Introduction
Ionic liquids (ILs) are a class of compounds of
great importance in modern science due to their extensive use in
major areas of research activity. For example, these liquids can be
used as: i) alternative solvents to volatile organic compounds
(1,2), ii) media for electrodeposition of
metals (3), iii) catalysts and
biocatalysts (4,5), iv) potential corrosion inhibitors
(6,7), or v) solvents in the food industry
(8). ILs have several key
physicochemical properties for such applications, including: high
thermal stability, increased ionic conductivity, low vapor
pressure, non-flammability, tenability and high polarity (9). As a result, they have attracted
increasing research interest from the pharmaceutical industry.
ILs can affect biological systems at different
biochemical levels, starting from simple macromolecules to complex
metabolic mechanisms in prokaryotic and eukaryotic cells. In this
context, investigation into the antimicrobial activity of different
ILs classes on microorganisms of clinical and environmental
importance has emerged as an important direction of research during
the past few decades. Recent studies have shown that many types of
ILs have the potential to inhibit the growth of different bacteria
and fungi, rendering them useful for various applications in
medicine and industry (1,10,11-13).
It has also been reported that antimicrobial activity is linked to
the length of the substituent chain, with the mention that
short-chain compounds exert a weaker antimicrobial efficacy
compared to long-chain compounds (14-16).
This antimicrobial effect was studied on both standardized
bacterial strains and bacterial strains isolated from the hospital
environment, and some of the strains of interest were found to
display antibiotic resistance (17,18).
However, to fully evaluate the clinical applicability of ILs,
further studies are required; one important topic being the
deciphering of the toxicological, antimicrobial and
antiproliferative effect of ILs.
The cytotoxicity of ILs has been extensively
analyzed using in vitro 2D monolayer culture systems and
different cell lines as study systems-both normal cells
(fibroblasts, osteoblasts, macrophages, keratinocytes, rat glial
cells) and neoplastic cell lines (breast, colon, lung, liver
carcinoma and leukemia cells) (19). However, our knowledge concerning the
possibility of using ILs as anticancer agents is yet to be fully
understood. In addition, the use of the aforementioned culture
systems has several drawbacks which may lead to the obtaining of
unrealistic results. Thus, the substance of interest is applied in
a uniform layer to the surface of the 2D culture. This contrasts
with the three-dimensional architecture of tumors in vivo
and also with the heterogeneous distribution of nutrients and drugs
in tumor cells. This environment can more realistically be
replicated via the use of an in vitro 3D culture, which
generates multilayer cell aggregates with a complex tissue
organization, similar to what can be observed in vivo
(20).
The present study was designed to investigate the
antibacterial and antiproliferative effect of tetrahexylammonium
bromide (THABr) ionic liquid formulation. To evaluate the
antibacterial effect of THABr_IL, the following standardized
bacterial strains were used: i) Gram-positive bacteria
(Gram+): Staphylococcus aureus, Streptococcus
pneumoniae and Enterococcus faecalis; and ii)
Gram-negative bacteria (Gram-): Escherichia coli,
Salmonella typhimurium, Proteus miriabilis,
Klebsiella pneumoniae, Pseudomonas aeruginosa and
Haemphilus influenzae. The cell line Caco-2 (colorectal
adenocarcinoma) served as a study system and was grown in
vitro using both 2D and 3D culture systems.
Materials and methods
Tetrahexylammonium bromide
[CH3(CH2)5]4N(Br) and
distilled water were purchased from Sigma-Aldrich (Merck KGaA).
Cell culture specific reagents [Dulbecco's modified Eagle's medium
(DMEM), fetal bovine serum (FBS), dimethyl sulfoxide (DMSO),
trypsin-EDTA solution, penicillin-streptomycin mixture, and
phosphate-buffered saline (DMSO)] and cytotoxicity assays
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and Alamar blue] were obtained from Invitrogen (Thermo Fisher
Scientific, Inc.).
Antimicrobial susceptibility tests
Microorganism testing
The antimicrobial activity was studied for
Gram+ bacteria, represented by Staphylococcus
aureus (ATCC 25923), Streptococcus pneumoniae (ATCC
49619) and Enterococcus faecalis (ATCC 29212) and
Gram- bacteria, represented by Escherichia coli
(ATCC 25922), Salmonella typhimurium (ATCC 14028),
Proteus miriabilis (ATCC 12453), Klebsiella
pneumoniae (ATCC 1388), Pseudomonas aeruginosa (ATCC
27853) and Haemphilus influenzae (ATCC 49247). The tested
THAB_IL concentrations were: c1, 13.6 mg/ml; c2, 6.8 mg/ml; c3, 3.4
mg/ml; c4, 1.7 mg/ml; and c5, 0.85 mg/ml. Concentrations of
interest were prepared using sterile distilled water.
Determination of the minimum
inhibitory concentration (MIC)
Antimicrobial tests (antibiogram and ionic liquid
testing) for the microorganisms studied were carried out using the
Disk Diffusion method as previously described (21). A small amount of each microbial
culture was diluted in sterile 0.9% sodium chloride solution until
the turbidity was equivalent to the 0.5 McFarland standard. These
suspensions were further diluted 1:10 in medium CHROM agar for
bacteria (Oxoid) and then spread on sterile Petri plates. Sterile
micro-compresses were applied on the agar surface (in Petri
plates), and after that 10 µl of each sample was added into the
micro-compresses. Commercially available discs containing
antibiotics were used as positive controls. The plates were
incubated at 37˚C for 24 h.
The inhibition zones were assessed by comparatively
testing THABr_IL vs. gentamicin (Gn) and THABr_IL vs.
trimethoprim/sulfamethoxazole (Sxt). Gentamycin and
trimethoprim/sulfamethoxazole were used as controls for
Gram+ and Gram- bacteria, respectively.
Cellular viability test from bacterial
strain
An amount 100 µl of microbial cultures in Mueller
Hinton broth (with a turbidity was equivalent to 0.5 McFarland
standard) was transferred into 96-well plates. Next, 50 µl THABr_IL
at different concentrations of interest were loaded into wells-with
Sxt and Gn used as controls- and the plates were incubated at 37˚C
for 6 h. After adding 10 µl of solution of
2,3,5-tripheniltetrazolium chlorine 0.5% (TTC) into the wells, the
plates were incubated at 37˚C for another 2 h. Absorbance was
measured at 460 nm (Tecan Sunrise spectrophotometer). The rate of
inhibition (expressed as a percentage) was determined as the ratio
between the difference between absorbance values of the control and
sample and absorbance value of the control. All experiments were
conducted in triplicate.
In vitro cytotoxicity testing 2D cell
culture
Human colon adenocarcinoma Caco-2 cells
(ATCC® HTB-37™) were used as a cell line model. Briefly,
the cells were seeded in 75 cm2 flasks in DMEM GlutaMax
medium supplemented with 10% FBS and antibiotics in a 5% humidified
atmosphere at 37˚C. At 80-90% confluence, the Caco-2 cells were
detached by trypsinization, and the cell number was assessed using
the Countess II Automated Cell Counter (Thermo Fisher Scientific,
Inc.). Caco-2 cells (100 µl, 2x105 cells/ml) were next
seeded in a 96-well plate. After 12 h, fresh complete medium
containing different concentration of THABr_IL dissolved in DMSO
(ranging from 0.1 to 10 mM) was added, with 0.1% DMSO being used as
vehicle control. The cytotoxic effect was assessed with MTT
reagent. After 24, 48 or 72 h, MTT solution (5 mg/ml in PBS) was
added, followed by incubation at 37˚C for 4 h. Then the medium was
removed and 50 µl DMSO was added to solubilize the formazan
crystals. The absorbance was read at 570 nm (Tecan Sunrise
spectrophotometer). The cells treated only with DMSO (0.1%) were
used as control to calculate the inhibition rate (%) as described
above. All experiments were run in triplicate.
