Introduction
Rheumatoid arthritis (RA) is a common inflammatory
disease characterized by inflammatory cell infiltration that leads
to degeneration of the synovium, articular cartilage and bone
(1). The occurrence and development
of RA involves the release of a large number of inflammatory
cytokines and mediators, which causes irreversible destruction of
cartilage and bone in the joint (2). With the increase of an ageing
population in human society, the incidence of RA has shown an
increased trend in developed countries from 1995 to 2001 (3,4).
Currently, RA treatment relies on non-steroidal anti-inflammatory
drugs, glucocorticoids, methotrexate and other symptomatic methods
of controlling the disease. These drugs can improve the symptoms of
RA, but long-term use has strong toxic side effects, such as
inhibiting the immune function of the body (5). Therefore, finding safe and effective
drugs for the treatment of RA is of great importance.
Quercetin is the most important bioflavonoid in the
human diet, and is widely found in the flowers, leaves and fruits
of plants (6,7). Quercetin has anti-inflammatory,
anti-oxidation and immune regulation activities (8,9), and
therefore shows high medicinal value. Clinical studies have shown
that quercetin has a good effect on relieving knee osteoarthritis,
as well as rheumatoid joint and body pain after exercise (10-12).
Fibroblast-like synoviocytes (FLSs) are the main effector cells and
can release a variety of inflammatory cytokines, aggravating the
progression of RA (13,14). In addition, TNF-α can promote the
activation and bone resorption of osteoclasts, and can induce the
expression of other inflammatory factors, adhesion molecules and
proteases associated with bone and cartilage; thus, causing the
thickening of the synovial lining layer and further exacerbating
the inflammatory response of the joints (15). The present study therefore aimed to
observe the effect of quercetin on FLSs in RA and explore the
possible mechanism of quercetin in the treatment of RA.
Materials and methods
Cell culture
The primary cells RAFLSs were attained from The Cell
Bank of the Type Culture Collection of The Chinese Academy of
Sciences (cat. no. 408-05a). All cells were maintained in DMEM
(Invitrogen; Thermo Fisher Scientific, Inc.) containing 10% FBS
(HyClone; Cytiva) 100 U/ml penicillin and 100 µg/ml streptomycin in
an incubator at 37˚C. After culturing for 24 h, RAFLSs were
pretreated with 50 nmol/l quercetin (MilliporeSigma) for 2 h at
37˚C and then were stimulated using 50 ng/ml TNF-α for 24 h at 37˚C
for the following experiments.
Cell transfection
Small interfering (si)-RNAs targeting XIST (si-XIST;
final concentration, 20 nM) and its scrambled negative control (NC)
siRNA (final concentration, 20 nM), miR-485 inhibitor (final
concentration, 30 nM) and scrambled inhibitor control (miR-NC;
final concentration, 30 nM) and miR-485 mimics (final
concentration, 15 nM) and scrambled mimic control (miR-NC mimics;
final concentration, 15 nM) were obtained from Shanghai GenePharma
Co., Ltd. A total of 1x106 RAFLSs were plated into
six-well plates for 12 h, then the constructs were transfected into
RAFLSs by mixing with Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37˚C for 15 min
following the manufacturer's instructions. A total of 24-72 h after
transfection, the transfected cells were collected for subsequent
experiments. The sequences of the transfected molecules were as
follows: si-XIST, 5'-GCAAAUGAAAGCUACCAAU-3'; si-NC,
5'-AAUUCUCCGAACGUGUCACGU-3'; miR-485 inhibitor,
5'-UCACGCGAGCCGAACGAACAAA-3'; anti-miR-NC,
5'-CAGUACUUUUGUGUAGUACAAA-3'; miR-485 mimic,
5'-GUCAUACACGGCUCUCCUCUCU-3'; miR-NC,
5'-UUCUCCGAACGUGUCACGUTT-3'.
Enzyme-linked immunosorbent assay
(ELISA)
The concentration of inflammatory cytokines in
transfected and non-transfected RAFLSs (IL-1β, IL-6 and IL-8) was
determined using ELISA kits (cat. nos. KE00021, KE00139 and
KE00006; Proteintech Group, Inc.) following the manufacturer's
protocol. A microplate reader was used to read the absorbance at
450 nm.
