Introduction
Cerebral ischemia-reperfusion injury (CIRI) refers
to an acute cerebral dysfunction that occurs after cerebral
ischemia for a certain period of time, followed by the restoration
of the infarction-associated blood supply (1). The cure rate associated with CIRI is
very low, accompanied by a high disability rate and a high
mortality rate; and the prognosis is very poor, with the condition
seriously affecting individual's health (2). CIRI is a complex multi-factorial
process whose underlying mechanism is poorly understood. The
current consensus is that post-ischemia reperfusion causes
pathophysiological cascade reactions, including the explosive
release of intracellular Ca2+ ions, excessive production
of reactive oxygen species and neutrophil recruitment, which in
turn leads to an acceleration of the inflammatory process, sustains
ischemia and causes cell damage (3). The underlying pathological mechanisms
include energy metabolism disorder, the inflammatory response,
oxidative stress, excitatory amino acid toxicity and apoptosis
(4).
Recently, a large number of studies have shown that
apoptosis induced by endoplasmic reticulum stress (ERS) fulfills an
important role in CIRI (5-7).
ERS occupies a central role in CIRI and can initiate neuronal
apoptosis (8). Hypoxia, energy
depletion, acidosis, damage to Ca2+ homeostasis and the
generation of a large number of free radicals caused by ischemia
and reperfusion can all induce ERS, and stress-protective effects
in cells may be generated in cells by ERS itself. However,
excessive or persistent ERS is able to activate apoptotic signaling
pathways, induce apoptosis and aggravate CIRI (9,10). A
previously published study reported that the ER-associated protein
reticulon 1 is able to mediate CIRI through ERS and
mitochondria-associated apoptotic pathways (11). In addition, a vascular endothelial
growth factor antagonist was demonstrated to attenuate
ischemic/reperfusion (I/R)-induced injury through inhibiting
ERS-mediated apoptosis (12).
Therefore, an increasing body of evidence supports that ERS has an
important role in CIRI.
Among the therapeutic drugs that are currently
available for cerebral infarction, statins are often used for
primary and secondary prevention, as these have been shown to be
important drugs for the treatment of cerebral infarction and to
significantly decrease the risk of patients with cerebral
infarction (13). In addition to
its powerful lipid-lowering effects, rosuvastatin is also useful in
terms of its anti-inflammatory, antioxidant and immunological
regulation effects, and for its ability to improve vascular
endothelial function (14). A
previous study revealed that rosuvastatin in combination with
resveratrol is able to protect the synergic nerve in cases of CIRI
(15). Rosuvastatin preconditioning
exerts a marked protective effect on focal CIRI in rats, and its
mechanism of action is predominantly to decrease oxidative stress
and the inflammatory response (16). Rosuvastatin in combination with
other drugs has been shown to decrease the size of cerebral
infarction (17). In addition, it
has been reported in other diseases that rosuvastatin may protect
umbilical venous endothelial cells from damage induced under
high-glucose conditions by decreasing ERS (18). Rosuvastatin was also shown to
inhibit the apoptosis of endothelial cells in ApoE-/-
mice via inhibiting ERS, and through the alleviation of
atherosclerosis (19). However, to
the best of our knowledge, the effect of rosuvastatin on ERS in
CIRI has not yet been reported.
In the present study, PC12 cells were induced to
establish a cell damage model by hypoxia/reoxygenation (H/R); the
apoptosis of the model cells was subsequently detected after
administering rosuvastatin, and the underlying mechanism was
investigated.
Materials and methods
Cell culture and model induction
PC12 cells were obtained from the American Type
Culture Collection and cultured in Dulbecco's Modified Eagle Medium
(Gibco; Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.). The PC12 cells were
cultured at 37˚C in an atmosphere containing 5% CO2 in a
humidified incubator. For induction of the H/R model, after culture
at 37˚C with 5% CO2 for 24 h, PC12 cells were placed
under hypoxic conditions (3% O2/5% CO2/92%
N2) for 2 h. The cells were then cultured under normal
conditions for 12 h. In terms of cell administration, PC12 cells
were pretreated with different concentrations of rosuvastatin
(0.01, 0.1, 1 and 10 µm, cat. no. HY-17504A, MedChemExpress) for 12
h at 37˚C before model induction.
Cell viability assay
Cell viability was measured using a Cell Counting
Kit (CCK)-8 assay according to the manufacturer's instructions
(Merck KGaA). Briefly, PC12 cells were seeded into 96-well plates
(1x104 cells/well). After the cells had reached 80%
confluence, they were treated accordingly, and CCK-8 solution (10
µl) was subsequently added to each well for an additional 4 h at
37˚C. The absorbance at 450 nm was measured with a microplate
reader (Biotek Instruments, Inc.). Cell viability was expressed as
a percentage of the control group cells.
Lactate dehydrogenase (LDH) release
assay
LDH detection is usually performed for evaluating
cytotoxicity. A commercial LDH assay kit was purchased from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China) and the LDH
assay was performed strictly according to the manufacturer's
instructions. The absorbance of each sample was detected at 440 nm
with a microplate reader. The percentage of cell death was
calculated according to the following formula: Viability
(%)=(ODTreatment-ODTreatment
blank)/(ODMax LDH activity-ODMax LDH activity
blank)x100%.
TUNEL assay
The extent of apoptosis was detected by TUNEL assay
(Promega Corporation), according to the manufacturer's
instructions. The PC12 cells (1x104 cells/well) were
incubated in 6-well plates for 24 h. Cells were subsequently fixed
using 4% paraformaldehyde for 30 min at 37˚C, and washed three
times with cold PBS. The TUNEL reaction mix was added to the cells
and incubated at 37˚C for 1 h. Cells were subsequently rinsed with
PBS, streptavidin-HRP was added and the mixture was incubated for 1
h in the dark. Finally, DAB solution was added to develop the color
reaction. Cells were observed under a light microscope
(magnification, x200), and images were captured. The number of
apoptotic cells was calculated as the mean proportion of positive
cells out of the total number of cells in six fields of view per
slide.
