Introduction
Lung cancer remains the dominant cause of
cancer-associated deaths worldwide, and its incidence has been
increasing year by year (1). Lung
cancer is separated into two clinical types: Small cell lung cancer
and non-small cell lung cancer (NSCLC). NSCLC is the most common
subtype, accounting for 85% of confirmed cases (1). For patients with intermediate and
advanced lung cancer, who have lost the opportunity for surgery,
radiotherapy is one of the most effective treatment methods, which
can prolong the survival time of patients (2). However, NSCLC, especially lung
adenocarcinoma, is less sensitive to radiotherapy than other types
of lung cancer (3). Radiation
resistance can significantly affect treatment efficiency.
Therefore, research on radiotherapy sensitizers has gradually
become a focus in the field of radiobiology study in recent years
(4). In this regard, considering
the requirements of biosafety, low toxicity, antioxidant properties
and public acceptance, natural compounds are regarded as ideal
research targets for choosing a sensitizer (5). Resveratrol is a non-flavonoid
polyphenol compound extracted from Polygonum cuspidatum,
grapes and peanuts (6). It has
antitumor, antioxidant, antiaging and immune regulation effects
(7). A number of studies have shown
that resveratrol has the potential as a radiotherapy sensitizer as
it can increase the radiosensitivity of tumor cells and promote
tumor cell death via different subcellular pathways (8-10).
However, the mechanisms are complex and researchers have not
reached a consensus. Recently, studies have found that resveratrol
can trigger calcium homeostasis imbalance inside and outside of the
cell, thereby reducing mitochondrial membrane potential (Δψm) and
triggering pro-apoptotic protein release, eventually promoting
tumor cell death (11-13).
Calcium, as a signal transduction molecule, not only
regulates a series of cell activities including transcription,
proliferation, migration and death processes but also affects the
development and metastasis of tumors (14). There have been an increasing number
of studies focusing on store-operated calcium channel (SOCC) and
its function in regulating calcium homeostasis (15-17).
Store-operated calcium entry (SOCE) mediated by SOCC exists in
several types of tumor cells and plays an important role in tumor
migration and invasion (18). SOCE
is an essential way to mediate extracellular Ca2+ to
flow inside the cell, following endoplasmic reticulum (ER) calcium
depletion regulated by matrix stromal interaction molecule (STIM1)
located on the ER, and a calcium release-activated calcium channel
protein (Orai1) located on the cell membrane (19). After the depletion of ER calcium
storage, STIM1 protein can sense the change in Ca2+
concentration through its structure and activate reactions such as
rapid translocation and polymerization (20). STIM1 subsequently couples with Orai1
protein on the plasma membrane to achieve functional opening of the
SOCE pathway and promote Ca2+ influx from the outside
(21). When Ca2+ is
replenished in the calcium pool, STIM1 protein and Orai1 protein
are slowly dissociated and inactivated, and the pathway is closed.
In mammals, STIM proteins often express two homologs, STIM1 and
STIM2, and Orai has three homologs including Orai1, Orai2 and
Orai3(22). Resveratrol activated
autophagic cell death in prostate, colorectal and myeloma cancer
cells via the downregulation of STIM1 and SOCE (23,24).
However, to the best of our knowledge, the effects of resveratrol
on SOCE or SOCC in NSCLC have not been thoroughly studied.
The present study aimed at investigating the effects
of resveratrol on the radiosensitization of lung adenocarcinoma
A549 cells and demonstrating whether it can regulate SOCE activity
to achieve the sensitization effect by affecting STIM or Orai
expression. The findings may provide a basis for improving
radiotherapy effects and prognosis of patients with advanced lung
cancer.
Materials and methods
Cell culture, drug administration and
radiation therapy
Lung adenocarcinoma A549 cells were kindly donated
by The Cell Bank of Type Culture Collection of The Chinese Academy
of Sciences. Cells were cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) containing a mixture of 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 1% penicillin and 1%
streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at 37˚C and 5%
CO2, as previously described (9). A549 cells at the logarithmic growth
phase were seeded (6x105 cells/well) into six-well
culture dishes. Once the cells have adhered, resveratrol
(Sigma-Aldrich; Merck KGaA) was dissolved in dimethylsulfoxide
(Sigma-Aldrich; Merck KGaA) to produce a stock solution of 10,000
µM, and stored at -20˚C. Different concentrations of resveratrol
(0, 10, 50, 100, 150 and 200 µmol/l) were added into each dish and
incubated for 24 h at 37˚C, and the resveratrol-containing medium
was removed with a pipette. The same resveratrol treatment groups
were prepared and irradiated with 0, 2, 4 and 6 Gy X-rays (room
temperature, 6 MV X-rays, 300 cGy/min, the distance from the source
to the specimen was 100 cm and the size of the irradiation field
was 20x20 cm). The culture was continued for 4 h at 37˚C after
irradiation for the following experiments.
Cell counting
Cell counting was performed to perform an
approximate evaluation of cell damage from irradiation and
resveratrol treatment. In total, 0.8x106 A549 cells were
seeded into 60-mm culture dish to conduct the following experiment.
After receiving corresponding experimental treatments, the culture
medium was replaced to wash out dead cells and the remaining A549
cells growing on the bottom of the 60-mm culture dish were placed
under a light microscope (magnification, x40) to check the overall
growth state. In order to quantify the cell number, A549 cells were
resuspended in 1X PBS and 100 µl cell suspension was added to the
corresponding chip, which was inserted into the cell counting
machine (Scepter2.0; Sigma-Aldrich; Merck KGaA) in order to
calculate the concentration of the remaining cells within each
100-µl A549 cell suspension. Together, the data were used to
calculate the total remaining A549 cell number within an entire
50-mm culture dish.
Cell counting Kit-8 (CCK-8) assay
CCK-8 assay was performed to check the cell
viability after respective experimental treatments. A549 cells in
the logarithmic growth phase were dissociated with trypsin and
5x103 cells/well were added to a 96-well plate. Once the
cells had adhered to the well, they were divided into different
groups and treated with various concentrations of resveratrol and
different doses of irradiation, as aforementioned. After incubation
at 5% CO2 and 37˚C for 4 h, 10 µl CCK-8 solution
(Beyotime Institute of Biotechnology) was added to each well. After
2 h of incubation, the optical density of each well was measured at
a wavelength of 450 nm on an enzyme-linked immunosorbent detector
(Beyotime Institute of Biotechnology).
Δψm
A549 cells (2.5x105 cells/ml) were seeded
onto each well of a 24-well plate and incubated for 24 h. After
treatment with different doses of resveratrol (0, 10, 50, 100, 150
and 200 μM) combined with different doses of irradiation (0, 2, 4
and 6 Gy), the culture medium was removed from each well and the
cells were washed twice with PBS at room temperature. Rhodamine 123
powder (Dalian Meilun Biology Technology Co., Ltd.) was prepared
into a 1 mg/ml stock solution with methanol and stored at -20˚C.
The cells were incubated with 5 µg/ml rhodamine 123 for 30 min in a
5% CO2 cell incubator at room temperature, then washed
with PBS two or three times, and finally 0.5 ml PBS was added to
each well. The plate was placed under a fluorescent microscope
(Nikon M50; Nikon Corporation) with parameters initially set to
detect fluorescence of rhodamine 123 (480/40 excitation filter, 505
long pass dichroic mirror and 535/40 emission filter;
magnification, x20) and red fluorescence dots indicated
mitochondria inside the cells (10). Briefly, once a clear visual field
with 200-300 cells is achieved, images were captured at five
different and qualified visual fields randomly within one well for
each experimental group. The fluorescence intensity was reversely
associated with Δψm and mean fluorescent intensity within an image
was analyzed using ImageJ 1.8.0 software (National Institutes of
Health).
Western blotting
RIPA buffer (Beyotime Institute of Biotechnology)
was used to extract total cell protein in different dose groups,
and the protein concentration was determined spectrophotometrically
using a BCA assay (Beyotime Institute of Biotechnology). After
boiling and denaturing, 30 µg proteins were separated by 10%
SDS-PAGE and transferred to a PVDF film (EMD Millipore). TBS-T (50
mM Tris-HCl, pH 7.6, 150 mM NaCl, and 0.1% Tween-20) containing 5%
skim milk was used to block the membranes for 2 h room temperature.
The following primary antibodies (diluted 1:1,000; all ProteinTech
Group, Inc.), were added and incubated at 4˚C overnight: Rabbit
anti-human STIM1 (cat. no. 11565-1-AP), STIM2 (cat. no.
