Introduction
At present, the incidence and mortality of
cardiovascular disease are increasing worldwide (1,2) and
acute myocardial infarction (AMI) is a major cause of death
(3-5).
In addition, AMI exhibits a trend of rapid growth in young and
low-income groups (6-8).
According to statistics, it is estimated that one-third of patients
with AMI die outside the hospital (5). Therefore, an early diagnosis is
important for the treatment and prognosis of patients with AMI
(9,10). At present, the diagnostic methods
for AMI mainly include electrocardiogram (ECG), coronary imaging
and biochemical markers for myocardial injury, but they all require
expensive testing equipment and complex operation procedures that
are time-consuming and laborious (5,11-13).
For patients with non-ST-segment elevation on ECG monitoring, a
series of serum tests are required (5). These tests consume a large quantity of
materials, resulting in a waste of medical resources and
environmental pollution. In addition, certain problems, such as low
sensitivity, late appearance and low specificity, exist in the
detection of existing biochemical markers (14-16).
Therefore, a rapid detection method with fast detection and
accurate results with an environmentally friendly, economical and
convenient application that may be performed at home for the rapid
detection of AMI has become a high priority (11-13,17).
Currently, early clinical markers of myocardial
infarction include myoglobin (Myo), creatine kinase-Mb (CK-MB),
cardiac troponin T (cTnT) and cardiac troponin I (cTnI) (18). A novel type of myocardial marker,
heart-type fatty acid-binding protein (H-FABP), has been introduced
for the early diagnosis of AMI (13). Under normal physiological
conditions, H-FABP does not appear in plasma or tissue fluids, and
it may be detected only in the case of myocardial injury; H-FABP
begins to increase 1-3 h after the occurrence of acute coronary
syndrome. It peaks after 6-8 h and returns to normal after 12-24 h.
It is one of the earliest markers released into the circulating
blood during myocardial injury (15).
Myo has a low molecular weight and appears early,
and CK-MB takes a long time (9-30 h) to reach a peak value (86
µg/l) and has poor sensitivity (19). Although the specificity is high,
this marker is mainly used for the diagnosis of myocardial
infarction with a recurrent attack in a short time period due to
its short duration (13,14). The cTnT and cTnI markers have high
specificity, particularly the appearance of hs-cTnI, but they are
limited by the sensitivity of current rapid detection technology;
thus, these markers cannot be detected with home detection methods.
In addition, due to the long persistence of cTnT and cTnI in the
blood, they are useful in the detection of initial ischaemic events
but are not reliable in the detection of a reinfarction (15,20).
Therefore, the abovementioned myocardial infarction markers are not
ideal for the early diagnosis of AMI.
By contrast, H-FABP is mainly found in
cardiomyocytes, with high specificity for AMI; furthermore, H-FABP
is rapidly released into the blood (14). Therefore, it is superior to other
major myocardial infarction markers and is an ideal early
diagnostic marker of AMI. In addition, H-FABP has the potential to
assist in other clinical diagnoses, such as detecting AMI
recurrence and assessing the size of myocardial infarction, to
serve as an independent indicator for predicting the relative risk
of cardiac events; it has a good prognostic value, for example
regarding the prediction of the long-term mortality of a patient
and the probability of reinfarction; it may also be used for
monitoring the success of reperfusion after AMI (14,21,22).
Lateral tomography is simple, fast, sensitive and
economical, and it is widely used for in-home self-tests and
real-time detection (23). The
choice of labelled probes is critical to the utility and
generalization of the detection method. Due to its good
biocompatibility, stability and relatively simple preparation,
colloidal gold is the most widely used coloured labelling probe and
the most developed domestic in vitro diagnostic marker
(24). The use of lateral
tomography eliminates the need for precision instrumentation,
requires no operator training and may produce results in 10 min
(25).
Therefore, in the present study, a lateral flow
immunoassay was developed through the use of H-FABP as a marker and
colloidal gold as a labelling probe to produce a rapid detection
kit for AMI; this kit is able to provide testing that is fast,
effective and easy, and may be performed at home.
Materials and methods
Preparation of colloidal gold
Colloidal gold nanoparticles (GNPs) were synthesized
using a sodium citrate reduction method (26,27).
GNPs were prepared by heating with an electric furnace (DK-98-Ⅱ,
Tianjin City TAISITE Instrument Co., Ltd.) and an agitator (RCT B
S025, IKA), respectively. The effects of ultrapure water from a
Millipak terminal filter and Biopak terminal filter (EMD Millipore)
on the properties of colloidal gold were also compared. The quality
of colloidal gold was controlled by full wavelength scanning,
centrifugation and resuspension, transmission electron microscopy
and dynamic light scattering (DLS). The solution was cooled and
stored at 4˚C.
Optimization of marker pH and
labelling concentration
In this experiment, a 10% NaCl colour reaction was
used to determine the optimal pH. Colloidal gold was bound by an
anti-H-FABP monoclonal antibody (mAb; cat. no. M020203; Hangzhou
Biogenome Biotechnology Co., Ltd.). Colloidal gold consists of a
gold core and a wrapped double-ion layer to maintain a suspended
state. When a strong ion (such as 10% NaCl) is added, the
negative-ion layer on the surface of the gold core is destroyed and
the precipitating gold sol that emerges turns the solution blue;
however, if a sufficient amount of protein binds to all active
sites on the surface of the colloidal gold, an additional
protective layer is formed on the surface. Thus, no coagulation
occurs when strong ions are added. As the concentration of the
protective protein decreases, the colour of the colloidal gold
changes from the original rose colour to purple and then to
blue-grey. The corresponding pH + 0.5 when the colloidal gold first
changed colour was determined as the optimum pH of the label.
Similar to the pH selection principle, the pH of the
colloidal gold was adjusted to the optimum value and the antibodies
at different concentrations were added to observe the colour
change. The optimal label amount of the corresponding antibody was
increased by 20% based on when the colloidal gold first changed
colour.
