Introduction
Glioblastoma multiforme (GBM) is the most lethal
primary brain tumor (1) worldwide,
with a mean survival time of ~8-12 months (2). The current clinical strategy for GBM
consists of surgical resection, radiation therapy and treatment
with adjuvant temozolomide (TMZ) chemotherapy (3). Although TMZ exhibits antitumor effects
against high-grade glioma (4),
previous studies have suggested that its efficacy is affected by
the development of drug resistance in tumor cells (5-7).
Therefore, identifying the mechanism underlying TMZ resistance and
developing a new adjuvant chemotherapy drug against GBM is
important.
Leucine-rich repeat-containing G-protein coupled
receptor 6 (LGR6) is involved in the growth and proliferation of
multiple types of cancer, including colon cancer and gastric cancer
(8-10),
and high levels of LGR6 have been correlated with colorectal
metastasis (8). LGR6 was initially
identified as a cognate receptor of R-spondin ligands, which serve
as enhancers of WNT signaling (11-13)
and was later identified as a stem cell marker (14-17).
Functioning as an oncogene or tumor suppressor, LGR6 modulates the
activation of signaling pathways, such as the zinc transporter
ZIP10-p63(18) and WNT (19) signaling pathways.
In addition, several signaling pathways, including
the STAT5 and PI3K/Akt signaling pathways, serve a vital role
during the progression of GBM (20-22).
Cytokine-induced Janus kinases initiate the STAT family or activate
mitogen-activated protein kinases PI3K and mTOR (23), which are all associated with the
progression of GBM (24-27);
therefore, assessing whether LGR6 can activate these signaling
pathways and serve as a potent therapeutic target for GBM requires
investigation.
Materials and methods
Cell culture
Human GBM cell lines T98G (accession no. CVCL_0556)
and U87 (glioblastoma of unknown origin; accession no. CVCL_0022)
were purchased from American Type Culture Collection. GBM cell
lines SHG-44, U251 and human normal glial HEB cells and human
embryonic kidney 293T cells were purchased from The Cell Bank of
Type Culture Collection of the Chinese Academy of Sciences. T98G
and U87 cells were maintained in modified Eagle's medium (MEM;
Hyclone; Cytiva), SHG-44 cells were maintained in RPMI-1640 medium
(Hyclone; Cytiva) and U251, HEB and 293T cells were maintained in
DMEM (Hyclone; Cytiva). All culture mediums were supplemented with
10% FBS (Hyclone; Cytiva). Cells were maintained at 37˚C in 5%
CO2 incubators. To establish TMZ-resistant cell lines,
SHG-44 and U251 cells were cultured and passaged over 8 weeks in
the presence of increasing concentrations of TMZ (30 to 300 µM;
Selleck Chemicals) to generate TMZ resistant lines at 37˚C in 5%
CO2 incubator, which were denoted as SHG-44TMZ+ and
U251TMZ+ as per a previous study (28) and the parental cells were denoted ad
SHG-44TMZ- and U251TMZ-.
Plasmid construction and cell
transfection
overexpression plasmids (LGR6) were constructed by
inserting the LGR6 coding sequence into a pcDNA3.1 plasmid (General
Biosystems, Inc.). An empty pcDNA3.1 vector was used as the
negative control (Vector). The small interfering (si)RNA targeting
LGR6 (siRNA-LGR6) and the control (siRNA-Ctrl) were purchased from
Shanghai GenePharma Co., Ltd. The microRNA (miR)-1236-3p mimic
(miR-1236-3p) and scrambled oligonucleotides (miR-Ctrl) were
purchased from Guangzhou RiboBio Co., Ltd. Sequences are presented
in Table I. The day prior to
transfection, ~2x105 cells were plated in growth medium
without antibiotics at a density of 30-50%. Both siRNAs and miRNAs
were transfected into cells at a final concentration of 100 nM
using Lipofectamine® 2000 (Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. DNA fragments
containing the wild-type (WT) or mutated (Mut) miR-1236-3p
3'-untranslated region (3'-UTR) and their complementary fragments
were cloned from SHG-44 cDNA. The annealed double-stranded DNA was
then cloned into the dual-luciferase reporter gene vector
psicheck-2 (Promega Corporation). The recombinant WT and Mut
reporter gene vectors were named LGR6-3' UTR-WT and LGR6-3'
UTR-Mut, respectively.
 | Table ISequences of siRNA-LGR6, miR-1236-3p
mimics and negative controls. |
Table I
Sequences of siRNA-LGR6, miR-1236-3p
mimics and negative controls.
| RNA | Sequence (5' to
3') |
|---|
| siRNA-LGR6 | Sense:
CCUGGAACUGUCUCACAAUTT |
| | Antisense:
AUUGUGAGACAGUUCCAGGTT |
| siRNA-Ctrl | Sense:
UUCUCCGAACGUGUCACGUTT |
| | Antisense:
ACGUGACACGUUCGGAGAATT |
| miR-1236-3p
mimics |
CCUCUUCCCCUUGUCUCUCCAG |
| miR-Ctrl |
UUCUCCGAACGUGUCACGUTT |
Potential microRNAs prediction
To investigate whether microRNAs regulated the
expression of LGR6, the available complementary-based algorisms
were predicted using TargetScan (www.targetscan.org/vert_72) and miRTarBase (mirtarbase.mbc.nctu.edu.tw/php/index.php). miR-1236-3p
displayed a low mirSVR score (-2.69) and was selected as a
prediction microRNA.
