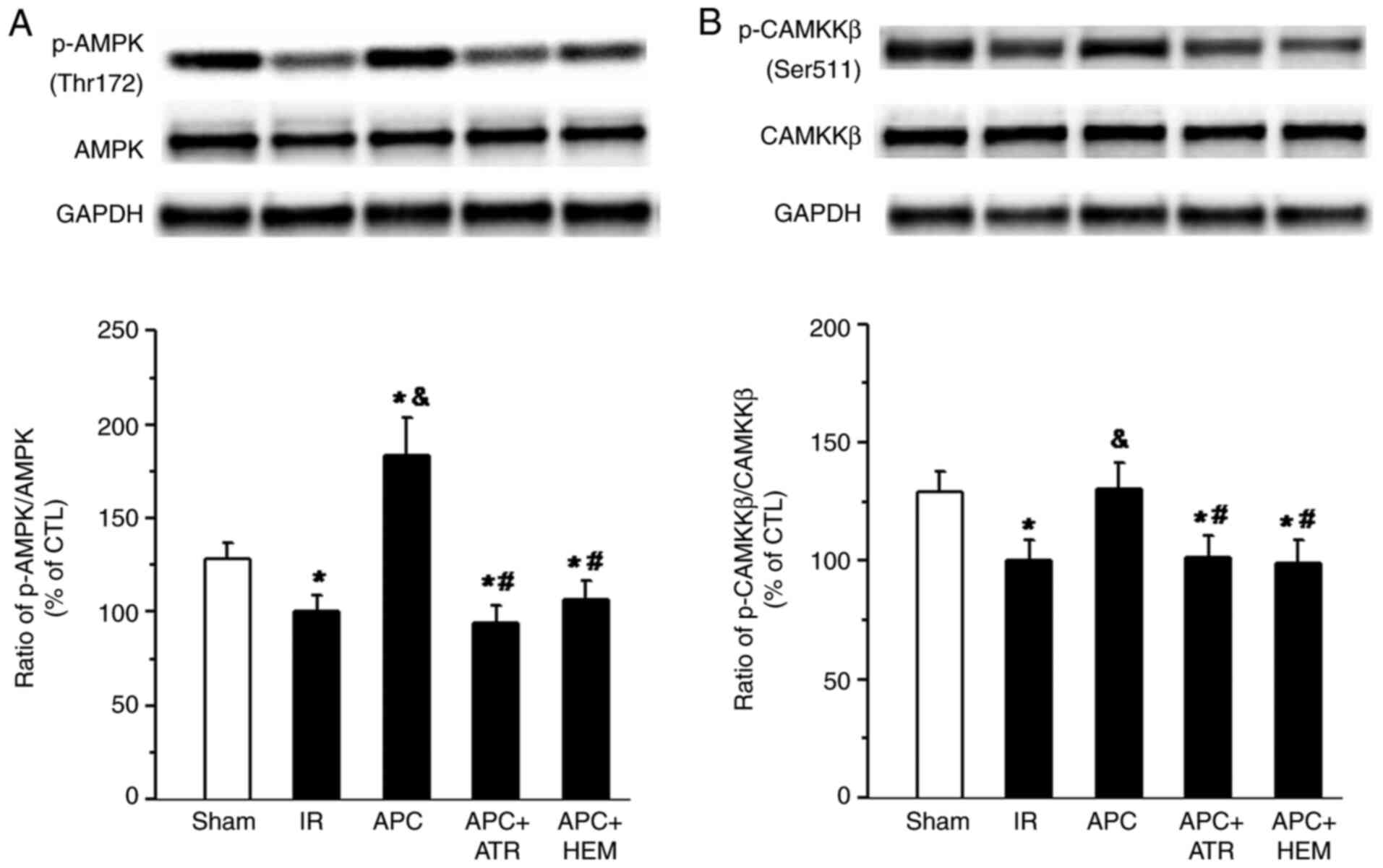 | Figure 5Western blot analysis of AMPK,
CAMKKβ, AMPK phosphorylation at Thr-172 and CAMKKβ phosphorylation
at Ser-511 in the homogenates of myocardial tissue from rat hearts.