Preparation of colloidal gold-labelled
mAb
According to the conditions determined above, the
coating concentration of the test line was optimized. Goat
anti-mouse IgG (1 mg/ml; cat. no. BA1054; Boster Biological
Technology Co., Ltd.) was coated on the control line and the test
line was coated with different concentrations of anti-H-FABP mAb 2
(cat. no. M020201; Hangzhou Biogenome Biotechnology Co., Ltd.). The
colour development of the test strip was observed and the lowest
antibody concentration at which the ideal colour developed was
selected as the optimal coating concentration.
For antibody conjugation, the adjusted colloidal
gold was added to anti-FABP mAb 1 (cat. no. M020203; Hangzhou
Biogenome Biotechnology Co., Ltd.). The mixtures were incubated for
30 min under stirring, after which an aqueous bovine serum albumin
(BSA; cat. no. A7906; Sigma-Aldrich; Merck KGaA) solution was added
to a final concentration of 1% (w/v). GNP-labelled anti-FABP mAb
conjugates were separated from unreacted antibodies by
centrifugation at 9,600 x g at 4˚C for 30 min. After the
supernatant was removed, the residue was resuspended in a buffer
comprising 0.02 M Tris-HCl (pH 7.6), 1.0% BSA, 5% sucrose and 0.3%
Tween-20 for storage at 4˚C for up to one year.
Preparation of the H-FABP test
kit
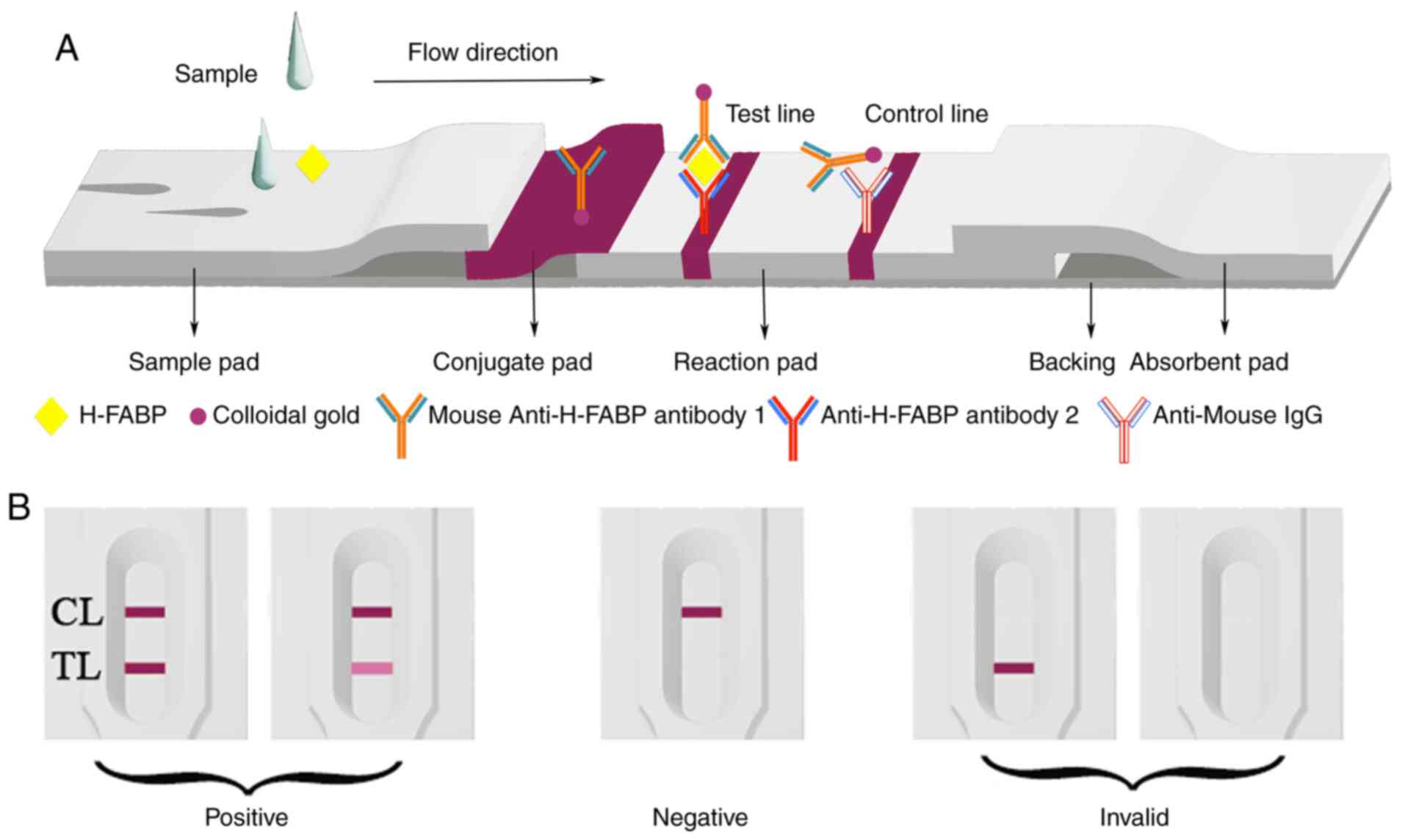
As presented in Fig.
1A, the immunoassay test device comprised four parts: A sample
pad, an absorption pad, a nitrocellulose (NC) membrane and a
polyvinylchloride (PVC) backing card. In brief, goat anti-mouse IgG
and anti-H-FABP mAb 2 were separately applied to the NC membrane in
10 mM PBS (pH 7.4) to be used as the control line and the test
line, respectively. The NC membrane was then dried for 4 h at 37˚C
to fix the antibody and the antigen. The NC membrane was pasted
onto the PVC strip with the adsorption pad on the top end, while
the colloidal gold-conjugated pad overlapped by the sample pad
adhered to the bottom end of the NC membrane. The colloidal
gold-conjugated pad was prepared by adding the anti-FABP mAb
1-coated GNPs to a glass fibre (50 µl/cm2). The
resultant conjugated pad was incubated at 37˚C for 1.5 h until
fully dried. The glass fibre sample pad was submerged in 10 mM PBS
(pH 7.4, 0.05% Tween-20) and dried at 37˚C for 1.5 h. Finally, the
test device was stored at room temperature (RT) prior to use.