21192-1-AP), mouse anti-human Orai1 (cat. no. 66223-1-lg), Orai2
(cat. no. 20592-1-AP) and Orai3 (cat. no. 25766-1-AP). β-actin
(cat. no. 66009-1-Ig) were used as an internal control. The
following day, TBST was used to wash the membrane for 10 min, and
HRP-labeled anti-rabbit or anti-mouse antibodies were added
(1:2,000; cat. nos. SA00001-1 and 11565-1-AP, respectively;
ProteinTech Group, Inc.). After 1 h incubation at room temperature,
the membranes were washed for 10 min three times with PBS-T, and
the bands were visualized using an ECL development gel imaging
system (ImageQuant LAS 500; Cytiva). Each experiment was repeated
at least three times. Densitometry for each graph was conducted
using ImageJ 1.8.0 software (National Institutes of Health).
Calcium imaging
After experimental treatment, a round coverslip was
placed on the bottom of a 12-well plate using tweezers, and 150 µl
100 µg/ml polylysine was added to the middle of each coverslip. The
polylysine was then removed by pipette after 10 min. A total of
4x104 cells were seeded on circular coverslips.
Following incubation for 8 h with culture medium, 10 µmol/l Fluo-8
AM (cat. no. ab112129; Abcam), a fluorescent probe for
intracellular Ca2+ ([Ca2+]i), was added and
incubated at 37˚C for 30 min. The sample was washed twice with
Ca2+-free PBS buffer (standard external solution
including 10 µM HEPES, 120 µM NaCl, 5.4 µM KCl, 1 µM
MgCl2 and 10 µM glucose; pH 7.4) and calcium
fluorescence was detected under a fluorescent microscope with
MetaFluor software 7.0 (Molecular Devices, LLC) (magnification,
x200). Briefly, stained cells were initially observed under white
light, by adjusting the focus, opening the fluorescence and
detecting the cells in darkness. The software tools were used to
capture each cell outline within the visual field and their
fluorescence intensity was recorded. When the fluorescence
intensity curve become stable for 3 min, Ca2+ release
was induced by 2 µmol/l thapsigargin (cat. no 12758; Cell Signaling
Technologies, Inc.) administration and left for another 5 min until
the fluorescent curve become stable. Extracellular Ca2+
solution (cat. no. c1016; Sigma-Aldrich; Merck KGaA; 1 mmol/l) was
then added to induce calcium influx with anther fluorescent peak
and left for 5 min until the fluorescent curve became stable again.
The process was observed under a fluorescence microscope (Nikon
M50; Nikon Corporation) at 488 nm excitation and 515 nm emission
wavelengths (magnification, x200). The change in [Ca2+]i
was compared with the fluorescence intensity (F0) before adding
extracellular Ca2+. SOCE was demonstrated as the ratio
of peak intensity (F1)/F0 and the intensity of each fluorescence
data was an average of 20-30 cells (11). Data were collected and analyzed with
MetaFluor software7.0 (Molecular Devices LLC).
Cell transfection
The Orai1 cDNA sequence was inserted into a
pCMVPuro01 expression vector (Guangzhou Sinogen Pharmaceutical Co.,
Ltd.). The forward and reverse primer sequences for constructing
the vectors were 5'-CTAGTCTAGAATGCATCCGGAGCCCGCCC-3' and
5'-TCCTTCGAACTAGGCATAGTGGCTGCCGG-3'. The STIM1 cDNA sequence was
also inserted into a pCMVPuro01 expression vector (Guangzhou
Sinogen Pharmaceutical Co., Ltd.). The forward and reverse primer
sequences for constructing the vectors were
5'-AAGAGTCTACCGAAGCAG-3' and 5'-GTGCTATGTTTCACTGTTGG-3'. Empty
vectors were used as a negative control. Briefly, an appropriate
number of cells (4x104) were seeded into each well
(12-well dish) with 1 ml medium and incubated at 37˚C and 5%
CO2 until the cells reached 80-90% confluence for
transfection. Orai1 overexpression vector (100 pmol) and/or STIM1
overexpression vector (100 pmol) were added to 100 µl Opti-MEM I
reducing serum medium (Invitrogen; Thermo Fisher Scientific, Inc.)
within an Eppendorf tube, and 5 µl Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) was added to the same
tube. The tube was gently agitated and incubated at room
temperature for 25 min to form Lipofectamine complexes. The old
medium was aspirated, and 1 ml serum-free medium was added. The
Lipofectamine complex (100 µl) was added to each well and mixed
gently. The cells were incubated at 37˚C and 5% CO2 for
8 h and the serum-free medium was replaced with normal medium.
Transfection efficiency was assessed via western blotting after 48
h.
Statistical analysis
All experiments were repeated independently at least
three times. Data are presented as the mean ± SD. Multiple
comparison analysis was conducted using one-way ANOVA followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference. All analyses were performed
with GraphPad Prism 5.0 (GraphPad Software, Inc.) and SPSS 19.0
(IBM Corp.).
Results
Effects of resveratrol combined with
irradiation treatment on proliferation and apoptosis of A549
cells
A549 cells at the logarithmic growth phase were
divided into different experimental groups, receiving a series of
concentrations of resveratrol (0, 10, 50, 100, 150 and 200 µmol/l)
combined with different doses (0, 2, 4 and 6 Gy) of X-ray
irradiation. After experimental treatment, cell growth was
observed, and cell numbers were counted and compared under a light
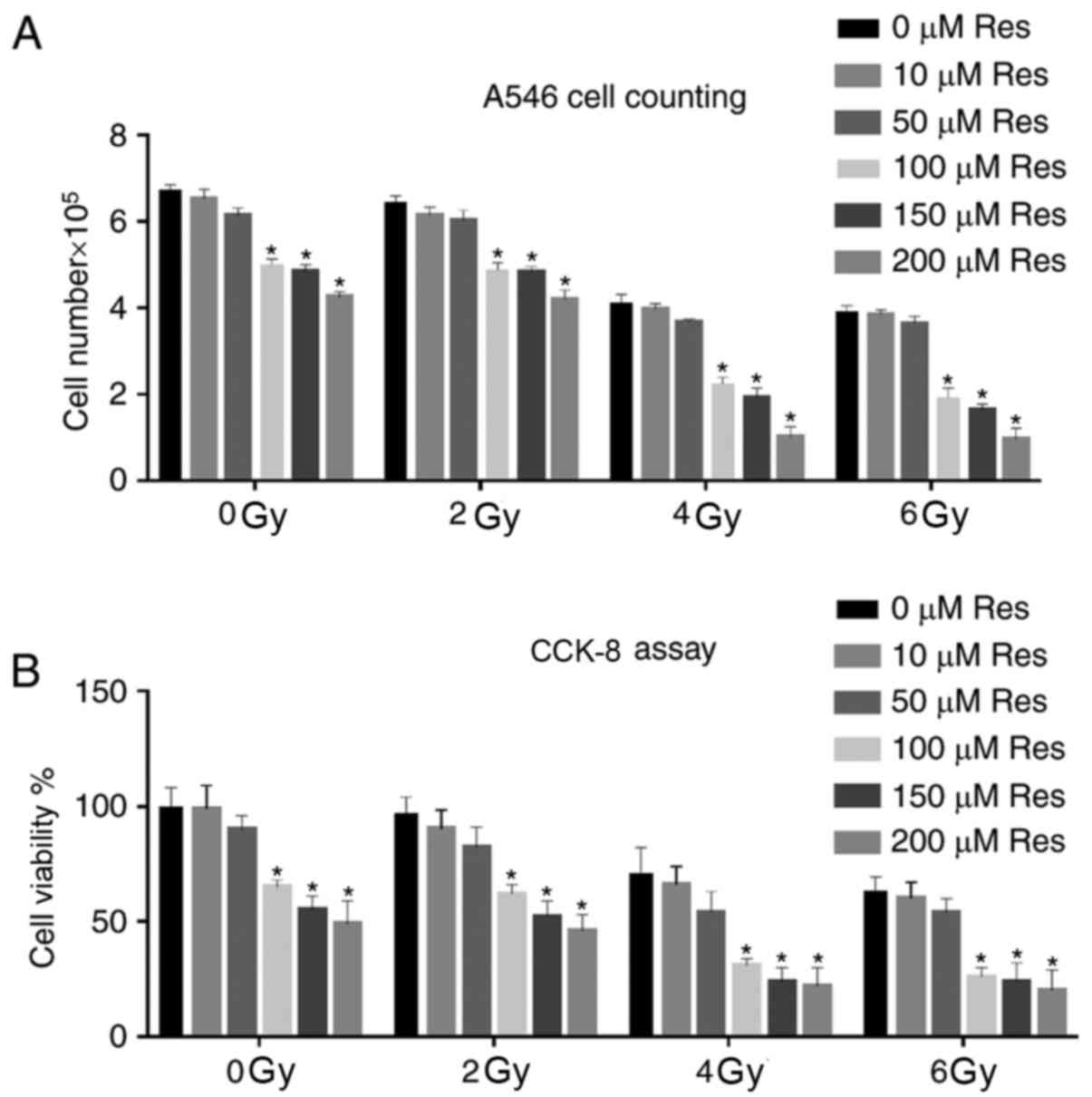
microscope. The data showed that increased cell death and reduced
cell numbers depended on resveratrol and irradiation dose. For each
irradiation dose, compared with the 0 µM Res group, a significant
cell number reduction was observed initially from 100 µM
resveratrol treatment group. Among all the irradiation doses with
100 µM resveratrol, a significant cell number reduction was
observed between 2 and 4Gy. Although combination of higher doses of
irradiation and resveratrol also significantly reduced the cell
number, based on the principle that the least but significant
dosage might be the best, the combination of 4 Gy irradiation and
100 µM resveratrol could be used as an ideal treatment strategy for
the following experiments (Fig.
