Introduction
Breast cancers often produce prostate-related
antigens, including prostate-specific antigen (PSA),
prostate-specific membrane antigen (PSMA), and prostate acid
phosphatase (PAP), and the serum level of PSA has been suggested to
be a breast cancer prognostic marker (1-4).
Although several studies have indicated that the presence of
antigen spreading response after the administration of a vaccine
against PSA could influence the outcomes of patients with prostate
cancer (5,6), the immune response to these
prostate-related antigens (PRAs) in patients with metastatic
recurrent breast cancer (mrBC) has rarely been investigated. In
2014 we conducted a phase II study of personalized peptide
vaccination (PPV) for mrBC patients, the results of which indicated
that the median progression-free survival (PFS) and median overall
survival (OS) were 7.5 and 15.9 months, respectively; in addition,
an enhanced number of cytotoxic T lymphocytes (CTLs) and/or an
increased IgG response was observed after the vaccination in most
of the patients, irrespective of the breast cancer subtype
(7).
Most of the peptides used for PPV therapy are
commonly expressed in various types of advanced cancers, and we
demonstrated the safety and feasibility of a PPV for patients with
advanced cancer in our previous phase II clinical trials (8-11).
The PPV regimen used individually selected vaccine antigens, chosen
from a panel of peptide candidates applicable for the human
leukocyte antigen (HLA)-A2, -A24, -A26, -A3, -A11, -A31 and -A33
patients, based on the patients' pre-existing host immunity and
HLA-A types. Although a panel used for the peptide vaccination in
the present study did not include PRA peptides since no expression
of PRA in mrBC had been suggested by our preliminary studies
(7), we analyzed the pre- and
postvaccination plasma levels of antigen-specific IgG to PRA
peptides of the original panel for common cancer vaccines and their
potential as prognostic biomarkers of cancer vaccine therapy for
mrBC patients. Our findings suggest that the plasma anti-PRA
peptide IgG is a possible prognostic marker for monitoring the
outcomes of peptide vaccine therapy in mrBC patients.
Patients and methods
Patients and datasets
A total of 79 mrBC patients with metastases who had
failed standard chemotherapy and/or hormonal therapy were
vaccinated as PPV therapy. A maximum of four HLA-matched peptides
showing high peptide-specific IgG responses in the prevaccination
plasma were selected from a panel of 31 peptides (Table SI) applicable for the four HLA-A2,
-A24, -A26, -A3, -A11, -A31 and -A33 types followed by subcutaneous
administration once a week for 6 weeks and once every 2 weeks
thereafter. All patients were positive for HLA-A2, -A24, -A26, -A3,
-A11, -A31, or -A33. Enrolled patients were required to show at
least two positive IgGs reactive to the different vaccine peptides
in prevaccination plasma, as reported (7-12).
We collected and analyzed the data from the 77 mrBC
patients who received PPV therapy. Eligible patients were aged 20
years or older with histologically confirmed advanced metastatic
breast cancer, and had an Eastern Cooperative Oncology Group (ECOG)
performance status (PS) of 0 or 1, life expectancy of at least 12
weeks, and adequate bone marrow function, hepatic function and
renal function. Exclusion criteria included acute infection,
history of severe allergic reactions, pulmonary, cardiac or other
systemic diseases, or other inappropriate conditions for enrollment
as judged by clinicians (7). We
divided these patients into three different intrinsic subtypes:
Estrogen-receptor-positive (ER+)/HER2-negative
(HER2-), HER2-positive (immunohistochemical score 3+ or
HER2 gene/chromosome 17 ratio >2.2 in fluorescence in
situ hybridization: HER2+), and triple-negative
(hormone-receptor-negative and HER2-negative: TNBC). A total of 77
patients were subgrouped as the TNBC (n=18),
ER+/HER2- (n=44), and HER2+ (n=15)
groups. The clinical evaluation of the disease progression, new
lesions of the recurrence was performed in each follow-up according
to the study protocol for all cases (7,8).
Measurement of peptide-reactive
IgG
We determined the plasma IgG levels reactive to the
31-vaccine peptide panel with CTL-epitope peptides, including
PSA-248, PSMA-624 and PAP-213, and PAP-248 by assessing the
fluorescence intensity unit (FIU) values obtained with a Luminex
system (Luminex) as described (13,14).
If the peptide-reactive IgG level in the postvaccination plasma was
more than twofold higher than that of prevaccination level, the
levels were considered increased, as reported (9-12).
Statistical analyses
The Mann-Whitney U test was used to examine the
statistical differences in continuous and categorical values,
respectively. A P-value <0.05 was considered significant. The
PFS and OS were calculated from the date of the first vaccination
until the date of disease progression or death, respectively, or
the last date when the patient was known to be alive. The survival
analysis was performed with the Kaplan-Meier method, and a
comparison of the survival curves was performed with the log-rank
test. Statistical tests were performed using JMP Pro software, ver.
11 (SAS Institute Inc.). The Cox proportional hazard analysis was
used for a univariate analysis to identify clinically relevant
factors: Age, performance status, anti-PRAs antibody IgG,
pathological intrinsic subtype, total number of metastases, the
median time to the first PPV from recurrence, and the median
duration of previous chemotherapies. All analyses were also
stratified for concurrent treatment: The regimen numbers of
previous chemotherapies, hormonal therapies, anti-HER2 therapies,
and bisphosphonate acid or anti-RANKL antibody therapies. The
interaction showed a univariate significant difference between the
outcomes and relevant factors, which we analyzed in a multivariable
model.
Results
Patient characteristics
Seventy-seven mrBC patients with a median age of 57
years (range 30-77 years) were comprised the patient series. After
the 6 and 12th vaccinations, the sum of the plasma levels of
anti-PRA IgG had significantly increased in 31 patients (the
‘anti-PRA increase group’), whereas these levels did not increase
in the remaining 46 patients (the ‘anti-PRA no-increase group’).