Western blot analysis
PC12 cells were washed three times with ice-cold
phosphate buffered saline, and lysis buffer (Merck KGaA) was added
to the cells for 30 min to isolate the total protein. Protein
concentration was determined using a bicinchoninic acid assay
protein assay kit (Bio-Rad Laboratories, Inc.). Proteins (25
µg/lane) were separated using 10% SDS-PAGE gel and transferred to a
polyvinylidene fluoride membrane, prior to blocking with 5% non-fat
milk in Tris-buffered saline/0.10% Tween-20 at room temperature for
2 h. The membranes were then incubated with the following primary
antibodies at 4˚C overnight (all antibodies were purchased from
Abcam): Anti-Bcl-2 (1:1,000; cat. no. ab182858), anti-Bax (1:1,000;
cat. no. ab182733), anti-cleaved caspase-3 (1:1,000; cat. no.
ab32042), anti-cleaved caspase-9 (1:1,000; cat. no. ab2324),
anti-caspase-3 (1:1,000; cat. no. ab32351), anti-caspase-9
(1:1,000; cat. no. ab32539), anti-phosphorylated (p)-protein kinase
R-like endoplasmic reticulum kinase (PERK; 1:1,000; cat. no.
ab192591), anti-p-eukaryotic initiation factor 2α (eIF2α; 1:1,000;
cat. no. ab32157), anti-eIF2α (1:1,000; cat. no. ab169528),
anti-PERK (1:1,000; cat. no. ab229912), anti-GRP78 (1:1,000; cat.
no. ab21685), anti-activating transcription factor 6 (ATF6;
1:1,000; cat. no. ab227830), anti-inositol-requiring transmembrane
kinase/endoribonuclease 1α (IRE1α; 1:1,000; cat. no. ab37073),
anti-X-box binding protein 1 (XBP1; 1:1,000; cat no. ab37152),
anti-C/EBP homologous protein (CHOP; 1:1,000; cat. no. ab11419) and
anti-GAPDH (1:1,000; cat. no. ab8245), and anti-cleaved
caspase-12/caspase 12 (1:1,000; cat. no. #2202; Cell Signaling
Technology, Inc.). After washing with Tris-buffered saline/0.10%
Tween-20, the blots were incubated with Goat Anti-Mouse IgG H&L
(Alexa Fluor® 488; 1:5,000; cat. no. ab150113; Abcam)
for 2 h at room temperature. Signals were visualized with enhanced
chemiluminescence (Beyotime Institute of Biotechnology), and ImageJ
software (Version1.8.0; National Institutes of Health) was used for
semi-quantification of the blots.
Statistical analysis
Data are expressed as the mean ± SD. Comparisons
among multiple groups were analyzed using a one-way ANOVA followed
by a Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Rosuvastatin inhibits decreased
cellular activity in H/R-induced PC12 cells
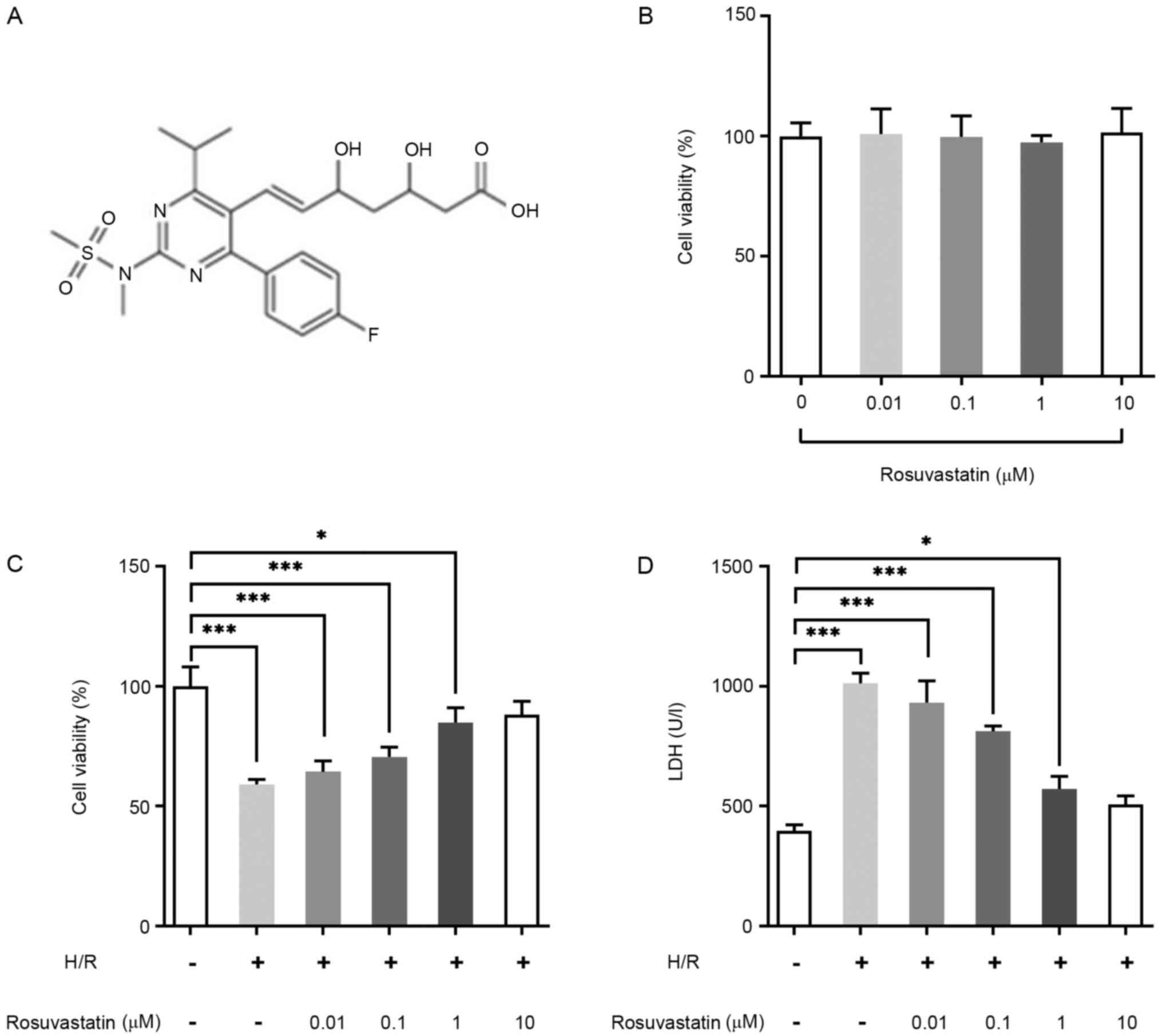
The chemical structure of rosuvastatin is shown in
Fig. 1A. PC12 cells were induced by
rosuvastatin at different concentrations (0, 0.01, 0.1, 1 and 10
µM). The results of the CCK-8 assay demonstrated that rosuvastatin
exerted no significant effect on the viability of PC12 cells
(Fig. 1B). Subsequently, CCK-8
assay was used to investigate the effect of rosuvastatin on the
viability of H/R-induced PC12 cells, and it was observed that, with
an increase in rosuvastatin concentration, the viability of
H/R-induced PC12 cells was significantly increased (Fig. 1C). LDH kit assay was then performed
for the detection of cytotoxicity, and these results showed that
rosuvastatin led to a marked decrease in the cytotoxicity of
H/R-induced PC12 cells (Fig. 1D).