(A) Western blot bands of AMPK and p-AMPK(Thr172) protein and the
ratio of p-AMPK(Thr172)/total AMPK. (B) Western blot bands of
CAMKKβ and p-CAMKKβ(Ser511) protein and the ratio of
p-CAMKKβ(Ser511)/total CAMKKβ. APC-induced increases of AMPK and
CAMKKβ phosphorylation were reduced by the muscarinic acetylcholine
receptor antagonist atropine (ATR, 100 nM) and nicotinic
acetylcholine receptor antagonist hexamethonium (HEM, 50 µM). The
data are presented as the mean ± standard deviation. n=5
hearts/group. *P<0.05 vs. Sham group;
&P<0.05 vs. IR group; #P<0.05 vs.
APC group. CAMKKβ, calcium/calmodulin-dependent protein kinase
kinase β; p, phosphorylated; IR, ischemia-reperfusion; APC,
anesthetic preconditioning; ATR, atropine; HEM, hexamethonium; CTL,
control. |
Discussion
Our recent study demonstrated that APC reduced
myocardial enzyme release and infarct size by enhancing the
recovery of cardiac function, thereby reducing myocardial damage
after IR (2). Possible mechanisms
underlying the role of cholinergic receptors in alleviating IR
injury have been proposed in several previous studies, including
NOS and ROS-mediated CaMKII pathways (14,15,21,22).
However, whether and how APC regulates the intrinsic cardiac
nervous system to improve cardiac function remains unknown. The
current study demonstrated that both mAChRs and nAChRs participate
in APC-induced cardioprotection in isolated rat hearts following
IR. ATR and HEM attenuated the protective effects of APC against IR
injury, highlighting the importance of cholinergic receptors in the
mechanism of APC-induced cardioprotection. Thus, the present
findings indicated that APC plays a cardioprotective role, in part,
by regulating neurohumoral pathways. In addition, NOS and
CaMKKβ/AMPK may be involved in shared pathways that mediate the
cardioprotective mechanisms of APC.
Previous studies suggested that increased vagal
nerve activity could reduce myocardial IR injury (3,4,7,23).
The main vagal neurotransmitter ACh can replicate the
cardioprotective effects of IPC (17,24,25).
Upon pharmacological or direct-current stimulation, both mAChR and
nAChR can trigger cardioprotective signaling cascades, which are
effective against I/R injury (21).
In addition, previous studies have demonstrated that cardiomyocytes
synthesized and secreted ACh, which provided further evidence for
the importance of non-neurocholinergic signaling cascades in the
maintenance of myocardial function in physiological and
pathological states (26-28).
A recent study examined the role of the intrinsic cardiac nervous
system in the classic myocardial IPC mechanism and demonstrated
that intrinsic cardiac ganglia remain intact in isolated hearts
subjected to IR injury (17). In
addition, IPC activated the intrinsic cardiac nerve reflex, leading
to the release of ACh in the ventricle and inducing protective
effects through the activation of cholinergic receptors. Treatment
with ATR and HEM also blocked the protective effects of IPC
(17). The present study was
consistent with these previous findings. APC reduced IR injury by
activating intrinsic cardiac cholinergic receptors, and this
protective effect was abrogated by ATR and HEM, indicating that APC
protected the heart against IR injury via intrinsic neuronal
mechanisms.
NO plays a number of beneficial roles during
myocardial reperfusion, including regulating myocardial
contractility, opening KATP channels to the sarcolemma
and mitochondria, antioxidant effects and oxygen free radical
production (29,30). Our previous study indicated that NO
played a vital role in the cardioprotective effects of APC
(11). APC alleviated cardiac
dysfunction caused by IR, reduced the area of infarction after
ischemia, and led to higher levels of eNOS and nNOS
phosphorylation, NOS content and NO production. The present study
is consistent with our previous reports (31,32).
NO, a neurosensor of parasympathetic nerves, had a significant
effect on the promotion of vagus nerves by increasing the release
of ACh and reducing the downstream effects of catecholamines on
heart rate and contractility (33).
Thus, control of cardiac contraction through eNOS activation may
represent an important function of cholinergic receptor activation
(34). In the present study, the
mAChR antagonist ATR and the nAChR antagonist HEM eliminated the
effects of APC on eNOS and nNOS phosphorylation, increased NOS
content and NO production and antagonized the protective effects of
APC on the heart. These results indicated that eNOS and nNOS
phosphorylation is one of the downstream pathways of APC-induced
cholinergic receptor activation and cardioprotection.
