Introduction
Cervical cancer ranks third in terms of incidence of
malignant tumors worldwide, and is the most frequent type of
gynecological cancer in developing countries (1-3).
The increasing trend in cervical cancer in developing countries is
attributed to the early beginning of sexual activities, certain
sexual behaviors such as high number of partners, early age at
first intercourse, infrequent use of condoms, multiple pregnancies
with Chlamydia association, and immunosuppression with human
immunodeficiency virus, which is associated with higher risk of
infection by human papillomavirus (HPV) (4,5). In
USA, the HPV16 and 18 types are detected in 70% of high-grade
squamous intraepithelial lesions, as well as in invasive cervical
cancer cases (6,7). To date, chemotherapy and surgery are
the two major strategies for treating cervical cancer (8). However, the prognosis of cervical
cancer remains poor. Thus, it is urgent to identify novel methods
for the treatment of cervical cancer.
Recent studies have shown that interleukin (IL)-17
plays an important role in cervical cancer (9,10).
IL-17A is a member of the IL-17 family, which has been regarded as
a pro-inflammatory cytokine (11).
In addition, IL-17 is secreted by various cells, including T helper
cells, CD8+ T cells, γδ T cells and natural killer cells
in the tumor microenvironment (12). IL-17 and its receptor are expressed
in a variety of cell types, including fibroblasts and tumor cells,
leading to the secretion of pro-inflammatory cytokines, such as
IL-6, various chemokines and metalloproteinases (13). Previous studies have shown that an
inflammatory environment may lead to tumor growth by generating
tumor-promoting cytokines, decreasing cytotoxic T cells and
developing myelogenous inhibitory cells, and further production can
promote tumor growth (14-16).
Besides, previous findings have revealed that the risk of cervical
cancer is associated with IL-17 polymorphism in both Chinese and
Western populations (17,18). However, the mechanism by which IL-17
modulates the development of cervical cancer remains unclear.
Thus, the present study aimed to explore the
potential molecular mechanism of IL-17 in cervical cancer. The
results may provide experimental basis for the possibility of using
IL-17 as a key marker to predict prognosis of cervical cancer.
Materials and methods
Sample collection
In total, 30 pairs of cervical cancer samples and
adjacent normal tissues were collected from Gansu Provincial Cancer
Hospital between June 2018 and June 2019. The clinical and
pathological data of these patients (n=30) were collected with
their written informed consent. The patient exclusion criteria were
as follows: i) Patients (women) who suffered from other diseases
and were currently under treatment; ii) pregnant and lactating
women; iii) patients allergic to probiotics or have used/are using
antibiotics recently; and iv) alcoholics (people who drink ≥5
bottles of beer at a time, or the alcohol content in the blood
reaches ≥0.08). The patient inclusion criteria were as follow:
Women (≥18 years old) who have been diagnosed with cervical cancer
and have undergone surgery. Each tissue sample was stored at -80˚C
until RNA extraction. In addition, the serum samples were collected
from the patients. The present study was approved by the Ethics
Committee of Gansu Provincial Cancer Hospital. The distribution of
age and sex (30 females; mean age, 52 years; age range, 32-68
years) among the patients with cervical cancer was presented in
Table I.
 | Table IDistribution of sex and age among
patients with cervical cancer. |
Table I
Distribution of sex and age among
patients with cervical cancer.
| Sex | Age, years | Stage |
|---|
| Female | 53 | IIA2 |
| Female | 52 | IIA2 |
| Female | 61 | IIA1 |
| Female | 55 | IIA1 |
| Female | 57 | IIA1 |
| Female | 48 | IIA2 |
| Female | 55 | IIA1 |
| Female | 61 | IB2 |
| Female | 49 | IB1 |
| Female | 61 | IIA1 |
| Female | 62 | IIA2 |
| Female | 52 | IB1 |
| Female | 46 | IB2 |
| Female | 61 | IIA1 |
| Female | 32 | IIA2 |
| Female | 42 | IIA1 |
| Female | 56 | IB1 |
| Female | 51 | IB2 |
| Female | 56 | IIA1 |
| Female | 45 | IIA1 |
| Female | 50 | IIA1 |
| Female | 34 | IB2 |
| Female | 55 | IIA1 |
| Female | 55 | IB1 |
| Female | 53 | IIA1 |
| Female | 44 | IB1 |
| Female | 68 | IIA1 |
| Female | 41 | IB1 |
| Female | 59 | IIA1 |
| Female | 50 | IIA1 |
Meanwhile, serum was also collected from healthy
donors (n=50; age, 21-58 years; sex, 28 males and 22 females). The
informed consent was also obtained from healthy individuals for
blood donation in the present study. The inclusion criteria of
individuals without cervical cancer as control blood donors were as
follows: i) Aged from 20-60 years old; and ii) no history of
cancer.
Cell culture
The HeLa cell line was obtained from the Shanghai
Cell Bank of Chinese Academy of Sciences. Cells were cultured in
DMEM (Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum
(FBS; Thermo Fisher Scientific, Inc.), 1% penicillin (Thermo Fisher
Scientific, Inc.) and streptomycin (Thermo Fisher Scientific, Inc.)
at 37˚C in the presence of 5% CO2.
Cell transfection
HeLa cells were seeded at a density of
3x105 cells/well in a 6-well plate and cultured until
70% confluence. Then, the cells were transfected with small
interfering RNA (si)STAT3 (10 nM), siJAK2 (10 nM) or negative
control (empty vector, siNC, 10 nM) using Lipofectamine®
2000 reagent (Thermo Fisher Scientific, Inc.). For siRNA knockdown,
the sequence of siRNA targeting JAK2 (siJAK2) or STAT3 (siSTAT3)
was designed and synthesized from Shanghai GenePharma Co., Ltd. The
efficiency of transfection was detected by reverse
transcription-quantitative PCR (RT-qPCR). The sequences of siRNAs
were as follows: siNC, 5'-ACGUGACACGUUCGGAGAAUU-3'; siJAK2,
5'-ATCATGUUUGAGACCUUAAA-3'; siSTAT3, 5'-CUUUGAGGUCAGCCGACUCU-3'.
After 24 h of transfection, transfected cells were used in
subsequent experiments.
RT-qPCR
Total RNAs were extracted from tissues or cell lines
with TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
RT-qPCR was conducted with PrimeScript RT Reagent kit (Takara Bio,
Inc.) and SYBR Premix Ex Taq II kit (Takara Bio, Inc.). The
temperature and duration of RT were as follows: 37˚C for 60 min and
85˚C for 5 min. The thermocycling conditions were as follows:
Initial denaturation for 10 min at 95˚C; 40 cycles of 95˚C for 15
sec and 60˚C for 30 sec; and final extension for 1 min at 60˚C. The
primers were purchased from Nanjing Jinsirui Biotechnology Co.,
Ltd. β-actin was used as the internal control. The primers were as
follows: STAT3, 5'-CATCCTGAAGCTGACCCAGG-3'; STAT3 reverse,
5'-TCCTCACATGGGGGAGGTAG-3'; JAK2 forward,
5'-GAGACAACTGTGACGGGCTT-3'; JAK2 reverse,
5'-GCTCAGCTCCCACTCACATC-3'; IL-17 forward,
5'-CCTTGGAATCTCCACCGCAA-3'; IL-17 reverse,
5'-GAGCTCTTAGGCCACATGGT-3'; IL-17A forward,
5'-CTACAACCGATCCACCTCACC-3'; IL-17A reverse,
5'-AGCCCACGGACACCAGTATC-3'; IL-17F forward,
5'-CTGTGCCAGGAGGTAGTATGA-3'; IL-17F reverse,
5'-TTGATGCAGCCCAAGTTCCTA-3'; β-actin forward,
5'-GTCCACCGCAAATGCTTCTA-3'; and β-actin reverse,
5'-TGCTGTCACCTTCACCGTTC-3'. The 2-ΔΔCq method (19) was used to measure relative
expression.
Wound-healing assay
HeLa cells (5x103 per well) were plated
into a 24-well Cell Culture Cluster. Once cells reached 80-90%
confluence, the layer of cells was scratched perpendicular with a
small pipette head. After washing with PBS for 3 times, serum-free
medium was used for further culture, and the scratch widths at 0
and 24 h were recorded under an optical light microscope
(magnification, x200). The experiment was repeated 3 times.