1A). CCK-8 assay was further used to detect A549 cell viability
in different dose groups. Similar to light microscopy results,
irradiation promoted the reduction of cell viability, and
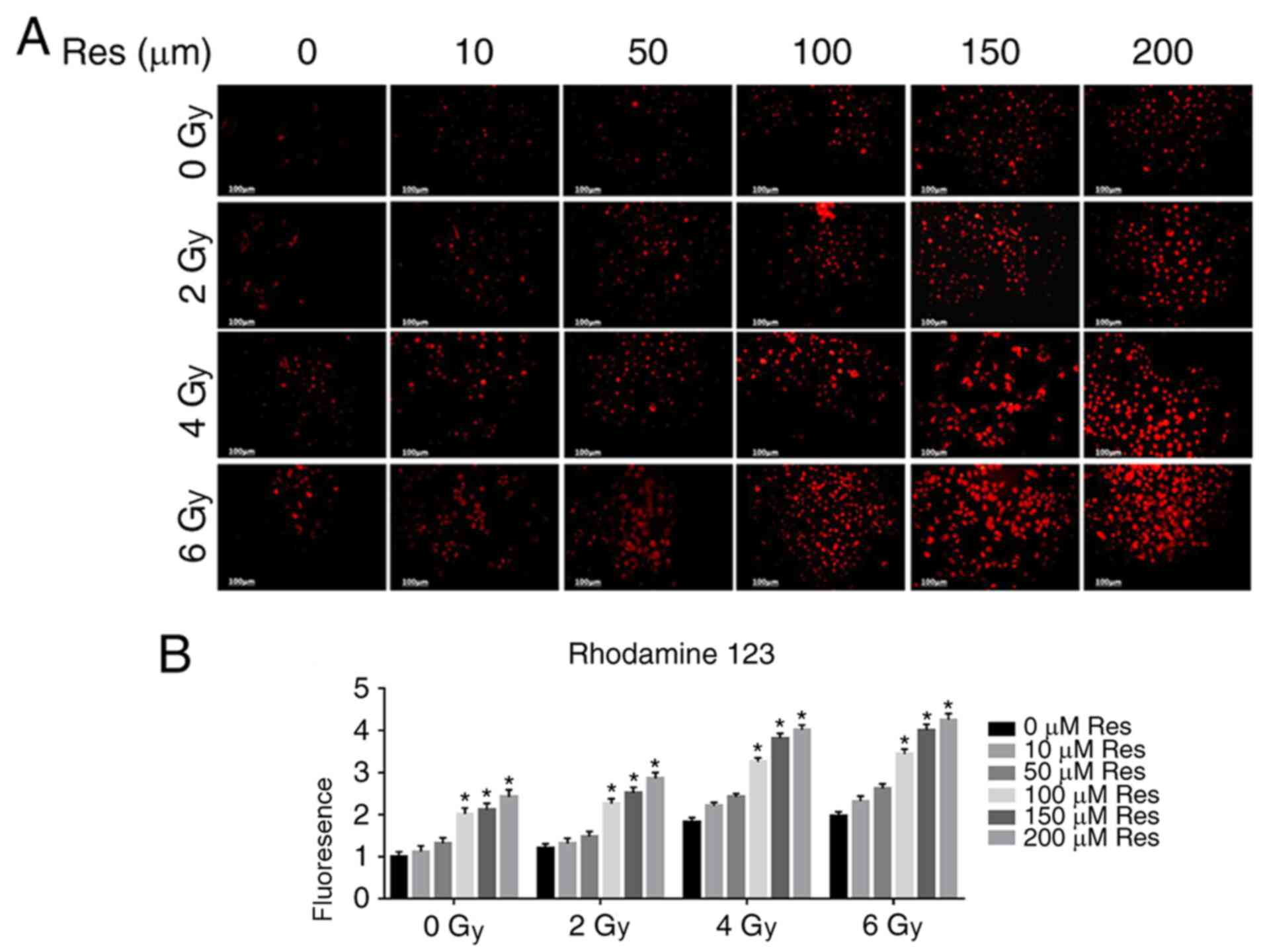
resveratrol pretreatment enhanced this effect (Fig. 1B). The Δψm is a key parameter of
mitochondrial function, and its decrease is often associated with
apoptosis (25). Therefore, the
fluorescent lipophilic cationic dye rhodamine 123 was used for Δψm
detection in each group. As a positively charged dye, rhodamine 123
will accumulate in the mitochondria in an inverse proportion to the
Δψm. The accumulation of fluorescent dyes in mitochondria was
detected by confocal microscopy to evaluate Δψm to compare
apoptosis (26). Fluorescent
microscopy showed that both resveratrol and irradiation treatment
reduced the Δψm of A549 cells in a dose-dependent manner and the
combination of resveratrol and irradiation enhanced this effect
(Fig. 2A and B), which was also consistent with
aforementioned experiments (Fig.
1).
Effects of resveratrol combined with
irradiation treatment on thapsigargin-induced SOCE in lung
adenocarcinoma A549 cells
The aforementioned results indicated that
resveratrol had the potential to be developed as an irradiation
sensitizer. Thus, in order to further investigate the subcellular
mechanism, the effects of resveratrol combined with irradiation
treatment on thapsigargin-induced SOCE in lung adenocarcinoma A549
cells were detected using calcium imaging in four experimental
groups: Blank control, 4 Gy irradiation, 100 µM resveratrol and 4
Gy irradiation + 100 µM resveratrol using Fluo-8, an intracellular
calcium fluorescent probe. When the fluorescence intensity curve
was detected to be stable, thapsigargin was utilized to cause
calcium storage depletion, and the level of intracellular calcium
rapidly increased and then decreased to normal levels.
Subsequently, extracellular calcium was added to trigger SOCE,
which demonstrated another instant increase in intracellular
fluorescence (Fig. 3B). Based on
the results, the peak level of fluorescence intensity was
significantly reduced by resveratrol and this reduction was
enhanced when combined with irradiation, indicating that SOCE was
significantly inhibited by the addition of resveratrol (Fig. 3A and C).