AMPK is a key cellular energy sensor and regulator
of metabolic homeostasis (15).
Increasing evidence suggested that AMPK dysfunction is associated
with the occurrence and development of a number of cardiovascular
diseases, including atherosclerosis, myocardial IR injury and
cardiac remodeling (35-37).
Activation of AMPK protected the myocardium from IR injury by
regulating mitochondrial function (38) and preventing myocardial necrosis and
systolic dysfunction (39). Other
studies reported that VNS activates AMPK and is accompanied by
CaMKKβ phosphorylation during myocardial ischemia (15). These findings suggested that the
intrinsic cardiac nervous system may be involved in APC-mediated
cardioprotective effects against IR injury through the cholinergic
receptor and CaMKKβ/AMPK signaling during myocardial IR. The
present study also demonstrated that APC significantly increased
the phosphorylation of AMPK and CaMKKβ, and that the administration
of ATR and HEM could suppress this increase in phosphorylation.
This indicated that the activation of the CaMKKβ/AMPK signaling
pathway by cholinergic receptors was involved in APC-mediated
cardioprotection. Taken together, these results represented an
important complement to the understanding of the role of
cholinergic receptors in APC-induced cardioprotection against IR
injury. Nonetheless, the myocardial protective properties of
volatile anesthetics, including sevoflurane, may also be due to
their cardiosuppressive nature. These cardiac depressant effects
decrease myocardial oxygen demand and may thus improve the
myocardial oxygen balance during ischemia (40). A previous study demonstrated that
both isoflurane and sevoflurane increased coronary blood flow and
decreased coronary vascular resistance, including resistance
through the collateral circulation (41). Therefore, further research is
required to ascertain the effects of APC on the microcirculation
under myocardium ischemia conditions.
The present study has its limitations. Although HEM
is widely used as a nAChR antagonist, it also has low affinity to
M2 receptors (17).
Therefore, in the present study, 50 µM was used in order to achieve
high specificity to nicotinic receptors. Nevertheless, the
potential nonspecific effects of HEM still remains a possibility.
Moreover, the interaction between NO and the CaMKKβ/AMPK signaling
pathway is still unclear. In addition, it was reported that
cholinergic receptor activation also plays a cardioprotective role
through other pathways, such as, phosphoinositide 3-kinase and
Erk1/2 signaling (42), Bcl-2
family proteins and caspase-3-related pathways (42), as well as Akt and GSK-3β enzyme
activity (43). The interaction
between these signal pathways is still poorly understand. Lastly,
cholinergic receptor activation has previously been reported to
mediate apoptosis and oxidative stress during I/R-induced cell
injury (21). However, how
apoptosis, oxidative stress and CaMKKβ/AMPK pathways regulate
cholinergic receptors to resist IR injury through APC was not
evaluated in the present study.
In summary, the present study demonstrated that APC
could protect cardiac function against IR injury through the
activation of cholinergic receptor-mediated eNOS, nNOS and
CaMKKβ/AMPK phosphorylation. The present findings may provide
insight into novel mechanisms of APC-induced cardioprotection
against IR injury.
Acknowledgements
The authors acknowledge Dr Tuanjie Che (Precision
Medicine and Translational Medicine Laboratory, Suzhou Science
& Technology Town Hospital, China) for his technical assistance
in the study.
