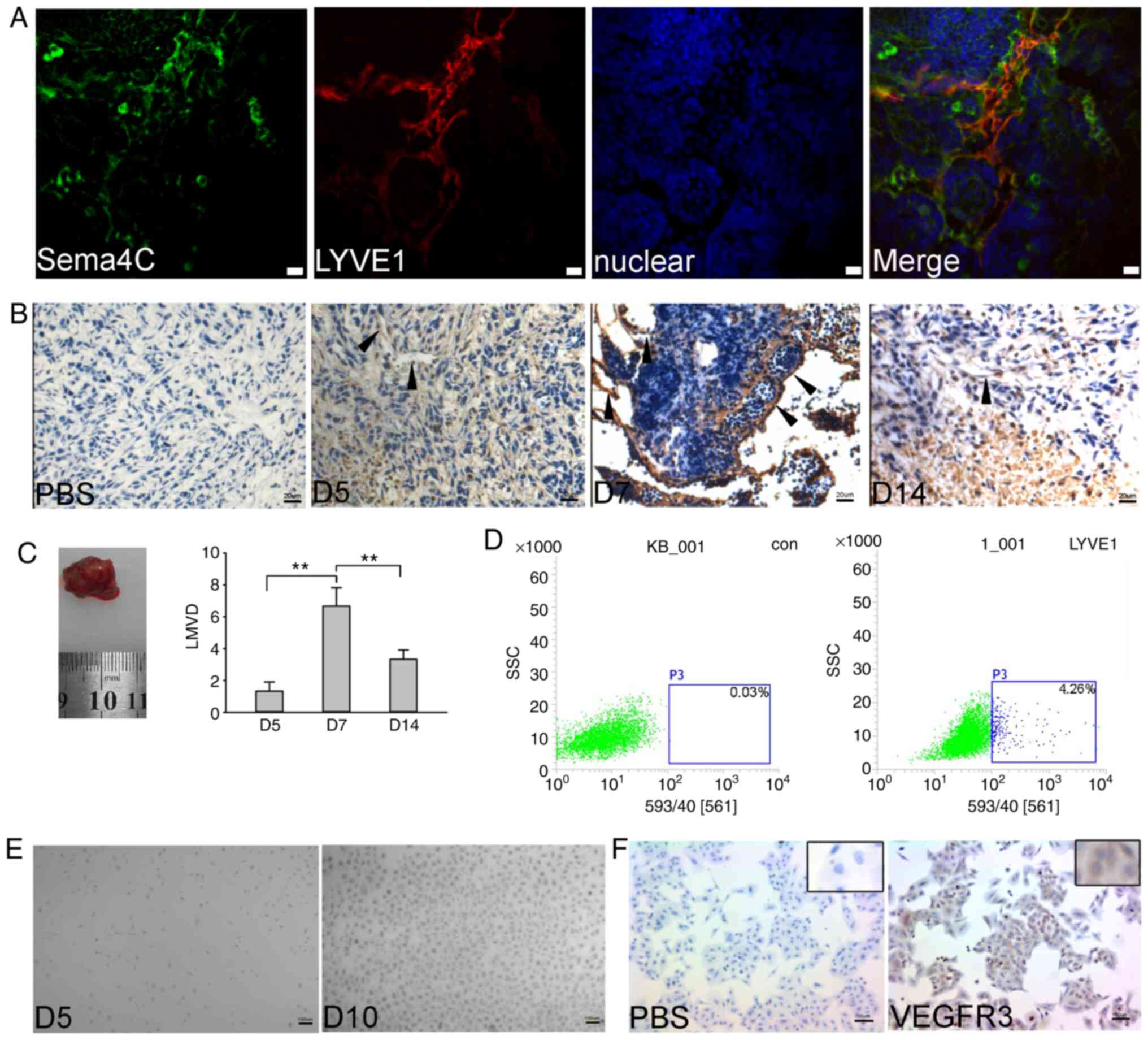
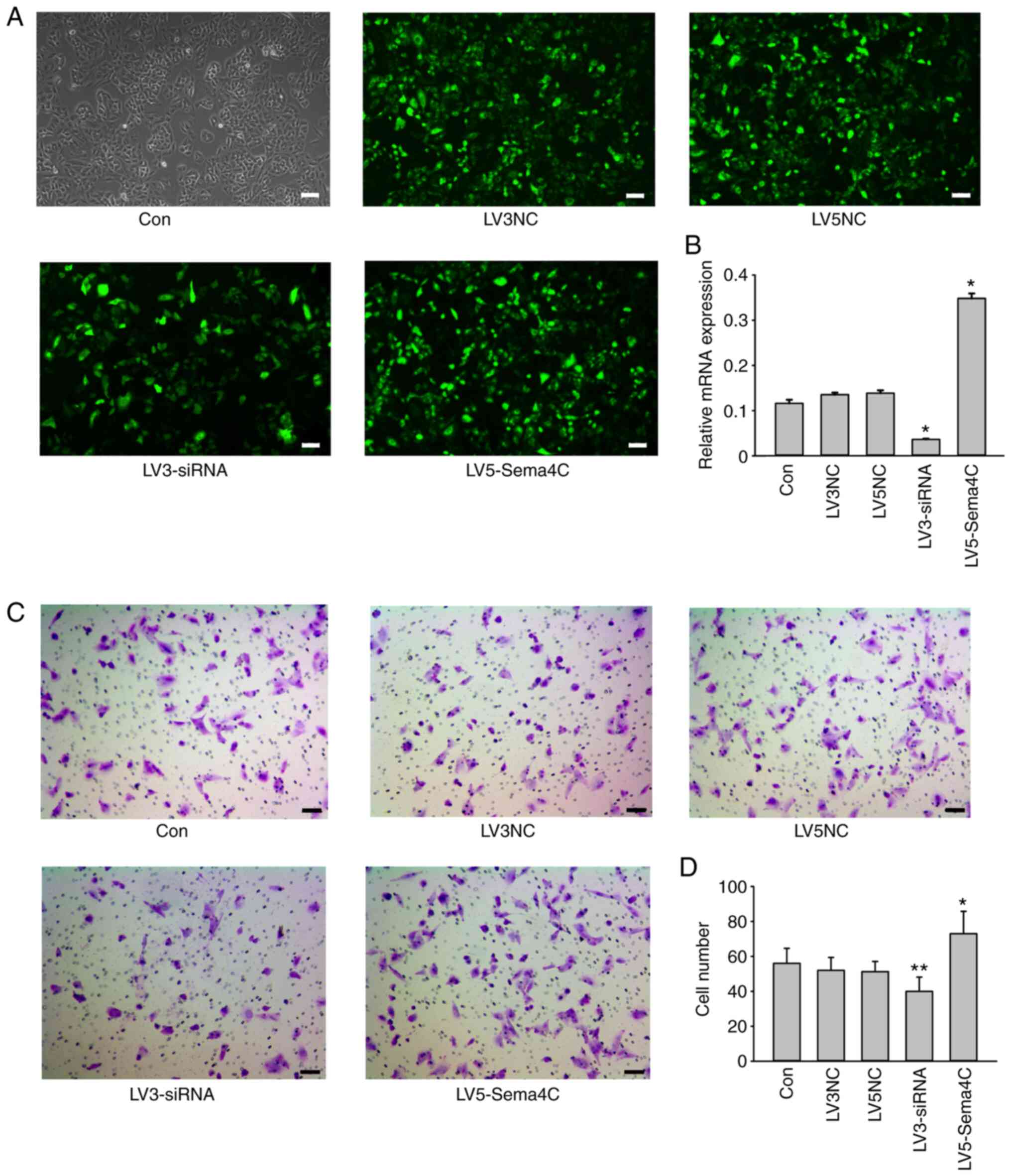