The patient characteristics of the two groups are summarized in
Table I.
 | Table ICharacteristics of patients with mrBC
for the anti-PRA increase group and the anti-PRA no-increase
group. |
Table I
Characteristics of patients with mrBC
for the anti-PRA increase group and the anti-PRA no-increase
group.
| Variable | Anti-PRA increase
group (n=31) | Anti-PRA
no-increase group (n=46) |
P-valuea |
|---|
| Median age (range),
years | 59 (35-74) | 55.5 (30-76) | 0.582 |
| Ductal
carcinoma | 29 | 42 | - |
| Lobular
carcinoma | 1 | 2 | - |
| Others | 1 | 2 | - |
| ER positive/HER2
negative, n (%) | 18 (58.1) | 30 (65.2) | 0.068 |
| HER-2 positive, n
(%) | 7 (22.5) | 5 (10.9) | 0.020 |
| Triple negative, n
(%) | 6 (19.4) | 11 (23.9) | 0.892 |
| Median number of
metastatic sites (range) | 3 (1-4) | 2 (1-4) | 0.573 |
| Median duration of
previous chemotherapies, months (range) | 12 (3-48) | 11 (2-148) | - |
| Number of previous
chemotherapy regimens, 1-3/≥4 | 6/18 | 12/19 | 0.300 |
| Chemotherapy, n
(oral/infusion) | 25 (18/7) | 34 (19/15) | 0.494 |
| Anti-HER2
therapy | 7 | 5 | 0.143 |
| Hormonal
therapy | 18 | 21 | 0.164 |
|
Bisphosphonate/anti-RANKL therapy | 14 | 7 | 0.004 |
| Median numbers of
peptide vaccination (range) | 12 (2-39) | 14 (2-30) | 0.885 |
The anti-PRA increase group consisted of 18 (58.1%)
ER+/HER2-patients, seven (22.5%) HER2+
patients, and six (19.4%) TNBC patients. In contrast, the anti-PRA
no-increase group consisted of 30 (65.2%)
ER+/HER2-patients, five (10.9%) HER2+ patients, and 11
(23.9%) TNBC patients (Table I).
The combined therapies included chemotherapy, anti-HER2 therapy,
hormone therapy, and bisphosphonate (Zometa®) or
anti-RANKL therapy (Ranmark®), also shown in Table I. Compared to the anti-PRA
no-increase group, the anti-PRA increase group included a
significantly large number of HER2+ patients (P=0.020)
and a significantly higher frequency of patients who received
concurrent combined bisphosphonate or anti-RANKL therapy (P=0.004).
There were no significant between-group differences in age
(P=0.582), intrinsic ER+/HER2- (P=0.068) or
triple-negative (P=0.892) subtype, the median number of metastases
(P=0.573), the median duration or number (P=0.300) of previous
chemotherapy treatments, the combined number of concurrent
chemotherapy regimens (P=0.494), anti-HER2 therapy (trastuzumab)
(P=0.143), or hormonal therapies (P=0.164).
Combination hormonal therapy was used for a total of
39 (50.7%) of the ER+/HER2-negative patients (18 anti-PRA increase
patients and 21 anti-PRA no-increase patients) using an aromatase
inhibitor such as anastrozole for 10 anti-PRA increase patients and
14 anti-PRA no-increase patients, and letrozole for four anti-PRA
increase patients and five anti-PRA no-increase patients.
Fulvestrant, a selective estrogen receptor downregulator, was used
for one patient in each group, and a high dose of toremifene was
given to one anti-PRA no-increase patient. In addition, the median
length of PPV therapy showed no significant difference (P=0.885)
between the two groups (Table
I).
Plasma IgG levels reactive to PSA,
PSMA, and PAP peptides
The plasma IgG reactive to the peptide panel
including the four PRA peptides (PSA-248, PSMA-624, PAP-213, and
PAP-248) were analyzed in the plasma samples from prevaccination
(n=77), the post-6th (n=75), and the post-12th vaccinations (n=53).
The plasma IgG levels against anti-PRA peptides showed a remarkable
increase in the 31 patients (anti-PRA increase group) at the 6 and
12th vaccination, even though these peptides had not been used for
the vaccinations. An increase in anti-PRA IgG was observed
irrespective of the intrinsic subtypes of mrBC (Fig. 1A and B). The total plasma anti-PRA IgG levels of
the post-6 and 12th vaccinations were markedly increased compared
to the prevaccination values (Fig.
1A). An increase in the sum of anti-PRA IgG after the 6 and
12th vaccinations was also observed in each intrinsic mrBC subtype
group (Fig. 1B).