Determination of the sensitivity,
specificity and stability of the H-FABP test kit
Plasma samples were provided by Huaihe Hospital of
Henan University (Kaifeng, China) and stored at -80˚C for further
use. Samples from healthy donors were confirmed to be negative for
H-FABP using an ELISA kit (cat. no. HK401; Hycult Biotech Inc.)
according to the manufacturer's instructions. A total of 12 samples
were collected at Huaihe Hospital from February to April 2019,
including 2 normal people (age 72, male; 66, female) and 10
myocardial infarction patients (4 males and 6 females; age range
from 69 to 90, the average age was 75.1 years old). Standard H-FABP
was added to the negative samples at concentrations of 0, 1, 2, 5
or 10 ng/ml, and the samples were mixed completely and stored at RT
for 30 min prior to use. Next, 70-µl samples containing H-FABP were
tested using the H-FABP test strips in triplicate; If the sample
was blood, and dilute with saline. Due to differences in the visual
ability between individual observers, particularly for determining
low-intensity colour reactions, the strips were analysed with
ImageJ v2 software (National Institutes of Health) to evaluate the
results.
The cTnI (cat. no. 921807; Beckman Coulter, Inc.),
CK-MB (cat. no. 921957; Beckman Coulter, Inc.) and Myo (cat. no.
921849; Beckman Coulter, Inc.) markers (5 µg/ml) were added to
different samples and 70 µl of each sample was added to the strip
sample region for a specificity assay.
A kit was prepared for H-FABP detection with
different storage conditions (4˚C, RT and 40˚C) after 30 days for
stability verification.
Clinical samples were collected and detected using
the H-FABP test kit and the results were revised with the ELISA kit
for H-FABP. Information on the patients is presented in Table I. The consistent rate was calculated
according to the following formula: Total consistent rate=(samples
with true positive result + true negatives)/all samples x100%. The
results were able to be determined with the naked eye within 5 min
(Fig. 1B). The mAb-conjugated GNPs
reacted with goat anti-mouse IgG and were captured in the control
zone, while the mAb combined with GNPs reacted with H-FABP and were
able to be captured in the test zone. The positive result was
judged by the presence of two rose lines in the reaction area. A
negative result was determined by the presence of a single line in
the control zone. If no line was present in the reaction area or
only one line was present in the test zone, the test was considered
invalid.
 | Table IBasic information of donors of
clinical samples. |
Table I
Basic information of donors of
clinical samples.
| Donor no. | Sex | Age, years | Myocardial
infarction (Yes/No) | Result by the test
strip (+/-) | Result by ELISA
(ng/ml) | Year-month of
sampling |
|---|
| 1 | Male | 69 | Yes | + | 1.92 | 2019-03 |
| 2 | Female | 71 | Yes | + | 2.38 | 2019-03 |
| 3 | Male | 81 | Yes | + | 1.78 | 2019-03 |
| 4 | Male | 78 | Yes | + | 3.96 | 2019-03 |
| 5 | Female | 78 | Yes | + | 4.10 | 2019-03 |
| 6 | Female | 79 | Yes | + | 2.42 | 2019-03 |
| 7 | Male | 72 | No | - | 0.26 | 2019-03 |
