Introduction
The introduction of femtosecond laser-assisted small
incision lenticule extraction (SMILE) surgery has been a great
advance in corneal refractive surgery in recent years (1-3).
In order to correct the refractive error, SMILE makes use of a
femtosecond laser to create a lenticule in the corneal stroma under
the corneal cap, and then the lenticule is removed by a small
peripheral incision (4-6).
As a type of ultra-short pulse laser based on chirped pulse
amplification technology, the femtosecond laser is considered to
have high instantaneous power and minimal thermal effects, which
makes it safer and more accurate compared with the excimer laser
and the micromechanical knife (7).
However, studies that have been performed to
investigate the surface quality of the corneal stroma following
femtosecond laser ablation during SMILE surgery are currently
limited. It has been hypothesized that the surface quality of the
corneal stroma after laser ablation, in terms of smoothness and
regularity, may directly affect visual quality following corneal
refractive surgery (8,9). In general, good surface quality after
surgery may alleviate aberration and help achieve a reasonable
visual quality. Due to the limited sources of human corneal tissue,
the majority of previous studies investigating the surface quality
after corneal refractive surgery focused on animal eyes (10). As an alternative to this, the
corneal stromal lenticule extracted during SMILE surgery offers the
possibility to study surface quality directly after femtosecond
laser ablation.
Therefore, the present study aimed to preliminarily
investigate the surface quality of the corneal stromal lenticule by
using optical instruments, to explore corresponding influencing
factors and to provide a morphological basis for the future
improvement of femtosecond laser-assisted corneal refractive
surgery.
Materials and methods
Subjects and surgical procedure
A total of 20 patients with myopia who underwent
SMILE surgery were enrolled in the present study, including 9 males
and 11 females aged between 18 and 30 years. The present study was
approved by the Ethics Committee of Xiangya Hospital (Changsha,
China). The surgery was performed in October 2018 at the Laser
Treatment Center of the Ophthalmology Department of Xiangya
Hospital (Changsha, China). The spherical diopter (D) was between
-6.00 and -8.75 D (-7.01±0.71 D) and the columnar D was between
-0.25 and -2.50 D (-1.33±0.53 D). Prior to surgery, written
informed consent was obtained from the patients with myopia in
accordance with the institutional guidelines following full
comprehension of the benefits and risks of the surgery as well as
the use of their cornea tissues for experimental purpose.
The surgery was performed by an experienced surgeon
using the VisuMax femtosecond laser system (Zeiss AG). The surgery
methods were as follows: i) The patients' refractive degrees and
ablating thickness were checked, the surgery area was
conventionally disinfected and the eyelid opener was placed
following surface anesthesia with Alcaine; ii) accurate suction of
the cornea was performed with a negative pressure suction device,
the corneal stromal lenticule was created and an incision was made
using a femtosecond laser; and iii) the lenticule was separated and
extracted using the operating microscope. The surgery parameters
were as follows: Cutting diameter, 6.0-6.8 mm; thickness of the
corneal cap, 120 µm; point spacing, 4.5 µm; row spacing, 4.5 µm;
laser energy, 130 nJ. Following surgery, bitobramycin and
dexamethasone eye drops were applied.
After extraction, the front surfaces of the corneal
stromal lenticules were stained with gentian violet solution.
Subsequently, the lenticules were flattened and kept in glass vials
with 4% polyformaldehyde phosphate buffer solution for light
microscopy or 2.5% glutaraldehyde for electron microscopy.
Afterwards, the lenticules were immediately sent to the laboratory
for further microstructural observation.
Histological section and microscopy
examination
A total of six corneal stromal lenticules were fixed
in 4% polyformaldehyde phosphate buffer solution for 24 h at 4˚C.
Conventional dehydration, paraffin-embedding and slicing were
performed, with a slice thickness of 4.5 µm. After H&E
staining, dehydration, transparentization with xylene and sealing,
the slices were observed under a light microscope.
Electron microscopic observation and
surface evaluation
For electron microscopic examination, 14 corneal
stromal lenticules were fixed in 2.5% glutaraldehyde at room
temperature for 24 h and in 2% osmium acid for 2 h with washing
steps in between. The specimens were dehydrated in a gradient
series of aqueous ethanol solution (50, 70, 90 and 100%) and then
in pure acetone. For the scanning electron microscopy (SEM)
examination, the specimens were then placed in amyl acetate. After
dehydration, the lenticule specimens were dried using a critical
point dryer (model, HCP-2; Hitachi, Ltd.) with liquid
CO2. Subsequently, the specimens were mounted on
aluminum stubs and sputtered with gold. Finally, the Hitachi HT7700
transmission electron microscope and the Hitachi S-3400nN SEM
(Hitachi, Ltd.) were used to observe the slices.