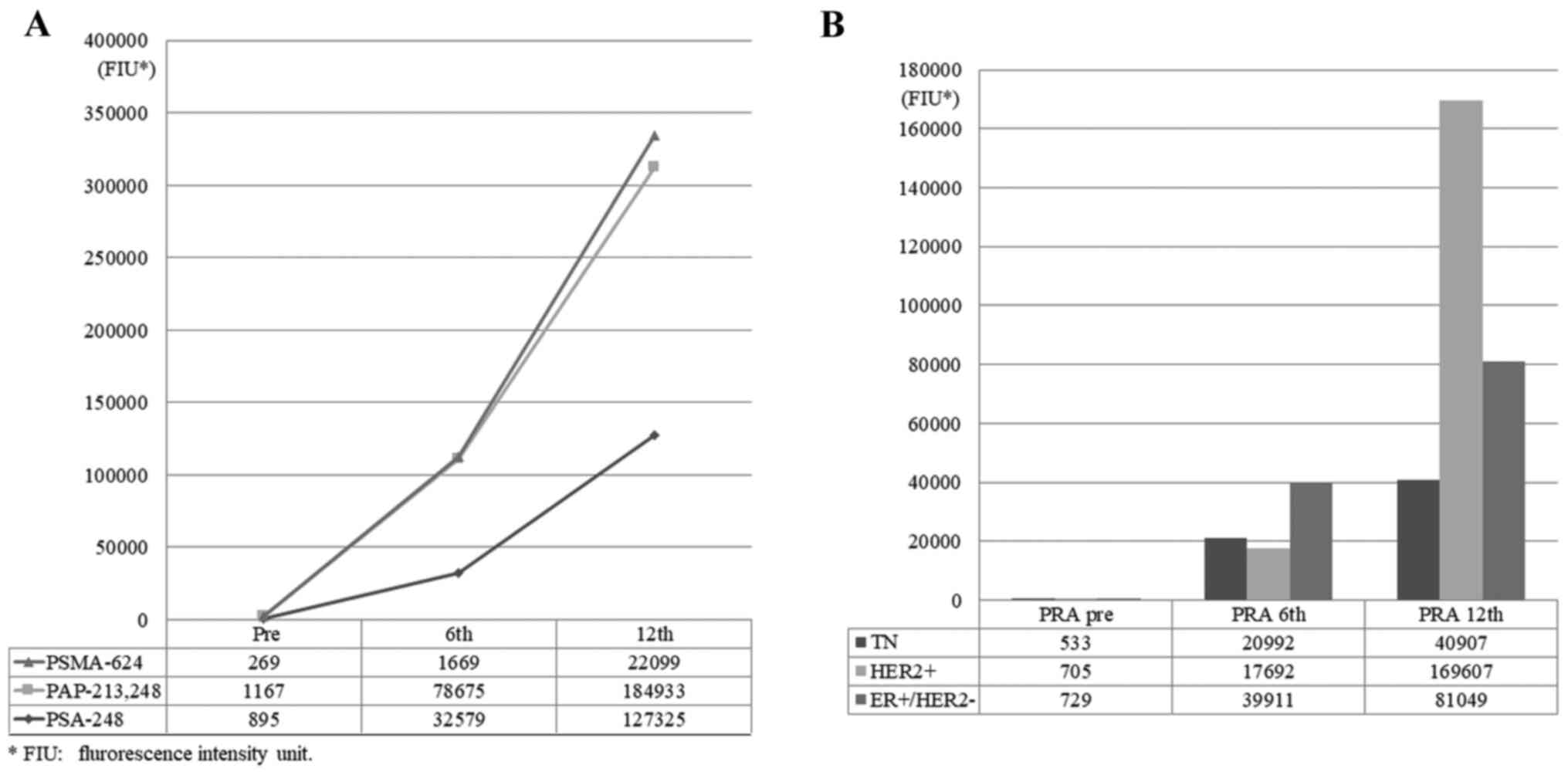 | Figure 1Anti-PRA IgG responses before and
after PPV treatment. After 6 and 12 cycles of PPV therapy, total
plasma levels of anti-PRA IgG significantly increased in 31
patients with mrBC (anti-PRA increase group). (A) Plasma IgG levels
for anti-PMSA-624, anti-PAP-213/-248 and anti-PSA-248 before and
after the 6 and 12th vaccinations. (B) Total plasma IgG levels
including anti-PMSA-624, anti-PAP-213/-248 and anti-PSA-248 in
patients with TN, HER2+ and ER+/HER2-subtypes before and after the
6 and 12th vaccinations. PRA, prostate related antigen; PPV,
personalized peptide vaccine; mrBC, metastatic recurrent breast
cancer; PMSA, prostate-specific membrane antigen; PAP, prostatic
acid phosphatase; PSA, prostate specific antigen; TN, triple
negative; ER, estrogen receptor; FIU, fluorescence intensity
unit. |
An increase in IgG reactive to the PAP (PAP-213
and/or -248), PSA248, and PSMA624 peptides after the 12th
vaccination was observed in 17 of 31 (54.8%), 11 of 31 (35.5%), and
three of 31 (9.7%) patients, respectively. The rates of increase
for each subtype of mrBC are as follows: 58.1% (18 of the 31)
patients in the ER+/HER2-subtype, 22.5% (seven of the
31) in the HER2+ subtype, and 19.4% (six of the 31) in
the TN subtype. There was no significant correlation between the
subtype and the increase of anti-IgG response (Table SII). On the other hand, no
augmentation of the anti-PRA response was observed in the remaining
46 patients (data not shown).
Survival analyses by total anti-PRA
IgG level and intrinsic mrBC subtype
At the time of the present analyses, the median
duration of follow-up was 33.5 months, the median PFS was 7.4
months, and the median OS was 13.4 months. No significant
differences in PFS or OS were observed among the intrinsic mrBC
subtypes, i.e., the ER+/HER2-, HER2+, and TN subtypes, which is
consistent with our previous study (7).
