Introduction
Tuberculosis (TB) is a major global health problem
associated with significant morbidity and potential mortality
(1,2). Diagnostic bronchoscopy studies have
suggested that 10-50% of patients with pulmonary TB have
tracheobronchial TB (TBTB) (3,4).
Airway stenosis is the most common complication of TBTB. Despite
adequate anti-TB therapy, patients with TBTB develop different
degrees of airway stenosis, resulting in marked narrowing of the
bronchial lumen, dyspnoea on exertion and obstructive pneumonia
(5,6). In the long term, >90% of patients
develop stenosis (6). The damage
after TBTB is residual and long-term (7,8).
Therefore, in addition to anti-TB treatment, monitoring and
determining the degree of post-TB tracheobronchial stenosis (PTTS)
are important for early diagnosis and treatment, and for improving
the survival of patients and their quality of life. In the early
stages, the disease is difficult to diagnose based on clinical
presentation alone and is frequently missed (6). Bronchoscopy is thus far the major
strategy for diagnosing and monitoring PTTS (9-11).
In general, bronchoscopy is selectively performed when PTTS is
suspected in patients with severe cough, wheezing or haemoptysis.
Furthermore, bronchoscopy is invasive and tends to increase the
risk of occupational exposure among medical staff, and unvaccinated
patients with TB may try to avoid hospital visits during the time
of the COVID-19 pandemic (12-17).
These limitations have essentially forced the early diagnosis and
non-invasive monitoring program to shift from in person to remote
follow-up, which involves telemedicine and remote laboratory
monitoring (18-20).
These challenges in diagnosing and monitoring the stenosis status
have created an urgent need for reliable biomarkers to predict the
progression of PTTS (20,21).
The exact pathogenesis of PTTS has remained to be
fully elucidated; one possible underlying mechanism involves
diffuse fibrosis in the trachea and bronchus (6,22-25).
Molecular changes usually require less time to manifest than
morphological changes. Fibrosis is frequently accompanied by
changes in serum levels of the markers (26). Thus, circulating biomarkers that
allow the non-invasive assessment of the existence and degree of
PTTS have immense clinical value. However, only a few biomarkers
have been proposed for predicting PTTS. An isoform of transforming
growth factor-β (TGF-β), TGF-β1, is a master regulator of tissue
repair, inflammation and fibrosis (27). Current pathological evidence
suggests that TGF-β1 is a central mediator driving the fibrotic
process induced by multiple profibrotic stimuli (27,28).
Previous studies suggested that as a non-invasive biomarker, serum
TGF-β1 is a marker of fibrotic involvement in systemic sclerosis
(29) and is related to the
development of moderate to severe radiation-induced fibrosis
(30). Furthermore, serum TGF-β1 is
an independent predictor of recurrence of atrial fibrillation in
patients with paroxysmal atrial fibrillation (31). Procollagen type I N-propeptide
(PINP) is cleaved from type I pro-collagen during its extracellular
processing (32). As a profibrotic
biomarker, serum PINP has been used to reflect the healing of
Achilles' tendon rupture (33).
High serum PINP levels have been reported in pathological fibrotic
conditions. One study suggested that changes in serum PINP levels
in patients with liver diseases may reflect the severity of liver
cirrhosis (34). Furthermore, the
baseline serum PINP levels in patients with chronic heart failure
are higher than those in the controls and are decreased after
therapy (26).
Although serum TGF-β1 and PINP are implicated in the
development of multiple fibrosis, their utility in PTTS is not well
established. Hence, in the present study, the potential of
utilising serum TGF-β1 and PINP levels as diagnosing/monitoring
biomarkers for PTTS was assessed.
Materials and methods
Study design and population
A total of 119 patients with TBTB after the
condition was treated for at least 6 months (59 patients with
airway stenosis and 60 patients with no stenosis) from the
Department of Respiratory and Critical Care Medicine at The First
Affiliated Hospital of Chongqing Medical University (Chongqing,
China) between March and September 2020 were included in the study.
All of these patients had no positive acid-fast bacilli, positive
Mycobacterium tuberculosis culture or pathological diagnosis
of TB. In addition, bone disease, cardiovascular disease, liver
fibrosis, kidney fibrosis, evidence of other systemic diseases
causing fibrosis, malignancy and other infectious diseases were
also excluded. For the airway stenosis group, data regarding
clinical characteristics, such as demographic information, symptoms
at presentation, chest high-resolution computed tomography (HRCT)
and bronchoscopic findings were collected. These clinical
characteristics and serum TGF-β1 and PINP levels in paired blood
samples were analysed. All subjects provided written informed
consent for participation in the study. The study was performed in
accordance with the Declaration of Helsinki and the Uniform
Requirements for Manuscripts Submitted to Biomedical Journals. The
protocol was approved by the Ethics Committee of The First
Affiliated Hospital of Chongqing Medical University (approval no.
2020-147).
Measurement of serum TGF-β1 and PINP
levels
Venous blood samples (2.0 ml) were collected from
each subject in tubes without anticoagulant. Serum was separated by
centrifugation of blood samples at room temperature for 10 min at
2,000 x g, and samples to be used for biomarker assays were
refrigerated at 4˚C and transferred to the Department of Central
Laborarory at The First Affiliated Hospital of Chongqing Medical
University (Chongqing, China), where they were stored at -80˚C
until further use. This process was performed within 1 h of blood
collection. Commercially available ELISA kits were used for TGF-β1
(cat. no. EK0513; Wuhan Boster Biological Technology, Ltd.) and
PINP (cat. no. EH1092; Wuhan Fine Biotech Co., Ltd.) assays as per
the manufacturers' protocols. The lower limits of detection were
15.6 pg/ml for TGF-β1 and 1.563 ng/ml for PINP. The absorbance
(optical density) was measured at 450 nm using a microplate reader
(Infinite M Plex; Tecan Trading AG).
Chest HRCT
Chest HRCT images were obtained with shallow
breathing using a 64-slice helical HRCT system (Siemens AG). Two
radiologists blinded to the clinical data interpreted all HRCT
findings by consensus. Atelectasis and mucus plugging on chest HRCT
were used to assess PTTS.
Diagnostic criteria for PTTS and
interventional bronchoscopy
PTTS was diagnosed based on the detection of lesions
using a bronchoscope and microbiological testing for TB bacilli.
The types of PTTS are described as inflammatory infiltration,
ulceration necrosis, granulation hyperplasia, cicatrices stricture,
bronchomalacia and lymph fistula (4). The degree of stenosis was classified
into five grades according to the cross-sectional area of the
trachea (35). The patients were
divided into two groups: Mild-to-moderate stenosis group (<75%)
and severe stenosis group (≥75%). All patients with PTTS were
treated with interventional bronchoscopy according to treatment
guidelines (9) using a bronchoscope
(CV-290; Olympus Corp.), an electrocautery needle knife (VIO 300S;
Erbe Elektromedizin GmbH), a multi-use cryosurgery system (Erbokryo
CA; Erbe Elektromedizin GmbH) and a dilatation balloon (Endo-Flex
GmbH). An airway stent (Micro-Tech Europe GmbH) was considered to
relieve symptoms of patients with dyspnoea only if the conventional
airway interventional therapy was not effective. Patients were
followed up for 1 month after the bronchoscopic intervention
treatment.
Histological analysis
During interventional bronchoscopy an electrocautery
needle knife was first used to release airway cicatrix tissues and
subsequently, cryotherapy or forceps biopsy was performed to obtain
the cicatrix tissues. Tissue sections (5 µm in thickness) were
subjected to haematoxylin and eosin (H&E) and Masson staining
to enable the histological evaluation of airway cicatrix tissue
fibrosis. Morphometric analysis was performed using five
measurements randomly taken in five different fields independently
by two pathologists blinded to the clinical data. The scores of the
two pathologists were combined in an average score and the final
results were obtained.