| 8 | Female | 66 | No | - | 0 | 2019-03 |
| 9 | Female | 79 | Yes | - | 1.21 | 2019-04 |
| 10 | Female | 90 | Yes | + | 6.01 | 2019-03 |
| 11 | Female | 73 | Yes | + | 2.23 | 2019-02 |
| 12 | Male | 83 | Yes | + | 2.75 | 2019-03 |
Results
Characterization of the colloidal
gold
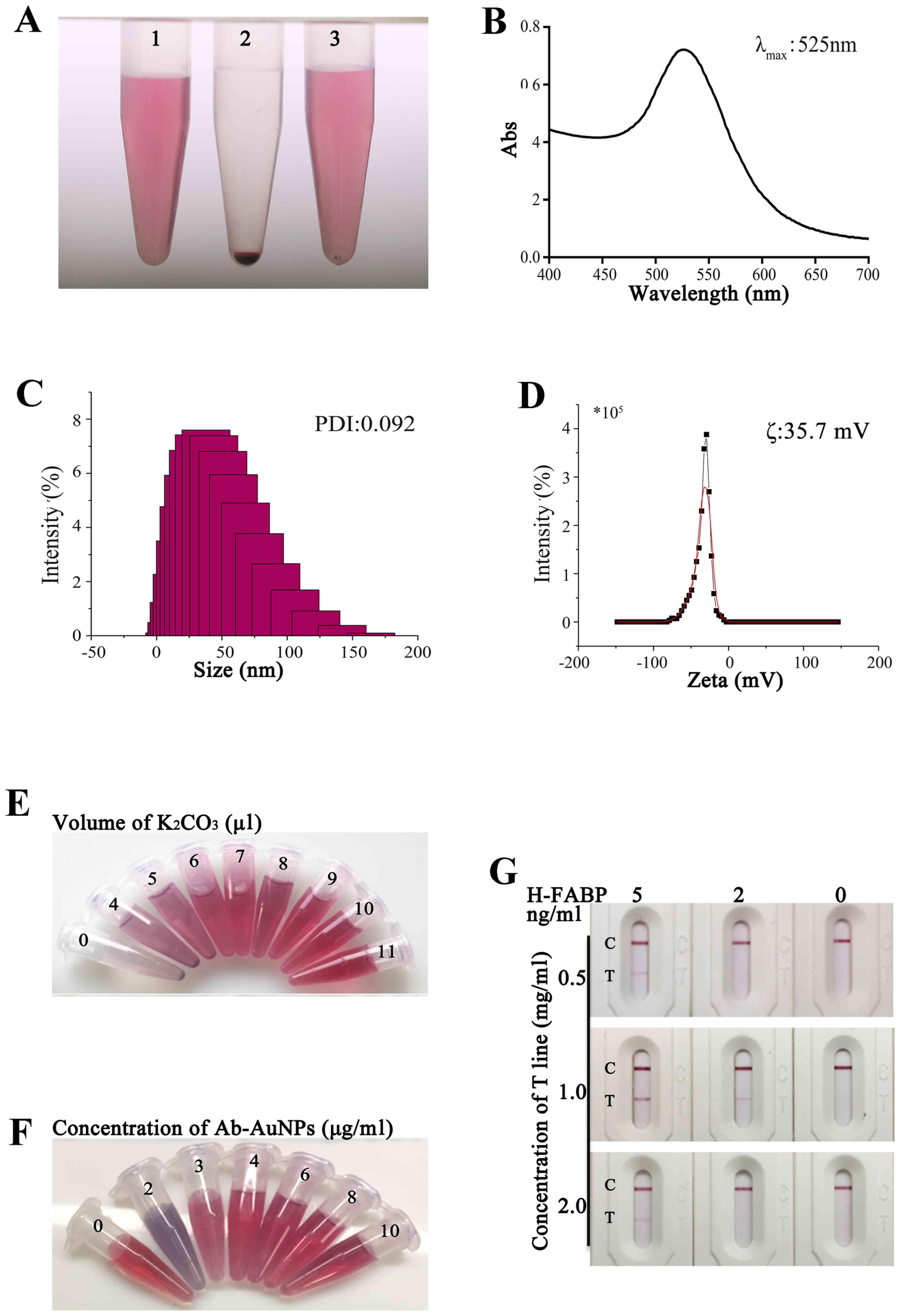
The apparent characteristics of a high-quality
colloidal gold solution are a lack of floating material and
impurity precipitation in a clear and transparent liquid. In the
present experiment, colloidal gold was prepared by reducing 1%
HAuCl4 with 1.5% sodium citrate and it had a clear and
transparent red-rose colour (Fig.
2A-1). After centrifugation, the gold particles were loosely
concentrated at the bottom of the Eppendorf (EP) tube; in this
state, the clear liquid was colourless and the separation was
superior (Fig. 2A-2). After the EP
tube was rotated, the precipitate was observed to be in the form of
loose sand and was able to be completely suspended, indicating that
the colloidal gold had a good resuspension ability (Fig. 2A-3).
Fig. 2B indicates
the waveform in a range of 400-700 nm. The wavelength range
corresponding to the maximum absorption peak was narrow, suggesting
that the particle size of the gold nanoparticles was uniform and
the polydispersity index (PDI) of the gold nanoparticles, as
measured by DLS, was 0.092 (Fig.
2C). As presented in Fig. 2D,
the ζ potential of colloidal gold was -35.7 mV, indicating that
colloidal gold may exist stably in a liquid-phase environment. In
summary, the custom colloidal gold met the preparation requirements
of the kit.
Determination of the optimal labelling
pH, concentration and coating concentration
As presented in Fig.
2E, the colour of colloidal gold in the samples no. 1-9, from 0
to 11 µl of K2CO3 volume, changed from
grey-blue to rose-red. As the volume of added
K2CO3 increased, the pH gradually increased
and the no. 7 (K2CO3, 9 µl) tube did not
initially change colour; i.e., it initially turned a rose-red
colour. At this point, the pH of the tube was 6.5; thus, the
optimal label of the monoclonal antibody was pH (6.5+0.5), which is
7.0.
As presented in Fig.
2F, the colour of colloidal gold in samples no. 2-7, from 0 to
10 µg/ml of antibody concentration, changed from grey-blue to
rose-red; no. 1 was the stock solution. As the amount of added
protein increased, the no. 4 EP tube did not initially change
colour. At first, it was close to rose-red, and at this point, the
labelling concentration of the tube was 4 µg/ml. Therefore, the
optimal labelling concentration of the monoclonal antibody was
(4x120%) µg/ml, which is 4.8 µg/ml.
When H-FABP at 0.5 and 2 mg/ml was coated on the
test strip, the kit detection zone was lighter and the sensitivity
was lower. When the test strip was coated with 1 mg/ml H-FABP, the
quality control strip and the detection strip were superior to
those with the other coating concentrations and the sensitivity was
2 ng/ml (Figs. 2G and S1). Therefore, the 1 mg/ml coating was
the optimal coating concentration for the H-FABP colloidal gold kit
test strip.
