Introduction
Microglia are widely distributed in the brain and
spinal cord. Immune abnormalities occurring in degenerative
diseases of the central nervous system are mainly characterized by
excessive activation of microglia and elevated levels of
inflammatory factors in specific brain regions (1). Excessive activation and proliferation
of microglia in numerous neurodegenerative diseases are important
manifestations of inflammatory responses of central nervous system
diseases such as Parkinson's disease, Alzheimer's disease,
amyotrophic lateral sclerosis, cerebellar atrophy and multiple
sclerosis. According to previous studies, excessive activation of
microglia and continuous releases of nitric oxide (NO), inducible
NO synthase (iNOS), interleukin-1β (IL-1β) and other
pro-inflammatory factors are causes of neuronal cell death
(2-4).
These inflammatory factors have strong toxic effects on neurons,
ultimately leading to degeneration and necrosis. Therefore,
inhibiting microglia hyperactivation is currently an important
strategy for treating neurological diseases. Microglia may be
activated by lipopolysaccharide (LPS), interferon-γ and β-amyloid,
resulting in the production of inflammatory cell mediators.
Mecasin (KCHO-1, Gamijakyakgamchobuja-tang), has
anti-inflammatory and antioxidant properties. It is composed of
Curcuma longa, Salvia miltiorrhiza, Gastrodia
elata, Chaenomeles sinensis, Polygala tenuifolia, Paeonia japonica,
Glycyrrhiza uralensis, Atractylodes japonica and processed
Aconitum carmichaeli (5,6).
Through continuous research in recent years, various medical
effects of mecasin have been discovered. For instance, it was
demonstrated to have a role in reducing pain, the regeneration of
gamma-aminobutyric acid neurons and NO reduction in neuropathic
pain of rats (5). It also has
anti-seizure, analgesic, antipyretic, anti-inflammatory,
anti-ulcer, osteoarthritis-suppressing, neuro-protective and
anti-neuroinflammatory effects. Its safety has also been
demonstrated in both in vitro and in vivo trials
(7-15).
Previous studies have indicated that mecasin acts by
inducing heme oxygenase 1 expression and suppressing nuclear factor
κB (NF-κB)-mediated production of proinflammatory mediators,
cytokines and proteins in LPS-stimulated microglia (10). However, the contribution of each
herbal medicine constituent to the effect of mecasin has remained
elusive. Therefore, the objective of the present study was to
determine whether constituents of mecasin may have synergistic
anti-neuroinflammatory effects using LPS-stimulated BV2 cells.
Materials and methods
Materials
Tissue culture reagents such as RPMI 1640 and fetal
bovine serum (FBS) were purchased from Gibco (Thermo Fisher
Scientific, Inc.). All chemicals were obtained from Sigma-Aldrich
(Merck KGaA). Primary antibodies including anti-cyclooxygenase
(COX)-2 (cat. no. SC-376861), anti-iNOS (cat. no. SC-7271) and
anti-β-actin (cat. no. SC-47778) were purchased from Santa Cruz
Biotechnology, Inc. Anti-rabbit (cat. no. AP132P) and anti-mouse
(cat. no. AP124P) secondary antibodies were purchased from EMD
Millipore.
Cell culture and cell viability
assay
BV2 microglia were donated by Youn-Chul Kim,
Wonkwang University (Iksan, Korea). BV2 microglia were seeded at
5x105 cells/ml in RPMI 1640 supplemented with 10%
heat-inactivated FBS, penicillin G (100 units/ml), streptomycin
(100 mg/ml), and L-glutamine (2 mM) and incubated at 37˚C in a
humidified atmosphere containing 5% CO2. For the
determination of cell viability, 50 mg/ml of MTT was added to 0.1
ml of each well (1x105 cells/ml in 96-well plates) for 4
h. The resulting formazan was dissolved in DMSO, after which the
optical density of the solution was measured at 540 nm using an
ELISA microplate reader (Model 550; Bio-Rad Laboratories Inc.). The
optical density of the formazan solution from control (untreated)
cells was considered to represent 100% viability.
Proportions and concentrations of
individual ingredients of mecasin
Mecasin and its herbal medicine constituents were
obtained from Hanpoong Pharm & Foods. The concentration of the
individual ingredients was determined based on concentrations of
mecasin at 25, 50, 100 and 200 µg/ml (Table I).
 | Table IComposition of mecasin and proportions
of its constituents. |
Table I
Composition of mecasin and proportions
of its constituents.
| Individual
ingredient | Ratio (%) | Treatment
concentration by ratio of mecasin concentration (µg/ml) |
|---|
| Curcuma
longa | 17.4 | 4.35, 8.7, 17.4,
34.8 |
| Salvia
miltiorrhiza | 17.4 | 4.35, 8.7, 17.4,
34.8 |
| Gastrodia
elata | 17.4 | 4.35, 8.7, 17.4,
34.8 |
| Chaenomeles
sinensis | 8.7 | 2.17, 4.35, 8.7,
17.4 |
| Polygala
tenuifolia | 8.7 | 2.17, 4.35, 8.7,
17.4 |
| Paeonia
japonica | 8.7 | 2.17, 4.35, 8.7,
17.4 |
| Glycyrrhiza
uralensis | 8.7 | 2.17, 4.35, 8.7,
17.4 |
| Atractylodes
japonica | 8.7 | 2.17, 4.35, 8.7,
17.4 |
| Processed
Aconitum carmichaeli | 4.35 | 1.09, 2.17, 4.35,
8.7 |
Nitrite assay
BV2 microglia were cultured in 48-well plates at a
density of 5x105 cells/well (100 µl) for 12 h.
Subsequently, the cells were pre-treated with indicated
concentrations of each test sample for 3 h and then stimulated with
LPS at 1 µg/ml for 24 h. The amount of NO released from cells into
the medium was then measured using Griess reagent [0.1% wv
N-(1-naphathyl)-ethylenediamine and 1% (wv) sulfanilamide in 5%
(vv) phosphoric acid]. The absorbance of each well was then
measured at 570 nm using an ELISA microplate reader (Model 550;
Bio-Rad Laboratories Inc.). NO was quantified using a standard
curve.
Western blotting analysis
The pelleted BV2 microglia were washed with PBS, and
then lysed using RIPA buffer (Thermo Fisher Scientific, Inc.; cat.
no. 89900). Equal quantities of proteins (30 µg) were quantified
using Protein Assay Dye Reagent Concentrate obtained from Bio-Rad
Laboratories, mixed in the sample loading buffer and separated by
7.5% SDS-PAGE. Separated proteins were transferred to a
nitrocellulose membrane. Non-specific binding to the membrane was
blocked by incubating with a solution of 5% skimmed milk at 37˚C
for 1 h. The membrane was incubated with primary antibodies
including COX-2 (1:1,000), iNOS (1:1,000) and β-actin (1:1,000) at
4˚C overnight, and then reacted with horseradish
peroxidase-conjugated rabbit (goat anti-rabbit IgG; 1:5,000) and
mouse (goat anti-mouse IgG; 1:5,000) secondary antibodies at 37˚C
for 1 h, followed by ECL detection (GENDEPOT Laboartories; cat. no.
W3652-020). Quantitative densitometric analysis was conducted using
ImageJ software 1.47v (National Institutes of Health).