Reverse transcription-quantitative
(RT-q)PCR analysis
Total cellular RNAs were extracted from transfected
and non-transfected RAFLSs using the TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). cDNA synthesis was
performed using Maxima Probe qPCR Master mix (Fermentas; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. Quantitative analysis of XIST and PSMB8 expression
was performed using SYBR® Premix Ex Taq™
reagent (Takara Bio, Inc.) and GAPDH was used as the endogenous
control. Thermocycling conditions were as follows: 94˚C for 3 min;
40 cycles of 94˚C for 30 sec, 55˚C for 30 sec and 72˚C for 1 min;
and 72˚C for 10 min. The expression level of miR-485 was analyzed
using SYBR® PrimeScript™ miRNA RT-PCR kit
(Takara Biotechnology Co., Ltd.) and U6 small nuclear RNA was used
as the internal reference. Thermocycling conditions were as
follows: Denaturation at 95˚C for 10 sec; 40 cycles of 95˚C for 5
sec and 60˚C for 20 sec; followed by dissociation curve analysis at
95˚C for 60 sec, 55˚C for 30 sec and 95˚C for 30 sec. Primers were
purchased from Sangon Biotech Co., Ltd., and primer sequences were
as follows: GAPDH Forward, 5'-CTTTGGTATCGTGGAAGGACTC-3' and
reverse, 5'-GTAGAGGCAGGGATGATGTTCT-3'; U6 forward,
5'-GCTTCGGCAGCACATATACTAAAAT-3' and reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3'; lncRNA XIST forward,
5'-AATGGAACGGGCTGAGTTTTAC-3' and reverse,
5'-TCATCCGCTTGCGTTCATAG-3'; miR-485 forward,
5'-CAGAGGCTGGCCGTGAT-3' and reverse,
5'-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGAATTC-3'; PSMB8
forward, 5'-GCTGCCTTCAACATAACATCA-3' and reverse,
5'-CTGCCACCACCACCATTA-3'. The relative expression was analysis
using 2-ΔΔCq (16).
Western blotting
Total protein was isolated from RAFLSs using
pre-cold RIPA lysis buffer (Beyotime Institute of Biotechnology)
including protease inhibitor. Total protein was quantified using a
BCA protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). A
total of 30 µg protein sample per lane were separated by 10%
SDS-PAGE, and subsequently transferred onto a nitrocellulose
membrane (MilliporeSigma). The membranes were blocked with 5%
non-fat milk at room temperature for 1 h. Subsequently, the
membranes were incubated overnight at 4˚C with primary antibodies
targeted against: PSMB8 (1:1,000; cat. no. ab180606) and GAPDH
(1:1,000; cat. no. ab181602) (all from Abcam). The membranes were
incubated with a horseradish peroxidase-labeled anti-rabbit IgG
secondary antibody (1:1,000; sc-2027; Santa Cruz Biotechnology,
Inc.) at room temperature for 1 h. Protein bands were visualized
using an ECL detection kit (Pierce; Thermo Fisher Scientific, Inc.)
and analyzed with Quantity One v4.6.2 software (Bio-Rad
Laboratories, Inc.). GAPDH was used as the loading control.
Luciferase reporter assay
The XIST fragments and PSMB8 3'-untranslated regions
containing wild-type (WT) or mutant (MUT) miR-485 binding sites
were attained from Shanghai GenePharma Co., Ltd. They were cloned
into the firefly luciferase gene reporter vector pmiRGLO (Promega
Corporation). The plasmid was synthesized by Invitrogen; Thermo
Fisher Scientific, Inc. Then 48 h after transfection using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37˚C with 5% CO2, the luciferase
assay was performed using the dual-luciferase reporter assay system
kit (Promega Corporation) according to the manufacturer's
instructions. The luciferase activity was analyzed using a Modulus
single-tube multimode reader (Promega Corporation) in comparison
with Renilla luciferase activity. The following sequences
were used: XIST-WT Forward, 5'-CGCCAGCTTCGACAATTCGAC-3', and
reverse, 5'-ACTTGAAGCGGCCATACGTTGACAA-3'; XIST-MUT forward,
5'-TCAAAACCCAACTAGAGGACGATTTGTGA-3', and reverse,
5'-AACCCAGAAAACCGTACGGCAATCGT-3'; PSMB8-WT forward,
5'-CCGCTAAGTCCGAGTAGTCA-3', and reverse,
5'-ATAAGAATGCGGCCGAATTCATGGGAA-3'; and PSMB8-MUT forward,
5'-TTAGGACACGCATCGGATTACGCTATTAT-3', and reverse,
5'-GACACCTGTTTACGGCTACCCGCAAATATGCCAA-3'.
Bioinformatics analysis
The binding sites between miR-485 and XIST, as well
as miR-485 and PSMB8 were predicted by StarBase v2.0 (http://www.microrna.gr/lncBase) or TargetScan
software v7.1 (http://www.targetscan.org/vert_71/), respectively.