Sensitivity of the H-FABP test kit in
human plasma and blood
The sensitivity of a kit is a key parameter for the
early diagnosis of AMI markers. Therefore, the sensitivity of
H-FABP in both plasma and blood matrices was first examined
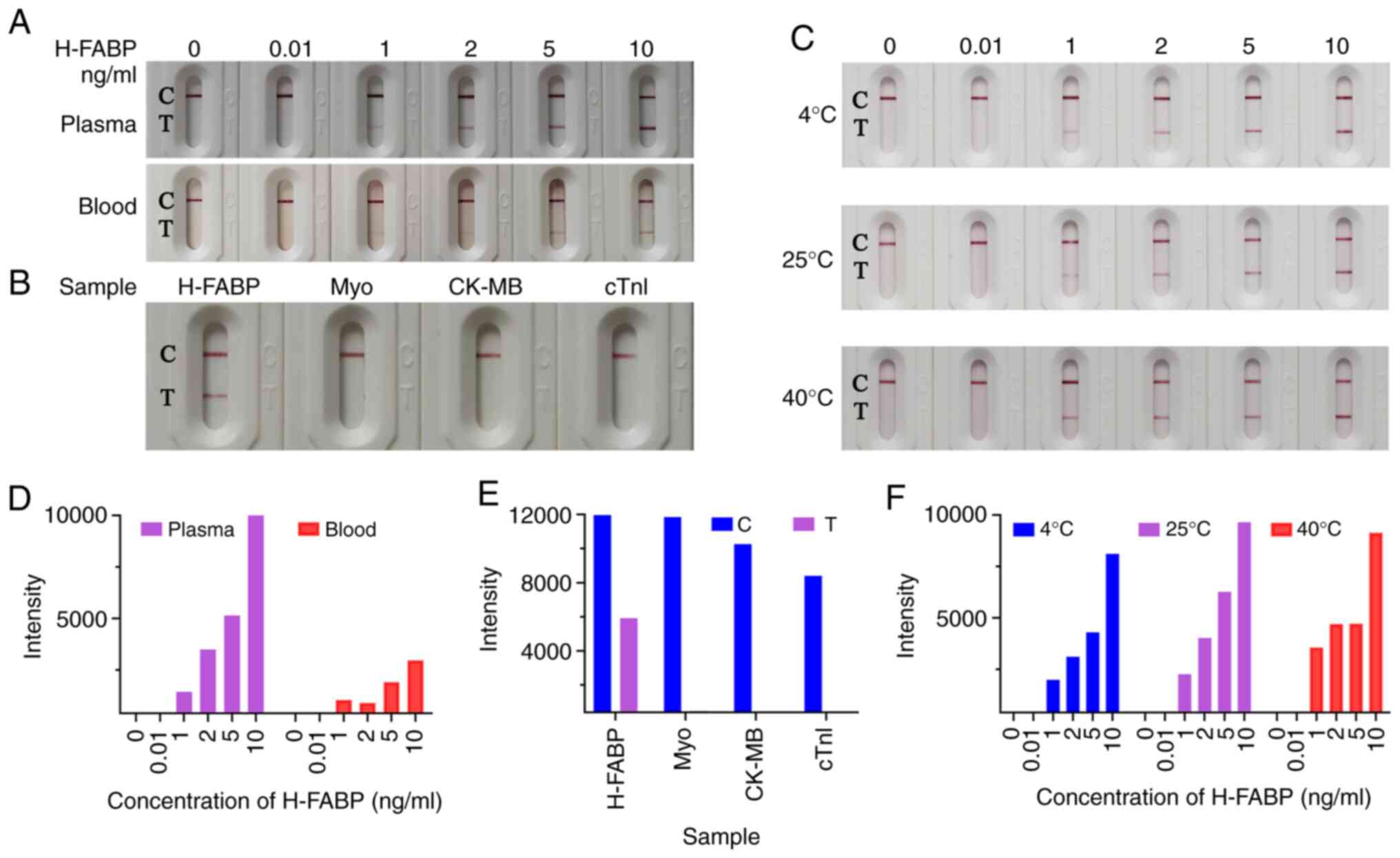
(Fig. 3).
Plasma test
The H-FABP standard was diluted to 10, 5, 2, 1 and
0.01 ng/ml with no H-FABP plasma and the negative control was free
of H-FABP plasma. Subsequently, 70 µl of standard solution at
different concentrations were added to the sample well to test the
sensitivity of the kit in plasma (Figs.
3A and S2). When a negative
sample was detected, only the quality control band was coloured and
when the sample concentration was 10 ng/ml, the colour of the
detection band became most clear to the naked eye and most strongly
detected by ImageJ software. With a decrease in sample
concentration, the test strip exhibited a positive line with a
gradual decrease in the colour intensity of the test lines, and the
colour of the test lines was still clearly visible when the sample
concentration was 1 ng/ml. However, negative results appeared when
the concentration of the sample was 0.01 ng/ml. Therefore, the
H-FABP limit of detection for this kit is 1 ng/ml. When the
concentration of H-FABP in blood reaches 5 ng/ml, it may be
preliminarily judged as myocardial injury (15,28).
The sensitivity of this experiment may reach 1 ng/ml, which
indicated that the kit is sensitive and has promising clinical
application value.
Blood test
The H-FABP standard was diluted with healthy human
whole blood to 20, 10, 4, 2 and 0.02 ng/ml, and the negative
control was healthy human whole blood. To test the sensitivity of
the kit with blood, different concentrations of standard solution
(50 µl) were added to the sample hole of the kit containing a
filter membrane with 50 µl of normal saline for a blood
chromatography test. The actual concentrations of the samples were
10, 5, 2, 1 and 0.01 ng/ml healthy human blood. When the
concentration of the sample was 0.01 ng/ml negative control, the
detection band did not appear, presenting a negative result. When
the concentration of the sample was 1 ng/ml, the positive line was
visible with the naked eye; as the concentration of the sample was
gradually increased to 10 ng/ml, the colour of the detection belt
gradually increased (Fig. 3A), the
auxiliary judgment result was shown in Fig. 3D. The repeated trial results were
concordant with initial results.
Specificity and stability of the
H-FABP test kit
In general, specificity is also an important
parameter for characterizing the performance of a kit. The
specificity of the kit was verified by diluting 5 µg/ml of H-FABP,
cTnI, Myo and CK-MB standards without H-FABP plasma. The colour
change of the quality control line was not ideal when the test kit
detected a large concentration of cTnI. This may be because H-FABP
and cTnI have similar epitopes and have certain cross-reactivity.
However, as cTnI is also unique to myocardial tissue, its
cross-reaction with H-FABP does not affect the diagnosis of AMI
(29-31).
To make the results more direct, the cTnI samples were redetected
at a concentration of 500 ng/ml. As presented in Figs. 3B, E
and S3, only H-FABP was used for
detection and the difference between the blank and the sample was
evident. When the other samples were tested, the quality control
band was ideal and the detection band was not coloured; the
negative results indicated that the specificity of the kit is
excellent.