Statistical analysis
The normality of distribution of continuous
variables was assessed using the Shapiro-Wilk test, which indicated
that they were not normally distributed. The data were then
expressed as the median (interquartile range). Differences between
any two groups were evaluated using the Mann-Whitney U-test, while
differences between two related samples were assessed using the
Wilcoxon matched-pair signed-rank test. Correlations were tested
for significance by calculating Spearman's rank correlation
coefficient. Categorical data were expressed as n (%) and
comparisons for testing statistically significant differences were
made using the χ2-test (minimum expected values ≥5) or
Fisher's exact test (minimum expected values <5). Receiver
operating characteristic (ROC) curves generated by plotting
sensitivity against 1-specificity were used to assess the
diagnostic performances of serum TGF-β1 and PINP levels for
distinguishing between airway stenosis and non-stenosis. For each
ideal cut-off value (at the point of the highest Youden index),
sensitivity, specificity, positive predictive value (PPV), negative
predictive value (NPV) and accuracy were reported. Statistical
analyses were performed using SPSS version 20.0 software (IBM
Corp.) and P<0.05 was considered to indicate statistical
significance (all P-values are from two-sided tests). Graphical
representation was performed using GraphPad Prism 6.05 software
(GraphPad Software, Inc.).
Results
Clinical information
The relevant clinical characteristics of the
subjects are summarised in Table I.
Of all the subjects (14 male and 105 female participants), the
median (interquartile range) age was 27 (23-32)
years. The significant clinical characteristics of all patients
included age, sex, smoking, symptoms at presentation and chest HRCT
features. Compared to patients with non-stenosis, those with
stenosis more frequently presented with the symptoms of cough,
dyspnoea and wheezing at presentation, as well as chest HRCT
features of atelectasis and mucus plugging (all P<0.05; Table I). There were no significant
differences in age, sex and smoking between patients with stenosis
and those with non-stenosis (P>0.05).
 | Table IClinical characteristics of study
subjects. |
Table I
Clinical characteristics of study
subjects.
| Characteristic | Stenosis
(n=59) | Non-stenosis
(n=60) |
Z/χ2 | P-value |
|---|
| Age (years) | 26.0
(22.0-32.0) | 28.0
(24.0-32.8) | -0.477 | 0.634a |
| Sex | | | 0.287 | 0.592b |
|
Male | 6 (10.17) | 8 (13.33) | | |
|
Female | 53 (89.83) | 52 (86.67) | | |
| Smoking | | | NA | 0.679c |
|
Yes | 3 (5.08) | 2 (3.33) | | |
|
No | 56 (94.92) | 58 (96.67) | | |
| Symptoms at
presentation (overlapped, certain patients exhibited two or three
symptoms) | | | | |
|
Cough | | | 11.773 | 0.001b |
|
Yes | 23 (38.98) | 7 (11.67) | | |
|
No | 36 (61.02) | 53 (88.33) | | |
|
Dyspnoea | | | 19.855 |
<0.001b |
|
Yes | 21 (35.59) | 2 (3.33) | | |
|
No | 38 (64.41) | 58 (96.67) | | |
|
Wheezing | | | 20.169 |
<0.001b |
|
Yes | 17 (28.81) | 0 (0) | | |
|
No | 42 (71.19) | 60(100) | | |
| Chest HRCT features
(overlapped) | | | | |
|
Atelectasis | | | 18.442 |
<0.001b |
|
Yes | 20 (33.90) | 2 (3.33) | | |
|
No | 39 (66.10) | 58 (96.67) | | |
|
Mucus
plugging | | | 10.847 | 0.001b |
|
Yes | 16 (27.12) | 3 (5.00) | | |
|
No | 43 (72.88) | 57 (95.00) | | |
| Bronchoscopic
features (overlapped) | | | | |
|
Tracheal
stenosis | | | NA | NA |
|
Yes | 15 (25.42) | NA | | |
|
No | 44 (74.58) | NA | | |
|
Main
bronchus stenosisd | | | NA | NA |
|
Left | 32 (54.24) | NA | | |
|
Right | 27 (45.76) | NA | | |
|
Stenosis
degree | | | NA | NA |
|
Mild-to-moderate | 20 (33.90) | NA | | |
|
Severe | 39 (66.10) | NA | | |
|
Bronchomalacia | | | NA | NA |
|
Yes | 33 (55.93) | NA | | |
|
No | 26 (44.07) | NA | | |
The bronchoscopic features of stenosis are also
summarised in Table I. All of the
patients with airway stenosis enrolled in the present study had
cicatrices stricture and 33 (55.93%) of them also had
bronchomalacia; however, no other types of stenosis were detected.
The chest HRCT and bronchoscopy features of representative cases of
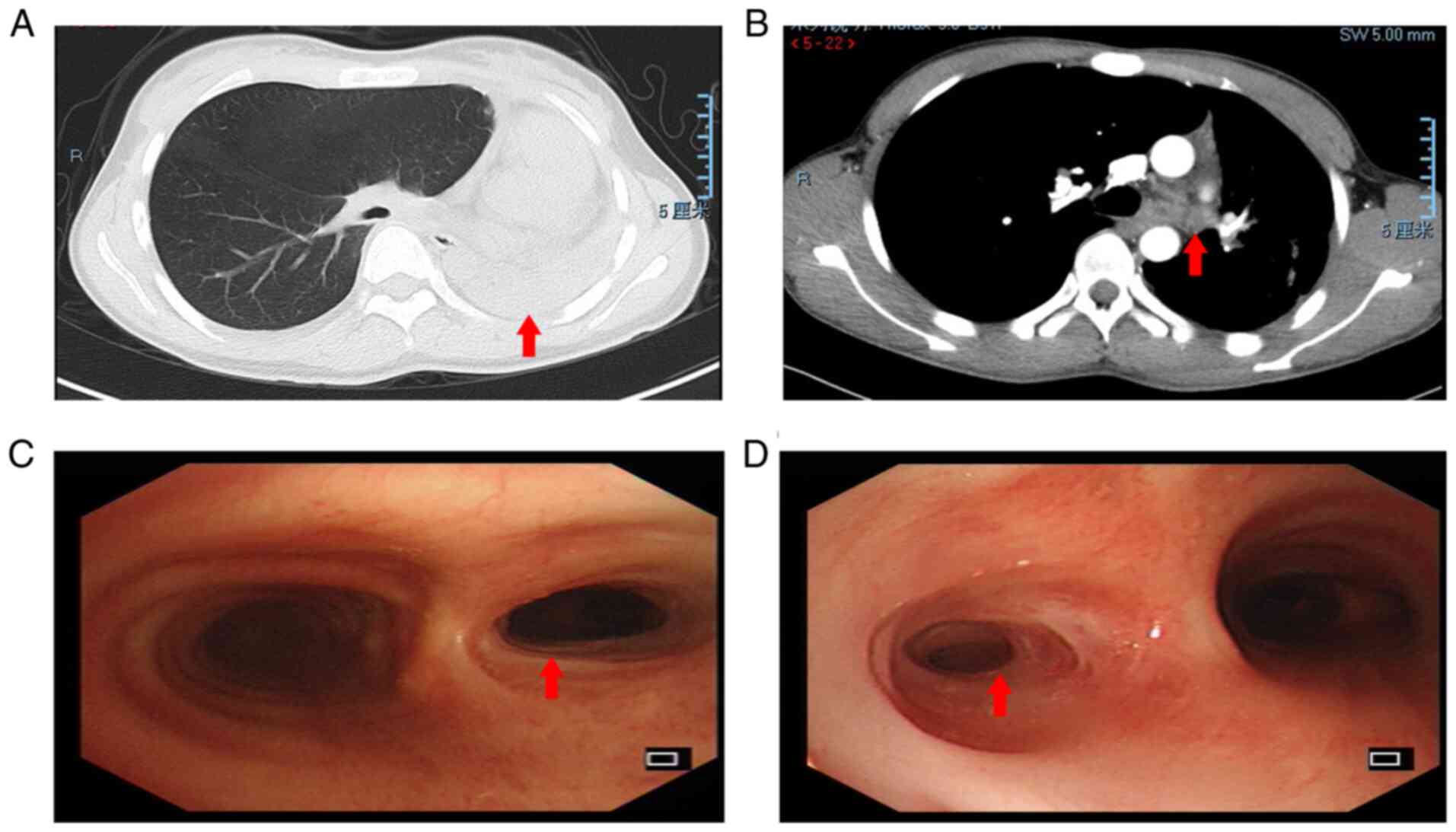
patients with PTTS are presented in Fig. 1. Chest HRCT indicated left lung
atelectasis (A) and left main bronchus mucus plugging (B), and
diagnostic bronchoscopy revelaed moderate (C) and severe (D) airway
stenosis due to TBTB, respectively.