Statistical analysis
Quantitative data are expressed as the mean ±
standard deviation of at least three independent experiments. To
compare among three groups, one-way analysis of variance was used
followed by Dunnett's test. All statistical analyses were performed
using GraphPad Prism software, version 5.01 (GraphPad Software
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of cell viability
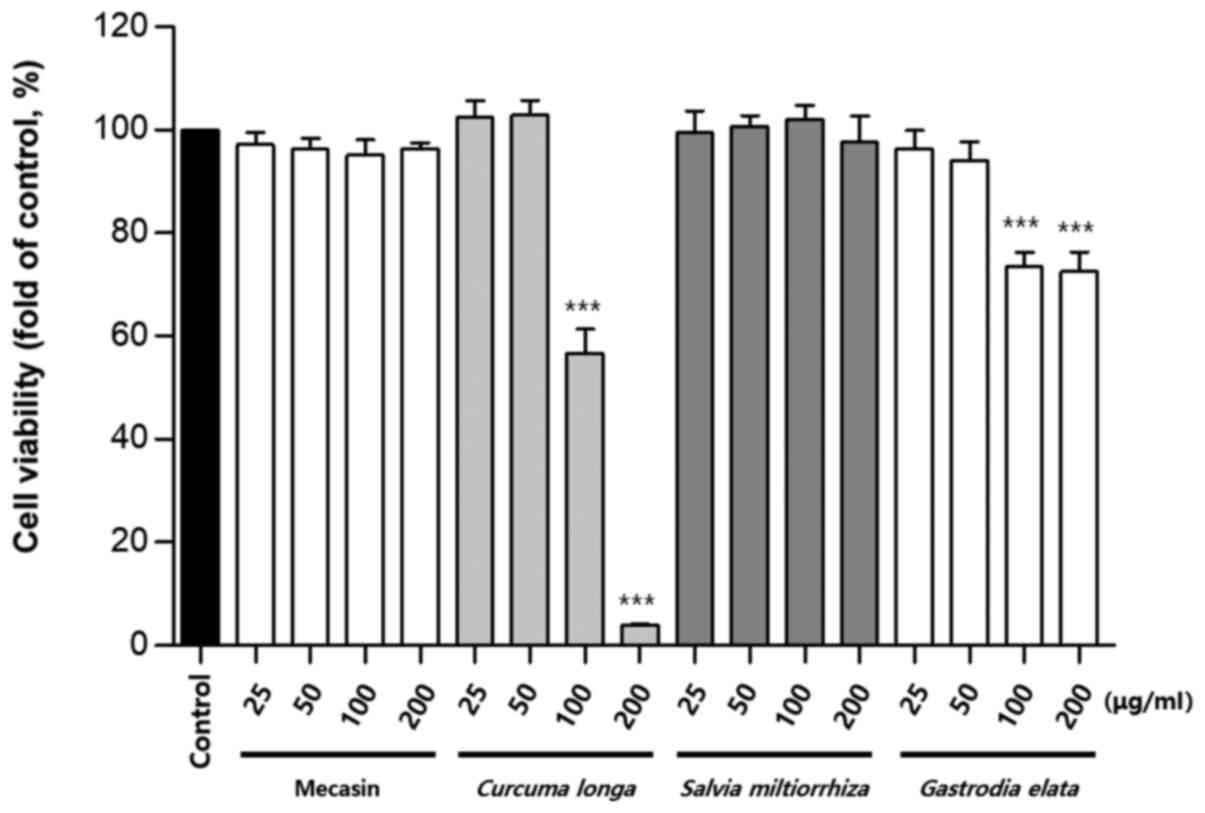
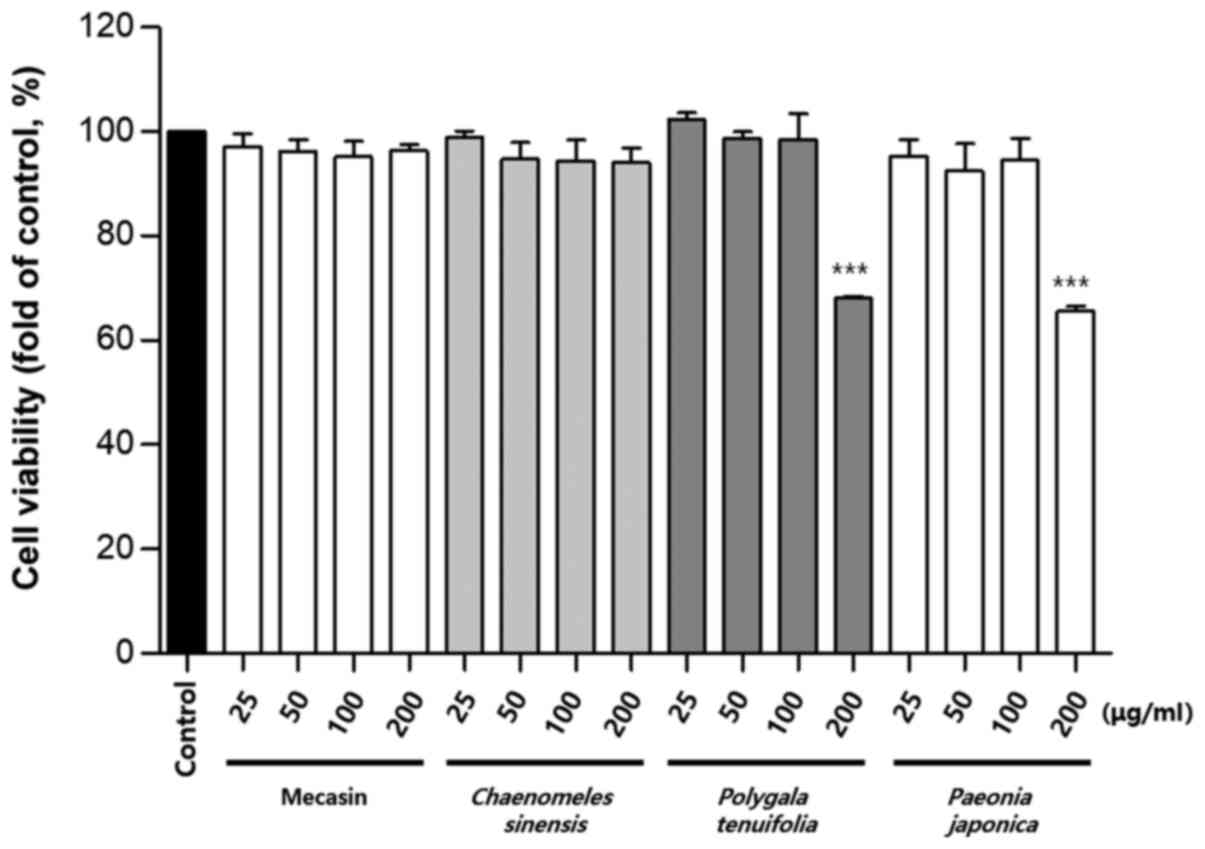
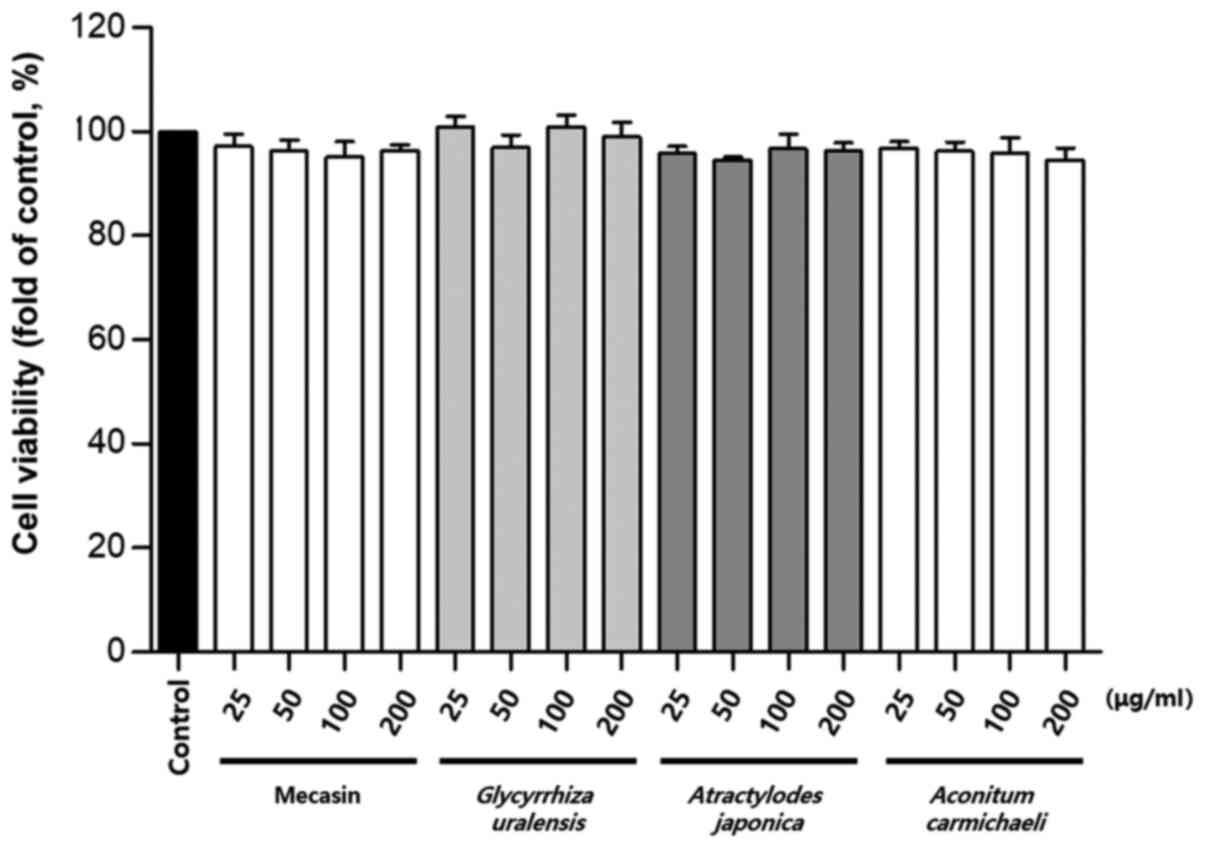
Cytotoxicity was measured using the MTT assay. The
results indicated that C. longa and G. elata were
cytotoxic at a concentration of 100 µg/ml or higher, while P.
tenuifolia and P. japonica were cytotoxic at a
concentration of 200 µg/ml or higher. No toxicity was observed for