Enzyme-linked immunosorbent assay
(ELISA)
The levels of IL-17 in tissues of patients were
detected using an ELISA kit (Hangzhou Multisciences Biotech Co.,
Ltd., cat. no. 70-EK117/2-96), according to the manufacturer's
instructions.
Transwell assay
For cell invasion analysis, Transwell assay was
performed. The upper chamber was pre-treated with 100 µl Matrigel.
HeLa cells were seeded into the upper chamber in medium with 1%
FBS, and the density was adjusted to ~1.0x106 cells per
chamber. RPMI-1640 medium with 10% FBS was added to the lower
chamber. After 24 h of incubation at 37˚C, the Transwell chamber
was rinsed twice with PBS (5 min each time), fixed with 5%
glutaraldehyde at 4˚C, stained with 0.1% crystal violet at room
temperature for 30 min, washed twice with PBS and observed under an
optical light microscope (magnification, x200). The number of cells
invading the Matrigel was regarded to represent the invasion
ability.
Cell Counting Kit-8 (CCK-8) assay
HeLa cells were seeded in 96-well plates
(5x103 per well) overnight. Then, cells were treated
with 0, 5, 10, 50 or 50 ng/ml IL-17 for 72 h. Next, 10 µl CCK-8
reagent was added to each well and further incubated for 2 h at
37˚C. Finally, the absorbance was measured at 450 nm using a
microplate reader (Thermo Fisher Scientific, Inc.).
Western blotting
Total protein was isolated from tissue or cell
lysates using RIPA buffer (Shanghai GenePharma Co., Ltd.), and
quantified using a BCA protein assay kit (Beyotime Institute of
Biotechnology). Proteins (30 µg/lane) were resolved on 10% SDS-PAGE
gel, and then transferred to PVDF membranes (Bio-Rad Laboratories,
Inc.). After blocking with 5% skimmed milk for 1 h at room
temperature, the membranes were incubated with primary antibodies
at 4˚C overnight, and then incubated with an HRP-conjugated
secondary anti-rabbit antibody (1:5,000; cat. no. ab7090; Abcam) at
room temperature for 1 h. Membranes were scanned using an Odyssey
Imaging System and analyzed with Odyssey v2.0 software (LI-COR
Biosciences). The visualization was performed using an ECL
chemiluminescent kit (Beyotime Institute of Biotechnology)
according to the manufacturer's instructions. The primary
antibodies used in the present study were as follows: Anti-JAK2
(1:1,000; cat. no. ab108596; Abcam), anti-IL-17A (1:1,000; cat. no.
ab79056; Abcam), anti-IL-17F (1:1,000; cat. no. ab168194; Abcam),
anti-STAT3 (1:1,000; cat. no. ab68153; Abcam), anti-vascular
endothelial growth factor (VEGF; 1:1,000; cat. no. ab32152; Abcam),
anti-Akt (1:1,000; cat. no. ab8805; Abcam), anti-p65 (1:1,000; cat.
no. ab32536; Abcam), anti-p-STAT3 (1:1,000; cat. no. ab267373;
Abcam), p-p65 (1:1,000; cat. no. ab76302; Abcam), anti-p-JAK2
(1:1,000; cat. no. ab32101; Abcam), anti-p-Akt (1:1,000; cat. no.
ab76302; Abcam) and anti-GAPDH (1:1,000; cat. no. ab8245; Abcam).
GAPDH was used as an internal control.
Immunofluorescence
Cervical cancer cells (1x104 per well)
were seeded in 24-well plates overnight and treated as following:
Control, IL-17, IL-17 plus siRNA-NC, IL-17 plus siRNA-STAT3 or
IL-17 plus siRNA-JAK2 for 72 h. After that, the cells were prefixed
in 4% paraformaldehyde at 4˚C for 10 min, and fixed in pre-cold
methanol at 4˚C for another 10 min. Next, cells were incubated with
primary antibodies overnight at 4˚C: Anti-Ki67 (Abcam; cat. no.
ab15580; 1:1,000). The nuclei were stained with DAPI (Beyotime
Institute of Biotechnology). Goat anti-rabbit IgG antibody (Abcam;
cat. no. ab150077; 1:5,000) was used as the secondary antibody. The
samples were visualized by fluorescence microscope (magnification,
x200; Olympus CX23; Olympus Corporation) immediately.
Statistical analysis
In total, 3 independent experiments were performed,
and all data are expressed as the mean ± standard deviation.
GraphPad Prism 7 (GraphPad Software, Inc.) was used for all
statistical analyses. The comparison between two groups was
analyzed using paired Student's t-test (Figs. 1A and 2) or unpaired Student's t-test (Fig. 1B). One-way ANOVA followed by Tukey's
test was used for comparisons between multiple groups. P<0.05
was considered to indicate a statistically significant
difference.
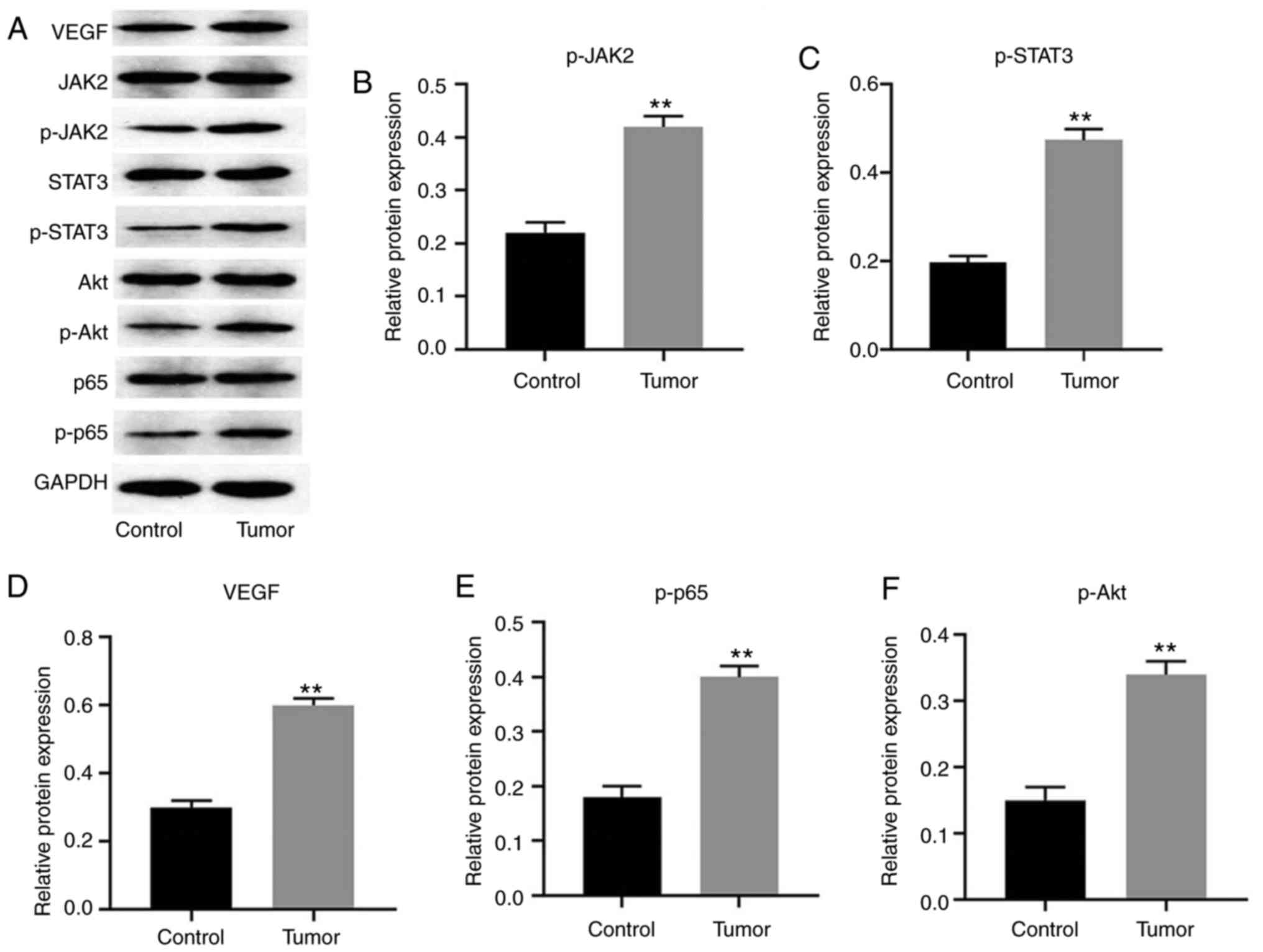 | Figure 2JAK2/STAT3, NF-κB, VEGF and PI3K are
involved in tumorigenesis of cervical cancer. (A) Protein
expression levels of JAK2, p-JAK2, STAT3, p-STAT3, p-p65, Akt,
p-Akt and VEGF in tissues detected by western blotting. Relative
expression levels of (B) p-JAK2 (normalized to JAK2), (C) p-STAT3
(normalized to STAT3), (E) p-p65 (normalized to p65) and (F) p-Akt
(normalized to Akt) were quantified. Relative expression level of
(D) VEGF was normalized to GAPDH. **P<0.01 vs.
control. The data were analyzed using paired Student's t-test.