3D cell culture Development of 3D
cellular aggregates
A total of 1x104 cells/ml in 100 µl
medium (1x103 cells/well) were seeded in 96-well plates
(NunclonSphera, Thermo Fisher Scientific, Inc.). After 72 h and
after adding fresh specific medium with different concentrations of
THABr_IL or 0.1% DMSO, the plates were maintained in an incubator
at 37˚C with 5% CO2 for 24 and 72 h. The Alamar blue
assay was used to assess the effects of THABr_IL on Caco-2 cell
viability and proliferation. Alamar blue reagent [10% (v/v)] was
added in each well, and then the absorbance was measured at 570 and
600 nm (Tecan Sunrise spectrophotometer) to calculate cell
proliferation. Cell morphology was analyzed by using a Zeiss Axio
Observer A1 Inverted Phase Contrast Microscope (Zeiss).
Statistical analyses
Statistical analyses were performed using Excel from
MS Office Pro Plus 2019 and GraphPad Prism 8 (GraphPad Software,
Inc.); the differences were assessed by one-way ANOVA. The level of
P<0.1 was considered statistically significant and P≤0.0001 was
considered highly statistically significant. The following
notations were used on the graphs: *P≤0.1,
**P≤0.01, ***P≤0.001 and
****P≤0.0001.
Results
Antimicrobial activity
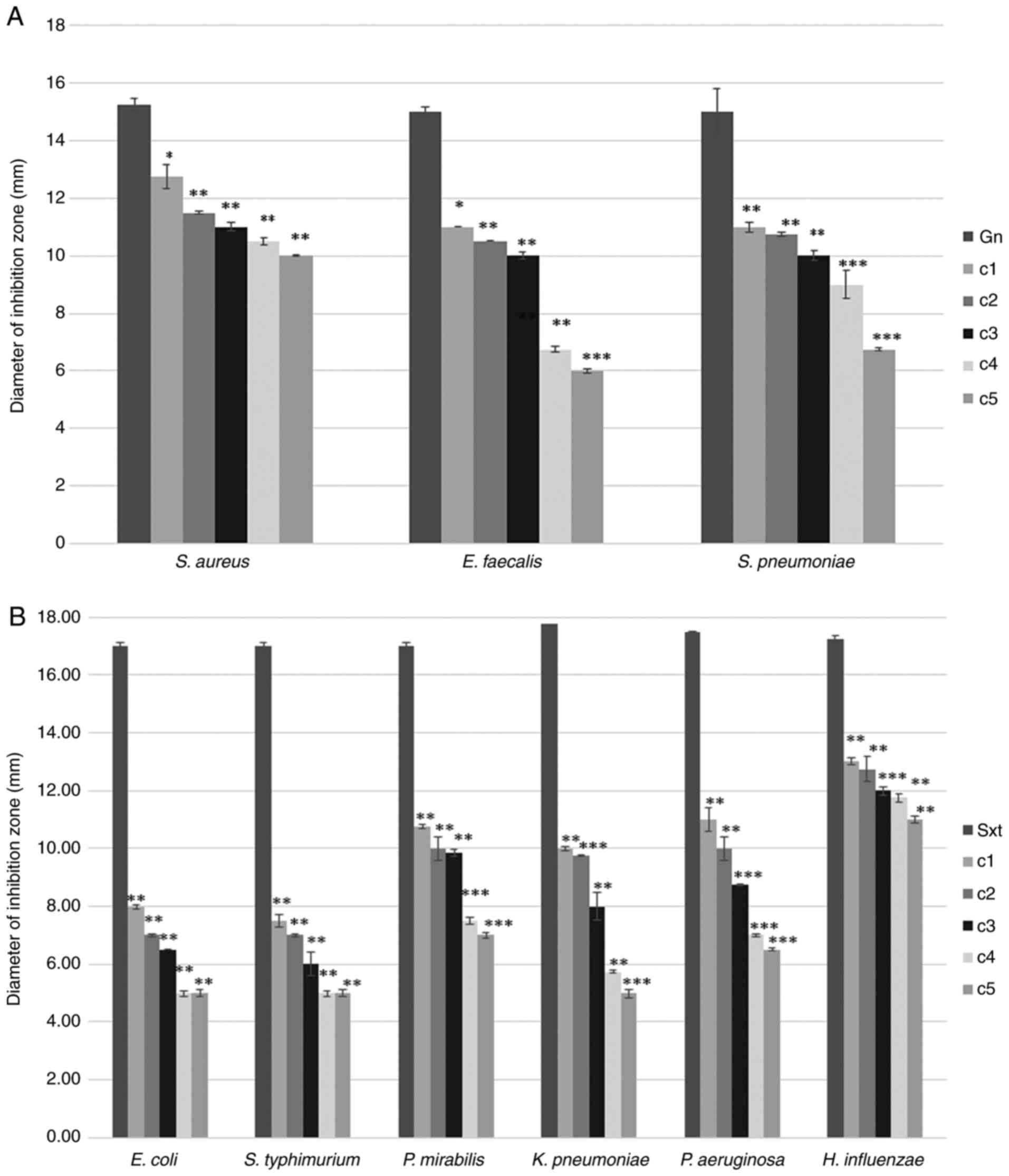
The antimicrobial effect of tetrahexylammonium
bromide (as IL) on three different Gram+ bacteria was
investigated. The growth of S. aureus was affected by all
five tested concentrations, while the corresponding diameter of the
zone of inhibition was recorded between 10 and 12.75 mm. This
indicates a moderate antibacterial effect of tetrahexylammonium
bromide on the aforementioned bacterial strain. In the case of
E. faecalis, this antibacterial effect was pronounced only
for the highest concentrations tested (c1-c3) and in the case of
S. pneumoniae, this effect occurred at c1-c4 concentrations
tested (Fig. 1A).
In contrast to Gram+ bacteria, a quite
homogenous pattern of response to tetrahexylammonium bromide
exposure was found in the Gram- bacteria, with E.
coli, S. typhimurium and K. pneumoniae showing an
intermediate sensitivity to this THABr_IL formulation. These
bacteria were inhibited when exposed to the three highest
concentrations, but were not affected by the two lowest doses.
Concerning P. mirabilis and P. aeruginosa, the
measured values of the inhibition areas were between 9.85-10.75 and
8.75-11 mm, respectively, again with an inhibitory effect being
detected at the highest treatment doses. The strongest
antibacterial activity of this THABr_IL formulation was identified
for H. influenzae, with a diameter of zone of inhibition
around 11-13 mm (Fig. 1B).