Luciferase activity analysis
293T cells (~5x103 cells/well) were
plated in 96-well plates and co-transfected with 25 ng luciferase
reporter gene vector and 50 nM miR-1236-3p or miR-Ctrl using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Following culturing at 37˚C for 48 h in a 5% CO2
incubator, luciferase activity were detected using the
Dual-Luciferase Reporter assay system (Promega Corporation). The
results were normalized to Renilla luciferase and analyzed,
according to the manufacturer's protocol.
Cell viability
Cells were plated in 96-well plates
(~5x103) and transfected with siRNA-LGR6, siRNA-Ctrl,
LGR6 overexpression plasmids or empty pcDNA3.1 vector for 24 h at
37˚C in 5% CO2 incubator, then TMZ was added to culture
medium at final concentrations of 0, 100, 200, 300, 400 or 500 µM.
At 0, 24, 48 and 72 h post-transfection, Cell Counting Kit-8
reagent (10 µl; Beyotime Institute of Biotechnology) was added to
each well for 4 h at 37˚C. The absorbance of each well was measured
at a wavelength of 450 nm using the Multiskan GO plate reader
(Thermo Fisher Scientific, Inc.).
Inhibitor treatment
Selective inhibitors of Akt1/2/3 (MK-2206) were
purchased from Selleck Chemicals. Frozen aliquots (-80˚C) were
melted and dissolved in DMSO (Sigma-Aldrich; Merck KgaA) and
diluted in growth medium (RPMI-1640 medium for SHG-44 cells; DMEM
medium for U251 cells). A total of 5 µM MK-2206 was added to SHG-44
and U251 cells for 0, 24, 48 or 72 h following transfection with
LGR6 overexpression plasmids. Cell Counting Kit-8 reagent (10 µl;
Beyotime Institute of Biotechnology) was added to each well for 4 h
at 37˚C. The absorbance of each well was measured at a wavelength
of 450 nm using a Multiskan GO plate reader (Thermo Fisher
Scientific, Inc.).
Cell invasion
For cell invasion assays, Corning®
Transwell® polycarbonate membrane cell culture inserts
containing polycarbonate membranes with 8-µm pores (Corning, Inc)
were precoated with Matrigel® (BD Biosciences) for 30
min at 37˚C. Cells (~5x104 cells/well) were suspended in
culture medium supplemented with 5% FBS and plated into the upper
chambers. The lower chambers were filled with culture medium
supplemented with 20% FBS. Following incubation for 24 h at 37˚C in
5% CO2 incubators, cells were washed with PBS and fixed
with cold 99.9% methanol for 30 min at room temperature. After
staining with 1% crystal violet for 30 min at room temperature,
cells on the upper surface of the membrane were removed using
cotton swabs. Stained cells were counted using a light microscope
and analyzed using Image J software (v18.0; National Institutes of
Health).
Cell cycle assay
At 48 h post-transfection, cells were washed twice
with cold PBS and harvested using trypsin. Cells were fixed with
cold 75% (v/v) ethanol overnight at -20˚C. After washing twice with
PBS, cells were suspended in staining buffer containing 5 µl PI and
5 µl RNAase A inhibitor for 30 min in the dark at room temperature
using a Cell Cycle Analysis kit (Shanghai Yeasen Biotechnology Co.,
Ltd.), according to the manufacture's protocol. Stained cells were
analyzed via ACEA NovoCyte flow cytometry instrument (ACEA
Bioscience, Inc.) and cell cycle distribution was assessed using
Novo Express software (https://www.aceabio.com.cn/support/software_download#edit-group-novocyte-software-download;
ACEA Bioscience, Inc.).
Western blot analysis
Transfected cells were washed with cold PBS and
total protein was extracted using RIPA lysis buffer (Beyotime
Institute of Biotechnology) supplemented with phosphatase
inhibitors (Roche Applied Science). Total protein was quantified
using a bicinchoninic acid assay kit (Thermo Fisher Scientific,
Inc.). Protein (30 µg per lane) was separated via 10% SDS-PAGE and
transferred onto PVDF membranes (Bio-Rad Laboratories, Inc.), which
were blocked with 5% non-skimmed milk for 1 h at room temperature.
The membranes were incubated overnight at 4˚C with primary
antibodies targeted against: Phosphorylated (p)-Akt (Ser473;
dilution, 1:1,000; cat. no. 4060; Cell Signaling Technology, Inc.),
Akt (dilution, 1:500; cat. no. OM238722; OmnimAbs), LGR6 (dilution,
1:1,000; cat. no. ab126747; Abcam) and β-actin (dilution, 1:8,000;
cat. no. 60008-1; ProteinTech Group, Inc.). Following primary
incubation, the membranes were incubated with goat anti-rabbit
(dilution, 1:6,000, cat. no. SA00001-2; ProteinTech Group, Inc.) or
donkey anti-mouse (dilution, 1:8,000, cat. no. 715-005-150; Jackson
ImmunoResearch) IgG horseradish peroxidase-conjugated secondary
antibodies for 1 h at room temperature. Immunoreactive bands were
visualized using a chemiluminescence kit (Thermo Fisher Scientific,
Inc.). Protein expression was quantified using ImageJ software
(National Institutes of Health) with β-actin as the loading
control.
RNA isolation and reverse
transcription-quantitative PCR
Total RNA was extracted from transfected cells using
TRIzol® (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. Total RNA was reverse transcribed into
cDNA using the Hifair® II 1st Strand cDNA Synthesis kit
(Shanghai Yeasen Biotechnology Co., Ltd.) or Hairpin-it™
miRNA RT-PCR Quantitation kit (Shanghai GenePharma Co., Ltd.).