NF-κB, nuclear factor-κB; VEGF, vascular endothelial growth factor;
p-, phosphorylated. |
Results
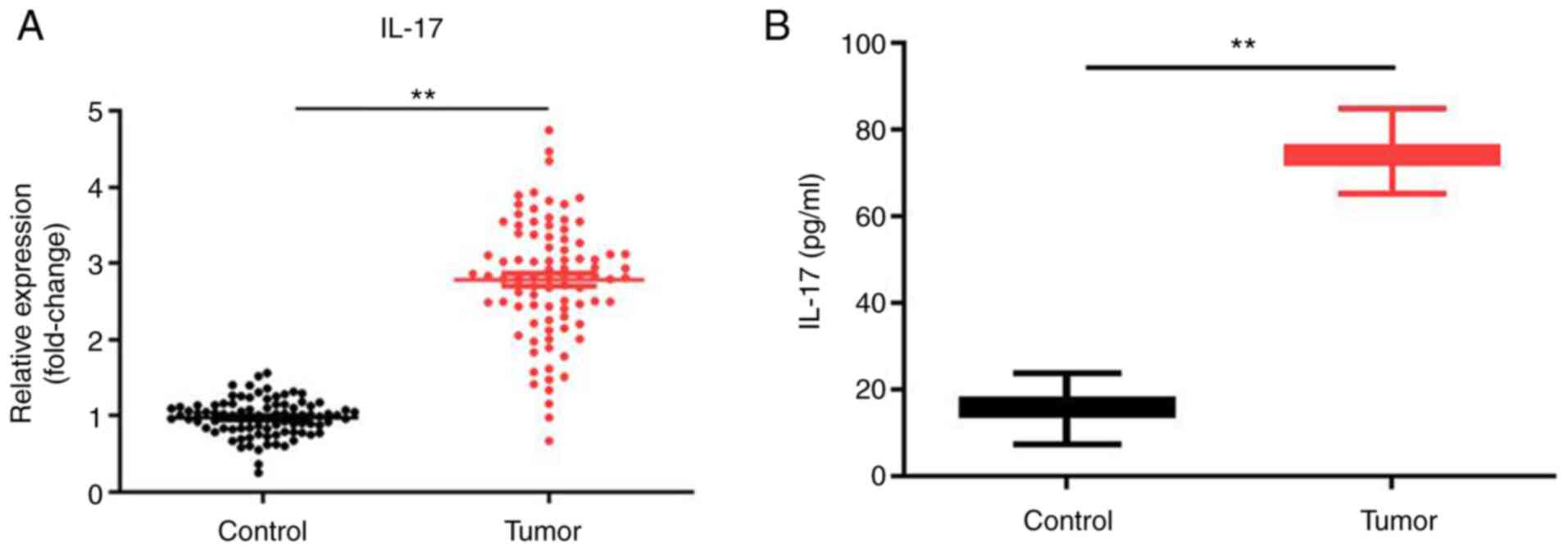
IL-17 mRNA expression level is
upregulated in cervical tumor tissues compared with that in normal
tissues
In order to investigate the role of IL-17 in the
progression of cervical cancer, RT-qPCR was employed. As indicated
in Fig. 1A, the expression level of
IL-17 was significantly upregulated in tumor tissues compared with
that in normal tissues; to verify this result, ELISA was performed.
The results demonstrated that the level of IL-17 in serum of
patients with cervical cancer was significantly increased (Fig. 1B). Taken together, the results
showed that IL-17 was upregulated during the tumorigenesis of
cervical cancer.
JAK2/STAT3, NF-κB, VEGF and PI3K
signaling are involved in the tumorigenesis of cervical cancer
For the purpose of exploring the role of JAK2/STAT3,
NF-κB, VEGF and PI3K signaling in development of cervical cancer,
western blotting was used. As shown in Fig. 2A-C, the expression levels of JAK2
and STAT3 in tumor tissues was notably increased compared with that
in normal tissues. These data suggested that JAK2/STAT3 was
involved in the pathogenesis of cervical cancer. Similarly, NF-κB,
VEGF and PI3K/Akt signaling were also upregulated in tumor tissues
(Fig. 2A and D-F). These results indicated that
JAK2/STAT3, NF-κB, VEGF and PI3K/Akt were activated in the
tumorigenesis of cervical cancer.
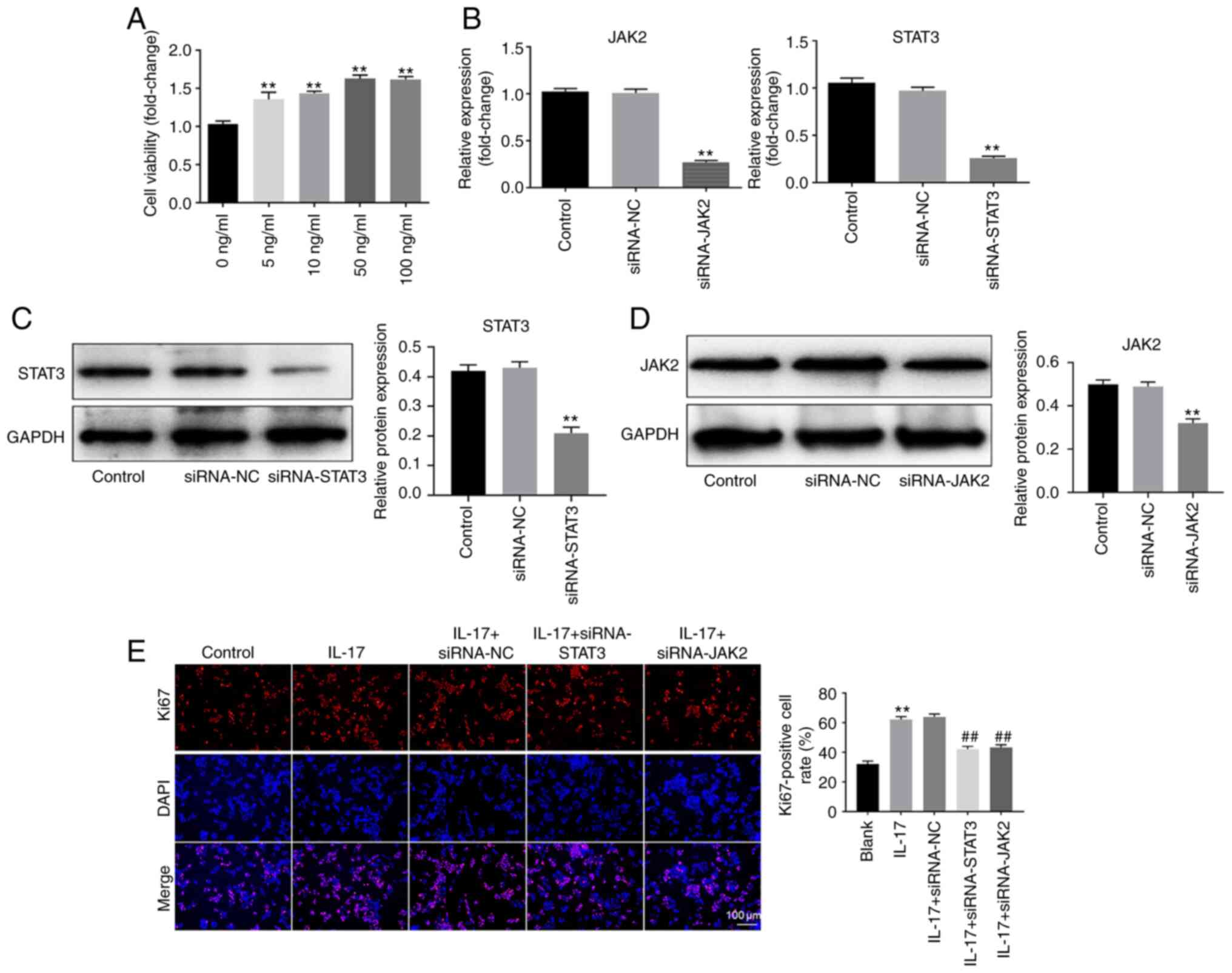
IL-17 promotes the growth of HeLa
cells
To verify the function of IL-17 in the progression
of cervical cancer, CCK-8 assay was performed. As shown in Fig. 3A, IL-17 notably increased the
proliferation of HeLa cells. Moreover, a concentration of 50 ng/ml
exhibited the most proliferative effect. Therefore, 50 ng/ml was
used in the following experiments. Next, RT-qPCR and western
blotting were used to detect the transfection efficiency. The data
demonstrated that the expression of JAK2 in HeLa cells was
significantly decreased by knockdown of JAK2 (Fig. 3B-D). Similarly, the expression of
STAT3 in cervical cancer cells was notably inhibited after STAT3
silencing (Fig. 3B-D). These
results suggested that JAK2 and STAT3 siRNA were stably transfected
into HeLa cells. Then, the results of Ki-67 staining demonstrated
that IL-17 significantly promoted the proliferation of cervical
cancer cells. However, knockdown of JAK2 or STAT3 partially rescued
the proliferative effect of IL-17 (Fig.