The diameters of the corresponding zones of
inhibition were 15 mm (for gn) and 17 mm (for Sxt). Compared with
standard reference antibiotics (gn and Sxt), tetrahexylammonium
bromide (as IL) exerted a weaker antimicrobial effect expressed by
smaller inhibition areas. These changes were found to be
statistically significant (P<0.1, P<0.01, P<0.001), but
this antimicrobial effect was found to vary depending on the tested
concentration and bacteria (Fig.
1).
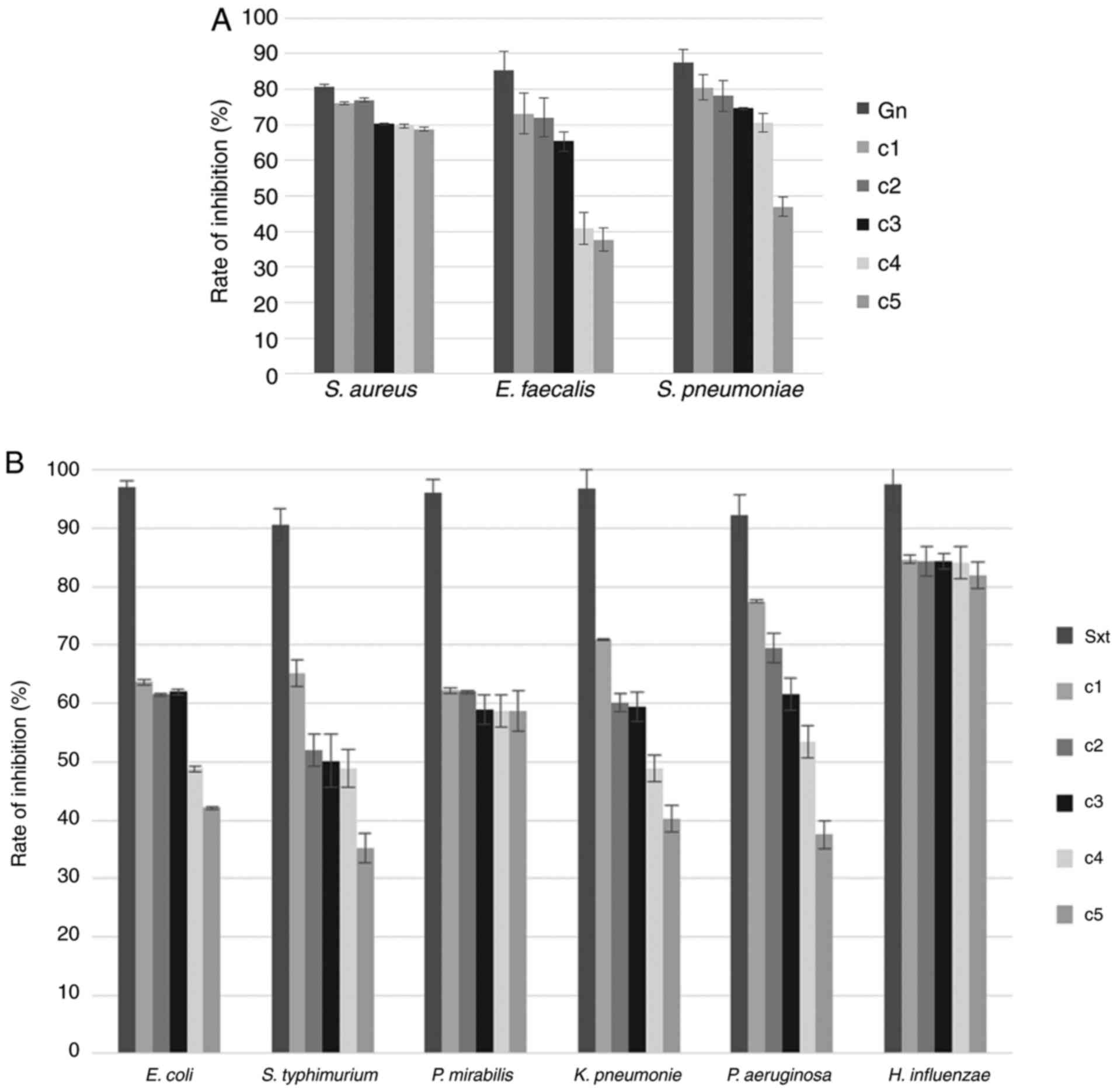
Concerning the rate of inhibition (%) of
Gram+ bacteria, the measured values ranged between 68.80
and 76.08% for S. aureus; between 46.98 and 80.47% for S.
pneumoniae; and between 37.63 and 73.11% for E.
faecalis. A clear bacteriolytic activity of tetrahexylammonium
bromide was identified for all concentrations tested, the highest
four concentrations and the highest three concentrations for S.
aureus, S. pneumoniae and E. faecalis,
respectively (Fig. 2A).
In the case of Gram- bacteria, the
measured rates of inhibition were also diferent among bacteria and
treatment groups. Thus, the bacteriolytic potential against E.
coli, K. pneumoniae, P. mirabilis and P.
aeruginosa was observed when these bacteria where exposed to
the highest three tetrahexylammonium bromide concentrations. For
S. typhimurium, by contrast, this potential was noted only
for the highest two treatment groups. In the case of H.
influenzae, the rates of inhibition were 82.01-84.70%, with the
tested IL showing a bacteriolytic action for all analyzed doses
(Fig. 2B).
