Introduction
The dental pulp is vulnerable to infection resulting
from dental caries and injuries. Most dental pulp infections are
irreversible due to ischemia induced by anatomic factors, since the
dental pulp is surrounded by rigid dentin walls and has limited
collateral circulation. The survival of inflamed vital pulp is
closely associated with the process of angiogenesis (1). Angiogenesis provides blood supply,
oxygen and nutrients, which are indispensable during dental pulp
repair and regeneration.
Angiogenesis is a multi-sequential-step procedure
involving the interaction between endothelial or stem cells,
extracellular matrix components and mediation of various
pro-angiogenic and anti-angiogenic factors (2). Dental pulp cells (DPCs) are a
heterogeneous population of dental pulp stem cells (DPSCs) and
other progenitors that possess mesenchymal stem cell (MSC)-like
properties (3). DPSCs have been
reported to induce angiogenesis in a paracrine fashion by secreting
angiogenic molecules (4). Under
extreme circumstances, such as serum deprivation, the secretome of
DPSCs was observed to enhance the pulp repair ability, but this
effect was not obvious under normal serum conditions (5). Furthermore, a recent study indicated
that DPSC-conditioned medium (CM) accelerated the adhesion,
proliferation, migration and tubulogenesis of human umbilical vein
endothelial cells (HUVECs) (6).
Accumulating evidence suggests that DPCs or DPSC-derived CM has
angiogenic potential (7). However,
the mechanisms underlying the angiogenesis of dental pulp have
remained to be completely elucidated.
Erythropoietin (EPO) is mainly synthesized in the
fetal liver and adult kidney and was initially highlighted for its
action on the hematopoietic system (8). Extensive studies indicated that EPO
and EPO receptor (EPOR) may be detected in a wide range of
nonhematopoietic tissues and cells and have an important role in
angiogenesis, anti-inflammation and anti-apoptosis (9-11).
For instance, EPO enhanced the angiogenic capacity in a model of
cerebral unilateral hypoxia-ischemia, bone repair and burn injury
(12-14).
Previous studies suggested that EPO was expressed in inflamed
dental pulp but rarely detected in healthy dental pulp. EPOR was
detected in both healthy and inflamed dental pulp. EPO and EPOR
expressions in DPCs were increased under hypoxia (15). Given that dental pulp is susceptible
to ischemia conditions (hypoxia and serum deprivation) when exposed
to trauma or inflammation, it was hypothesized that EPO may have a
role in enhancing the angiogenic capacity of DPCs.
MAPKs regulate cellular proliferation, development,
differentiation and apoptosis through cascade amplification, and
respond to a variety of extracellular stimuli, such as
inflammation, physical and chemical stress. The activation of MAPK
pathways is involved in angiogenesis by regulating the secretion of
cytokines. Furthermore, it was revealed that EPO binding to EPOR
activates pathways downstream of MAPK (16). However, it is unknown whether MAPK
is involved in the angiogenesis of EPO-induced DPCs.
In the present study, the effects of EPO on the
proliferation and angiogenesis of DPCs were studied. To explore how
EPO works on DPCs, the cytokine profile of DPCs in response to EPO
was investigated by using an angiogenic cytokine array under serum
deprivation conditions. Furthermore, the involvement of signaling
pathways was investigated by detecting proteins of the MAPK
signaling pathway. The present study supplements the previously
known angiogenesis effect of EPO on DPCs and may also provide novel
perspectives in pulp repair and regeneration.
Materials and methods
Isolation and culture of DPCs
Integral premolars and impacted third molars were
collected from young patients (10 males and 8 females; 18-25 years
of age; recruited between January 2014 and January 2018) who
underwent surgical orthodontic treatment at the Hospital of
Stomatology of Sun Yat-sen University (Guangzhou, China). None of
the patients had clinically significant medical history, nor were
they receiving medication. Ethics approval for the present study
was obtained from the Research Ethics Committee of Hospital of
Stomatology, Guanghua School of Stomatology, Sun Yat-sen University
and informed consent was obtained from each patient prior to
obtainment of the samples. The DPCs were isolated and cultured as
previously reported (15). DPCs
cultured from the third to sixth passages were used in the
subsequent experiments.
Cell counting Kit-8 (CCK-8) assay
DPCs were seeded into 96-well plates at a density of
4x10³ cells/well. After overnight incubation, different
concentrations of EPO (0.1, 1, 5, 10, 20 and 40 U/ml; R&D
Systems, Inc.) were added to each well of cells in the treatment
groups, while cells in serum-free DMEM only served as the control
group. After being cultured for 1, 2 or 3 days, CCK-8 stain
(Dojindo Molecular Technologies, Inc.) was added to each well
followed by incubation at 37˚C in the dark for 3 h, according to
the manufacturer's protocol. The optical density was measured at
450 nm using a microplate reader (BioTek). The results were
calculated as the mean of three replicates of three experiments
under the same conditions.
Tube formation assay on
matrigel®
CM was collected according to the following
procedure: DPCs were cultured in 25-cm2 flasks at 90%
confluence in 1 ml serum-free DMEM with 40 U/ml EPO for 24 h.
Subsequently, the CM was collected, centrifuged for 20 min at
104 x g at 4˚C and used in the subsequent experiments.
HUVECs (cat. no. 8000; ScienCell Research Laboratories, Inc.) were
cultured in complete endothelial cell medium (ScienCell Research
Laboratories, Inc.). For the tube formation assay, HUVECs
(4x104 cells per well) were seeded onto each well
precoated with Matrigel® (350 µl/well; BD Biosciences)
in 48-well plates. CM with or without 40 U/ml EPO neutralization
antibody (cat. no. AB-286-NA; R&D Systems, Inc.) was added to
HUVECs after plating and cultured for 4 h. Tube formation images
were obtained using an inverted microscope (Zeiss AG) and 3 fields
per well were randomly selected for analysis of tube lengths branch
points and amounts of loops with Quantitative Tube Formation Image
Analysis software (WimTube, Wimasis; https://www.wimasis.com/en/products/13/WimTube).
