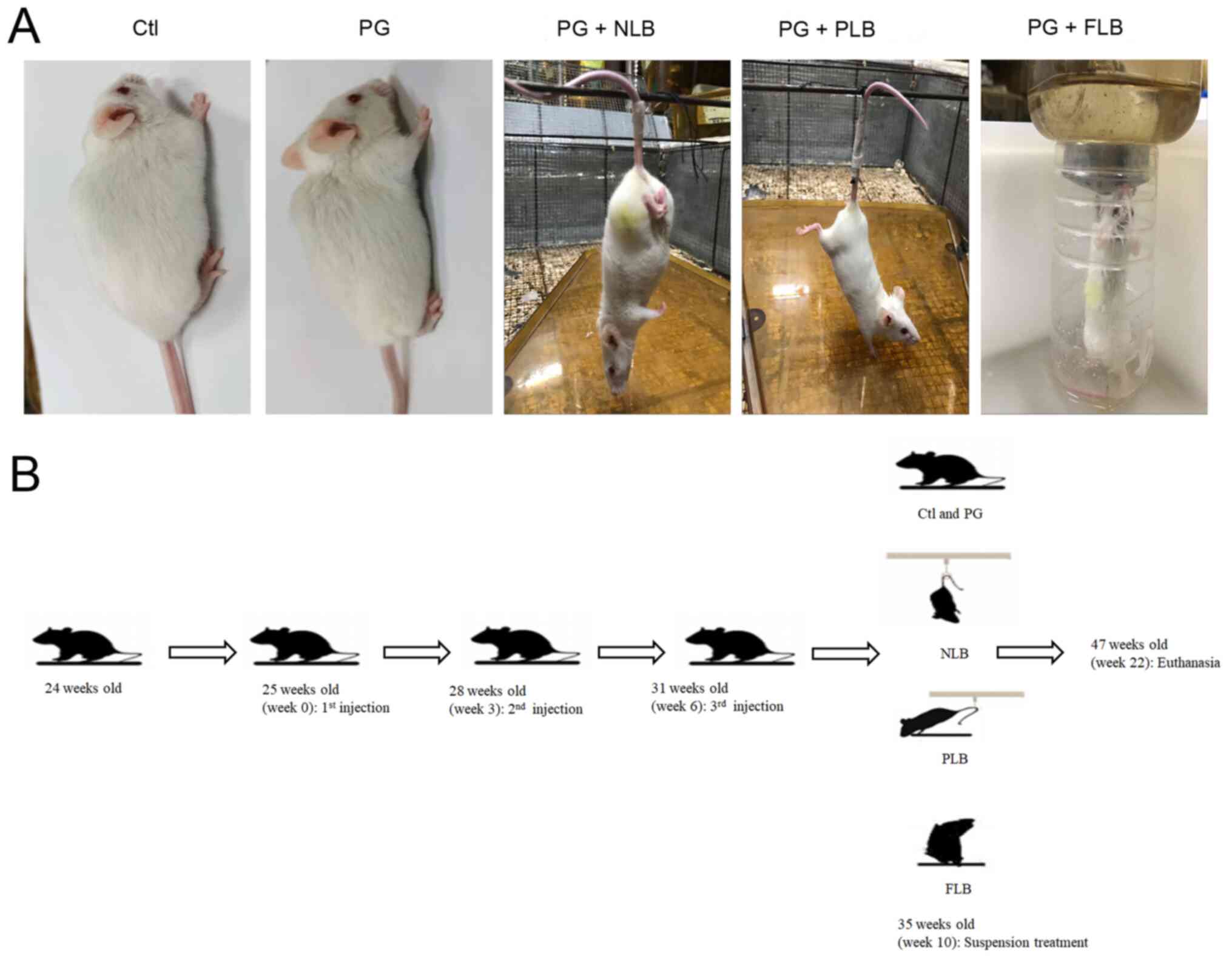
Introduction
Ankylosing spondylitis (AS) is a typical chronic
autoimmune disease characterized by sacroiliac and spinal arthritis
(1). New bone formation at the
sacroiliac joint and spine are diagnostic features of AS (2). In addition to ectopic bone formation,
chronic inflammation and bone erosion are the dominant
pathophysiological manifestations of AS (3,4). In
AS, immune inflammation has been demonstrated to stimulate new bone
formation at the attachment site, causing joint stiffness and
mobility problems (4). The
heterotopic ossification of various attachment points is the
leading cause of disability in patients with AS (5). Excessive axial myofascial stress under
overload conditions increases pain and the occurrence of joint
movement disorders (6). Thus, an
increased mechanical load may be a significant cause of ectopic new
bone formation in AS. Previous studies have shown that mechanical
stress is a major driving factor of ectopic osteogenic
proliferation via the activation of the ρ/ρ-associated
coiled-coil-containing protein kinase (ROCK) pathway (7) and the phosphorylation of the
Erk1/2/MAPK signaling pathway (8).
Biomechanical factors also play an notable role in the occurrence
and development of AS, especially when the joints are overworked
(9). When articular bone tissues
are subjected to excessive biomechanical stress, heterotopic
ossification of the cartilage and ligaments surrounding these
tissues can be accelerated (4).
Indeed, inflammation-induced ectopic bone formation requires the
activation of the classical Wnt/β-catenin pathway, which may be
positively associated with heterotopic ossification. (10). Wnt/β-catenin signaling is increased
in patients with AS (11). When the
biomechanical load is increased, the ρ/ROCK pathway can also play a
notable role in activating the Erk1/2 MAPK signaling pathway,
stimulating heterotopic ossification (12).
Treatments for AS include not only drugs, but also
non-pharmacological therapy. Because mechanical load stimulation
plays an important role in disease progression, exercise therapy
allows for training under reduced mechanical load, which may slow
the progression of AS (13). A
previous study reported that therapeutic exercise, such as spa
exercise, can relieve AS symptoms in adults (13). Suspension also appears to be a
promising modality; this method has been regularly applied to
mechanical load reduction experiments (14,15).
In recent years, tail suspension to the point of no load-bearing
has become popular as a microgravity model (16).
MicroRNAs (miRNAs/miRs) are small non-coding RNAs
involved in a wide range of biological regulatory processes; a
number of studies have shown that numerous miRNAs play important
roles in mechanical regulation (14,17)
Under microgravity, the mechanosensory miR-103 is upregulated and
may inhibit bone formation by targeting runt-related transcription
factor 2 (Runx2) during osteoblast differentiation (18). Coincidentally, miR-103 inhibits
osteoblast proliferation and bone formation mainly by suppressing
the expression of the calcium channel voltage-dependent L type a 1C
subunit, which encodes Cav1.2 under reduced load conditions
(19). Thus, under conditions of
mechanical load reduction, bone formation may be reduced by changes
in miR-103 levels (20). On the
other hand, activating the Wnt/β-catenin signaling pathway promotes
bone formation (21). In AS, the
level of dickkopf Wnt signaling pathway inhibitor 1 (DKK1), an
inhibitor of the Wnt pathway, is reduced, causing excessive
cartilage and bone formation (22).
It is unclear whether reducing mechanical load via suspension
alleviates the heterotopic ossification of the sacroiliac joint in
AS by inhibiting the Wnt/β-catenin pathway through miR-103
expression.
The present study aimed to explore the pathogenesis
of mechanical load in AS disease and to investigate whether
reducing mechanical load, such as suspension, could delay the
heterotopic ossification of AS mice. To the best of our knowledge,
this is the first study to investigate the therapeutic effect and
mechanism of suspension for AS.
Materials and methods
Animal modeling and suspension for
mechanical load reduction intervention
All animal experiments were performed following The
Laboratory Animal Care and Use Guidelines of Southern Medical
University (23). The experimental
scheme was approved by The Institutional Animal Care and Use
Committee of Southern Medical University (Guangzhou, China). The
reduction of animal suffering during experiments was ensured by
continuously monitoring the health status of the animals and
applying humane endpoints. The body weight of the experimental
animals was measured every month till the end of the experiment. A
20% loss in body weight over any time period was the humane
endpoint criterion used in accoradance with the guidance of
Institutional Animal Care and Use Committees (IACUCs) (24).
A total of 50 female specific pathogen-free BALB/c
mice (24-week-old; 25.02±3.36 g; Beijing Vital River Laboratory
Animal Technology Co., Ltd.) were used in the present study. The
proteoglycan (PG)-induced spondylitis (PGIS) mouse model was used
(19,20). When the BALB/c mice were 25 weeks
old (week 0), they received an intraperitoneal (i.p.) injection
with 100 mg of bovine PG (MilliporeSigma) (25,26) as
the antigen, combined with Freund's complete adjuvant
(MilliporeSigma) in a 1:1 (vol:vol) ratio. Subsequently, the same
amount of antigen was injected at weeks 3 and 6(27). The blank control mice were injected
with the same volume of Freund's adjuvant. The mice were maintained
in an air-conditioned room (60±5% relative humidity) with a 12-h
light/dark cycle. Water and food were offered ad libitum and
the room temperature was maintained at 22-24˚C with adequate
ventilation. After one week of adaptive feeding, female BALB/c mice
were randomly assigned to each of the five experimental groups
(n=10/group), including: i) The mice without PGIS induction
(control group); ii) the mice with PGIS induction (PG group); iii)
the PGIS mice with tail suspension and no load-bearing (NLB)
treatment (PG + NLB group); iv) the PGIS mice with tail suspension
and forelimbs touching the floor with the torso ~60˚ from the
ground to achieve a partial load-bearing (PLB) treatment (PG + PLB
group); and v) the PGIS mice subjected to vertical full
load-bearing (FLB) intervention, with a small amount of water
beneath them so that they remained upright to avoid having their
abdomens touching the water (PG + FLB group; Fig. 1).
