Introduction
Asthma is a highly prevalent chronic airway disease,
which affects >300 million individuals worldwide (1). Its morbidity and mortality rate are
increasing gradually with the development of the society. The
symptoms include shortness of breath, cough and chest tightness
(2). To date, the pathological
mechanisms of asthma have remained to be fully elucidated. Studies
have indicated that the disease is an inflammatory disorder of the
conducting airways, which has a strong association with allergic
sensitization (3). It is
classically recognized as a typical type 2 T-helper cell (Th2)
disease, with increased immunoglobulin E (IgE) levels and
eosinophilic inflammation in the airways. Resulting from airway
inflammation, airway remodeling leads to airway wall thickening and
induces increased airway smooth muscle mass, which generates
asthmatic symptoms (4). Chronic
airway inflammation, airway hyperresponsiveness and airway
remodeling are the central pathogenic processes of this disease
(5).
Liver X receptors (LXRs) belong to the nuclear
receptor superfamily and occur in two forms, known as LXRα and LXRβ
(6). Previous studies have
established LXRs as the key modulators of both lipid metabolism and
inflammation (7-10).
When combined with natural ligands, such as oxysterols
ordesmosterol (11), or synthetic
ligands, including GW3965 or T0901317(12), LXRs regulate the transcription of
genes involved in cholesterol efflux and macrophage-mediated
inflammation. The LXR ligand exertsinhibitory roles in
lipopolysaccharide-induced lung inflammatory responses and
anti-proliferative effects on airway smooth muscle (13-16).
However, LXRs fail to attenuate acute allergic airway inflammation
and Th2 cytokines in bronchoalveolar lavage fluid (BALF) (17). In chronic allergic asthma, LXRs have
the ability to reduce serum IgE. Furthermore, the LXR agonist
T0901317 is able to inhibit the airway remodeling process (12,18,19).
However, T0901317 is not highly selective for LXRs and it isalso
able to activate other nuclear receptors, such as the Farnesoid X
receptor (20,21). To the best of our knowledge, there
are currently no data regarding chronic allergic asthma in
LXR-deficient mice. Therefore, it was necessary to performan
in-depth study on this topic. Thus, the present study used
LXR-deficient mice and a highly selective agonist of LXRs to
investigate the role of LXRs in allergen-induced chronic
asthma.
Materials and methods
Mice and ethics statement
A total of 36C57BL/6 female mice (age, 8 weeks;
weight, 19±2g) were obtained from the Mice Breeding Service
Department of GemPharmatech. Animals were given free access to
sterilized tap water and a standard rodent chow in a specific
pathogen-free biosafety level 3 facility at a temperature of
22-24˚C and a relative humidity of 50-60%. An artificial 12 h
light/dark cycle with suitable ventilation was maintained in the
animal room.
The present experiment was approved by The
Institutional Animal Care and Use Committee of Nanjing First
Hospital Affiliated to Nanjing Medical University Animal Center
(Nanjing, China). Animal experiments were performed in strict
accordance with the provisions of the Guidance on Feeding and Use
of Experimental Animals by the Ministry of Science and Technology
of the P.R. China. All operations were performed under anesthesia
and all efforts were made to minimize suffering.
Establishment of the mouse model and
drug intervention
Mice were randomly divided into six groups (n=6
mice/group) as follows: Control group, ovalbumin (OVA) group,
GW3965 group, LXRα-/- group, LXRβ-/- group
and LXRα/β knockout (KO) group. Certainwild-type mice received
intervention with the LXR agonist GW3965 (Cayman Chemical Company)
at 20 mg/kg by intraperitoneal injection. LXR-KOmice and wild-type
mice were used to establish a chronic asthma model (18). Mice were intraperitoneally injected
with 0.1 ml sensitizing solution [0.1 mg OVA; 0.07 ml Imject™ Alum
Adjuvant (Beijing Biolead Biotechnology Co., Ltd.); 0.03 ml PBS] on
days 0, 7 and 14. The mice were stimulated with 1% OVA solution
using an ultrasonicnebulizer (402AI; Jiangsu Yuyue Medical
Equipment & Supply Co., Ltd.) from day 21 for 30 min each time,
three times a week, for 8 consecutive weeks. Mice treated with the
LXR agonist were administered an intraperitoneal injection of
GW3965 at 20 mg/kg (10,22) prior to each stimulation.
Airway physiology
Airway responsiveness was measured 24 h after the
last OVA treatment using an AniRes 2005 Lung Function system
(Bestlab). The mice were anesthetized with Nembutal (60 mg/kg;
intraperitoneal). The trachea was then surgically exposed and
connected to a computer-controlled ventilator using an
intratracheal tube. The respiration rate and the
expiratory/inspiratory time ratio were preset at 90/min, 1.5:1.
Mice were stabilized for 5 min. Subsequently, various doses of
acetylcholine chloridewere administeredby atomizing inhalation for
20 sec at increasing concentrations, with intervals of 5 min from
10 to 200 µg/kg. The values of airway resistance were recorded by
the system after the administration of acetylcholine chloride
(18).
Analysis of serum and BALF
After the airway physiology analysis, blood was
collected from the hearts of the mice. The collected blood was left
in a centrifuge tube at room temperature for 2 h and was
centrifuged at 4,000 x g for 10 min at 4˚C. The supernatant was
transferred into a clean 1.5-ml centrifuge tube and stored at
-80˚C. Mice were sacrificed by cervical dislocation. When the
spinal cord and brain were severed, spontaneous respiration
disappeared and there was no response to external stimuli,
indicating that the mouse had died instantly. In order to obtain
the BALF, ice-cold PBS (0.5 ml) was infused into the lungs and
withdrawn thrice via tracheal cannulation (total volume, 1.5 ml).
The supernatant obtained from the BALF was stored at -80˚C for
subsequent biochemical analysis. The levels of IL-4 (cat. no.
JM-02448M2), IL-5 (cat. no. JM-11729M2) and IL-13 (cat. no.
JM-02456M2) in the BALF and the level of IgE (cat. no. JM-02339M2)
(all Jiangsu Jingmei Biotech, Co., Ltd.) in serum were quantified
using ELISAs according to the manufacturer's protocols.
The total cell count and differential cell type
count in BALF from the slides and the stains was performed using
Wright-Giemsa stain (Nanjing KeyGen Biotech Co., Ltd.) were
determined using an optical microscope (Olympus Corporation). In
total, ≥200 cells were counted for each mouse and were identified
as macrophages, eosinophils, lymphocytes and neutrophils according
to standard pathological morphology under x400 magnification.
Histology and Masson trichrome
staining
After the BALF samples were obtained, the left lung
tissue was fixed in 10% (v/v) neutral buffed formalin. The tissue
was then embedded in paraffin, sectioned at 5-µm thickness and
subjected to H&E or periodic acid-Schiff (PAS) staining to
estimate inflammation or mucus production, respectively. The
numbers of PAS-negative and PAS-positive epithelial cells (goblet
cells) in individual bronchioles were counted. In total, ≥10 random
bronchioles were counted in each slide.