To further clarify the MAPK signaling pathway in the
angiogenic capacity of DPCs, cells were preconditioned with U0126,
SB203580 and SP600125 (10 µmol/ml; Gene Operation) prior to
addition of EPO and subsequent experiments were performed as
described above.
Protein array analysis
The Human Cytokine Antibody Array Kit (RayBiotech,
Inc) was used to identify angiogenesis-related factors within the
CM of DPCs, which were treated and collected as described above.
The assay was performed strictly according to the manufacturer's
protocol. The chip was scanned with a GenePix 4000B Microarray
Scanner (Molecular Devices, LLC) using Cy3 as the fluorophore. The
binding signals were acquired and analyzed by applying the
RayBio® software tool (AAH-CUST-G; RayBiotech, Inc).
ELISA
To confirm the results of the protein array using
quantitative analysis, the protein concentrations of MMP-3 (cat.
no. DMP300), angiopoietin-1 (Ang-1) (cat. no. DY923), IL-6 (cat.
no. D6050) and TGF-β (cat. no. DB100B) were detected using ELISA
kits (R&D Systems, Inc.) according to the manufacturer's
protocol. ELISAs were performed on the CM of DPCs treated with
different concentrations of EPO.
Western blot analysis
DPCs were preconditioned with 40 U/ml EPO for 1 h
and lysed in RIPA buffer containing proteinase inhibitor cocktail
(Beyotime Institute of Biotechnology). The protein was quantified
using a BCA Protein Assay Kit. Western blot analysis was performed
as instructed by the manufacturer using a capillary-based automated
system WES (Protein Simple) which uses capillary electrophoresis to
identify and quantify a protein of interest. In brief, protein
samples of 0.5 mg/ml/lane were loaded. Next, primary and secondary
antibodies and luminal peroxide mix were dispensed into the assay
plate per the manufacturer's protocol. Primary antibodies included
rabbit anti-human ERK1/2 (cat. no. 4695), phospProtein
Simplep-p38(cat. no. 4511) (1:100; R&D Systems, Inc.), JNK
(cat. no. 9252), p-JNK (cat. no. 9255) (1:100; Cell Signaling
Technology, Inc.) and β-actin (cat. no. 31-1013-00, 1:1,000;
EarthOx Life Sciences). The secondary antibodies were included in
the Simple Wes Kit (cat. no. 042-205 and cat. no. 042-206; (Protein
Simple). Analysis of detected proteins was performed with Compass
Software (version: 2.6.7; Protein Simple).
Statistical analysis
The experiments were performed in triplicate unless
otherwise stated and quantitative results are expressed as the mean
± standard deviation. Data were analyzed using the SPSS software
(version 13.0; SPSS, Inc.). One-way ANOVA was performed for
multiple-group comparisons, with post-hoc Bonferroni correction.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of EPO on the proliferation
and angiogenesis of DPCs
The effect of EPO on the proliferation of DPCs was
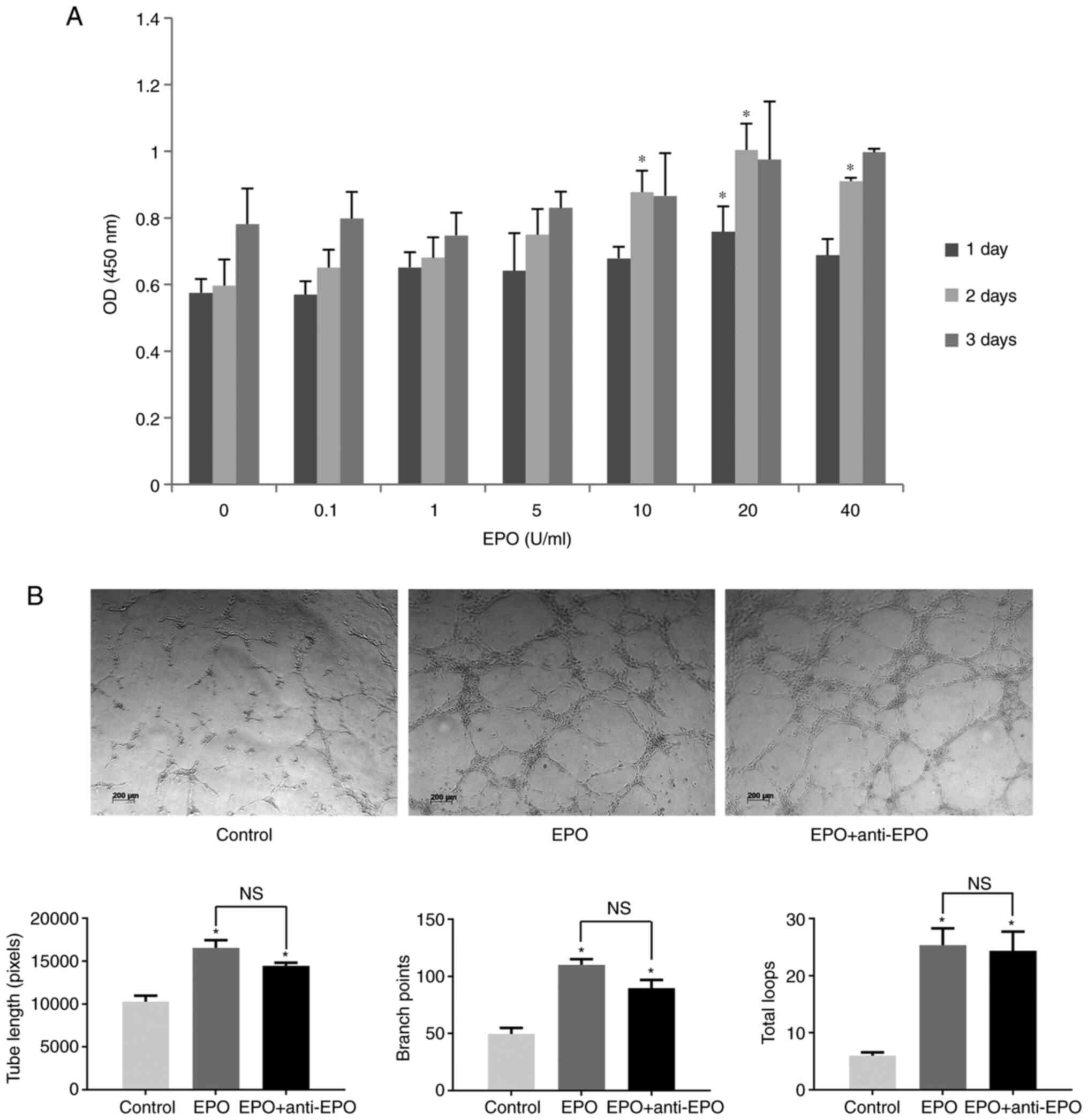
assessed with a CCK-8 assay. As presented in Fig. 1A, low concentrations of EPO (0.1, 1
or 5 U/ml) had no effect on DPC proliferation at any time-point.