The experiment was initiated at week 10 after PG
induction, followed by continuous intervention for 12 weeks for 6
h/day. The reduced mechanical load experiments were based on
studies that observed simulating microgravity could cause bone loss
(28,29). The experiment lasted 22 weeks, and
all the mice were euthanized at the end (Fig. 1). CO2 euthanasia was
performed using an in-house-designed euthanasia system with a rate
of 30% of the chamber volume/min.
microCT analysis
At week 12 of PGIS model induction, the mice in the
model and control groups were anesthetized via i.p. injection of
sodium pentobarbital (50 mg/kg). microCT imaging and histological
analysis was performed to assess the bone morphology in the PGIS
mice and to observe the typical characteristics of these mice.
Scans of the sacroiliac joint were conducted using a microCT
instrument (SCANCO Medical AG) with 80 kV scanning voltage, 180 µA,
9 W and 12-µm scan thickness. For the sacroiliac joint, a 3.1-mm
region consisting of 209 slices at the center of the joint were
scanned at 15 µm nominal voxel size. The two-dimensional images of
the joint center were reconstructed using the software in the
microCT system. The outcome variables were also analyzed using the
microCT.
Histology
After PGIS modeling and experimental interventions,
the PG-induced mice were sacrificed. The sacroiliac joint tissue
was collected, fixed in 4% paraformaldehyde for 24-48 h at 4˚C and
decalcified for 3-4 weeks (30).
Next, the tissues were embedded in paraffin and cut into 5-µm
sections. The sections were then dewaxed with xylene and gradient
alcohol (100, 95, 90, 80 and 70%), and stained with hematoxylin and
eosin (cat. no. G1120; Beijing Solarbio Science & Technology
Co., Ltd.) at room temperature for 30 min. Hematoxylin and eosin
(H&E) staining of the sacroiliac joint demonstrated the typical
characteristic changes in the AS model mice (31). Three discontinuous fields of per
sample were captured at a magnification of x100-200 using a digital
light microscope (Nikon ECLIPSE Ti-S; Nikon Corporation). The
percentage of hypertrophic cartilage-like cells was quantified
using ImageJ software v.1.51, (National Institutes of Health).
Transfection
The sequence for miR-103 mimic was
5'-AGCAGCAUUGUACAGGGCUAUGA-3', and the nonsense sequence for
miR-103 mimic negative control (NC-mimic) was
5'-UUCUCCGAACGUGUCACGUTT-3'. The sequence for miR-103 inhibitor was
5'-TCATAGCCCTGTACAATGCTGCT-3' and the nonsense sequence for miR-103
inhibitor negative control (NC-inhibitor) was
5'-CAGUACUUUUGUGUAGUACAA-3'. The sequences were all designed and
synthesized by Guangzhou RiboBio Co., Ltd. The 293T cells (China
Center for Type Culture Collection) were transfected with 100
nmol/l miR-103 mimic or NC-mimic using Lipofectamine®
3000 transfection reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The 293T cells were cultured in DMEM (HyClone; Cytiva) with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific Inc.) at
37˚C and 5% CO2 for 24 h. For miR-103 inhibitor groups,
the 293T cells were transfected with 100 nmol/l miR-103 inhibitor
and NC-inhibitor using Lipofectamine® 3000 transfection
reagent for 12 h at 37˚C. The medium was changed after 12 h and the
cells were incubated for additional 2 days.
Dual luciferase assay
The microRNA.org
site (http://www.microrna.org/; v.3.0)
predicted that a binding site for hsa-miR-103 was contained within
the 3'-untranslated region (UTR) of the Rock1 mRNA. A 3'-UTR
luciferase reporter plasmid psiCHECK-2 (Promega Corporation) was
constructed for Rock1, and DNA was extracted using a QIAGEN
Plasmid kit (QIAGEN GmbH) to obtain endotoxin-free plasmid and
stored at -20˚C until use. 293T cells were co-transfected with 20
ng reporter expressing the Rock1-wild-type (WT) 3'-UTR and
100 nmol/l miR-103 mimics using Lipofectamine® 3000
transfection reagent (Invitrogen; Thermo Fisher Scientific Inc.). A
Rock1-mutant (Mu) recombinant vector was used as a positive
control. The target genes and hsa-miR-103 were co-transfected into
293T cells using the cationic liposome method. The interaction
between has-miR-103 and the target genes was determined based on
luciferase activity. After transfection for 48 h, the fluorescence
intensity was detected with dual-luciferase reporter assay system
kit (cat. no. K801-200; BioVision, Inc.) and Glomax 20/20
luminometer (Promega, Inc.) according to the manufacturer's
protocols and was normalized to that of Renilla
luciferase.
Reverse transcription-quantitative
(RT-q)PCR
The sacroiliac bone tissues from the experimental
and control groups were flash-frozen upon collection and stored at
-80˚C until total RNA extraction using TRIzol® reagent
(Takara Bio, Inc.). RNA was reversed transcribed into cDNA and
amplified using the systems from Vazyme Biotech Co., Ltd. The cDNA
Synthesis kit (cat. no. FSQ-101; Toyobo Life Science) was used to
perform RT-qPCR using the following conditions: 15 min at 37˚C; 5
min at 98˚C; and 4˚C hold (32).
The RT of miRNA was carried out using the stem-loop method with the
same cDNA Synthesis Kit. qPCR was conducted using SYBR Green Master
Mix (cat. no. QPK-201; Toyobo Life Science) (32) for the target osteogenic genes
(Table I). The thermocycling
conditions used were as follows: 95˚C for 60 sec, followed by 40
cycles at 95˚C for 15 sec, 60˚C for 15 sec, and 72˚C for 45 sec.
The gene expressions were quantified using the comparative
threshold cycle (2-ΔΔCq) method (33) and the relative mRNA levels were
normalized to the level of Gapdh mRNA. The small nuclear RNA
U6 was used as a control for miRNA samples.
 | Table IPrimers used for quantitative
PCR. |
Table I
Primers used for quantitative
PCR.
| Gene name | Forward, 5'-3' | Reverse, 5'-3' |
|---|
| Bmp2 |
TCTTCCGGGAACAGATACAGG |
TGGTGTCCAATAGTCTGGTCA |
| Runx2 |
GACTGTGGTTACCGTCATGGC |
ACTTGGTTTTTCATAACAGCGGA |
| Bglap |
GAAGCCCAGCGGTGCA |
CACTACCTCGCTGCCCTCC |
| Rock1 |
GACTGGGGACAGTTTTGAGAC |
GGGCATCCAATCCATCCAGC |
| miR-103 |
GCGAGCAGCATTGTACAGGG |
AGTGCAGGGTCCGAGGTATT |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
| Gapdh |
TCCACCACCCTGTTGCTGTA |
ACCACAGTCCATGCCATCAC |
IHC
After the PGIS mice underwent 12 weeks of
intervention with different mechanical loads, they were sacrificed
using CO2. The tissues surrounding the sacroiliac joints
were harvested to preserve their integrity. The samples were fixed
in 4% paraformaldehyde at 4˚C for 24 h, decalcified in EDTA
solution for 4 weeks, dehydrated in xylene and descending ethanol
(100, 95, 90, 80 and 70%), paraffin-embedded and cut into 4-µm
sections. For anti-DKK1 IHC, antigen retrieval was performed using
proteinase K at 20 µg/ml and room temperature for 15 min. After the
quenching of endogenous peroxidase with 3%
H2O2 (cat. no. AR1108; Wuhan Boster
Biological Technology, Ltd.), the slides were blocked in TNB buffer
(Perkin-Elmer, Inc.) at 37˚C for 30 min and stained with the
anti-DKK1 antibody (1:200; cat. no. ab61275, Abcam) for 1 h at room
temperature. The sections were washed and incubated with
HRP-coupled goat anti-mouse IgG antibodies (1:500; cat. no. BM3894;
Wuhan Boster Biological Technology, Ltd.) at for 30 min at 37˚C;
their signals were amplified using tyramide signal amplification.