To compare the quality of the front and rear
surfaces of the lenticules, the electron microscopy images of the
samples with a scale bar of 500 µm were rated according to unified
criteria (Table I) (11). The surface quality of the lenticules
was graded as follows: 1, very smooth; 2, smooth; 3, rough; 4, very
rough. All electron microscopy images were anonymized and judged in
a double-blinded manner by three technicians independently who were
trained in electron microscopy image analysis. The average of the
score determined by the three technicians was considered to be the
final rating of each electron microscopy image.
 | Table IEvaluation criteria of the surface
quality of corneal stromal lenticules observed under a scanning
electron microscope. |
Table I
Evaluation criteria of the surface
quality of corneal stromal lenticules observed under a scanning
electron microscope.
| Smoothness score | Microscopic
observation |
|---|
| 1 | Very rough: No
obvious area without burrs, curls or tissue bridges |
| 2 | Rough: More than half
of the surface has burrs, curls or tissue bridges |
| 3 | Smooth: Over half the
surface has no obvious burrs, curls or tissue bridges |
| 4 | Very smooth: No
obvious burrs or curls |
Statistical analysis
Measurement data were expressed as the mean ±
standard deviation. The SPSS v23.0 statistical software package
(IBM Corp.) and a paired t-test were used for the statistical
analysis of the front and rear surface quality scores. P<0.05
was considered to indicate a statistically significant
difference.
Results
Histological appearance of the corneal
stromal lenticules under the light microscope
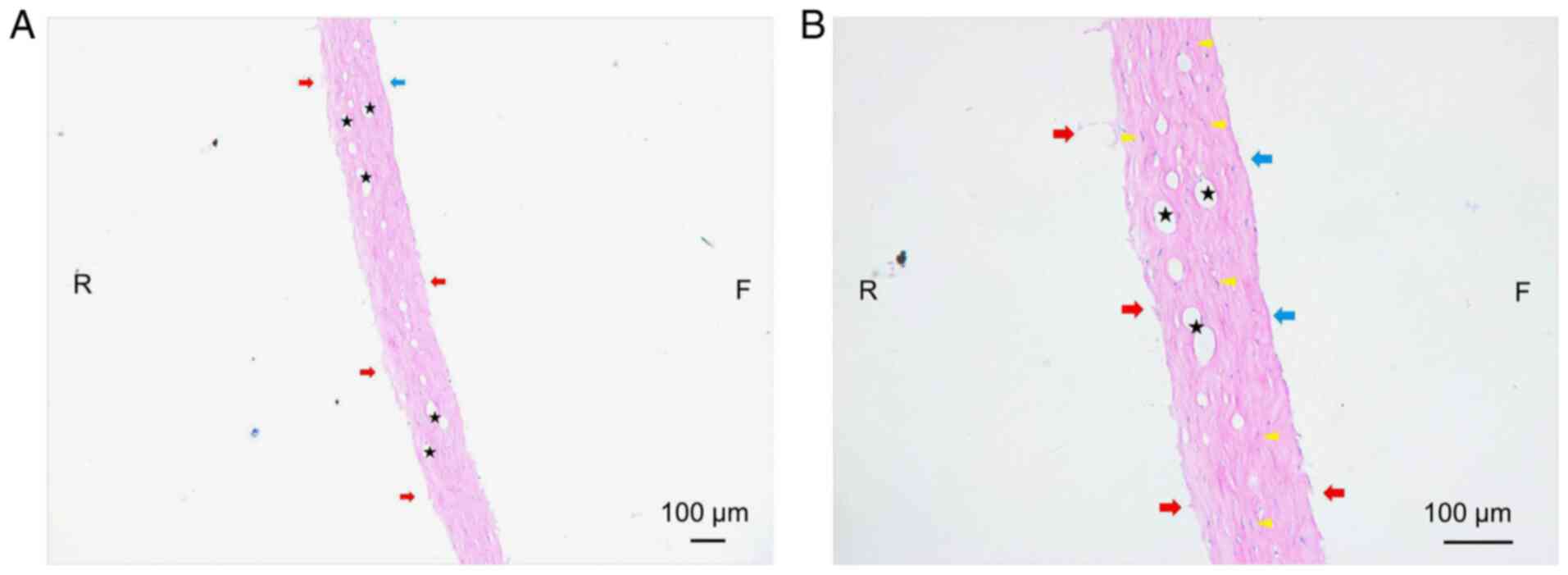
Using a light microscope, the cross section of the
lenticule was observed as illustrated in Fig. 1. The following was observed: i) The
thickness of the lenticules was inconsistent, which may have been
caused by the swelling of collagen fibers to varying degrees; ii)
the edge of the lenticule, particularly the rear surface, was
irregular with burrs and broken fibers; iii) the edge of the
lenticule, particularly the anterior surface, was deeply stained,
which indicated that the laser energy had damaged the corneal
stroma; iv) the collagen fibers were distributed irregularly and
stained unevenly, and certain nucleated corneal stromal cells were
sprinkled throughout the collagen bundles; and v) bubbles of
different sizes were visible throughout the collagen bundles
without distribution differences and certain bubbles were connected
with others.