However, the proliferation of DPCs was significantly above that of
the control (P<0.05) when exposed to 10, 20 or 40 U/ml EPO for 2
days. These results indicated that high concentrations of EPO
promoted the proliferation of DPCs.
In the Matrigel assay, vessel-like structures of
HUVECs were observed in the EPO group. By contrast, HUVECs in the
control group assembled into vessel wall analogs but hardly formed
any loops. Furthermore, addition of EPO neutralization antibody did
not significantly attenuate tube formation compared with the EPO
group. Quantitative data indicated that the tube lengths, branch
points and total loop amounts significantly increased in the EPO
group and EPO with antibody group (P<0.05). In addition, no
significant differences were observed between the EPO group and EPO
group with antibodies (Fig.
1B).
Expression of various
angiogenesis-related factors in CM of DPCs stimulated by EPO
The relative expression of 35 angiogenesis-related
factors, including 29 pro-angiogenic cytokines and 6
anti-angiogenic factors in the CM of DPCs, was detected by the
human angiogenesis protein array. As indicated in Fig. 2A-D and Table I, the expression of vascular
endothelial cell growth factor (VEGF), MMP-3, Ang-1/2 and
hepatocyte growth factor (HGF) significantly increased by >50%,
while the secretion of TGF-β, TNF-α, IL-2, IL-6 and IL-10 decreased
to ~40-50% compared with CM from DPCs untreated with EPO. In
addition, the expression of 4 out of 6 anti-angiogenic factors
(IL-10, IL-12p70, TIMP-1 and TIMP-4) decreased in the CM of DPCs
after EPO stimulation.
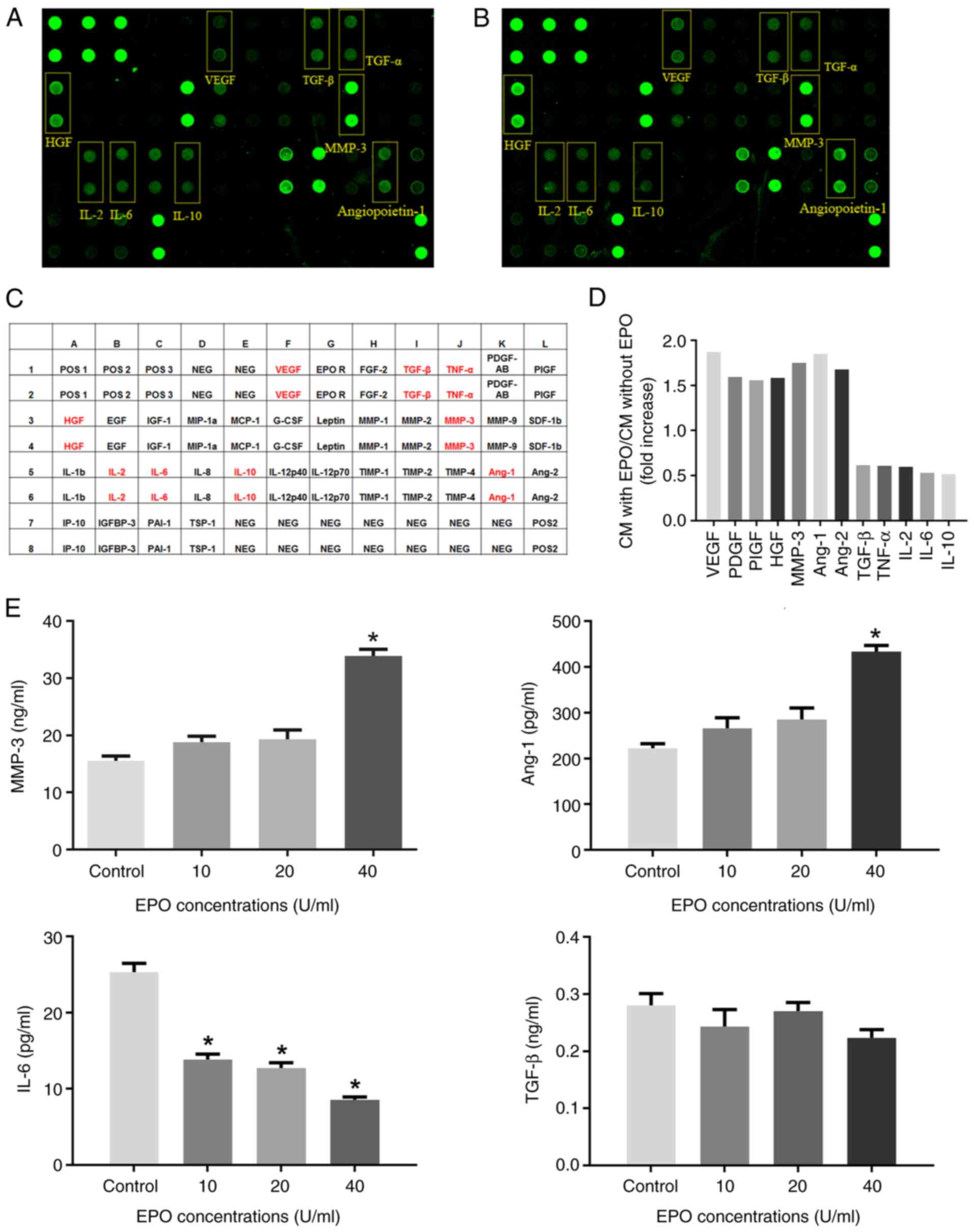 | Figure 2Protein array and ELISA of CM of DPCs.