mecasin at concentrations of ≤200 µg/ml and the maximum
concentration used in the present study was within the
concentration range that caused no toxicity (Fig. 1, Fig.
2, Fig. 3).
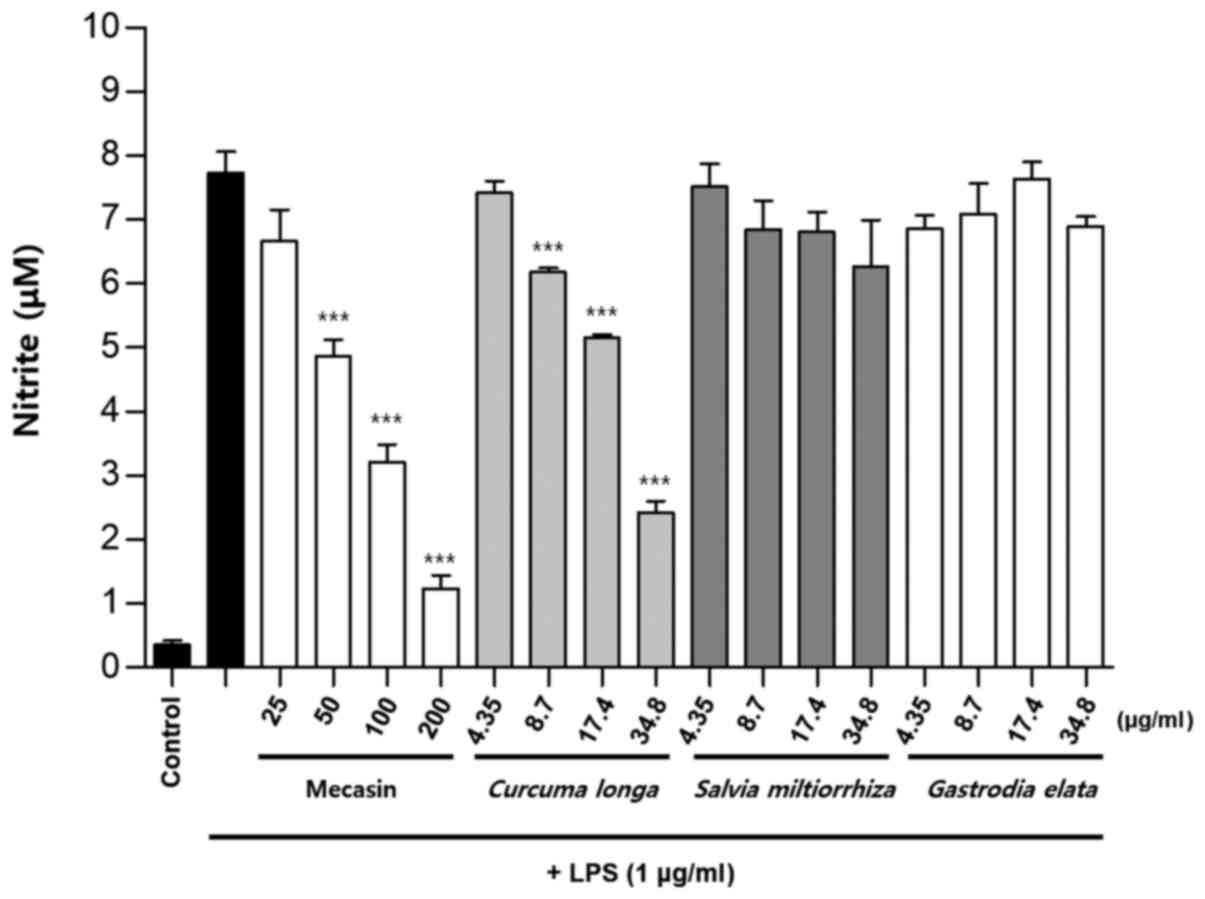
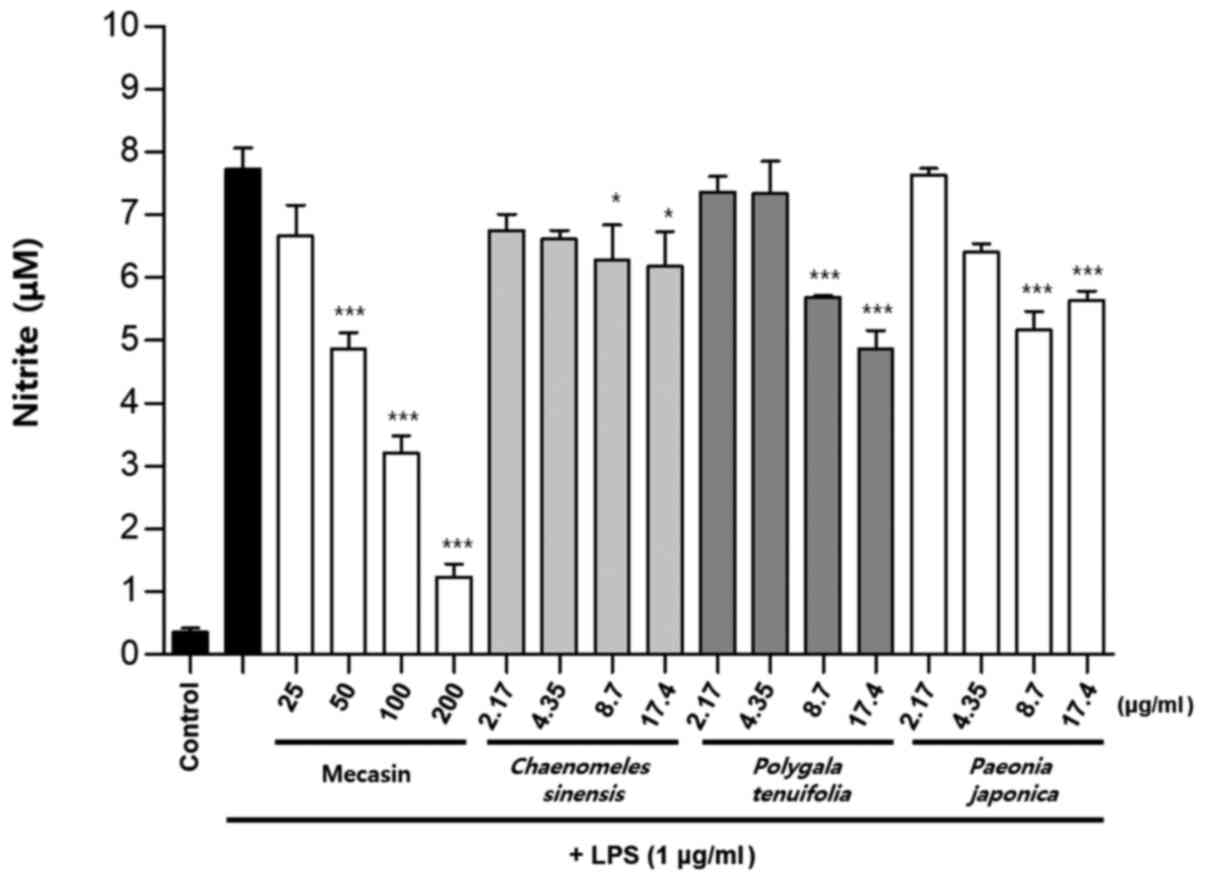
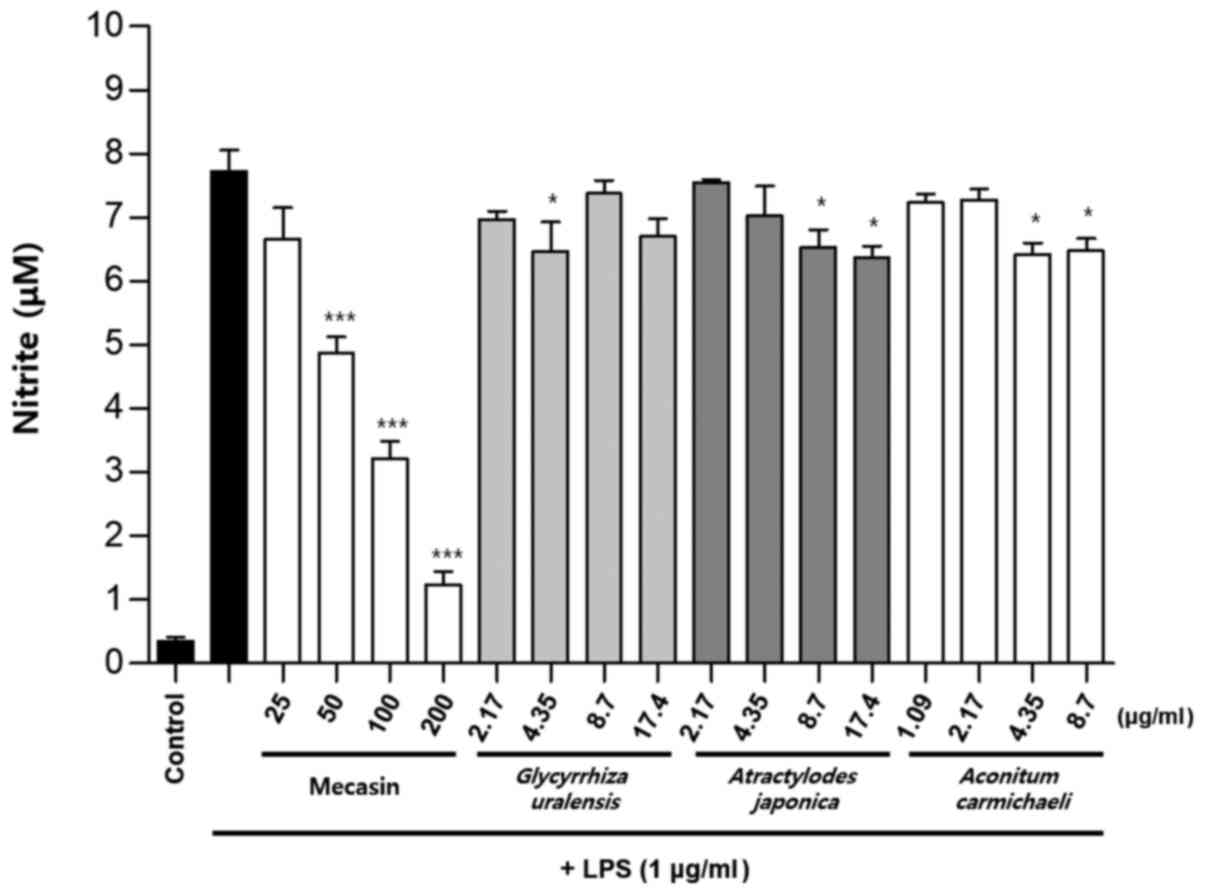
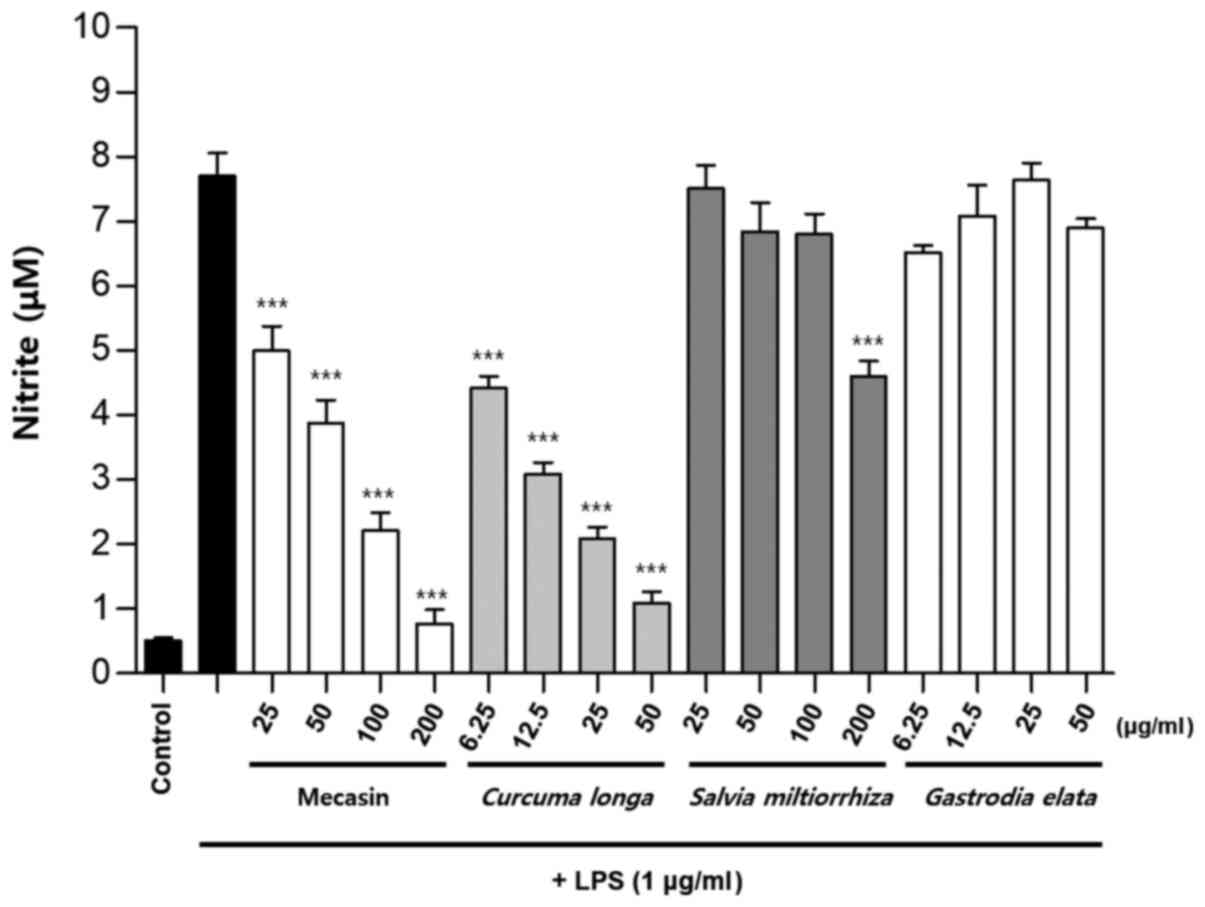
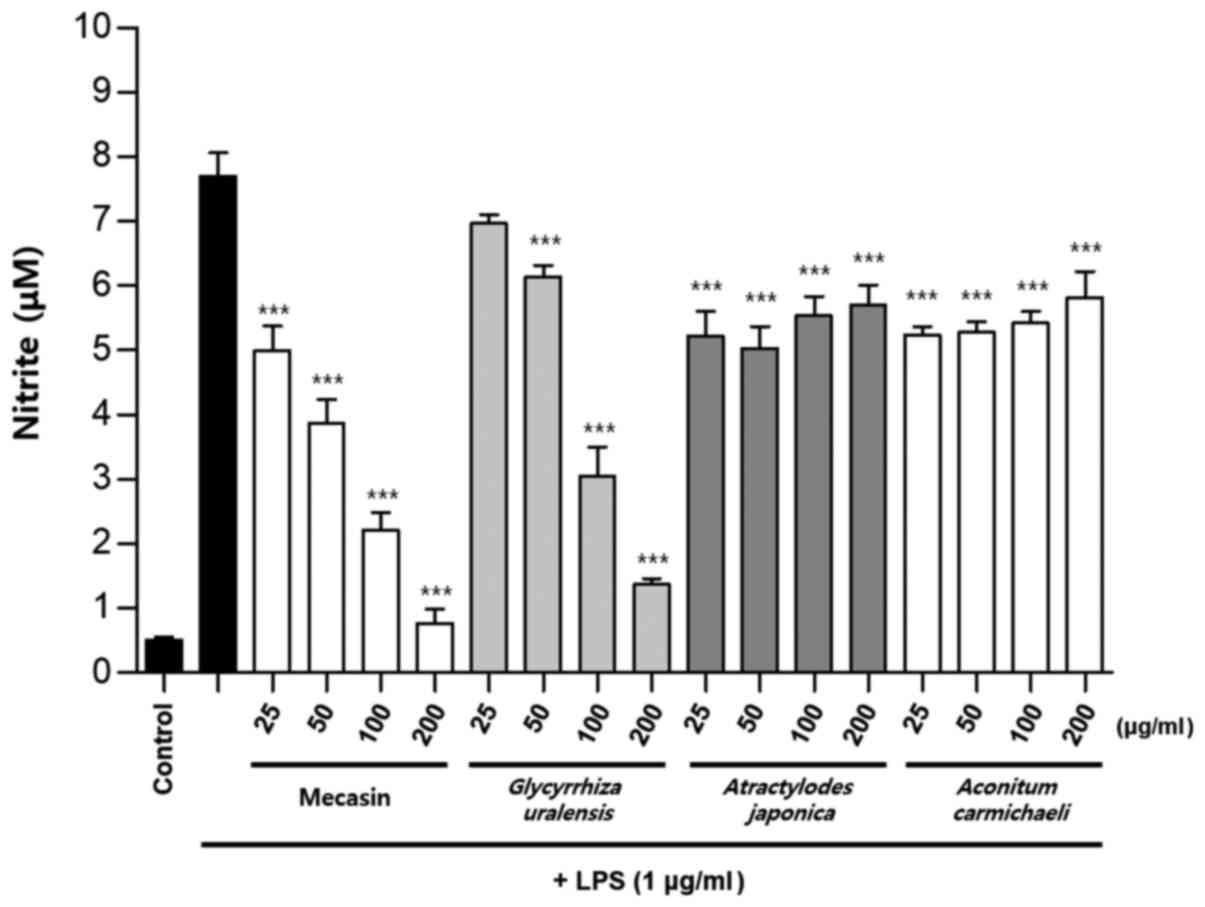
Inhibition rate of nitrite
production
BV2 cells were pre-treated with each test sample at
the indicated concentrations for 3 h and then stimulated with LPS
at 1 µg/ml for 24 h. The concentration of individual ingredients
was set based on concentrations of mecasin at 25, 50, 100 and 200
µg/ml (Table I). The inhibition
rate of nitrite production was compared after this concentration
was set. Mecasin at 25, 50, 100 and 200 µg/ml contained C.