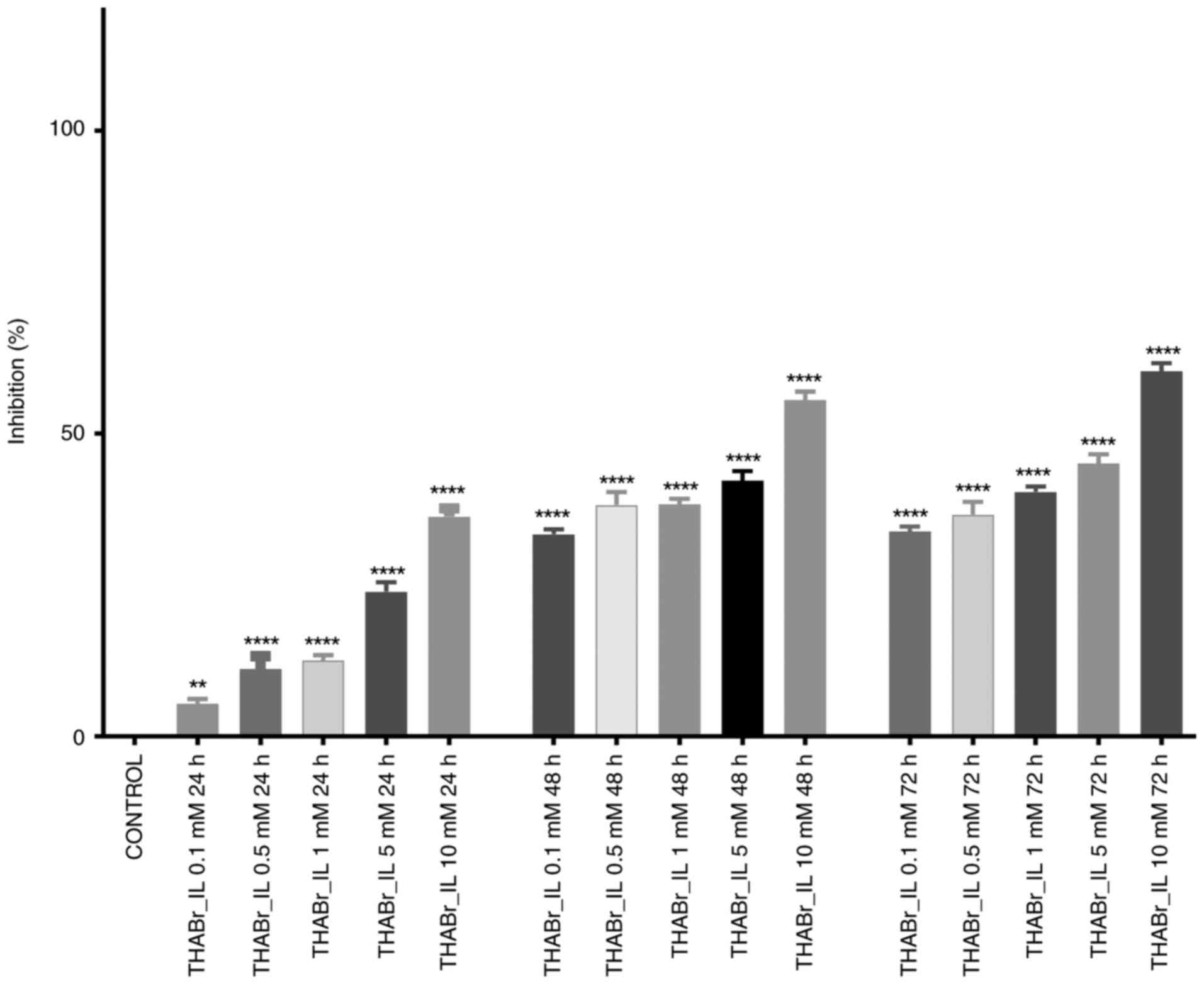
Cytotoxic capacity
The density of cultured cells was 4,000 cells/well
for the 3D cell culture and 20,000 cells/well for the 2D cell
culture. Determination of the antiproliferative potential was
performed by incubating cells in specific media containing
different concentrations of tetrahexylammonium bromide in DMSO.
First, the IL was solubilized in DMSO (stock solution), and then
the final concentrations (10, 5, 1, 0.5 and 0.1 mM) were obtained
by dilution in culture medium. The concentration of DMSO in culture
medium was 0.1%, a concentration at which cell viability is not
affected. After one day of incubation, the 2D cell cultures
revealed a slight decrease in cell proliferation. However, the
inhibition rate was dose-dependent, with statistically significant
differences (P≤0.01) being observed between different treatments.
The half maximal inhibitory concentration (IC50) was not
reached, irrespective of the tested concentrations and bacterial
strain. More than half of cells showed reduced growth following 48
and 72 h of continuous exposure to 10 mM tetrahexylammonium
bromide, more precisely 55.57 and 60.26% at 48 and 72 h,
respectively (Fig. 3). Exposure of
Caco-2 cells to the THABr_IL resulted in significantly different
cellular responses between the 10 mM treatment and the other
treatments used. Were also noticed a similar pattern for the 0.5 mM
treatment and the 1 mM treatment (P≤0.0001).
Assessment of tetrahexylammonium bromide effect on
3D culture neoplastic colon cells was conducted after exposing the
cells to THABr_IL at different concentrations for 72 h. Aiming to
create optimal conditions for the development of cell aggregates,
the cells were first cultured in a specific medium without IL for 4
days (Fig. 4).
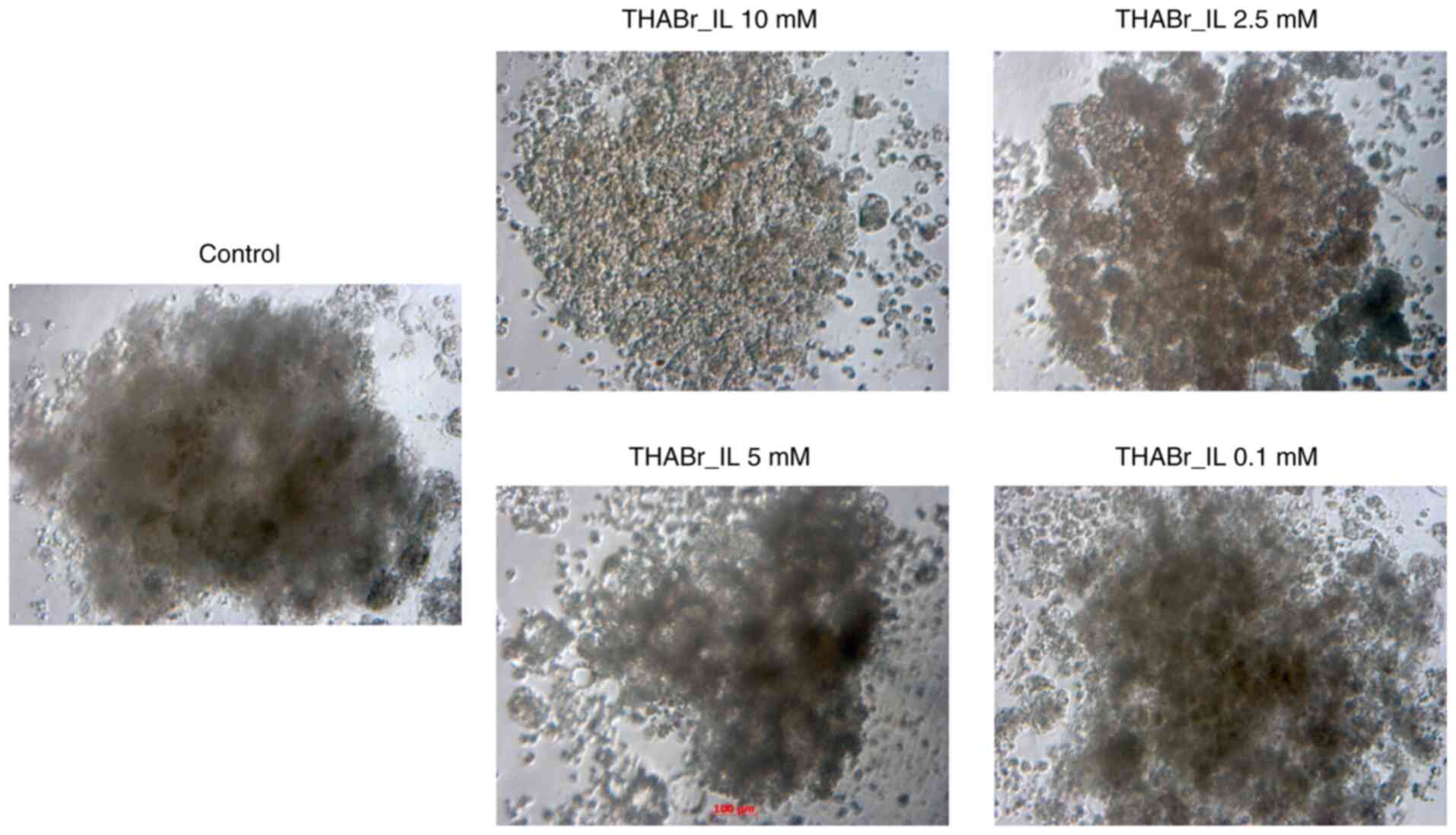
Evaluation of morphology of mature cellular
aggregates was performed at 7 days, while 0.1% DMSO was used as
reference. It was observed that the cell aggregates in the control
groups tended to have round/oval shape. In contrast, cell cultures
exposed to tetrahexylammonium bromide showed alterations in cell
shape and dimensions. In fact, none of the cells from these
treatment groups had a round shape, which is characteristic to
spheroids in the control group. In addition, at high IL doses the
formation of multiple, uneven cellular aggregates was observed
(Fig. 5).