Observation of the corneal stromal
lenticules under the electron microscope
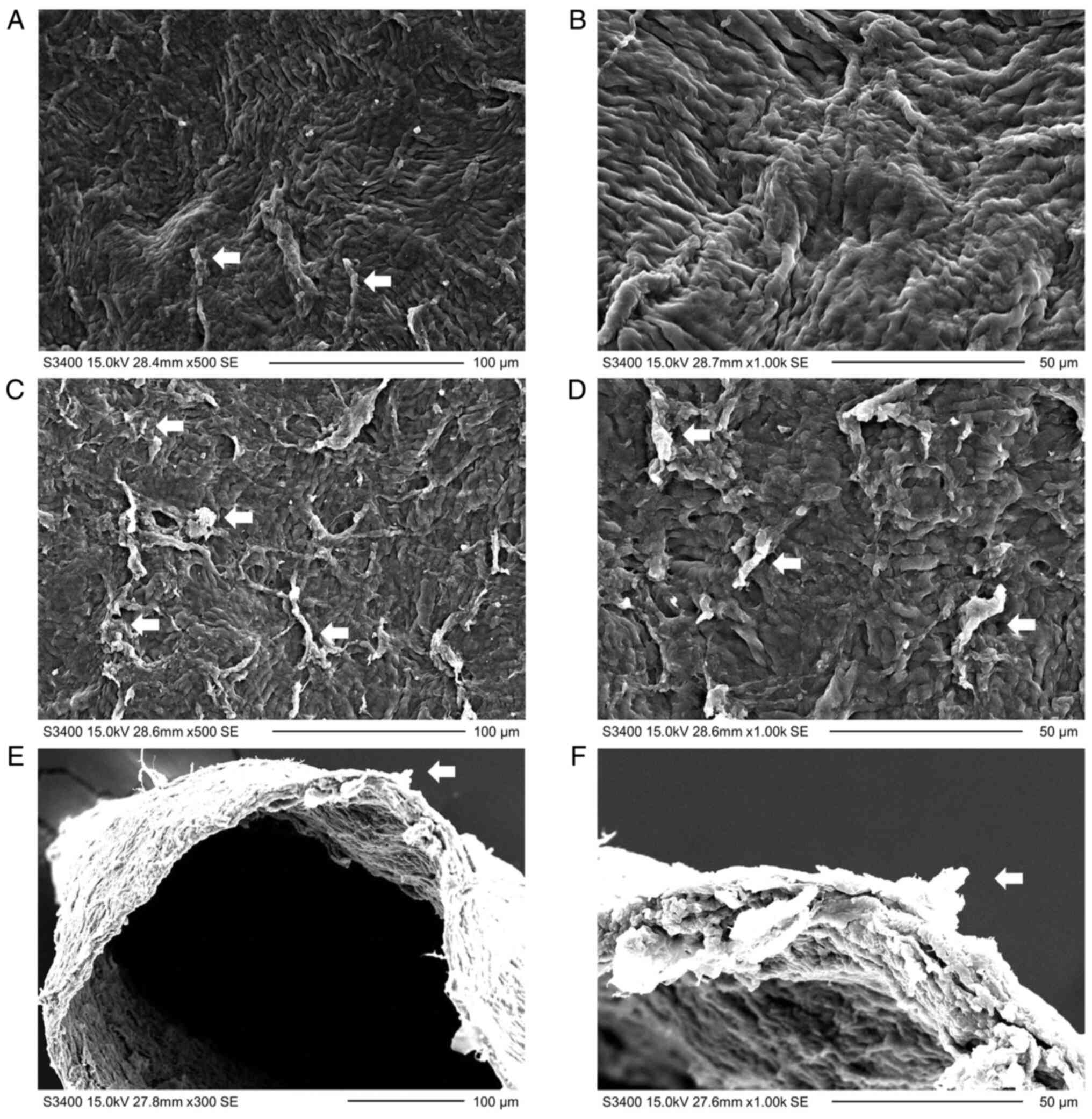
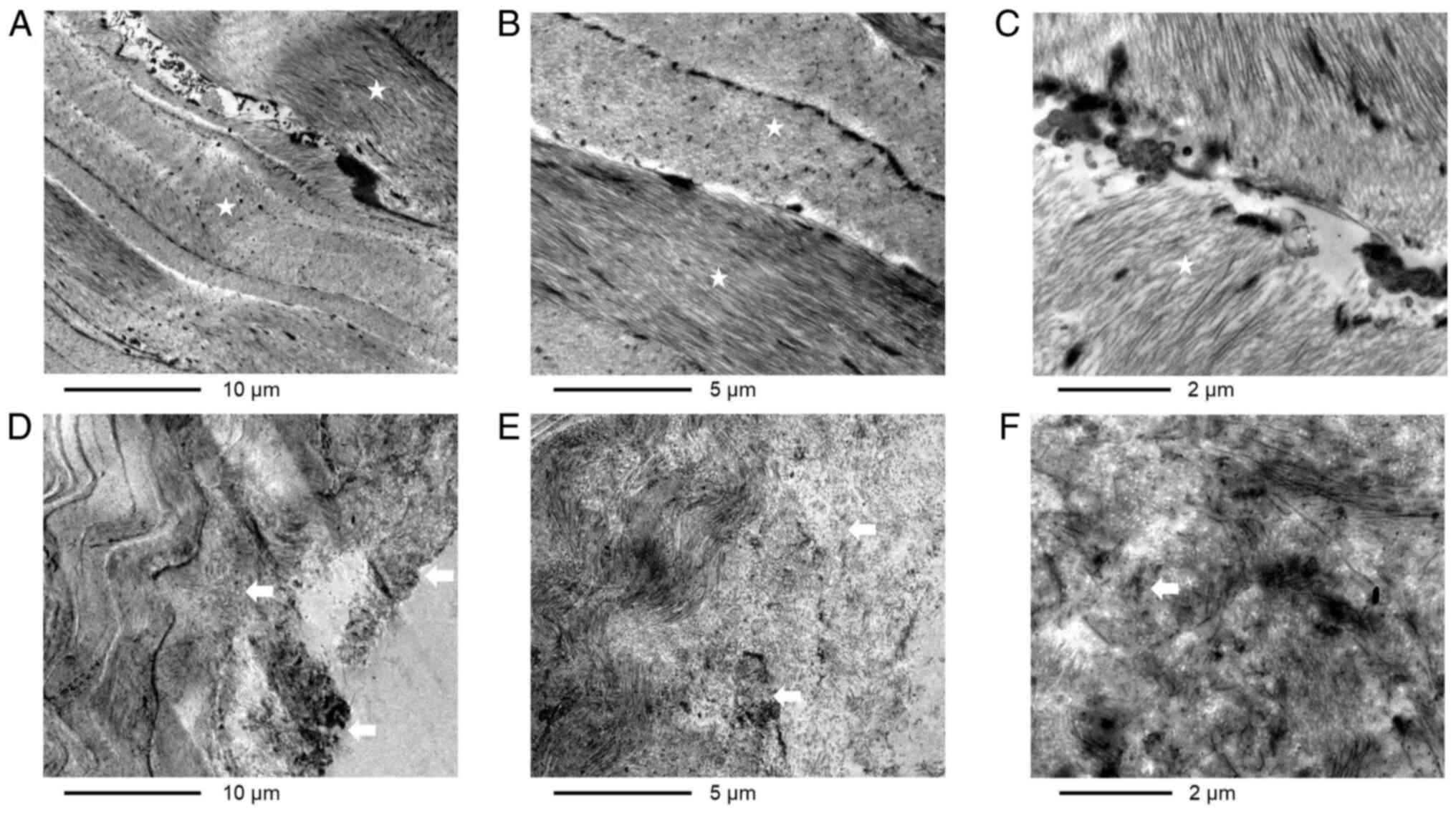
The microstructure, as observed under an electron
microscope, is presented in Figs. 2
and 3. The following observations
were made: i) Linear uplifts, short wrinkles and curls were present
on the front and rear surfaces (Fig.
2A-D); ii) the front surface was relatively smooth (Fig. 2A and B), whereas the rear surface exhibited a
certain number of tissue bridges and fractured collagen fibers
(Fig. 2C and D); iii) the side edge of the lenticule was
rough with short wrinkles, curls and fractured collagen fibers
(Fig. 2E and F); iv) in the middle of the lenticule, the
corneal stromal fibers were normally distributed in parallel to
each other like waves and the collagen bundles were mutually
arranged crosswise (Fig. 3A-C); and
v) the collagen fibers near the surface were disordered and
exhibited short protuberances, lysis and necrosis, and transited to
be normal towards the center (Fig.
3D-F).
Quantitative evaluation of the surface
quality of the corneal stromal lenticules under the electron
microscope
The quality of the front and rear surface of the
corneal stromal lenticules was rated based on unified evaluation
standards (Table I). As presented
in Table II, the smoothness scores
of the front surface were markedly higher than those of the rear
surface (P<0.001).
 | Table IIQuantitative evaluation of the surface
quality of corneal stromal lenticules. |
Table II
Quantitative evaluation of the surface
quality of corneal stromal lenticules.