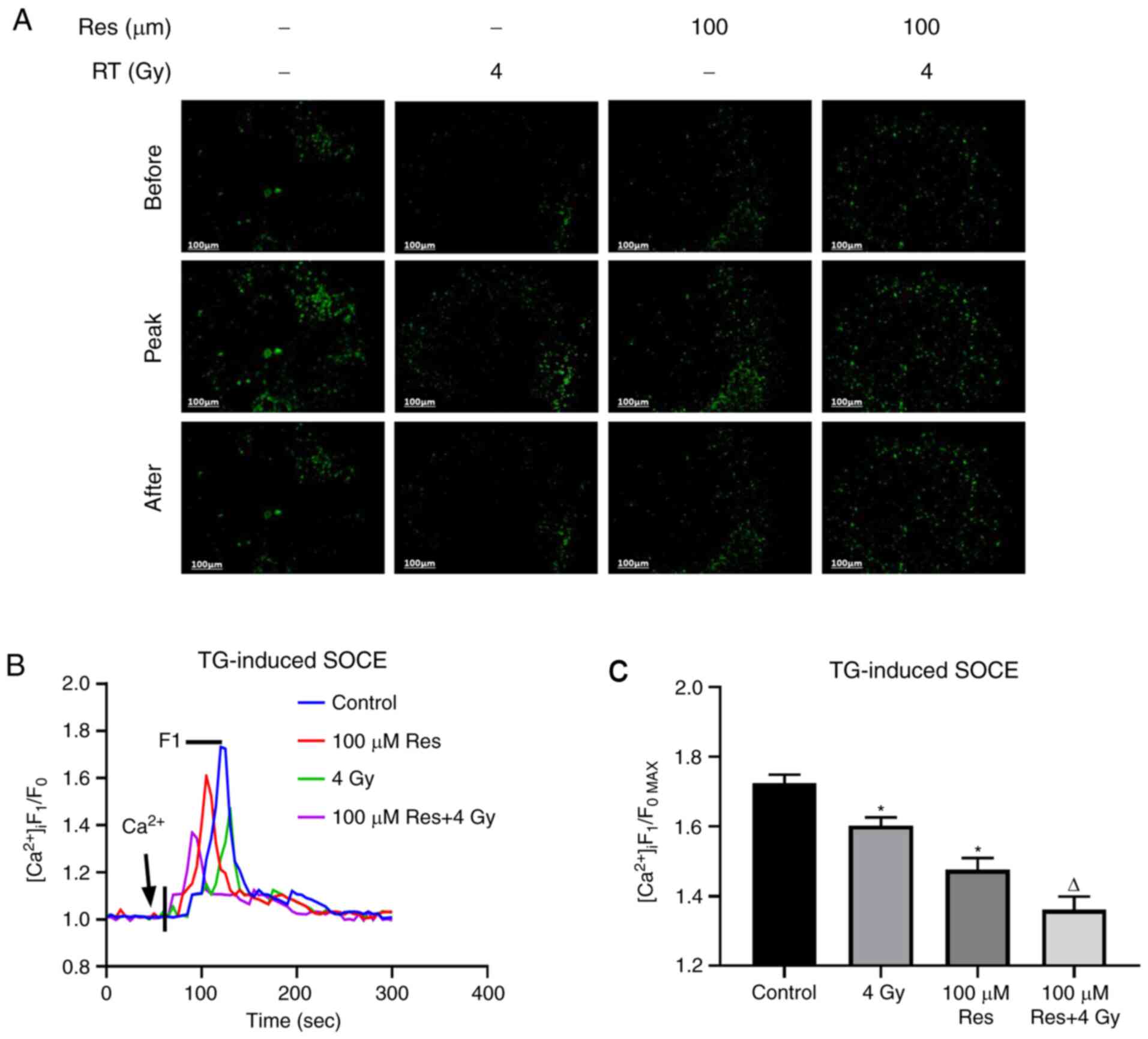 | Figure 3Effects of a range of doses of
resveratrol and irradiation treatment on SOCE in A549 cells. Lung
cancer A549 cells were treated with 100 µM resveratrol, 4 Gy
irradiation or both, and untreated. [Ca2+]i was labeled
with sensitive fluorescent indicator Fluo-8 AM and its real-time
fluorescence intensity was detected. After Ca2+ storage
depletion in A549 cells of different dose groups induced by 2
µmol/l TG, 1 mmol/l extracellular Ca2+ was added to
trigger SOCE. (A) Representative fluorescence images, (B)
fluorescence intensity curve and (C) summarized data of SOCE are
shown. The change in [Ca2+]i was illustrated as the
ratio of F1 and F0. SOCE was demonstrated as the
F1/F0max and the intensity of fluorescence was an
average of 20-30 cells. Magnification, x200. Values are shown as
the mean ± standard deviation. n=3. *P<0.05. vs.
control; ∆P<0.05. vs. 4 Gy. Before, images of
Fluo-8-labeled A549 cells before addition of extracellular
Ca2+; Peak, images of Fluo-8-labeled A549 cells giving
off strongest fluorescence following addition of extracellular
Ca2+; After, images of Fluo-8-labeled A549 cells at 5
min after addition of extracellular Ca2+; STOCE,
store-operated calcium entry; RT, radiotherapy; TG, thapsigargin;
Res, resveratrol; [Ca2+]i, intracellular
Ca2+; F1, real-time fluorescence intensity; F0,
fluorescence intensity before the addition of extracellular
calcium; F1/F0max, ratio of peak intensity. |
Effects of resveratrol combined with
irradiation treatment on SOCCs in lung adenocarcinoma A549
cells
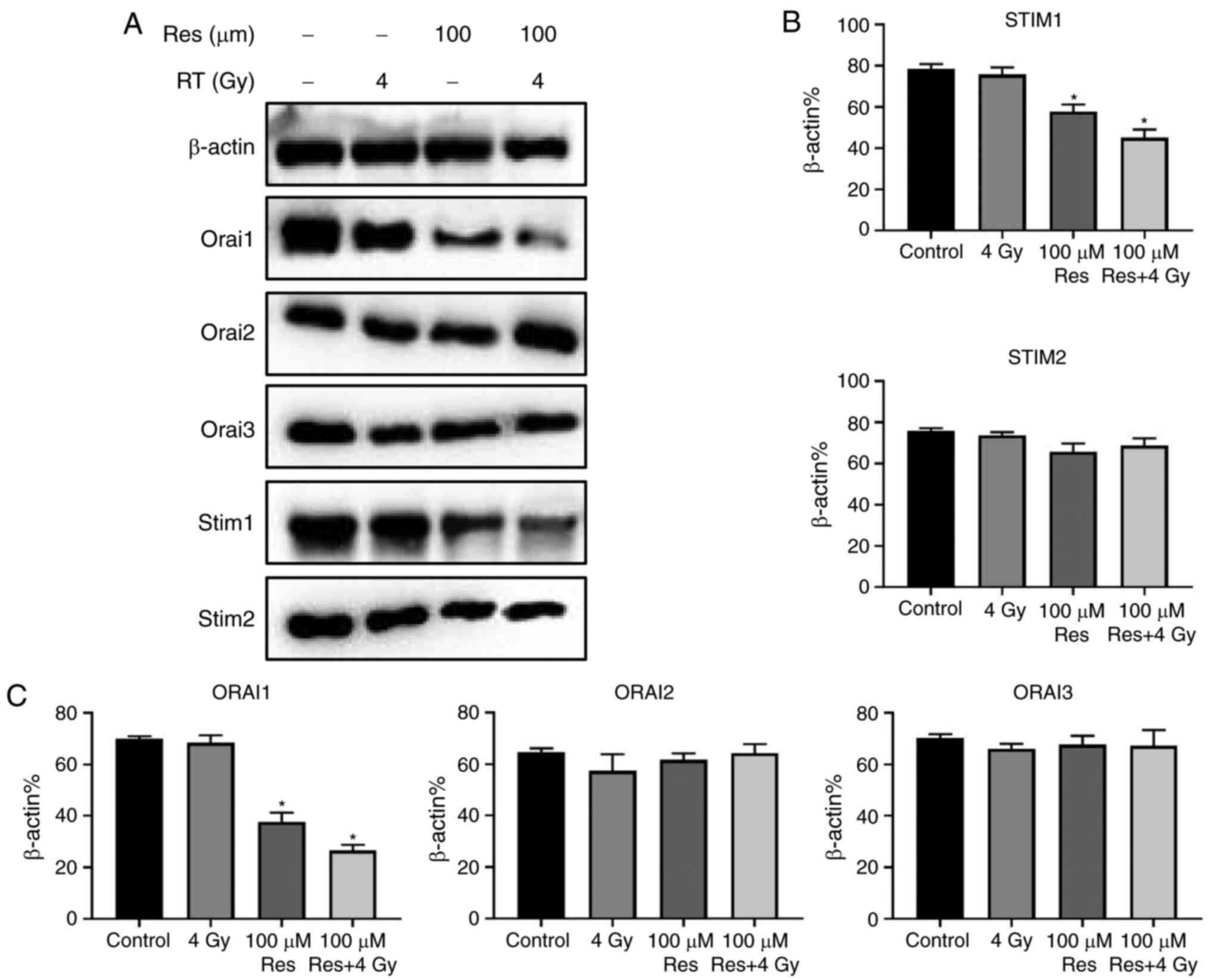
STIM and Orai family members are the SOCCs mediating
the SOCE process in non-excited cells, responsible for inflow,
storage and consumption of calcium ions (13). Thus, western blotting was used to
detect the expression of STIM1, STIM2, Orai1, Orai2 and Orai3
proteins in four experimental groups: Blank control, 4 Gy
irradiation, 100 µM resveratrol and 4 Gy irradiation + 100 µM
resveratrol. The results showed that compared with controls,
resveratrol alone or resveratrol combined with irradiation
treatment had no significant effect on the expression of STIM2,
Orai2 and Orai3 in A549 cells but significantly suppressed the
expression of STIM1 and Orai1. Among the four groups, combined
treatment exerted the strongest effects (Fig. 4A and B). To some extent, these results indicated
that resveratrol-sensitized irradiation damage in A549 cells may be
associated with STIM1 and Orai1 downregulation that leads to SOCE
suppression.