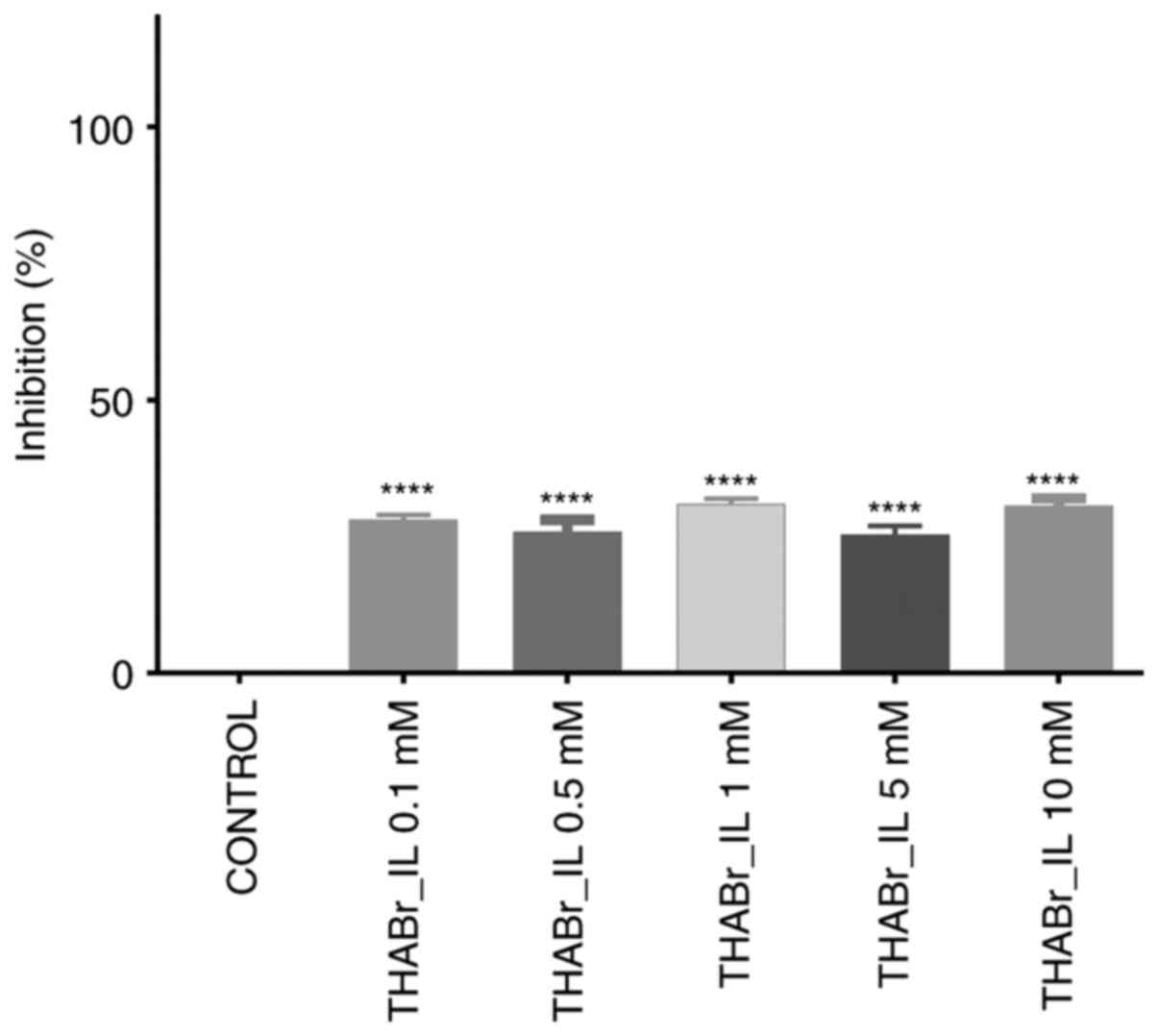
Analysis of the cell proliferation using the Alamar
blue test also revealed no consistent effect on cell proliferation
in the exposed groups. Inhibition of cell multiplication appeared
to be independent of tetrahexylammonium bromide dose and ranged
from 31.01% (0.5 mM concentration) to 25.33% (1 mM
concentration).
In the cells cultured in the 3D system, THABr_IL did
not cause an increased inhibition of cell growth (IC50
was not reached at any applied concentration), however, this ionic
fluid appeared to inhibit cell aggregation in the 3D
structures.
Discussion
To the best of our knowledge, in vitro
cytotoxicity studies for ionic liquids (ILs) have been performed
only using 2D cell cultures. This is hence the first study to use
3D-cultured cells to study the cytotoxic effect of
tetrahexylammonium bromide, an IL. By using a comparative approach,
our paper also extends from a bidirectional point of view the
previous knowledge existing on the potential clinical importance of
these compounds. The biological activity of ILs depends primarily
on their hydration status (22,23);
although their mechanisms of action can vary among different
organisms, water being essential for all living systems. Therefore,
the main factors modulating the biological activity of ILs are
their solubility and their interaction with water (24). These liquids can dissolve a wide
array of chemical compounds (25)
and many of them can act against cell systems. Various ILs are
highly toxic for cellular systems and can induce changes in
intracellular osmotic pressure, structure alterations and fluidity
of the cell membrane, as well as inhibition of enzymatic activity
via changes in their folding. The dynamic interplay underlying such
changes is now a subject of intense research interest (26).
There are many different mechanisms of action by
which chemical compounds can affect bacterial cells, including
protein denaturation, disruption of nucleo-protein complexes, cell
membrane alteration, oxidation of sulph-hydryl groups and reactions
with amino groups (27,28). Our team has conducted studies
regarding the therapeutic potential of some biologically active or
synthetic compounds, including the chemical characterization of
plant compounds, some of which have confirmed antitumor and
antibacterial potential (29,30).
Certainly, the applicability of these compounds in medical practice
requires additional studies on their physicochemical
characterization, their bioavailability and other pharmacokinetic
parameters. The antibacterial activity of ILs is based on the
interaction of the alkyl chain with membrane lipids, leading to the
formation of ion channels, disturbance of the corresponding
transmembrane potential, and finally, to the death of bacteria
(31,32). Quaternary ammonium halides, which
serve as precursors for ILs, exhibit notable antimicrobial
properties. They have a relatively low toxicity to organisms and
they are used in sterilization, disinfection, as well as in the
preparation of compounds with bactericidal and fungicidal
properties (33). Among this
category of compounds, quaternary ammonium chlorides and bromides
are the most frequently used quaternary ammonium halides as
antiseptics despite having some potential drawbacks (34,35).