Taken together, the results from these assays revealed that
rosuvastatin treatment led to decreased cellular activity in
H/R-induced PC12 cells. In addition, 1 µM Rosuvastatin had
significant effects on cell viability and cell LDH release, thus, 1
µM rosuvastatin was selected as the concentration for subsequent
experiments.
Rosuvastatin inhibits apoptosis of
H/R-induced PC12 cells
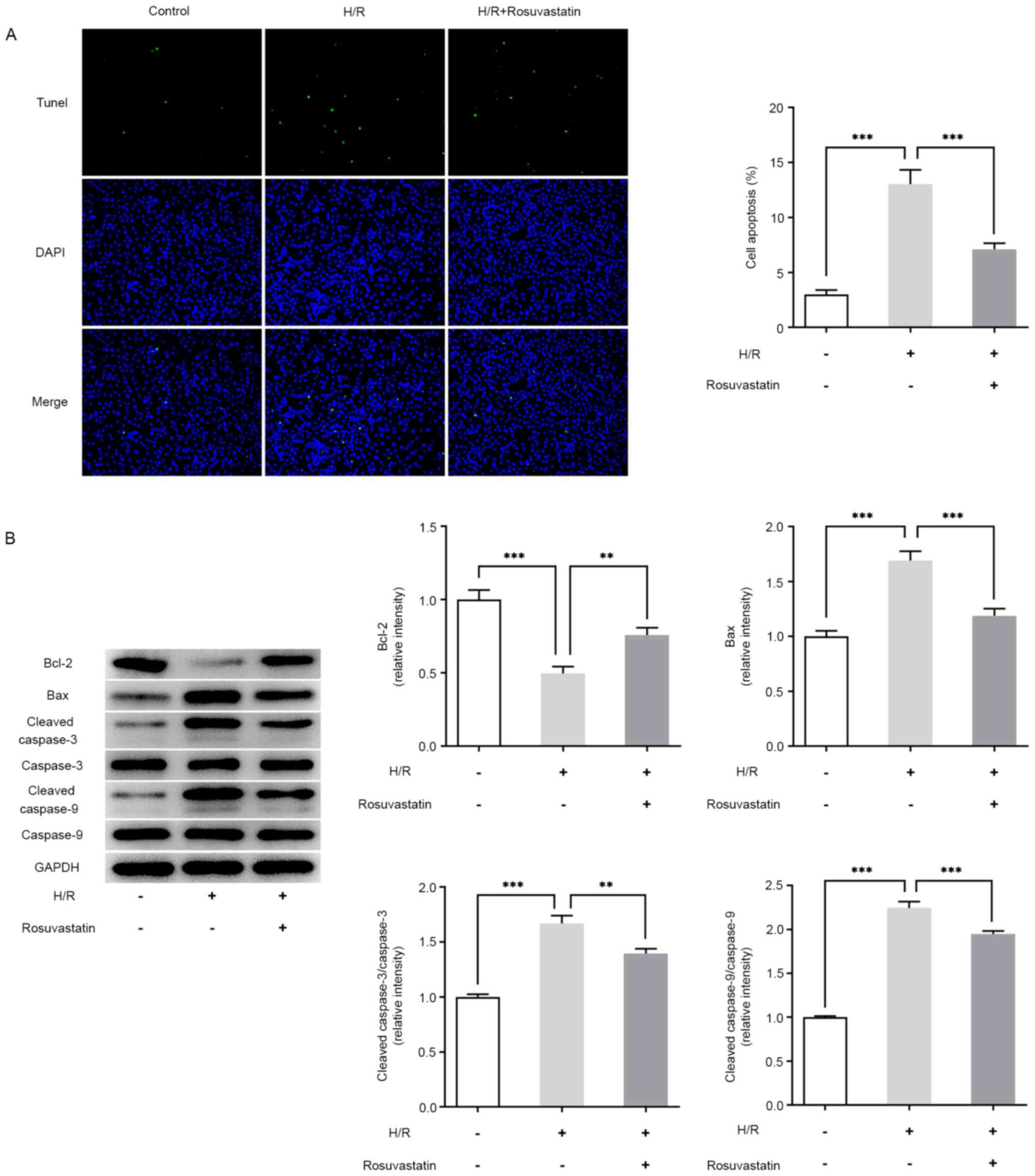
Apoptosis was detected using a TUNEL assay. Compared
with the control group, apoptosis was found to be significantly
increased following H/R induction (Fig.
2A), accompanied by an increased expression of the Bax, cleaved
caspase-3, cleaved caspase-9 proteins, whereas the expression level
of the Bcl-2 protein was decreased (Fig. 2B). Compared with the H/R group, the
apoptotic rate of the H/R + rosuvastatin group was decreased,
accompanied by a decreased expression of cleaved caspase-3, cleaved
caspase-9, and an increased expression of Bcl-2. These results
showed that rosuvastatin is able to inhibit apoptosis in
H/R-induced PC12 cells.