longa at 4.35, 8.7, 17.4 and 34.8 µg/ml, respectively. For
mecasin, the inhibition rate of nitrite production was 14.5% at 25
µg/ml, 38.8% at 50 µg/ml, 61.2% at 100 µg/ml and 88.0% at 200
µg/ml. For C. longa, which had the greatest inhibitory
effects on nitrite production among individual constituents, the
inhibition rate of nitrite production was 4.2% at 4.35 µg/ml, 21.1%
at 8.7 µg/ml, 34.8% at 17.4 µg/ml and 71.9% at 34.8 µg/ml. When
inhibition rates of nitrite generation were compared, mecasin had a
higher rate than its individual constituents. Its inhibition effect
on nitrite production was dose-dependent. Among its ingredients,
C. longa had the highest inhibition rate, followed by P.
tenuifolia and P. japonica. The remaining six individual
herbal components did not exert any significant inhibition on
nitrite production (Table II;
Fig. 4, Fig. 5, Fig.
6) .
 | Table IINitrite inhibition percentages of
mecasin and its constituents. |
Table II
Nitrite inhibition percentages of
mecasin and its constituents.
| | Nitrite inhibition
(%) |
|---|
| Item | Concentration
1 | Concentration
2 | Concentration
3 | Concentration
4 |
|---|
| Mecasin | 14.5 | 38.8 | 61.2 | 88.0 |
| Curcuma
longa | 4.2 | 21.1 | 34.8 | 71.9 |
| Salvia
miltiorrhiza | 3.0 | 12.1 | 12.6 | 19.8 |
| Gastrodia
elata | 11.9 | 8.8 | 1.3 | 11.4 |
| Chaenomeles
sinensis | 13.3 | 15.2 | 19.6 | 21.0 |
| Polygala
tenuifolia | 5.0 | 5.4 | 27.7 | 38.8 |
| Paeonia
japonica | 1.4 | 18.0 | 34.7 | 28.4 |
| Glycyrrhiza
uralensis | 10.4 | 17.1 | 4.8 | 14.0 |
| Atractylodes
japonica | 2.4 | 9.5 | 16.2 | 18.4 |
| Processed
Aconitum carmichaeli | 6.7 | 6.2 | 17.8 | 16.9 |
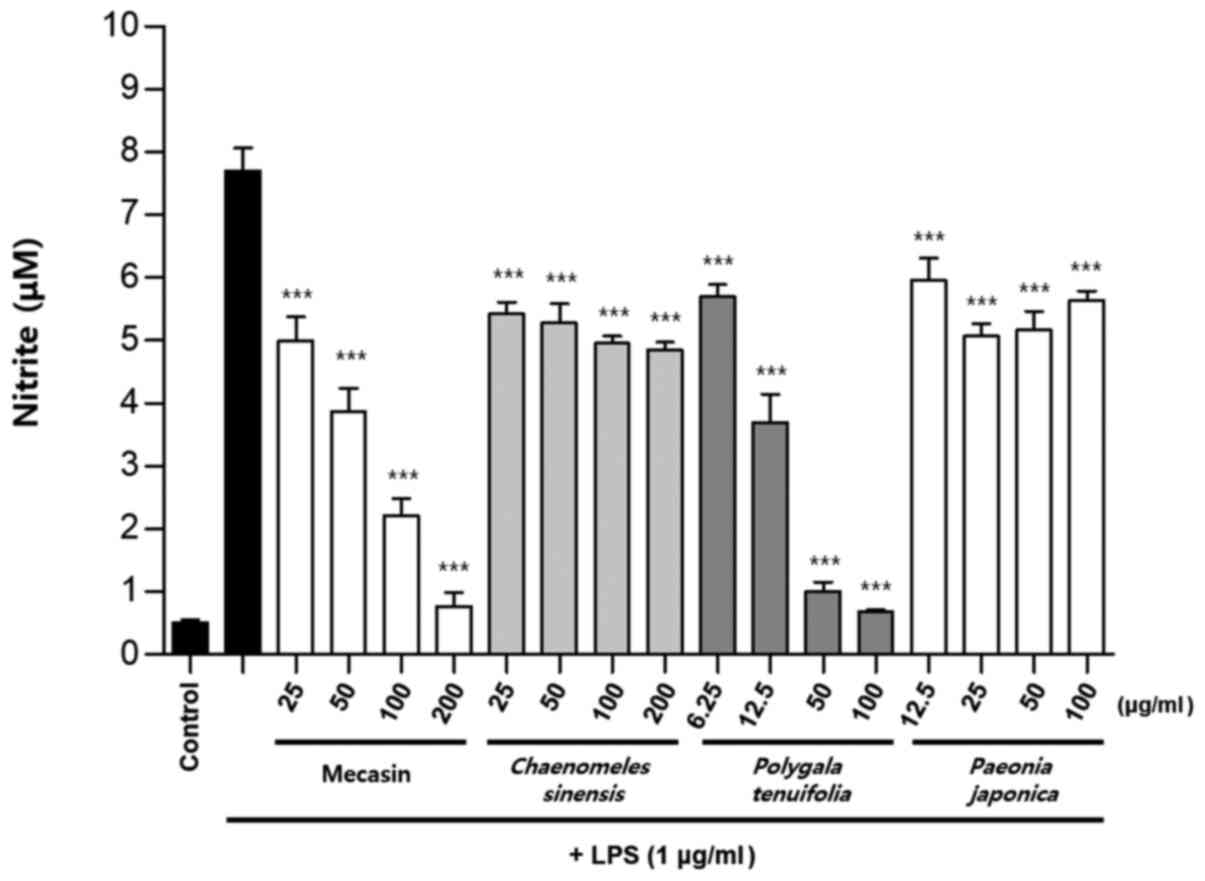
Comparison of nitrite production by
mecasin and its constituents at the same concentration
BV2 microglia were cultured for 12 h. After 12 h,
cells were pre-treated with each test sample at the indicated
concentration for 3 h and then stimulated with LPS for 24 h. Next,
the concentration-dependent nitrite reduction effect of mecasin was
compared with that of each constituent at the same concentration.
The results indicated that C. longa, P. tenuifolia
and G. uralensis had similar inhibitory effects on nitrite
production, while S. miltiorrhiza had a relatively weak
inhibitory effect on nitrite production. However, the remaining
five individual constituents had no significant effect on nitrite
reduction Fig. 7, Fig. 8, Fig.
9).