The sections were then subjected to Masson trichrome
staining to evaluate collagen deposition according to the standard
protocol recommended by the manufacturer. All histologic
examinations were performed in a double-blinded manner under x200
magnification.
Immunostaining for α-smooth muscle
actin (α-SMA)
Immunohistochemistry was performedto detectα-SMA.
The paraffin slices were dewaxed inxylene followed by rehydration
and were then incubated in 3% H2O2 at room
temperature for 10 min to eliminate the activity of endogenous
peroxidase. Subsequently, the slices were rinsed with distilled
water and soaked twice in PBS for 5 min. These slices were blocked
with 5% normal goat serum [Hangzhou Multisciences (Lianke) Biotech,
Co., Ltd.] in PBS at room temperature for 10 min, after which the
serum was removed but slices were not washed. Next, the mouse
anti-human α-SMA monoclonal antibody (1:50 dilution; cat. no.
GA085; Dako; Agilent Technologies, Inc.) was added and the slices
were incubated for 2 h at 37˚C or overnight at 4˚C. Following
rinsing three times with PBS for 5 min, a peroxidase-labeled goat
anti-mouse polyclonal IgG antibody (1:1,000 dilution; cat. no.
AP124ASigma-Aldrich; Merck KGaA) was added and the slices were
incubated at 37˚C for 30 min. This was followed by rinsing with PBS
and staining with diaminobenzidine at -20˚C for 5 min. (Santa Cruz
Biotechnology, Inc.). After washing, re-dyeingwith hematoxylin at
37˚C for 5 min and dehydration, the slides were made transparent
and sealed with neutral gum. Image Pro Plus 6.0 software (Media
Cybernetics, Inc.) was used for analysis of the immunostained
sections. Results werepresented as the area of α-SMA immunostaining
per µm length of the basement membrane of the bronchioles that had
a 150-200 µm internal diameter. In total, ≥10 bronchioles were
randomly selected in each of the slides.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Comparisons among the different groups were performed by
one-way ANOVA (with nonparametric or mixed test) followed by
Tukey's post-hoc test for multiple comparisons (GraphPad Prism
8.0). Statistical comparisons used SPSS 16.0 (SPSS, Inc.).
P<0.05 was considered to indicate statistical significance.
Results
LXRs increase airway
hyperresponsiveness in the chronic model of asthma
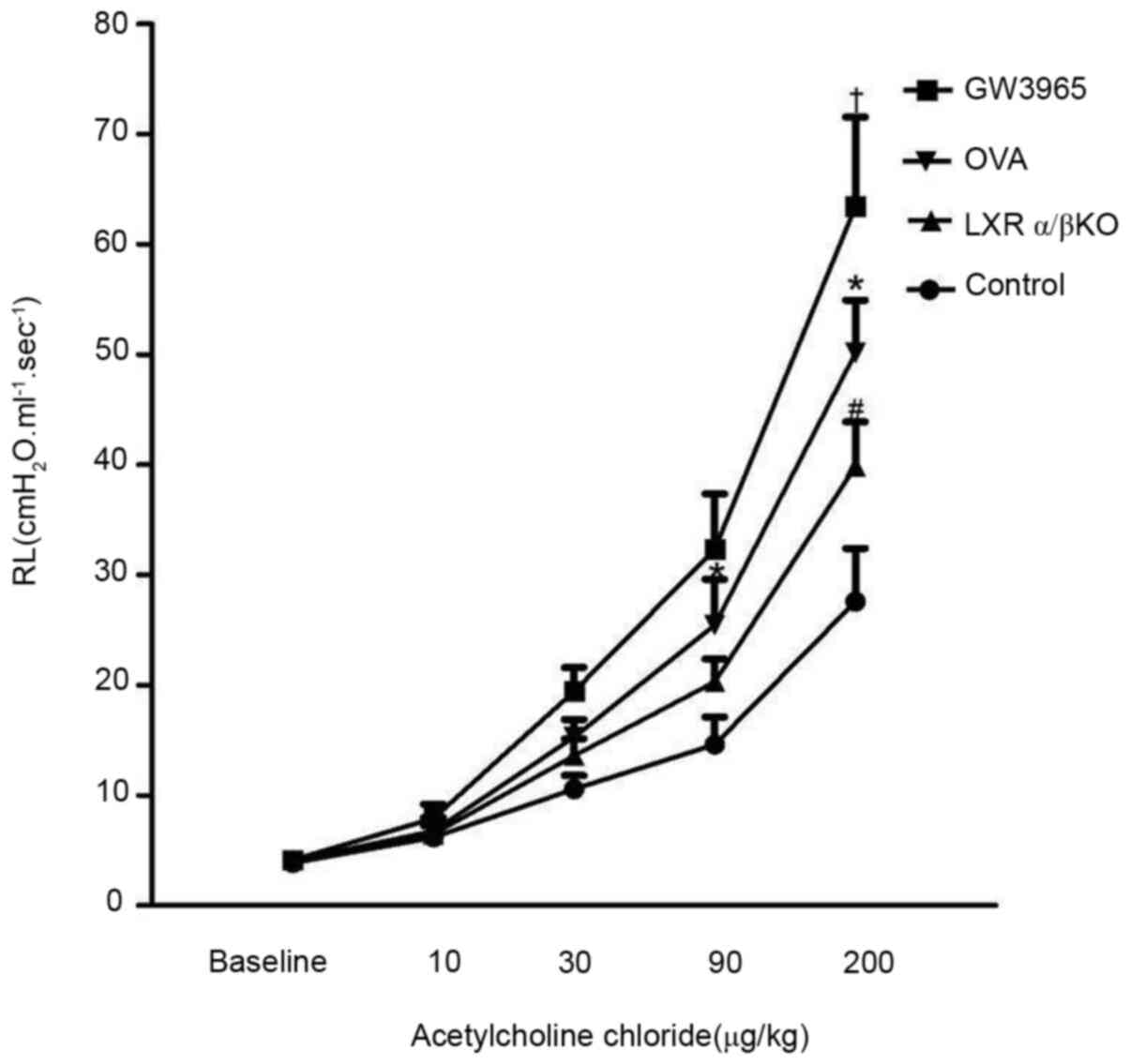
After the administration of the indicated doses of
aerosolized acetylcholine for 20 sec, at 24 h after the last OVA
challenge, lung resistance (RL) was recorded (Fig. 1). There was no difference in basic
airway resistance between the groups. Administration of
acetylcholine induced a concentration-dependent increase in RL in
each group. When the stimulation dose reached 200 µg/kg, the airway
resistance was higher in the GW3965 group compared with that in the
OVA group (P<0.05). Furthermore, the airway resistance was lower
in the LXRα/β KO group compared with that in the OVA group
(P<0.05). However, there was no decrease in the RL of
LXRα-/- and LXRβ-/- mice compared with that
in the mice in the OVA group (data not shown).