Subsequently, qPCR was performed using SYBR Green Select Master Mix
(Thermo Fisher Scientific, Inc.) and an ABI 7500 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions were as follows: 95˚C for 5 min followed by 40 cycles at
95˚C for 10 sec, 58˚C for 20 sec, 72˚C for 20 sec, followed by
melting curve detection at 95˚C for 15 sec, 60˚C for 1 min and 95˚C
for 15 sec. The sequences of the primers used for qPCR are listed
in Table II. miRNA and mRNA
expression levels were quantified using the 2-∆∆Cq
method (29) and normalized to the
internal reference genes U6 and β-actin.
 | Table IISequences of primers used for reverse
transcription-quantitative PCR. |
Table II
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene | Sequence
(5'-3') |
|---|
| LGR6 | F:
ACCCCCTGACGGCTTACCT |
| | R:
GCTTGTCCTGGGATGTGTGAG |
| miR-1236-3p | F:
CCAATCAGCCTCTTCCCCTT |
| | R:
TATGGTTGTTCACGACTCCTTCAC |
| U6 | F:
ATTGGAACGATACAGAGAAGATT |
| | R:
GGAACGCTTCACGAATTTG |
| β-actin | F:
CTTAGTTGCGTTACACCCTTTCTTG |
| | R:
CTGTCACCTTCACCGTTCCAGTTT |
Statistical analysis
Data are presented as the mean ± SD. Experiments
were performed in triplicate. One-way ANOVA followed by Tukey's
post hoc test was used to analyze comparisons among multiple
groups. Comparisons between two groups were analyzed using the
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
LGR6 promotes GBM cell viability and
invasion
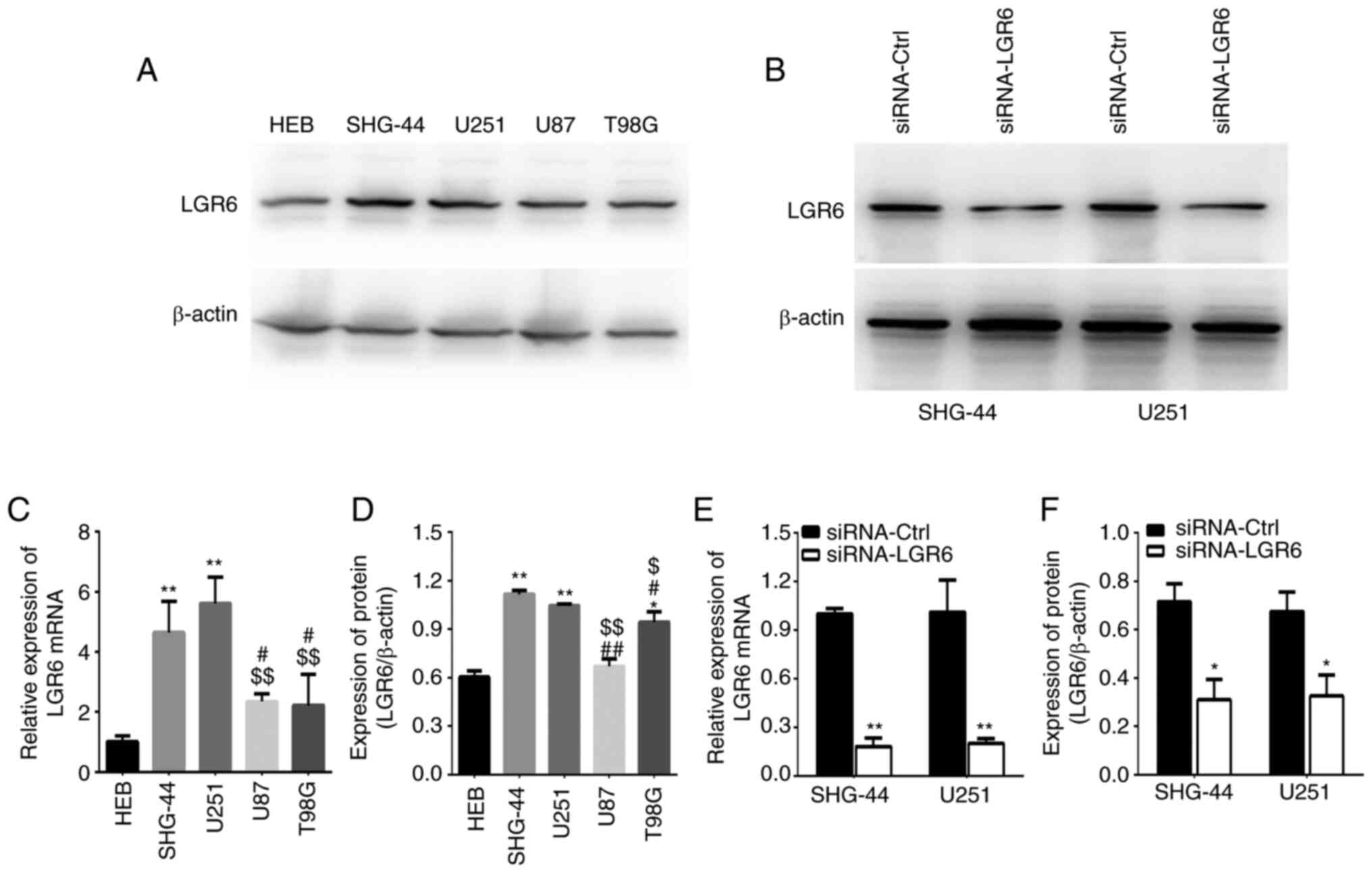
The expression of LGR6 was detected in GBM cells and
normal glial HEB cells. The results indicated that LGR6 mRNA levels
were significantly increased in U251 and SHG-44 cells compared with
HEB cells (Fig. 1A) and that LGR6
protein expression was increased in SHG-44, U251 and T98G cells
compared with HEB cells (Fig. 1B).