Confirmation of hyperplasia of fibrous
tissue using histopathology
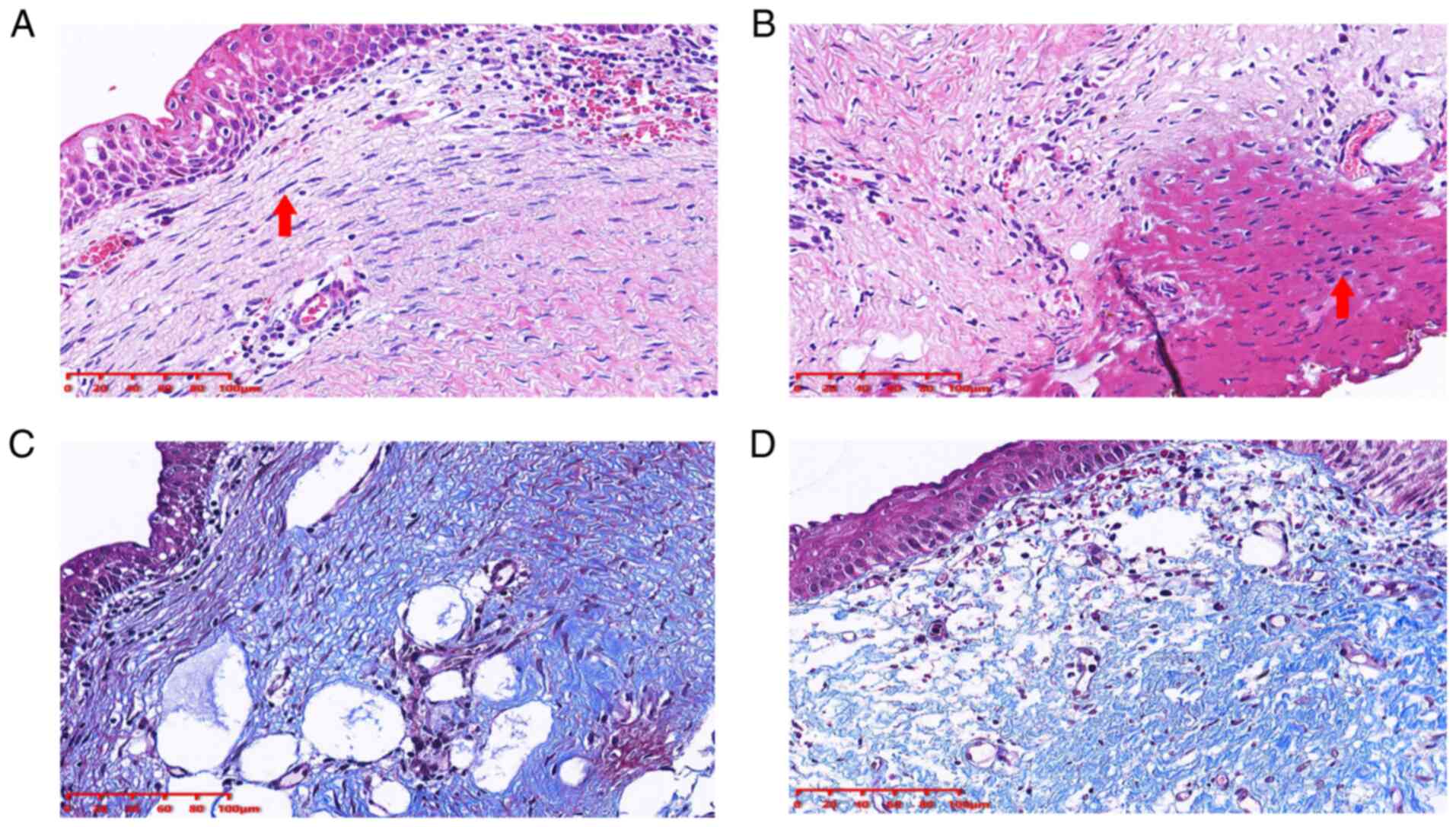
The airway cicatrix tissue was evaluated using
H&E and Masson staining. The principal histological finding was
squamous epithelialisation of the bronchial epithelial cells
(Fig. 2A) and fibrotic lesions with
thickened submucosal layers (Fig.
2A). Furthermore, electrocautery needle knife therapy resulted
in marked cell coagulative necrosis (Fig. 2B). In addition, the presence of
fibrosis was verified by Masson staining (Fig. 2C and D).
Serum TGF-β1 and PINP levels in the
airway stenosis and non-stenosis groups
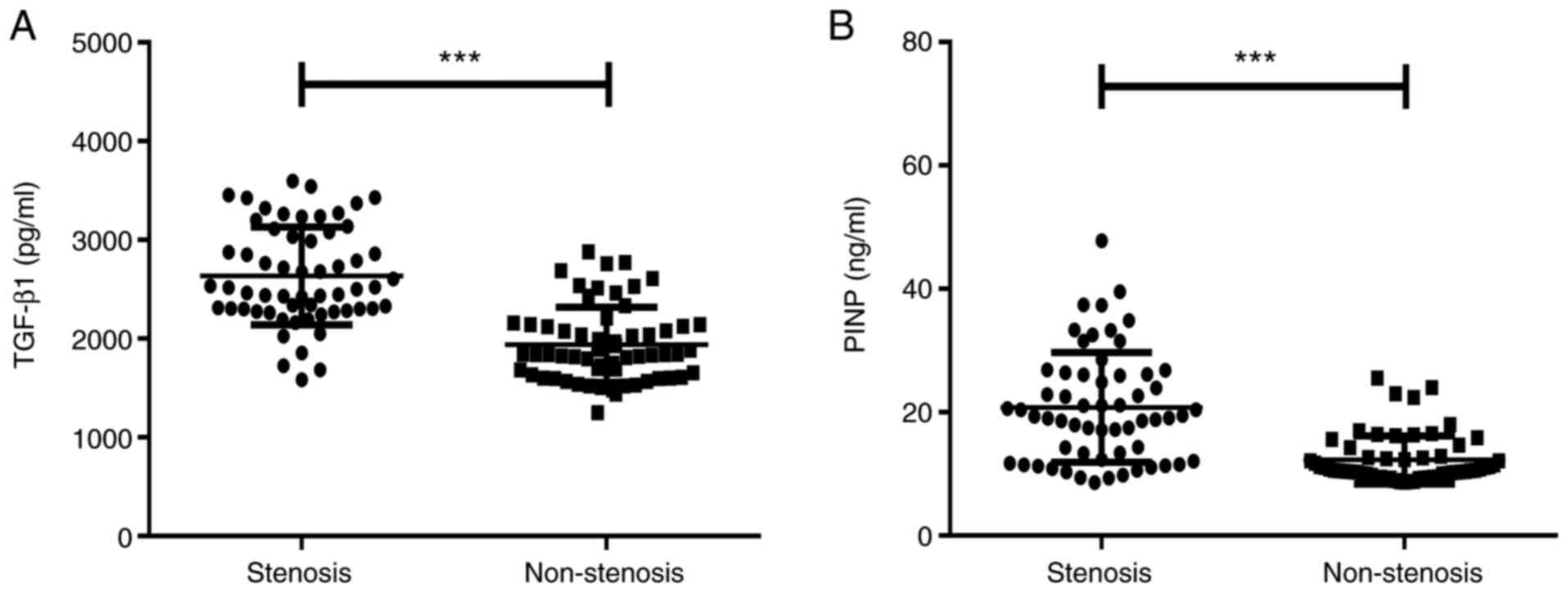
As presented in Fig.
3A, the serum TGF-β1 levels in the airway stenosis group were
significantly higher than those in non-stenosis group [2,518.98
(2,302.58-3,080.27) vs. 1,839.62 (1,616.82-2,141.38) pg/ml,
Z=-6.830, P<0.001]. As presented in Fig. 3B, the serum PINP levels in the
airway stenosis group were significantly higher than those in the
non-stenosis group [19.39 (12.37-26.14) vs. 10.63 (9.86-12.85)
ng/ml, Z=-6.102, P<0.001].