Funding
The present study was supported by the Suzhou
Science and Technology Development Plan (grants nos. SS201756 and
SS201613); the Suzhou New District Science and Technology Project
(grant no. 2017Z004) and the National Science and Technology
Development Plan (grant no. NSFC 81703501).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY, YL, CW and JA were responsible for experimental
design, data collection, data analysis and manuscript writing. YY,
YL, JW, LH and SQ performed the experiments. JA revised manuscript
critically for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Institutional
Animal Care and Use Committee of the Affiliated Suzhou Science
& Technology Town Hospital of Nanjing Medical University (grant
no. IRB2018032).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
An J, Varadarajan SG, Novalija E and Stowe
DF: Ischemic and anesthetic preconditioning reduces cytosolic
[Ca2+] and improves Ca(2+) responses in intact hearts. Am J Physiol
Heart Circ Physiol. 281:H1508–H1523. 2001.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang C, Qiao S, Hong L, Sun J, Che T, An J
and Camara AKS: NOS cofactor tetrahydrobiopterin contributes to
anesthetic preconditioning induced myocardial protection in the
isolated ex vivo rat heart. Int J Mol Med. 45:615–622.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang J, Yong Y, Li X, Hu Y, Wang J, Wang
YQ, Song W, Chen WT, Xie J, Chen XM, et al: Vagal modulation of
high mobility group box-1 protein mediates
electroacupuncture-induced cardioprotection in ischemia-reperfusion
injury. Sci Rep. 5(15503)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nuntaphum W, Pongkan W, Wongjaikam S,
Thummasorn S, Tanajak P, Khamseekaew J, Intachai K, Chattipakorn
SC, Chattipakorn N and Shinlapawittayatorn K: Vagus nerve
stimulation exerts cardioprotection against myocardial
ischemia/reperfusion injury predominantly through its efferent
vagal fibers. Basic Res Cardiol. 113(22)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhao M, He X, Bi XY, Yu XJ, Gil Wier W and
Zang WJ: Vagal stimulation triggers peripheral vascular protection
through the cholinergic anti-inflammatory pathway in a rat model of
myocardial ischemia/reperfusion. Basic Res Cardiol.
108(345)2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang Z, Yu L, Wang S, Huang B, Liao K,
Saren G, Tan T and Jiang H: Chronic intermittent low-level
transcutaneous electrical stimulation of auricular branch of vagus
nerve improves left ventricular remodeling in conscious dogs with
healed myocardial infarction. Circ Heart Fail. 7:1014–1021.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Uitterdijk A, Yetgin T, te Lintel Hekkert
M, Sneep S, Krabbendam-Peters I, van Beusekom HM, Fischer TM,
Cornelussen RN, Manintveld OC, Merkus D and Duncker DJ: Vagal nerve
stimulation started just prior to reperfusion limits infarct size
and no-reflow. Basic Res Cardiol. 110(508)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang R, Wugeti N, Sun J, Yan H, Guo Y,
Zhang L, Ma M, Guo X, Jiao C, Xu W, et al: Effects of vagus nerve
stimulation via cholinergic anti-inflammatory pathway activation on
myocardial ischemia/reperfusion injury in canine. Int J Clin Exp
Med. 7:2615–2623. 2014.PubMed/NCBI
|
|
9
|
Calvillo L, Vanoli E, Andreoli E, Besana
A, Omodeo E, Gnecchi M, Zerbi P, Vago G, Busca G and Schwartz PJ:
Vagal stimulation, through its nicotinic action, limits infarct
size and the inflammatory response to myocardial ischemia and
reperfusion. J Cardiovasc Pharmacol. 58:500–507. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kiss A, Tratsiakovich Y, Mahdi A, Yang J,
Gonon AT, Podesser BK and Pernow J: Vagal nerve stimulation reduces
infarct size via a mechanism involving the -7 nicotinic
acetylcholine receptor and downregulation of cardiac and vascular
arginase. Acta Physiol. 221:174–181. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qiao SG, Sun Y, Sun B, Wang A, Qiu J, Hong
L, An JZ, Wang C and Zhang HL: Sevoflurane postconditioning
protects against myocardial ischemia/reperfusion injury by
restoring autophagic flux via an NO-dependent mechanism. Acta
Pharmacol Sin. 40:35–45. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Balligand JL, Kobzik L, Han X, Kaye DM,
Belhassen L, O'Hara DS, Kelly RA, Smith TW and Michel T: Nitric
oxide-dependent parasympathetic signaling is due to activation of
constitutive endothelial (type III) nitric oxide synthase in
cardiac myocytes. J Biol Chem. 270:14582–14586. 1995.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hao M, Zhu S, Hu L, Zhu H, Wu X and Li Q:
Myocardial ischemic postconditioning promotes autophagy against