Additionally, SHG-44 and U251 cells exhibited higher LGR6 mRNA and
protein expression levels compared with U87 and T98G cells
(Fig. 1A, C and D);
therefore, SHG-44 and U251 cells were selected for further
experiments. In addition, siRNA-LGR6 significantly reduced LGR6
mRNA and protein expression levels compared with siRNA-Ctrl
(Fig. 1B, E and F).
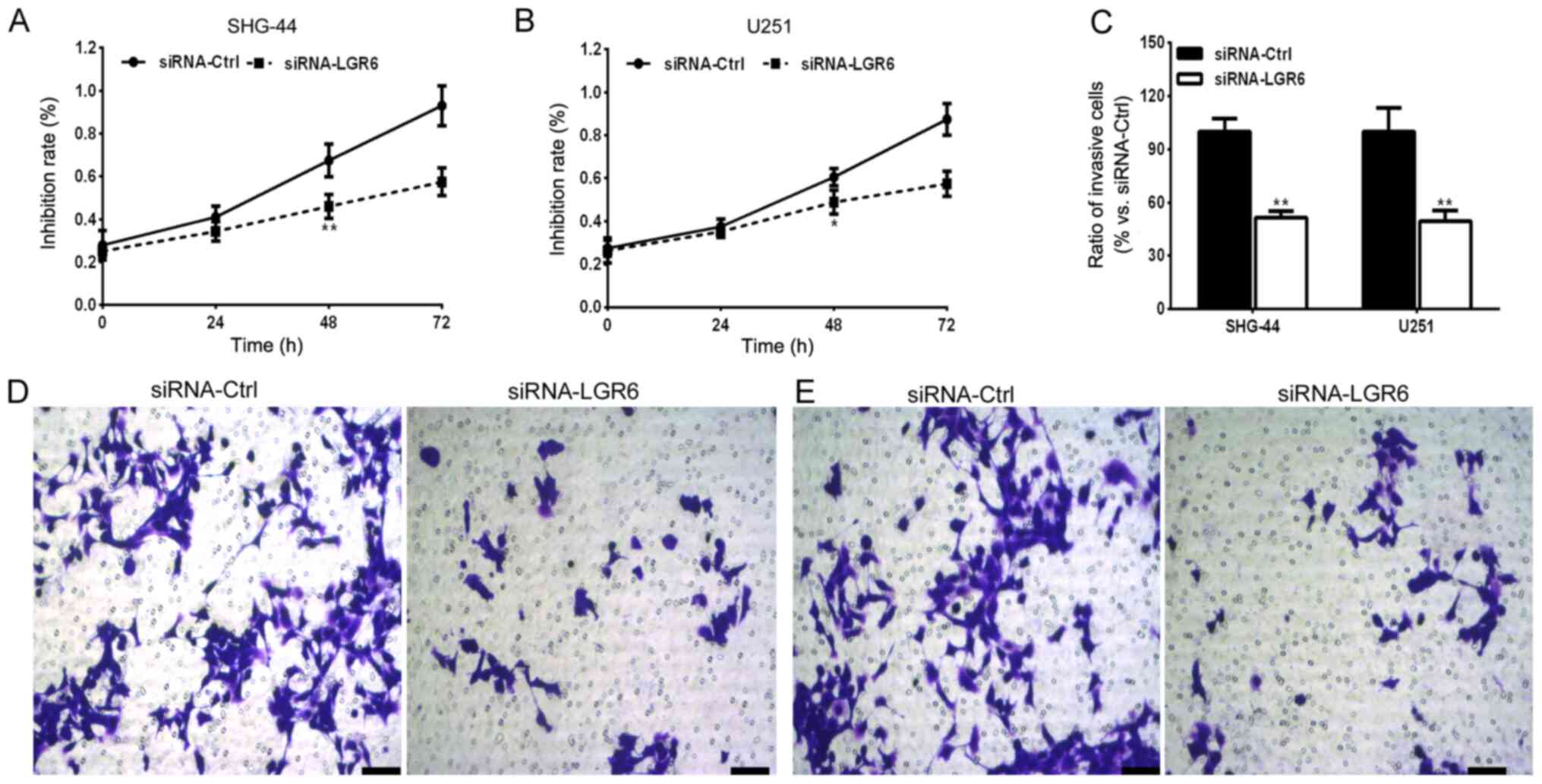
The effect of LGR6 knockdown on SHG-44 and U251 cell
viability was investigated. The results indicated that LGR6
knockdown significantly reduced SHG-44 and U251 cell viability at
48 h compared with the siRNA-Ctrl group (Fig. 2A and B). Additionally, due to the invasive
capability of glioma cells that induce malignancy or intracranial
metastasis (30), the effect of
LGR6 on cell invasion was assessed. The results suggested that LGR6
knockdown significantly reduced the number of invasive cells
compared with the siRNA-Ctrl group (Fig. 2C-E).