HRP detection was performed using 3,3'-diaminobenzidine (DAB kit;
Invitrogen; Thermo Fisher Scientific Inc.) at room temperature for
2-3 min. The slides were briefly counterstained with hematoxylin at
room temperature for 1 min before mounting. Three discontinuous
images per sample were captured at a magnification of x100-200
using a digital light microscope (Nikon ECLIPSE Ti-S; Nikon
Corporation). The percentage DKK1-positive cells was quantified
using ImageJ software v.1.51 (National Institutes of Health)
(34).
Western blotting
The ligaments in the sacroiliac joint obtained from
the five different groups and were flash-frozen in liquid nitrogen.
RIPA lysis and extraction buffer (cat. no. 89901; Thermo Fisher
Scientific, Inc.) containing 50X protease phosphatase inhibitor mix
(cat. no. P1045; Beyotime Institute of Biotechnology) was used to
lyse the ligament tissues. The samples were then centrifuged at
10,000 x g and 4˚C for 10 min to collect the supernatant, which
contained the target proteins. A bicinchoninic acid assay kit (cat.
no. KGP902; Nanjing KeyGen Biotech Co., Ltd.) was used to detect
and adjust protein concentrations.
A total of 10 µg/lane target protein were separated
via 10-15% SDS-PAGE gels and transferred to a polyvinylidene
difluoride membrane. The membrane was blocked with 5% BSA (Wuhan
Boster Biological Technology, Ltd.) at room temperature for 1 h and
incubated with primary antibodies against ROCK1 (1:1,000; cat. no.
ab134181), β-catenin (1:1,000; cat. no. ab32572), DKK1 (1:1,000;
cat. no. ab61275), β-tubulin (1:1,000; cat. no. ab6046), or Erk1/2
(1:1,000; cat. no. ab17942; all Abcam) or phosphorylated (p-)Erk1/2
(1:1,000; cat. no. 4377; Cell Signaling Technology, Inc.) at 4˚C
overnight. Subsequently, the membrane was incubated with the
appropriate horse-radish peroxidase (HRP)-conjugated Goat
Anti-Rabbit IgG secondary antibodies (1:5,000; cat. no. BM3894;
Wuhan Boster Biological Technology, Ltd.) for 1 h at room
temperature. The level of β-tubulin was used as a standard internal
control. Finally, an enhanced chemiluminescence detection system
(Thermo Fisher Scientific Inc.) and ChemiDoc Touch Imaging System
(Bio-Rad Laboratories, Inc.) were used to identify the relative
levels of proteins.
Statistical analysis
Each experiment was performed in triplicate and data
were expressed as the mean ± SD. The statistically significant
differences between experimental groups were determined using
GraphPad Prism v6.02 (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Analysis of the relative protein levels and gene expression among
the experimental groups was conducted using one-way ANOVA followed
by multiple comparisons with Bonferroni's post hoc test.
Results
Different mechanical interventions
cause varying histopathological changes in AS mice
Representative images of different groups in an AS
animal model and mechanical interventions are presented below
(n=10/group; Fig. 1). The H&E
and microCT results indicated the typical characteristic changes of
the AS model. Sacroiliac inflammation and hyperplasia in PG-induced
mice were noticeable compared with the control group, manifesting
as infiltration of inflammatory cell and synovial cells, bone
erosion, gap fusion between the bone and cartilage, and significant
hypertrophy of cartilage-like cells (Fig. 2A). Furthermore, representative
high-resolution microCT images of the sacroiliac spine joint of
mice demonstrated that, in the AS model, the sacroiliac space was
narrowed and the edges were unclear. The sacroiliac joint bone
erosion and joint fusion in tail suspension groups (PG + NLB and PG
+ PLB) were partly relieved compared with the PG group (Fig. 2B); however, the vertical full
load-bearing group (PG + FLB) exhibited aggravated properties. The
bone morphometry parameters (BV/TV, Tt.Ar, Ct.Ar and Ct.Th)
indicated that bone formation induced by PG could be alleviated by
suspension therapy, but exacerbated by full load bearing treatment
(Fig. 2B). Taken together, these
results suggested a role for mechanical interventions in AS.
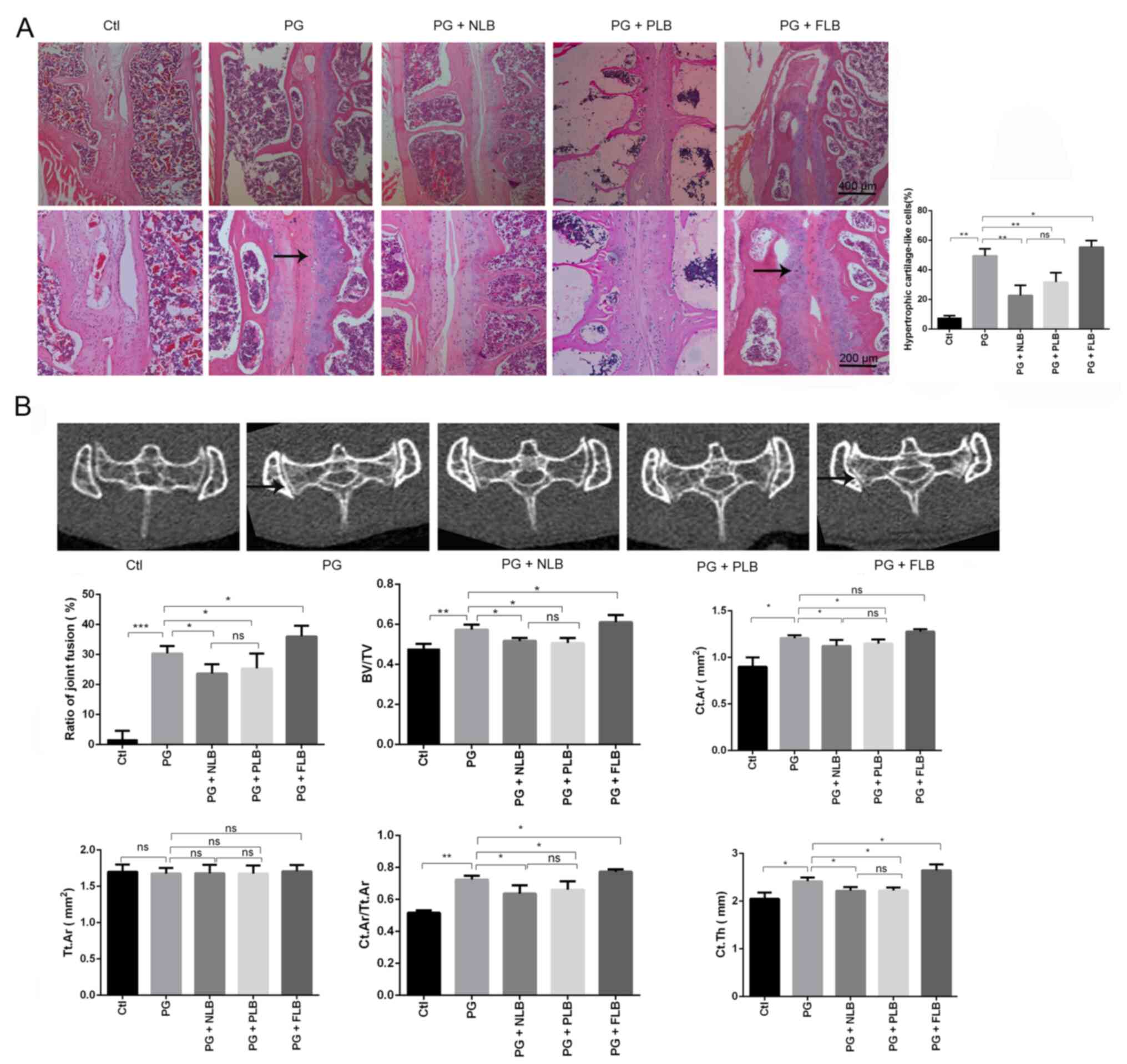 | Figure 2Mechanical load reduction relieves
the typical characteristic changes of the AS model. (A) Ctl:
H&E Staining of the sacroiliac joint of control group
demonstrated little inflammatory infiltration. PG: Representative
H&E staining of the sacroiliac joints of AS mice demonstrated
synovitis, cartilage hyperplasia and bone erosion. Local bone and
hypertrophic cartilage hyperplasia filled the entire joint space
(arrow; n=4). PG + NLB/PLB: Bone erosion and joint fusion were
partially relieved. PG + FLB: Bone erosion of the sacroiliac joint
increased. (B) microCT images of the sacroiliac joint from each
group. Arrows show the narrowed sacroiliac space and unclear edges
(n=4) *P<0.05, **P<0.01,
***P<0.001. H&E, hematoxylin and eosin; BV/TV,
ratio of the segmented bone volume to the total volume of the
region of interest; Tt.Ar, total cross-sectional area; Ct.Ar,
cortical bone area; Ct.Th, average cortical thickness; Ctl,
control; PG, proteoglycan; NLB, no load bearing; PLB, partial load
bearing; FLB, full load bearing; AS, ankylosing spondylitis; ns,
not significant. |
The various mechanical interventions were
demonstrated to cause significant differences in the levels of the
mechanosensory miR-103 compared with the control groups, as
presented in Fig. 3A. The PG + NLB
and PG + PLB groups exhibited significantly elevated expression of
miR-103 compared with the PG-immunized control group, while the PG
+ FLB group exhibited decreased expression of miR-103. There was a
statistically significant difference between the two suspension
groups with a reduced mechanical load, the NLB and PLB groups
(Fig. 3A). Luciferase assay results
indicated that hsa-miR-103 bound to the 3'-UTR end of the mRNA of
Rock1-WT in 293T cells, thus reducing luciferase activity;
conversely, hsa-miR-103 failed to bind to the Rock1-Mu,
leaving its fluorescence intensity unchanged (Fig. 3B and C). 293T cells were successfully
transfected with miR-103 inhibitor and miR-103 mimics. Further
findings indicated that the mRNA expression level of Rock1
was significantly upregulated in 293T cells transfected with the
miR-103 inhibitor but significantly reduced in cells transfected
with miR-103 mimics when compared with their respective NC
(Fig. 3D). Meanwhile, the
observational PCR results demonstrated that the expression level of
Rock1 was inhibited by miR-103 in the suspension groups
(Fig. 3A and D). Hence, the suspension exerted a
significant protective effect against AS through miR-103 and
Rock1.