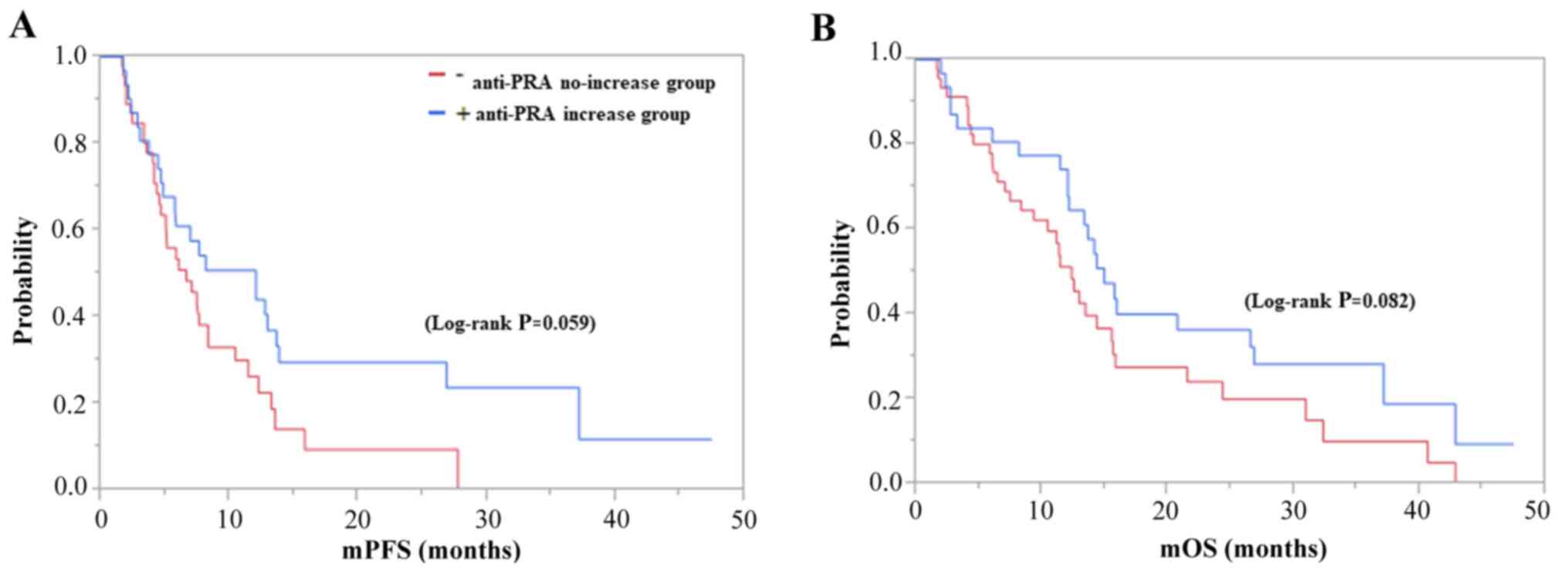
The PFS and OS of the anti-PRA increase group were
8.1 and 14.3 months, and those of the anti-PRA no-increase group
were 5.1 and 10.8 months (log-rank P=0.059 and P=0.082),
respectively, with no significant between-group differences
(Fig. 2A and B). In contrast, the PFS and OS of the
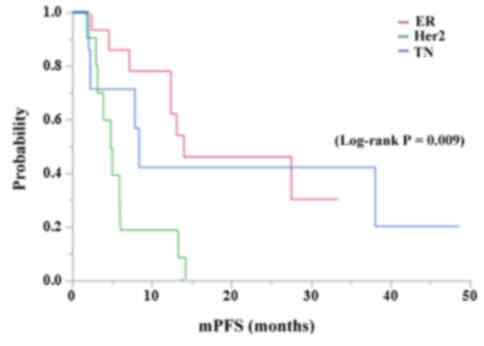
patients with the ER+/HER2-,
HER2+, and TN subtypes were 13.6 and 26.5, 4.8 and 13.7,
and 8.1 and 12.1 months, respectively, in the anti-PRA increase
group, whereas those of the anti-PRA no-increase group were 7.4 and
14.3, 10.4 and 10.7, and 5.0 and 6.4 months, respectively (Fig. 3). The survival curve for PFS
(log-rank P=0.009; Fig. 3) but not
OS (log-rank P=0.154) of the ER+/Her2-subtype was significantly
longer than those of other subtypes in the anti-PRA increase group.
In contrast, such significance was not observed in the anti-PRA
no-increase group, regardless of the mrBC subtypes in PFS (P=0.169)
and OS (P=0.144). In addition, no significant difference was found
among the IgG levels against each single PRA in PFS and/or OS.
Association of plasma anti-PRA IgG and
clinical factors with the patients' prognoses
Cox regression for survival analysis was performed
to investigate the effect of multiple variables included anti-PRA
IgG and clinical factors associated with the events that happened.
As shown in Table II, the
multivariate analyses for the PFS of all 77 patients showed that
age over 60 years, anti-PRA IgG, HER2 positivity, number of
previous chemotherapy regimens, and duration of vaccine therapy
were each prognostic factors for PFS (P=0.03, 0.039, 0.023, 0.029,
and 0.001, respectively). The analysis of OS showed that the age,
duration of vaccine therapy, anti-HER2 therapy, concurrent standard
hormonal therapy, and bisphosphonate and/or anti-RANKL therapy were
each prognostic factors for OS (P=0.025, 0.0001, 0.033, 0.033, and
0.05, respectively) (Table
II).
 | Table IICox analysis in patients with mrBC
who received PPVs for PFS and OS. |
Table II
Cox analysis in patients with mrBC
who received PPVs for PFS and OS.
| | PFS | OS |
|---|
| | | Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
Characteristics | No. of
patients | P-value | HR | 95% CI | P-value | P-value | HR | 95% CI | P-value |
|---|
| Age, <59 vs.
>60 years | 50/27 | 0.303 | 0.49 | 0.25-0.94 | 0.03 | 0.037 | 2.1 | 1.09-4.28 | 0.025 |
| Performance status,
0 vs. 1 | 69/8 | 0.451 | | | | 0.225 | | | |
| Post-PPV anti-PRA
mAb boosting, + vs. - a | 31/46 | 0.023 | 0.46 | 0.22-0.96 | 0.039 | 0.463 | | 0.41-1.58 | 0.542 |
| ER+ vs. HER2- | 44 | 0.337 | | | | 0.592 | | 0.21-1.49 | 0.267 |
| HER-2 positive | 15 | 0.049 | 9.17 | 1.33-95.2 | 0.023 | 0.843 | | | |
| Triple
negative | 18 | 0.635 | | | | 0.507 | | | |
| 1-3 regimens vs.