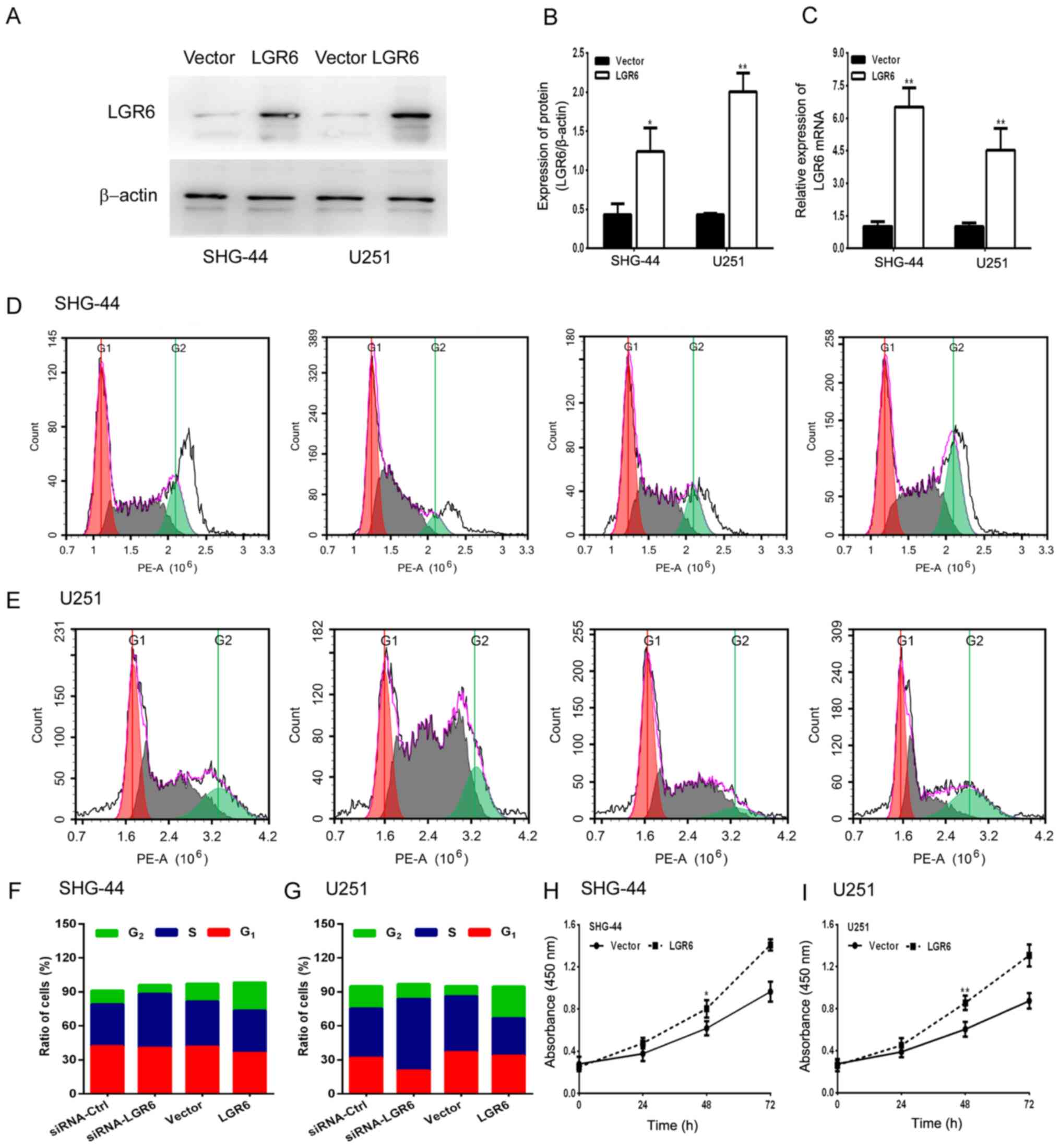
Conversely, LGR6 overexpression significantly
increased the expression levels of LGR6 in SHG-44 and U251 cells
compared with the vector group (Fig.
3A-C). Furthermore, LGR6 overexpression significantly increased
cell viability compared with the vector group (Fig. 3H and I) and promoted cell cycle progression. By
contrast, LGR6 knockdown arrested the cell cycle at the S phase
(Fig. 3D-G). The results suggested
that LGR6 served a vital role in regulating GBM cell viability and
invasion.
LGR6 mediates TMZ sensitivity in GBM
cells
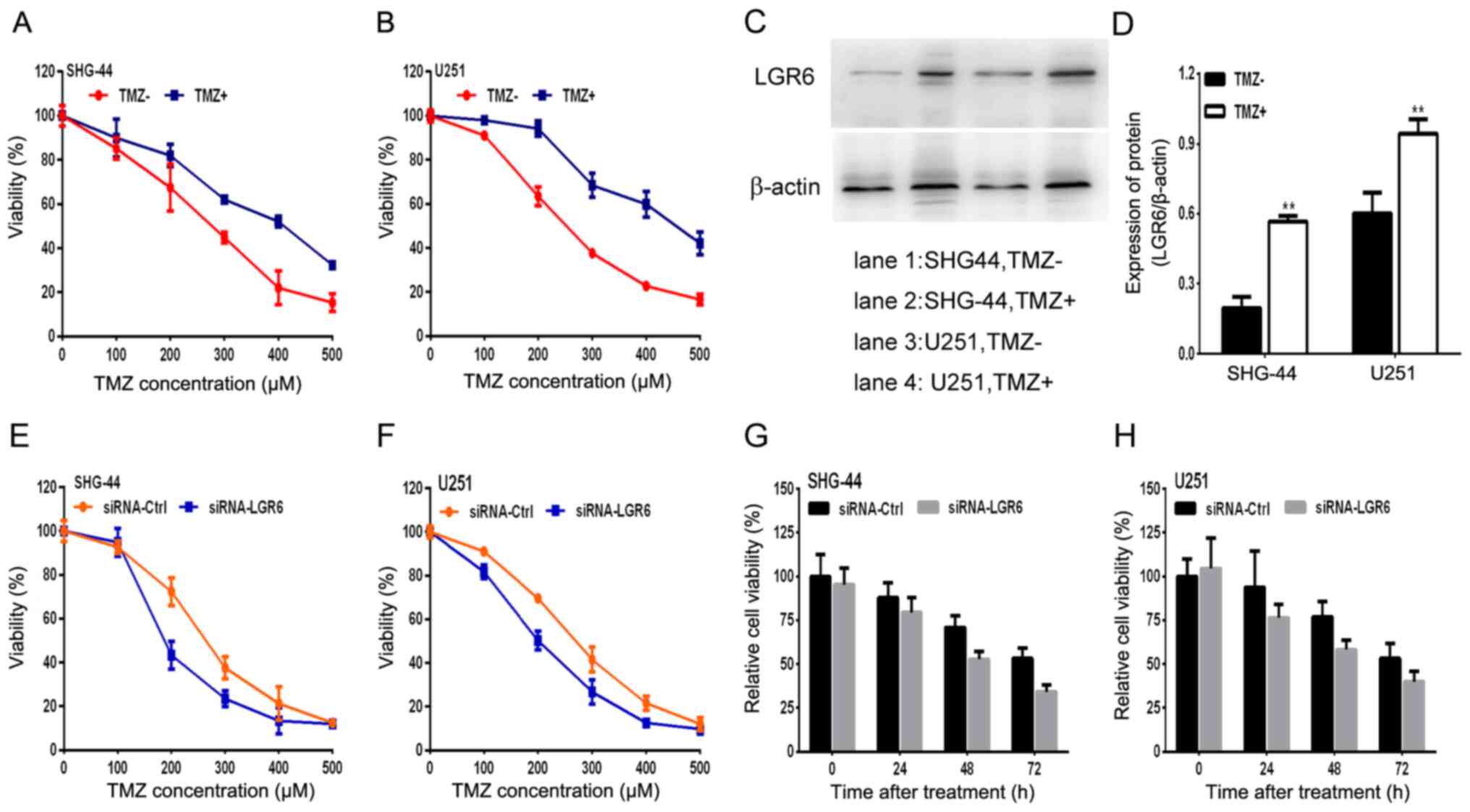
A TMZ-resistant GBM cell model was successfully
established and used to investigate TMZ sensitization. A total of 2
TMZ-resistant human glioma cell sublines, SHG-44TMZ+ and U251TMZ+,
were generated by increasing TMZ concentrations for 6 months. The
IC50 of SHG-44TMZ+ and U251TMZ+ exhibited a >2-fold
increase compared with parental TMZ-sensitive cell lines
(SHG-44TMZ- and U251TMZ- cells; Fig.