ischemia reperfusion injury via the activation of the
nNOS/AMPK/mTOR pathway. Int J Mol Sci. 18(614)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bi X, He X, Xu M, Zhao M, Yu X, Lu X and
Zang W: Acetylcholine ameliorates endoplasmic reticulum stress in
endothelial cells after hypoxia/reoxygenation via M3 AChR-AMPK
signaling. Cell Cycle. 14:2461–2472. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xue RQ, Sun L, Yu XJ, Li DL and Zang WJ:
Vagal nerve stimulation improves mitochondrial dynamics via an M3
receptor/CaMKKbeta/AMPK pathway in isoproterenol-induced myocardial
ischaemia. J Cell Mol Med. 21:58–71. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mavropoulos SA, Khan NS, Levy ACJ, Faliks
BT, Sison CP, Pavlov VA, Zhang Y and Ojamaa K: Nicotinic
acetylcholine receptor-mediated protection of the rat heart exposed
to ischemia reperfusion. Mol Med. 23:120–133. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pickard JMJ, Burke N, Davidson SM and
Yellon DM: Intrinsic cardiac ganglia and acetylcholine are
important in the mechanism of ischaemic preconditioning. Basic Res
Cardiol. 112(11)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
An J, Rhodes SS, Jiang MT, Bosnjak ZJ,
Tian M and Stowe DF: Anesthetic preconditioning enhances
Ca2+ handling and mechanical and metabolic function
elicited by Na+/Ca2+ exchange inhibition in
isolated hearts. Anesthesiology. 105:541–549. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fang L, Xu Z, Lu J, Hong L, Qiao S, Liu L
and An J: Cardioprotective effects of triiodothyronine
supplementation against ischemia reperfusion injury by preserving
calcium cycling proteins in isolated rat hearts. Exp Ther Med.
18:4935–4941. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sasamori J, Abe Y, Marunouchi T, Manome Y,
Uchibori T and Tanonaka K: Effects of 2-octynyladenosine (YT-146)
on mitochondrial function in ischemic/reperfused rat hearts. Biol
Pharm Bull. 38:1946–1953. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Intachai K, C Chattipakorn S, Chattipakorn
N and Shinlapawittayatorn K: Revisiting the cardioprotective
effects of acetylcholine receptor activation against myocardial
ischemia/reperfusion injury. Int J Mol Sci. 19(2466)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhao M, Sun L, Yu XJ, Miao Y, Liu JJ, Wang
H, Ren J and Zang WJ: Acetylcholine mediates AMPK-dependent
autophagic cytoprotection in H9c2 cells during
hypoxia/reoxygenation injury. Cell Physiol Biochem. 32:601–613.
2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shinlapawittayatorn K, Chinda K, Palee S,
Surinkaew S, Kumfu S, Kumphune S, Chattipakorn S, KenKnight BH and
Chattipakorn N: Vagus nerve stimulation initiated late during
ischemia, but not reperfusion, exerts cardioprotection via
amelioration of cardiac mitochondrial dysfunction. Heart Rhythm.
11:2278–2287. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Palee S, Apaijai N, Shinlapawittayatorn K,
Chattipakorn SC and Chattipakorn N: Acetylcholine attenuates
hydrogen peroxide-induced intracellular calcium dyshomeostasis
through both muscarinic and nicotinic receptors in cardiomyocytes.
Cell Physiol Biochem. 39:341–349. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li DL, Liu BH, Sun L, Zhao M, He X, Yu XJ
and Zang WJ: Alterations of muscarinic acetylcholine receptors-2, 4
and α7-nicotinic acetylcholine receptor expression after
ischaemia/reperfusion in the rat isolated heart. Clin Exp Pharmacol
Physiol. 37:1114–1119. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Roy A, Fields WC, Rocha-Resende C, Resende
RR, Guatimosim S, Prado VF, Gros R and Prado MA:
Cardiomyocyte-secreted acetylcholine is required for maintenance of
homeostasis in the heart. FASEB J. 27:5072–5082. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kakinuma Y, Akiyama T, Okazaki K, Arikawa
M, Noguchi T and Sato T: A non-neuronal cardiac cholinergic system
plays a protective role in myocardium salvage during ischemic
insults. PLoS One. 7(e50761)2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Oikawa S, Kai Y, Tsuda M, Ohata H, Mano A,
Mizoguchi N, Sugama S, Nemoto T, Suzuki K, Kurabayashi A, et al:
Non-neuronal cardiac cholinergic system influences CNS via the
vagus nerve to acquire a stress-refractory propensity. Clin Sci
(Lond). 130:1913–1928. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pang L, Cai Y, Tang EH, Yan D, Kosuru R,
Li H, Irwin MG, Ma H and Xia Z: Cox-2 inhibition protects against
hypoxia/reoxygenation-induced cardiomyocyte apoptosis via
Akt-dependent enhancement of iNOS expression. Oxid Med Cell Longev.