Statistical analysis
All measured data are expressed as the mean ±
standard deviation. Differences were calculated with paired
Student's t-test for comparisons between two groups or one-way
ANOVA followed by Dunnett's or Tukey's post hoc test for multiple
comparisons. All statistical analyses were performed using GraphPad
Prism 5.0 (GraphPad Software, Inc.) and SPSS 14.0 (SPSS, Inc.).
P<0.05 was considered to indicate a statistically significant
difference. Experiments were repeated three times.
Results
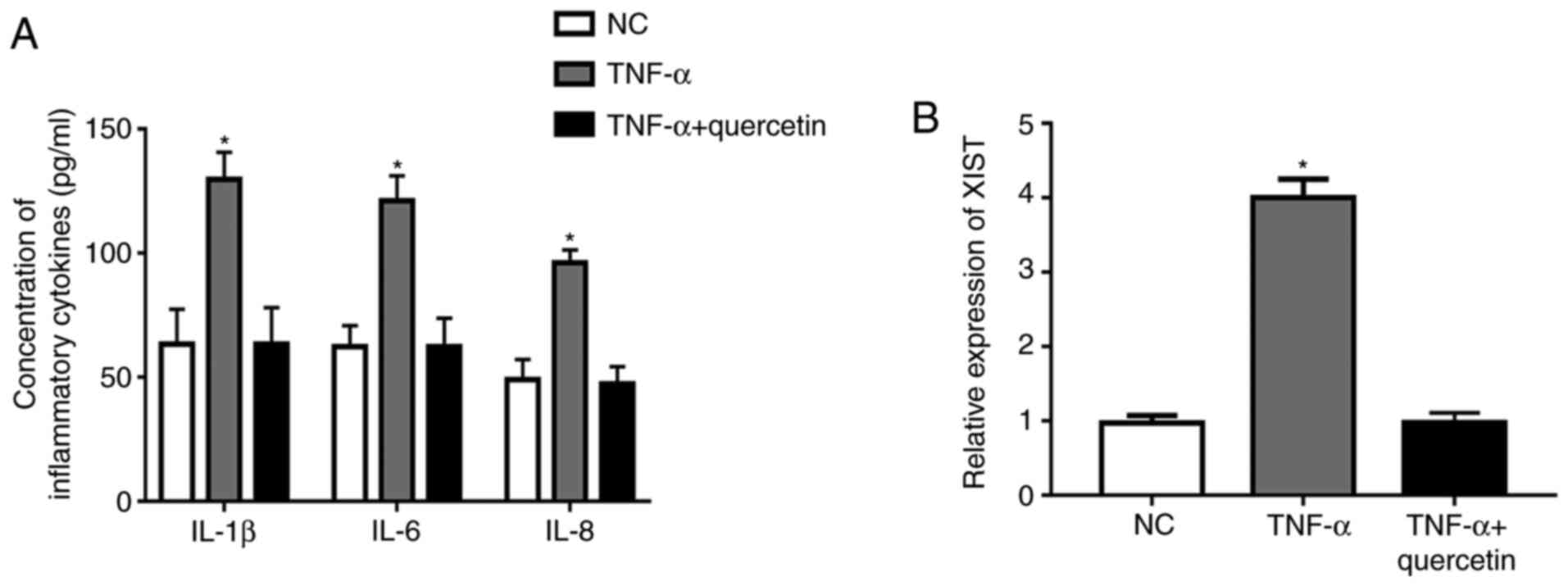
Quercetin inhibits the production of
inflammatory cytokines and the expression of XIST in RAFLSs induced
by TNF-α
Under TNF-α stimulation, the concentration of
inflammatory cytokines (IL-1β, IL-6 and IL-8) and the expression
level of XIST were significantly upregulated in RAFLSs compared
with those in the NC (Fig. 1A and
B). However, treatment with
quercetin could markedly suppress the promotion of TNF-α in the
production of inflammatory cytokines, and the expression level of
XIST was markedly decreased in the FLSs (Fig. 1A and B). These results indicated that quercetin
could suppress the inflammation reaction in RAFLSs induced by
TNF-α.