4A and B). Moreover,
TMZ-resistant SHG-44 and U251 GBM cells displayed increased
expression levels of LGR6 compared with TMZ-sensitive SHG-44 and
U251 GBM cells (Fig. 4C and
D). TMZ-resistant GBM cells
displayed higher viability rates compared with TMZ-sensitive cells
following treatment with a series of TMZ concentrations (0, 100,
200, 300, 400 and 500 µM; Fig. 4C
and D). U251 and SHG-44 cell
viability decreased in a time-dependent manner, whereas LGR6
knockdown decreased U251 and SHG-44 cell viability compared with
the siRNA-Ctrl group (Fig. 4E-H).
Based on the results, it was hypothesized that LGR6 participated in
the failure of TMZ chemotherapy in GBM, which might indicate a new
therapeutic target for the disease.
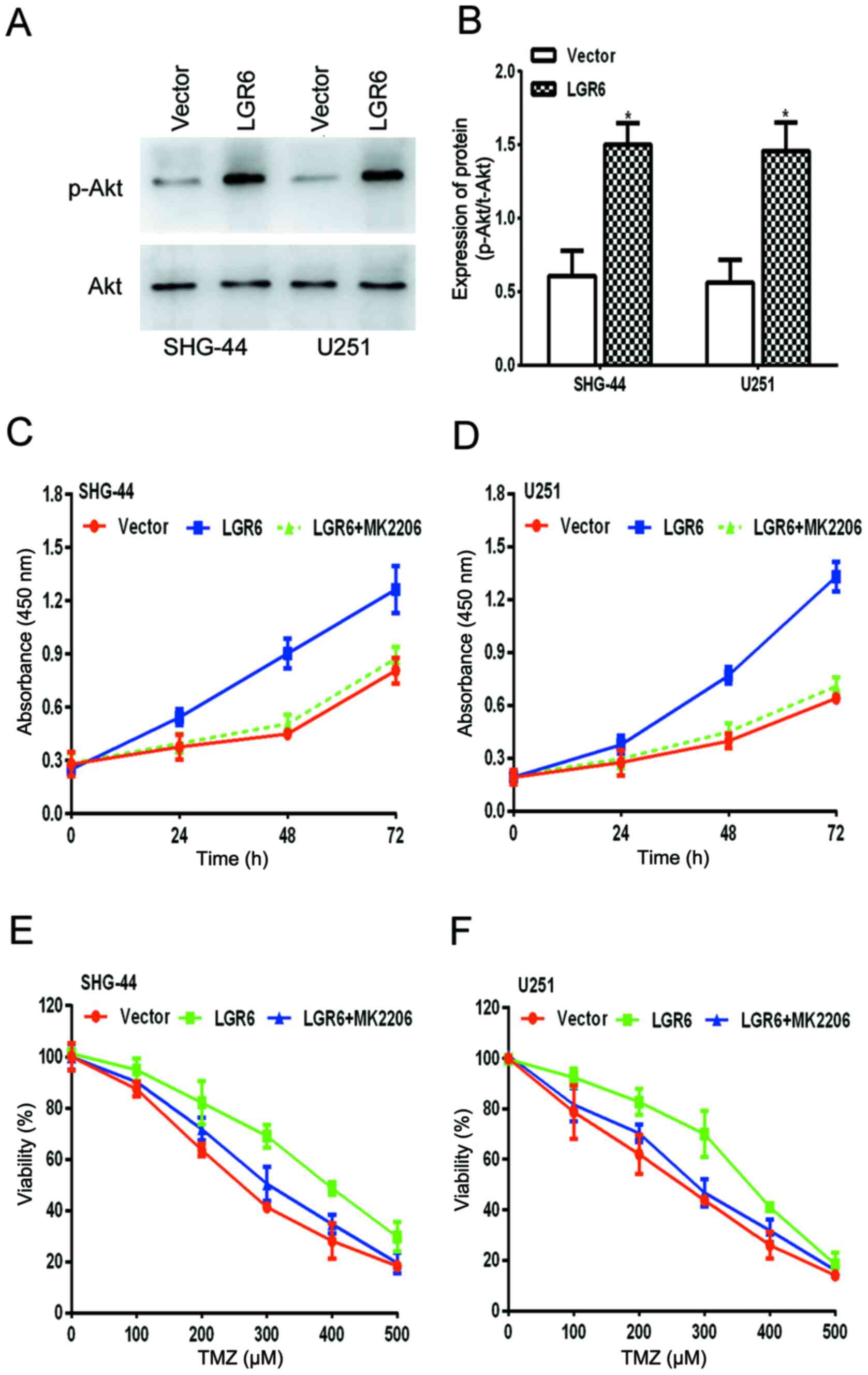
LGR6 promotes GBM viability and
chemoresistance by activating Akt signaling
The Akt signaling pathway is involved in numerous
types of cancer, including GBM (31,32);
therefore, the levels of p- and total Akt in transfected GBM cells