Effect of LXRs on lung histology in
the chronic model of asthma
Rare inflammatory cells were observedaround the
airways in the control group. Furthermore, mice in the OVA group
presented with severe infiltration of inflammatory cells around the
respiratory tract and mice in the GW3965 group presented with a
marked increase in inflammatory cells around the airways. In
addition, LXRα-/- mice and LXRβ-/- mice did
not exhibit anyreduced OVA-induced inflammation of the airways. It
was indicated that LXRα/β KO mice presented with reduced
inflammation of the airways compared with mice in the OVA group
(Fig. 2A-F).
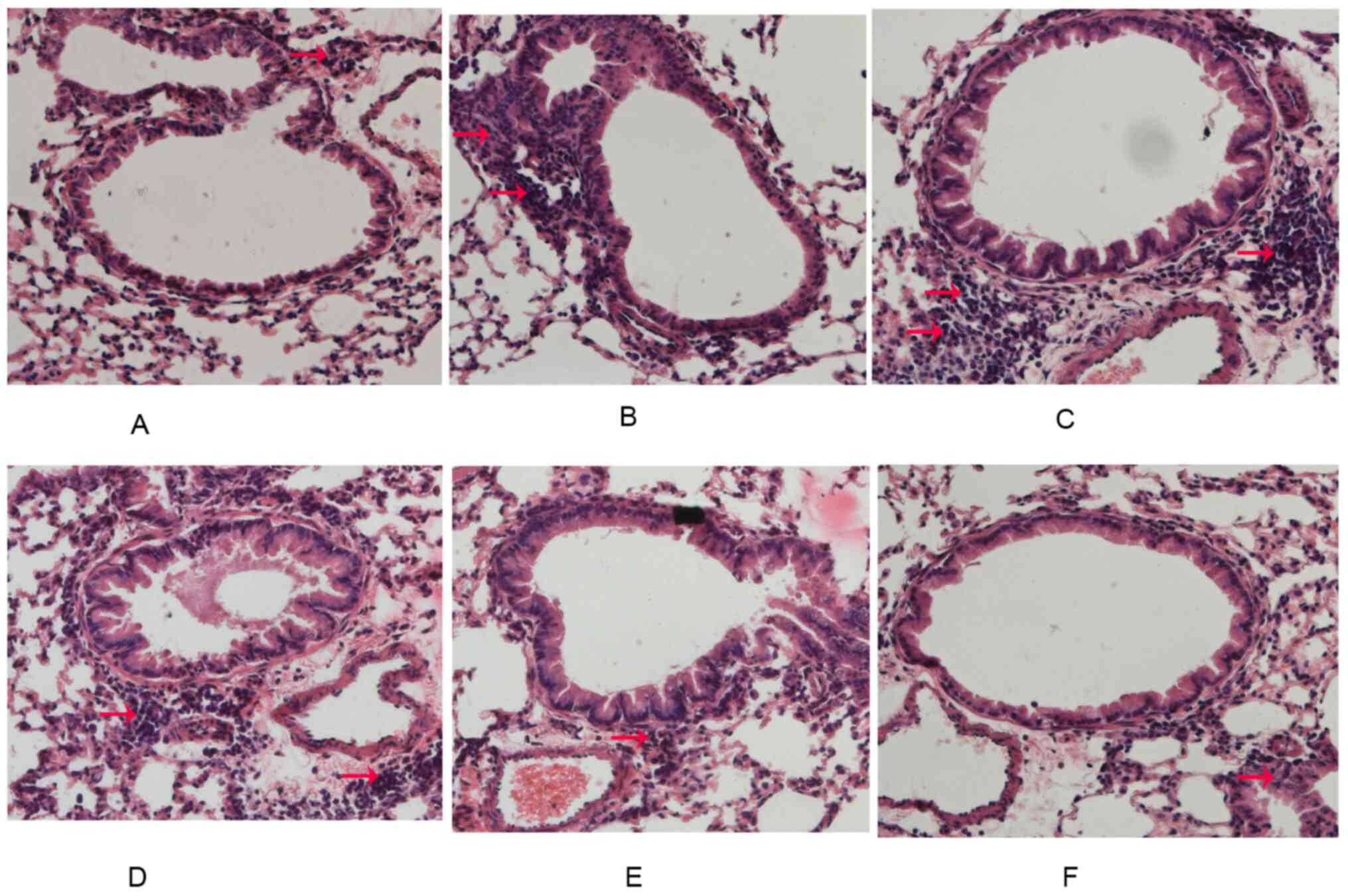 | Figure 2LXR-deficient, chronic asthmatic mice
display reduced airway inflammation. After the last OVA challenge,
lung tissues were fixed, sectioned and stained with H&E. The
inflammatory cells around the airways and vessels were observed by
pathologists (red arrows). Representative images for the (A)
Control group, (B) OVA group, (C) GW3965 group, (D)
LXRα-/- group, (E) LXRβ-/- group and (F)
LXRα/β KO group (magnification, x200). LXR, liver X receptor; OVA,
ovalbumin; KO, knockout. |
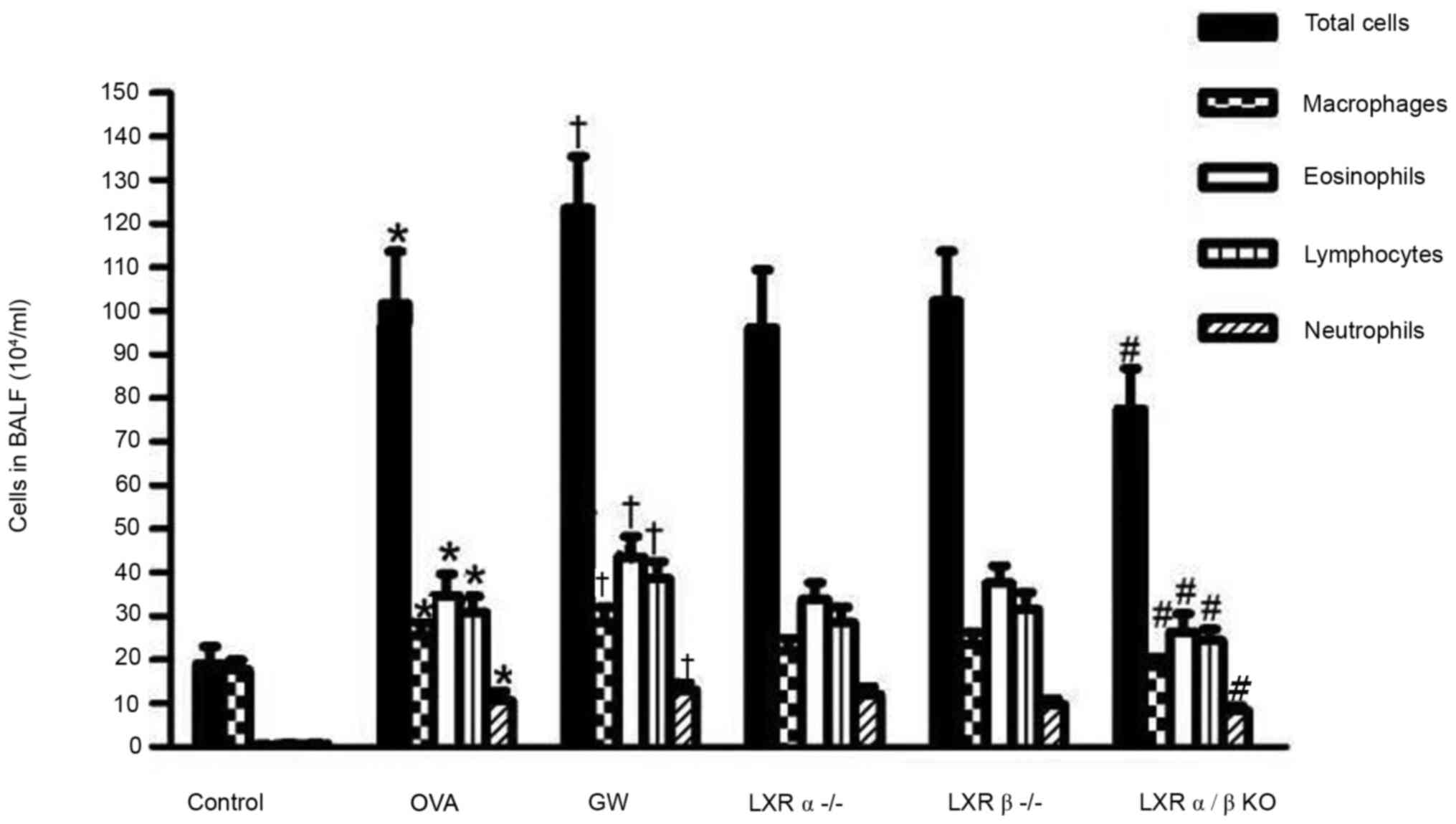