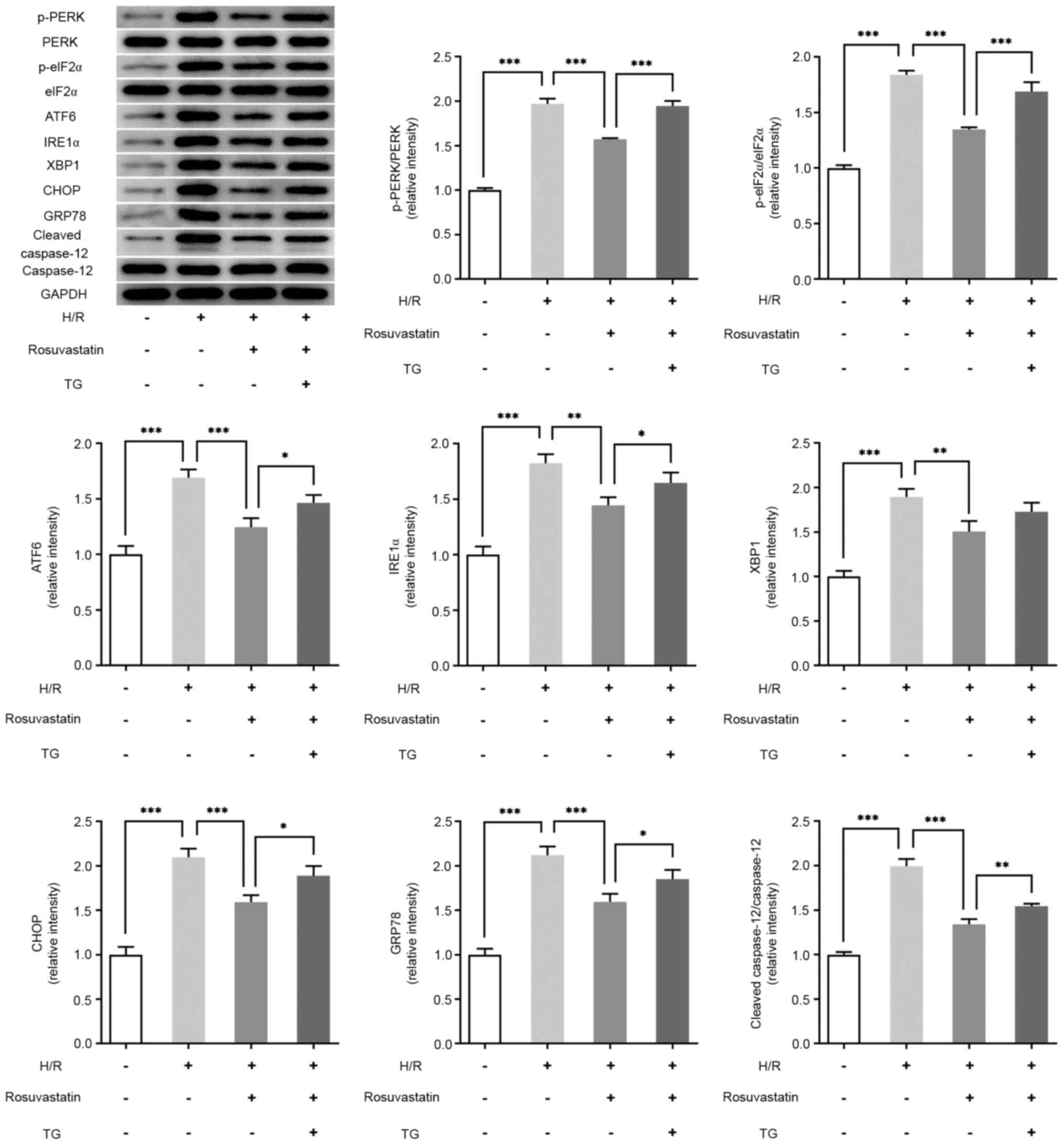
Rosuvastatin inhibits ERS levels in
H/R-induced PC12 cells
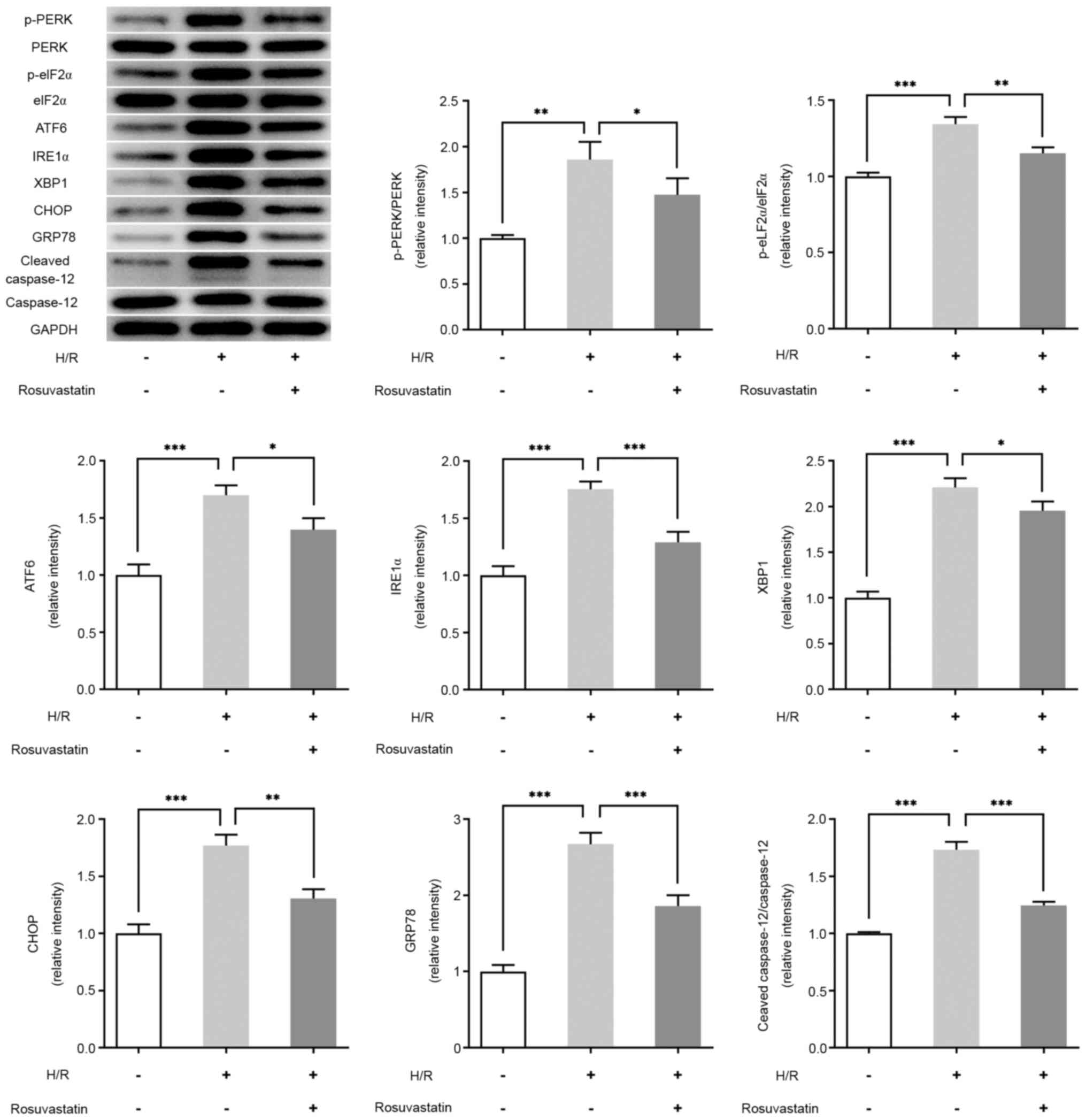
In order to detect the effect of rosuvastatin on ERS
levels in H/R-induced PC12 cells, western blot analysis was used to
investigate the expression levels of ERS-associated proteins.