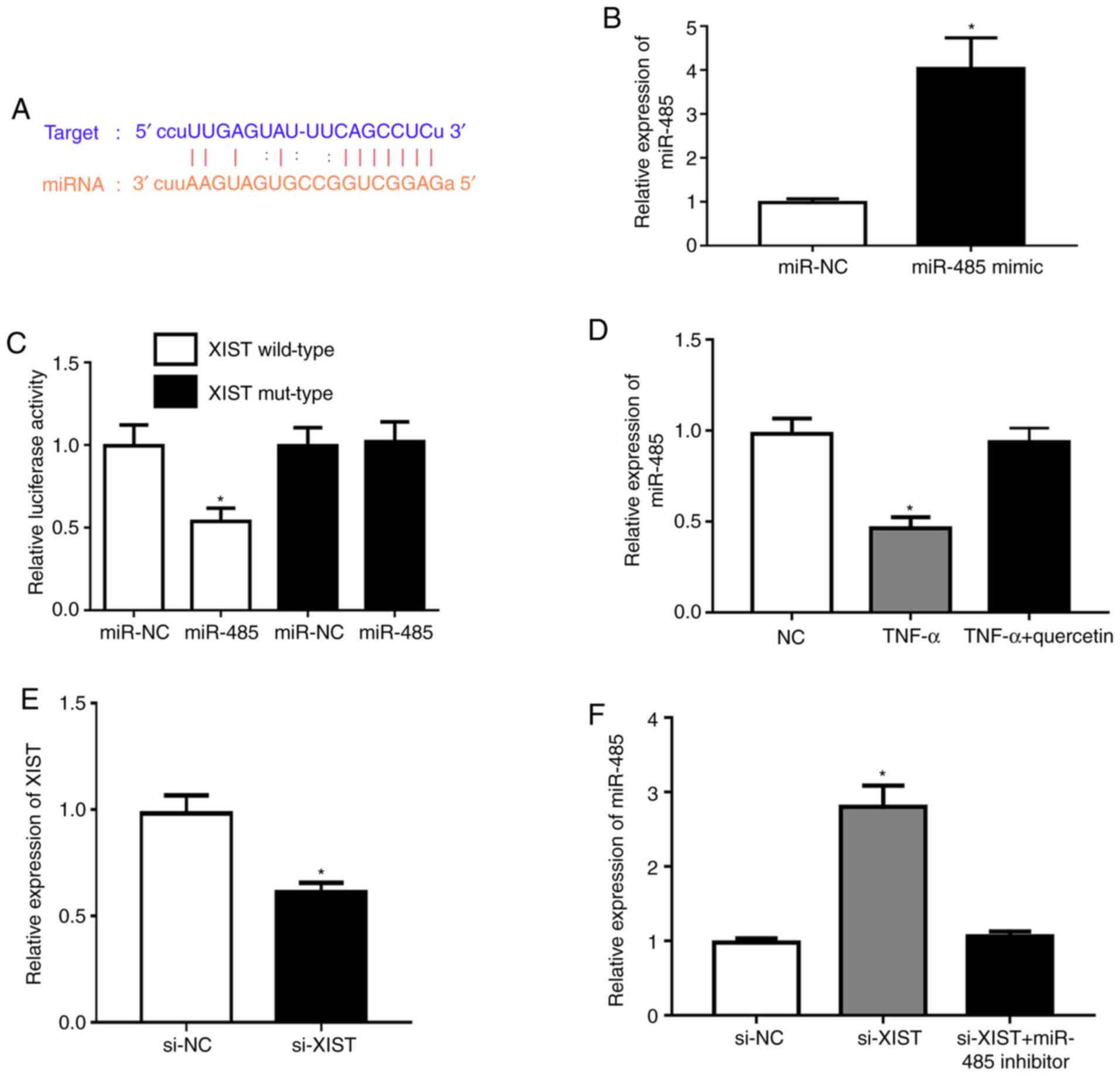
XIST acts as a sponge for miR-485
Subsequently, the potential molecular mechanisms
were explored and the possible targets of XIST were predicted using
the StarBase database v2.0. The bioinformatics analysis indicated
that miR-485 had a potential binding site with the XIST sequence
(Fig. 2A). Transfection with the
miR-485 mimic was demonstrated to upregulate the expression of
miR-485 in RAFLSs (Fig. 2B).
Meanwhile, the miR-485 mimic markedly repressed luciferase activity
in the XIST wild-type compared with that in the NC (Fig. 2C). TNF-α could significantly
downregulate the expression level of miR-485 in RAFLSs, which was
rescued by quercetin (Fig. 2D).
XIST was significantly underexpressed in RAFLSs after transfection
with si-XIST (Fig. 2E). In
addition, XIST-silencing significantly promoted the expression of
miR-485, which was reversed by the miR-485 inhibitor in RAFLSs
treated with TNF-α (Fig. 2F). These
results indicated that XIST interacted with miR-485.