2016(3453059)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li XD, Yang YJ, Geng YJ, Zhao JL, Zhang
HT, Cheng YT and Wu YL: Phosphorylation of endothelial NOS
contributes to simvastatin protection against myocardial no-reflow
and infarction in reperfused swine hearts: Partially via the PKA
signaling pathway. Acta Pharmacol Sin. 33:879–887. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cao J, Xie H, Sun Y, Zhu J, Ying M, Qiao
S, Shao Q, Wu H and Wang C: Sevoflurane post-conditioning reduces
rat myocardial ischemia reperfusion injury through an increase in
NOS and a decrease in phopshorylated NHE1 levels. Int J Mol Med.
36:1529–1537. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ge ZD, Pravdic D, Bienengraeber M, Pratt
PF Jr, Auchampach JA, Gross GJ, Kersten JR and Warltier DC:
Isoflurane postconditioning protects against reperfusion injury by
preventing mitochondrial permeability transition by an endothelial
nitric oxide synthase-dependent mechanism. Anesthesiology.
112:73–85. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sun L, Lu J, Yu XJ, Li DL, Xu XL, Wang B,
Ren KY, Liu JK and Zang WJ: Adenine sulfate improves cardiac
function and the cardiac cholinergic system after myocardial
infarction in rats. J Pharmacol Sci. 115:205–213. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Waid DK, Chell M and El-Fakahany EE: M(2)
and M(4) muscarinic receptor subtypes couple to activation of
endothelial nitric oxide synthase. Pharmacology. 61:37–42.
2000.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Dong Y, Zhang M, Liang B, Xie Z, Zhao Z,
Asfa S, Choi HC and Zou MH: Reduction of AMP-activated protein
kinase alpha2 increases endoplasmic reticulum stress and
atherosclerosis in vivo. Circulation. 121:792–803. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kataoka Y, Shibata R, Ohashi K, Kambara T,
Enomoto T, Uemura Y, Ogura Y, Yuasa D, Matsuo K, Nagata T, et al:
Omentin prevents myocardial ischemic injury through AMP-activated
protein kinase- and Akt-dependent mechanisms. J Am Coll Cardiol.
63:2722–2733. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Viollet B, Horman S, Leclerc J, Lantier L,
Foretz M, Billaud M, Giri S and Andreelli F: AMPK inhibition in
health and disease. Crit Rev Biochem Mol Biol. 45:276–295.
2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mukherjee D, Ghosh AK, Bandyopadhyay A,
Basu A, Datta S, Pattari SK, Reiter RJ and Bandyopadhyay D:
Melatonin protects against isoproterenol-induced alterations in
cardiac mitochondrial energy-metabolizing enzymes, apoptotic
proteins, and assists in complete recovery from myocardial injury
in rats. J Pineal Res. 53:166–179. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Groenendyk J, Sreenivasaiah PK, Kim DH,
Agellon LB and Michalak M: Biology of endoplasmic reticulum stress
in the heart. Circ Res. 107:1185–1197. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lin E and Symons JA: Volatile anaesthetic
myocardial protection: A review of the current literature. HSR Proc
Intensive Care Cardiovasc Anesth. 2:105–109. 2010.PubMed/NCBI
|
|
41
|
Turek Z, Sykora R, Matejovic M and Cerny
V: Anesthesia and the microcirculation. Semin Cardiothorac Vasc
Anesth. 13:249–258. 2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Harvey KL, Hussain A and Maddock HL:
Ipratropium bromide-mediated myocardial injury in in vitro models
of myocardial ischaemia/reperfusion. Toxicol Sci. 138:457–467.
2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Buchholz B, Donato M, Perez V, Deutsch
ACR, Höcht C, Del Mauro JS, Rodríguez M and Gelpi RJ: Changes in
the loading conditions induced by vagal stimulation modify the
myocardial infarct size through sympathetic-parasympathetic
interactions. Pflugers Arch. 467:1509–1522. 2015.PubMed/NCBI View Article : Google Scholar
|