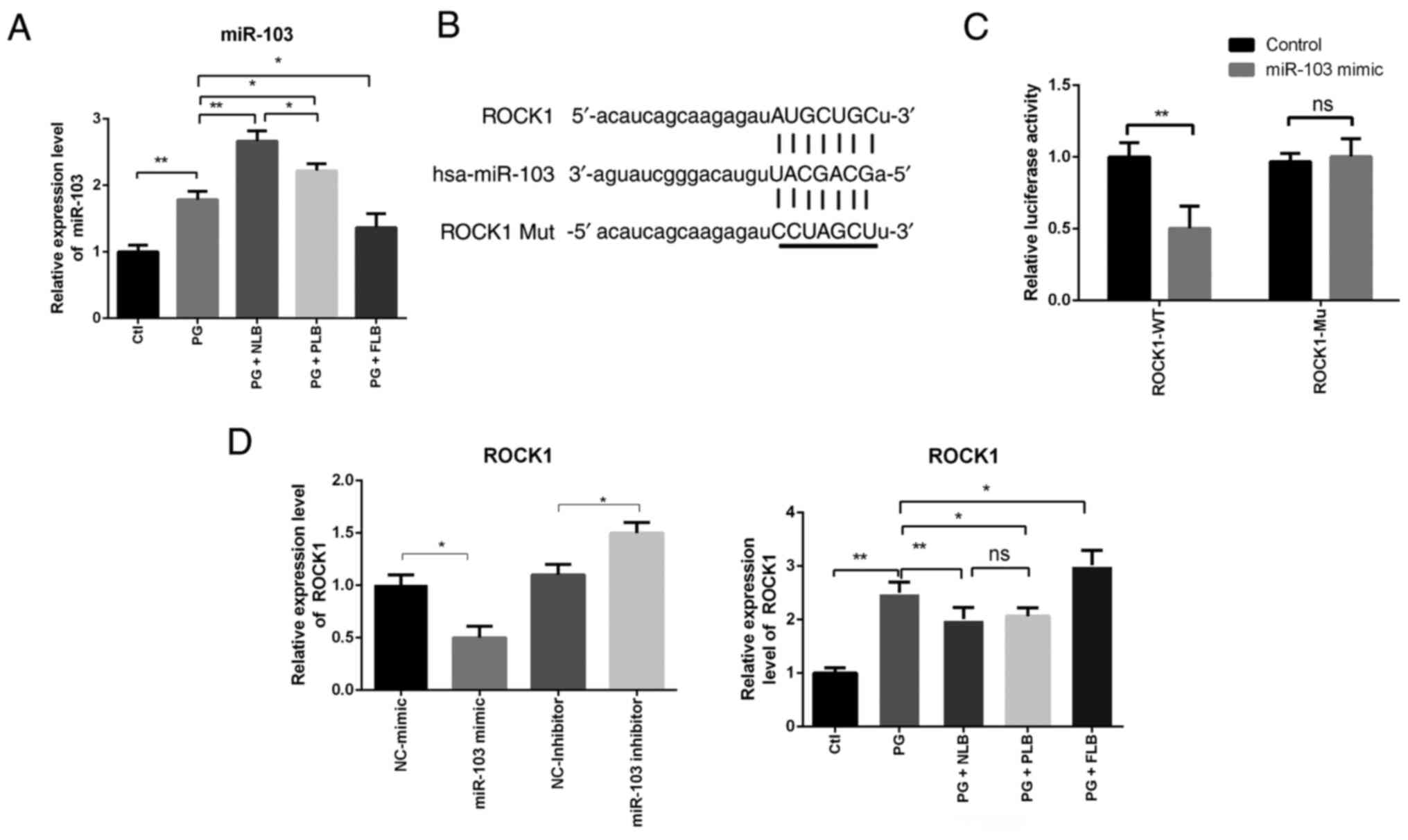 | Figure 3miR-103 expression is increased in
the tail suspension group and may affect the pathological
ossification of AS by combining with the 3'-UTR of ROCK1. (A)
Expression levels of miR-103 were increased in the PG + NLB and PG
+ PLB groups compared with the PG group, while the PG + FLB group
demonstrated decreased expression levels. (B) Alignment of miR-103
with the 3'-UTR of Rock1. (C) 293T cells were co-transfected
with a reporter expressing the Rock1-WT 3'-UTR and miR-103
mimics. Rock1-Mu recombinant vector was used as a positive
control and targeting effect was measured using luciferase
activity. (D) mRNA expression of Rock1 in 293T cells (left)
and in vivo (right) transfected with miR-103 mimics or
miR-103 inhibitor. *P<0.05, **P<0.01.
miR, microRNA; Rock1, ρ-associated coiled-coil containing
protein kinase 1; UTR, untranslated region; WT, wild-type; Mu,
mutant; AS, ankylosing spondylitis; PG, proteoglycan; Ctl, control;
NLB, no load bearing; PLB, partial load bearing; FLB, full load
bearing; ns, not significant; NC, negative control. |
Suspension and load reduction
decreases expression of bone formation indicator genes
The expression levels of essential
osteogenesis-related genes, such as bone morphogenetic protein-2
(Bmp2), runt-related transcription factor-2 (Runx2)
and osteocalcin (Bglap), were analyzed in sacroiliac bone
tissues of the five experimental groups using RT-qPCR. Abnormal
reductions in bone formation were observed (Fig. 4). The decreased expression of these
osteogenesis-related genes in the experimental groups followed a
notably similar pattern. The tail suspension groups (PG + NLB and
PG + PLB) exhibited significantly downregulated expression of
Bmp2, Runx2 and Bglap, which may be associated
with the reduced bone formation in sacroiliac joint tissues.
Meanwhile, the PG + FLB group exhibited upregulated expression.
Although the expression levels of the osteogenic genes were
markedly lower in the PG + NLB group compared with the PG + PLB
group, the difference between the two groups was not statistically
significant for any gene. The above data suggested that suspension
and load reduction decreased expression of bone formation indicator
genes (Bmp2, Runx2 and Bglap).
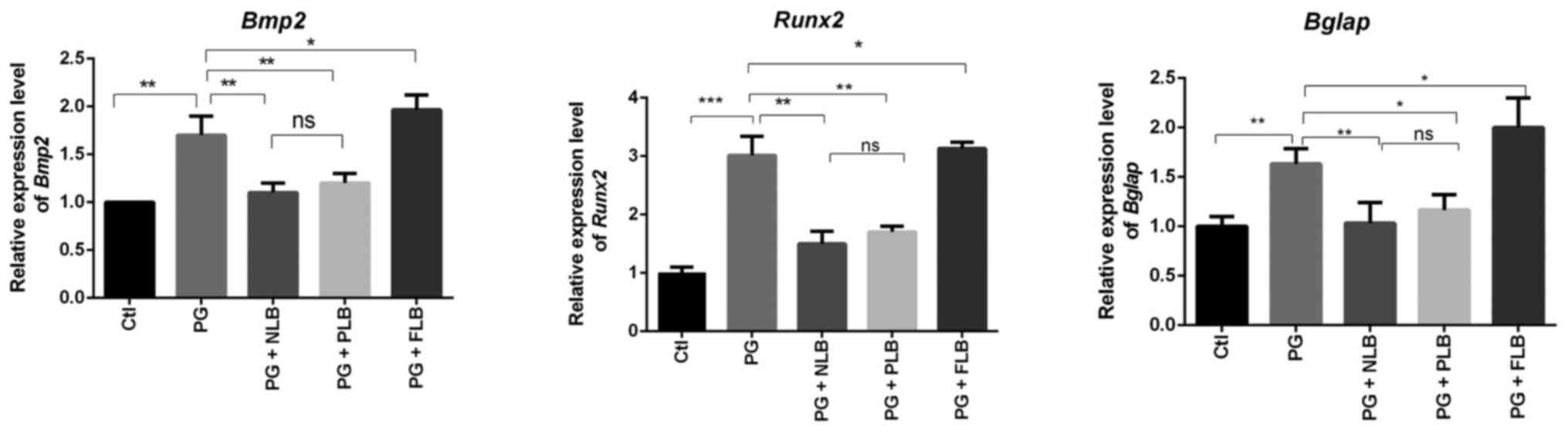 | Figure 4RT-qPCR results of sacroiliac joint
bone tissue indicate that tail suspension reduces osteogenesis,
while upright intervention increases osteogenesis. RT-qPCR analysis
demonstrated that the expression levels of osteo-specific genes
(Bmp2, Runx2 and Bglap) were decreased in the
PG + NLB and PG + PLB groups, whereas the expression of osteogenic
genes in the PG + FLB group exhibited opposing trends (n=3).