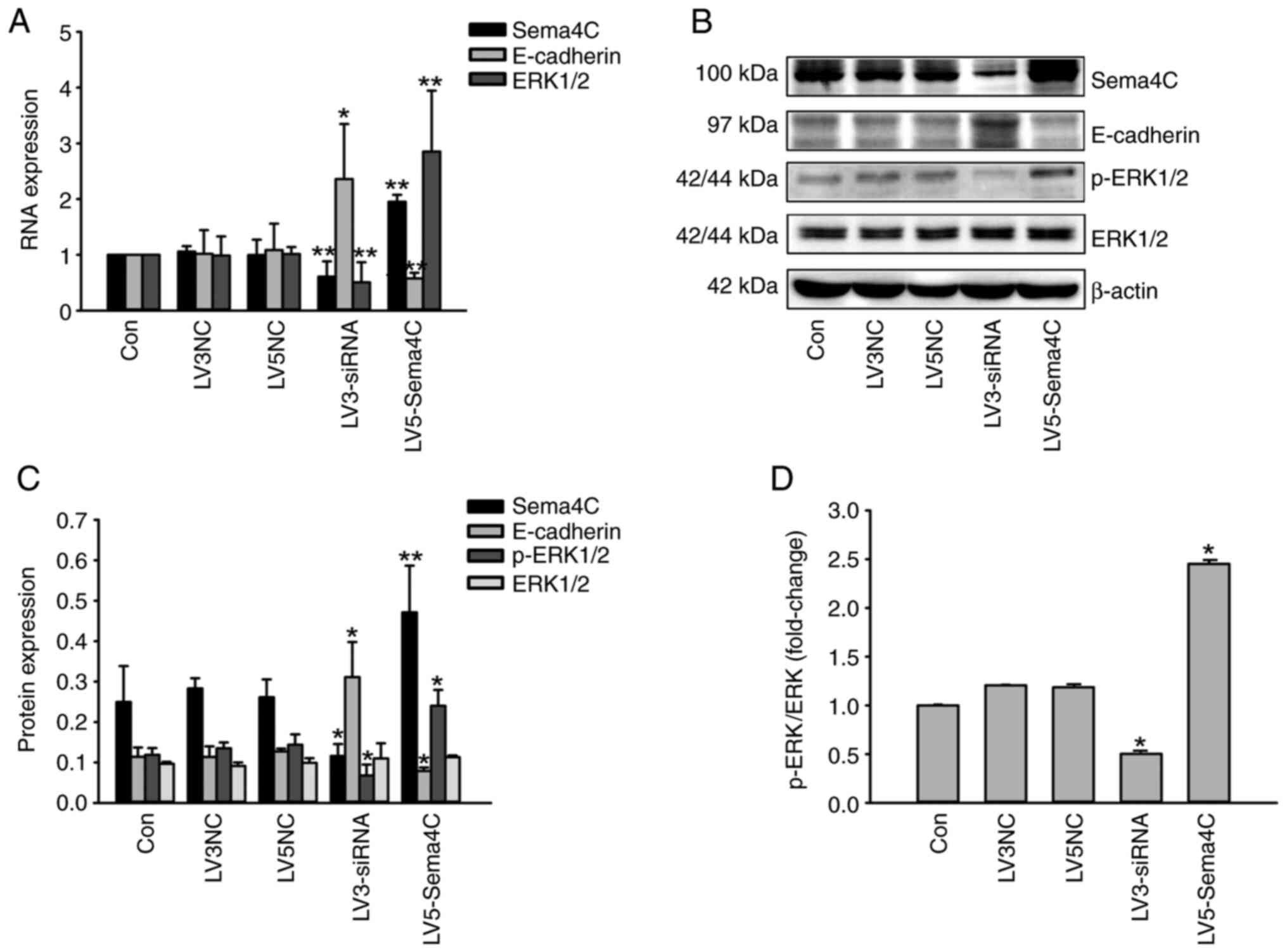