(A) Protein array image of CM from DPCs; (B) protein array image of
CM from DPCs treated with EPO. (C) Map of the protein array;
positive proteins are marked in red. (D) Fold changes of the
fluorescence signals of positive proteins. (E) ELISA was used to
determine angiogenesis-associated cytokines MMP3, Ang-1, IL-6 and
TGF-β in the CM of DPCs treated with graded concentrations of EPO.
Values are expressed as the mean ± standard deviation of three
independent experiments. *P<0.05 vs. Control group.
EPO, erythropoietin; DPCs, dental pulp cells; CM, conditioned
medium; NEG, negative control; POS, positive control; VEGF,
vascular endothelial growth factor; PDGF, platelet-derived growth
factor; HGF, hepatocyte growth factor; Ang-1, angiopoietin-1; PIGF,
phosphatidylinositol glycan anchor biosynthesis class F; IGF,
insulin-like growth factor; IGFBP, IGF binding protein; EGF,
epidermal growth factor; MIF, macrophage migration inhibitory
factor; MCF, monocyte chemoattractant protein; G-CSF, granulocyte
colony-stimulating factor; FGF, fibroblast growth factor; TIMP1,
TIMP metallopeptidase inhibitor 1; SDF, stromal cell-derived
factor; PAI-1, plasminogen activator inhibitor- 1; IP-10,interferon
inducible protein-10; MIP-1a, macrophage inflammatory protein-1a;
TSP, thrombin-sensitivity protein. |
 | Table IProfile of cytokines in conditioned
medium of dental pulp cells induced by EPO. |
Table I
Profile of cytokines in conditioned
medium of dental pulp cells induced by EPO.
| A, Pro-angiogenic
factors and receptors |
|---|
| Cytokine | EPO/control
ratio |
|---|
| VEGF | 1.871 |
| EPOR | 1.268 |
| bFGF | 0.849 |
| TGF-β | 0.616 |
| TNF-α | 0.609 |
| PDGF-AB | 1.595 |
| PIGF | 1.557 |
| HGF | 1.585 |
| EGF | 1.299 |
| IGF-1 | 0.777 |
| Ang-1 | 1.849 |
| Ang-2 | 1.677 |
| IGFBP-3 | 1.200 |
| Leptin | 0.931 |
| MMP-1 | 0.834 |
| MMP-2 | 1.078 |
| MMP-3 | 1.750 |
| MMP-9 | 1.302 |
| IL-1β | 1.241 |
| IL-2 | 0.597 |
| IL-6 | 0.531 |
| IL-8 | 0.705 |
| SDF-1β | 1.001 |
| PAI-1 | 0.896 |
| IL-12p40 | 0.930 |
| MIP-1α | 0.950 |
| MCP-1 | 0.858 |
| G-CSF | 0.682 |
| IP-10 | 0.921 |
| B, Anti-angiogenic
cytokines |
| Cytokine | EPO/control
ratio |
| IL-10 | 0.518 |
| IL-12p70 | 0.950 |
| TIMP-1 | 0.687 |
| TIMP-2 | 1.272 |
| TIMP-4 | 0.898 |
|
Thrombospondin-1 | 1.403 |
Furthermore, ELISA indicated that 40 U/ml EPO
significantly increased the secretion of MMP-3 and Ang-1 in
comparison with the control group (P<0.05). However, lower
concentrations (10 and 20 U/ml) of EPO had no significant effect on
MMP-3 and Ang-1 production in DPCs. Furthermore, EPO significantly
decreased the secretion of IL-6 but had no obvious effect on TGF-β
secretion (Fig. 2E).