Compared with the control group, the expression levels of p-PERK,
p-eIF2α, ATF6, IRE1α, XBP1, CHOP, GRP78 and cleaved caspase-12 were
significantly increased in the H/R group. Further administration of
rosuvastatin, however, reversed the expression levels of p-PERK,
p-eIF2α, ATF6, IRE1α, XBP1, CHOP, GRP78, CHOP and cleaved
caspase-12 (Fig. 3).
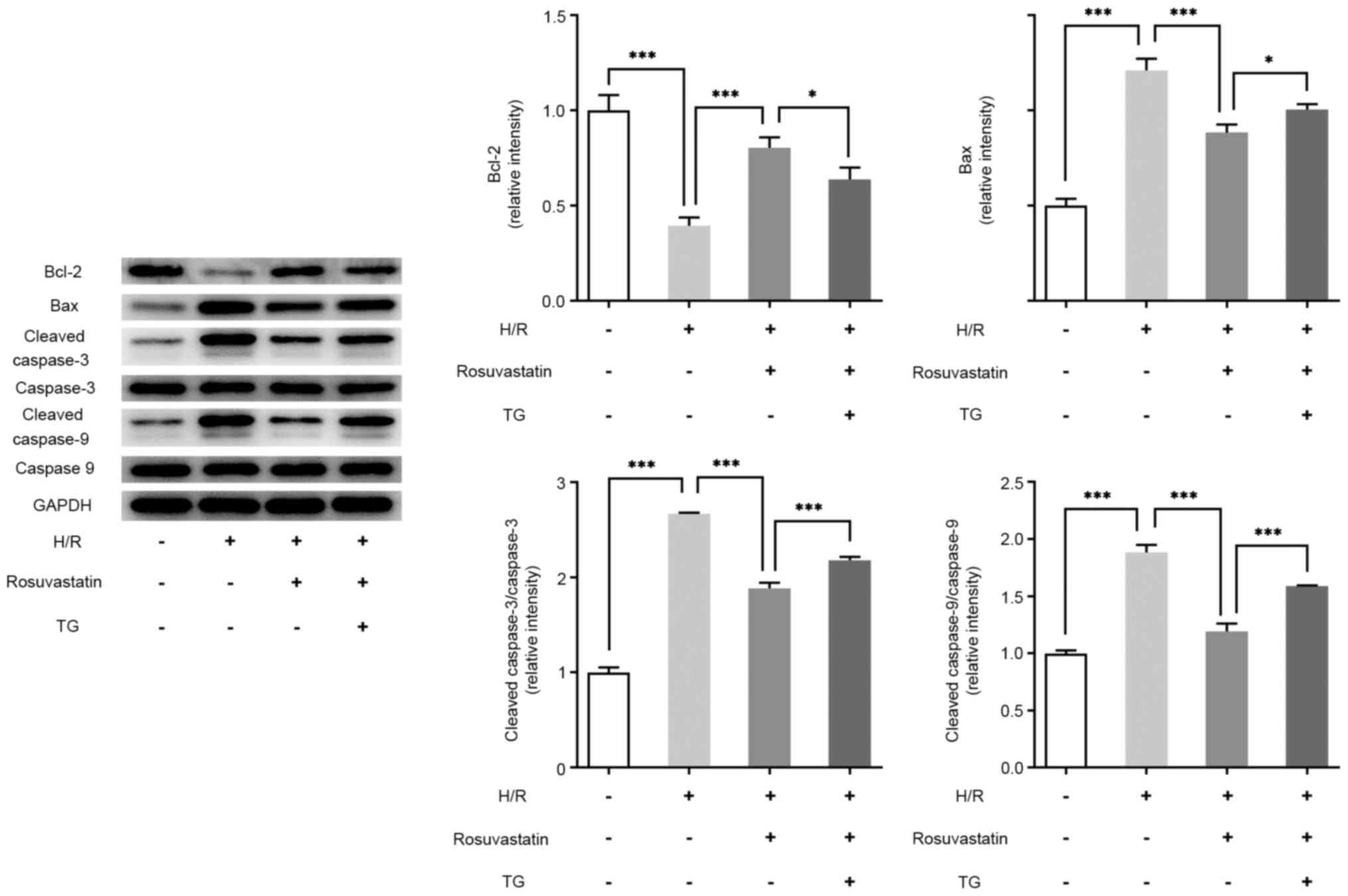
TG reverses the protective effects of
rosuvastatin on H/R-induced PC12 cell injury
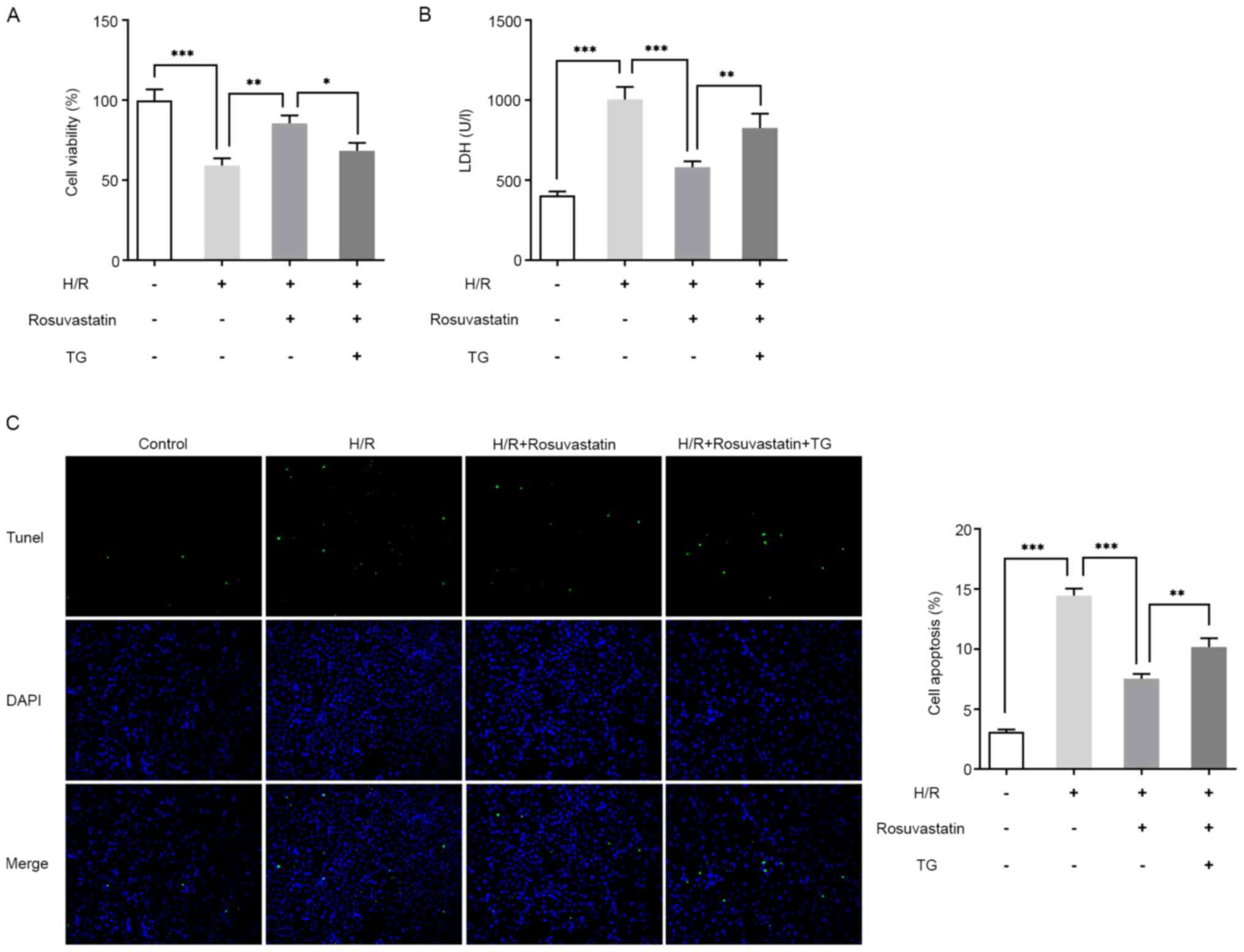
Subsequently, the mechanism of rosuvastatin action
on H/R-induced PC12 cells by adding the ERS activator, thapsigargin
(TG), was investigated. To this end, the cells were divided into
control, H/R, H/R + rosuvastatin and H/R + rosuvastatin + TG
groups. The results of CCK-8 assay showed that, compared with the
H/R + rosuvastatin group, the cell survival rate was significantly
reversed following TG addition (Fig.
4A). The results of the LDH kit assay revealed that, compared
with the H/R + rosuvastatin group, the cytotoxicity of the H/R +
rosuvastatin + TG group was significantly increased (Fig. 4B). The results from the TUNEL assay
experiments revealed that TG administration was able to
significantly reverse the apoptotic rate of H/R-induced PC12 cells
inhibited by rosuvastatin (Fig.
4C). Subsequently, the expression levels of
apoptosis-associated proteins were investigated, and the trend in
expression level changes was consistent with those of the TUNEL
assay (Fig. 5). In addition, the
expression levels of ERS-associated proteins were also
investigated, and these experiments showed that, compared with the
H/R + rosuvastatin group, the expression levels of p-PERK, p-eIF2α,
ATF6, IRE1α, XBP1, CHOP, GRP78 and cleaved caspase-12 were
significantly increased (Fig. 6).
Taken together, the aforementioned experimental results showed that
TG reversed the protective effect of rosuvastatin on H/R-induced
PC12 cell injury.
Discussion
PC12 cells are a differentiated cell line of
pheochromocytoma in the adrenal medulla of rats. PC12 cells have
the general characteristics of neuroendocrine cells and are widely
used in neurophysiological and pharmacological studies due to their
ability of passage. In addition, a study has used
hypoxia-reoxygenation-induced PC12 cells to form a model of
cerebral ischemia reperfusion injury model, which has been
recognized as a model (20).
Therefore, in the present study, PC12 cells were induced to
establish a damage model through H/R and carried out relevant
experiments.
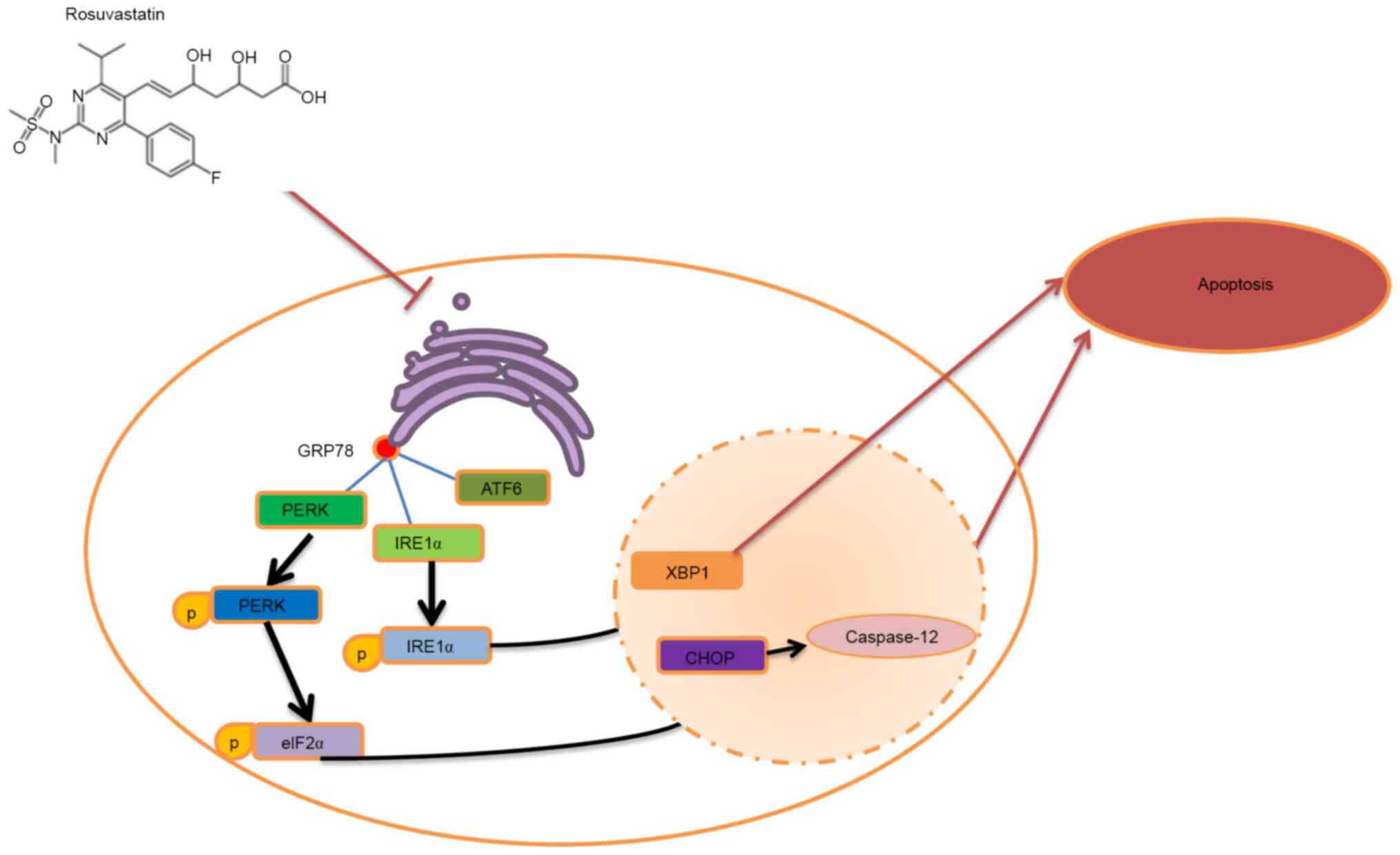
ERS mediates three different signaling pathways at
both the transcriptional and translational levels, predominantly
through ER transmembrane proteins, namely, the three unfolded
protein response signaling receptor molecules, IRE-1, PERK and