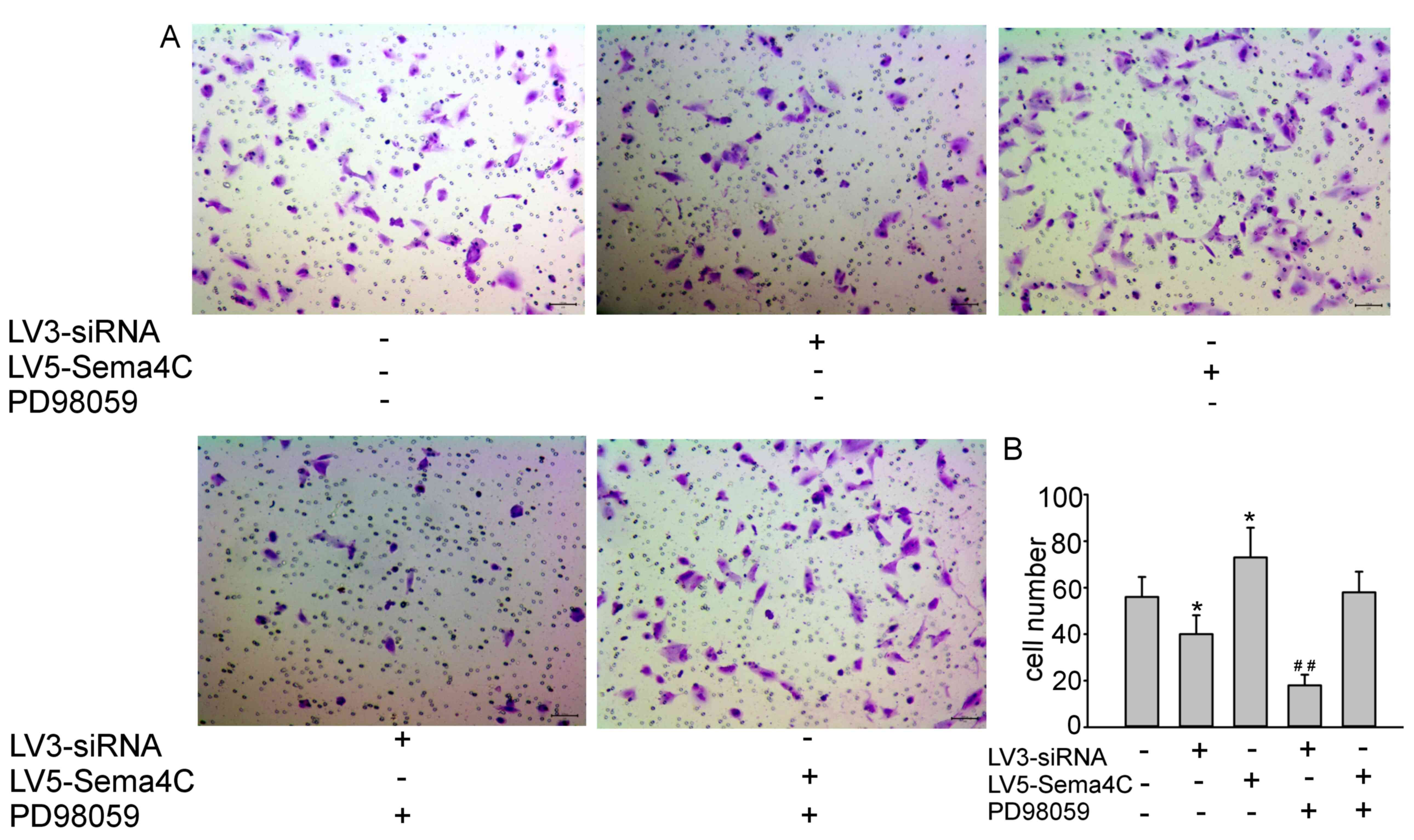
|
1
|
Frumovitz M: Sentinel lymph node biopsy
for cervical cancer patients-what's it gonna take? Gynecol Oncol.