Effect of LXRs on inflammatory cells
in the BALF of the chronic model of asthma
Following sensitization and treatment challenges,
the total number of leukocytes, as well as macrophages,
eosinophils, lymphocytes and neutrophils in the BALF of the OVA
group were significantly increased compared with those in the
control group. It was indicated that treatment with GW3965
significantly enhanced the number of eosinophils and lymphocytes in
the BALF compared with those in the OVA group. Furthermore, LXRα/β
KO mice had fewer total leukocytes, macrophages, lymphocytes and
eosinophils as compared with mice in the OVA group. However, there
was no significant depression of inflammatory cells in the BALF of
LXRα-/- mice and LXRβ-/- mice compared with
those in the OVA group (Fig.
3).
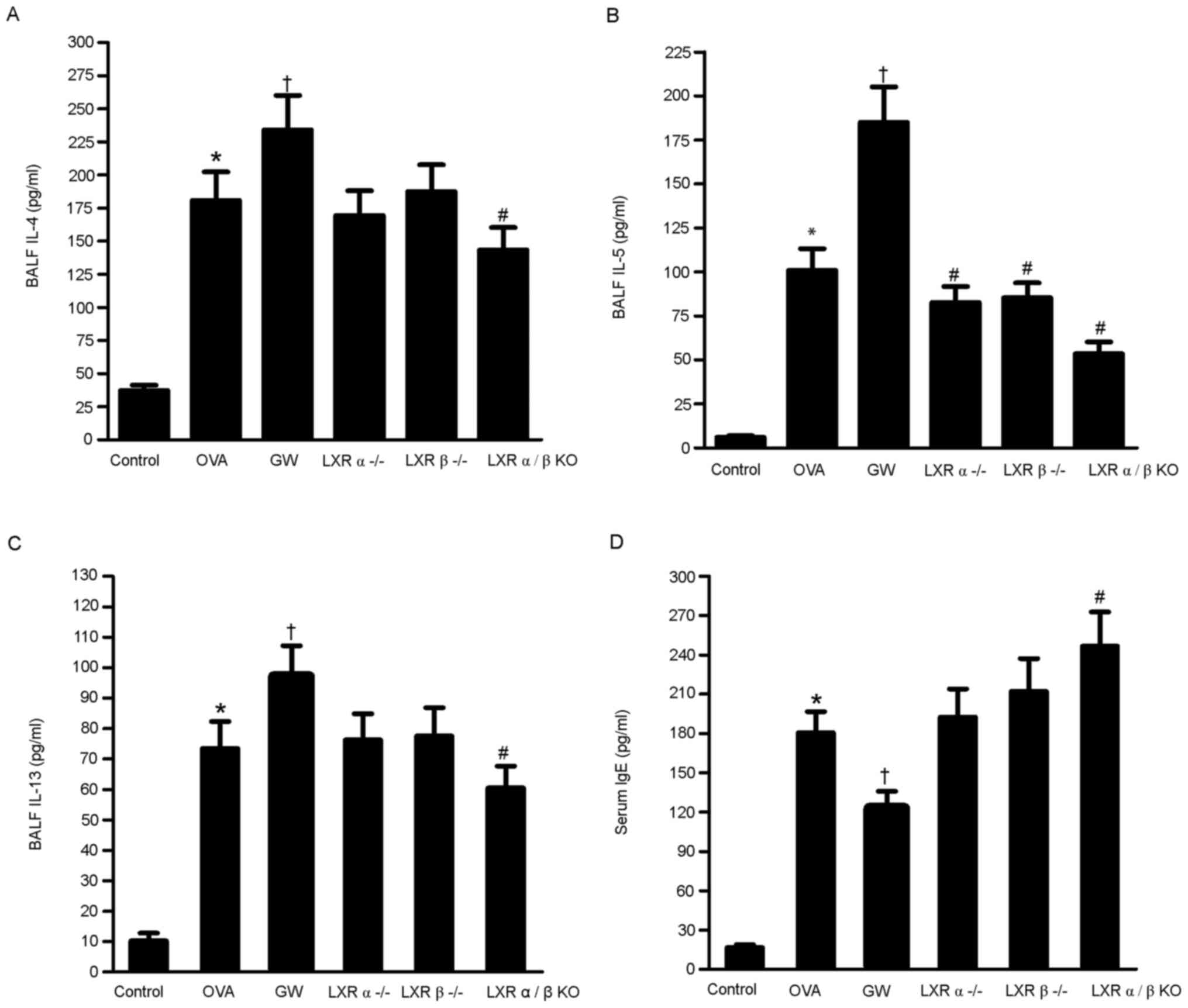
Effect of LXRs on IL-4, IL-5 and IL-13
levels in the BALF and IgE levels in serum of the chronic model of
asthma
The levels of IL-4, IL-5 and IL-13 in the BALF and
the levels of IgE in serum were quantified using ELISAs. The levels
of IL-4, IL-5 and IL-13 in the BALF were significantly higher in
the OVA group compared with those in the control group.