3E). Overall, these data suggested that IL-17 could promote the
growth of cervical cancer cells via activation of JAK2/STAT3
signaling.
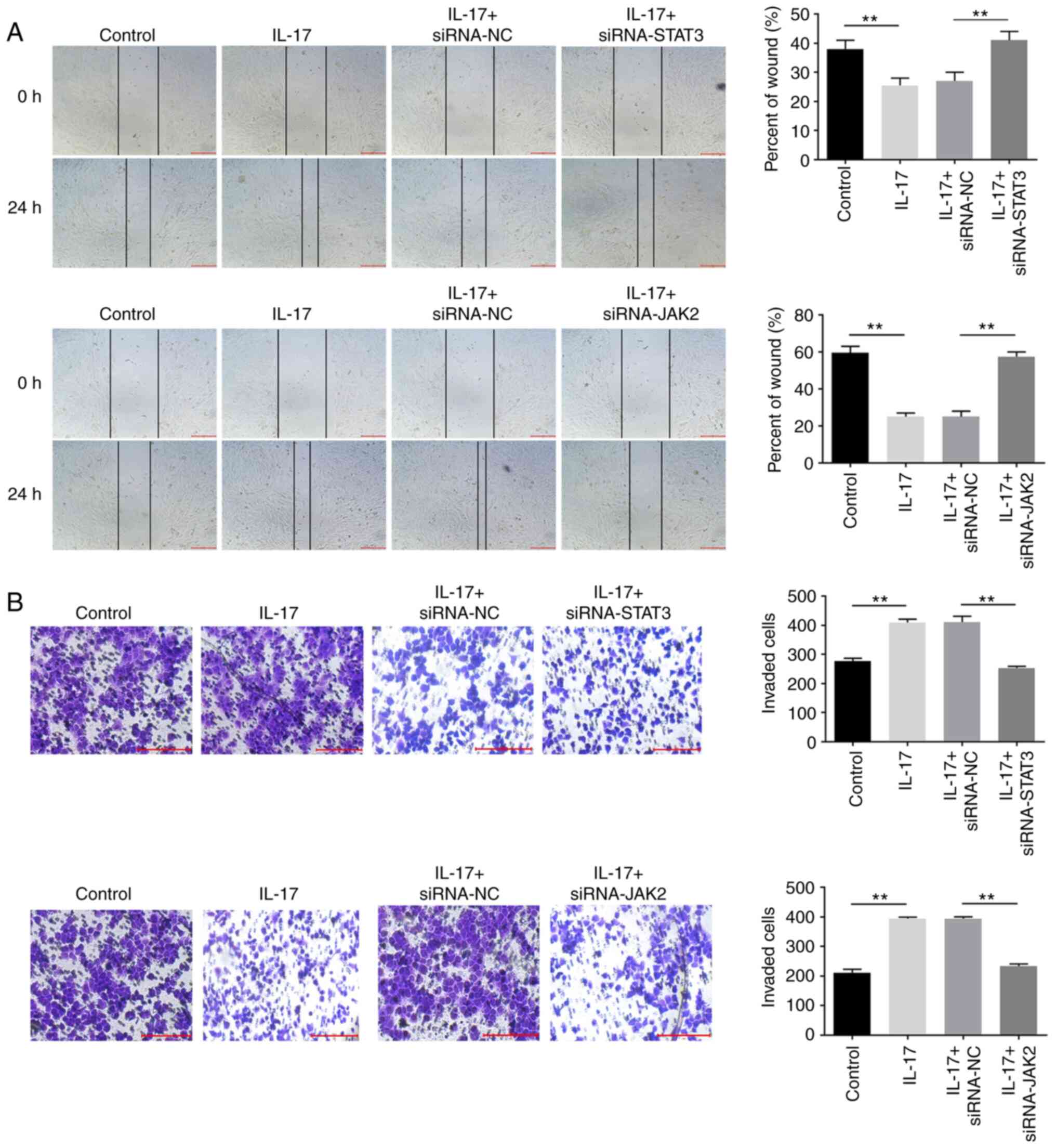
IL-17 significantly promotes the
migration and invasion of cervical cancer cells
To further investigate the effect of IL-17 on the
migration and invasion of cervical cancer cells, wound-healing and
Transwell assays were performed. As shown in Fig. 4A and B, the migration and invasion of cervical
cancer cells were notably inhibited in the presence of IL-17, which
were partially reversed by downregulation of JAK2 or STAT3. These
data confirmed that IL-17 could promote the migration and invasion
of cervical cancer cells via the JAK2/STAT3 signaling pathway.
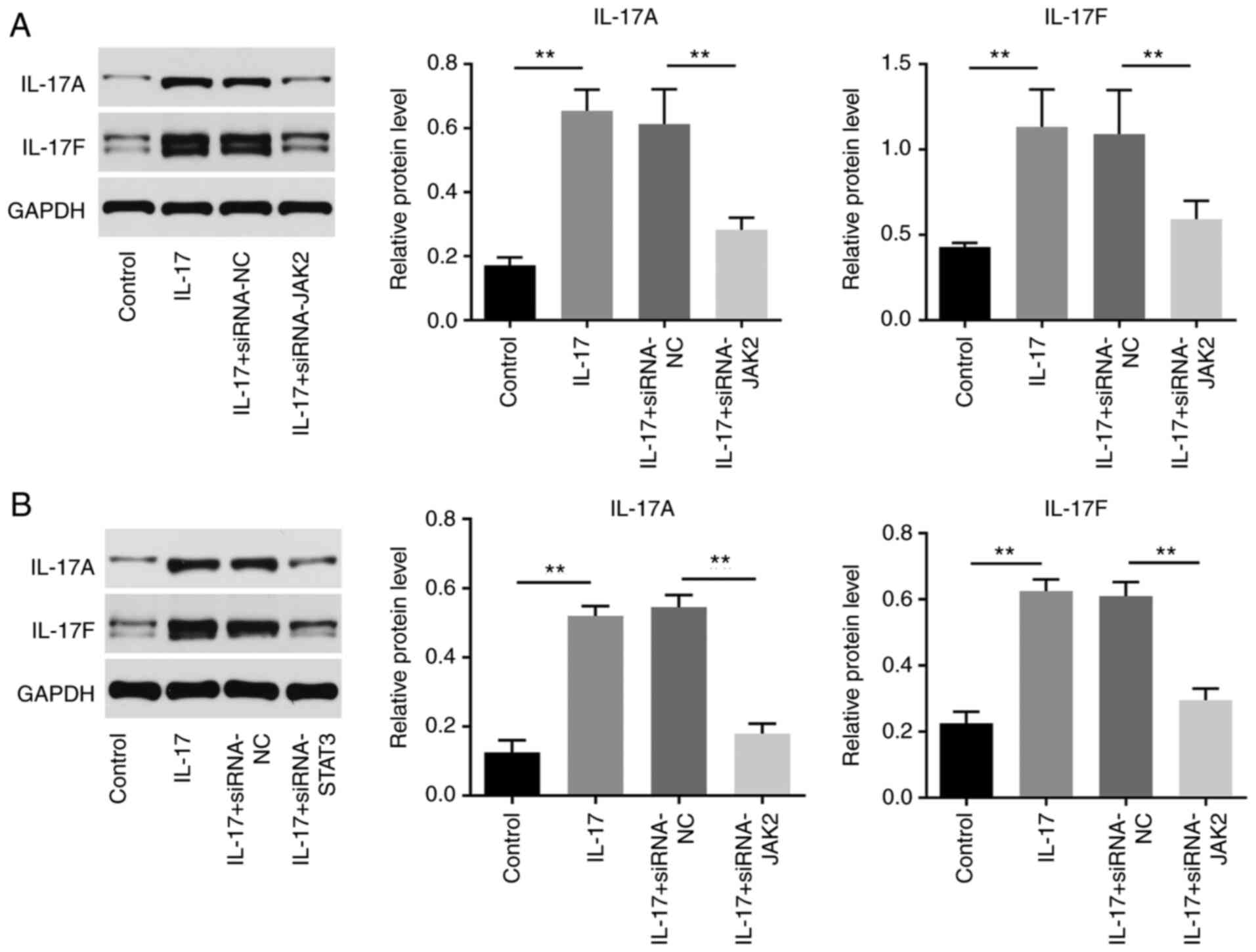
IL-17 promotes the tumorigenesis of
cervical cancer via upregulation of IL-17A and IL-17F
IL-17A and IL-17F are two major isoforms of IL-17
that can regulate the cancer tumorigenesis (20,21).