activating transcription factor 6(21). These three signaling molecules are
normally inactive. When ERS occurs, heat shock protein family A
(Hsp70) member 5 (GRP78) is isolated from these signal receptor
molecules, which assists in the process of enabling unfolded or
misfolded proteins to recover their correct conformation, and
activating these signal receptor molecules, affecting the
downstream CHOP levels (22).
Previous studies have shown that the inhibition of ERS-mediated
apoptosis leads to a decrease in CIRI-induced injury (12,23,24).
Therefore, the ER should serve an important role in the development
of CIRI. In the present study, a damage model of PC12 cells induced
by H/R was established. It was found that the expression levels of
ERS-associated proteins were significantly increased, and the cell
apoptotic rate was also increased, indicating that ERS-induced
apoptosis has an important role in H/R-induced cell damage.
A previous study reported that rosuvastatin is able
to alleviate myocardial IR injury by upregulating
miR-17-3s-mediated autophagy (25).
Rosuvastatin protects isolated hearts from IR injury through the
Akt/GSK-3β signaling axis, the metabolic environment and
mitochondrial permeability transition hole (26). Pretreatment with rosuvastatin has
been shown to decrease oxidative stress and the inflammatory
response associated with focal CIRI in rats (16). In addition, rosuvastatin has been
demonstrated to have an important neuroprotective role in animal
models of CIRI through decreasing inflammatory damage, regulating
thrombosis and improving endothelial cell function. Furthermore,
rosuvastatin has been widely recommended for primary and secondary
defense treatment of CIRI (27-29).
Rosuvastatin alleviates endothelial cell apoptosis
and atherosclerosis of ApoE-/- mice by inhibiting ERS
(19). It has also been shown that
rosuvastatin may protect endothelial cells from
hyperglycemia-induced damage by decreasing ERS (18). Rosuvastatin and simvastatin
attenuate cisplatin-induced cardiotoxicity through the disruption
of ERS-mediated apoptotic death in rats via targeting the
ER-chaperone GRP78 and calpain-1 pathways (30). However, the regulatory effect of
rosuvastatin on ERS in CIRI has not yet been reported, to the best
of our knowledge. In the present study, it was shown that
rosuvastatin could inhibit the cell activity decline, decrease the
cytotoxicity and inhibit the apoptotic rate and ERS level of
H/R-induced PC12 cells. By adding the ERS activator TG, the
protective effect of rosuvastatin on H/R-induced PC12 cell injury
was found to be significantly reversed. The aforementioned findings
suggested that rosuvastatin may protect PC12 cells from H/R-induced
injury through inhibiting apoptosis induced by ERS.
In conclusion, the present study has shown that