Serum TGF-β1 and PINP levels in
patients with airway stenosis and different clinical
characteristics
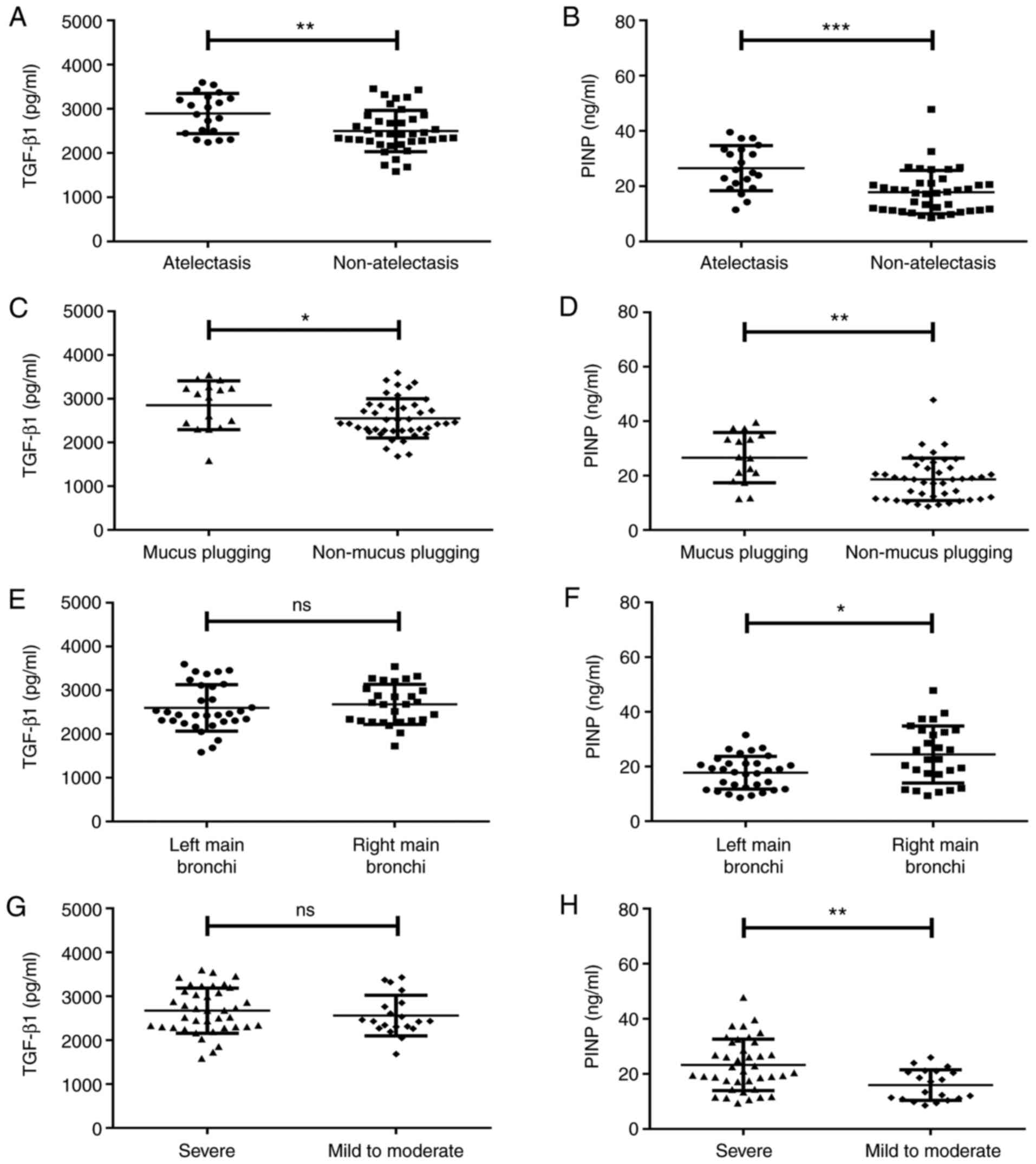
Patients with atelectasis exhibited significantly
higher serum TGF-β1 [2,954.85 (2,462.82-3,265.12) vs. 2,430.92
(2,262.60-2,766.27) pg/ml, Z=-2.746, P=0.038; Fig. 4A] and PINP [25.43 (19.81-33.35) vs.
17.53 (11.38-21.07) ng/ml, Z=-3.691, P<0.001; Fig. 4B] levels than those without
atelectasis. Patients with mucus plugging had significantly higher
TGF-β1 [3,075.18 (2,367.28-3,265.59) vs. 2,438.27
(2,271.83-2,851.15) pg/ml, Z=-2.106, P=0.035; Fig. 4C] and PINP [26.65 (18.74-34.54) vs.
18.65 (11.55-22.91) ng/ml, Z=-3.060, P=0.002; Fig. 4D] levels than those without mucus
plugging.
Patients with left main bronchus stenosis and those
with right main bronchus stenosis had comparable TGF-β1 levels
(Z=-0.533, P=0.594, P>0.05; Fig.
4E), while patients with right main bronchus stenosis [22.71
(17.18-33.32) vs. 18.29 (11.92-21.15) ng/ml, Z=-2.404, P=0.016;
Fig. 4F] had higher serum PINP
levels than those with left main bronchus stenosis. Serum TGF-β1
levels were comparable between severe airway tracheal stenosis and
mild-to-moderate airway tracheal stenosis (Z=-0.793, P=0.428,
P>0.05; Fig. 4G), while serum
PINP levels were higher in severe airway tracheal stenosis than
those in mild-to-moderate airway tracheal stenosis [21.07
(17.18-31.55) vs. 15.33 (10.94-20.96) ng/ml, Z=-3.060, P=0.003;
Fig. 4H]. Serum TGF-β1 and PINP
levels did not vary significantly with age, sex, smoking, symptoms
at presentation or bronchomalacia (P>0.05).
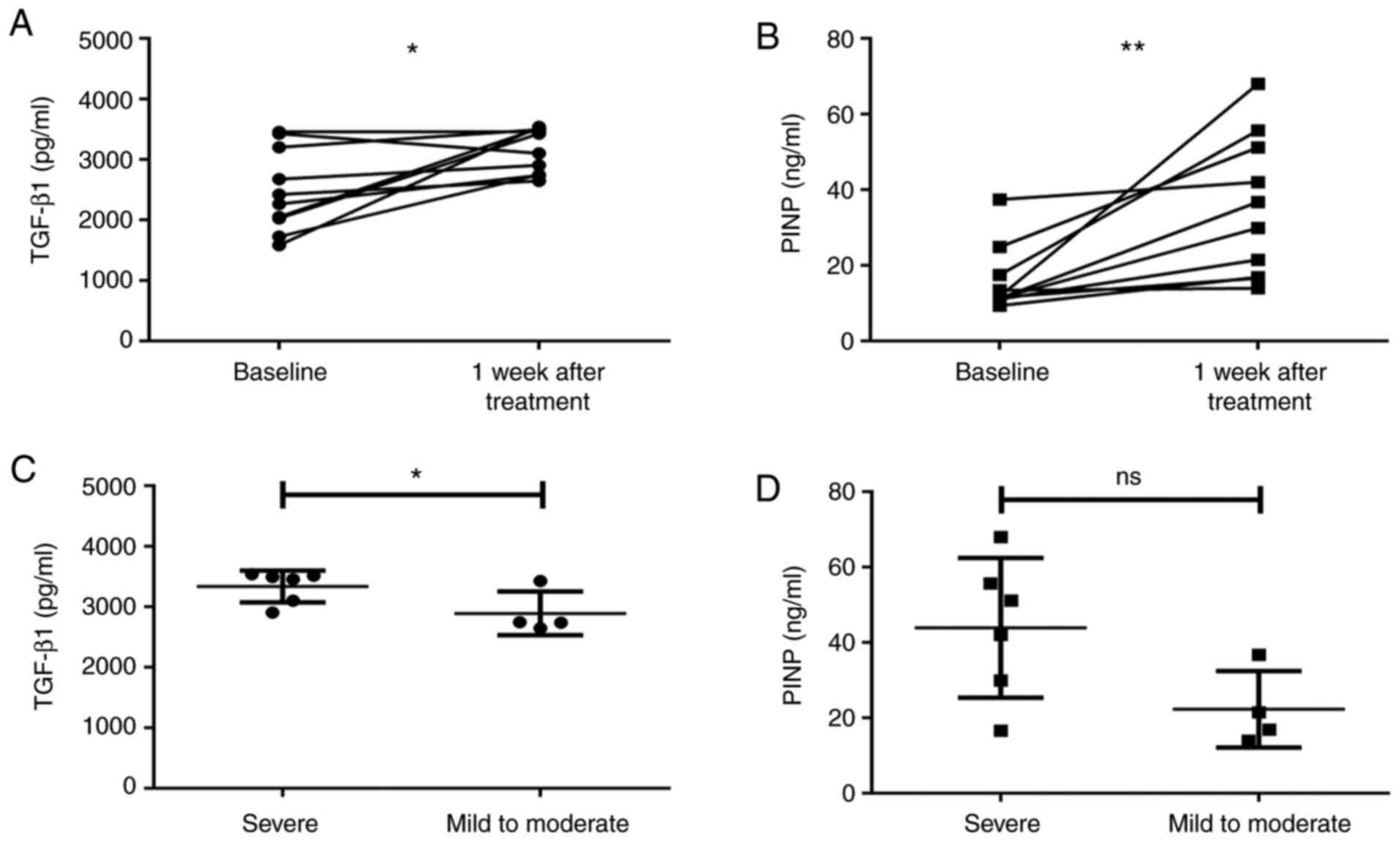
Changes in serum TGF-β1 and PINP
levels after interventional bronchoscopy
Considering the invasive nature of interventional
bronchoscopy therapy, samples from only 10 patients with airway
stenosis were used to study the effect of interventional
bronchoscopy therapy on serum TGF-β1 and PINP levels. As presented
in Fig. 5, the levels of serum
TGF-β1 (Z=-2.293, P=0.022; Fig. 5A)
and PINP (Z=-2.803, P=0.005; Fig.