Thus, these two isoforms were selected for investigations. As
indicated in Figs. 5A and B and S1A-D, the levels of IL-17A and IL-17F in
cervical cancer cells were significantly upregulated by IL-17,
while JAK2 or STAT3 knockdown reversed this phenomenon. Taken
together, IL-17 promotes the tumorigenesis of cervical cancer via
the upregulation of IL-17A and IL-17F.
IL-17 promotes the progression of
cervical cancer through JAK2/STAT3, PI3K/Akt and NF-κB
signaling
To further verify the mechanism by which IL-17
modulates the progression of cervical cancer, western blotting was
used. The results indicated that the expression levels of VEGF,
phosphorylated (p)-JAK2, p-STAT3, p-Akt and p-p65 were
significantly upregulated by IL-17, which was notably rescued by
silencing of JAK2 or STAT3 (Figs.
6A and B, 7A and B).
Taken together, the results confirmed that IL-17 promoted the
progression of cervical cancer through the upregulation of
JAK2/STAT3 signaling.
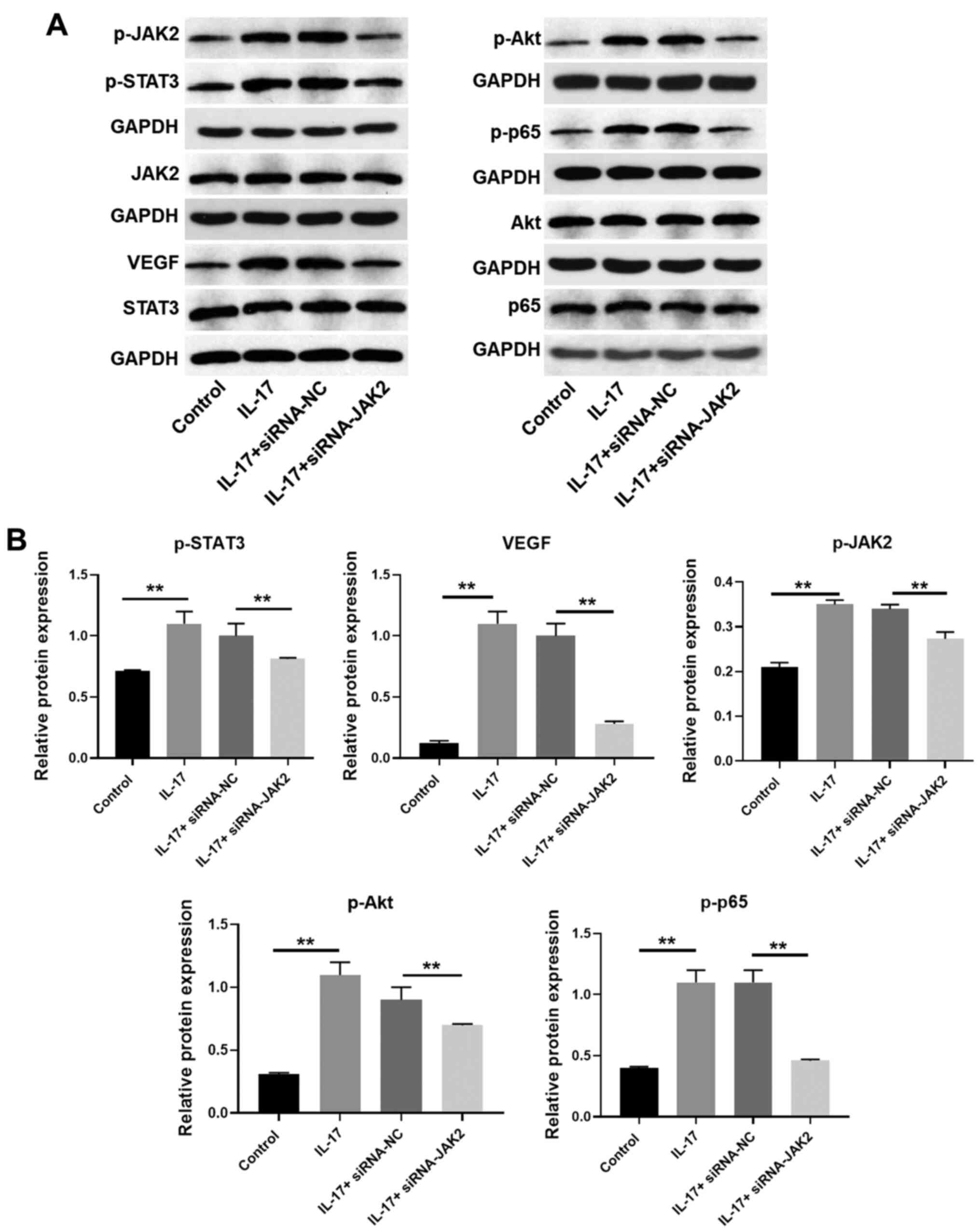 | Figure 6IL-17 promotes the progression of
cervical cancer through JAK2/STAT3, PI3K/Akt and NF-κB signaling.