Intracellular pathways of the DPCs
activated by EPO
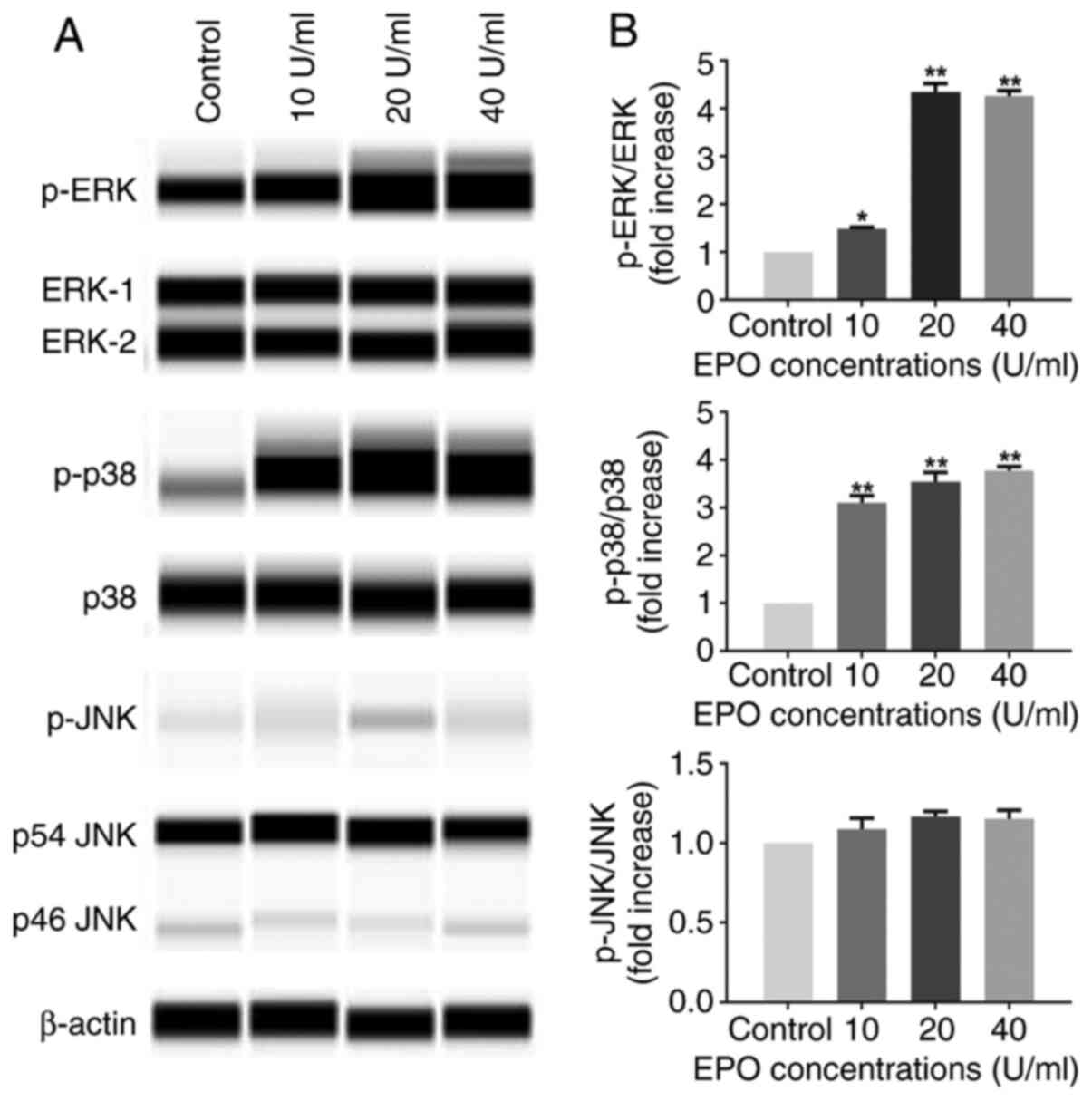
For evaluation of the activity of MAPK, the ratio of
phosphorylated to total protein was quantified. As indicated in
Fig. 3, the levels of p-ERK and
p-p38 were significantly increased in DPCs after exposure to EPO
for 1 h, whereas the phosphorylation levels of JNK were not
enhanced compared with the control group. However, the total
protein levels of ERK1/2, p38 and JNK were maintained at the same
level under EPO stimulation.
Signaling pathways involved in
EPO-induced angiogenesis of DPCs
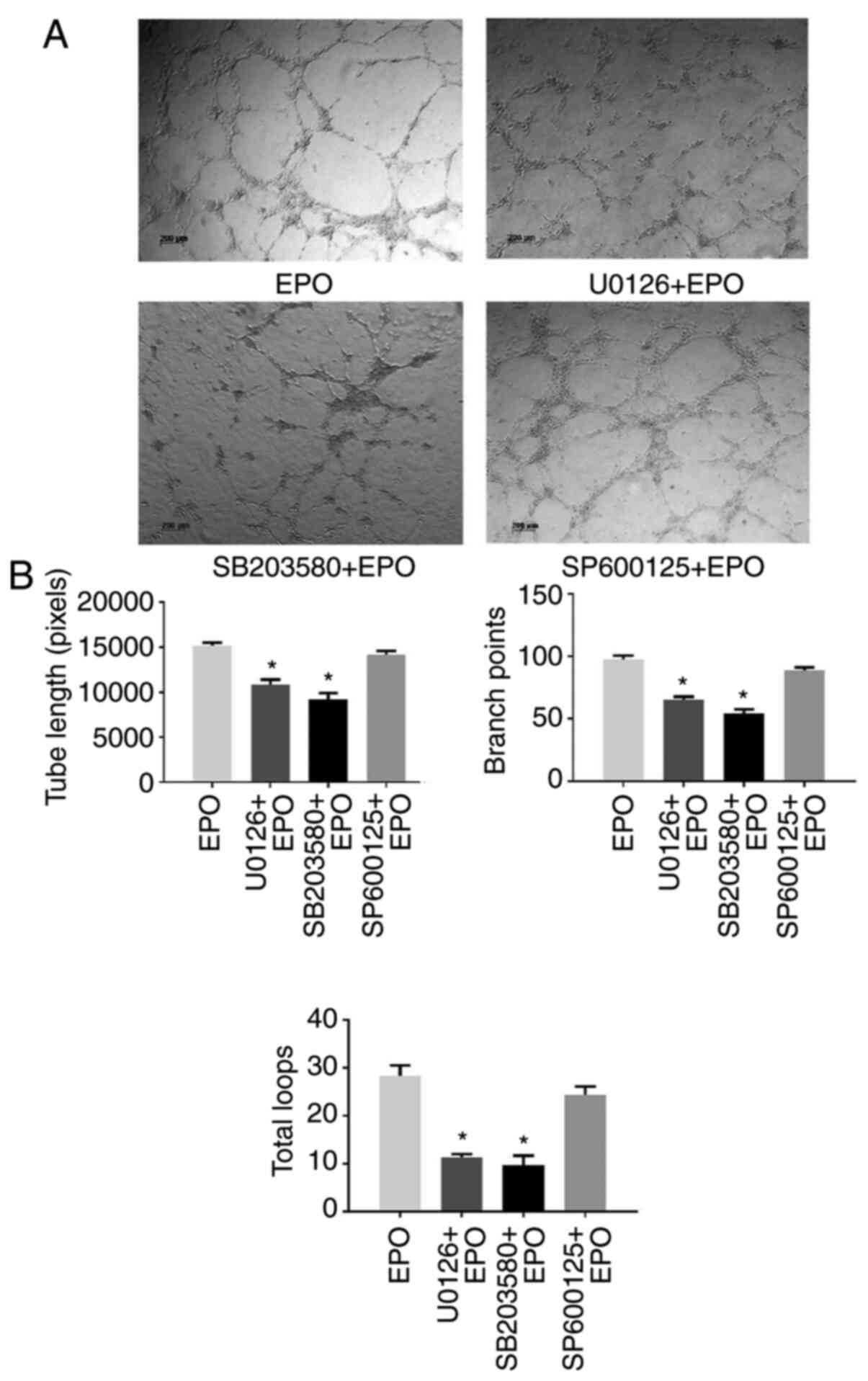
To further determine the molecular mechanisms of
EPO-induced angiogenesis, DPCs were pretreated with MAPK signaling
pathway inhibitors and then subjected to EPO stimulation.