were measured. The results indicated that LGR6 overexpression
significantly increased the levels of p-Akt compared with the
vector group, but did not alter the total levels of Akt (Fig. 5A and B), which suggested that LGR6 might
activate Akt signaling to mediate GBM malignancy. Therefore, it was
hypothesized that as LGR6 induced the activation of Akt signaling
during GBM progression, the loss of Akt activity may abolish the
regulatory ability of LGR6.
Further experiments were conducted to investigate
whether MK-2206, a specific inhibitor of Akt signaling, reversed
LGR6-induced cell viability and reduced cell viability in response
to TMZ treatment in SHG-44 and U251 cells. The results were
consistent with the hypothesis (Fig.
5C-F), which suggested that LGR6 promoted GBM viability and
chemoresistance by activating Akt signaling.
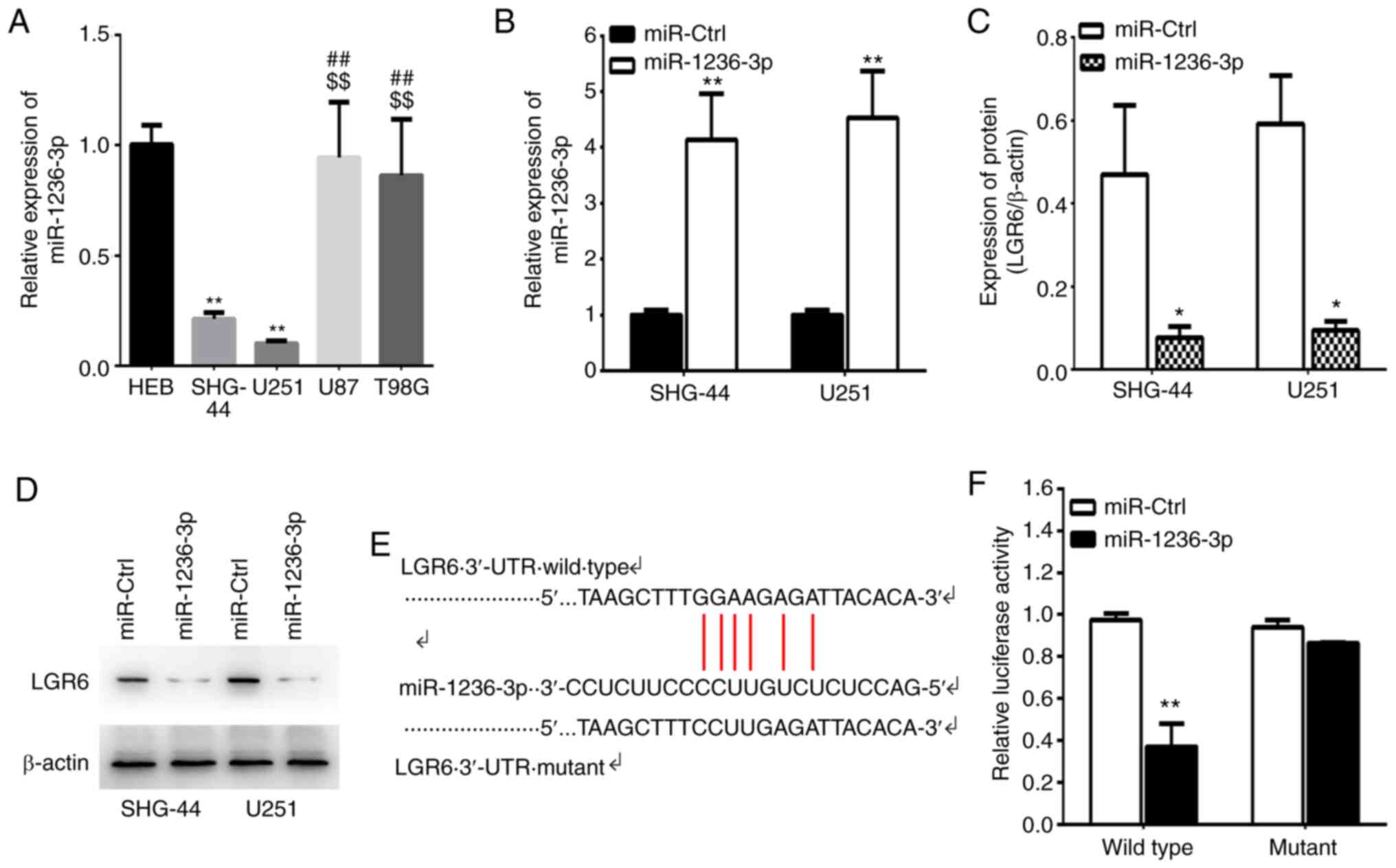
LGR6 is a target of miR-1236-3p
miR-1236-3p serves as a tumor suppressor in various
types of cancer (33-35).