| Score | Front surface | Rear surface | P-value |
|---|
| Smoothness | 3.50±0.33 | 2.52±0.24 |
<0.001a |
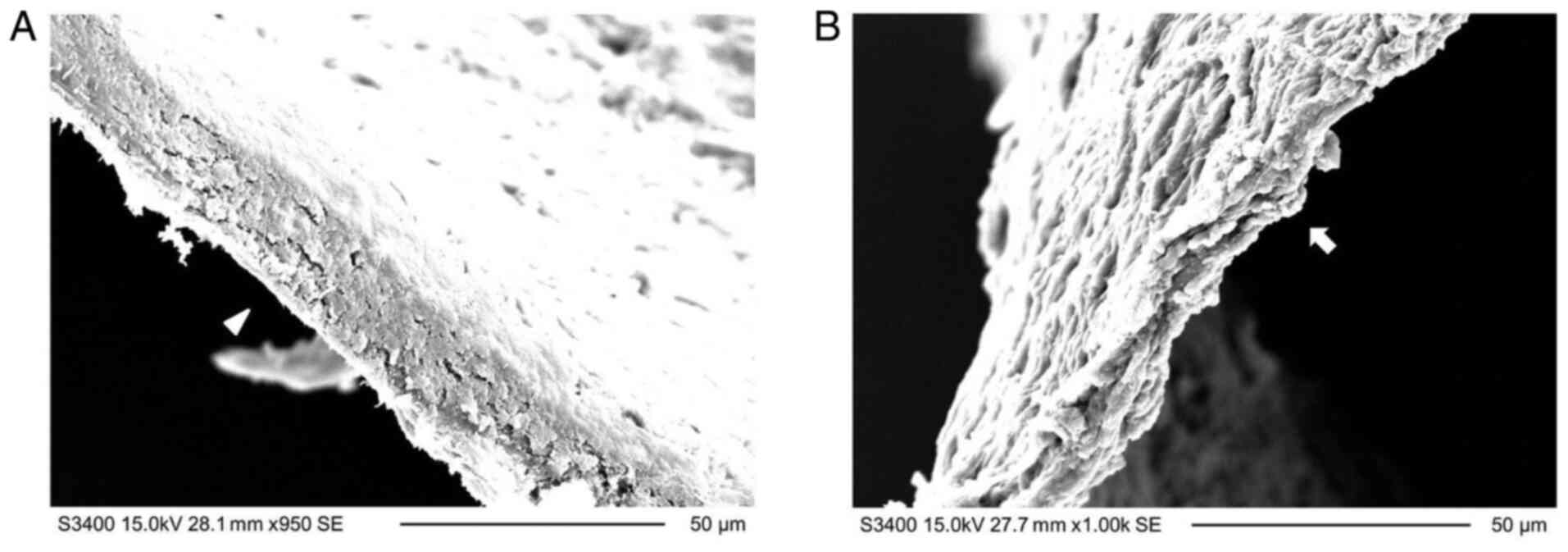
Comparison of the side edge cut using
a femtosecond laser or microscissors
The corneal stromal lenticule was cut by sharp
microscissors and then the transection was compared with the side
edge of the lenticule. As presented in Fig. 4, the transection cut by the
microscissors (Fig. 4B) was
smoother than the side edge of the lenticule ablated using a laser
(Fig. 4A).
Discussion
Based on technological improvements, femtosecond
lasers have been widely applied in SMILE surgery and have been
tentatively used for other corneal surgeries, such as keratoplasty
(12,13). In addition, the corneal stromal
lenticules extracted during SMILE surgery have been considered in
the surgical treatment of hyperopia, corneal ulcers and even
corneal perforation, all of which have produced promising clinical
results (14-16).
It has been reported that the surface quality of the corneal tissue
influences wound healing and post-operative visual quality
(8,17,18).
However, so far, morphological studies regarding the surface
quality of human corneal tissue after femtosecond laser scanning
are limited. Therefore, it is useful to evaluate the effect of
femtosecond lasers on the corneal stromal lenticules by light and
electron microscopy, which may help to form a foundation for the
future improvement of medical laser technology and corneal surgery
using medical lasers.
In the present study, the surface of corneal stromal
lenticules was observed to exhibit burrs, broken fibers and tissue
bridges under both light and electron microscopes, and was not
perfectly smooth at the micro-scale, which was consistent with the
results of previous studies (8,19). The
underlying mechanisms may be attributed to the effects of
femtosecond laser on the corneal stroma. During SMILE surgery, the
lenticule surface is the ablating focus of the femtosecond laser
and the photo-decomposing function of the femtosecond laser makes
it possible to break the chemical bonds between molecules and to
produce a number of bubbles. Once the bubbles expand and merge to
form a separate plane in the corneal tissue, the corneal stroma may
be separated (20,21). However, due to the existence of
discontinuous cavitation bubbles, certain tissue bridges remain and
the collagen fibers tend to curl and wrinkle, which results in a
not-so-smooth surface of the corneal stromal bed. Certain bubbles
may be partially absorbed by the surrounding corneal tissue or
spread into the air (22,23). The remaining bubbles tend to be
fixed in the tissue when preparing the lenticule samples, as was
observed in the cross-sections of the lenticules under the light
microscope. The observed trends in bubble distribution revealed in
the present study may not necessarily be the same as those reported
in a previous study (24), in which
the bubbles were observed to be preferably located in the anterior
layer of the corneal stroma. The latter may be ascribed to the
dense collagen fibers in the anterior stroma that markedly limit
the movement of bubbles (25).
However, in the present study, the lenticule was thin. Thus, the
difference in collagen fiber density between the anterior and
posterior layers of the lenticules was not significant. Therefore,
the preferential bubble distribution could hardly be observed.