Subsequently, CM from DPCs was collected to stimulate HUVECs. As
presented in Fig. 4, both U0126
(ERK1/2 inhibitor) and SB203580 (p38 inhibitor) impaired the
formation of capillary-like structures, while SP600125 (JNK
inhibitor) had no significant effect on tube formation, which was
also confirmed by the quantitative data.
Discussion
Dental pulp regeneration is challenging due to the
special structure of the enamel-dentin-pulp complex and the limited
blood and oxygen supply. Numerous studies have focused on the key
role of DPCs, as they have multidifferentiation potential and
affect stem cells in a paracrine manner. EPO has been indicated to
protect against hypoxia and ischemia and exert anti-inflammatory,
anti-apoptosis and pro-angiogenesis effects within numerous organs
(17-19).
Koutsoumparis et al (20)
indicated that recombinant human EPO promoted endothelial
transdifferentiation of stem cells from the apical papilla, which
may be of clinical value in the treatment of ischemic disorders.
The present study was the first, to the best of our knowledge, to
report that EPO promoted the angiogenic potential of DPCs in a
paracrine manner. EPO stimulated the secretion of various
angiogenic cytokines in DPCs and thus promoted tube formation of
endothelial cells through ERK1/2 and p38 MAPK signaling.
Proliferation and migration are the primary steps of
dental pulp repair. Lin et al (21) demonstrated that EPO induced the
proliferation of MSCs and overexpression of EPO enhanced the
migration capacity of MSCs. Zwezdaryk et al (22) revealed that low levels of EPO
promoted human MSC proliferation, whereas high levels of EPO
(>30 U/ml) more successfully affected cell migration and
angiogenesis potential. Previous research by our group also
indicated that EPO increased C-X-C motif chemokine receptor 4 and
stromal cell-derived factor-1 expression and enhanced DPC migration
in vitro (data not shown). Consistently, it was observed in
the present study that the proliferation of DPCs increased the most
in the 20 U/ml EPO group, while a lower concentration of EPO had no
such effect. However, other studies reported that EPO had no
effects on bone marrow stromal cell proliferation (23,24).
Therefore, it was speculated that the inconsistency of these
studies was due to different types of EPO, cell types and
experimental conditions; and EPO was potent only if its
concentration reached a certain magnitude and sufficient EPOR
requires to be activated to achieve biological efficiency.
Various studies confirmed that EPO promoted tissue
repair by supporting or inducing angiogenesis (25,26),
but whether EPO has similar effects on DPCs has remained elusive.
Previous studies have demonstrated that autocrine and/or paracrine
factors are the secretion mechanism of MSCs within tissue
regeneration (27). Likewise,
different research groups performed studies on paracrine effects of
DPSC-CM on endothelial cells and angiogenesis. Shen et al
(28) reported that DPSC-derived CM
significantly promoted angiogenesis in hind limb ischemia,
suggesting that paracrine effects are of great importance in
angiogenesis. In the present study, it was observed that CM from
DPCs stimulated by EPO significantly promoted the formation of
vessel-like structures by HUVECs. Addition of EPO antibody did not
significantly attenuate angiogenesis, which not only excluded the
direct effects of EPO but also demonstrated that EPO enhanced tube
formation by HUVECs through the cytokines secreted by DPCs.
However, the molecular mechanisms by which EPO
promotes angiogenesis through DPCs have remained to be fully
elucidated. Thus, a protein array was performed in the present
study to examine the differential expression profile of cytokines
in CM of DPCs treated with EPO. Serum-free CM was used to simulate
the inflammatory environment of injured pulp. Among all the
angiogenesis-related factors selected, the secretion of
pro-angiogenesis factors in CM, such as VEGF, HGF, MMP-3, Ang-1 and
Ang-2, were all markedly upregulated, whereas TGF-β, TNF-α, IL-2,
IL-6 and IL-10 were downregulated after EPO treatment. Since the
downregulated cytokines are also pro-inflammatory cytokines, these
changes may be speculated to be related to the possible
anti-inflammatory effects of EPO (29-31).
Subsequently, ELISA confirmed that MMP-3 and Ang-1 in the CM was
upregulated in an EPO dose-dependent manner, while downregulation
of IL-6 secretion was detected by the protein array. According to
previous studies, MMP-3 and Ang-1 are vital factors involved in the
process of angiogenesis in dental pulp and other tissues (32,33).
IL-6, as an inflammatory cytokine, has a critical role in dental
pulp inflammation (34). These
results suggested that EPO may exert angiogenic potential and
anti-inflammatory activity in dental pulp repair. Xu et al
(35) indicated that hydrogels
loaded with aspirin/EPO are effective in the control of
inflammation and regeneration of the periodontium. A recent study
also demonstrated that an EPO-impregnated collagen scaffold
promoted new bone formation in an alveolar defect through coupled
angiogenesis/osteogenesis (36).