Antimicrobial activity depends on the length and the
number of alkyl chains within the molecule (27,28,36).
There is strong evidence that, for ILs containing methylimidazolium
cation, the toxicity is mainly related to the length of the n-alkyl
radical, but not to the anion type (37). Several studies have also
demonstrated that compounds with an alkyl radical exceeding four
carbon atoms exert marked toxic effects on different bacteria, such
as Escherichia coli, Staphylococcus aureus and Bacillus
subtilis, and prokaryotes, e.g. cladoceres such as Daphnia
magna and green algae such as Oocystis submarina,
Chlorella vulgaris, Scenedesmus vacuolatus and
Chlamidomonas reinhardtii (38,39).
The bactericidal activity of ILs is only known for
vegetative forms of bacteria, with gram-positive bacteria being
more sensitive than gram-negative. The strongest effect was
identified to occur against gram-positive bacteria belonging to the
genus Staphylococcus and Streptococcus, gram-negative
bacteria belonging to the genus Lactobacillus and vegetative
forms of Bacillus subtilis. The biological activity of ILs
is high for compounds containing up to 12 carbon atoms (40). Moreover, certain yeasts, such as
Candida albicans, and filamentous fungi (Aspergillus
niger, Chaetomiumglobosum, Myrotheciumverrucaria,
Trichodermaviridae, Coniophoraputean, Trametes
versicolor) were found to be sensitive to exposure to
quaternary ammonium halides (40).
The incidence of infections and especially their recurrence, in
most medical fields, with a maximum recorded in cases involving
surgery, is increasing despite the constant improvement of
treatment protocols (41). There
are still a number of gaps and these new approaches send positive
signals about the possibility of combating these deficiencies.
Although many studies have been conducted over time on topics
related to antimicrobial effect of different ILs (17,18,42,43),
the mechanisms underlying these effects or the clinical relevance
of these results are still far from being completely understood.
Further studies are hence required, not only to expand on the
clinical significance of the antibacterial effect of ILs, but also
to refine our knowledge of how different ILs interact with
different types of bacteria.
For in vitro evaluation of the cytotoxicity
of ILs, cell lines of different organisms (humans, rats, mice,
Chinese hamsters) have been utilized. Concerning the Caco-2 cell
line (colorectal adenocarcinoma), ILs based on imidazolium,
guanidinium, ammonium, phosphonium, pyridinium, pyrrolidinium and
choline have been tested (44-47).
To the best of our knowledge, in vitro cytotoxicity studies
of ILs have been performed on 2D cell cultures The aim of our work
was to evaluate the effect of different concentrations of
tetrahexyl ammonium bromide on Caco-2 cells, seeded in 2D and 3D
systems.
3D culture cell systems grow naturally in an
environment allowing them to interact with each other, with MEC
(microbial electrolysis cell) and their microenvironment. In turn,
these interactions affect cell functioning, including cell
proliferation, cell differentiation, cell morphology, gene
expression, protein synthesis and cellular responses to external
stimuli. Monolayer (2D) cell cultures consist of living,
proliferating cells, as necrotic cells are usually detached from
the surface of the monolayer and can be easily removed during
change in cell media. Abnormal cell morphology in 2D culture also
influences many of the aforementioned cellular processes. Despite
these disadvantages, the 2D cell culture is still the most common
in vitro testing platform. Unlike 2D culture, 3D culture
cells form aggregates or spheroids on a matrix or while being
suspended in growth medium. In cell aggregates, the cell-cell and
cell-MEC interactions allow scientists to realistically replicate
in vivo conditions. In addition, 3D constructs are composed
of cells at various developmental stages, including proliferative
cells, senescent cells, apoptotic cells, hypoxic cell, and necrotic
cells. The outer layers of a spheroid, which is highly exposed to
the environment, include mainly viable, proliferating cells. The
heterogeneity of 3D cells is very similar to those seen in tissues
in vivo, especially in tumors. In the present study,
although the inhibition of proliferation in cells cultured in the
3D system was not significant compared to the control group, we
observed an alteration in the shape of cell aggregates at high
doses of the IL.
Several recent studies have reported that different
types of ILs have antitumor potential. Various cell lines were used
to assess the cytotoxic potential. For instance, Kumar and Malhotra
evaluated the cytotoxic effect of ammonium and phosphonium-based
ILs on 60 different cell lines. Their study showed that the length
of the alkyl chain is directly related to antitumor and cytotoxic
activity; the increase in the length of the alkyl chain being
accompanied by an increase in antitumor activity. On the other
hand, they reported that anions have a significant effect on
cytotoxicity, while cations appear to play a major role in
cytostatic activity. Ammonium-based ILs had a higher cytotoxic
potential than those based on phosphonium (48). At present, the data reported by most
studies indicate that the length of the carbon atom chain has a key
role in the anticancer effect of ILs. In 2015, Li et al
reported that imidazolium-based IL and bromide anion
(1-methyl-3-octylimidazolium bromide) exert a cytotoxic action on
HepG2 cells, inducing caspase-mediated apoptosis (49). As evidenced by the reported data,
the presence of the bromide anion does not appear to influence the
antitumor activity of ILs (49,50).
However, in some ILs, the anionic moiety influences the cytotoxic
action (44,51). The clinical future of these
compounds looks promising. Formulations for topical application
based on ILs have shown promising results in some in vivo
tests and have even reached clinical trials (52,53).
The antibacterial effect of the tetrahexylammonium
bromide IL displayed variations depending on the tested
concentrations and on the bacterial strain studied; in general the
effect was highlighted when testing the highest concentrations
(c1-c3). In the case of Gram+ bacteria, the sensitivity
to the tested IL decreased as follows: S. aureus > S.
pneumoniae > E. faecalis. In Gram-
bacterial strains, the antibacterial effect of the IL decreased as
follows: H. influenzae > P. mirabilis > P.
aeruginosa > K. pneumoniae > E. coli >
S. typhimurium. Regarding the cytotoxic potential on Caco-2
neoplastic colon cells, moderate results were noted. Future studies
should be conducted to assess the impact of THABr_IL on a
three-dimensional aggregation of tumor cells.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Authors may make available to the publisher the data
and materials presented in the manuscript.
Authors' contributions
Conceptualization of the research study was achieved
by RP, MNF and IP. Data curation was conducted by DCV, DV, KG and
GD. Formal analysis was conducted by RP, AM, GM, LPC and GD.
Funding acquisition was the responsibility of RP and DCV.
Investigation was carried out by IVC, CAD, KG, AM and GM. Research
methodology was the responsibility of MNF, IP, DV and IVC. Project
administration was the responsibility of RP, MNF and GD. Resources
were obtained by KG and LPC. Software was the responsibility of IP,
CAD and DCV. Supervision was undertaken by RP, MNF, IP, and GD.
Validation was carried out by DV, AM, CAD and GM. Visualization was
undertaken by IVC, IP and LPC. Writing of the original draft was
conducted by DCV, DV, KG and GM; writing, review and editing was
conducted by IP, AM, IVC and CAD. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study respected norms of professional ethics and
deontology.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Busetti A, Crawford DE, Earle MJ, Gilea
MA, Gilmore BF, Gorman SP, Laverty G, Lowry AF, McLaughlin M and
Seddon KR: Antimicrobial and antibiofilm activities of
1-alkylquinolinium bromide ionic liquids. Green Chem. 12:420–425.