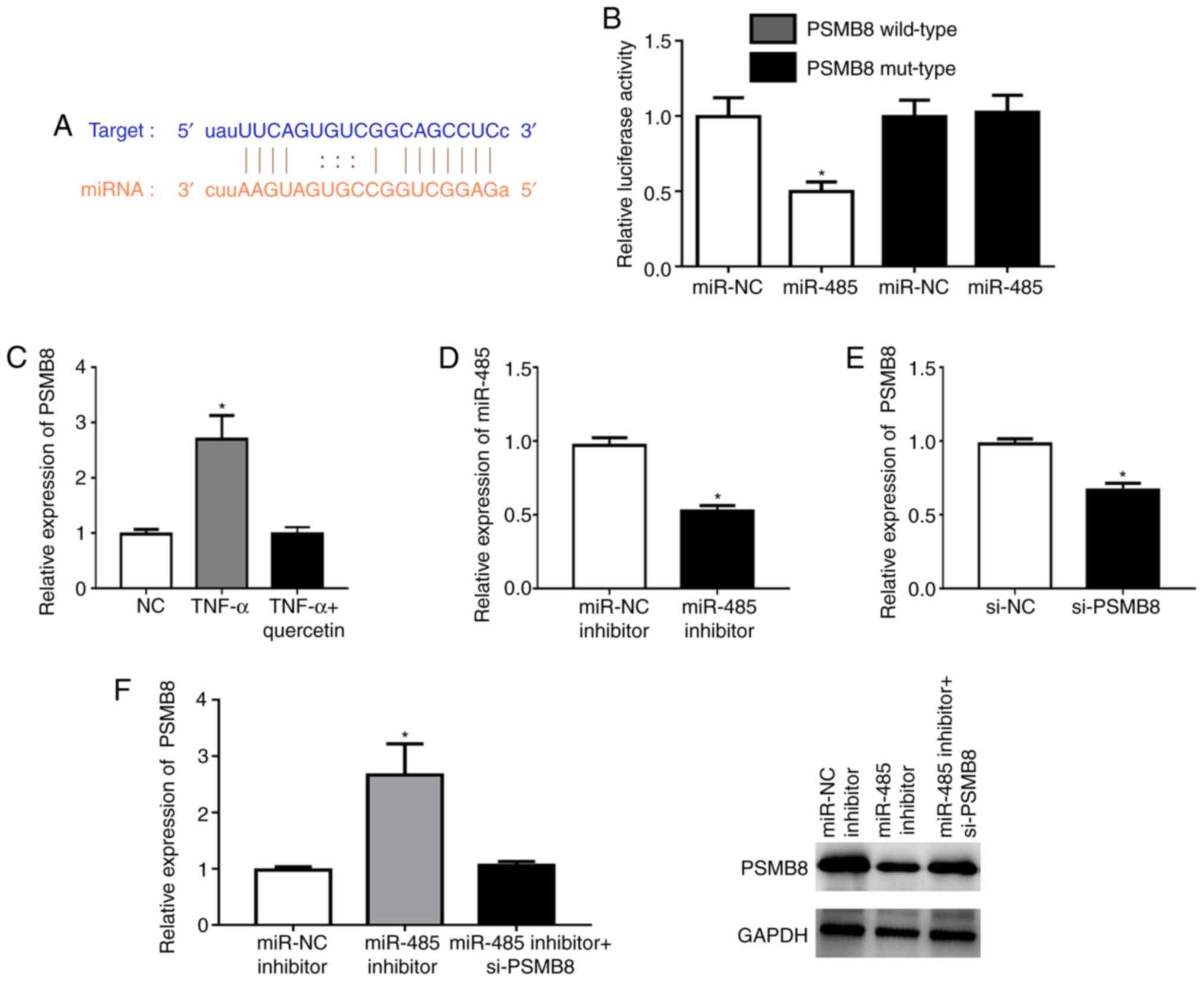
PSMB8 is a direct target of
miR-485
According to TargetScan analysis, a highly conserved
putative binding site with miR-485 was identified at the PSMB8
3'-untranslated region (Fig. 3A).
The miR-485 mimic significantly repressed the fluorescence in the
PSMB8 wild-type compared with that in the NC (Fig. 3B). TNF-α could significantly
upregulate the expression level of PSMB8 in RAFLSs, which was
reversed by quercetin (Fig. 3C).