rosuvastatin protects PC12 cells from H/R-induced injury by
inhibiting ERS-induced apoptosis (Fig.
7). The findings may provide a theoretical basis for the
clinical treatment of CIRI with rosuvastatin.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by Hunan Province
Traditional Chinese Medicine Research Project (grant no. 202015)
and Scientific Research Project of Hunan Provincial Health and
Family Planning Commission (grant no. 20200308).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ and YG wrote the manuscript and analyzed the
data. LL and JY performed the experiments and supervised the study.
YG searched the literature and revised the manuscript for important
intellectual content. ZZ and YG confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang M, Wang J, Liu Z, Guo X, Wang N, Jia
N, Zhang Y and Yuan J: Effects of intermedin on autophagy in
cerebral ischemia/reperfusion injury. Neuropeptides. 68:15–21.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Deng Y, Zou W, Chen G, Shangguan S, Zhou
F, Jiang W and Li X: Comparative studies on the effects of
different doses of atorvastatin combined with aspirin on
inflammatory cytokines and carotid plaques in patients with
ischemic cerebrovascular disease. Int J Neurosci. 129:1133–1138.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng
YL, Cheng PW, Li CY and Li CJ: Current mechanistic concepts in
ischemia and reperfusion injury. Cell Physiol Biochem.
46:1650–1667. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sun MS, Jin H, Sun X, Huang S, Zhang FL,
Guo ZN and Yang Y: Free radical damage in ischemia-reperfusion
injury: An obstacle in acute ischemic stroke after
revascularization therapy. Oxid Med Cell Longev.
2018(3804979)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xu F, Ma R, Zhang G, Wang S, Yin J, Wang
E, Xiong E, Zhang Q and Li Y: Estrogen and propofol combination
therapy inhibits endoplasmic reticulum stress and remarkably
attenuates cerebral ischemia-reperfusion injury and OGD injury in
hippocampus. Biomed Pharmacother. 108:1596–1606. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lin YW, Chen TY, Hung CY, Tai SH, Huang
SY, Chang CC, Hung HY and Lee EJ: Melatonin protects brain against
ischemia/reperfusion injury by attenuating endoplasmic reticulum
stress. Int J Mol Med. 42:182–192. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Feng D, Wang B, Wang L, Abraham N, Tao K,
Huang L, Shi W, Dong Y and Qu Y: Pre-ischemia melatonin treatment
alleviated acute neuronal injury after ischemic stroke by
inhibiting endoplasmic reticulum stress-dependent autophagy via
PERK and IRE1 signalings. J Pineal Res. 62:2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nakka VP, Gusain A and Raghubir R:
Endoplasmic reticulum stress plays critical role in brain damage
after cerebral ischemia/reperfusion in rats. Neurotox Res.