2010.
|
|
2
|
Myles L, Gore R, Špulák M, Gathergood N
and Connon SJ: Highly recyclable, imidazolium derived ionic liquids
of low antimicrobial and antifungal toxicity: A new strategy for
acid catalysis. Green Chem. 12:1157–1162. 2010.
|
|
3
|
Endres F: Ionic liquids: Solvents for the
electrodeposition of metals and semiconductors. Chemphyschem.
3:144–154. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Moniruzzaman M, Nakashima K, Kamiya N and
Goto M: Recent advances of enzymatic reactions in ionic liquids.
Biochem Eng J. 48:295–314. 2010.
|
|
5
|
Ferlin N, Courty M, Gatard S, Spulak M,
Quilty B, Beadham I, Ghavre M, Haiss A, Kümmerer K, Gathergood N
and Bouquillon S: Biomass derived ionic liquids: Synthesis from
natural organic acids, characterization, toxicity, biodegradation
and use as solvents for catalytic hydrogenation processes.
Tetrahedron. 69:6150–6161. 2013.
|
|
6
|
Messali M: A green microwave-assisted
synthesis, characterization and comparative study of new
pyridazinium-based ionic liquids derivatives towards corrosion of
mild steel in acidic environment. J Mater Environ Sci. 2:174–185.
2011.
|
|
7
|
Messali M, Bousskri A, Anejjar A, Salghi R
and Hammouti B: Electrochemical studies of new pyridazinium-based
ionic liquid, (1-4-nitro phenyl-1-ethanone) pyridazinium bromide,
on carbon steel corrosion in hydrochloric acid medium. Int J
Electrochem Sci. 10:4532–4551. 2015.
|
|
8
|
Biswas A, Shogren RL, Stevenson DG,
Willett JL and Bhowmik PK: Ionic liquids as solvents for
biopolymers: Acylation of starch and zein protein. Carbohydr Polym.
66:546–550. 2006.
|
|
9
|
Talavera-Prieto NM, Ferreira AG, Simões
PN, Carvalho PJ, Mattedi S and Coutinho JA: Thermophysical
characterization of N-methyl-2-hydroxyethylammoniumcarboxilate
ionic liquids. J Chem Thermodyn. 68:221–234. 2014.
|
|
10
|
Petkovic M, Ferguson J, Bohn A, Trindade
J, Martins I, Carvalho MB, Leitão MC, Rodrigues C, Garcia H,
Ferreira R, et al: Exploring fungal activity in the presence of
ionic liquids. Green Chem. 11:889–894. 2009.
|
|
11
|
Iwai N, Nakayama K, Oku J and Kitazume T:
Synthesis and antibacterial activity of alaremycin derivatives for
the porphobilinogen synthase. Bioorg Med Chem Lett. 21:2812–2815.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Alberto EE, Rossato LL, Alves SH, Alves D
and Braga AL: Imidazolium ionic liquids containing selenium:
Synthesis and antimicrobial activity. Org Biomol Chem. 9:1001–1003.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Coleman D, Špulák M, Garcia MT and
Gathergood N: Antimicrobial toxicity studies of ionic liquids
leading to a ‘hit’ MRSA selective antibacterial imidazolium salt.
Green Chem. 14:1350–1356. 2012.
|
|
14
|
Tavares AP, Rodriguez O, Raquel Cristóvão
and Macedo EA: Applications and perspectives ionic liquids:
Alterna-tive reactive media for oxidative enzymes. In: Ionic
Liquids, Kokorin A (ed). Rijeka, InTech, pp449-516, 2011.
|
|
15
|
Rodríguez O, Cristovao RO, Tavares APM and
Macedo EA: Study of the alkyl chain length on laccase stability and
enzymatic kinetic with imidazolium ionic liquids. Biotechnol Appl
Bioc. 164:524–533. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cieniecka-Roslonkiewicz A, Pernak J,
Kubis-Feder J, Ramani A, Robertson AJ and Seddo KR: Synthesis,
anti-microbial activities and anti-electrostatic properties of
phosphonium-based ionic liquids. Green Chem. 7:855–862. 2005.
|
|
17
|
Carson L, Chau PK, Earle MJ, Gilea MA,
Gilmore BF, Gorman SP, McCann MT and Seddon KR: Antibiofilm
activities of 1-alkyl-3-methylimidazolium chloride ionic liquids.
Green Chem. 11:492–497. 2009.
|
|
18
|
Messali M: Eco-friendly synthesis of a new
class of pyridinium-based ionic liquids with attractive
antimicrobial activity. Molecules. 20:14936–14949. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Egorova KS, Gordeev EG and Ananikov VP:
Biological activity of ionic liquids and their application in
pharmaceutics and medicine. Chem Rev. 117:7132–7189.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kapałczyńska M, Kolenda T, Przybyła W,
Zajączkowska M, Teresiak A, Filas V, Ibbs M, Bliźniak R, Łuczewski
L and Lamperska K: 2D and 3D cell cultures-a comparison of
different types of cancer cell cultures. Arch Med Sci. 14:910–919.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
CLSI. Performance standards for
antimicrobial susceptibility testing. 27th edition. CLSI supplement
M100. Wayne, PA: Clinical and Laboratory Standards Institute,
2017.
|
|
22
|
Kurnia KA, Sintra TE, Neves CM, Shimizu K,
Canongia Lopes JN, Goncalves F, Ventura SP, Freire MG, Santos LM
and Coutinho JA: The effect of the cation alkyl chain branching on
mutual solubilities with water and toxicities. Phys Chem Chem Phys.
16:19952–19963. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ohno H, Fujita K and Kohno Y: Is seven the
minimum number of water molecules per ion pair for assured
biological activity in ionic liquid-water mixtures? Phys Chem Chem
Phys. 17:14454–14460. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang Y, Li H and Han SA: A theoretical
investigation of the interactions between water molecules and ionic
liquids. J Phys Chem B. 110:24646–24651. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zheng Z, Xu Q, Guo J, Qin J, Mao H, Wang B
and Yan F: Structure-antibacterial activity relationships of
imidazolium-type ionic liquid monomers, poly(ionic liquids) and
poly(ionic liquid) membranes: Effect of alkyl chain length and
cations. ACS Appl Mater Interfaces. 8:12684–12692. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Khudyakov JI, D'Haeseleer P, Borglin SE,
Deangelis KM, Woo H, Lindquist EA, Hazen TC, Simmons BA and Thelen
MP: Global transcriptome response to ionic liquid by a tropical
rain forest soil bacterium, Enterobacter lignolyticus. Proc Natl
Acad Sci USA. 109:E2173–E2182. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Borkowski A, Kowalczyk P, Czerwonka G,
Cieśla J, Cłapa T, Misiewicz A, Szala M and Drabik M: Interaction
of quaternary ammonium ionic liquids with bacterial
membranes-studies with Escherichia coli r1-r4-type
lipopolysaccharides. J Mol Liquids. 246:282–289. 2017.
|
|
28
|
Kowalczyk P, Borkowski A, Czerwonka G,
Cłapa T, Cieśla J, Misiewicz A, Borowiec M and Szala M: The
microbial toxicity of quaternary ammonium ionic liquids is
dependent on the type of lipopolysaccharide. J Mol Liquids.