Overexpression of STIM1 and Orai1
partly reduces resveratrol-sensitized lung adenocarcinoma A549 cell
damage under irradiation
Downregulation of SOCE was associated with apoptosis
in various cell types, including osteosarcoma, skin cancer and
renal cancer (27-29).
According to the aforementioned results, it was found that
resveratrol can inhibit SOCE by downregulating the expression of
STIM1 and Orai1, further leading to A549 cell death. To further
verify this result, STIM1 and Orai1 were overexpressed, following
resveratrol treatment and irradiation, in order to observe whether
the recovery of STIM1 and Orai1 could restore SOCE and cell death
in A549 cells. Western blotting images (Fig. S1A and B) and corresponding analysis (Fig. S1C and D) demonstrated that the vectors were
transfected successfully. The expression levels of STIM1 and Orai1
proteins, following respective or combined treatment with Orai1 and
STIM1 overexpression vectors after administration of both
resveratrol and irradiation, were significantly increased compared
with the pure combined treatment group with resveratrol and
irradiation (Fig. 5A and B). Calcium imaging (Fig. 6A and B) and corresponding analysis of SOCE
(Fig. 6C) in each group were partly
consistent with our hypothesis. Although the results were not
statistically significant, the SOCE of the combined resveratrol and
irradiation treatment group was partly restored after adding both
STIM1 and Orai1 overexpression vectors compared with single
treatment groups. Additionally, CCK-8 results indicated that cell
viability of the resveratrol combined with irradiation treatment
group was partly restored after adding both STIM1 and Orai1
overexpression vectors compared with single treatment groups
(Fig. 5C). Based on the
aforementioned findings, the role of resveratrol in sensitizing
A549 cells to irradiation treatment may be associated with
downregulating the expression of STIM1 and Orai1, inhibiting the
SOCE process and triggering cell death.
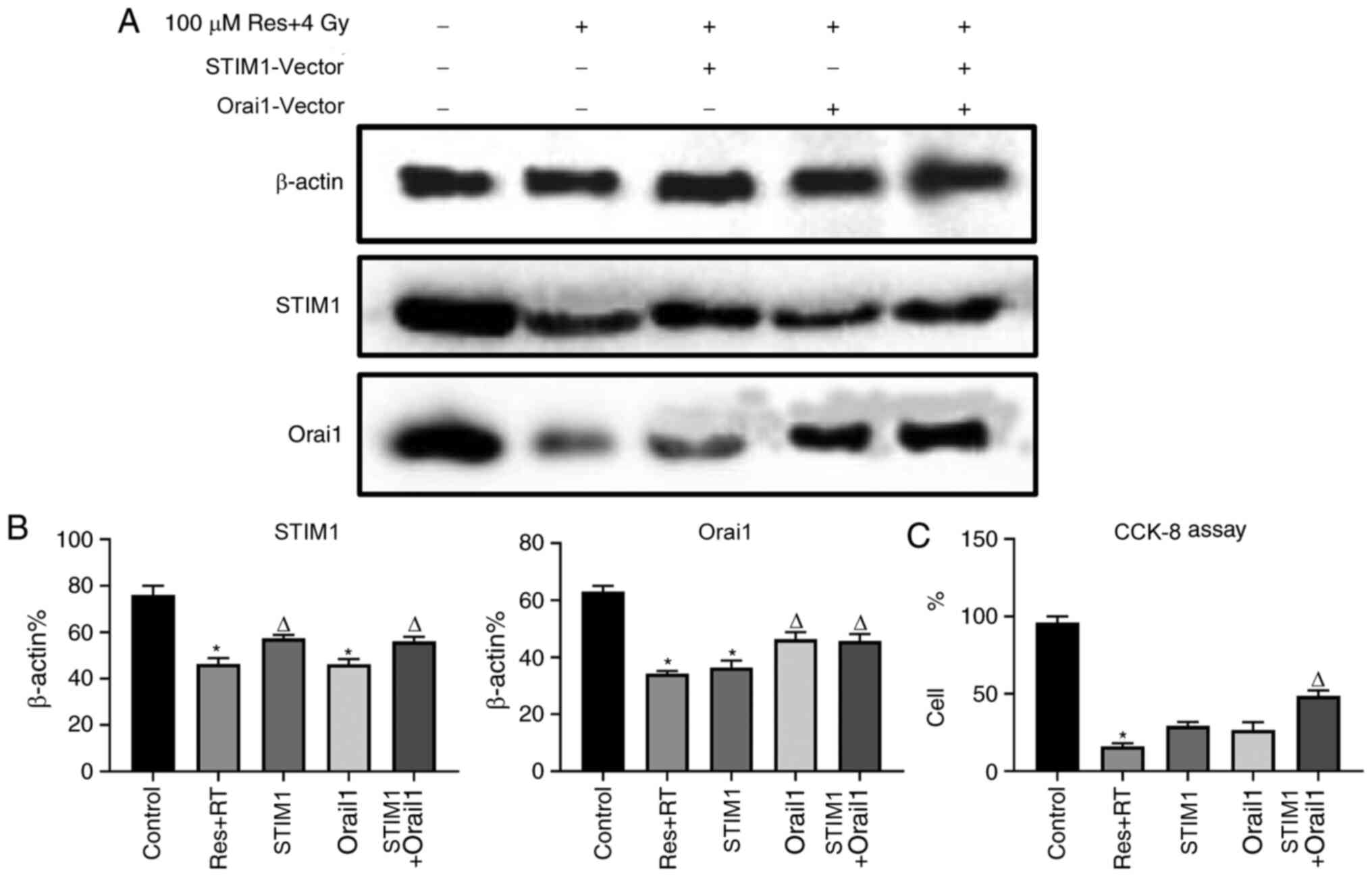 | Figure 5Effects of various doses of
resveratrol and irradiation treatment following Orai1, STIM1 or
dual overexpression of STIM1 or Orai1 in A549 cells. After the
cells were cultured to the logarithmic phase, they were divided
into five experimental groups: Untreated, 4 Gy irradiation + 100 µM
resveratrol, 4 Gy irradiation + 100 µM resveratrol + STIM1
overexpression vector, 4 Gy irradiation + 100 µM resveratrol +
Orai1 overexpression vector group and 4 Gy irradiation + 100 µM
resveratrol + STIM1 + Orai1 overexpression vector. Following
treatment, western blotting was used to detect the levels of STIM1
and ORAI1 protein expression in each group. (A) Corresponding
images and (B) analysis show the expression levels of STIM1 and
Orai1 protein after experimental treatment. (C) Cell viability in
each group was detected using a CCK-8 assay. Values are shown as
the mean ± standard deviation n=3. *P<0.05. vs.