>4 regimensb | 36/41 | 0.009 | 2.2 | 1.09-4.57 | 0.029 | 0.088 | | | |
| Total site of
metastases (range: 1-4): <2/>2 | | 0.171 | | | | 0.234 | | | |
| Median times of
peptide vaccination (months): <3/>3 | 40/37 | 0.009 | 0.1 | 0.04-0.26 | 0.001 | <0.0001 | 0.08 | 0.03-0.21 | <0.0001 |
| Anti-Her2
therapy | 15 | 0.88 | | | | 0.255 | 0.37 | 0.14-0.92 | 0.033 |
| Hormonal
therapy | 30 | 0.299 | 0.48 | 0.22-0.99 | 0.048 | 0.131 | 0.48 | 0.23-0.94 | 0.033 |
|
Bisphosphonate/Anti-RNAKL therapy | 21 | 0.261 | | | | 0.001 | 0.48 | 0.22-1.01 | 0.05 |
We then analyzed the PFS and OS of the 77 patients
by the Kaplan-Meier method. Patient age (older or ≤60 years), the
duration of PPV therapy lasting more or ≤3 months, and concurrent
conventional hormonal therapies were each significantly associated
with both PFS and OS, age over 60 years (Fig. S1A and B), duration of PPV therapy over 3 months
(Fig. S1C and D), and concurrent hormonal therapies
(Fig. S1E and F) were significantly associated with
better prognosis. (P=0.0419, <0.0001, 0.0002 in PFS, and
P=0.0019, <0.0001, 0.0006 in OS).
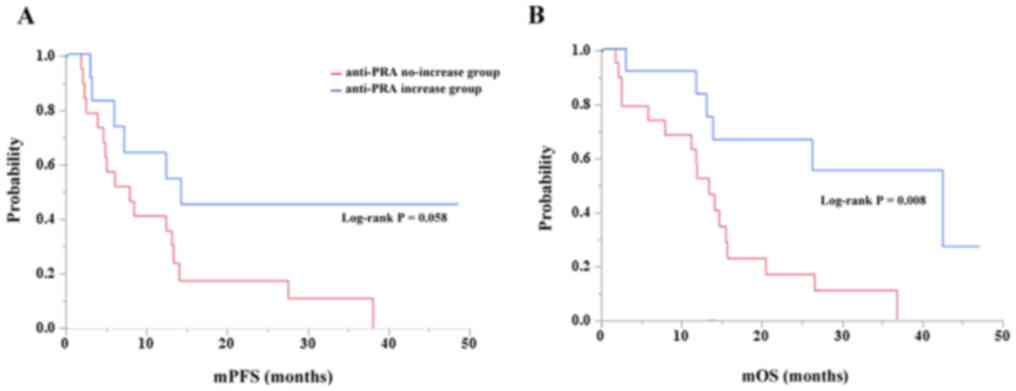
We further analyzed the association of anti-PRA IgG
in age subgroups (Fig. 4). We
determined the survival curves comparing patients ≤60 years old and
those >60 years old who did or did not exhibit increased
anti-PRA IgG after PPV therapy. Although it was marginally
associated with PFS (Fig. 4A,
log-rank P=0.058), an increase in anti-PRA IgG was significantly
associated with OS in the >60-year-old patients (Fig. 4B, log-rank P=0.008). In contrast,
this significance was not observed in the patients ≤60 years old
for PFS (P=0.422) or OS (P=0.127).
Discussion
The serum PSA level is one of the most valuable
serum tumor markers used for the standard diagnosis and clinical
management of prostate cancer (6,15,16).
In contrast, the predictive potential of the PRA expression in
breast cancer (particularly the expression of PSA) for prognosis is
still controversial. Several research groups have reported that PSA
positivity was significantly associated with normal breast tissues,
with benign, smaller tumors, and with progesterone and/or androgen
receptor positivity. Those researchers proposed that PRA could be a
valuable tool for the prediction of a favorable breast cancer
outcome (2,17), whereas those were inversely
associated with stage III or IV advanced breast cancer (1). In addition, we observed the lesser or
lower expression of those antigens on refractory mrBC specimens in
our preliminary study (7). Taken
together, the above-described results suggest that plasma anti-PRA
IgG levels could be an alternate biomarker for the prediction of
breast cancer progression.
However, recent research has indicated that
immunologic factors, such as tumor-infiltrating lymphocytes (TILs)
and PD-1/PD-L1 expression, have a significant impact on the
clinical outcome of patients with early-stage breast cancer
(18-20).
Novel immunotherapeutic strategies, including PPV therapy, have
also showed considerable promise in the immune system response to
breast tumors in the majority of patients with mrBC (7,21,22).
Several studies showed that the IgG response to antigens of mucin-1
(MUC-1) or other tumor-associated antigens on breast cancer might
contribute to a better prognosis (23-25).
To the best of our knowledge, there has been no
research investigating the clinical significance associated with
anti-PRA IgG for metastatic recurrent breast cancer. We thus
focused in the present study on patients with refractory mrBC, and
we examined their plasma pre- and postvaccination levels of
anti-PRA IgG. The results of our analyses revealed that anti-PRA
IgG levels were increased in 31 of the 77 (40.3%) patients after
PPV therapy.
It is well recognized that the cancer immunity cycle
consists of several steps, including the release of cancer antigens
from cell death, their presentation by antigen-presenting cells to
T cells, the activation of T cells, their infiltration to the
cancer tissues, the elimination of cancer cells, and the release of
cancer antigens. The newly released cancer antigens have been
described as antigen spreading phenomena after peptide vaccination.
This epitope-spreading responses have been observed in
HER2+ patients following immunization with the HER2
peptide vaccine (26,27), and Gulley et al (6) found that using the vaccine (Prostvac)
against PSA in combination with radiation therapy caused antigen
spreading immune responses to a number of prostate antigens, and
this vaccine showed evidence of improved survival (5). We also reported the PPV-induced
antigen spreading was a favorable biomarker for gynecological
cancers (28,29).