144:3–4. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li H, Wu X and Cheng X: Advances in
diagnosis and treatment of metastatic cervical cancer. J Gynecol
Oncol. 27(e43)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hoshino A and Lyden D: Metastasis:
Lymphatic detours for cancer. Nature. 546:609–610. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Lucas ED and Tamburini BAJ: Lymph node
lymphatic endothelial cell expansion and contraction and the
programming of the immune response. Front Immunol.
10(36)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Saito A: EMT and EndMT: Regulated in
similar ways? J Biochem. 153:493–495. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kourtidis A, Lu R, Pence LJ and
Anastasiadis PZ: A central role for cadherin signaling in cancer.
Exp Cell Res. 358:78–85. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang SH, Chang JS, Hsiao JR, Yen YC, Jiang
SS, Liu SH, Chen YL, Shen YY, Chang JY and Chen YW: Tumour
cell-derived WNT5B modulates in vitro lymphangiogenesis via
induction of partial endothelial-mesenchymal transition of
lymphatic endothelial cells. Oncogene. 36:1503–1515.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee HR, Li F, Choi UY, Yu HR, Aldrovandi
GM, Feng P, Gao SJ, Hong YK and Jung JU: Deregulation of HDAC5 by
viral interferon regulatory factor 3 plays an essential role in
Kaposi's sarcoma-associated herpesvirus-induced lymphangiogenesis.
mBio. 9:e02217–17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jiang L, Hao C, Li Z, Zhang P, Wang S,
Yang S, Wei F and Zhang J: miR-449a induces EndMT, promotes the
development of atherosclerosis by targeting the interaction between
AdipoR2 and E-cadherin in Lipid Rafts. Biomed Pharmacother.
109:2293–2304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wu H, Wang X, Liu S, Wu Y, Zhao T, Chen X,
Zhu L, Wu Y, Ding X, Peng X, et al: Sema4C participates in myogenic
differentiation in vivo and in vitro through the p38 MAPK pathway.
Eur J Cell Biol. 86:331–344. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Elder AM, Tamburini BAJ, Crump LS, Black
SA, Wessells VM, Schedin PJ, Borges VF and Lyons TR: Semaphorin 7A
promotes macrophage-mediated lymphatic remodeling during postpartum
mammary gland involution and in breast cancer. Cancer Res.
78:6473–6485. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Acker DWM, Wong I, Kang M and Paradis S:
Semaphorin 4D promotes inhibitory synapse formation and suppresses
seizures in vivo. Epilepsia. 59:1257–1268. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sakurai A, Doci CL and Gutkind JS:
Semaphorin signaling in angiogenesis, lymphangiogenesis and cancer.
Cell Res. 22:23–32. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Doçi CL, Mikelis CM, Lionakis MS, Molinolo
AA and Gutkind JS: Genetic identification of SEMA3F as an
antilymphangiogenic metastasis suppressor gene in head and neck
squamous carcinoma. Cancer Res. 75:2937–2948. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nasarre P, Gemmill RM and Drabkin HA: The
emerging role of class-3 semaphorins and their neuropilin receptors
in oncology. Onco Targets Ther. 7:1663–1687. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wei JC, Yang J, Liu D, Wu MF, Qiao L, Wang
JN, Ma QF, Zeng Z, Ye SM, Guo ES, et al: Tumor-associated lymphatic
endothelial cells promote lymphatic metastasis by highly expressing
and secreting SEMA4C. Clin Cancer Res. 23:214–224. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Albini A, Mirisola V and Pfeffer U:
Metastasis signatures: Genes regulating tumor-microenvironment
interactions predict metastatic behavior. Cancer Metastasis Rev.