control. ∆P<0.05. vs. Res + RT. RT, radiotherapy;
STIM, stromal interaction molecule; CCK-8, Cell Counting Kit-8;
Orai, calcium release-activated calcium channel protein; Res,
resveratrol. |
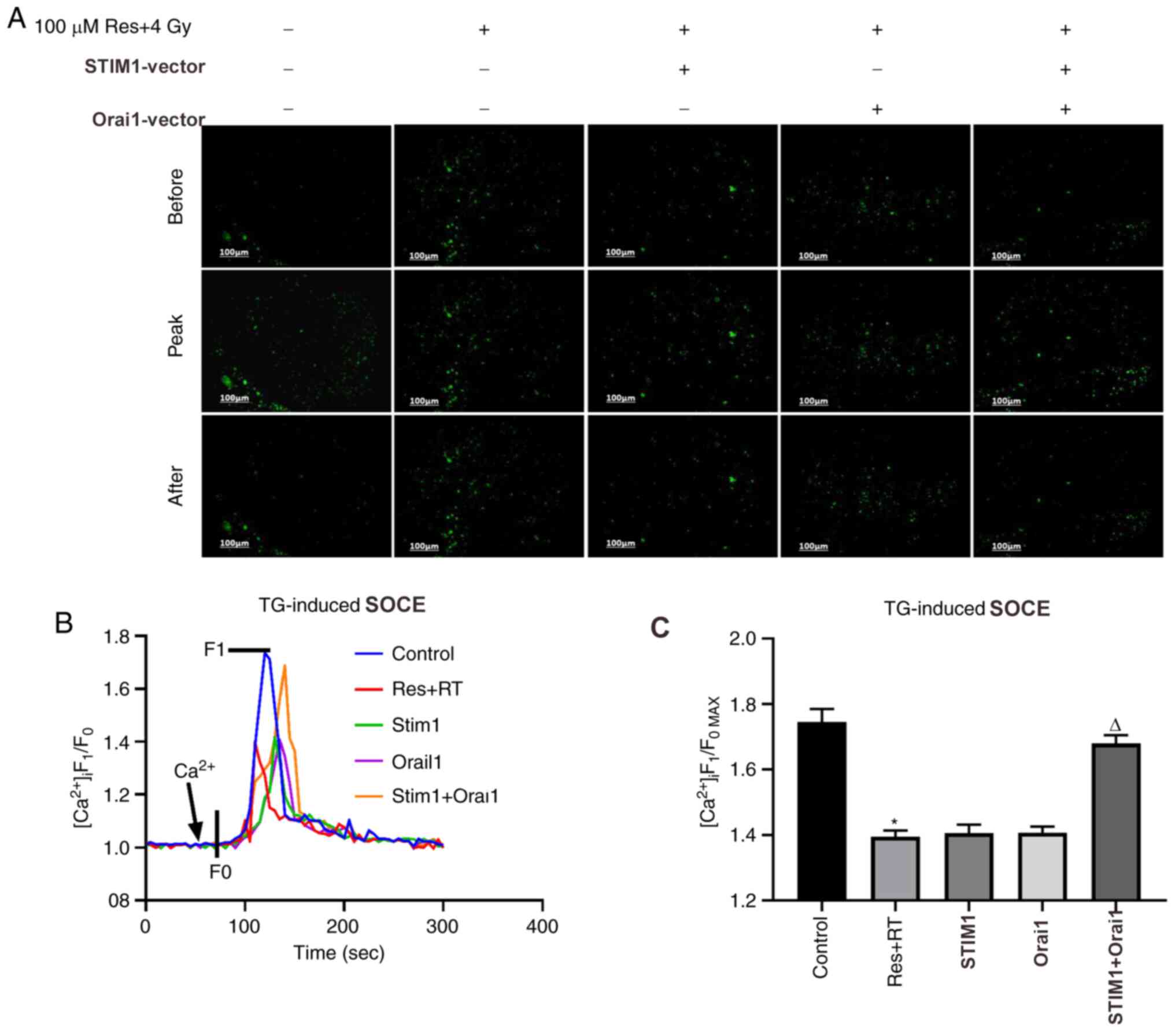 | Figure 6Effects of various doses of
resveratrol and irradiation treatment following Orai1, STIM1 or
dual overexpression on SOCE in A549 cells. After the cells were
cultured to the logarithmic phase, they were divided into five
experimental groups: Untreated, 4 Gy irradiation + 100 µM
resveratrol, 4 Gy irradiation + 100 µM resveratrol + STIM1
overexpression vector, 4 Gy irradiation + 100 µM resveratrol +
Orai1 overexpression vector group and 4 Gy irradiation + 100 µM
resveratrol + STIM1 + Orai1 overexpression vector group.
[Ca2+]i was labeled with sensitive fluorescence
indicator Fluo-8 AM and its real-time fluorescent intensity was
detected. Following Ca2+ store depletion in lung cancer
A549 cells of different dose groups induced by the addition of 2
µmol/l TG, extracellular calcium was added to observe the SOCE. (A)
Representative fluorescence images, (B) fluorescence intensity
curves and (C) summarized data of SOCE are shown. The change in
[Ca2+]i is illustrated as the ratio of F1 and F0. SOCE
was demonstrated as the F1/F0max and the intensity of
fluorescence data is an average of 20-30 cells. Magnification,
x200. Values are shown as the mean ± standard deviation. n=3.
*P<0.05. vs. control; ∆P<0.05. vs. Res
+ RT. Before, images of Fluo-8-labeled A549 cells before addition
of extracellular Ca2+; Peak, images of Fluo-8-labeled
A549 cells giving off strongest fluorescence following addition of
extracellular Ca2+; After, images of Fluo-8-labeled A549
cells at 5 min after addition of extracellular Ca2+;
SOCE, store-operated calcium entry; STIM, stromal interaction
molecule; Orai, calcium release-activated calcium channel protein;
RT, radiotherapy; Res, resveratrol; TG, thapsigargin;
[Ca2+]i, intracellular Ca2+; F1, real-time
fluorescence intensity; F0, fluorescence intensity before the
addition of extracellular calcium; F1/F0max,
ratio of peak intensity. |
Discussion
Most patients with NSCLC are diagnosed at advanced
stages (30). Traditionally, for
inoperable stage III NSCLC and other medically inoperable diseases,
high-dose external radiotherapy is generally considered to be a
suitable treatment for the disease (31). However, in terms of long-term tumor
control, the effects of radiation therapy have been poor (32). The median survival of patients
receiving radiation therapy is <10 months, and the 5-year
survival rate varies between 5 and 10%. The two main reasons for
its low efficacy are limited radiosensitivity of the tumor and the
presence of systemic micrometastases at the time of diagnosis
(1). Therefore, enhancing the
sensitivity of NSCLC is essential.
In order to confirm whether resveratrol had
radiosensitization effects on lung adenocarcinoma A549 cells, the
present study used microscopic observation, cell counting, CCK-8
assay and Δψm detection to compare cell damage in different dose
groups of resveratrol combined with radiation. Compared with single
resveratrol or irradiation treatment, enhanced cell death occurred
in the resveratrol combined irradiation treatment group, indicating
that resveratrol enhanced the irradiation damage of lung
adenocarcinoma A549 cells. Considering that the optimal dose
combination should be as little as possible but also trigger
maximum cell damage, experiments found that the radiation dose of 4
Gy combined with 100 µM resveratrol was the most suitable choice
for following experiments. An increase in cell damage was observed
following 2 to 4 Gy irradiation and 50 to 100 µM resveratrol
administration. Western blotting results showed that the expression
levels of STIM1 and Orai1 in the resveratrol combined with
irradiation treatment group were evidently downregulated compared
with the single resveratrol or irradiation treatment group, while
little effects were observed on STIM2, Orai2 and Orai3.
Concurrently, in the calcium imaging experiment, SOCE in the
resveratrol combined with irradiation treatment group was
significantly inhibited.
Aside from resveratrol, irradiation may also
influence SOCC expression and activity of SOCE, and it has been
previously reported that radiation inhibited salivary gland
function by promoting STIM1 cleavage by caspase-3 and loss of SOCE
via a transient receptor potential cation channel subfamily M
member 2-dependent pathway (19).
However, to the best of our knowledge, no other study has
illustrated the effects of irradiation on SOCE, and the present
results indicated that compared with the control group, irradiation
itself did not result in statistically significant up- or
downregulation on STIM, Orai family members and SOCE activity,
indicating that loss of SOCE, STIM1 and Orai1 was exclusively
caused by adding resveratrol. Therefore, it was concluded that the
effects of resveratrol-sensitized irradiation damage were
associated with downregulating the expression of STIM1 and Orai1
and subsequent loss of SOCE process. To further verify this result,
an overexpression vector was constructed to reverse the effects.
The results of western blotting and calcium imaging experiments
showed that the effects of resveratrol combined irradiation
treatment were restored to a certain extent after co-overexpression
of both STIM1 and Orai1, although it was not completely reversed.
This experiment determined that resveratrol could sensitize A549
cells to irradiation damage, and this effect may be associated with
the downregulation of STIM1 and Orai1 expression, leading to the
inhibition of SOCE, and then triggering cell death.
Published studies demonstrated that the mechanism of
resveratrol-sensitized irradiation damage mainly includes: i)
Affecting the balance of pro- and anti-apoptotic molecules,
promoting the increase of ROS, affecting tumor cell metabolism and
promoting autophagy to induce tumors cell death (33-36);
ii) inhibition of related cyclins, transcription factors and
pathways to inhibit proliferation (37-39);
iii) mediating anti-inflammatory and anti-angiogenic effects to
inhibit tumorigenesis and development (40-43);
and iv) protection of surrounding tissues to decrease the side
effects of radiation (44-46).
However, few studies associated this mechanism with SOCE (37,47,48).
The present study proposed a new hypothesis and verified that the
effects of resveratrol-sensitized irradiation damage may be
associated with SOCE. Compared with single treatment, resveratrol
combined with irradiation treatment significantly affected the
expression of STIM1 and Orai1, and these two proteins are
associated with SOCE. Intracellular calcium serves a number of
regulatory functions as an intracellular bio-signal messenger
(49). When the ER releases
Ca2+, STIM1 can sense Ca2+ consumption,
triggering structural changes and combine with Orai1 to form
Ca2+ channels, guide extracellular Ca2+
inflow and compensate for Ca2+ consumption. Compared
with the single irradiation treatment group, the resveratrol
combined irradiation treatment group significantly downregulated
the expression of STIM1 and Orai1, leading to a decrease in calcium
channels, decrease in calcium influx and depletion of calcium
storage, thereby causing calcium imbalance inside the cell leading
to cell dysfunction, and eventually cell death (Figs.