In addition to the influence of existing bubbles,
other factors, including the laser parameters (26-28),
surgical technique (29,30), cap thickness (31-33)
and mechanical separation (34),
may also affect the surface smoothness. For instance, Kunert et
al (26) reported that the
surface regularity of corneal lenticules tends to decrease with the
increase in laser energy. Serrao et al (27) demonstrated that the stromal
interface quality may be improved by decreasing the pulse energy
and spot distance. In addition, it has been noticed that a smooth
appearance of the lenticular surface exists when a thin cap or a
shallow ablating depth is created (31-33).
When a laser beam passes through a thick corneal tissue, the focus
of the beam is distorted and this weakens the laser efficacy;
therefore, additional tissue bridges are created and these make the
ablating surface more irregular. Furthermore, it has been
speculated that the mechanical separation of the corneal lenticules
during SMILE surgery after laser ablation may cause damage to the
corneal tissue (34).
The present study further indicated that the front
surface of the lenticule was more regular than the rear surface by
subjective evaluation according to the unified criteria. This
rating method was rapid and economical for evaluating the surface
quality and had been widely applied by certain other similar
studies (11,26). As to the different regularity
between the front and rear surfaces, the primary reason may be that
the collagen fibers in the anterior corneal stroma are denser than
those in the posterior corneal stroma (25). Therefore, the posterior corneal
stroma has less of a restrictive effect on the geometrical shape of
bubbles produced by light blasting. Accordingly, the bubbles in the
posterior stroma are wider in the vertical direction than those in
the anterior stroma; therefore, the bubbles in the posterior stroma
are closer to a wide ellipse rather than a long ellipse or even a
long shuttle-like shape. Therefore, given the condition of a fixed
spot distance, the ability of the bubbles in the posterior stroma
to diffuse and fuse with each other along the horizontal direction
and form a separation plane was limited, which finally resulted in
the formation of tissue bridges, curled fibers and an irregular
rear surface of the lenticule. Furthermore, when numerous tissue
bridges remain in the posterior stroma after laser scanning, the
mechanical separation of the corneal lenticule encounters great
resistance, which may cause additional damage to the rear surface
of the corneal lenticule.
In addition, the present study revealed that the
side edge of the stromal lenticule ablated by a femtosecond laser
was not as regular as the transection cut by microscissors, which
was similar to the results of a previous study, which compared the
cutting surface generated using a femtosecond laser with that
generated using a microlamellar knife (7). Due to the damage induced by laser
energy, the stromal fibers broke, crinkled and shrunk, making the
cutting surface irregular at the micro-level. According to a
previous study, no differences in surface roughness were observed
between the mechanically resected tissues and those ablated using
0.50 µJ pulse laser energy (35).
However, when the laser pulse energy was >0.50 µJ, the surface
smoothness was no longer predictable and controllable. In addition,
the surgical operation of artificially removing the corneal
lenticule through a small incision may introduce additional damage
to the side edge of the lenticule.
Concerning the wavy change of the stromal fibers and
deep-stained margin of the lenticule, as observed under the
electron microscope, the biological reactions and thermal injury
caused by laser energy may be the primary reasons (36). The high-density free electrons
produced by the laser may shrink and expand in the focusing area,
which leads to the formation of shock waves that generate the wavy
change of superficial fibers (37).
Furthermore, the electron motions led to an increase in the local
temperature and caused thermal damage. Due to thermal injury, the
edge was edematous and deep staining was observed under a
microscope. The high energy of the laser may also destroy the
molecular skeleton and produce bio-molecular fragments, such as
free radicals, which tend to destroy or even kill the cells around
the laser focusing area (37).
Therefore, disordered and necrotic collagen fibers were observed at
the edge of the lenticules. Since the thermal radiation range of
the femtosecond laser was <1 µm, there was no obvious tissue
reaction in the deep corneal stroma.
Similar to a previous study (31), the cap thickness was set as 120 µm
and microscopes were used to observe the lenticule surface quality
in the present study. To determine the histological and
morphological characteristics of the lenticules, H&E staining
was applied to help observe the changes of collagen fibers and
scoring criteria for evaluating the smoothness from the SEM images
were applied. Furthermore, the side edge of the lenticule ablated
by a femtosecond laser was compared with the side edge cut using
the microscissor and certain differences in results were observed.
However, there were certain limitations to the present study that
should be improved in future studies. First, due to the shrinkage
and swelling of the cornea specimens, it was hard to actually
effectively evaluate and compare the surface smoothness between
different corneal lenticules. It was also difficult to distinguish
the causes of lenticule edema between the laser energy and the
saving condition of the extracted lenticule, as well as the
occurrence of edema prior to or after the femtosecond laser
ablation. Furthermore, in the present study, the smoothness of the
corneal surfaces was not compared between different laser energies,
spot distances, lenticule thicknesses and corneal cap thicknesses.
Finally, in the present study, the software applied by Weng et
al (31) was not used to
evaluate the surface quality of the lenticules, which may have led
to the evaluation of the results being affected by the technicians.
Additional studies with larger sample sizes are required to obtain
a sophisticated comprehension of the most important factors that
influence the smoothness of the ablated surface.