Furthermore, the levels of the inflammatory cytokines were higher
in the GW3965 group compared with those in the OVA group. However,
the levels of these inflammatory cytokines were lower in the LXRα/β
KO group compared with those in the OVA group. Compared with the
control group, the OVA, GW3965 and LXR α/β KO groups had
significantly higher IgE levels. The IgE levels in the LXR α/β KO
group were significantly higher compared with those in the OVA
group, while the GW3965 group had significantly lower levels
(Fig. 4A-D).
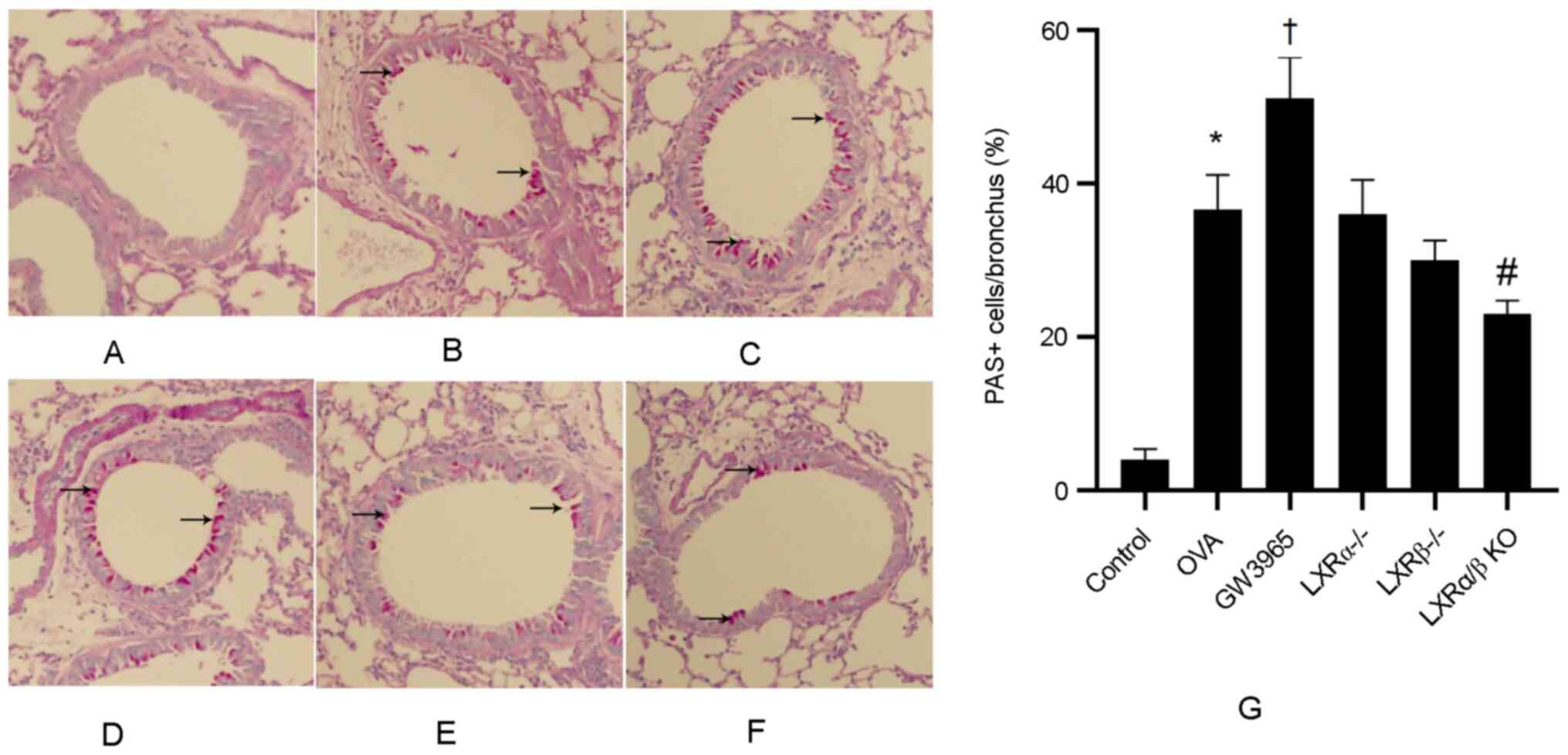
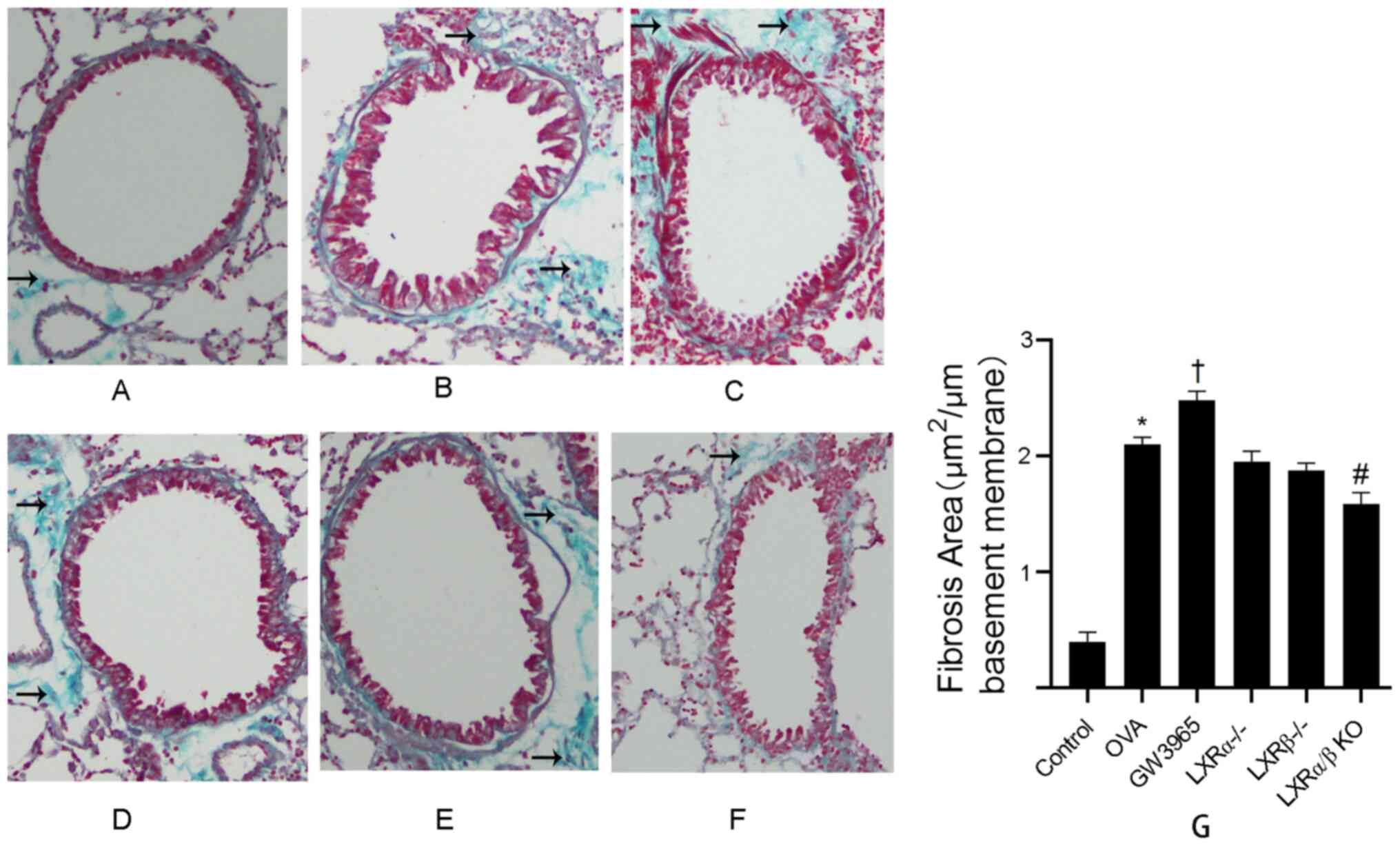
LXRs inhibit airway remodeling in
chronic experimental asthma
The level of airway metaplasia and mucus production
was assessed via PAS staining of lung tissues (Fig. 5A-G). There was a low number of
PAS-positive goblet cells in the control group, while the number of
PAS-positive goblet cells was markedly increased in the OVA group
and further increased in the GW3965 group. In addition, the number
of PAS-positive goblet cells was significantly lower in the LXRα/β
KO group compared with that in the OVA group.