5B) increased significantly at 1 week after the interventional
bronchoscopy therapy. As not all patients had been subjected to
routine TGF-β1 and PINP examinations after each bronchoscopy, the
data of the 10 patients were used to study the correlation between
the degree of stenosis at baseline and biomarker levels after
interventional bronchoscopy. In the post-interventional
bronchoscopy period, the serum TGF-β1 (Z=-2.132, P=0.033; Fig. 5C) and PINP (Z=-1.706, P=0.088, not
statistically significant; Fig. 5D)
levels in the severe stenosis group (n=6) at the baseline were also
higher than those in the mild-to-moderate stenosis group (n=4).
The relative changes in the post- vs.
pre-interventional bronchoscopy levels of serum TGF-β1 and PINP are
presented in Table II. The
variables included the number of biopsies taken and the duration of
the bronchoscopy procedure per patient. When comparison of post-
and pre-interventional bronchoscopy data was performed in each
group, the increase in TGF-β1 and PINP levels was revealed to be
greater in patients with≥3 biopsies than in those with<3
biopsies (Z=-1.358, P=0.175 and Z=-1.567, P=0.117, respectively).
It was also greater when the median duration of the bronchoscopy
procedure per patient was ≥60 min as compared to <60 min
(Z=-1.919, P=0.055 and Z=-1.706, P=0.088, respectively; none of
these was statistically significant).
 | Table IIChanges in serum TGF-β1 and PINP
levels between the post- and pre-interventional bronchoscopy. |
Table II
Changes in serum TGF-β1 and PINP
levels between the post- and pre-interventional bronchoscopy.
| Variable | TGF-β1 (pg/ml) | Z | P-value | PINP (ng/ml) | Z | P-value |
|---|
| Number of biopsies
per patient | | -1.358 | 0.175 | | -1.567 | 0.117 |
|
<3
(n=5) | 231.10
(-49.80-748.49) | | | 7.51
(2.81-17.81) | | |
|
≥3
(n=5) | 1,400.41
(145.61-1,708.64) | | | 25.50
(11.56-47.21) | | |
| Duration of
biopsies per patient (min) | | -1.919 | 0.055 | | -1.706 | 0.088 |
|
<60
(n=4) | 227.55
(-186.70-275.75) | | | 4.85
(1.54-20.96) | | |
|
≥60
(n=6) | 1,210.70
(357.14-1,585.24) | | | 22.01
(9.66-42.69) | | |
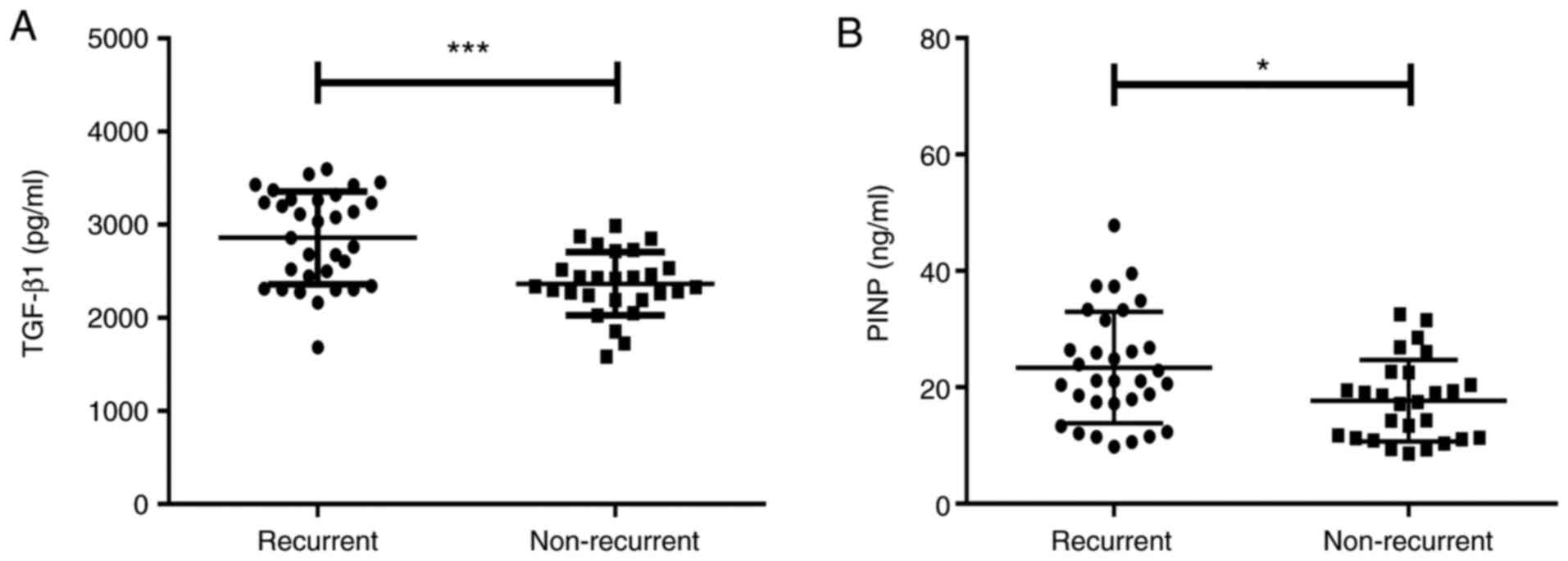
Relationships between baseline levels
of serum TGF-β1 and PINP and recurrence of stenosis
All of the patients with airway stenosis were
treated using interventional bronchoscopy and longitudinally
followed up for 1 month. As presented in Fig. 6, 32 (54.24%) patients were confirmed
to have recurrent stenosis using bronchoscopy. The baseline levels
of serum TGF-β1 [2,947.29 (2,371.74-3,272.32) vs. 2,339.71
(2,194.94-2,536.76) pg/ml, Z=-3.743, P<0.001; Fig. 6A] and PINP [21.13 (17.30-30.37) vs.
17.47 (11.27-22.56) ng/ml, Z=-2.419, P=0.016; Fig. 6B] in the subgroup with recurrence
were significantly higher than those in the non-recurrent subgroup.
Only two patients in the present study underwent serum TGF-β1 and
PINP examinations while receiving repeated interventional
bronchoscopy therapy. The serum TGF-β1 and PINP levels in the two
patients fluctuated prior to each therapy, as presented in Fig. S1.