(A and B) HeLa cells were transfected with JAK2 siRNA. Then, the
protein expression of p65, p-p65 (normalized to p65), STAT3,
p-STAT3 (normalized to STAT3), Akt, p-Akt (normalized to Akt),
JAK2, p-JAK2 (normalized to JAK2) and VEGF in HeLa cells were
detected by western blotting. The relative expression of VEGF was
quantified normalized to GAPDH. **P<0.01. IL,
interleukin; NF-κB, nuclear factor NF-κB; p-, phosphorylated-;
siRNA, small interfering RNA; VEGF, vascular endothelial growth
factor; NC, negative control. |
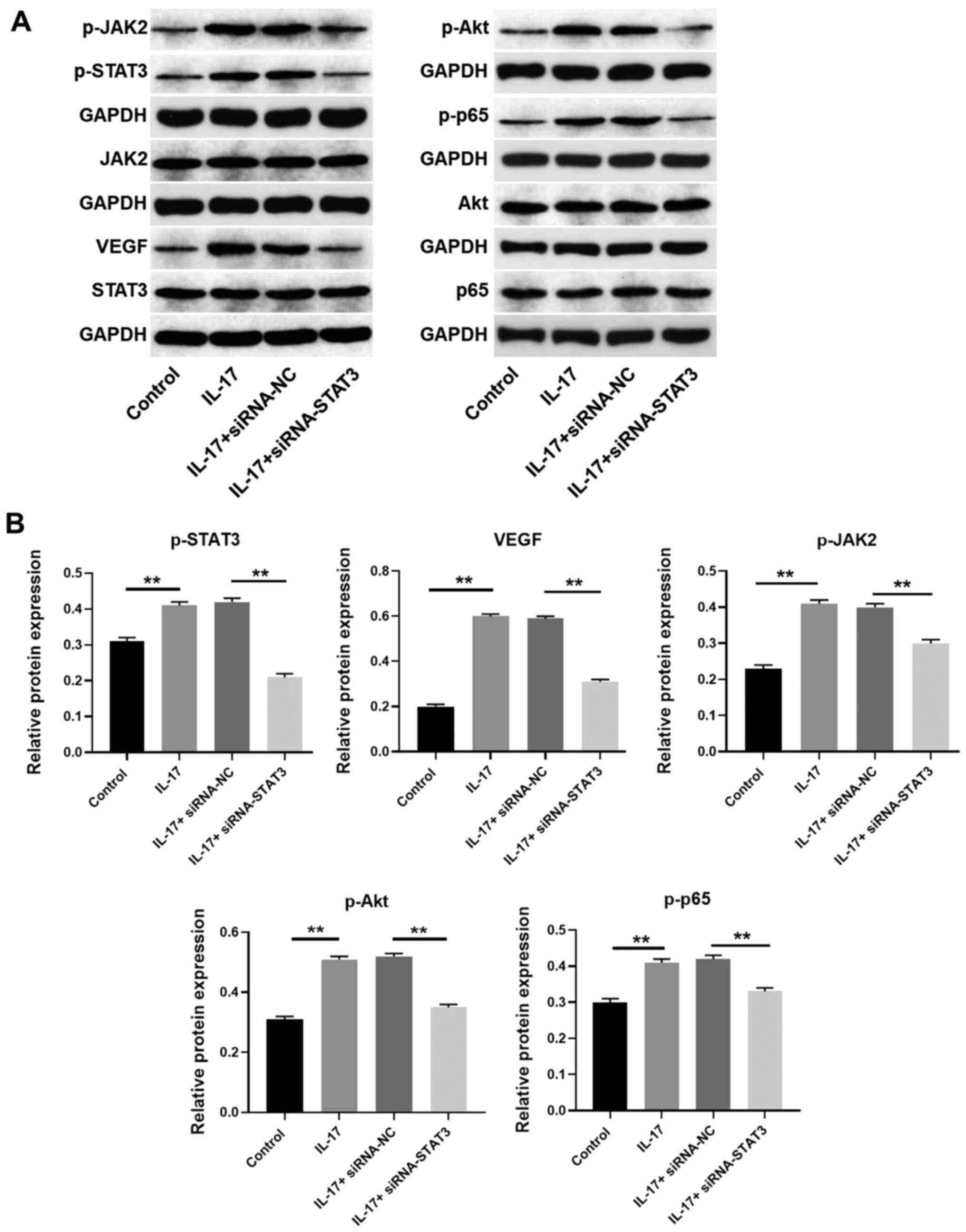 | Figure 7STAT3 siRNA reversed the effect of
IL-17 on JAK2/STAT3, PI3K/Akt and NF-κB signaling. (A and B) HeLa
cells were transfected with STAT3 siRNA. Then, the protein
expression of p65, p-p65 (normalized to p65), STAT3, p-STAT3
(normalized to STAT3), Akt, p-Akt (normalized to Akt), JAK2, p-JAK2
(normalized to JAK2) and VEGF in HeLa cells were detected by
western blotting. The relative expression of VEGF was quantified
normalized to GAPDH. **P<0.01. IL, interleukin;
NF-κB, nuclear factor NF-κB; p-, phosphorylated-; siRNA, small
interfering RNA; VEGF, vascular endothelial growth factor; NC,
negative control. |
Discussion
The IL-17 family was identified in 1993 by gene
screening of mouse T cells. It was originally named CTLA-8, and was
regarded as a cytokine called IL-17 in 1995(22). The IL-17 family contains 6 members
(23). IL-17 is an important
inflammatory regulator that may activate tissue responses and guide
immune defense (24). Previous
studies have reported that IL-17 could be involved in tumorigenesis
due to the fact that inflammatory factors in the tumor
microenvironment can promote tumors to produce cytokines and
decrease cytotoxic T cells, thereby promoting tumor growth
(12,25). However, the mechanism by which IL-17
regulates the development of cervical cancer remains unclear. The
present study is the first to identify that IL-17 could promote the
progression of cervical cancer via the activation of JAK2/STAT3.
Moreover, Zhang et al (26)
found that IL-17 could be closely associated with the progression
of thyroid cancer. Besides, a previous study indicated that IL-17
significantly promoted the occurrence of biliary tract cancer via
self-producing cytokines (27). The
present data further supplemented these previous results,
indicating that IL-17 could act as a JAK2/STAT3 promoter during the
occurrence of multiple diseases. According to Song et al
(20), IL-17 could act as an
oncogene in laryngeal cancer via the activation of
PI3K/AKT/FAS/FASL signaling. Consistently, the present finding
suggested that PI3K/Akt signaling could be activated by IL-17 in
cervical cancer. It has been reported that IL-17 could activate
PI3K/AKT in malignant tumors (28,29).
Thereby, the function of IL-17 might contribute to the consistence.
On the other hand, IL-12, IL-23 and IL-17 are known to be
pro-inflammatory cytokines in immunology (30), and TGF-β is an immunosuppressive
cytokine (31). A previous report
indicated that IL-12, IL-23 and IL-17 could be upregulated in acute
lymphoblastic leukemia, while TGF-β was downregulated (32). Consistently, the present data
indicated that IL-17 was upregulated in cervical cancer. Meanwhile,
IL-12, IL-23 and TGF-β will be investigated in the future.
According to the literature, the JAK2/STAT3
signaling pathway is commonly associated with the metastasis of
malignant tumors (33-35).
Moreover, the JAK2/STAT3 signaling pathway has been also found to
regulate the process of cancer metastasis (36,37).
Therefore, it was hypothesized that the knockdown of IL-17 could
mediate the JAK2/STAT3 signaling pathway and suppress the growth of
cervical cancer cells. As expected, IL-17 could activate JAK2/STAT3
signaling. The present findings are consistent with those from
previous studies (38,39), indicating that JAK2/STAT3 signaling
could play a key role during tumorigenesis.
The present study also revealed that IL-17 could
activate NF-κB and PI3K/Akt signaling. Various evidence suggest
that numerous signaling pathways are involved in the regulation of
tumorigenesis, and the JAK2/STAT3, PI3K/Akt and NF-κB signaling
axes have been shown to play important roles in this process
(40,41). It has been previously confirmed that
the STAT3 transcription factor could be constitutively activated
through phosphorylation by upstream JAK kinases in various cancer
types, including gastric cancer, glioma and esophageal cancer, in
response to various stimuli such as cytokines and growth factors
(42,43). STAT3 was activated to translocate to
the nucleus, where it promotes cell proliferation and cell cycle
progression, and inhibits apoptosis by activating the transcription
of downstream oncogenes, such as Bax and Bcl-2 (44,45).
The PI3K/Akt signaling pathway is also involved in the regulation
of multiple cellular functions. Once activated, AKT phosphorylates
a variety of substrates, resulting in cell cycle progression and
inhibition of apoptosis (46,47).
NF-κB is a major transcription factor that is involved in the
inflammatory regulation of cells by responding to pro-inflammatory
stimuli (48). In the present
study, IL-17 increased the expression of p-Akt and p-p65. However,
the knockdown of JAK2 or STAT3 significantly reversed the effect of
IL-17 on these two signaling pathways. Increasing reports have
indicated that the STAT3, NF-κB and PI3K/Akt signaling pathways
could exert their function interactively or independently in
different cellular contexts (49-51).
These data were similar to the results of the present study, which
indicated that IL-17 was the upstream factor of the aforementioned
three signaling pathways in the tumorigenesis of cervical cancer.
The present findings also indicated that knockdown of JAK2 or STAT3
could reverse the effect of IL-17 on the expression levels of PI3K-
and NF-κB-associated proteins. Therefore, it is urgent to determine
whether there is an association between STAT3, Akt and NF-κB
signaling pathways in IL-17-treated cervical cancer cells.
Epigenetic modifications often play important roles
in cancer tumorigenesis (52). In
the present study, STAT3 was demonstrated to play a key role in
IL-17-mediated cervical cancer progression. According to Zhang
et al (53), STAT3 could
induce the transcription of the DNA methyltransferase 1 gene
(DNMT1) in malignant T lymphocytes. Based on the aforementioned
study (53), STAT3 activation might
promote the transcription of DNMT1 in cervical cancer.