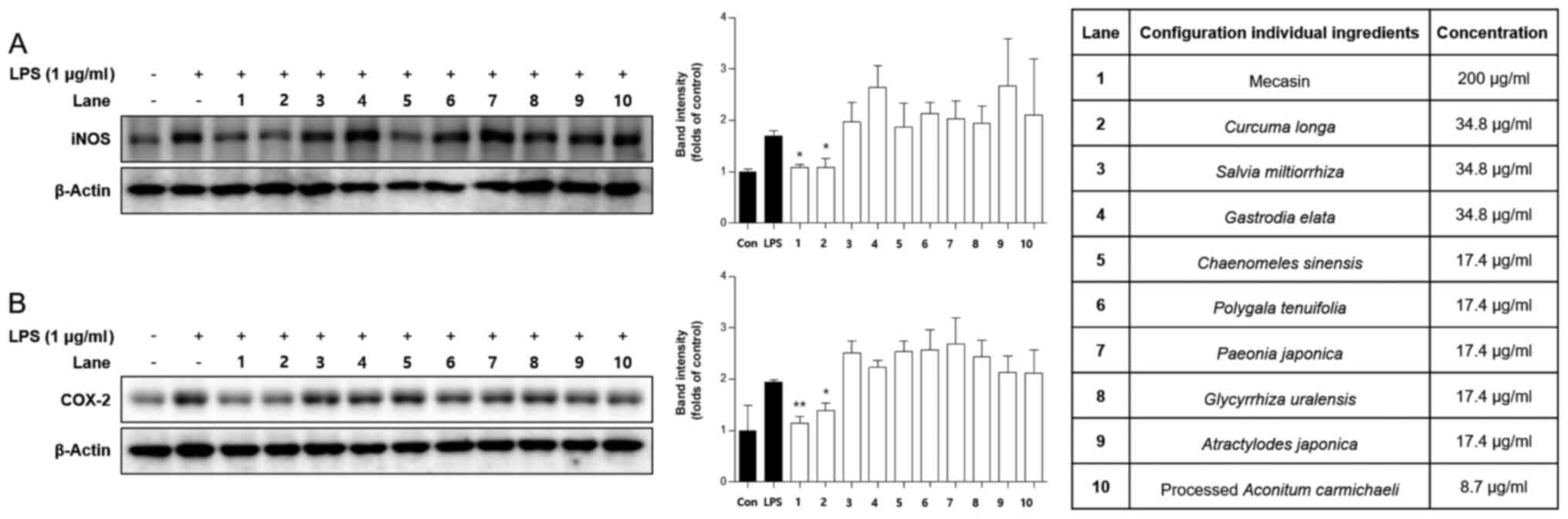
Comparison of inhibitory effects of
mecasin and its constituents on iNOS and COX-2 expression
It is well known that iNOS and COX-2 are responsible
for the production of nitrite in LPS-induced microglia cells. Thus,
it was examined whether the mecasin exerted its inhibitory effect
on LPS-induced nitrite production through regulating iNOS and COX-2
expression by using western blot analysis. BV2 microglia were
pre-treated with each sample at the indicated concentration for 3 h
and then stimulated with LPS at 1 µg/ml for 24 h. As presented in
Fig. 10, pre-treatment with
mecasin or certain constituents thereof suppressed the expression
of iNOS and COX-2 in a pattern similar to the inhibition rate of
nitrite production.
Discussion
BV2 microglia are main immune effector cells in the
brain. They have a major role in the pathogenesis of a wide range
of chronic neuroinflammation and neurodegenerative diseases. A
general feature of BV2 microglia in the inflammation of the central
nervous system is the long-term activation of BV2 microglia and
consequently increased inflammatory expansion, leading to increased
release of inflammatory mediators. Therefore, BV2 microglia have a
crucial role in the development of neuroinflammation and
neurodegenerative diseases. BV2 microglia are known to secrete NO,
tumor necrosis factor-α (TNF-α), IL-1β and other active substances
involved in the pathogenesis of nervous system diseases (16,17).
The expression of NOS is significantly increased in lesions of
nervous system diseases. NOS is known to promote the production of
NO. NO is able to form a strong oxidant that causes tissue
oxidative damage after a series of reactions (18). TNF-α and IL-1β are strong
pro-inflammatory factors that may increase the release of NO and
aggravate inflammatory response and neuronal damage (19). Therefore, in the present study, BV2
microglia were chosen as an in vitro cell model. NO acts as
a highly reactive free radical in the body. It is both a second
messenger and a neurotransmitter. It is also an effector molecule
that is widely used in the body. It has various physiological
effects, such as relaxing vascular smooth muscle, inhibiting
platelet aggregation, regulating cerebral blood flow, mediating
cytotoxic effects and regulating the immune system. NO itself has a
short half-life. NO in the blood is mainly produced by vascular
endothelial cells, vascular smooth muscle cells, platelets and
macrophages in the form of nitrite and nitrate. According to the
principle that NO will form nitrite when it is dissolved in water,
the content of NO was indirectly reflected by the nitrite content
measured in the present study (19,20).
A previous study by our group have suggested that
mecasin has anti-inflammatory and anti-oxidant effects (10). Jakyak-Gamcho-tang, a prescription
that is the origin of mecasin, has been used mainly for alleviating
pain, muscle spasms and cold syndrome due to blood deficiency for
centuries in traditional oriental medicine (21). A previous study has indicated that
mecasin is able to reduce the release of NO in activated microglial
cells (10). However, the role of
each ingredient regarding the total effect of mecasin has remained
elusive. The results of the present study suggested that
constituents of mecasin have synergistic anti-neuroinflammatory
effects.
First, the concentration of individual ingredients
was set based on mecasin concentrations of 25, 50, 100 and 200
µg/ml. Next, the toxicities of mecasin and its nine individual
medicinal herb constituents were measured using an MTT assay.
LPS-stimulated BV2 cells were selected as an experimental model to
study effects of mecasin and its constituents on the release of
inflammatory products. The results indicated that mecasin had the
most potent effect. Among the single herbs, C. longa, P.
tenuifolia and P. japonica also exhibited marked
effects. Joseph et al (22)
suggested that C. longa may act as a pro-drug, as its
metabolite has anti-inflammatory activity. The metabolite produced
by the oxidation of curcumin derived from C. longa is able
to bind to NF-κB in a covalent bond, thereby further suppressing
inflammatory reactions (22).
Mecasin demonstrated a higher inhibitory effect on nitrite
generation than its nine medicinal herb constituents. Next, we also
checked that the effect of mecasin and its constituents (nine
individual medicinal herbs) on iNOS and COX-2 expression was
assessed. As presented in Fig. 10,
mecasin and C. longa demonstrated the most prominent
inhibitory effects. Furthermore, mecasin demonstrated higher
inhibitory effects compared with C. longa. The results
suggested that treatment with mecasin inhibited the protein
expression of iNOS and COX-2 to a greater extent than its nine
herbal constituents separately. These results demonstrated that the
most potent inhibitor of nitrite production and iNOS and COX-2
expression was mecasin, probably due to synergistic effects of its
herbal medicinal constituents.
The effects of mecasin and its constituents on
LPS-stimulated nitrite production and expression levels of iNOS and
COX-2 on BV2 microglia cells at the same concentration were
examined. Mecasin is not a simple combination of nine herbs with
pharmacological effects. The first experiment was performed to
observe neuroinflammatory effects of mecasin and its constituents
at corresponding concentrations. It was indicated that mecasin
(mixture of 9 components) had a higher effect than its individual
herbal constituents. The second experiment was performed to observe
their neuroinflammatory effects at the same concentration. The
results suggested that three single individual herbal constituents
had similar effects to those of mecasin. However, these three
constituents exhibited higher cytotoxicity than mecasin at the same
concentration. This result indicated that synergistic effects of
constituents of mecasin may increase the effectiveness and safety
of mecasin, indicating that mecasin has potential for treating
neuroinflammation. To draw any reliable conclusions regarding the
potential value of mecasin in neurological diseases, the results
should be reperformed at least in another microglial cell line such
as HMC3. Therefore, in a follow-up study, other microglial cells
such as HMC3 cells will be used.