In conclusion, it was demonstrated that the surface
quality of corneal stromal lenticules ablated using a femtosecond
laser was imperfect under a microscope. Additional attention should
be focused on achieving an improved laser scanning protocol that
may help improve the histology and morphology of the corneal
surface following laser ablation. This would be promising, since
numerous corneal materials may be used for the treatment of corneal
diseases once the corneal stromal lenticules have a perfect surface
quality.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Natural Science
Foundation of Hunan Province, China (grant no. 2015JJ4093) and the
National Natural Science Foundation of China (grant no.
81900890).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DW, YY and XW (author position 7) designed the study
and revised the manuscript. DW also performed the surgery. YY
finished the drafting of the manuscript. TH, AX, YF and YZ analyzed
and interpreted the data. The histological and microscopic
examinations were performed by XW (author position 6). All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
Xiangya Hospital (Changsha, China). Prior to surgery, informed
consent was obtained from the myopic patients in accordance with
the institutional guidelines after being given a full understanding
of the benefits and risks of the surgery as well as the use of
their cornea tissues for experimental purpose.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aristeidou A, Taniguchi EV, Tsatsos M,
Muller R, McAlinden C, Pineda R and Paschalis EI: The evolution of
corneal and refractive surgery with the femtosecond laser. Eye Vis
(Lond). 2(12)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Reinstein DZ, Archer TJ and Gobbe M: Small
incision lenticule extraction (SMILE) history, fundamentals of a
new refractive surgery technique and clinical outcomes. Eye Vis
(Lond). 1(3)2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yan H, Gong LY, Huang W and Peng YL:
Clinical outcomes of small incision lenticule extraction versus
femtosecond laser-assisted LASIK for myopia: A Meta-analysis. Int J
Ophthalmol. 10:1436–1445. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang D, Liu M, Chen Y, Zhang X, Xu Y, Wang
J, To CH and Liu Q: Differences in the corneal biomechanical
changes after SMILE and LASIK. J Refract Surg. 30:702–707.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shah R, Shah S and Sengupta S: Results of
small incision lenticule extraction: All-in-one femtosecond laser
refractive surgery. J Cataract Refract Surg. 37:127–137.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen Y, Yin YW, Zhao Y, Wu XY, Young K,
Song WT, Xia XB and Wen D: Differentiation of human embryonic stem
cells derived mesenchymal stem cells into corneal epithelial cells
after being seeded on decellularized SMILE-derived lenticules. Int
J Ophthalmol. 12:717–724. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Soong HK and Malta JB: Femtosecond lasers
in ophthalmology. Am J Ophthalmol. 147:189–197.e2. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sumioka T, Miyamoto T, Takatsuki R, Okada
Y, Yamanaka O and Saika S: Histological analysis of a cornea
following experimental femtosecond laser ablation. Cornea. 33
(Suppl 11):S19–S24. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Miao H, He L, Shen Y, Li M, Yu Y and Zhou
X: Optical quality and intraocular scattering after femtosecond
laser small incision lenticule extraction. J Refract Surg.
30:296–302. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Heichel J, Blum M, Duncker GI, Sietmann R
and Kunert KS: Surface quality of porcine corneal lenticules after
Femtosecond Lenticule Extraction. Ophthalmic Res. 46:107–112.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cheng YY, Kang SJ, Grossniklaus HE, Pels
E, Duimel HJ, Frederik PM, Hendrikse F and Nuijts RM: Histologic
evaluation of human posterior lamellar discs for femtosecond laser
Descemet's stripping endothelial keratoplasty. Cornea. 28:73–79.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yoo SH and Hurmeric V: Femtosecond
laser-assisted keratoplasty. Am J Ophthalmol. 151:189–191.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chamberlain WD, Rush SW, Mathers WD,
Cabezas M and Fraunfelder FW: Comparison of femtosecond
laser-assisted keratoplasty versus conventional penetrating
keratoplasty. Ophthalmology. 118:486–491. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhao J, Shang J, Niu L, Xu H, Yang D, Zhao
Y, Fu D and Zhou X: Two-year outcome of an eye that underwent
hyperopic LASIK following inadvertent myopic SMILE lenticule in
situ implantation. BMC Ophthalmol. 19(176)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jin H, He M, Liu H and Zhong X, Wu J, Liu
L, Ding H, Zhang C and Zhong X: Small-incision femtosecond
laser-assisted intracorneal concave lenticule implantation in
patients with keratoconus. Cornea. 38:446–453. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Abd Elaziz MS, Zaky AG and El SaebaySarhan
AR: Stromal lenticule transplantation for management of corneal
perforations; one year results. Graefes Arch Clin Exp Ophthalmol.
255:1179–1184. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Piñero-Llorens DP, Murueta-Goyena
Larrañaga A and Hanneken L: Visual outcomes and complications of
small-incision lenticule extraction: A review. Exp Rev Ophthalmol.