The effect of the LXR agonist GW3965 on the area of
collagen deposition around the duct wall was determined using
Masson's trichrome staining analysis of lung tissues (Fig. 6A-G). In the control group, collagen
deposition around the airways was minimal. The results demonstrated
that the collagen deposition around the airways of mice in the OVA
group was significantly increased compared with that in the control
group (P<0.05). In addition, compared with that in the OVA
group, the collagen deposition area was increased in the GW3965
group (P<0.05), while this area was decreased in the LXRα/β KO
group (P<0.05).
The results further demonstrated that α-SMA
expression was increased in the peribronchiolar region of
OVA-challenged mice compared with that in control mice. In
addition, administration of GW3965 markedly increased the area of
the α-SMA-stained smooth muscle layer compared with that in the OVA
group. However, the area of smooth muscle layer stained with α-SMA
was reduced in the LXRα/β KO group as compared with that in the OVA
group (Fig. 7A-G). These results
indicated that LXRs maypromote airway remodeling.
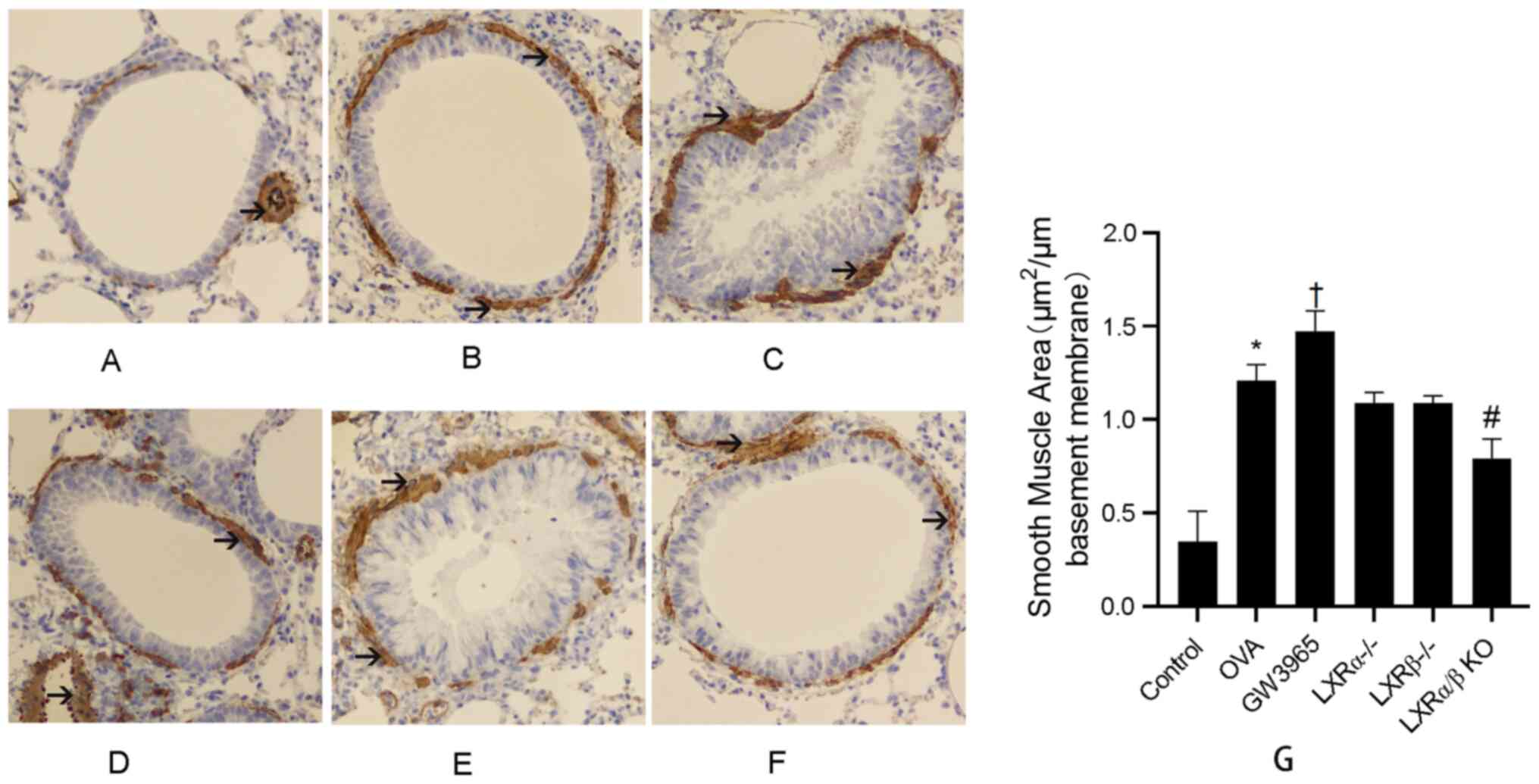 | Figure 7LXR-deficient, chronic asthmatic mice
have a smaller area of α-SMA-stained smooth muscle layer. The area
of α-SMA-stained smooth muscle layer (brown stained) was examined
using immunostaining analysis of lung tissues. Black arrows
indicate theimmunostained areas around bronchioles and vessels.