17:189–202. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xin Q, Ji B, Cheng B, Wang C, Liu H, Chen
X, Chen J and Bai B: Endoplasmic reticulum stress in cerebral
ischemia. Neurochem Int. 68:18–27. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ishige K, Osada N, Kosuge Y and Ito Y:
Involvement of endoplasmic reticulum stress in neurodegeneration
after transient global ischemia-reperfusion. Nihon Yakurigaku
Zasshi. 142:9–12. 2013.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
11
|
Gong L, Tang Y, An R, Lin M, Chen L and Du
J: RTN1-C mediates cerebral ischemia/reperfusion injury via ER
stress and mitochondria-associated apoptosis pathways. Cell Death
Dis. 8(e3080)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Feng SQ, Zong SY, Liu JX, Chen Y, Xu R,
Yin X, Zhao R, Li Y and Luo TT: VEGF antagonism attenuates cerebral
ischemia/reperfusion-induced injury via inhibiting endoplasmic
reticulum stress-mediated apoptosis. Biol Pharm Bull. 42:692–702.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gutiérrez-Vargas JA, Cespedes-Rubio A and
Cardona-Gómez GP: Perspective of synaptic protection after
post-infarction treatment with statins. J Transl Med.
13(118)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cortese F, Gesualdo M, Cortese A,
Carbonara S, Devito F, Zito A, Ricci G, Scicchitano P and Ciccone
MM: Rosuvastatin: Beyond the cholesterol-lowering effect. Pharmacol
Res. 107:1–18. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu Y, Yang H, Jia G, Li L, Chen H, Bi J
and Wang C: The synergistic neuroprotective effects of combined
rosuvastatin and resveratrol pretreatment against cerebral
ischemia/reperfusion injury. J Stroke Cerebrovasc Dis.
27:1697–1704. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ma M, Uekawa K, Hasegawa Y, Nakagawa T,
Katayama T, Sueta D, Toyama K, Kataoka K, Koibuchi N, Kuratsu J and
Kim-Mitsuyama S: Pretreatment with rosuvastatin protects against
focal cerebral ischemia/reperfusion injury in rats through
attenuation of oxidative stress and inflammation. Brain Res.
1519:87–94. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Engelhorn T, Doerfler A, Heusch G and
Schulz R: Reduction of cerebral infarct size by the AT1-receptor
blocker candesartan, the HMG-CoA reductase inhibitor rosuvastatin
and their combination. An experimental study in rats. Neurosci
Lett. 406:92–96. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu JZ, Chai YL and Zhang YL: Effect of
rosuvastatin on high glucose-induced endoplasmic reticulum stress
in human umbilical vein endothelial cells. Genet Mol Res.
15:2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Geng J, Xu H, Fu W, Yu X, Xu G, Cao H, Lin
G and Sui D: Rosuvastatin protects against endothelial cell
apoptosis in vitro and alleviates atherosclerosis in
ApoE-/- mice by suppressing endoplasmic reticulum
stress. Exp Ther Med. 20:550–560. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen H and Li X: LncRNA ROR is involved in
cerebral hypoxia/reoxygenation-induced injury in PC12 cells via
regulating miR-135a-5p/ROCK1/2. Am J Transl Res. 11:6145–6158.
2019.PubMed/NCBI
|
|
21
|
Oakes SA and Papa FR: The role of
endoplasmic reticulum stress in human pathology. Annu Rev Pathol.
10:173–194. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang K and Kaufman RJ: The unfolded
protein response: A stress signaling pathway critical for health
and disease. Neurology. 66 (2 Suppl 1):S102–S109. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Han ZW, Chang YC, Zhou Y, Zhang H, Chen L,
Zhang Y, Si JQ and Li L: GPER agonist G1 suppresses neuronal
apoptosis mediated by endoplasmic reticulum stress after cerebral
ischemia/reperfusion injury. Neural Regen Res. 14:1221–1229.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhong CJ, Chen MM, Lu M, Ding JH, Du RH
and Hu G: Astrocyte-specific deletion of Kir6.1/K-ATP channel
aggravates cerebral ischemia/reperfusion injury through endoplasmic
reticulum stress in mice. Exp Neurol. 311:225–233. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang X, Chen J and Huang X: Rosuvastatin
attenuates myocardial ischemia-reperfusion injury via upregulating
miR-17-3p-mediated autophagy. Cell Reprogram. 21:323–330.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Vélez DE, Mestre-Cordero VE, Hermann R,
Perego J, Harriet S, Fernandez-Pazos MLM, Mourglia J and
Marina-Prendes MG: Rosuvastatin protects isolated hearts against
ischemia-reperfusion injury: Role of Akt-GSK-3β, metabolic
environment, and mitochondrial permeability transition pore. J
Physiol Biochem. 76:85–98. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Griffin JM, Kho D, Graham ES, Nicholson LF
and O'Carroll SJ: Statins inhibit fibrillary β-amyloid induced
inflammation in a model of the human blood brain barrier. PLoS One.
11(e0157483)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rodriguez-Perea AL, Gutierrez-Vargas J,
Cardona-Gómez GP, Guarin CJ, Rojas M and Hernández PA: Atorvastatin
modulates regulatory T cells and attenuates cerebral damage in a
model of transient middle cerebral artery occlusion in rats. J
Neuroimmune Pharmacol. 12:152–162. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sohn HM, Hwang JY, Ryu JH, Kim J, Park S,
Park JW and Han SH: Simvastatin protects ischemic spinal cord
injury from cell death and cytotoxicity through decreasing
oxidative stress: In vitro primary cultured rat spinal cord model
under oxygen and glucose deprivation-reoxygenation conditions. J
Orthop Surg Res. 12(36)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Saleh DO, Mansour DF and Mostafa RE:
Rosuvastatin and simvastatin attenuate cisplatin-induced
cardiotoxicity via disruption of endoplasmic reticulum
stress-mediated apoptotic death in rats: Targeting ER-chaperone
GRP78 and calpain-1 pathways. Toxicol Rep. 7:1178–1186.
2020.PubMed/NCBI View Article : Google Scholar
|