The present study is the first to explore the
function of IL-17 in cervical cancer, and the first to identify
that IL-17 could promote the progression of cervical cancer via the
activation of JAK2/STAT3. In addition, IL-17 was demonstrated to
activate JAK2/STAT3, PI3K/Akt and NF-κB in cervical cancer.
However, the present study has the following limitations: i) More
IL-17 isoforms need to be detected; ii) some rescue experiments are
needed to further verify the association between IL-17 and NF-κB
signaling. Thereby, more investigations are required in the
future.
In conclusion, IL-17 significantly promoted the
progression of cervical cancer, which may serve as a potential
novel target for the treatment of cervical cancer.
Supplementary Material
IL-17 upregulates the level of IL-17A
and IL-17F. (A and B) HeLa cells were transfected with JAK2 siRNA.
Then, the expression of IL-17A and IL-17F in HeLa cells were
detected by RT-qPCR. (C and D) HeLa cells were transfected with
STAT3 siRNA. Then, the expression of IL-17A and IL-17F in HeLa
cells were detected by RT-qPCR. **P<0.01. IL,
interleukin; siRNA, small interfering RNA; NC, negative control;
p-, phosphorylated.; RT-qPCR, reverse transcription-quantitative
PCR.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Study design, literature research, experimental
study was performed by YB, HL and RL. Data acquisition, data
analysis and statistical analysis were performed by YB. RL and YB
confirmed the authenticity of all the raw data. All authors were
responsible for guarantor of integrity of entire study, manuscript
preparation and manuscript editing, and all authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was carried out in accordance with the
World Medical Association Declaration of Helsinki approved by Gansu
Provincial Cancer Hospital (approval no. GPCH20190220). The
clinical and pathological data of patients were collected with
their written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tsikouras P, Zervoudis S, Manav B, Tomara
E, Iatrakis G, Romanidis C, Bothou A and Galazios G: Cervical
cancer: Screening, diagnosis and staging. J BUON. 21:320–325.
2016.PubMed/NCBI
|
|
2
|
Benard VB, Thomas CC, King J, Massetti GM,
Doria-Rose VP and Saraiya M: Centers for Disease C and Prevention
(CDC). Vital signs: Cervical cancer incidence, mortality, and
screening-United States, 2007-2012. MMWR Morb Mortal Wkly Rep.
63:1004–1009. 2014.PubMed/NCBI
|
|
3
|
Zeng SY, Liang MR, Li LY, Li L, Jiang W
and Zhong ML: Application of transvaginal external fascia
trachelectomy in the treatment of CIN and micro-invasive cervical
cancer. Zhonghua Zhong Liu Za Zhi. 35:543–546. 2013.PubMed/NCBI(In Chinese).
|
|
4
|
Gilham C, Sargent A, Kitchener HC and Peto
J: HPV testing compared with routine cytology in cervical
screening: Long-term follow-up of ARTISTIC RCT. Health Technol
Assess. 23:1–44. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Hong DK, Kim SA, Lim KT, Lee KH, Kim TJ
and So KA: Clinical outcome of high-grade cervical intraepithelial
neoplasia during pregnancy: A 10-year experience. Eur J Obstet
Gynecol Reprod Biol. 236:173–176. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cheng X, Feng Y, Wang X, Wan X, Xie X and
Lu W: The effectiveness of conization treatment for post-menopausal
women with high-grade cervical intraepithelial neoplasia. Exp Ther
Med. 5:185–188. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ostojic DV, Vrdoljak-Mozetic D,
Stemberger-Papic S, Finderle A and Eminovic S: Cervical cytology
and HPV test in follow-up after conisation or LLETZ. Coll Antropol.
34:219–224. 2010.PubMed/NCBI
|
|
8
|
Apgar BS, Kittendorf AL, Bettcher CM, Wong
J and Kaufman AJ: Update on ASCCP consensus guidelines for abnormal
cervical screening tests and cervical histology. Am Fam Physician.
80:147–155. 2009.PubMed/NCBI
|
|
9
|
Guo N, Shen G, Zhang Y, Moustafa AA, Ge D
and You Z: Interleukin-17 promotes migration and invasion of human
cancer cells through upregulation of MTA1 expression. Front Oncol.
9(546)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Alves JJ, De Medeiros Fernandes TAA, De
Araujo JM, Cobucci RN, Lanza DC, Bezerra FL, Andrade VS and
Fernandes JV: Th17 response in patients with cervical cancer. Oncol
Lett. 16:6215–6227. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Karabulut M, Usul Afsar C, Serimez M and
Karabulut S: Serum IL-17 levels can be diagnostic for gastric
cancer. J BUON. 24:1601–1609. 2019.PubMed/NCBI
|
|
12
|
Iwakura Y, Ishigame H, Saijo S and Nakae
S: Functional specialization of interleukin-17 family members.
Immunity. 34:149–162. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lotti F, Jarrar AM, Pai RK, Hitomi M,
Lathia J, Mace A, Gantt GA Jr, Sukhdeo K, DeVecchio J, Vasanji A,
et al: Chemotherapy activates cancer-associated fibroblasts to
maintain colorectal cancer-initiating cells by IL-17A. J Exp Med.
210:2851–2872. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Flies EJ, Mavoa S, Zosky GR, Mantzioris E,
Williams C, Eri R, Brook BW and Buettel JC: Urban-associated
diseases: Candidate diseases, environmental risk factors, and a
path forward. Environ Int. 133(105187)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Alshammari TK, Alghamdi H, Green TA, Niazy
A, Alkahdar L, Alrasheed N, Alhosaini K, Alswayyed M, Elango R,
Laezza F, et al: Assessing the role of toll-like receptor in
isolated, standard and enriched housing conditions. PLoS One.
14(e0222818)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nagarkoti S, Dubey M, Sadaf S, Awasthi D,
Chandra T, Jagavelu K, Kumar S and Dikshit M: Catalase
S-Glutathionylation by NOX2 and mitochondrial-derived ROS adversely
affects mice and human neutrophil survival. Inflammation.
42:2286–2296. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cong J, Liu R, Wang X, Sheng L, Jiang H,
Wang W, Zhang Y, Yang S and Li C: Association between
interluekin-17 gene polymorphisms and the risk of cervical cancer
in a Chinese population. Int J Clin Exp Pathol. 8:9567–9573.
2015.PubMed/NCBI
|
|
18
|
Miranda LN, Reginaldo FP, Souza DM, Soares
CP, Silva TG, Rocha KB, Jatoba CA, Donadi EA, Andrade JM, Goncalves
AK and Crispim JC: Greater expression of the human leukocyte
antigen-G (HLA-G) and interleukin-17 (IL-17) in cervical
intraepithelial neoplasia: Analytical cross-sectional study. Sao
Paulo Med J. 133:336–342. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Song Y, Yang M, Zhang H, Sun Y, Tao Y, Li
H, Zhang J, Li Y and Yang J: IL-17 affects the progression,
metastasis, and recurrence of laryngeal cancer via the inhibition
of apoptosis through activation of the PI3K/AKT/FAS/FASL pathways.
J Immunol Res. 2020(2953191)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lu W, He F, Lin Z, Liu S, Tang L, Huang Y
and Hu Z: Dysbiosis of the endometrial microbiota and its
association with inflammatory cytokines in endometrial cancer. Int
J Cancer. 148:1708–1716. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liao C, Zhang C, Jin L and Yang Y: IL-17
alters the mesenchymal stem cell niche towards osteogenesis in
cooperation with osteocytes. J Cell Physiol. 235:4466–4480.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Guo JQ, Liu J and Lu B: Expression of
gamma-delta T cells in immune microenvironment in children with
Henoch-Schonlein purpura. Zhongguo Dang Dai Er Ke Za Zhi.
21:960–965. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
24
|
Siefker DT, Vu L, You D, McBride A, Taylor
R, Jones TL, DeVincenzo J and Cormier SA: Respiratory syncytial
virus disease severity is associated with distinct CD8+
T-cell profiles. Am J Respir Crit Care Med. 201:325–334.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang J, Lu L, Luo Z, Li W, Lu Y, Tang Q
and Pu J: MiR-383 inhibits cell growth and promotes cell apoptosis
in hepatocellular carcinoma by targeting IL-17 via STAT3 signaling
pathway. Biomed Pharmacother. 120(109551)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang N, Wang Q, Tian Y, Xiong S, Li G and
Xu L: Expressions of IL-17 and TNF-α in patients with Hashimoto's
disease combined with thyroid cancer before and after surgery and
their relationship with prognosis. Clin Transl Oncol. 22:1280–1287.