27:75–83. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Olmeda D, Cerezo-Wallis D,
Riveiro-Falkenbach E, Pennacchi PC, Contreras-Alcalde M, Ibarz N,
Cifdaloz M, Catena X, Calvo TG, Cañón E, et al: Whole-body imaging
of lymphovascular niches identifies pre-metastatic roles of
midkine. Nature. 546:676–680. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Young YK, Bolt AM, Ahn R and Mann KK:
Analyzing the tumor microenvironment by flow cytometry. Methods Mol
Biol. 1458:95–110. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lukacs-Kornek V: The role of lymphatic
endothelial cells in liver injury and tumor development. Front
Immunol. 7(548)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Faustino-Rocha A, Oliveira PA,
Pinho-Oliveira J, Teixeira-Guedes C, Soares-Maia R, da Costa RG,
Colaço B, Pires MJ, Colaço J, Ferreira R and Ginja M: Estimation of
rat mammary tumor volume using caliper and ultrasonography
measurements. Lab Anim (NY). 42:217–224. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Humbert M, Hugues S and Dubrot J: Shaping
of peripheral T cell responses by lymphatic endothelial cells.
Front Immunol. 7(684)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ji RC: Lymph nodes and cancer metastasis:
New perspectives on the role of intranodal lymphatic sinuses. Int J
Mol Sci. 18(51)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Potenta S, Zeisberg E and Kalluri R: The
role of endothelial-to-mesenchymal transition in cancer
progression. Br J Cancer. 99:1375–1379. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cho JG, Lee A, Chang W, Lee MS and Kim J:
Endothelial to mesenchymal transition represents a key link in the
interaction between inflammation and endothelial dysfunction. Front
Immunol. 9(294)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Harris AR, Perez MJ and Munson JM:
Docetaxel facilitates lymphatic-tumor crosstalk to promote
lymphangiogenesis and cancer progression. BMC Cancer.
18(718)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mumblat Y, Kessler O, Ilan N and Neufeld
G: Full-length semaphorin-3C Is an inhibitor of tumor
lymphangiogenesis and metastasis. Cancer Res. 75:2177–2186.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lawson CD and Ridley AJ: Rho GTPase
signaling complexes in cell migration and invasion. J Cell Biol.
217:447–457. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee G, Kim HJ and Kim HM: RhoA-JNK
regulates the E-cadherin junctions of human gingival epithelial
cells. J Dent Res. 95:284–291. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Connor AL, Kelley PM and Tempero RM:
Lymphatic endothelial lineage assemblage during corneal
lymphangiogenesis. Lab Invest. 96:270–282. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hou WH, Liu IH, Tsai CC, Johnson FE, Huang
SS and Huang JS: CRSBP-1/LYVE-1 ligands disrupt lymphatic
intercellular adhesion by inducing tyrosine phosphorylation and
internalization of VE-cadherin. J Cell Sci. 124:1231–1244.
2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhou QD, Ning Y, Zeng R, Chen L, Kou P, Xu
CO, Pei GC, Han M and Xu G: Erbin interacts with Sema4C and
inhibits Sema4C-induced epithelial-mesenchymal transition in HK2
cells. J Huazhong Univ Sci Technolog Med Sci. 33:672–679.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Marchese V, Juarez J, Patel P and
Hutter-Lobo D: Density-dependent ERK MAPK expression regulates
MMP-9 and influences growth. Mol Cell Biochem. 456:115–122.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tang FY, Chiang EP, Chung JG, Lee HZ and
Hsu CY: S-allylcysteine modulates the expression of E-cadherin and
inhibits the malignant progression of human oral cancer. J Nutr
Biochem. 20:1013–1020. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kawata K, Yugi K, Hatano A, Kokaji T,
Tomizawa Y, Fujii M, Uda S, Kubota H, Matsumoto M, Nakayama KI and
Kuroda S: Reconstruction of global regulatory network from
signaling to cellular functions using phosphoproteomic data. Genes
Cells. 24:82–93. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wick N, Saharinen P, Saharinen J,
Gurnhofer E, Steiner CW, Raab I, Stokic D, Giovanoli P, Buchsbaum
S, Burchard A, et al: Transcriptomal comparison of human dermal
lymphatic endothelial cells ex vivo and in vitro. Physiol Genomics.
28:179–192. 2007.PubMed/NCBI View Article : Google Scholar
|