Our present findings consequently showed that
peptide vaccines derived from tumor-associated antigens (TAAs)
induced a humoral IgG response to a variety of PRAs including PSA,
PSMA, and PAP in patients with mrBC, and that this
treatment-associated anti-PRA IgG response demonstrates potential
prognostic significance for monitoring the outcome of peptide
vaccine treatment for patients with mrBC.
Although the mechanisms by which high plasma IgG
levels against PRA are associated with better survival have not
been fully explained, it has been suggested that PRA, in particular
plasma PSA, is associated with a favorable prognosis and that its
induction is an unfavorable factor for breast cancer patients with
ER+ cancer, but not with androgens and progestins
(30-32).
Nevertheless, we did not analyze the expression of androgen
receptor (AR), which is widely expressed in breast cancer. As is
the case for ER, AR expression is associated with a more favorable
prognosis among patients with ER+ breast cancer
(33,34).
Our results showed that higher post-vaccinated
plasma IgG antibody levels to PRA were associated with better PFS
and OS, and it should be noted that our results provide the first
evidence that the plasma anti-PRA IgG level might be a useful
prognostic biomarker for peptide vaccine therapy in patients with
mrBC.
Our analyses revealed that patients who underwent a
longer duration of PPV therapy had significantly better PFS and OS
outcomes, as did the ER+/HER2-patients (n=18; 58.1%) in
the anti-PRA increase group who were simultaneously given a peptide
vaccine with an aromatase inhibitor (Table I, Fig.
S1). In contrast, the ER+ mrBC patients without an
increased anti-PRA IgG response (the anti-PRA no-increase group)
showed that AI treatment along with the vaccination could not
improve the outcome for these patients, as there was no significant
difference in survival between the ER+ patients and the
HER2+ or TN patients (Fig.
3). Consequently, our findings suggest that conventional
hormonal therapy combined with peptide vaccines for postmenopausal
mrBC patients, particularly for those over 60 years old, might be a
novel and effective treatment strategy.
Recent evidence has also shown that the monoclonal
antibody trastuzumab can kill HER2+ breast cancer cells
not only by blocking HER2 signaling, but also through immune
mechanisms that include antibody-dependent cellular cytotoxicity
and complement-dependent cytotoxicity. In addition, the
administration of trastuzumab was observed to induce adaptive
immunity including both T-cell and antibody responses in patients
with HER2+ breast cancer (35,36).
Clinical observations have also demonstrated that peptide-based
HER2 vaccines administered concurrently with trastuzumab resulted
in potent and specific immune activation and were associated with
better survival (21,37).
However, there is evidence suggesting that
anti-RANKL therapy (with denosumab, a fully human IgG2 monoclonal
antibody specific to RANKL) may induce divergent effects in the
immune system beyond the effects on bone, which is also true of the
bisphosphonate Zometa® (zoledronic acid) (38-40).
Moreover, combination therapies targeting RANKL-RANK signaling can
be used to prevent subsequent metastatic disease in breast cancer
(41).
Therefore, our results are consistent with the
supposition that immunotherapeutic strategies using peptide
vaccines, such as PPV therapy, can be efficiently combined with
conventional therapies such as hormonal, anti-HER2, and
bisphosphonate/anti-RANKL therapies for mrBC patients whose cancer
has been resistant to previous standard cytotoxic chemotherapies.
Notably, this novel complementary integrative treatment might be
more effective in older postmenopausal mrBC patients (≥60 years
old).
Although we analyzed 77 vaccinated patients and
observed the prognostic predictive possibility of anti-PRA IgG, our
study has some limitations. These include the presence of multiple
confounding factors in the examination of prognostic biomarkers,
the small sample size with more HER2+ patients in the
PRA response group, the single-arm data set, and, finally, the
combination treatment with standard chemotherapy, endocrine
therapy, and/or bisphosphonate/anti-RANKL therapy. In summary, our
data show that plasma IgG antibodies to PRA increased in patients
with mrBC, and the presence of these antibodies was associated with
better survival in the patients who were treated using personalized
peptide vaccines, particularly in the older postmenopausal
ER+ mrBC patients. Additional prospective studies with
larger numbers of patients are needed in order to confirm the
clinical importance of anti-PRA IgG in patients with mrBC in
relation to tumor progression and therapeutic implications.
Supplementary Material
Survival curve analyses with patient
age, duration of PPV therapy and concurrent hormonal therapy
status. (A-F) There were significant survival advantages in the
patients ≥60 years old, those who underwent >3 months of PPV
therapy, and those who were treated with combined standard hormonal
therapy, compared to the patients <60 years, those who received
<3 months of PPV therapy, and those without combined hormonal
therapy. (A and B) By patient's age (<60/≥60 year old); PFS and
OS, P=0.0419 and P=0.0019, respectively. (C and D) The duration of
PPV therapy (<3 months/≥3 months); both PFS and OS, P<0.0001.
(E and F) With or without combined hormonal therapy; PFS and OS,
P=0.0002 and P=0.0006, respectively.
Information on the peptide candidates
used for PPV.
Pearson's correlation coefficient for
PRAs and the subtypes of mrBC.
Acknowledgements
Professor Tatsuyuki Kakuma of Kurume University
Biostatistics Center was responsible for the supervision of the
statistical analysis.
Funding
Funding: The present study was supported by grants from the
Ministry of Education, Culture, Sports, Science and Technology of
Japan.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SSaku, UT and KI are responsible for the study
design, analysis of data and drafting the manuscript. UT, AY and YA
are responsible for the supervision of analysis of data. S Sakurai,
YT and SShichijo are responsible for the acquisition and
interpretation of data. SSaku is responsible for the material and
technical support. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Kurume
University Ethical Committee and registered in the UMIN Clinical
Trials Registry (no. UMIN000001844). All patients were given a full
explanation of the protocol, and provided their informed consent
prior to enrollment in the clinical trial of PPV therapy and
subsequent data analysis.