2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kinoshita M, Kobayashi S, Gotoh K, Kubo M,
Hayashi K, Iwagami Y, Yamada D, Akita H, Noda T, Asaoka T, et al:
Heterogeneity of Treg/Th17 according to cancer progression and
modification in biliary tract cancers via self-producing cytokines.
Dig Dis Sci. 65:2937–2948. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Amara S, Majors C, Roy B, Hill S, Rose KL,
Myles EL and Tiriveedhi V: Critical role of SIK3 in mediating high
salt and IL-17 synergy leading to breast cancer cell proliferation.
PLoS One. 12(e0180097)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Varikuti S, Oghumu S, Elbaz M, Volpedo G,
Ahirwar DK, Alarcon PC, Sperling RH, Moretti E, Pioso MS, Kimble J,
et al: STAT1 gene deficient mice develop accelerated breast cancer
growth and metastasis which is reduced by IL-17 blockade.
Oncoimmunology. 6(e1361088)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zou Y, Dai SX, Chi HG, Li T, He ZW, Wang
J, Ye CG, Huang GL, Zhao B, Li WY, et al: Baicalin attenuates
TNBS-induced colitis in rats by modulating the Th17/Treg paradigm.
Arch Pharm Res. 38:1873–1887. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Song KH, Jung SY, Kang SM, Kim MH, Ahn J,
Hwang SG, Lee JH, Lim DS, Nam SY and Song JY: Induction of
immunogenic cell death by radiation-upregulated karyopherin alpha 2
in vitro. Eur J Cell Biol. 95:219–227. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Perez-Figueroa E, Sanchez-Cuaxospa M,
Martinez-Soto KA, Sanchez-Zauco N, Medina-Sanson A,
Jimenez-Hernandez E, Torres-Nava JR, Felix-Castro JM, Gomez A,
Ortega E, et al: Strong inflammatory response and Th1-polarization
profile in children with acute lymphoblastic leukemia without
apparent infection. Oncol Rep. 35:2699–2706. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Souza CM, do Amaral CL, Souza SC, de Souza
ACP, de Cassia Alves Martins I, Contieri LS, Milanski M, Torsoni
AS, Ignacio-Souza LM and Torsoni MA: JAK2/STAT3 pathway is required
for alpha7nAChR-dependent expression of POMC and AGRP neuropeptides
in male mice. Cell Physiol Biochem. 53:701–712. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fogg KC, Olson WR, Miller JN, Khan A,
Renner C, Hale I, Weisman PS and Kreeger PK: Alternatively
activated macrophage-derived secretome stimulates ovarian cancer
spheroid spreading through a JAK2/STAT3 pathway. Cancer Lett.
458:92–101. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wei L, Chen Y, Zhang C, Liu M and Xiong H:
Leptin induces IL-6 and IL-8 expression through leptin receptor
Ob-Rb in human dental pulp fibroblasts. Acta Odontol Scand.
77:205–212. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Song Q, Liu B, Li X, Zhang Q, Cao L, Xu M,
Meng Z, Wu X and Xu K: MiR-26a-5p potentiates metastasis of human
lung cancer cells by regulating ITGβ8-JAK2/STAT3 axis. Biochem
Biophys Res Commun. 501:494–500. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang L, Lu P, Guo X, Liu T, Luo X and Zhu
YT: Inhibition of JAK2/STAT3 signaling pathway protects mice from
the DDP-induced acute kidney injury in lung cancer. Inflamm Res.
68:751–760. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wu D, Dong W, Fang K and Wang M:
As4S4 exhibits good killing effect on
multiple myeloma cells via repressing SOCS1 methylation-mediated
JAK2/STAT3 signaling pathway. Technol Cancer Res Treat.
18(1533033819896806)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jing W, Guo X, Wang G, Bi Y, Han L, Zhu Q,
Qiu C, Tanaka M and Zhao Y: Breast cancer cells promote
CD169+ macrophage-associated immunosuppression through
JAK2-mediated PD-L1 upregulation on macrophages. Int
Immunopharmacol. 78(106012)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang X, Yin H, Zhang L, Zheng D, Yang Y,
Zhang J, Jiang H, Ling X, Xin Y, Liang H, et al: The construction
and analysis of the aberrant lncRNA-miRNA-mRNA network in non-small
cell lung cancer. J Thorac Dis. 11:1772–1778. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li X, Mak VCY, Zhou Y, Wang C, Wong ESY,
Sharma R, Lu Y, Cheung ANY, Mills GB and Cheung LWT: Deregulated
Gab2 phosphorylation mediates aberrant AKT and STAT3 signaling upon
PIK3R1 loss in ovarian cancer. Nat Commun. 10(716)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Deng F, Wang S, Zhang L, Xie X, Cai S, Li
H, Xie GL, Miao HL, Yang C, Liu X and Xia Z: Propofol through
upregulating caveolin-3 attenuates post-hypoxic mitochondrial
damage and cell death in H9C2 cardiomyocytes during hyperglycemia.
Cell Physiol Biochem. 44:279–292. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li YL, Gao L, Zucker IH and Schultz HD:
NADPH oxidase-derived superoxide anion mediates angiotensin
II-enhanced carotid body chemoreceptor sensitivity in heart failure
rabbits. Cardiovasc Res. 75:546–554. 2007.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wei Z, Jiang X, Qiao H, Zhai B, Zhang L,
Zhang Q, Wu Y, Jiang H and Sun X: STAT3 interacts with Skp2/p27/p21
pathway to regulate the motility and invasion of gastric cancer
cells. Cell Signal. 25:931–938. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Vona R, Gambardella L, Cittadini C,
Straface E and Pietraforte D: Biomarkers of oxidative stress in
metabolic syndrome and associated diseases. Oxid Med Cell Longev.
2019(8267234)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Li K: Iron pathophysiology in friedreich's
ataxia. Adv Exp Med Biol. 1173:125–143. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Cho TM, Kim JY, Kim YJ, Sung D, Oh E, Jang
S, Farrand L, Hoang VH, Nguyen CT, Ann J, et al: C-terminal HSP90
inhibitor L80 elicits anti-metastatic effects in triple-negative
breast cancer via STAT3 inhibition. Cancer Lett. 447:141–153.
2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ucci M, Di Tomo P, Tritschler F, Cordone
VGP, Lanuti P, Bologna G, Di Silvestre S, Di Pietro N, Pipino C,
Mandatori D, et al: Anti-inflammatory role of carotenoids in
endothelial cells derived from umbilical cord of women affected by
gestational diabetes mellitus. Oxid Med Cell Longev.
2019(8184656)2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Li Y, Cui N, Zheng PS and Yang WT: BMX/Etk
promotes cell proliferation and tumorigenicity of cervical cancer
cells through PI3K/AKT/mTOR and STAT3 pathways. Oncotarget.
8:49238–49252. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Steelman LS, Abrams SL, Whelan J, Bertrand
FE, Ludwig DE, Basecke J, Libra M, Stivala F, Milella M, Tafuri A,
et al: Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and
Jak/STAT pathways to leukemia. Leukemia. 22:686–707.
2008.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yang X, Yan H, Jiang N, Yu Z, Yuan J, Ni Z
and Fang W: IL-6 Trans-signaling drives a STAT3-dependent pathway
that leads to structural alterations of peritoneal membrane. Am J
Physiol Renal Physiol. 318:F338–F353. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Buttura JR, Provisor Santos MN, Valieris
R, Drummond RD, Defelicibus A, Lima JP, Calsavara VF, Freitas HC,
Cordeiro de Lima VC, Fernanda Bartelli T, et al: Mutational
signatures driven by epigenetic determinants enable the
stratification of patients with gastric cancer for therapeutic
intervention. Cancers (Basel). 13(490)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zhang Q, Wang HY, Woetmann A, Raghunath
PN, Odum N and Wasik MA: STAT3 induces transcription of the DNA
methyltransferase 1 gene (DNMT1) in malignant T lymphocytes. Blood.
108:1058–1064. 2006.PubMed/NCBI View Article : Google Scholar
|