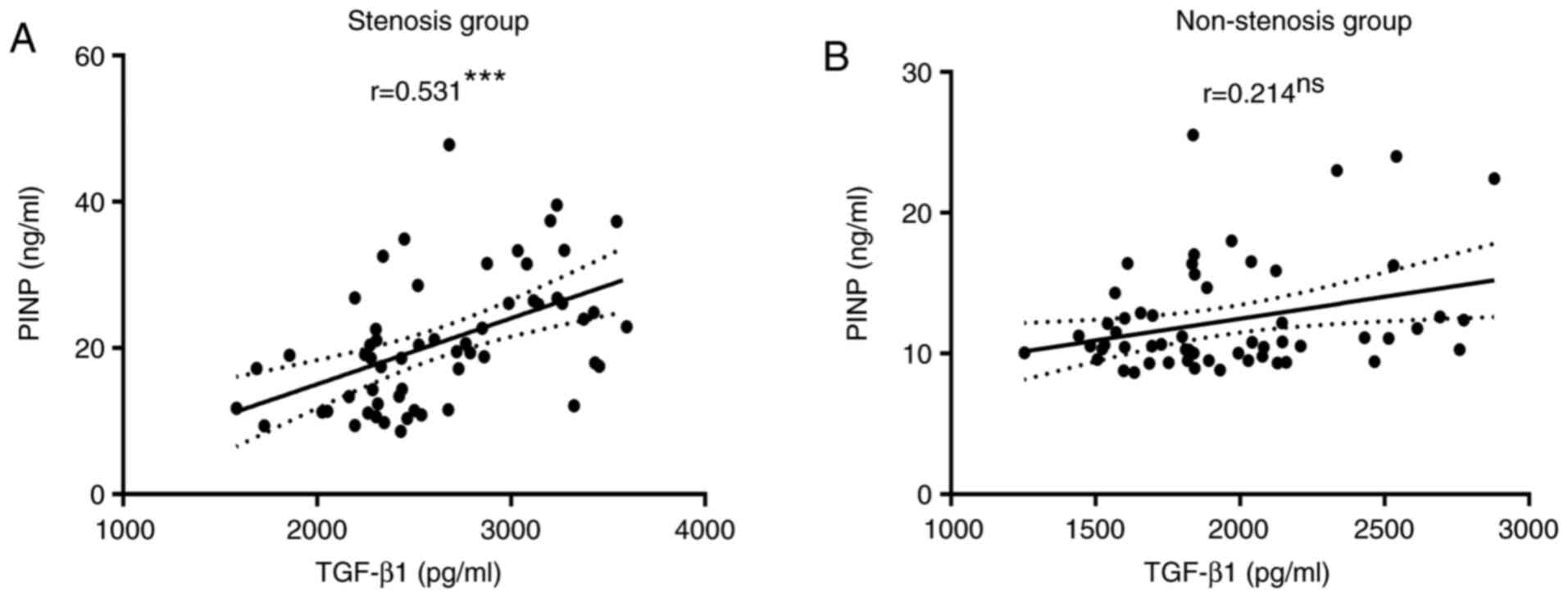
Correlation between serum TGF-β1 and
PINP levels in the stenosis and non-stenosis groups
The serum PINP levels were positively correlated
with TGF-β1 levels in patients with airway stenosis (P<0.001;
Fig. 7A), whereas the correlations
were not significant in the non-stenosis group (P=0.101; Fig. 7B).
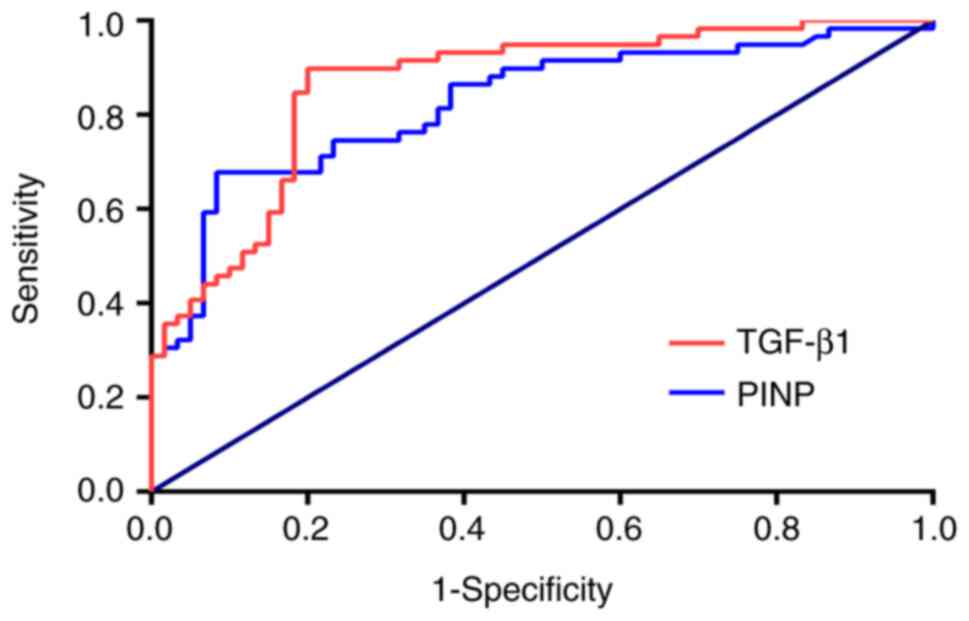
Performance of serum TGF-β1 and PINP
levels in distinguishing between airway stenosis and
non-stenosis
The area under the ROC curve for serum TGF-β1 levels
to distinguish patients with airway stenosis from those without
stenosis was 0.824 (95% CI: 0.748-0.900) and that for PINP was
0.863 (95% CI: 0.796-0.930; Fig.
8). Thus, TGF-β1 and PINP levels performed well in
distinguishing airway stenosis from non-stenosis. The cut-off
value, 95% CI, P-value, sensitivity, specificity, PPV, NPV and
accuracy associated with the highest Youden index are also
presented in Table III. Serum
TGF-β1 levels exhibited a sensitivity of 89.83% and specificity of
79.32%, while serum PINP levels exhibited a sensitivity of 67.80%
and specificity of 91.77%. These results were all acceptable.
 | Table IIISummary of the results of the
receiver operating characteristic curve analysis. |
Table III
Summary of the results of the
receiver operating characteristic curve analysis.
| Parameter | Cut-off point | 95% CI | P-value | HYI | Sensitivity
(%) | Specificity
(%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|
| TGF-β1 | >2,162.16
pg/ml | 0.748-0.900 | <0.001 | 0.698 | 89.83 | 79.32 | 81.54 | 88.89 | 84.87 |
| PINP | >17.10
ng/ml | 0.796-0.930 | <0.001 | 0.595 | 67.80 | 91.77 | 86.96 | 74.32 | 79.83 |
Discussion
TBTB is frequently followed by the development of
airway stenosis, which is usually detected via invasive methods,
such as bronchoscopy. However, bronchoscopy is performed
selectively, particularly owing to the COVID-19 pandemic, and is
associated with side effects (10,11,15).
Hence, the present study aimed to assess the feasibility of using
serum TGF-β1 and PINP levels as biomarkers for distinguishing
between airway stenosis and non-stenosis and for monitoring the
degree of PTTS. To the best of our knowledge, the present study was
the first to demonstrate the clinical significance of serum TGF-β1
and PINP levels in PTTS.
TGF-β has been indicated to have an important
function in local immunity in bronchial airway stenosis in active
TBTB (27). However, the
relationship between TGF-β and fibrosis post-TBTB had so far
remained elusive. TGF-β1 has been suggested as a biomarker for
fibrosis (28-31).
In laboratory studies, TGF-β1 is used to promote collagen
expression and fibrosis (36-42).
In the present study, the serum levels of TGF-β1 were significantly
higher in patients with airway stenosis than in those who did not
have stenosis. This indicates that TGF-β1 may be involved in the
development and/or consequences of fibrosis after TBTB. The
clinical characteristics of mucus plugging and atelectasis are also
indicators of airway stenosis. In airway stenosis, the tracheal
scar may cause partial or whole lung atelectasis and may even
destroy the lung (3-6,7,43-44). In the present study, it was
observed that patients with atelectasis or mucus plugging had
significantly higher serum TGF-β1 levels than those in the control
group. Biomarkers should reliably predict not only the extent of
airway stenosis but also the response to therapy. The present
results suggested that serum TGF-β1 levels higher than the baseline
were related to worse response and prognosis for patients with
airway stenosis treated using interventional bronchoscopy during
the follow-up. Consistent with these results, previous studies have
reported that a high level of serum TGF-β1 is associated with the
development of moderate-to-severe radiation-induced fibrosis due to
intracavitary accelerated partial breast irradiation (30) and is an independent predictor of
atrial fibrillation recurrence after catheter ablation (31). All of the above results suggest that
TGF-β1 contributes considerably to airway scar hyperplasia and that
its serum levels may be used to monitor the exacerbation rate of
airway scarring for PTTS.
The serum PINP level is a biomarker reflecting
collagen synthesis in pathological fibrotic processes (26,34);
it not only indicates the severity of liver cirrhosis (34) but may also be used to monitor
therapeutic efficacy for chronic heart failure (26). Therefore, serum PINP levels may be
clinically valuable for the non-invasive assessment of the presence
and extent of the profibrotic state of PTTS. In the present study,
histopathological examination demonstrated the proliferation of
fibroblasts in the bronchial submucosa and airway scar tissue.