The miR-485 inhibitor could significantly downregulate the
expression level of miR-485 in RAFLSs (Fig. 3D). In addition, the expression of
PSMB8 was significantly decreased in RAFLSs after transfection with
si-PSMB8 (Fig. 3E). miR-485
inhibitor could significantly enhance the expression of PSMB8 at
the mRNA and protein levels, which was abolished by si-PSMB8 in
RAFLSs treated with TNF-α (Fig.
3F). These results indicated that miR-485 directly targeted
PSMB8.
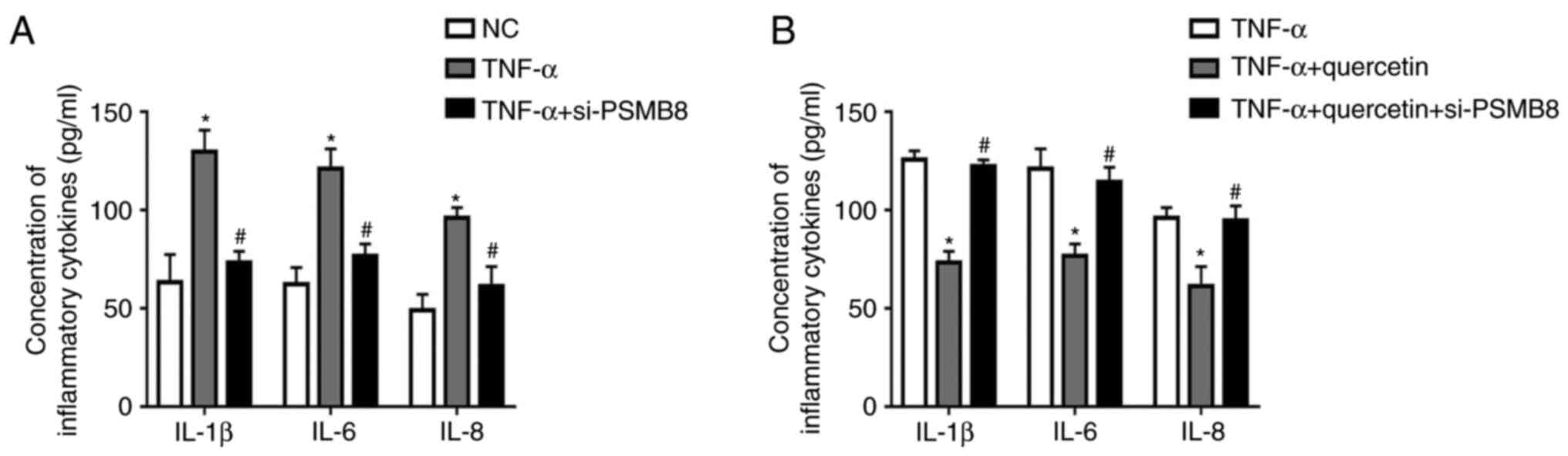
PSMB8 functions as a suppressor in the
production of inflammatory cytokines in RAFLSs induced by
TNF-α
After transfection with si-PSMB8, the concentrations
of inflammatory cytokines (IL-1β, IL-6 and IL-8) were significantly
decreased in transfected RAFLSs treated with TNF-α compared with
the untransfected cells treated with TNF-α (Fig. 4A). Furthermore, quercetin suppressed
the inflammatory reaction in RAFLSs under TNF-α stimulation, which
was rescued by transfection with si-PSMB8 (Fig. 4B). These results indicated that
PSMB8 could suppress the inflammation reaction in RAFLSs induced by
TNF-α.
Discussion
As a natural active product from plants, quercetin
is a secondary metabolite isolated and extracted from fagaceae
plants by natural medicinal chemistry (17). In previous years, quercetin has been
verified to inhibit the inflammatory response in a number of
diseases, including malignant tumors (18), diabetic mellitus (19) and atherosclerosis (20). The present study assessed the role
of quercetin on the development of RA. It is hypothesized that once
fibroblasts are activated in the pathogenesis of RA, a series of
inflammatory factors and extracellular matrix degrading enzymes
will be produced to mediate the inflammatory response, causing the
progressive destruction of bone and cartilage, and influencing the
quality of life severely (21,22).
The present study observed that quercetin suppressed the
inflammatory cytokine production of FLSs induced by TNF-α.
As a class of non-coding transcripts, lncRNAs are
>200 nucleotides in length and regulate gene expression at the
epigenetic, transcriptional and post-transcriptional levels
(23,24). lncRNA XIST was originally identified
in the regulation of X inactivation in the female mammal somatic
cell (25). Previous research
suggests that deregulated expression of XIST is involved in the
inflammatory reaction of some diseases including inflammatory
neuralgia and acute pneumonia (26,27).