266:540–547. 2018.
|
|
29
|
Dauborca VC, Dumitrascu V, Popescu R,
Cimporescu A, Vlad CS, Flangea C, Grecu DS, Vágvölgyi C, Papp T and
Horhat FG: Gas chromatography-mass spectrometry evidences for new
chemical insights of momordica charantia. Rev Chim. 66:1914–1920.
2015.
|
|
30
|
Moatar AE, Vlad CS, Vlad DC, Verdes DM,
Filimon MN, Bloju O, Borcan F, Dehelean CA and Dumitrascu V:
Evaluation of antiproliferative potential of Myrmecodia pendans and
its activity on il-8 secretion in colon cancer cell. Farmacia.
68:710–714. 2020.
|
|
31
|
O'Toole GA, Wathier M, Zegans ME, Shanks
RM, Kowalski R and Grinstaff MW: Diphosphonium ionic liquids as
broad-spectrum antimicrobial agents. Cornea. 31:810–816.
2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hou XD, Liu QP, Smith TJ, Li N and Zong
MH: Evaluation of toxicity and biodegradability of cholinium amino
acids ionic liquids. PLoS One. 8(e59145)2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Docherty KM, Joyce MV, Kulacki KJ and
Kulpa CF: Microbial biodegradation and metabolite toxicity of three
pyridinium-based cation ionic liquids. Green Chem. 12:701–712.
2010.
|
|
34
|
Hough-Troutman WL, Smiglak M, Griffin S,
Matthew Reichert W, Mirska I, Jodynis-Liebert J, Adamska T, Nawrot
J, Stasiewicz M, Rogers RD and Pernak J: Ionic liquids with dual
biological function: Sweet and anti-microbial, hydrophobic
quaternary ammonium-based salts. New J Chem. 33:26–33. 2009.
|
|
35
|
Stoimenovski J, MacFarlane DR, Bica K and
Rogers RD: Crystalline vs. ionic liquid salt forms of active
pharmaceutical ingredients: A position paper. Pharm Res.
27:521–526. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tran CD, Prosenc F and Franko M: Facile
synthesis, structure, biocompatibility and antimicrobial property
of gold nanoparticle composites from cellulose and keratin. J
Colloid Interface Sci. 510:237–245. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gilmore BF: Antimicrobial ionic liquids,
In: Ionic Liquids: Applications and Perspectives, Kokorin A (ed).
InTech, Rijeka, pp587-604, 2011.
|
|
38
|
Yu Y and Nie Y: Toxicity and antimicrobial
activities of ionic liquids with halogen anion. J Environ Prot.
2:298–303. 2011.
|
|
39
|
Ventura SPM, Gurbisz M, Ghavre M, Ferreira
FMM, Gonçalves F, Beadham I, Quilty B, Coutinho JAP and Gathergood
N: Imidazolium and pyridinium ionic liquids from mandelic acid
derivatives: Synthesis and bacteria and algae toxicity evaluation.
ACS Sustain Chem Eng. 1:393–402. 2013.
|
|
40
|
Payagala T and Armstrong DW: Chiral ionic
liquids: A compendium of syntheses and applications (2005-2012).
Chirality. 24:17–53. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Haragus H, Vermesan D, Lazureanu V,
Ferdean N, Radu D, Prejbeanu R and Niculescu M: Antibiotic loaded
cement spacers for two stage treatment of periprosthetic joint
infections. Rev Chim (Bucharest). 67:764–767. 2016.
|
|
42
|
Messali M, Aouad MR, El-Sayed WS, Ali AA,
Ben Hadda T and Hammouti B: New eco-friendly
1-alkyl-3-(4-phenoxybutyl) imidazolium-based ionic liquids
derivatives: A green ultrasound-assisted synthesis,
characterization, antimicrobial activity and POM analyses.
Molecules. 19:11741–11759. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Miskiewicz A, Ceranowicz P, Szymczak M,
Bartuś K and Kowalczyk P: The use of liquids ionic fluids as
pharmaceutically active substances helpful in combating nosocomial
infections induced by Klebsiella pneumoniae New Delhi
strain, Acinetobacter baumannii and Enterococcus
species. Int J Mol Sci. 19(2779)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Egorova KS, Seitkalieva MM, Posvyatenko AV
and Ananikov VP: An unexpected increase of toxicity of amino
acid-containing ionic liquids. Toxicol Res. 4:152–159. 2015.
|
|
45
|
Frade RFM, Matias A, Branco LC, Afonsoc
CAM and Duarte CMM: Effect of ionic liquids on human colon
carcinoma HT-29 and CaCo-2 cell lines. Green Chem. 9:873–877.
2007.
|
|
46
|
Frade RFM, Rosatella AA, Marques CS,
Branco LC, Kulkarni PS, Mateus NMM, Afonso CAM and Duarte CMM:
Toxicological evaluation on human colon carcinoma cell line
(CaCo-2) of ionic liquids based on imidazolium, guanidinium,
ammonium, phosphonium, pyridinium and pyrrolidinium cations. Green
Chem. 11:1660–1665. 2009.
|
|
47
|
García-Lorenzo A, Tojo E, Tojo J, Teijeira
M, Rodríguez-Berrocal FJ, Pérez González M and Martínez-Zorzano VS:
Cytotoxicity of selected imidazolium-derived ionic liquids in the
human Caco-2 cell line. Sub-structural toxicological interpretation
through a QSAR study. Green Chem. 10:508–516. 2008.
|
|
48
|
Kumar V and Malhotra SV: Study on the
potential anti-cancer activity of phosphonium and ammonium-based
ionic liquids. Bioorg Med Chem Lett. 19:4643–4646. 2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Li X, Ma J and Wang J: Cytotoxicity,
oxidative stress, and apoptosis in HepG2 cells induced by ionic
liquid 1-methyl-3-octylimidazolium bromide. Ecotoxicol Environ Saf.
120:342–348. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ranke J, Mölter K, Stock F, Bottin-Weber
U, Poczobutt J, Hoffmann J, Ondruschka B, Filser J and Jastorff B:
Biological effects of imidazolium ionic liquids with varying chain
lengths in acute vibrio fischeri and WST-1 cell viability assays.
Ecotoxicol Environ Saf. 58:396–404. 2004.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Flieger J and Flieger M: Ionic liquids
toxicity-benefits and threats. Int J Mol Sci.
21(6267)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zakrewsky M, Lovejoy KS, Kern TL, Miller
TE, Le V, Nagy A, Goumas AM, Iyer RS, Del Sesto RE, Koppisch AT, et
al: Ionic liquids as a class of materials for transdermal delivery
and pathogen neutralization. Proc Natl Acad Sci USA.
111:13313–13318. 2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Miwa Y, Hamamoto H, Hikake S and Kuwabara
Y: A phase I, randomized, open-label, cross-over study of the
pharmacokinetics, dermal tolerability, and safety of MRX-7EAT
etodolac-lidocaine topical patch in healthy volunteers. J Pain. 14
(Suppl 4)(S72)2013.
|