Indeed, the mineralization of the dentin matrix during
dentinogenesis is similar to the osteogenesis processes. Based on
the present and previous results, it was hypothesized that
engineered scaffolds loaded with EPO may open new avenues for
future research in dental pulp regeneration and provide a promising
strategy for clinical applications for the treatment of
pulpitis.
Activation of EPOR usually triggers three major
intracellular signaling cascades: JAK2/STAT5, PI3K/Akt and
RAS/MAPK. The activation of MAPK pathways is known to respond to
various exogenous stresses by ischemia and is involved in
angiogenesis. Kwon et al (37) indicated that EPO induced the gradual
elevation of ERK1/2 and p38 expression as time progressed in C6
glioma cells and the protective effects of EPO against cytotoxicity
were significantly attenuated with pretreatment with p-ERK1/2 or
p-p38 inhibitor. In the present study, the phosphorylation of
ERK1/2, p38, and JNK was investigated and the results demonstrated
that EPO increased the angiogenesis capacity of DPCs through ERK1/2
and p38 signaling but not JNK. However, contrary to the present
results, p-JNK protein levels decreased significantly in the EPO
group in a rat model of ischemia-reperfusion injury (38). It may be inferred that the
activation of signaling pathways may vary from species to
species.
In conclusion, the present results revealed that EPO
enhanced the angiogenic potential of DPCs through the secretion of
angiogenic cytokines under serum deprivation conditions. In
addition, ERK1/2 and p38 MAPK signaling pathways participate in
this process. Although whether EPO is effective in vivo
remains to be determined, these results indicated that EPO has an
essential role in dental pulp repair and may be applied in pulp
regeneration.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the National Natural
Science Foundation of China (grant nos. 81870750 and 81700957), the
Natural Science Foundation of Guangdong Province (grant no.
2016A030310197) and the Fundamental Research Funds for the Central
Universities (grant no. 12ykpy65).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL conceived the present study. QG, JZ, YH, CC and
JQ performed the experiments. QG, JZ and XZ designed the study,
analyzed the data and wrote the manuscript. All authors read and
approved the final manuscript. QG and JZ confirm the authenticity
of the raw data.
Ethics approval and consent to
participate
All experiments were approved by The Research Ethics
Committee of Hospital of Stomatology, Guanghua School of
Stomatology, Sun Yat-sen University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saghiri MA, Asatourian A, Sorenson CM and
Sheibani N: Role of angiogenesis in endodontics: contributions of
stem cells and proangiogenic and antiangiogenic factors to dental
pulp regeneration. J Endod. 41:797–803. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liekens S, De Clercq E and Neyts J:
Angiogenesis: Regulators and clinical applications. Biochem
Pharmacol. 61:253–270. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu L, Ling J, Wei X, Wu L and Xiao Y:
Stem cell regulatory gene expression in human adult dental pulp and
periodontal ligament cells undergoing odontogenic/osteogenic
differentiation. J Endod. 35:1368–1376. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bronckaers A, Hilkens P, Fanton Y, Struys
T, Gervois P, Politis C, Martens W and Lambrichts I: Angiogenic
properties of human dental pulp stem cells. PLoS One.
8(e71104)2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Paschalidis T, Bakopoulou A, Papa P,
Leyhausen G, Geurtsen W and Koidis P: Dental pulp stem cells'
secretome enhances pulp repair processes and compensates
TEGDMA-induced cytotoxicity. Dent Mater. 30:e405–e418.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gharaei MA, Xue Y, Mustafa K, Lie SA and
Fristad I: Human dental pulp stromal cell conditioned medium alters
endothelial cell behavior. Stem Cell Res Ther. 9(69)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kichenbrand C, Velot E, Menu P and Moby V:
Dental pulp stem cell-derived conditioned medium: An attractive
alternative for regenerative therapy. Tissue Eng Part B Rev.
25:78–88. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tilbrook PA and Klinken SP: Erythropoietin
and erythropoietin receptor. Growth Factors. 17:25–35.
1999.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yan F, Zhang M, Meng Y, Li H, Yu L, Fu X,
Tang Y and Jiang C: Erythropoietin improves hypoxic-ischemic
encephalopathy in neonatal rats after short-term anoxia by
enhancing angiogenesis. Brain Res. 1651:104–113. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Park SL, Won SY, Song JH, Kambe T, Nagao
M, Kim WJ and Moon SK: EPO gene expression promotes proliferation,
migration and invasion via the p38MAPK/AP-1/MMP-9 pathway by
p21WAF1 expression in vascular smooth muscle cells. Cell Signal.
27:470–478. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lu H, Wu X, Wang Z, Li L, Chen W, Yang M,
Huo D, Zeng W and Zhu C: Erythropoietin-activated mesenchymal stem
cells promote healing ulcers by improving microenvironment. J Surg
Res. 205:464–473. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhu L, Bai X, Wang S, Hu Y, Wang T, Qian L
and Jiang L: Recombinant human erythropoietin augments angiogenic
responses in a neonatal rat model of cerebral unilateral
hypoxia-ischemia. Neonatology. 106:143–148. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wan L, Zhang F, He Q, Tsang WP, Lu L, Li
Q, Wu Z, Qiu G, Zhou G and Wan C: EPO promotes bone repair through
enhanced cartilaginous callus formation and angiogenesis. PLoS One.
9(e102010)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Irrera N, Bitto A, Pizzino G, Vaccaro M,
Squadrito F, Galeano M, Stagno d'Alcontres F, Stagno d'Alcontres F,
Buemi M, Minutoli L, et al: Epoetin alpha and epoetin zeta: A
comparative study on stimulation of angiogenesis and wound repair
in an experimental model of burn injury. Biomed Res Int.