1-3). At present, there are few studies indicating that the
target of resveratrol may be associated with ERK1/2. When
intracellular calcium is reduced to a certain limit, STIM1 protein
is activated (50). This activation
is mainly achieved through the phosphorylation of STIM1 protein at
Ser575, Ser608 and Ser621, which are ERK1/2 target sites (51). Under the quiescent state, STIM1
binds to end-binding protein 1, a major regulator of microtubule
growing ends. ERK1/2 can promote STIM1 unbinding activation during
calcium consumption, polymerize SOCC in plasma membrane and
activate SOCE (52). Resveratrol
can interfere with SOCE by inhibiting ERK1/2 activity and
inhibiting STIM1 phosphorylation (53).
Apart from cell or animal level investigations,
numerous resveratrol-based clinical trials have been performed and
found to have promising effects on cancer inhibition (54,55).
This therapeutic effect was found to exist in a wide range of human
cancer types including bowel, breast, bladder, prostate, liver,
thyroid and lung, with a 0.5-5-g daily oral intake of resveratrol
(56). It was reported that plasma
levels of 14.7 µmol/l resveratrol led to slightly suppressed
hepatic cancer metastasis in patients with colorectal cancer, which
is substantially lower than the 100 µmol/l dose used in the present
study, indicating that more studies may be required to determine
the most effective dosage of resveratrol (57).
Although resveratrol was shown to have a
radiosensitizing effect, its poor water solubility and low
stability limit its application (58). New derivatives are required to be
further developed by researchers to enhance their preventive and
therapeutic efficiency. The present study only used resveratrol for
cell-level research and animal experiments and clinically relevant
resveratrol and irradiation treatment cases were not presented.
Therefore, the in vivo therapeutic effect need to be further
studied.
In conclusion, this study demonstrated that
resveratrol can significantly enhance the effect of irradiation
damage on lung adenocarcinoma A549 cells, and this effect was
related with suppression of SOCE caused by reduction of both STIM1
and Orai1. As a safe bioactive antioxidant, resveratrol has already
been applied in health care and cosmetic industry. Once its
sensitizing effects, mechanism and pharmacokinetic feature are
confirmed in animal level and random clinical trials, this
beneficial polyphenol can be quickly put into practical
pretreatment ahead of radioactive therapy for cancer patients to
minimize the dose-dependent irradiation damage without disturbing
the efficacy.
Supplementary Material
Confirmation of validity of STIM1 and
Orai1 overexpression vector. (A) A representative image of western
blot analysis performed for the analysis (B) of the expression of
STIM1 in lung cancer A549 cells transfected with or without STIM1
overexpression vector. (C) A representative image of western blot
performed for the analysis (B) of the expression of Orai1 in lung
cancer A549 cells transfected with or without Orai1 overexpression
vector. β-actin was used as loading control. Values are shown as
the mean ± standard deviation. n=3. *P<0.05 vs.
control group. STIM, stromal interaction molecule; Orai, calcium
release-activated calcium channel protein.
Acknowledgements
The authors would like to thank Dr Jinsen Lu
(Department of Orthopedics of Anhui Provincial Hospital; Hefei,
China), who instructed on training group members to detect the
fluctuation of intracellular calcium intensity and SOCE via
fluorescent microscopy.
Funding
Funding: This study was supported by a grant from the Key
Research and Development Projects of Anhui Province (grant no.
1704a0802157).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL, LL and QL designed the experiments. WLL and LL
performed the experiments and the data analysis. WLL contributed to
data acquisition and analysis. WLL drafted the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Atagi S, Kawahara M, Yokoyama A, Okamoto
H, Yamamoto N, Ohe Y, Sawa T, Ishikura S, Shibata T, Fukuda H, et
al: Thoracic radiotherapy with or without daily low-dose
carboplatin in elderly patients with non-small-cell lung cancer: A
randomised, controlled, phase 3 trial by the Japan Clinical
Oncology Group (JCOG0301). Lancet Oncol. 13:671–678.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jonna S and Subramaniam DS: Molecular
diagnostics and targeted therapies in non-small cell lung cancer
(NSCLC): An update. Dis Med. 27:167–170. 2019.PubMed/NCBI
|
|
3
|
Brown S, Banfill K, Aznar MC, Whitehurst P
and Faivre Finn C: The evolving role of radiotherapy in non-small
cell lung cancer. Br J Radiol. 92(20190524)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li YL, Hu X, Li QY, Wang F, Zhang B, Ding
K, Tan BQ, Lin NM and Zhang C: Shikonin sensitizes wild-type EGFR
NSCLC cells to erlotinib and gefitinib therapy. Mol Med Rep.
18:3882–3890. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Crooker K, Aliani R, Ananth M, Arnold L,
Anant S and Thomas SM: A review of promising natural
chemopreventive agents for head and neck cancer. Cancer Prev Res
(Phila). 11:441–450. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Breuss JM, Atanasov AG and Uhrin P:
Resveratrol and its effects on the vascular system. Int J Mol Sci.
20(1523)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Athar M, Back JH, Kopelovich L, Bickers DR
and Kim AL: Multiple molecular targets of resveratrol:
Anti-carcinogenic mechanisms. Arch Biochem Biophys. 486:95–102.
2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ko JH, Sethi G, Um JY, Shanmugam MK,
Arfuso F, Kumar AP, Bishayee A and Ahn KS: The role of resveratrol
in cancer therapy. Int J Mol Sci. 18(2589)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jiang Z, Chen K, Cheng L, Yan B, Qian W,
Cao J, Li J, Wu E, Ma Q and Yang W: Resveratrol and cancer
treatment: Updates. Ann N Y Acad Sci. 1403:59–69. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vervandier-Fasseur D and Latruffe N: The
potential use of resveratrol for cancer prevention. Molecules.
24(4506)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pan X, Chen J, Wang W, Chen L, Wang L, Ma
Q, Zhang J, Chen L, Wang G, Zhang M, et al: Resveratrol-induced
antinociception is involved in calcium channels and
calcium/caffeine-sensitive pools. Oncotarget. 8:9399–9409.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xu H, Cheng J, Wang X, Liu H, Wang S, Wu
J, Xu B, Chen A and He F: Resveratrol pretreatment alleviates
myocardial ischemia/reperfusion injury by inhibiting STIM1-mediated
intracellular calcium accumulation. J Physiol Biochem. 75:607–618.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Stephan LS, Almeida ED, Markoski MM,
Garavaglia J and Marcadenti A: Red Wine, resveratrol and atrial
fibrillation. Nutrients. 9(1190)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
So CL, Saunus JM, Roberts-Thomson SJ and
Monteith GR: Calcium signalling and breast cancer. Semin Cell Dev
Biol. 94:74–83. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang Y, He J, Jiang H, Zhang Q, Yang H, Xu
X, Zhang C, Xu C, Wang J and Lu W: Nicotine enhances store-operated
calcium entry by upregulating HIF-1α and SOCC components in
non-small cell lung cancer cells. Oncol Rep. 40:2097–2104.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tang X, Qian LL, Dang SP, Wu Y, Liu XY,
Zhang ZY, Miu LF and Wang RX: Effects of n-3 polyunsaturated fatty
acid on store-operated calcium channels in coronary artery smooth
muscle cells derived from diabetic rat. Zhonghua Xin Xue Guan Bing
Za Zhi. 47:640–646. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
17
|
Wu H: Calcium signaling in platelet
activation. Sheng Li Ke Xue Jin Zhan. 43:417–421. 2012.PubMed/NCBI(In Chinese).
|
|
18
|
Pan Z and Ma J: Open Sesame: Treasure in
store-operated calcium entry pathway for cancer therapy. Sci China
Life Sci. 58:48–53. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ambudkar IS, de Souza LB and Ong HL:
TRPC1, Orai1, and STIM1 in SOCE: Friends in tight spaces. Cell
Calcium. 63:33–39. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lopez JJ, Jardin I, Sanchez-Collado J,
Salido GM, Smani T and Rosado JA: TRPC Channels in the SOCE
Scenario. Cells. 9(126)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rosenberg P, Katz D and Bryson V: SOCE and
STIM1 signaling in the heart: Timing and location matter. Cell
Calcium. 77:20–28. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Prakriya M and Lewis RS: Store-operated
calcium channels. Physiol Rev. 95:1383–1436. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Collins HE, Zhu-Mauldin X, Marchase RB and
Chatham JC: STIM1/Orai1-mediated SOCE: Current perspectives and
potential roles in cardiac function and pathology. Am J Physiology
Heart Circ Physiol. 305:H446–H458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lopez E, Frischauf I, Jardin I, Derler I,
Muik M, Cantonero C, Salido GM, Smani T, Rosado JA and Redondo PC:
STIM1 phosphorylation at Y316 modulates its interaction
with SARAF and the activation of SOCE and ICRAC.