11:59–75. 2016.
|
|
18
|
Ang M, Chaurasia SS, Angunawela RI, Poh R,
Riau A, Tan D and Mehta JS: Femtosecond lenticule extraction
(FLEx): Clinical results, interface evaluation, and intraocular
pressure variation. Invest Ophthalmol Vis Sci. 53:1414–1421.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhao J, Miao H, Han T, Shen Y, Zhao Y, Sun
L and Zhou X: A Pilot Study of SMILE for Hyperopia: Corneal
Morphology and Surface Characteristics of Concave Lenticules in
Human Donor Eyes. J Refract Surg. 32:713–716. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sugar A: Ultrafast (femtosecond) laser
refractive surgery. Curr Opin Ophthalmol. 13:246–249.
2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Vossmerbaeumer U and Jonas JB: Structure
of intracorneal femtosecond laser pulse effects in conical incision
profiles. Graefes Arch Clin Exp Ophthalmol. 246:1017–1020.
2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kaiserman I, Maresky HS, Bahar I and
Rootman DS: Incidence, possible risk factors, and potential effects
of an opaque bubble layer created by a femtosecond laser. J
Cataract Refract Surg. 34:417–423. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kanellopoulos AJ and Asimellis G: Digital
analysis of flap parameter accuracy and objective assessment of
opaque bubble layer in femtosecond laser-assisted LASIK: A novel
technique. Clin Ophthalmol. 7:343–351. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hurmeric V, Yoo SH, Fishler J, Chang VS,
Wang J and Culbertson WW: In vivo structural characteristics of the
femtosecond LASIK-induced opaque bubble layers with
ultrahigh-resolution SD-OCT. Ophthalmic Surg Lasers Imaging. 41
(Suppl):S109–S113. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Winkler M, Chai D, Kriling S, Nien CJ,
Brown DJ, Jester B, Juhasz T and Jester JV: Nonlinear optical
macroscopic assessment of 3-D corneal collagen organization and
axial biomechanics. Invest Ophthalmol Vis Sci. 52:8818–8827.
2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kunert KS, Blum M, Duncker GI, Sietmann R
and Heichel J: Surface quality of human corneal lenticules after
femtosecond laser surgery for myopia comparing different laser
parameters. Graefes Arch Clin Exp Ophthalmol. 249:1417–1424.
2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Serrao S, Buratto L, Lombardo G, De Santo
MP, Ducoli P and Lombardo M: Optimal parameters to improve the
interface quality of the flap bed in femtosecond laser-assisted
laser in situ keratomileusis. J Cataract Refract Surg.
38:1453–1459. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Winkler von Mohrenfels C, Khoramnia R,
Maier MM, Pfäffl W, Hölzlwimmer G and Lohmann C: Cut quality of a
new femtosecond laser system. Klin Monatsbl Augenheilkd.
226:470–474. 2009.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
29
|
Sarayba MA, Maguen E, Salz J, Rabinowitz Y
and Ignacio TS: Femtosecond laser keratome creation of partial
thickness donor corneal buttons for lamellar keratoplasty. J
Refract Surg. 23:58–65. 2007.PubMed/NCBI
|
|
30
|
Riau AK, Angunawela RI, Chaurasia SS, Tan
DT and Mehta JS: Effect of different femtosecond laser-firing
patterns on collagen disruption during refractive lenticule
extraction. J Cataract Refract Surg. 38:1467–1475. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Weng S, Liu M, Yang X, Liu F, Zhou Y, Lin
H and Liu Q: Evaluation of Human Corneal Lenticule Quality After
SMILE With Different Cap Thicknesses Using Scanning Electron
Microscopy. Cornea. 37:59–65. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang C, Bald M, Tang M, Li Y and Huang D:
Interface quality of different corneal lamellar-cut depths for
femtosecond laser-assisted lamellar anterior keratoplasty. J
Cataract Refract Surg. 41:827–835. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Soong HK, Mian S, Abbasi O and Juhasz T:
Femtosecond laser-assisted posterior lamellar keratoplasty: Initial
studies of surgical technique in eye bank eyes. Ophthalmology.
112:44–49. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhao Y, Li M, Sun L, Zhao J, Chen Y and
Zhou X: Lenticule Quality After Continuous Curvilinear
Lenticulerrhexis in SMILE Evaluated With Scanning Electron
Microscopy. J Refract Surg. 31:732–735. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lombardo M, De Santo MP, Lombardo G,
Schiano Lomoriello D, Desiderio G, Ducoli P, Barberi R and Serrao
S: Surface quality of femtosecond dissected posterior human corneal
stroma investigated with atomic force microscopy. Cornea.
31:1369–1375. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ziebarth NM, Lorenzo MA, Chow J, Cabot F,
Spooner GJ, Dishler J, Hjortdal JØ and Yoo SH: Surface quality of
human corneal lenticules after SMILE assessed using environmental
scanning electron microscopy. J Refract Surg. 30:388–393.
2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Aoki S, Murata H, Matsuura M, Fujino Y,
Nakakura S, Nakao Y, Kiuchi Y and Asaoka R: The effect of air
pulse-driven whole eye motion on the association between corneal
hysteresis and glaucomatous visual field progression. Sci Rep.
8(2969)2018.PubMed/NCBI View Article : Google Scholar
|