2015(968927)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gong Q, Jiang H, Wei X, Ling J and Wang J:
Expression of erythropoietin and erythropoietin receptor in human
dental pulp. J Endod. 36:1972–1977. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ammarguellat F, Llovera M, Kelly PA and
Goffin V: Low doses of EPO activate MAP kinases but not JAK2-STAT5
in rat vascular smooth muscle cells. Biochem Biophys Res Commun.
284:1031–1038. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Suresh S, Rajvanshi PK and Noguchi CT: The
many facets of erythropoietin physiologic and metabolic response.
Front Physiol. 10(1534)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hu R, Cheng Y, Jing H and Wu H:
Erythropoietin promotes the protective properties of transplanted
endothelial progenitor cells against acute lung injury via PI3K/Akt
pathway. Shock. 42:327–336. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Weng S, Zhu X, Jin Y, Wang T and Huang H:
Protective effect of erythropoietin on myocardial infarction in
rats by inhibition of caspase-12 expression. Exp Ther Med.
2:833–836. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Koutsoumparis A, Vassili A, Bakopoulou A,
Ziouta A and Tsiftsoglou AS: Erythropoietin (rhEPOa) promotes
endothelial transdifferentiation of stem cells of the apical
papilla (SCAP). Arch Oral Biol. 96:96–103. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lin H, Luo X, Jin B, Shi H and Gong H: The
effect of EPO gene overexpression on proliferation and migration of
mouse bone marrow-derived mesenchymal stem cells. Cell Biochem
Biophys. 71:1365–1372. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zwezdaryk KJ, Coffelt SB, Figueroa YG, Liu
J, Phinney DG, LaMarca HL, Florez L, Morris CB, Hoyle GW and
Scandurro AB: Erythropoietin, a hypoxia-regulated factor, elicits a
pro-angiogenic program in human mesenchymal stem cells. Exp
Hematol. 35:640–652. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang D, Zhang F, Zhang Y, Gao X, Li C, Ma
W and Cao K: Erythropoietin enhances the angiogenic potency of
autologous bone marrow stromal cells in a rat model of myocardial
infarction. Cardiology. 108:228–236. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Koh SH, Noh MY, Cho GW, Kim KS and Kim SH:
Erythropoietin increases the motility of human bone
marrow-multipotent stromal cells (hBM-MSCs) and enhances the
production of neurotrophic factors from hBM-MSCs. Stem Cells Dev.
18:411–421. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kimáková P, Solár P, Solárová Z, Komel R
and Debeljak N: Erythropoietin and its angiogenic activity. Int J
Mol Sci. 18(1519)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mizukami T, Iso Y, Sato C, Sasai M, Spees
JL, Toyoda M, Umezawa A, Miyazaki A and Suzuki H: Priming with
erythropoietin enhances cell survival and angiogenic effect of
mesenchymal stem cell implantation in rat limb ischemia. Regen
Ther. 4:1–8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Baraniak PR and McDevitt TC: Stem cell
paracrine actions and tissue regeneration. Regen Med. 5:121–143.
2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shen CY, Li L, Feng T, Li JR, Yu MX, Lu Q
and Li H: Dental pulp stem cells derived conditioned medium
promotes angiogenesis in hindlimb ischemia. Tissue Eng Regen Med.
12:59–68. 2015.
|
|
29
|
Kai-Ian W and Si Z: Pretreatment with
erythropoietin attenuates intestinal ischemia reperfusion injury by
further promoting PI3K/Akt signaling activation. Transplant Proc.
47:1639–1645. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu QS, Cheng ZW, Xiong JG, Cheng S, He XF
and Li XC: Erythropoietin pretreatment exerts anti-inflammatory
effects in hepatic ischemia/reperfusion-injured rats via
suppression of the TLR2/NF-κB pathway. Transplant Proc. 47:283–289.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cravedi P, Manrique J, Hanlon KE,
Reid-Adam J, Brody J, Prathuangsuk P, Mehrotra A and Heeger PS:
Immunosuppressive effects of erythropoietin on human alloreactive T
cells. J Am Soc Nephrol. 25:2003–2015. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zheng L, Amano K, Iohara K, Ito M,
Imabayashi K, Into T, Matsushita K, Nakamura H and Nakashima M:
Matrix metalloproteinase-3 accelerates wound healing following
dental pulp injury. Am J Pathol. 175:1905–1914. 2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Koh GY: Orchestral actions of
angiopoietin-1 in vascular regeneration. Trends Mol Med. 19:31–39.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Elsalhy M, Azizieh F and Raghupathy R:
Cytokines as diagnostic markers of pulpal inflammation. Int Endod
J. 46:573–580. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xu X, Gu Z, Chen X, Shi C, Liu C, Liu M,
Wang L, Sun M, Zhang K, Liu Q, et al: An injectable and
thermosensitive hydrogel: Promoting periodontal regeneration by
controlled-release of aspirin and erythropoietin. Acta Biomater.
86:235–246. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Pandya M, Saxon M, Bozanich J, Tillberg C,
Luan X and Diekwisch TGH: The glycoprotein/cytokine erythropoietin
promotes rapid alveolar ridge regeneration in vivo by promoting new
bone extracellular matrix deposition in conjunction with coupled
angiogenesis/osteogenesis. Int J Mol Sci. 22(2788)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kwon MS, Kim MH, Kim SH, Park KD, Yoo SH,
Oh IU, Pak S and Seo YJ: Erythropoietin exerts cell protective
effect by activating PI3K/Akt and MAPK pathways in C6 cells. Neurol
Res. 36:215–223. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pappo O, Ben-Ari Z, Shevtsov E, Avlas O,
Gassmann M, Ravid A, Cheporko Y and Hochhauser E: The role of
excessive versus acute administration of erythropoietin in
attenuating hepatic ischemia-reperfusion injury. Can J Physiol
Pharmacol. 88:1130–1137. 2010.PubMed/NCBI View
Article : Google Scholar
|