Furthermore, serum PINP levels were significantly higher in the
airway stenosis than in the non-stenosis group. In addition, all
patients with airway stenosis accompanied by atelectasis, mucus
plugging or severe main bronchus stenosis had higher serum PINP
levels. Of note, serum PINP levels were higher in patients with
right main bronchus stenosis than in those with left main bronchus
stenosis, possibly owing to the higher proportion of tracheal
stenosis in patients with right main bronchus stenosis. This
difference may be due to the different diameter and angle of the
right main bronchus, which is conducive to the excretion of sputum
containing TB bacilli to the trachea. The present results also
suggested that serum PINP levels higher than the baseline were
related to worse response and prognosis. Collectively, these
results suggest that collagen synthesis increases in airway
stenosis and that serum PINP levels are able to predict the
severity of PTTS.
The present study also demonstrated the side effects
of interventional bronchoscopy in terms of histopathological
studies and serum biomarkers. Cell coagulative necrosis was induced
by interventional bronchoscopy therapy, as evidenced by
histopathological examinations and confirmed by the increased serum
levels of TGF-β1 and PINP 1 week after interventional bronchoscopy.
These results indicated that interventional bronchoscopy may induce
the secretion of TGF-β1 by destroying the local microenvironment
and may thus accelerate the exacerbation of scarring, which may
explain the rapid post-therapy exacerbation of the scarring and
airway restenosis. Statistical analysis revealed a positive
correlation between the serum TGF-β1 and PINP levels of patients
with airway stenosis, indicating that serum TGF-β1 and PINP may be
used as complementary markers for evaluating airway stenosis in
patients with PTTS. Furthermore, serum TGF-β1 and PINP levels had
good diagnostic potential for distinguishing between airway
stenosis and non-stenosis. Taken together, these results indicated
that the serum TGF-β1 and PINP levels are potential clinical
markers for diagnosing and real-time monitoring of PTTS.
The present study has several limitations. First,
PTTS is mostly observed among females owing to the smaller
bronchial lumen size and less expectorated sputum than in males,
resulting in prolonged exposure to TB bacilli in the bronchi
(3-5,45);
therefore, the patients enrolled in this study were predominantly
females. Further studies should be performed in males. Furthermore,
the small number of patients reduced the power of the statistical
calculations and increased the risk for type 2 statistical errors.
However, the patients were selected from a larger cohort, the
clinical characteristics reflecting airway stenosis were
comprehensive, and cases of PTTS were closely monitored during the
treatment. In addition, serum biomarker levels may be affected by
post-TB pulmonary fibrosis (46).
As another limitation, serum TGF-β1 and PINP measurements were not
performed prior to each bronchoscopy and a further study should be
performed using a larger sample size to obtain more representative
results. Finally, longer periods of observation and measurement of
serum biomarker levels after interventional bronchoscopy are
required to evaluate the predictive value of TGF-β1 and PINP for
the recurrence of airway stenosis.
In conclusion, non-invasive strategies for
monitoring PTTS are clinically important but lacking. The present
study was the first to report that increased serum TGF-β1 and PINP
levels have the potential to act as biomarkers for diagnosing PTTS.
In future studies, more patients will be enrolled in a multi-centre
study to provide sufficient data to confirm the results of the
present study. These practically measurable markers may assist in
monitoring the treatment response. At present, a prospective
clinical study involving the use of a combination of drugs and
interventional bronchoscopy for airway scar stenosis is underway at
our hospital (registration no. ChiCTR1900024441). As this clinical
trial was influenced by the COVID-19 pandemic, telemedicine and
remote laboratory monitoring will be used to validate the utility
of serum TGF-β1 and PINP levels as biomarkers in patients with
PTTS. This approach is convenient with the advantage of reducing
the risk of staff being infected and the financial costs, and may
be used for the entire management of patients with TBTB in the
future.
Supplementary Material
Changes in serum (A) TGF-β1 and (B)
PINP levels in two patients with airway stenosis prior to each
bronchoscopy. The time interval between the bronchoscopies was 1
month. TGF-β1, transforming growth factor β1; PINP, procollagen
type I N-propeptide.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the National Major Science
and Technology Projects of China (grant no. 2018ZX10302302003).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SG contributed to the conception and design of the
study and the manuscript preparation. YW acquired serum samples,
analysed the demographic data and prepared the manuscript. YSL and
YB performed the interventional bronchoscopy, analysed the
measurement results and drafted components of the manuscript. JJ
and XHW acquired and analysed the chest HRCT data and drafted
components of the manuscript. YC, XW and GH assisted in the
experiments, analysed the experimental data and drafted components
of the manuscript. YG and YL contributed to the design of the
study, confirmed and approved the authenticity of the raw data and
drafted components of the manuscript. All authors read and approved
the final manuscript. All authors agreed to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The First Affiliated Hospital of Chongqing Medical University
(Chongqing, China). Informed consent was obtained from all the
subjects whose sera were used in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wingfield T, Cuevas LE, MacPherson P,
Millington KA and Squire SB: Tackling two pandemics: A plea on
world tuberculosis day. Lancet Respir Med. 8:536–538.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dara M, Sotgiu G, Reichler MR, Chiang CY,
Chee CBE and Migliori GB: New diseases and old threats: Lessons
from tuberculosis for the COVID-19 response. Int J Tuberc Lung Dis.
24:544–545. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jung SS, Park HS, Kim JO and Kim SY:
Incidence and clinical predictors of endobronchial tuberculosis in
patients with pulmonary tuberculosis. Respirology. 20:488–495.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Su Z, Cheng Y, Wu Z, Zhang P, Chen W, Zhou
Z, Zhong M, Luo W, Guo W and Li S: Incidence and predictors of
tracheobronchial tuberculosis in pulmonary tuberculosis: A
multicentre, large-scale and prospective study in Southern China.
Respiration. 97:153–159. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lee KCH, Tan S, Goh JK, Hsu AAL and Low
SY: Long-term outcomes of tracheobronchial stenosis due to
tuberculosis (TSTB) in symptomatic patients: Airway intervention
vs. conservative management. J Thorac Dis. 12:3640–3650.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pathak V, Shepherd RW and Shojaee S:
Tracheobronchial tuberculosis. J Thorac Dis. 8:3818–3825.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Meghji J, Lesosky M, Joekes E, Banda P,
Rylance J, Gordon S, Jacob J, Zonderland H, MacPherson P, Corbett
EL, et al: Patient outcomes associated with post-tuberculosis lung
damage in Malawi: A prospective cohort study. Thorax. 75:269–278.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Allwood B, van der Zalm M, Makanda G and
Mortimer K: The long shadow post-tuberculosis. Lancet Infect Dis.
19:1170–71. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang T, Zhang J, Qiu XJ, Wang J, Pei YH
and Wang YL: Scarring airway stenosis in Chinese adults:
Characteristics and interventional bronchoscopy treatment. Chin Med
J (Engl). 131:276–281. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Low SY, Hsu A and Eng P: Interventional
bronchoscopy for tuberculous tracheobronchial stenosis. Eur Respir
J. 24:345–347. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Khvilivitzky K, Trivedi PN and McFadden
PM: Tuberculous tracheobronchial stenosis: Avoiding resection-when
less is more. J Thorac Dis. 9:E779–E782. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pang Y, Liu Y, Du J, Gao J and Li L:
Impact of COVID-19 on tuberculosis control in China. Int J Tuberc
Lung Dis. 24:545–547. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pritchett MA, Oberg CL, Belanger A, De
Cardenas J, Cheng G, Nacheli GC, Franco-Paredes C, Singh J, Toth J,
Zgoda M and Folch E: Society for advanced bronchoscopy consensus
statement and guidelines for bronchoscopy and airway management
amid the COVID-19 pandemic. J Thorac Dis. 12:1781–1798.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Luo F, Darwiche K, Singh S, Torrego A,
Steinfort DP, Gasparini S, Liu D, Zhang W, Fernandez-Bussy S, Herth
FJF and Shah PL: Performing bronchoscopy in times of the COVID-19
pandemic: Practice statement from an international expert panel.
Respiration. 99:417–422. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Reddy PD, Nguyen SA and Deschler D:
Bronchoscopy, laryngoscopy, and esophagoscopy during the COVID-19
pandemic. Head Neck. 42:1634–1637. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lentz RJ and Colt H: Summarizing societal
guidelines regarding bronchoscopy during the COVID-19 pandemic.