*P<0.05, **P<0.01;
***P<0.001. Bmp2, bone morphogenetic protein
2; Runx2, runt-related transcription factor 2; Bglap,
osteocalcin; ns, not significant; Ctl, control; PG, proteoglycan;
NLB, no load bearing; PLB, partial load bearing; FLB, full load
bearing; RT-qPCR, reverse transcription-quantitative PCR. |
Suspension and mechanical load
reduction affects the classical osteogenic Wnt/β-catenin pathway in
AS
The expression level of DKK1 in various experimental
groups was analyzed using IHC. It was revealed that the expression
of DKK1, a Wnt/β-catenin pathway inhibitor, was elevated in the two
tail suspension groups (PG + NLB and PG + PLB), but was reduced in
the upright PG + FLB group (Fig.
5). Although the expression level of DKK1 in the PG + NLB group
was markedly higher compared with the PG + PLB group, the
difference between the two groups was not statistically
significant.
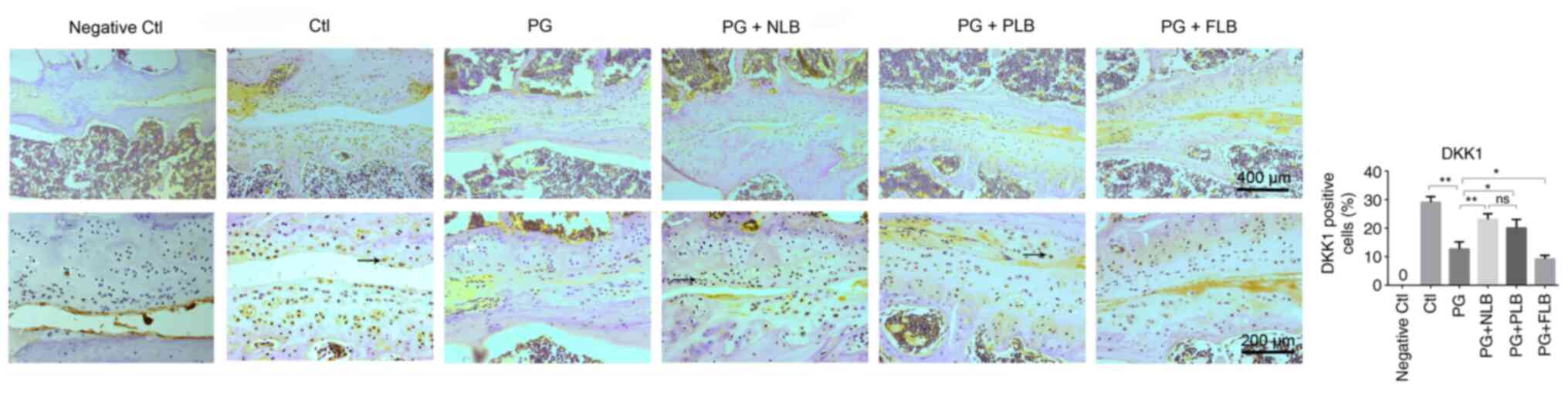 | Figure 5Reduced mechanical load upregulates
the expression of Wnt pathway inhibitor DKK1. DKK1-positive cells
were quantified using ImageJ software. Immunohistochemistry
analysis indicated that PG + NLB and PG + PLB groups increased
DKK1-positive expression compared with the PG group, while the PG +
FLB group exhibited lower DKK1 expression levels (n=4). Scale bar:
Top row, 400 µm; bottom row, 200 µm; *P<0.05,
**P<0.01. DKK1, dickkopf Wnt signaling pathway
inhibitor 1; Ctl, control; PG, proteoglycan; NLB, no load bearing;
PLB, partial load bearing; FLB, full load bearing; ns, not
significant. |
Western blotting revealed significantly decreased
expression levels of β-catenin, an essential component of the
Wnt/β-catenin signaling pathway, in the two tail suspension groups
(PG + NLB and PG + PLB) compared with the PG group (Fig. 6). In addition, a significant
increase in β-catenin was observed in the PG + FLB group compared
with the PG model group. The level of β-catenin is positively
associated with abnormal bone formation in AS (35). The expression levels of ROCK1 and
p-Erk1/2 in the ligaments of the sacroiliac joint were
significantly decreased in the groups with suspension with a
reduced mechanical load compared with the PG model group, and the
opposite trend was observed in the upright group. The expression
levels of β-catenin, ROCK1 and p-Erk in the PG + NLB group appeared
to be markedly decreased compared with the PG + PLB group; however,
the difference between the two groups was not statistically
significant (Fig. 6). Overall,
these data suggested that suspension and mechanical load reduction
affected the classical osteogenic Wnt/β-catenin pathway in AS in
vivo.
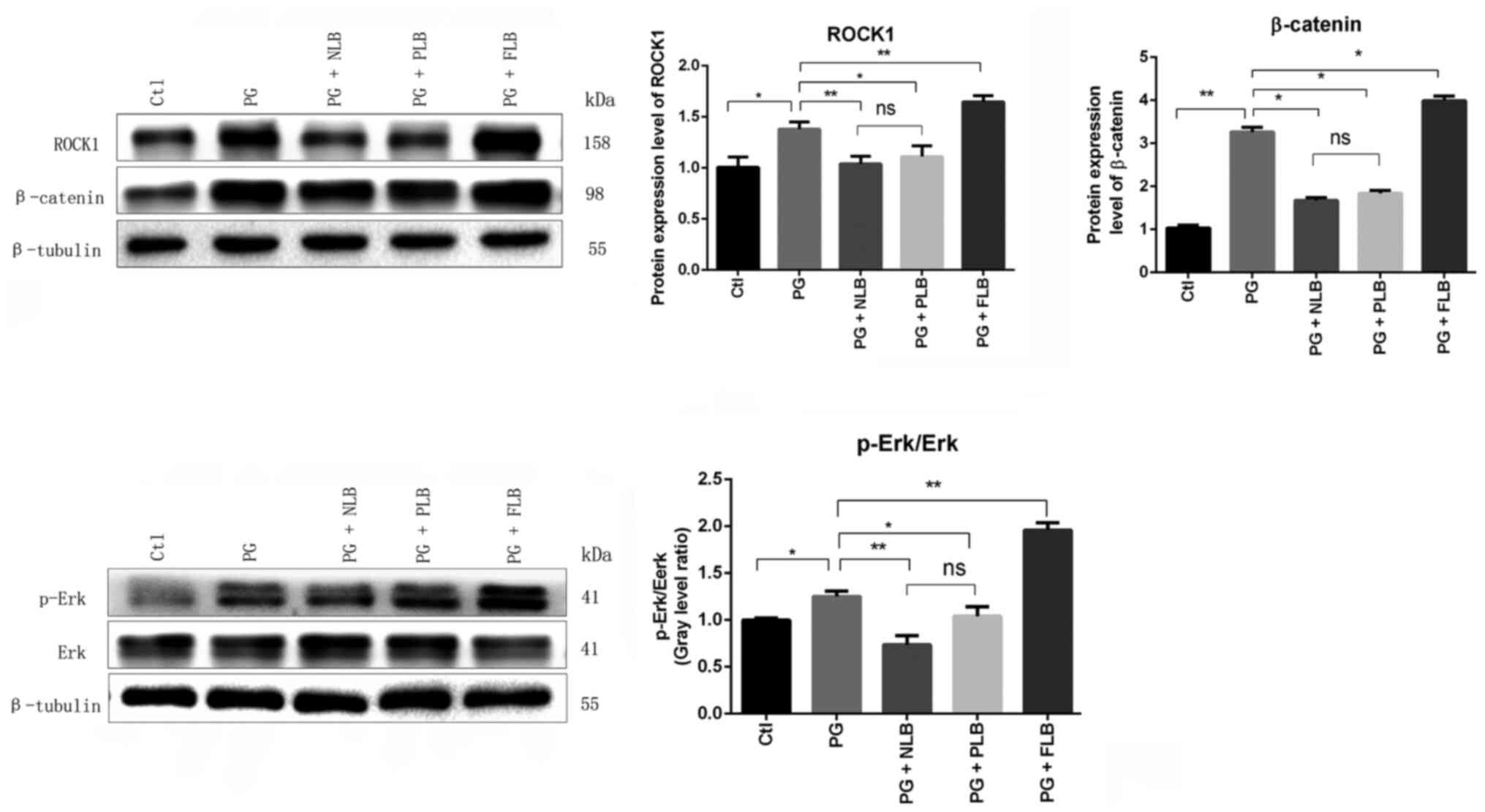 | Figure 6Changes in the mechanical pathway
proteins ROCK1 and p-Erk1/2 MAPK and osteogenic Wnt/β-catenin
pathway proteins. Western blotting and quantitative data
demonstrated reduced expression levels of ROCK1, p-Erk/Erk and
β-catenin in the PG + NLB and PG + PLB groups, and an increase in
these expressions in the PG + FLB group compared with the PG group.