Consistent with previous research, the present study found that the
expression level of XIST was upregulated in FLSs induced by TNF-α,
which was abolished by quercetin. lncRNAs can regulate gene
expression by sponging miRNAs (28). Bioinformatics analysis indicated
that miR-485 had a potential binding site with the XIST sequence.
In addition, the level of miR-485 expression was downregulated in
FLSs treated with TNF-α. Additionally, XIST-silencing effectively
promoted miR-485 expression in cells after transfection.
miRNAs can target the 3'-untranslated region of
mRNAs to control gene expression (29). The present study revealed that PSMB8
is a direct target of miR-485. PSMB8 can function as a subunit of
the immunoproteasome to induce an inflammatory response as follows.
Yamagishi (30) reported that
specific inhibition of PSMB8 can decrease the secretion of
inflammatory factors, thus delaying the inflammation response of
diabetes mellitus. Koerner et al (31) revealed that the involvement of PSMB8
in the inflammatory response is closely associated with the
development of colorectal cancer. The present study revealed that
TNF-α could significantly upregulate the expression level of PSMB8
in RAFLSs. Furthermore, si-PSMB8 could inhibit the concentration of
inflammatory cytokines in RAFLSs treated with TNF-α. In addition,
quercetin could not suppress the inflammatory reaction in RAFLSs
treated with si-PSMB8 under TNF-α stimulation. These data suggested
that PSMB8 functions as a suppressor in the production of
inflammatory cytokines in RAFLSs induced by TNF-α.
The present study observed that the suppression of
inflammatory cytokine production by quercetin in FLSs induced by
TNF-α is promising in vitro. However, the effect in
vivo and the effect of pharmacokinetics and penetration of the
synovial membrane on quercetin have yet to be investigated. Thus,
further studies are planned to attempt to study the effect of
quercetin on RA in vivo.
In conclusion, the present study observed
quercetin-inhibited production of inflammatory cytokines and XIST
expression in RAFLSs induced by TNF-α. Moreover, XIST-silencing
could suppress the inflammatory reaction by sponging miR-485 in
cells treated with TNF-α. Altogether, quercetin could suppress the
development of RA in vitro.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HTS and JPL designed experiments. WQQ and MFY
performed experiments. HY analyzed the data. GCH performed the
preliminary experiments and wrote the manuscript, JPL and GCH
revised the manuscript. HS and GCH confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All patients gave informed consent and this study
was approved by The Ethics Committee of Nanjing University of
Chinese Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smolen JS, Aletaha D and McInnes IB:
Rheumatoid arthritis. Lancet. 388:2023–2038. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Firestein GS: Evolving concepts of
rheumatoid arthritis. Nature. 423:356–361. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gabriel SE and Michaud K: Epidemiological
studies in incidence, prevalence, mortality, and comorbidity of the
rheumatic diseases. Arthritis Res Ther. 11(229)2009.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Klareskog L, Catrina AI and Paget S:
Rheumatoid arthritis. Lancet. 373:659–672. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tłustochowicz M, Śliwczyński AM,
Brzozowska M, Teter Z and Marczak M: Sequentiality of treatment in
the rheumatoid arthritis drug programme in the years 2009-2014.
Arch Med Sci. 14:569–571. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Garcia-Mateos R, Aguilar-Santelises L,
Soto-Hernández M and Nieto-Angel R: Flavonoids and antioxidant
activity of flowers of Mexican Crataegus spp. Nat Prod Res.
27:834–836. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Harnly JM, Doherty RF, Beecher GR, Holden
JM, Haytowitz DB, Bhagwat S and Gebhardt S: Flavonoid content of
U.S. fruits, vegetables, and nuts. J Agric Food Chem. 54:9966–9977.
2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Boots AW, Wilms LC, Swennen EL, Kleinjans
JC, Bast A and Haenen GR: In vitro and ex vivo anti-inflammatory
activity of quercetin in healthy volunteers. Nutrition. 24:703–710.
2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
McAnulty LS, Miller LE, Hosick PA, Utter
AC, Quindry JC and McAnulty SR: Effect of resveratrol and quercetin
supplementation on redox status and inflammation after exercise.
Appl Physiol Nutr Metab. 38:760–765. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mamani-Matsuda M, Kauss T, Al-Kharrat A,
Rambert J, Fawaz F, Thiolat D, Moynet D, Coves S, Malvy D and
Mossalayi MD: Therapeutic and preventive properties of quercetin in
experimental arthritis correlate with decreased macrophage
inflammatory mediators. Biochem Pharmacol. 72:1304–1310.