Respirology. 25:574–577. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang H, Chen H, Gao B, Xiong W, Zhang X,
Hogarth DK, Sun J, Ke M and Herth FJF: Expert panel consensus
statement on the applications and precaution strategies of
bronchoscopy in patients with COVID-19. Endosc Ultrasound.
9:211–219. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Visca D, Tiberi S, Pontali E, Spanevello A
and Migliori GB: Tuberculosis in the time of COVID-19: Quality of
life and digital innovation. Eur Respir J.
56(2001998)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Burzynski J, Macaraig M, Nilsen D and
Schluger NW: Transforming essential services for tuberculosis
during the COVID-19 pandemic: Lessons from New York City. Int J
Tuberc Lung Dis. 24:735–736. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Verna EC, Serper M, Chu J, Corey K, Fix
OK, Hoyt K, Page KA, Loomba R, Li M, Everson GT, et al: Clinical
research in hepatology in the COVID-19 pandemic and post-pandemic
era: Challenges and the need for innovation. Hepatology.
72:1819–1837. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Schindler SE, Jicha GA, Nelson PT, Keene
CD, Blennow K, Molinuevo JL, Masters CL, Hansson O, Teunissen CE,
Galasko D, et al: Maximizing safety in the conduct of Alzheimer's
disease fluid biomarker research in the era of COVID-19. J
Alzheimers Dis. 76:27–31. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Albert RK and Petty TL: Endobronchial
tuberculosis progressing to bronchial stenosis. Fiberoptic
bronchoscopic manifestations. Chest. 70:537–539. 1976.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mark EJ, Meng F, Kradin RL, Mathisen DJ
and Matsubara O: Idiopathic tracheal stenosis: A clinicopathologic
study of 63 cases and comparison of the pathology with
chondromalacia. Am J Surg Pathol. 32:1138–1143. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Enyuan Q, Mingpeng X, Luoman G, Jinghua G,
Yu L, Wentao L, Changchun H, Lihua L, Xiaoyan M, Lei Z and Guangnan
L: Erythromycin combined with corticosteroid reduced inflammation
and modified trauma-induced tracheal stenosis in a rabbit model.
Ther Adv Respir Dis. 12(1753466618773707)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xiao Y, Zhou L, Zhang T, Qin C, Wei P, Luo
L, Luo L, Huang G, Chen A and Liu G: Anti-fibrosis activity of
quercetin attenuates rabbit tracheal stenosis via the
TGF-β/AKT/mTOR signaling pathway. Life Sci.
250(117552)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zile MR, O'Meara E, Claggett B, Prescott
MF, Solomon SD, Swedberg K, Packer M, McMurray JJV, Shi V,
Lefkowitz M and Rouleau J: Effects of sacubitril/valsartan on
biomarkers of extracellular matrix regulation in patients with
HFrEF. J Am Coll Cardiol. 73:795–806. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lodyga M and Hinz B: TGF-β1-A truly
transforming growth factor in fibrosis and immunity. Semin Cell Dev
Biol. 101:123–139. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kim Y, Kim K, Joe J, Park H, Lee M, Kim Y,
Choi Y and Park S: Changes in the levels of interferon-gamma and
transforming growth factor-beta influence bronchial stenosis during
the treatment of endobronchial tuberculosis. Respiration.
74:202–207. 2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dantas AT, Gonçalves SM, de Almeida AR,
Gonçalves RS, Sampaio MC, Vilar KM, Pereira MC, Rêgo MJ, Pitta ID,
Marques CD, et al: Reassessing the role of the active TGF-β1
as a biomarker in systemic sclerosis: Association of serum levels
with clinical manifestations. Dis Markers.
2016(6064830)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Boothe DL, Coplowitz S, Greenwood E,
Barney CL, Christos PJ, Parashar B, Nori D, Chao KS and Wernicke
AG: Transforming growth factor β-1 (TGF-β1) is a
serum biomarker of radiation induced fibrosis in patients treated
with intracavitary accelerated partial breast irradiation:
Preliminary results of a prospective study. Int J Radiat Oncol Biol
Phys. 87:1030–1036. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tian Y, Wang Y, Chen W, Yin Y and Qin M:
Role of serum TGF-β1 level in atrial fibrosis and outcome
after catheter ablation for paroxysmal atrial fibrillation.
Medicine (Baltimore). 96(e9210)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Szulc P, Naylor K, Hoyle NR, Eastell R and
Leary ET: National Bone Health Alliance Bone Turnover Marker
Project. Use of CTX-I and PINP as bone turnover markers: National
Bone Health Alliance recommendations to standardize sample handling
and patient preparation to reduce pre-analytical variability.
Osteoporos Int. 28:2541–2556. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Abdul Alim M, Domeij-Arverud E, Nilsson G,
Edman G and Ackermann PW: Achilles tendon rupture healing is
enhanced by intermittent pneumatic compression upregulating
collagen type I synthesis. Knee Surg Sports Traumatol Arthrosc.
26:2021–2029. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gudowska-Sawczuk M, Wrona A, Gruszewska E,
Cylwik B, Panasiuk A, Flisiak R and Chrostek L: Serum level of
interleukin-6 (IL-6) and N-terminal propeptide of procollagen type
I (PINP) in patients with liver diseases. Scand J Clin Lab Invest.
78:125–130. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Freitag L, Ernst A, Unger M, Kovitz K and
Marquette CH: A proposed classification system of central airway
stenosis. Eur Respir J. 30:7–12. 2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kenyon NJ, Ward RW, McGrew G and Last JA:
TGF-beta1 causes airway fibrosis and increased collagen I and III
mRNA in mice. Thorax. 58:772–777. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang B, Komers R, Carew R, Winbanks CE, Xu
B, Herman-Edelstein M, Koh P, Thomas M, Jandeleit-Dahm K,
Gregorevic P, et al: Suppression of microRNA-29 expression by
TGF-β1 promotes collagen expression and renal fibrosis. J Am
Soc Nephrol. 23:252–265. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Iglesias-de la Cruz MC, Ziyadeh FN, Isono
M, Kouahou M, Han DC, Kalluri R, Mundel P and Chen S: Effects of
high glucose and TGF-beta1 on the expression of collagen IV and
vascular endothelial growth factor in mouse podocytes. Kidney Int.
62:901–913. 2002.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Giménez A, Duch P, Puig M, Gabasa M,
Xaubet A and Alcaraz J: Dysregulated collagen homeostasis by matrix
stiffening and TGF-β1 in fibroblasts from idiopathic
pulmonary fibrosis patients: Role of FAK/Akt. Int J Mol Sci.
18(2431)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Selvarajah B, Azuelos I, Platé M,
Guillotin D, Forty EJ, Contento G, Woodcock HV, Redding M, Taylor
A, Brunori G, et al: mTORC1 amplifies the ATF4-dependent de novo
serine-glycine pathway to supply glycine during
TGF-β1-induced collagen biosynthesis. Sci Signal.
12(eaav3048)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tian M, Chang X, Zhang Q, Li C, Li S and
Sun Y: TGF-β1 mediated MAPK signaling pathway promotes
collagen formation induced by Nano NiO in A549 cells. Environ
Toxicol. 34:719–727. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hwang HS, Lee MH and Kim HA:
TGF-β1-induced expression of collagen type II and ACAN is
regulated by 4E-BP1, a repressor of translation. FASEB J.
34:9531–9546. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sucena M, Amorim A, Machado A, Hespanhol V
and Magalhães A: Endobronchial tuberculosis-clinical and
bronchoscopic features. Rev Port Pneumol. 10:383–391.
2004.PubMed/NCBI View Article : Google Scholar : (In
Portuguese).
|
|
44
|
Li Z, Mao G, Gui Q and Xu C: Bronchoplasty
for treating the whole lung atelectasis caused by endobronchial
tuberculosis in main bronchus. J Thorac Dis. 10:4000–4005.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lee JH, Park SS, Lee DH, Shin DH, Yang SC
and Yoo BM: Endobronchial tuberculosis. Clinical and bronchoscopic
features in 121 cases. Chest. 102:990–994. 1992.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Christine T, Tarigan AP and Ananda FR: The
correlation between levels of transforming growth factor-β
with Pulmonary Fibrosis In Post Pulmonary Tuberculosis In Medan,
North Sumatera-Indonesia. Open Access Maced J Med Sci. 7:2075–2078.
2019.PubMed/NCBI View Article : Google Scholar
|