*P<0.05, **P<0.01. ROCK1, ρ-associated
coiled-coil containing protein kinase 1; MAPK, mitogen-activated
protein kinase; p-, phosphorylated; Ctl, control; PG, proteoglycan;
NLB, no load bearing; PLB, partial load bearing; FLB, full load
bearing; ns, not significant. |
Discussion
The present study explored whether mechanical load
reduction induced by tail suspension could delay the heterotopic
ossification following enthesitis in AS mice. The results suggested
that tail suspension with reduced mechanical load could alleviate
pathological bone formation. Furthermore, tail suspension could
inhibit the activation of mechanical ROCK1 kinase and the p-Erk1/2
MAPK signaling pathway, and upregulate mechanosensitive miR-103.
Meanwhile, the Wnt/β-catenin pathway is positively associated with
ectopic osteogenesis in AS; furthermore, DKK1, an inhibitor of the
Wnt pathway, is increased with a reduced mechanical load, thus
inhibiting the activation of the Wnt pathway associated with AS
osteogenesis (36).
AS, a chronic autoimmune disease, is characterized
by inflammation of the axial skeleton and attachment points, which
eventually leads to pathological ossification at attachment sites,
including ligaments, tendons and joint capsules (37). This causes the patient to lose
normal range of activity (38).
Enthesitis and subchondral osteogenesis are the principal factors
of inflammation and ossification associated with AS (39). The primary pathological site of AS
is the sacroiliac joint; then, it gradually develops and begins to
affect the axial spinal joints (40). The axial joints bear heavy loads
while maintaining the balance of the pelvis and trunk during daily
activities (41). Long-term axial
overload will increase micro-injury of the sacroiliac and spinal
joints, leading to a repair process that causes synovitis, joint
destruction and late-stage subchondral bone rigidity (42). The increased mechanical load could
be a significant cause of ectopic bone formation, which often
occurs in AS, and can lead to joint fusion and stiffness (6). Career-associated high-intensity
mechanical stress has been reported to accelerate immune joint
injury (6). Studies indicated that
an excessive mechanical load can lead to the progression of AS in
patients with an active background, such as athletes (37), according to several lines of
evidence (6,37). For example, spinal overloading could
predispose patients to enhanced sacroiliac joint damage and repair
pathways, leading to synovitis, erosions, and pathological
ossification (6,43,44).
On the other hand, previous studies have shown that patients who
remain in bed for an extended period may experience bone loss due
to disuse and reduced bone formation (45,46).
The pathogenesis of AS has not yet been fully
elucidated. Inflammation is generally associated with tissue
destruction, such as ankylosis following new cartilage and bone
formation (47). Also, the
activation of TNF-α (48) and the
IL-23/IL-17 pathway (49) are known
to play critical roles in the inflammatory response in AS (50). An autoimmune disorder marker, human
leukocyte antigen B27, exhibits the strongest association with AS
(51,52), but its relationship with AS has not
been firmly established. Thus, the treatment of AS has utilized
non-steroidal anti-inflammatory drugs and anti-rheumatic biologics
in recent years (53). The
biological agents approved for the treatment of AS include TNF-α
and IL-17 inhibitors, which are the subjects of intense research
focus (53). On the other hand,
severe dysfunction is treated with orthopedic surgery. However, no
method has been shown to delay the progression of AS (47). Furthermore, the impact of
biomechanical stress on AS, and the link between AS and
biomechanics, remain unclear. Early space flight experiments have
indicated that the musculoskeletal system is highly sensitive to
gravity loading (54). Recently,
mouse tail suspension used as a model to simulate the reduction of
mechanical stress has also been reported to reduce bone formation
in mice (55). Therefore, the
present study aimed to understand if tail suspension could be used
to delay the progress of AS.
The effect of tail suspension on the progression of
AS was investigated by monitoring changes in the expression of
genes involved in osteogenesis. RT-qPCR analysis revealed that the
expression levels of bone formation-related marker genes, including
Bmp2, Runx2 and Bglap, in sacroiliac bone
tissues were decreased in the tail suspension groups. By contrast,
the expression levels of these markers were increased in the
vertical FLB group. In AS, the molecules involved in bone
formation, such as Wnt and its antagonist DKK1, may drive
syndesmophyte formation (56). The
Wnt/β-catenin signaling pathway is known to respond to the
mechanical loading of bones (57).
Reduced DKK1 expression levels cause hyperplasia of the tissues
surrounding the joint, which is associated with stiffness
presenting as sacroiliitis (58).
In the present study, IHC analysis revealed that the expression of
DKK1 was upregulated in the tail suspension groups (PG + NLB and PG
+ PLB), indicating suppression of the classical Wnt/β-catenin
pathway and thus inhibition of osteophyte formation at sacroiliac
joints in AS. The upregulation of DKK1 under reduced mechanical
stress indicates remission of AS (59). β-catenin is a critical protein in
the classical Wnt signaling pathway that plays a central role in
the formation of ectopic bone (10). Moreover, the production of β-catenin
is positively associated with AS osteogenesis (60). The present study revealed that
β-catenin levels were upregulated in the AS model (PG) and
downregulated in the groups with a reduced mechanical load (PG +
NLB and PG + PLB), which was consistent with previous studies
(10). These data suggested that
reducing mechanical load could inhibit AS heterotopic bone
formation.
Available evidence indicates that increasing
mechanical stress can significantly promote the activation of the
p-Erk1/2 MAPK and Wnt/β-catenin signaling pathways (18). Also, the mechanical stress-induced
cell response depends on the effector ROCK1/2 and Erk1/2 pathways
(61). ρ kinase activates
phosphorylation of Erk1/2 in MAPK signaling by regulating ROCK1/2
with increasing biological load (62). Thus, ROCK1 is an upstream regulator
of the MAPK signaling pathway, and p-Erk1/2 activates the
Wnt/β-catenin pathway by promoting GSK-3β phosphorylation (12,63).
Consistent with the known activation of the p-Erk1/2 MAPK and
Wnt/β-catenin signaling pathways by increasing mechanical stress
(18), western blotting during the
present study indicated that the levels of ROCK1, p-Erk1/2 and
β-catenin were decreased in the groups with reduced mechanical load
(PG + NLB and PG + PLB). This finding indicated that tail
suspension may inhibit MAPK and Wnt/β-catenin signaling pathways by
reducing the expression of ROCK1. In addition, the standing group
that was bearing a greater load demonstrated the opposing trend,
with increases in the expression levels of osteogenic genes and
mechanical pathway proteins.
As miR-103 mainly inhibits osteoblast proliferation
by inhibiting the expression of Cav1.2 and Runx2
under simulated microgravity conditions (18,19),
the expression of miR-103 was evaluated under different
experimental conditions. Tail suspension and simulating reduced
mechanical load upregulated the expression level of miR-103 and
reduced the expression of osteogenic genes, consistent with the
inhibition of bone formation in the sacroiliac joints in AS.
Dual-luciferase assays verified that miR-103 would bind to the
3'-UTR end of Rock1. Thus, the increased expression levels
of miR-103 in the tail suspension groups with reduced mechanical
load negatively regulated the activity of ROCK1, which affected the
pathological ossification in AS.
In summary, the present study performed various
interventions, including tail suspensions for reduced mechanical
load and full upright load-bearing, in AS model mice. It was
revealed that under reduced mechanical load conditions, the
expression levels of osteogenesis-related genes and mechanical
pathway signals were decreased, and the expression of the
osteogenic Wnt pathway inhibitor DKK1 was increased, indicating
reduced bone formation. However, the difference between the PG +
NLB and PG + PLB groups was not statistically significant.
Furthermore, tail suspension affected control of the mechanical
kinase ROCK1 and p-Erk1/2 in the MAPK pathway by upregulating the
expression of miR-103, further suppressing the osteogenic
Wnt/β-catenin pathway associated with AS. The results indicated
that reducing mechanical stress could delay ectopic osteogenesis in
the sacroiliac joint by regulating the Wnt pathway and its
inhibitor DKK1, thereby delaying the progression of AS.