2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Natarajan V, Krithica N, Madhan B and
Sehgal PK: Formulation and evaluation of quercetin polycaprolactone
microspheres for the treatment of rheumatoid arthritis. J Pharm
Sci. 100:195–205. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
O'Fallon KS, Kaushik D, Michniak-Kohn B,
Dunne CP, Zambraski EJ and Clarkson PM: Effects of quercetin
supplementation on markers of muscle damage and inflammation after
eccentric exercise. Int J Sport Nutr Exerc Metab. 22:430–437.
2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bustamante MF, Garcia-Carbonell R,
Whisenant KD and Guma M: Fibroblast-like synoviocyte metabolism in
the pathogenesis of rheumatoid arthritis. Arthritis Res Ther.
19(110)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mor A, Abramson SB and Pillinger MH: The
fibroblast-like synovial cell in rheumatoid arthritis: A key player
in inflammation and joint destruction. Clin Immunol. 115:118–128.
2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Davis JM III and Matteson EL: American
College of Rheumatology; European League Against Rheumatism. My
treatment approach to rheumatoid arthritis. Mayo Clin Proc.
87:659–673. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang LL, Jiang MX, Xu SX, Sun QS, Zeng GY
and Zhou YJ: Two acylated flavonoid glycosides from the leaves of
Quercus dentata. Nat Prod Commun. 5:1597–1599. 2010.PubMed/NCBI
|
|
18
|
Lamson DW and Brignall MS: Antioxidants
and cancer, part 3: Quercetin. Altern Med Rev. 5:196–208.
2000.PubMed/NCBI
|
|
19
|
Fu J, Huang J, Lin M, Xie T and You T:
Quercetin promotes diabetic wound healing via switching macrophages
from M1 to M2 polarization. J Surg Res. 246:213–223.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tribolo S, Lodi F, Connor C, Suri S,
Wilson VG, Taylor MA, Needs PW, Kroon PA and Hughes DA: Comparative
effects of quercetin and its predominant human metabolites on
adhesion molecule expression in activated human vascular
endothelial cells. Atherosclerosis. 197:50–56. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Firestein GS: Invasive fibroblast-like
synoviocytes in rheumatoid arthritis. Passive responders or
transformed aggressors? Arthritis Rheum. 39:1781–1790.
1996.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang F, Zhang L and Zhang C: Long
noncoding RNAs and tumorigenesis: Genetic associations, molecular
mechanisms, and therapeutic strategies. Tumour Biol. 37:163–175.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Geisler S and Coller J: RNA in unexpected
places: Long non-coding RNA functions in diverse cellular contexts.
Nat Rev Mol Cell Biol. 14:699–712. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Mattick JS and Rinn JL: Discovery and
annotation of long noncoding RNAs. Nat Struct Mol Biol. 22:5–7.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Brown CJ, Ballabio A, Rupert JL,
Lafreniere RG, Grompe M, Tonlorenzi R and Willard HF: A gene from
the region of the human X inactivation centre is expressed
exclusively from the inactive X chromosome. Nature. 349:38–44.
1991.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Sun W, Ma M and Yu H and Yu H: Inhibition
of lncRNA X inactivate-specific transcript ameliorates inflammatory
pain by suppressing satellite glial cell activation and
inflammation by acting as a sponge of miR-146a to inhibit
Nav 1.7. J Cell Biochem. 119:9888–9898. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang Y, Zhu Y, Gao G and Zhou Z:
Knockdown XIST alleviates LPS-induced WI-38 cell apoptosis and
inflammation injury via targeting miR-370-3p/TLR4 in acute
pneumonia. Cell Biochem Funct. 37:348–358. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Zeisel MB, Druet VA, Wachsmann D and
Sibilia J: MMP-3 expression and release by rheumatoid arthritis
fibroblast-like synoviocytes induced with a bacterial ligand of
integrin alpha5beta1. Arthritis Res Ther. 7:R118–R126.
2005.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Yamagishi S: Role of advanced glycation
end products (AGEs) and receptor for AGEs (RAGE) in vascular damage
in diabetes. Exp Gerontol. 46:217–224. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Koerner J, Brunner T and Groettrup M:
Inhibition and deficiency of the immunoproteasome subunit LMP7
suppress the development and progression of colorectal carcinoma in
mice. Oncotarget. 8:50873–50888. 2017.PubMed/NCBI View Article : Google Scholar
|