Good stability has an important role in newly
developed kits and clinical applications (24). Kits from the same batch of kits were
placed in sealed zipper bags containing desiccant and then stored
away from light at 4˚C, RT and 40˚C for 30 days. The kits were then
separately used for measurements and the results are presented in
Figs. 3C, F and S4.
Based on the already mentioned findings, the threshold for
evaluating colour changes in the test band was a sample
concentration of 1 ng/ml. When the kits were used, the colour
changes were still clear. Therefore, the limit of sensitivity was
still reach 1 ng/ml, indicating that the kit has high
stability.
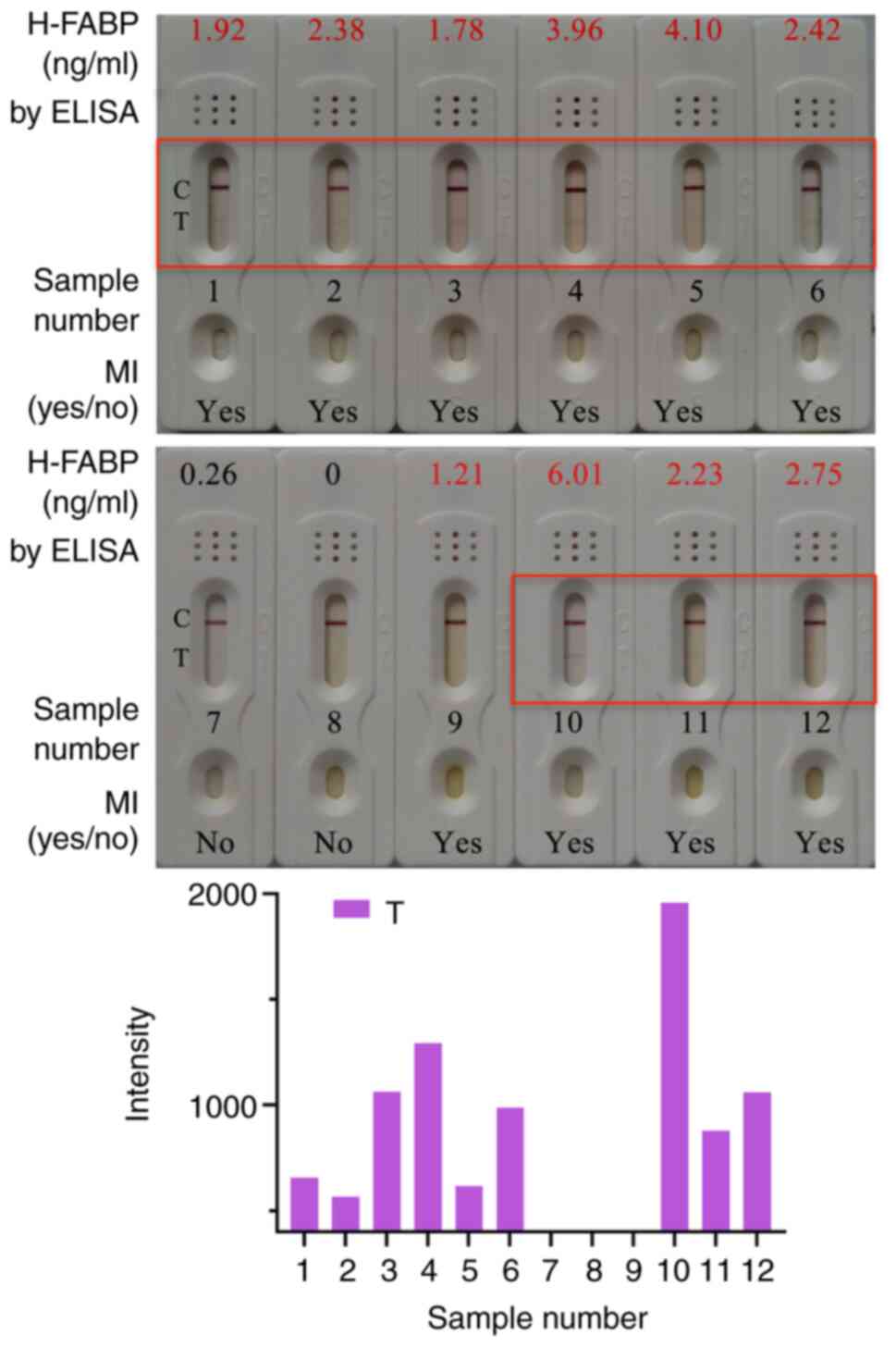
Clinical sample testing with the
H-FABP test kit
Fresh blood from 12 contributors, including 10
patients with myocardial infarction and 2 normal subjects (Table I), was used for clinical sample
testing. The plasma was separated and detected by ELISA and the
colloidal gold kit. Detection results obtained with the kit for
selected clinical samples are presented in Fig. 4. A total of 10 out of the 12 samples
had an H-FABP concentration >1 ng/ml, and the samples of the 2
normal subjects had an H-FABP concentration <1 ng/ml according
to ELISA detection. For the same cohort, 9 positive results and 3
negative results were obtained with the H-FABP rapid test kit based
on colloidal gold. Therefore, a total of 11 H-FABP kit results were
consistent with the ELISA results and the accuracy rate of the
statistical kit was 91.67%.
Discussion
Colloidal gold is one of the most commonly used
labelling probes due to its bright colour, easy preparation, good
biocompatibility and good optical properties. In this experiment,
to obtain stable colloidal gold for the preparation of test strips,
the effects of two different heating tools, an electric furnace and
an agitator, on the colloidal gold properties after the preparation
of colloidal gold were compared. The boiling of the electric
furnace was indicated to be more uniform when heated, which is
beneficial for the preparation of colloidal gold. The results
indicated that the colloidal gold prepared by the Millipak terminal
filter was superior to that prepared by the Biopak terminal filter
in terms of properties and colour rendering. In summary, aseptic
ultrapure water and an electric furnace were selected for the
preparation of colloidal gold. The PDI value was <0.2,
indicating that the distribution of colloidal gold particles was
uniform. Furthermore, the absolute value of the ζ potential of
colloidal gold indicated that the colloidal gold had a sufficient
repulsive force to maintain a dispersed state; i.e., it may exist
stably in a liquid-phase environment.