The current study investigated the effect of
reducing mechanical stress on AS mice and examined its potential as
a basis for clinical therapy. For humans, spinal traction is a
common clinical treatment for reducing mechanical stress, which is
similar to suspension (64). The
findings supported spinal traction as a novel therapeutic option
for AS treatment. However, further studies are required to
delineate the role of reducing mechanical stress in the pathology
of AS. In the future, it will be of interest to confirm these
findings in a clinical setting. Also, it will be valuable to
examine the immune-related pathological processes of osteogenesis.
However, the present study had several limitations. Although a
close relationship between miR-103 and ROCK1 by miR-103 mimic and
inhibitor was demonstrated in vitro, the effect of miR-103
expression on ROCK1 must be verified in vivo by future
studies. The present study used PG-induced mice rather than
patients with AS to assess the expression levels of miR-103 and
ROCK1.
In conclusion, the results demonstrated that
mechanical load played a notable role in ectopic bone formation and
AS progression. Tail suspension resulting in reduced mechanical
load alleviated ectopic osteogenesis in the sacroiliac joints of AS
mice by upregulating the expression of miR-103, suppressing
mechanical signaling pathways and inhibiting the classical
Wnt/β-catenin pathway involved in bone formation. In summary, tail
suspension to reduce mechanical stress offers a promising adjuvant
therapy for attenuating ectopic bone formation associated with AS
and requires further investigation.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by The Natural Science
Foundation of Guangdong Province (grant no. 2017A030313721), The
National Natural Science Foundation of China (grant no. 81774382)
and the Scientific Research Project of Traditional Chinese Medicine
Bureau of Guangdong Province (grant no. 20181175).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GL and YZ designed the experiments. ZZ and JZ
performed the experiments and wrote the manuscript. QL and YL
analyzed the data. DH analyzed the data and revised the manuscript.
GL and ZZ confirmed the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental scheme was approved by The
Institutional Animal Care and Use Committee of the Southern Medical
University (Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ma S, Wang DD, Ma CY and Zhang YD:
MicroRNA-96 promotes osteoblast differentiation and bone formation
in ankylosing spondylitis mice through activating the Wnt signaling
pathway by binding to SOST. J Cell Biochem. 120:15429–15442.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lories RJ and Haroon N: Evolving concepts
of new bone formation in axial spondyloarthritis: Insights from
animal models and human studies. Best Pract Res Clin Rheumatol.
31:877–886. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Carter S, Braem K and Lories RJ: The role
of bone morphogenetic proteins in ankylosing spondylitis. Ther Adv
Musculoskelet Dis. 4:293–299. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sieper J and Poddubnyy D: Axial
spondyloarthritis. Lancet. 390:73–84. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Voirin-Hertz M, Carvajal Alegria G,
Garrigues F, Simon A, Feydy A, Reijnierse M, van der Heijde D,
Loeuille D, Claudepierre P, Marhadour T and Saraux A: Associations
of lumbar scoliosis with presentation of suspected early axial
spondyloarthritis. Semin Arthritis Rheum. 50:48–53. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Masi AT: Might axial myofascial properties
and biomechanical mechanisms be relevant to ankylosing spondylitis
and axial spondyloarthritis? Arthritis Res Ther.
16(107)2014.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Hoch AI, Mittal V, Mitra D, Vollmer N,
Zikry CA and Leach JK: Cell-secreted matrices perpetuate the
bone-forming phenotype of differentiated mesenchymal stem cells.
Biomaterials. 74:178–187. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mu C, Lv T, Wang Z, Ma S, Ma J, Liu J, Yu
J and Mu J: Mechanical stress stimulates the osteo/odontoblastic
differentiation of human stem cells from apical papilla via erk 1/2
and JNK MAPK pathways. Biomed Res Int. 2014(494378)2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Van Mechelen M, Gulino GR, de Vlam K and
Lories R: Bone disease in axial spondyloarthritis. Calcif Tissue
Int. 102:547–558. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li X, Wang J, Zhan Z, Li S, Zheng Z, Wang
T, Zhang K, Pan H, Li Z, Zhang N and Liu H: Inflammation
intensity-dependent expression of osteoinductive Wnt proteins is
critical for ectopic new bone formation in ankylosing spondylitis.
Arthritis Rheumatol. 70:1056–1070. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zou YC, Yang XW, Yuan SG, Zhang P, Ye YL
and Li YK: Downregulation of dickkopf-1 enhances the proliferation
and osteogenic potential of fibroblasts isolated from ankylosing
spondylitis patients via the Wnt/β-catenin signaling pathway in
vitro. Connect Tissue Res. 57:200–211. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Appleton CT, Usmani SE, Mort JS and Beier
F: Rho/ROCK and MEK/ERK activation by transforming growth
factor-alpha induces articular cartilage degradation. Lab Invest.
90:20–30. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Millner JR, Barron JS, Beinke KM,
Butterworth RH, Chasle BE, Dutton LJ, Lewington MA, Lim EG, Morley
TB, O'Reilly JE, et al: Exercise for ankylosing spondylitis: An
evidence-based consensus statement. Semin Arthritis Rheum.
45:411–427. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen Y, Xu J, Yang C, Zhang H, Wu F, Chen
J, Li K, Wang H, Li Y, Li Y and Dai Z: Upregulation of miR-223 in
the rat liver inhibits proliferation of hepatocytes under simulated
microgravity. Exp Mol Med. 49(e348)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang J, Liu C, Li T, Wang Y and Wang D:
Proteomic analysis of pulmonary tissue in tail-suspended rats under
simulated weightlessness. J Proteomics. 75:5244–5253.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu J, Wang J and Guo Y: Effect of
collagen peptide, alone and in combination with calcium citrate, on
bone loss in tail-suspended rats. Molecules. 25(782)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Stadnik PS, Gilbert SJ, Tarn J, Charlton
S, Skelton AJ, Barter MJ, Duance VC, Young DA and Blain EJ:
Regulation of microRNA-221, -222, -21 and -27 in articular
cartilage subjected to abnormal compressive forces. J Physiol.
599:143–155. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Zuo B, Zhu J, Li J, Wang C, Zhao X, Cai G,
Li Z, Peng J, Wang P, Shen C, et al: microRNA-103a functions as a
mechanosensitive microRNA to inhibit bone formation through
targeting Runx2. J Bone Miner Res. 30:330–345. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sun Z, Cao X, Hu Z, Zhang L, Wang H, Zhou
H, Li D, Zhang S and Xie M: miR-103 inhibits osteoblast
proliferation mainly through suppressing Cav1.2 expression in
simulated microgravity. Bone. 76:121–128. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lv H, Yang H and Wang Y: Effects of
miR-103 by negatively regulating SATB2 on proliferation and
osteogenic differentiation of human bone marrow mesenchymal stem
cells. PLoS One. 15(e0232695)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zheng L, Hu F, Bian W, Li Y, Zhang L, Shi
L, Ma X, Liu Y, Zhang X and Li Z: Dickkopf-1 perpetuated synovial
fibroblast activation and synovial angiogenesis in rheumatoid
arthritis. Clin Rheumatol: May 19, 2021 (Epub ahead of print).
|
|
22
|
Haynes KR, Pettit AR, Duan R, Tseng HW,
Glant TT, Brown MA and Thomas GP: Excessive bone formation in a
mouse model of ankylosing spondylitis is associated with decreases
in Wnt pathway inhibitors. Arthritis Res Ther.
14(R253)2012.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Liu JH, Wang Q, You QL, Li ZL, Hu NY, Wang
Y, Jin ZL, Li SJ, Li XW, Yang JM, et al: Acute EPA-induced learning
and memory impairment in mice is prevented by DHA. Nat Commun.
11(5465)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sanders CJ, Johnson B, Frevert CW and
Thomas PG: Intranasal influenza infection of mice and methods to
evaluate progression and outcome. Methods Mol Biol. 1031:177–188.
2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Qin J, Li J, Yang H, Jia M, Li X, Yao Q,
Zhang Y, Zhu J and Li C: Values of intravoxel incoherent motion
diffusion weighted imaging and dynamic contrast-enhanced MRI in
evaluating the activity of sacroiliitis in ankylosing spondylitis
of rat model. Magn Reson Imaging. 68:30–35. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ishikawa LL, Colavite PM, da Rosa LC,
Balbino B, França TG, Zorzella-Pezavento SF, Chiuso-Minicucci F and
Sartori A: Commercial bovine proteoglycan is highly arthritogenic
and can be used as an alternative antigen source for PGIA model.
Biomed Res Int. 2014(148594)2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
He T, Huang Y, Zhang C, Liu D, Cheng C, Xu
W and Zhang X: Interleukin-17A-promoted MSC2 polarization related
with new bone formation of ankylosing spondylitis. Oncotarget.
8:96993–97008. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shanmugarajan S, Zhang Y,
Moreno-Villanueva M, Clanton R, Rohde LH, Ramesh GT, Sibonga JD and
Wu H: Combined effects of simulated microgravity and radiation
exposure on osteoclast cell fusion. Int J Mol Sci.