Patient consent for publication
Not applicable.
Competing interests
Akira Yamada is a part-time executive of Bright Pass
Biotherapeutics. Akira Yamada and Shigeki Shichijo have Bright Pass
Biotherapeutics stock. Kyogo Itoh received research funding from
Taiho Pharmaceutical Co. Ltd. The remaining authors declare that
they have no competing interests.
References
|
1
|
Yu H, Diamandis EP, Levesque M, Giai M,
Roagna R, Ponzone R, Sismondi P, Monne M and Croce CM: Prostate
specific antigen in breast cancer, benign breast disease and normal
breast tissue. Breast Cancer Res Treat. 40:171–178. 1996.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Black MH and Diamandis EP: The diagnostic
and prognostic utility of prostate-specific antigen for diseases of
the breast. Breast Cancer Res Treat. 59:1–14. 2000.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mannello F and Gazzanelli G:
Prostate-specific antigen (PSA/hK3): A further player in the field
of breast cancer diagnostics? Breast Cancer Res. 3:238–243.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Alanen KA, Kuopio T, Collan YU, Kronqvist
P, Juntti L and Nevalainen TJ: Immunohistochemical labelling for
prostate-specific antigen in breast carcinomas. Breast Cancer Res
Treat. 56:169–176. 1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kantoff PW, Schuetz TJ, Blumenstein BA,
Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R,
Schlom J, et al: Overall survival analysis of a phase II randomized
controlled trial of a Poxviral-based PSA-targeted immunotherapy in
metastatic castration-resistant prostate cancer. J Clin Oncol.
28:1099–1105. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gulley JL, Madan RA, Tsang KY, Jochems C,
Marté JL, Farsaci B, Tucker JA, Hodge JW, Liewehr DJ, Steinberg SM,
et al: Immune impact induced by PROSTVAC (PSA-TRICOM), a
therapeutic vaccine for prostate cancer. Cancer Immunol Res.
2:133–141. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Takahashi R, Toh U, Iwakuma N, Takenaka M,
Otsuka H, Furukawa M, Fujii T, Seki N, Kawahara A, Kage M, et al:
Feasibility study of personalized peptide vaccination for
metastatic recurrent triple-negative breast cancer patients. Breast
Cancer Res. 16(R70)2014.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Sasada T, Komatsu N, Suekane S, Yamada A,
Noguchi M and Itoh K: Overcoming the hurdles of randomised clinical
trials of therapeutic cancer vaccines. Eur J Cancer. 46:1514–1519.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Terasaki M, Shibui S, Narita Y, Fujimaki
T, Aoki T, Kajiwara K, Sawamura Y, Kurisu K, Mineta T, Yamada A and
Itoh K: Phase I trial of a personalized peptide vaccine for
patients positive for human leukocyte antigen-A24 with recurrent or
progressive glioblastoma multiforme. J Clin Oncol. 29:337–344.
2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yanagimoto H, Shiomi H, Satoi S, Mine T,
Toyokawa H, Yamamoto T, Tani T, Yamada A, Kwon AH, Komatsu N, et
al: A phase II study of personalized peptide vaccination combined
with gemcitabine for non-resectable pancreatic cancer patients.
Oncol Rep. 24:795–801. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hattori T, Mine T, Komatsu N, Yamada A,
Itoh K, Shiozaki H and Okuno K: Immunological evaluation of
personalized peptide vaccination in combination with UFT and UZEL
for metastatic colorectal carcinoma patients. Cancer Immunol
Immunother. 58:1843–1852. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Noguchi M, Mine T, Komatsu N, Suekane S,
Moriya F, Matsuoka K, Yutani S, Shichijo S, Yamada A, Toh U, et al:
Assessment of immunological biomarkers in patients with advanced
cancer treated by personalized peptide vaccination. Cancer Biol
Ther. 10:1266–1279. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Komatsu N, Shichijo S, Nakagawa M and Itoh
K: New multiplexed flow cytometric assay to measure anti-peptide
antibody: A novel tool for monitoring immune responses to peptides
used for immunization. Scand J Clin Lab Invest. 64:535–545.
2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Matsueda S, Komatsu N, Kusumoto K, Koga S,
Yamada A, Kuromatsu R, Yamada S, Seki R, Yutani S, Shichijo S, et
al: Humoral immune responses to CTL epitope peptides from
tumor-associated antigens are widely detectable in humans: A new
biomarker for overall survival of patients with malignant diseases.
Dev Comp Immunol. 41:68–76. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gulley JL, Arlen PM, Bastian A, Morin S,
Marte J, Beetham P, Tsang KY, Yokokawa J, Hodge JW, Ménard C, et
al: Combining a recombinant cancer vaccine with standard definitive
radiotherapy in patients with localized prostate cancer. Clin
Cancer Res. 11:3353–3362. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Noguchi M, Koga N, Moriya F and Itoh K:
Immunotherapy in prostate cancer: Challenges and opportunities.
Immunotherapy. 8:69–77. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yu H, Levesque MA, Clark GM and Diamandis
EP: Prognostic value of prostate-specific antigen for women with
breast cancer: A large United States cohort study. Clin Cancer Res.
4:1489–1497. 1998.PubMed/NCBI
|
|
18
|
Takenaka M, Seki N, Toh U, Hattori S,
Kawahara A, Yamaguchi T, Koura K, Takahashi R, Otsuka H, Takahashi
H, et al: FOXP3 expression in tumor cells and tumor-infiltrating
lymphocytes is associated with breast cancer prognosis. Mol Clin
Oncol. 1:625–632. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Okabe M, Toh U, Iwakuma N, Saku S, Akashi
M, Kimitsuki Y, Seki N, Kawahara A, Ogo E, Itoh K and Akagi Y:
Predictive factors of the tumor immunological microenvironment for
long-term follow-up in early stage breast cancer. Cancer Sci.