Finally, Chinese medicine is well known for reducing
side effects and enhancing the efficacy of several drugs (23,24).
In certain studies, some of the results regarding the lowering of
highly toxic drug side effects and the enhancing of drug efficacy
have been confirmed (23,24). Mecasin is a new combinational drug.
However, a detailed mechanistic study on the synergistic effect of
mecasin is required in future research.
Acknowledgements
Not applicable.
Funding
Funding: This research was supported by a grant from the Jeonbuk
Research & Development Program funded by Jeonbuk province
(grant no. RA202006-24-C4).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TW and WK designed the experiments. DSL, JYS, DC and
SK performed the nitrite and MTT assays. TW and WK drafted the
manuscript or revised it critically for important intellectual
content. DSL and SK gave final approval of the version to be
published. All the authors verified and approved the final version
of the manuscript. All the authors checked and approved the
authenticity of the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yoon HM, Jang KJ, Han MS, Jeong JW, Kim
GY, Lee JH and Choi YH: Ganoderma lucidum ethanol extract
inhibits the inflammatory response by suppressing the NF-κB and
toll-like receptor pathways in lipopolysaccharide-stimulated BV2
microglial cells. Exp Ther Med. 5:957–963. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Amor S, Peferoen LAN, Vogel DYS, Breur M,
van der Valk P, Baker D and van Noort JM: Inflammation in
neurodegenerative diseases-an update. Immunology. 142:151–166.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Griffiths MR, Gasque P and Neal JW: The
multiple roles of the innate immune system in the regulation of
apopyosis and inflammation in the brain. J Neuropathol Exp Neurol.
68:217–226. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hernagomez M, Carrillo-Salinas FJ, Mevha
M, Correa F, Mestre L, Loría F, Feliú A, Docagne F and Guaza C:
Brain innate immunity in the regulation of neuroinflammation:
Therapeutic strategies by modulating CD200-CD200R interaction
involve the cannabionoid system. Curr Pharm Des. 20:4707–4722.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kim DH: Effect of
Gamijakyakgamchobuja-tang on neuropathic pain in rats. Korean;
Jeollabuk-do, Wonkwang Univ, 2015.
|
|
6
|
Lee DS, Ko W, Song BK, Son I, Kim DW, Kang
DG, Lee HS, Oh H, Jang JH, Kim YC and Kim S: The herbal extract
KCHO-1 exerts a neuroprotective effect by ameliorating oxidative
stress via heme oxygenase-1 upregulation. Mol Med Rep.
13:4911–4919. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yu JW: Study on the ingredient of
Jakyakgamchotang. Jeollabuk-do. Wonkwang Univ. Korean, 2010.
|
|
8
|
Kim BW: Anti-inflammatory effect of
Jakyakgamcho-tang. J Int Korean Med. 31:365–371. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee JM, Hong SY and Oh MS: Effects of
Jakyakkamchobuja-tang on Papain-induced osteoarthritis in mice. J
Korean Med. 34:116–135. 2013.
|
|
10
|
Lee DS, Ko W, Yoon CS, Kim DC, Yun J, Lee
JK, Jun KY, Son I, Kim DW, Song BK, et al: KCHO-1, a novel
anti-neuroinflammatory agent, inhibits Lipopolysaccharide induced
neuroinflammatory responses through Nrf2-mediated heme oxygenase-1
expression in mouse BV2 microglia cells. Evid Based Complement
Alternat Med. 2014(357154)2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cha E, Lee J, Lee S, Park M, Song I, Son
I, Song BK, Kim D, Lee J and Kim S: A 4-week repeated dose oral
toxicity study of Mecasin in Sprague-Dawley rats to determine the
appropriate doses for a 13-week, repeated toxicity test. J
Pharmacopuncture. 18:45–50. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jeong H, Lee J, Cha E, Park M, Son I, Song
B and Kim S: A study on the oral toxicity of mecasin in rats. J
Pharmacopuncture. 17:61–65. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cha E, Jeong H, Lee J, Lee S, Park M and
Kim S: A study on single dose toxicity of Mecasin pharmacopuncture
injection in muscle. J Korean Med. 36:36–42. 2015.
|
|
14
|
Lee SJ, Jeong HH, Lee JC, Cha EH, Park MY
and Song BG: A study on single dose toxicity of intravenous
injection of Mecasin herbal acupuncture. J Korean Acupunct
Moxibustion Med. 33:1–7. 2016.
|
|
15
|
Kook MG, Choi SW, Seo Y, Kim DW, Song BK,
Son I, Kim S and Kang KS: KCHO-1, a novel herbal anti-inflammatory
compound, attenuates oxidative stress in an animal model of
amyotrophic lateral sclerosis. J Vet Sci. 18:487–497.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu HY, Chen CY and Hsueh YP: Innate
immune responses regulate morphogenesis and degeneration: Roles of
Toll-like receptors and Sarml in neurons. Neurosci Bull.
30:645–654. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
van Ham TJ, Brady CA, Kalicharan RD,
Oosterhof N, Kuipers J, Veenstra-Algra A, Sjollema KA, Peterson RT,
Kampinga HH and Giepmans BNG: Intravital correlated microscopy
reveals differential macrophage and microgial dynamics during
resolution of neuroinflammation. Dis Model Mech. 7:857–869.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Broholm H, Andersen B, Wanscher B,
Frederiksen JL, Rubin I, Pakkenberg B, Larsson HB and Lauritzen M:
Nitric oxide synthase expression and enzymatic activity in multiple
Sclerosis. Acta Neural Scand. 109:261–269. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Baud V and Karin M: Signal transduction by
tumor necrosis factor and its relatives. Trends Cell Biol.
11:372–377. 2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Braddock M and Quinn A: Targeting IL-1 in
inflammatory disease: New opportunities for therapeutic
intervention. Nat Rev Drug Discov. 3:330–339. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Guo L, Cho SY, Kang SS, Lee SH, Baek HY
and Kim YS: Orthogonal array design for optimizing extraction
efficiency of active constituents from Jakyak-Gamcho Decoction, the
complex formula of herbal medicines, Paeoniae Radix and
Glycyrrhizae Radix. J Ethnopharmacol. 113:306–311.
2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Joseph AI, Edwards RL, Luis PB, Presley
SH, Porter NA and Schneider C: Stability and anti-inflammatory
activity of the reduction-resistant curcumin analog, 2,6-dimethyl
curcumin. Org Biomol Chem. 16:3273–3281. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu J, Liu J, Shen F, Qin Z, Jiang M, Zhu
J, Wang Z, Zhou J, Fu Y, Chen X, et al: Systems pharmacology
analysis of synergy of TCM: An example using saffron formula. Sci
Rep. 8(380)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bhuyan DJ, Perera S, Kaur K, Alsherbiny
MA, Low M, Seto SW, Li CG and Zhou X: Synergistic Effects of
Chinese Herbal Medicine and Biological Networks. In: Approaching
Complex Diseases. Bizzarri M (ed). Springer, Cham, pp393-436,
2020.
|