The purpose of the kit developed in the present
study was for use in homes to achieve rapid detection of myocardial
infarction; thus, using a simple method is necessary. As blood is
thick, to achieve superior chromatographic and colour effects, a
diluent was selected to dilute it equally and increase the
chromatography speed. First, PBS was selected as the diluent, but
in the course of the experiment, false positives were at times
obtained. To avoid false positives, saline was used instead of PBS.
The osmotic pressure of normal saline is basically the same as the
osmotic pressure of a human plasma colloid and no haemolysis occurs
when used. In daily life, saline is easy to obtain and more
convenient to use. Therefore, saline was chosen as the diluent to
achieve more ideal results.
In the detection of clinical samples, the complex
plasma composition of the patients may affect the combination of
H-FABP and colloidal gold; therefore, the overall colour results of
the kit were less evident. Furthermore, the colour of the
high-concentration samples was lighter than that of the
low-concentration samples; however, it did not affect the
assessment of negative and positive results. In addition, the
difference in the colour discrimination ability between
individual's interpretations of the results compared with the
actual results, introduced variability to the results. Finally, to
observe the experimental results more intuitively and conveniently,
a greyscale analysis by ImageJ software was used to process the
colour depth and light colour of the images, and this provided more
accurate experimental results. This test kit has the potential to
assist in detection before the patient with AMI arrives at the
hospital, as the kit provides a rapid detection method.
In conclusion, in the present study, a rapid
detection kit for the AMI marker H-FABP was prepared based on
colloidal gold. The sensitivity of plasma detection and whole-blood
detection was 1 ng/ml, the specificity and stability were
excellent, the reproducibility was high, the operation was simple
and the detection was rapid; hence, it is suitable for rapid
self-examination of patients with suspected myocardial
infarction.
Supplementary Material
Optimization of the coating
concentration in the test line of the uncropped Fig. 2G. C, quality control line; T,
detection line; H-FABP, heart-type fatty acid-binding protein.
Plasma and whole-blood sensitivity
test results in the uncropped Fig.
3A. C, quality control line; T, detection line; H-FABP,
heart-type fatty acid-binding protein.
Specificity test results by the H-FABP
kit from the uncropped Fig. 3B. C,
quality control line; T, detection line; H-FABP, heart-type fatty
acid-binding protein; cTnI, cardiac troponin I; Myo, myoglobin;
CK-MB, creatine kinase-Mb.
Stability test results at
4˚C, room temperature and 40˚C for the H-FABP
kit from the uncropped Fig. 3C. C,
quality control line; T, detection line; H-FABP, heart-type fatty
acid-binding protein.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Science and Technology
Research Project of Kaifeng Science and Technology Bureau (grant
no. 1903097), the Science and Technology Department of Henan
Province (grant no. 182102310154) and the Key Scientific Research
Project Plan of Colleges and Universities in Henan province (grant
no. 19B320003).
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZW and YGR designed the study; MCL, MZJ, CC, PQY
and NYT performed the experiments; and ZXW, JMS, YJL, HZH, QWZ and
YFG collected and analysed the patient data. ZZW and MZJ confirm
the authenticity of all the raw data. All authors read and approved
the manuscript.
Ethics approval and consent to
participate
This study was approved by the Biomedical Research
Ethics Committee of Henan University (Kaifeng, China; approval no.
HUSOM2019-047) and the patients provided written, informed
consented to participate.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao D, Liu J, Wang M, Zhang X and Zhou M:
Epidemiology of cardiovascular disease in China: Current features
and implications. Nat Rev Cardiol. 16:203–212. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nemet I, Saha PP, Gupta N, Zhu W, Romano
KA, Skye SM, Cajka T, Mohan ML, Li L, Wu Y, et al: A cardiovascular
disease-linked gut microbial metabolite acts via adrenergic
receptors. Cell. 180:862–877.e22. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Roth GA, Johnson C, Abajobir A, Abd-Allah
F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, et al:
Global, regional, and national burden of cardiovascular diseases
for 10 causes, 1990 to 2015. J Am Coll Cardiol. 70:1–25.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bajaj A and Sethi A, Rathor P, Suppogu N
and Sethi A: Acute complications of myocardial infarction in the
current era: Diagnosis and management. J Investig Med. 63:844–855.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Boateng S and Sanborn T: Acute myocardial
infarction. Dis Mon. 59:83–96. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Guo X, Li Z, Vittinghoff E, Sun Y and
Pletcher MJ: Trends in rate of acute myocardial infarction among
patients aged <30 years. Nat Rev Cardiol. 15(119)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Arora S, Stouffer GA, Kucharska-Newton AM,
Qamar A, Vaduganathan M, Pandey A, Porterfield D, Blankstein R,
Rosamond WD, Bhatt DL and Caughey MC: Twenty year trends and sex
differences in young adults hospitalized with acute myocardial
infarction. Circulation. 139:1047–1056. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bob-Manuel T, Ifedili I, Reed G, Ibebuogu
UN and Khouzam RN: Non-ST elevation acute coronary syndromes: A
comprehensive review. Curr Probl Cardiol. 42:266–305.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Friess U and Stark M: Cardiac markers: A
clear cause for point-of-care testing. Anal Bioanal Chem.
393:1453–1462. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Morrow DA, Cannon CP, Jesse RL, Newby LK,
Ravkilde J, Storrow AB, Wu AH and Christenson RH: National Academy
of Clinical Biochemistry. National academy of clinical biochemistry
laboratory medicine practice guidelines: Clinical characteristics
and utilization of biochemical markers in acute coronary syndromes.