18(2443)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang Y, Zhao W, Shi J, Wang J, Hao J, Pang
X, Huang X, Chen X, Li Y, Jin R and Ge Q: Intestinal microbiota
contributes to altered glucose metabolism in simulated microgravity
mouse model. FASEB J. 33:10140–10151. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liang S, Wang ZG, Zhang ZZ, Chen K, Lv ZT,
Wang YT, Cheng P, Sun K, Yang Q and Chen AM: Decreased RIPK1
expression in chondrocytes alleviates osteoarthritis via the
TRIF/MyD88-RIPK1-TRAF2 negative feedback loop. Aging (Albany NY).
11:8664–8680. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ahmadvand Koohsari S, Absalan A and Azadi
D: Human umbilical cord mesenchymal stem cell-derived extracellular
vesicles attenuate experimental autoimmune encephalomyelitis via
regulating pro and anti-inflammatory cytokines. Sci Rep.
11(11658)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liang S, Zhang JM, Lv ZT, Cheng P, Zhu WT
and Chen AM: Identification of Skt11-regulated genes in
chondrocytes by integrated bioinformatics analysis. Gene.
677:340–348. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hao X, Wang S, Zhang J and Xu T: Effects
of body weight-supported treadmill training on
cartilage-subchondral bone unit in the rat model of posttraumatic
osteoarthritis. J Orthop Res. 39:1227–1235. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
He X and Dong Y: Ankylosis progressive
homolog upregulation inhibits cell viability and mineralization
during fibroblast ossification by regulating the Wnt/β-catenin
signaling pathway. Mol Med Rep. 22:4551–4560. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Klavdianou K, Liossis SN, Sakkas L and
Daoussis D: The role of dickkopf-1 in joint remodeling and
fibrosis: A link connecting spondyloarthropathies and scleroderma?
Semin Arthritis Rheum. 46:430–438. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Benjamin M, Toumi H, Ralphs JR, Bydder G,
Best TM and Milz S: Where tendons and ligaments meet bone:
Attachment sites (‘entheses’) in relation to exercise and/or
mechanical load. J Anat. 208:471–490. 2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Regnaux JP, Davergne T, Palazzo C, Roren
A, Rannou F, Boutron I and Lefevre-Colau MM: Exercise programmes
for ankylosing spondylitis. Cochrane Database Syst Rev.
10(CD011321)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jo S, Won EJ, Kim MJ, Lee YJ, Jin SH, Park
PR, Song HC, Kim J, Choi YD, Kim JY, et al: STAT3 phosphorylation
inhibition for treating inflammation and new bone formation in
ankylosing spondylitis. Rheumatology (Oxford): Nov 25, 2020 (Epub
ahead of print).
|
|
40
|
Soulard J, Vaillant J, Agier CT and
Vuillerme N: Gait characteristics in patients with ankylosing
spondylitis: A systematic review. Clin Exp Rheumatol. 39:173–186.
2021.PubMed/NCBI
|
|
41
|
Sturdy JT, Sessoms PH and Silverman AK: A
backpack load sharing model to evaluate lumbar and hip joint
contact forces during shoulder borne and hip belt assisted load
carriage. Appl Ergon. 90(103277)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Herrero-Beaumont G, Pérez-Baos S,
Sánchez-Pernaute O, Roman-Blas JA, Lamuedra A and Largo R:
Targeting chronic innate inflammatory pathways, the main road to
prevention of osteoarthritis progression. Biochem Pharmacol.
165:24–32. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Francois RJ, Gardner DL, Degrave EJ and
Bywaters EG: Histopathologic evidence that sacroiliitis in
ankylosing spondylitis is not merely enthesitis. Arthritis Rheum.
43:2011–2024. 2000.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Vleeming A, Schuenke MD, Masi AT, Carreiro
JE, Danneels L and Willard FH: The sacroiliac joint: An overview of
its anatomy, function and potential clinical implications. J Anat.
221:537–567. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bi D, Dai Z, Liu D, Wu F, Liu C, Li Y, Li
B, Li Z, Li Y and Ta D: Ultrasonic backscatter measurements of
human cortical and trabecular bone densities in a head-down
bed-rest study. Ultrasound Med Biol: May 26, 2021 (Epub ahead of
print).
|
|
46
|
Rolvien T, Milovanovic P, Schmidt FN, von
Kroge S, Wölfel EM, Krause M, Wulff B, Püschel K, Ritchie RO,
Amling M and Busse B: Long-term immobilization in elderly females
causes a specific pattern of cortical bone and osteocyte
deterioration different from postmenopausal osteoporosis. J Bone
Miner Res. 35:1343–1351. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Braem K and Lories RJ: Insights into the
pathophysiology of ankylosing spondylitis: Contributions from
animal models. Joint Bone Spine. 79:243–248. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lubrano E, Spadaro A, Amato G, Benucci M,
Cavazzana I, Chimenti MS, Ciancio G, D Alessandro G, Angelis R,
Lupoli S, et al: Tumour necrosis factor alpha inhibitor therapy and
rehabilitation for the treatment of ankylosing spondylitis: A
systematic review. Semin Arthritis Rheum. 44:542–550.
2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Gravallese EM and Schett G: Effects of the
IL-23-IL-17 pathway on bone in spondyloarthritis. Nat Rev
Rheumatol. 14:631–640. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
McGonagle DG, McInnes IB, Kirkham BW,
Sherlock J and Moots R: The role of IL-17A in axial
spondyloarthritis and psoriatic arthritis: Recent advances and
controversies. Ann Rheum Dis. 78:1167–1178. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Brown MA, Li Z and Cao KL: Biomarker
development for axial spondyloarthritis. Nat Rev Rheumatol.
16:448–463. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Pedersen SJ and Maksymowych WP: The
pathogenesis of ankylosing spondylitis: An update. Curr Rheumatol
Rep. 21(58)2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kiltz U and Braun J: Current treatment of
axial spondylarthritis: Clinical efficacy. Z Rheumatol. 79:13–22.
2020.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
54
|
Globus RK and Morey-Holton E: Hindlimb
unloading: Rodent analog for microgravity. J Appl Physiol (1985).
120:1196–1206. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Hu Y, Zhang Y, Ni CY, Chen CY, Rao SS, Yin
H, Huang J, Tan YJ, Wang ZX, Cao J, et al: Human umbilical cord
mesenchymal stromal cells-derived extracellular vesicles exert
potent bone protective effects by CLEC11A-mediated regulation of
bone metabolism. Theranostics. 10:2293–2308. 2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Heiland GR, Appel H, Poddubnyy D, Zwerina
J, Hueber A, Haibel H, Baraliakos X, Listing J, Rudwaleit M, Schett
G and Sieper J: High level of functional dickkopf-1 predicts
protection from syndesmophyte formation in patients with ankylosing
spondylitis. Ann Rheum Dis. 71:572–574. 2012.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Robinson JA, Chatterjee-Kishore M,
Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P,
Brown EL, et al: Wnt/beta-catenin signaling is a normal
physiological response to mechanical loading in bone. J Biol Chem.
281:31720–31728. 2006.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Uderhardt S, Diarra D, Katzenbeisser J,
David JP, Zwerina J, Richards W, Kronke G and Schett G: Blockade of
dickkopf (DKK)-1 induces fusion of sacroiliac joints. Ann Rheum
Dis. 69:592–597. 2010.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Xiong JH, Liu J and Chen J: Clinical
significance and prognostic value of tumor necrosis factor-α and
dickkopf related protein-1 in ankylosing spondylitis. World J Clin
Cases. 8:1213–1222. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
He C, Li D, Gao J, Li J, Liu Z and Xu W:
Inhibition of CXCR4 inhibits the proliferation and osteogenic
potential of fibroblasts from ankylosing spondylitis via the
Wnt/β-catenin pathway. Mol Med Rep. 19:3237–3246. 2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Blomme B, Deroanne C, Hulin A, Lambert C,
Defraigne JO, Nusgens B, Radermecker M and Colige A: Mechanical
strain induces a pro-fibrotic phenotype in human mitral valvular
interstitial cells through RhoC/ROCK/MRTF-A and Erk1/2 signaling
pathways. J Mol Cell Cardiol. 135:149–159. 2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Hamamura K, Swarnkar G, Tanjung N, Cho E,
Li J, Na S and Yokota H: RhoA-mediated signaling in
mechanotransduction of osteoblasts. Connect Tissue Res. 53:398–406.
2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Ding Q, Xia W, Liu JC, Yang JY, Lee DF,
Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, et al: Erk associates
with and primes GSK-3beta for its inactivation resulting in
upregulation of beta-catenin. Mol Cell. 19:159–170. 2005.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Abdollah V, Parent EC, Su A, Wachowicz K
and Battie MC: Could compression and traction loading improve the
ability of magnetic resonance imaging to identify findings related
to low back pain? Musculoskelet Sci Pract.
50(102250)2020.PubMed/NCBI View Article : Google Scholar
|