J Cell Sci. 132(jcs226019)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ren X, Zhang L, Zhang Y, Mao L and Jiang
H: Mitochondria response to camptothecin and
hydroxycamptothecine-induced apoptosis in Spodoptera exigua cells.
Pestic Biochem Physiol. 140:97–104. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Perry SW, Norman JP, Barbieri J, Brown EB
and Gelbard HA: Mitochondrial membrane potential probes and the
proton gradient: A practical usage guide. Biotechniques. 50:98–115.
2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xie J, Pan H, Yao J, Zhou Y and Han W:
SOCE and cancer: Recent progress and new perspectives. Int J
Cancer. 138:2067–2077. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jardin I and Rosado JA: STIM and calcium
channel complexes in cancer. Biochim Biophys Acta. 1863:1418–1426.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tanwar J and Motiani RK: Role of SOCE
architects STIM and orai proteins in cell death. Cell Calcium.
69:19–27. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Vokes EE, Govindan R, Iscoe N, Hossain AM,
San Antonio B, Chouaki N, Koczywas M and Senan S: The impact of
staging by positron-emission tomography on overall survival and
progression-free survival in patients with locally advanced NSCLC.
J Thorac Oncol. 13:1183–1188. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Velcheti V, Chandwani S, Chen X, Pietanza
MC and Burke T: First-line pembrolizumab monotherapy for metastatic
PD-L1-positive NSCLC: Real-world analysis of time on treatment.
Immunotherapy. 11:889–901. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wu YL, Yang JC, Kim DW, Lu S, Zhou J, Seto
T, Yang JJ, Yamamoto N, Ahn MJ, Takahashi T, et al: Phase II study
of crizotinib in east asian patients with ROS1-positive advanced
non-small-cell lung cancer. J Clin Oncol. 36:1405–1411.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu X, Gong B, de Souza LB, Ong HL, Subedi
KP, Cheng KT, Swaim W, Zheng C, Mori Y and Ambudkar IS: Radiation
inhibits salivary gland function by promoting STIM1 cleavage by
caspase-3 and loss of SOCE through a TRPM2-dependent pathway. Sci
Signal. 10(eaal4064)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Voellger B, Waldt N, Rupa R, Kirches E,
Melhem O, Ochel HJ, Mawrin C and Firsching R: Combined effects of
resveratrol and radiation in GH3 and TtT/GF pituitary adenoma
cells. J Neurooncol. 139:573–582. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fang Y, Bradley MJ, Cook KM, Herrick EJ
and Nicholl MB: A potential role for resveratrol as a radiation
sensitizer for melanoma treatment. J Surg Res. 183:645–653.
2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tak JK, Lee JH and Park JW: Resveratrol
and piperine enhance radiosensitivity of tumor cells. BMB Rep.
45:242–246. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen YA, Lien HM, Kao MC, Lo UG, Lin LC,
Lin CJ, Chang SJ, Chen CC, Hsieh JT, Lin H, et al: Sensitization of
radioresistant prostate cancer cells by resveratrol isolated from
arachis hypogaea stems. PLoS One. 12(e0169204)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tan Y, Wei X, Zhang W, Wang X, Wang K, Du
B and Xiao J: Resveratrol enhances the radiosensitivity of
nasopharyngeal carcinoma cells by downregulating E2F1. Oncol Rep.
37:1833–1841. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Assad DX, Borges GA, Avelino SR and Guerra
ENS: Additive cytotoxic effects of radiation and mTOR inhibitors in
a cervical cancer cell line. Pathol Res Pract. 214:259–262.
2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Said RS, El-Demerdash E, Nada AS and Kamal
MM: Resveratrol inhibits inflammatory signaling implicated in
ionizing radiation-induced premature ovarian failure through
antagonistic crosstalk between silencing information regulator 1
(SIRT1) and poly(ADP-ribose) polymerase 1 (PARP-1). Biochem
Pharmacol. 103:140–150. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Choi YJ, Heo K, Park HS, Yang KM and Jeong
MH: The resveratrol analog HS-1793 enhances radiosensitivity of
mouse-derived breast cancer cells under hypoxic conditions. Int J
Oncol. 49:1479–1488. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Aravindan S, Natarajan M, Herman TS,
Awasthi V and Aravindan N: Molecular basis of ‘hypoxic’ breast
cancer cell radio-sensitization: Phytochemicals converge on
radiation induced Rel signaling. Radiat Oncol. 8(46)2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Firouzi F, Khoei S and Mirzaei HR: Role of
resveratrol on the cytotoxic effects and DNA damages of
iododeoxyuridine and megavoltage radiation in spheroid culture of
U87MG glioblastoma cell line. Gen Physiol Biophys. 34:43–50.
2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sener TE, Tavukcu HH, Atasoy BM, Cevik O,
Kaya OT, Cetinel S, Dagli Degerli A, Tinay I, Simsek F, Akbal C, et
al: Resveratrol treatment may preserve the erectile function after
radiotherapy by restoring antioxidant defence mechanisms, SIRT1 and
NOS protein expressions. Int J Impot Res. 30:179–188.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Xu L, Yang X, Cai J, Ma J, Cheng H, Zhao
K, Yang L, Cao Y, Qin Q, Zhang C, et al: Resveratrol attenuates
radiation-induced salivary gland dysfunction in mice. Laryngoscope.
123:E23–E29. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhang H, Zhai Z, Wang Y, Zhang J, Wu H,
Wang Y, Li C, Li D, Lu L, Wang X, et al: Resveratrol ameliorates
ionizing irradiation-induced long-term hematopoietic stem cell
injury in mice. Free Radic Biol Med. 54:40–50. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Feng Y, Zhou J and Jiang Y: Resveratrol in
lung cancer-a systematic review. J BUON. 21:950–953.
2016.PubMed/NCBI
|
|
48
|
Yousef M, Vlachogiannis IA and Tsiani E:
Effects of resveratrol against lung cancer: In vitro and in vivo
studies. Nutrients. 9(1231)2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Boon N, Hul GB, Viguerie N, Sicard A,
Langin D and Saris WH: Effects of 3 diets with various calcium
contents on 24-h energy expenditure, fat oxidation, and adipose
tissue message RNA expression of lipid metabolism-related proteins.
Am J Clin Nutr. 82:1244–1252. 2005.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Chen X, Hu X, Li Y, Zhu C, Dong X, Zhang
R, Ma J, Huang S and Chen L: Resveratrol inhibits Erk1/2-mediated
adhesion of cancer cells via activating PP2A-PTEN signaling
network. J Cell Physiol. 234:2822–2836. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Thiel G and Rössler OG: Resveratrol
stimulates c-Fos gene transcription via activation of ERK1/2
involving multiple genetic elements. Gene. 658:70–75.
2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Soltoff SP and Lannon WA: Activation of
ERK1/2 by store-operated calcium entry in rat parotid acinar cells.
PLoS One. 8(e72881)2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Casas-Rua V, Alvarez IS, Pozo-Guisado E
and Martín-Romero FJ: Inhibition of STIM1 phosphorylation underlies
resveratrol-induced inhibition of store-operated calcium entry.
Biochem Pharmacol. 86:1555–1563. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Rauf A, Imran M, Butt MS, Nadeem M, Peters
DG and Mubarak MS: Resveratrol as an anti-cancer agent: A review.
Crit Rev Food Sci Nutr. 58:1428–1447. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Carter LG, D'Orazio JA and Pearson KJ:
Resveratrol and cancer: Focus on in vivo evidence. Endocr Relat
Cancer. 21:R209–R225. 2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Howells LM, Berry DP, Elliott PJ, Jacobson
EW, Hoffmann E, Hegarty B, Brown K, Steward WP and Gescher AJ:
Phase I randomized, double-blind pilot study of micronized
resveratrol (SRT501) in patients with hepatic metastases-safety,
pharmacokinetics, and pharmacodynamics. Cancer Prev Res (Phila).
4:1419–1425. 2011.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Patel KR, Brown VA, Jones DJ, Britton RG,
Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA,
et al: Clinical pharmacology of resveratrol and its metabolites in
colorectal cancer patients. Cancer Res. 70:7392–7399.
2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Galiniak S, Aebisher D and
Bartusik-Aebisher D: Health benefits of resveratrol administration.
Acta Biochim Pol. 66:13–21. 2019.PubMed/NCBI View Article : Google Scholar
|