Circulation. 115:e356–e375. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Leisy PJ, Coeytaux RR, Wagner GS, Chung
EH, McBroom AJ, Green CL, Williams JW Jr and Sanders GD: ECG-based
signal analysis technologies for evaluating patients with acute
coronary syndrome: A systematic review. J Electrocardiol. 46:92–97.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Takagi H, Tanaka R, Nagata K, Ninomiya R,
Arakita K, Schuijf JD and Yoshioka K: Diagnostic performance of
coronary CT angiography with ultra-high-resolution CT: Comparison
with invasive coronary angiography. Eur J Radiol. 101:30–37.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fung E, Järvelin MR, Doshi RN, Shinbane
JS, Carlson SK, Grazette LP, Chang PM, Sangha RS, Huikuri HV and
Peters NS: Electrocardiographic patch devices and contemporary
wireless cardiac monitoring. Front Physiol. 6(149)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pyati AK, Devaranavadagi BB, Sajjannar SL,
Nikam SV, Shannawaz M and Sudharani : Heart-type fatty acid
binding protein: A better cardiac biomarker than CK-MB and
myoglobin in the early diagnosis of acute myocardial infarction. J
Clin Diagn Res. 9:BC08–BC11. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ye XD, He Y, Wang S, Wong GT, Irwin MG and
Xia Z: Heart-type fatty acid binding protein (H-FABP) as a
biomarker for acute myocardial injury and long-term post-ischemic
prognosis. Acta Pharmacol Sin. 39:1155–1163. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen L, Guo X and Yang F: Role of
heart-type fatty acid binding protein in early detection of acute
myocardial infarction in comparison with cTnI, CK-MB and myoglobin.
J Huazhong Univ Sci Technolog Med Sci. 24:449–451, 459.
2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Neumann JT, Soerensen N, Schwemer T, Ojeda
F, Keller T, Renne T, Than M, Parsonage W, Schnabel R, Cullen L, et
al: Rapid measurement of a single troponin I combined with negative
ECG allow accurate rule-out of NSTEMI in patients with suspected
AMI. In: Coronary artery disease, acute coronary syndromes, acute
cardiac care. ESC Congress, Rome, Italy, p979, 2016.
|
|
18
|
Wang Y, Yang Y, Chen C, Wang S, Wang H,
Jing W and Tao N: One-step digital immunoassay for rapid and
sensitive detection of cardiac troponin I. ACS Sens. 5:1126–1131.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pöyhönen P, Kylmälä M, Vesterinen P,
Kivistö S, Holmström M, Lauerma K, Väänänen H, Toivonen L and
Hänninen H: Peak CK-MB has a strong association with chronic scar
size and wall motion abnormalities after revascularized
non-transmural myocardial infarction-a prospective CMR study. BMC
Cardiovasc Disord. 18(27)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Newby LK, Goldmann BU and Ohman EM:
Troponin: An important prognostic marker and risk-stratification
tool in non-ST-segment elevation acute coronary syndromes. J Am
Coll Cardiol. 41 (4 Suppl S):31S–36S. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Reiter M, Twerenbold R, Reichlin T,
Mueller M, Hoeller R, Moehring B, Haaf P, Wildi K, Merk S, Bernhard
D, et al: Heart-type fatty acid-binding protein in the early
diagnosis of acute myocardial infarction. Heart. 99:708–714.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Meng X, Ming M and Wang E: Heart fatty
acid binding protein as a marker for postmortem detection of early
myocardial damage. Forensic Sci Int. 160:11–16. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Henderson WA, Xiang L, Fourie NH, Abey SK,
Ferguson EG, Diallo AF, Kenea ND and Kim CH: Simple lateral flow
assays for microbial detection in stool. Anal Methods.
10:5358–5363. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Huang X, Aguilar ZP, Xu H, Lai W and Xiong
Y: Membrane-based lateral flow immunochromatographic strip with
nanoparticles as reporters for detection: A review. Biosens
Bioelectron. 75:166–180. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang Z, Zheng Z, Hu H, Zhou Q, Li X, Liu
W, Li X, Liu Z, Wang Y and Ma Y: A point-of-care selenium
nanoparticle-based test for the combined detection of
anti-SARS-CoV-2 IgM and IgG in human serum and blood. Lab Chip.
20:4255–4261. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Frens G: Controlled nucleation for the
regulation of the particle size in monodisperse gold suspensions.
Nat Phys Sci. 241:20–22. 1973.
|
|
27
|
Horisberger M and Rosset J: Colloidal
gold, a useful marker for transmission and scanning electron
microscopy. J Histochem Cytochem. 25:295–305. 1977.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xu LQ, Yang YM, Tong H and Xu CF: Early
diagnostic performance of heart-type fatty acid binding protein in
suspected acute myocardial infarction: Evidence from a
meta-analysis of contemporary studies. Heart Lung Circ. 27:503–512.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Anaya P and Moliterno DJ: The evolving
role of cardiac troponin in the evaluation of cardiac disorders.
Curr Cardiol Rep. 15(420)2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang Y, Zheng K, Zhan W, Huang L, Liu Y,
Li T, Yang Z, Liao Q, Chen R, Zhang C and Wang Z: Highly effective
stabilization of Cd and Cu in two different soils and improvement
of soil properties by multiple-modified biochar. Ecotoxicol Environ
Saf. 207(111294)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fathil MF, Md Arshad MK, Gopinath SC,
Hashim U, Adzhri R, Ayub RM, Ruslinda AR, Nuzaihan MNM, Azman AH,
Zaki M and Tang TH: Diagnostics on acute myocardial infarction:
Cardiac troponin biomarkers. Biosens Bioelectron. 70:209–220.
2015.PubMed/NCBI View Article : Google Scholar
|