Blue stain indicates smooth muscle cells. Representative images for
the (A) Control group, (B) OVA group, (C) GW3965 group, (D)
LXRα-/- group, (E) LXRβ-/- group and (F)
LXRα/β KO group (magnification, x200). (G) Smooth muscle area for
each group. *P<0.05 OVA group vs. control group;
†P<0.05 GW3965 group vs. OVA group;
#P<0.05 LXR-deficient groups vs. OVA group. LXR,
liver X receptor; OVA, ovalbumin; KO, knockout; α-SMA, α-smooth
muscle actin. |
Discussion
The present study investigated the effect of LXRs on
the pathophysiology of asthma by establishing an LXR-deficient
mouse model of chronic allergic asthma. The influence of LXRs on
the pathophysiological process of chronic asthma was
comprehensively evaluated by detecting airway inflammation, airway
hyperreactivity and airway remodeling.
The synthetic agonist used in the present study was
GW3965, which is a highly LXR-specific ligand. GW3965 increased the
eosinophilic airway inflammation by inducing high secretory levels
of Th2 cytokines. A previous study reported that LXR activation is
able to directly increase the transcription of IL-5 by improving
the promoter activity (23).
However, to the best of our knowledge, there are no previous
studies suggesting that LXRs are able to increase the transcription
of IL-4 and IL-13. The present study demonstrated that activation
of LXRs led to the production of IL-4 and IL-13 in the airways of
model mice with chronic asthma. These results indicated that LXRs
may affect T-cell function by increasing the Th2 cytokine
response.
An important feature of allergic asthma is the
increase in IgE levels in the serum. IgE-mediated type I allergy is
the primary mechanism of the asthma response and leads to airway
hyperresponsiveness (24),
particularly when asthmatic patients are exposed to allergens.
Allergens are captured by antigen-presenting cells and are
presented to T lymphocytes, which is a process that requires a
suitable Th2 cytokine environment to enable the B-cell type
conversion to a type that produces IgE (25-27).
Both IL-4 and IL-13 are able to transpose the mRNA
of B cells, thereby causing the transformation of B cells and the
production of IgG4 and IgE with the appropriate co-stimulus
signals. The production of IgE is largely dependent on T-cell
synthesis, while basophils and mast cells are able to express CD40
ligand (CD40L), interact with B cells and regulate IgE synthesis in
the absence of endogenous IL-4 and IL-13(28). Therefore, basophils and mast cells
are able to induce IgE syngenesis without T cells, which is another
important pathway for IgE synthesis (29-31).
It has been indicated that LXR activation limits anti-CD40 and
IL-4-induced differentiation of B cells into IgE-secreting
plasmablasts and this may be associated with the reduced
phosphorylation of JNK and the increased membrane expression of
CD23(25). In the present study, an
appropriate dose of LXR agonist was able to effectively reduce
serum IgE levels. On the basis that binding to LXR agonists
increased Th2 cytokines, it was suggested that LXR agonists may
inhibit IgE production by inhibiting the non-T-cell-dependent
pathways and this observation was in line with previous studies
(18,32).
Acetylcholine is a type of drug that causes
bronchoconstriction and increases airway resistance when inhaled.
The present study used different doses of acetylcholine to
stimulate the airways. The results demonstrated that LXRs were able
to increase airway hyperreactivity. This finding maybe explained by
the increase in airway inflammation caused by LXRs.
The specific manifestations of airway remodeling
include epithelial injury, hypertrophy of subepithelial fibrous
mucous glands, myofibroblast, smooth muscle cell proliferation and
angiogenesis (33). In the present
study, when comparing the LXR agonist group with the asthma group,
the aforementioned changes were more obvious. It was identified
that airway remodeling was decreased in LXRα/β KO mice, which
indicated that LXRs promote airway remodeling during chronic
asthma. While LXRs were previously indicated to
exertanti-proliferative effects on airway smooth muscle in
vitro (16), the role of LXRs
in mice with chronic asthma appeared to be more complex.
LXRs serve a key role in lipid metabolism and
exhibit anti-inflammatory roles in a number of animal models,
including those of atherosclerosis and acute lung injury (34). In the present study, it was observed
that LXRs aggravated antigen-induced eosinophilic airway
inflammation in mice with chronic asthma. However, asthma is a
heterogeneous disease with multiple phenotypes (1), and there are numerous non-allergic
asthmatic patients with neutrophilic airway inflammation (1,35). It
is suggested that LXRs may have favorable inhibitory effects on
airway inflammation in non-allergic asthma; however, further
research is required.
In conclusion, the present study demonstrated that
LXRs promote airway remodeling in allergic chronic asthma and this
may be a promising target for allergic asthma treatment. Inhibition
of LXR overactivation mayreduce allergic airway inflammation and
airway hyperresponsiveness, as well as decrease airway remodeling.
Therefore, inhibiting LXRs may be a potential treatment for
allergic asthma.
Acknowledgements
Not applicable.
Funding
Funding: The study was supported by the Nanjing Medical Science
and Technique Development Foundation (grant no. JQX16028).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS, LY and WG designed the experiments. JZ, ZW and
FY performed the experiments. JZ, FY and LW collected and analyzed
data. ZW and FY sacrificed mice and performed the ELISAs. FY and YT
took samples and performed immunohistochemistry. JZ and YT checked
and approved the authenticity of all the raw data. The manuscript
was written by JZ and YS. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present experiment was approved by The
Institutional Animal Care and Use Committee of Nanjing First
Hospital Affiliated to Nanjing Medical University Animal
Center.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boonpiyathad T, Sözener ZC, Satitsuksanoa
P and Akdis CA: Immunologic mechanisms in asthma. Semin Immunol.
46(101333)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Insuela DBR, Ferrero MR, Coutinho DS,
Martins MA and Carvalho VF: Could arachidonic acid-derived
pro-resolving mediators be a new therapeutic strategy for asthma
therapy? Front Immunol. 11:580–598. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Holgate ST, Arshad HS, Roberts GC, Howarth
PH, Thurner P and Davies DE: A new look at the pathogenesis of
asthma. Clin Sci (Lond). 118:439–450. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kudo M, Ishigatsubo Y and Aoki I:
Pathology of asthma. Front Microbiol. 4(263)2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Russell RJ and Brightling C: Pathogenesis
of asthma: Implications for precision medicine. Clin Sci (Lond).
131:1723–1735. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Birrell MA, De Alba J, Catley MC, Hardaker
E, Wong S, Collins M, Clarke DL, Farrow SN, Willson TM, Collins JL
and Belvisi MG: Liver X receptor agonists increase airway
reactivity in a model of asthma via increasing airway smooth muscle
growth. J Immunol. 181:4265–4271. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Castrillo A and Tontonoz P: Nuclear
receptors in macrophage biology: At the crossroads of lipid
metabolism and inflammation. Annu Rev Cell Dev Biol. 20:455–480.
2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Joseph SB, Castrillo A, Laffitte BA,
Mangelsdorf DJ and Tontonoz P: Reciprocal regulation of
inflammation and lipid metabolism by liver X receptors. Nat Med.