108:81–90. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sakamoto S, Matsueda S, Takamori S, Toh U,
Noguchi M, Yutani S, Yamada A, Shichijo S, Yamada T, Suekane S, et
al: Immunological evaluation of peptide vaccination for cancer
patients with the HLA-A11(+) or -A33(+) allele. Cancer Sci.
108:598–603. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Disis ML, Wallace DR, Gooley TA, Dang Y,
Slota M, Lu H, Coveler AL, Childs JS, Higgins DM, Fintak PA, et al:
Concurrent trastuzumab and HER2/neu-specific vaccination in
patients with metastatic breast cancer. J Clin Oncol. 27:4685–4692.
2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Higgins M, Curigliano G, Dieras V, Kuemmel
S, Kunz G, Fasching PA, Campone M, Bachelot T, Krivorotko P, Chan
S, et al: Safety and immunogenicity of neoadjuvant treatment using
WT1-immunotherapeutic in combination with standard therapy in
patients with WT1-positive Stage II/III breast cancer: A randomized
phase I study. Breast Cancer Res Treat. 162:479–488.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Isla Larrain MT, Colussi AG, Demichelis
SO, Barbera A, Creton A, Segal-Eiras A and Croce MV: Humoral immune
response against tumoral mucin 1 (MUC1) in breast cancer patients.
Int J Biol Markers. 28:318–325. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Clive KS, Tyler JA, Clifton GT, Holmes JP,
Ponniah S, Peoples GE and Mittendorf EA: The GP2 peptide: A
HER2/neu-based breast cancer vaccine. J Surg Oncol. 105:452–458.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mittendorf EA, Clifton GT, Holmes JP,
Schneble E, van Echo D, Ponniah S and Peoples GE: Final report of
the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine
with booster inoculations to prevent disease recurrence in
high-risk breast cancer patients. Ann Oncol. 25:1735–1742.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Disis ML, Goodell V, Schiffman K and
Knutson KL: Humoral epitope-spreading following immunization with a
HER-2/neu peptide based vaccine in cancer patients. J Clin Immunol.
24:571–578. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Disis ML, Schiffman K, Guthrie K, Salazar
LG, Knutson KL, Goodell V, dela Rosa C and Cheever MA: Effect of
dose on immune response in patients vaccinated with an her-2/neu
intracellular domain protein-based vaccine. J Clin Oncol.
22:1916–1925. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kawano K, Tsuda N, Matsueda S, Sasada T,
Watanabe N, Ushijima K, Yamaguchi T, Yokomine M, Itoh K, Yamada A
and Kamura T: Feasibility study of personalized peptide vaccination
for recurrent ovarian cancer patients. Immunopharmacol
Immunotoxicol. 36:224–236. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kawano K, Tsuda N, Waki K, Matsueda S,
Hata Y, Ushijima K, Itoh K, Yamada A and Kamura T: Personalized
peptide vaccination for cervical cancer patients who have received
prior platinum-based chemotherapy. Cancer Sci. 106:1111–1117.
2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zarghami N, Grass L and Diamandis EP:
Steroid hormone regulation of prostate-specific antigen gene
expression in breast cancer. Br J Cancer. 75:579–588.
1997.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yu H, Diamandis EP, Zarghami N and Grass
L: Induction of prostate specific antigen production by steroids
and tamoxifen in breast cancer cell lines. Breast Cancer Res Treat.
32:291–300. 1994.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zarghami N and Diamandis EP: Detection of
prostate-specific antigen mRNA and protein in breast tumors. Clin
Chem. 42:361–366. 1996.PubMed/NCBI
|
|
33
|
Hu R, Dawood S, Holmes MD, Collins LC,
Schnitt SJ, Cole K, Marotti JD, Hankinson SE, Colditz GA and Tamimi
RM: Androgen receptor expression and breast cancer survival in
postmenopausal women. Clin Cancer Res. 17:1867–1874.
2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Vera-Badillo FE, Templeton AJ, de Gouveia
P, Diaz-Padilla I, Bedard PL, Al-Mubarak M, Seruga B, Tannock IF,
Ocana A and Amir E: Androgen receptor expression and outcomes in
early breast cancer: A systematic review and meta-analysis. J Natl
Cancer Inst. 106(djt319)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bianchini G and Gianni L: The immune
system and response to HER2-targeted treatment in breast cancer.
Lancet Oncol. 15:e58–e68. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bianchini G, Pusztai L, Pienkowski T, Im
YH, Bianchi GV, Tseng LM, Liu MC, Lluch A, Galeota E, Magazzù D, et
al: Immune modulation of pathologic complete response after
neoadjuvant HER2-directed therapies in the NeoSphere trial. Ann
Oncol. 26:2429–2436. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Soliman H: Immunotherapy strategies in the
treatment of breast cancer. Cancer Control. 20:17–21.
2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gober HJ, Kistowska M, Angman L, Jenö P,
Mori L and De Libero G: Human T cell receptor gammadelta cells
recognize endogenous mevalonate metabolites in tumor cells. J Exp
Med. 197:163–168. 2003.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hewitt RE, Lissina A, Green AE, Slay ES,
Price DA and Sewell AK: The bisphosphonate acute phase response:
rapid and copious production of proinflammatory cytokines by
peripheral blood gd T cells in response to aminobisphosphonates is
inhibited by statins. Clin Exp Immunol. 139:101–111.
2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Cheng ML and Fong L: Effects of
RANKL-targeted therapy in immunity and cancer. Front Oncol.
3(329)2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tan W, Zhang W, Strasner A, Grivennikov S,
Cheng JQ, Hoffman RM and Karin M: Tumour-infiltrating regulatory T
cells stimulate mammary cancer metastasis through RANKL-RANK
signalling. Nature. 470:548–553. 2011.PubMed/NCBI View Article : Google Scholar
|