9:213–219. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Castrillo A, Joseph SB, Marathe C,
Mangelsdorf DJ and Tontonoz P: Liver X receptor-dependent
repression of matrix metalloproteinase-9 expression in macrophages.
J Biol Chem. 278:10443–10449. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Smet M, Van Hoecke L, De Beuckelaer A,
VanderBeken S, Naessens T, Vergote K, Willart M, Lambrecht BN,
Gustafsson JÅ, Steffensen KR and Grooten J: Cholesterol-sensing
liver X receptors stimulate Th2-driven allergic eosinophilic asthma
in mice. Immun Inflamm Dis. 4:350–361. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Bruemmer D, Collins AR, Noh G, Wang W,
Territo M, Arias-Magallona S, Fishbein MC, Blaschke F, Kintscher U,
Graf K, et al: Angiotensin II-accelerated atherosclerosis and
aneurysm formation is attenuated in osteopontin-deficient mice. J
Clin Invest. 112:1318–1331. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Ogawa D, Stone JF, Takata Y, Blaschke F,
Chu VH, Towler DA, Law RE, Hsueh WA and Bruemmer D: Liver x
receptor agonists inhibit cytokine-induced osteopontin expression
in macrophages through interference with activator protein-1
signaling pathways. Circ Res. 96:e59–e67. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Solan PD, Piraino G, Hake PW, Denenberg A,
O'Connor M, Lentsch A and Zingarelli B: Liver X receptor α
activation with the synthetic ligand T0901317 reduces lung injury
and inflammation after hemorrhage and resuscitation via inhibition
of the nuclear factor κB pathway. Shock. 35:367–374.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang D, Liu M, Wang Y, Luo M, Wang J, Dai
C, Yan P, Zhang X, Wang Y, Tang C and Xiao J: Synthetic LXR agonist
T0901317 attenuates lipopolysaccharide-induced acute lung injury in
rats. Int Immunopharmacol. 11:2098–2103. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
August A, Mueller C, Weaver V, Polanco TA,
Walsh ER and Cantorna MT: Nutrients, nuclear receptors,
inflammation, immunity lipids, PPAR, and allergic asthma. J Nutr.
136:695–699. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lee KS, Park SJ, Hwang PH, Yi HK, Song CH,
Chai OH, Kim JS, Lee MK and Lee YC: PPAR-gamma modulates allergic
inflammation through up-regulation of PTEN. FASEB J. 19:1033–1035.
2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zelcer N and Tontonoz P: Liver X receptors
as integrators of metabolic and inflammatory signaling. J Clin
Invest. 116:607–614. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Shi Y, Xu X, Tan Y, Mao S, Fang S and Gu
W: A liver-X-receptor ligand, T0901317, attenuates IgE production
and airway remodeling in chronic asthma model of mice. PLoS One.
9(e92668)2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ogawa S, Lozach J, Benner C, Pascual G,
Tangirala RK, Westin S, Hoffmann A, Subramaniam S, David M,
Rosenfeld MG and Glass CK: Molecular determinants of crosstalk
between nuclear receptors and toll-like receptors. Cell.
122:707–721. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Castrillo A, Joseph SB, Marathe C,
Mangelsdorf DJ and Tontonoz P: Liver X receptor-dependent
repression of matrix metalloproteinase-9 expression in macrophages.
J Biol Chem. 278:10443–10449. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Michael LF, Schkeryantz JM and Burris TP:
The pharmacology of LXR. Mini Rev Med Chem. 5:729–740.
2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhao Z, Xu D, Li S, He B, Huang Y, Xu M,
Ren S, Li S, Wang H and Xie W: Activation of liver X receptor
attenuates oleic acid-induced acute respiratory distress syndrome.
Am J Pathol. 186:2614–2622. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen Y, Duan Y, Kang Y, Yang X, Jiang M,
Zhang L, Li G, Yin Z, Hu W, Dong P, et al: Activation of liver X
receptor induces macrophage interleukin-5 expression. J Biol Chem.
287:43340–43350. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Asayama K, Kobayashi T,
D'Alessandro-Gabazza CN, Toda M, Yasuma T, Fujimoto H, Okano T,
Saiki H, Takeshita A, Fujiwara K, et al: Protein S protects against
allergic bronchial asthma by modulating Th1/Th2 balance. Allergy.
75:2267–2278. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Heine G, Dahten A, Hilt K, Ernst D,
Milovanovic M, Hartmann B and Worm M: Liver X receptors control IgE
expression in B cells. J Immunol. 182:5276–5282. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nunomura S, Endo K, Makishima M and Ra C:
Oxysterol represses high-affinity IgE receptor-stimulated mast cell
activation in Liver X receptor-dependent and -independent manners.
FEBS Lett. 584:1143–1148. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gould HJ and Sutton BJ: IgE in allergy and
asthma today. Nat Rev Immunol. 8:205–217. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Jabara HH, Fu SM, Geha RS and Vercelli D:
CD40 and IgE: Synergism between anti-CD40 monoclonal antibody and
interleukin 4 in the induction of IgE synthesis by highly purified
human B cells. J Exp Med. 172:1861–1864. 1990.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lala DS: The liver X receptors. Curr Opin
Investig. 6:934–943. 2005.PubMed/NCBI
|
|
30
|
Tontonoz P and Mangelsdorf DJ: Liver X
receptor signaling pathways in cardiovascular disease. Mol
Endocrinol. 17:985–993. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen S, Sims GP, Chen XX, Gu YY, Chen S
and Lipsky PE: Modulatory effects of 1,25-dihydroxyvitamin D3 on
human B cell differentiation. J Immunol. 179:1634–1647.
2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hatano Y, Man MQ, Uchida Y, Crumrine D,
Mauro TM, Feingold KR, Elias PM and Holleran WM: Murine atopic
dermatitis responds to peroxisome proliferator-activated receptors
alpha and beta/delta (but not gamma) and liver X receptor
activators. J Allergy Clin Immunol. 125:160–169.e1-e5.
2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Delvecchio CJ, Bilan P, Radford K, Stephen
J, Trigatti BL, Cox G, Parameswaran K and Capone JP: Liver X
receptor stimulates cholesterol efflux and inhibits expression of
proinflammatory mediators in human airway smooth muscle cells. Mol
Endocrinol. 21:1324–1334. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhao L, Lei W, Deng C, Wu Z, Sun M, Jin Z,
Song Y, Yang Z, Jiang S, Shen M and Yang Y: The roles of liver X
receptor α in inflammation and inflammation-associated diseases. J
Cell Physiol. 36:4807–4828. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hanashiro J, Muraosa Y, Toyotome T, Hirose
K, Watanabe A and Kamei K: Schizophyllum commune induces
IL-17-mediated neutrophilic airway inflammation in OVA-induced
asthma model mice. Sci Rep. 9(19321)2019.PubMed/NCBI View Article : Google Scholar
|