To investigate whether LGR6 was a potential target of miR-1236-3p,
the available complementary-based algorisms were predicted using
TargetScan and miRTarBase. The results indicated that miR-1236-3p
expression levels were significantly decreased in U251 and SHG-44
cells compared with HEB cells and SHG-44 and U251 cells displayed
lower miR-1236-3p levels compared with U87 and T98G cells (Fig. 6A). Additionally, miR-1236-3p mimic
significantly increased the expression of miR-1236-3p and
significantly decreased LGR6 expression levels at the mRNA and
protein level compared with miR-Ctrl (Fig. 6B-D). Based on the predicted
targeting sites of miR-1236-3p, LGR6 3'-UTR WT and Mut luciferase
reporter plasmids were constructed. The results indicated that
miR-1236-3p mimic significantly decreased the luciferase activity
of LGR6 WT 3'-UTR compared with miR-Ctrl, but did not alter the
luciferase activity of LGR6 Mut 3'-UTR (Fig. 6E and F). The results suggested that LGR6 was an
miR-1236-3p target, which may mediate its effects during cancer
development.
Discussion
As the most prevalent and malignant brain tumor in
the adult central nervous system (36), glioma results in a high number of
brain tumor-related deaths each year (37). Since the present curative efficiency
on glioma is limited, developing novel therapeutic targets and
understanding the molecular mechanism underlying glioma progression
is important. Accumulating evidence has demonstrated that LGR6 is a
contributing factor to cell proliferation in multiple types of
human cancer, including gastric cancer and colon cancer (8,10);
however, its role in glioma is not completely understood. In the
present study, although the expression of LGR6 in glioma tissues
was not investigated, in vitro experiments indicated that
LGR6 expression was higher in GBM cell lines compared with the
normal glial cell line and SHG-44 and U251 cells displayed higher
LGR6 expression levels compared with U87 and T98G cells. In
addition, LGR6 knockdown inhibited SHG-44 and U251 cell viability
compared with the siRNA-Ctrl group. Additionally, TMZ-resistant
SHG-44 and U251 cells displayed increased LGR6 expression levels
compared with TMZ-sensitive cells. To the best of our knowledge,
the present study was the first to suggest that LGR6 may be
associated with cell viability and TMZ resistance in GBM.
LGR4, LGR5 and LGR6 are receptors of the R-spondin
protein family (38-40).
In vitro experiments have demonstrated that the three
proteins could bind all types of R-spondins (40). Lebensohn and Rohatgi (41) indicated that R-spondin 1 binding to
LGR4/5/6 is essential for WNT signaling. Chong et al
(42) proposed that WNT can
activate Akt directly or via WNT1-induced secreted protein. Both
Akt and WNT/β-catenin signaling pathways may regulate cell
proliferation and migration (42-45),
and serve important roles in GBM (46). In accordance with the finding that
the Akt signaling pathway is activated in the TMZ-resistant U87
cell line (46), the present study
indicated that overexpression of LGR6 also increased the levels of
phosphorylated Akt in TMZ-resistant cell lines. The results of the
present study combined with the results of previous reports
indicated that LGR6 may serve an important role in TMZ-resistant
GBM, which may be mediated via the Akt signaling pathway.
Previous studies have reported that miRs serve
important roles in the majority of different types of cancer by
modulating key processes during tumorigenesis (47,48).
Through controlling the gene expression of target mRNAs, miRs can
serve as oncogenes or tumor suppressor genes (49). miR-1236-3p, an intronic miRNA, is
involved in multiple types of cancer, such as gastric (50,51),
ovarian (52), lung (34) and bladder (53) cancer. Wang et al (54) indicated that miR-1236-3p is
prominently downregulated in DDP-resistant A549 cells, the role of
which in lung cancer cells may be mediated by modulation of tumor
protein, translationally-controlled 1 and inhibition of the Pim-3
proto-oncogene, serine/threonine kinase signaling pathway. In the
present study, LGR6 was predicted as the potential target of
miR-1236-3p by bioinformatics analysis. The luciferase reporter
assays indicated that miR-1236-3p regulated LGR6 expression levels
by targeting its 3'-UTR sequence and miR-1236-3p was downregulated
in GBM cells compared with HEB cells. Moreover, miR-1236-3p
overexpression decreased LGR6 expression levels compared with
control cells, which suggested that LGR6 might be a downstream
effect effector of miR-1236-3p. Similarly, a previous study
indicated miR-1236-3p suppressed the progression of glioma by
targeting homeobox B7 (HOXB7), a key factor for tumor-associated
angiogenic switch (55,56). Previous studies have indicated that
HOXB7 is involved in cancer stem cell biology by regulating the
expression of the stem cell-related gene, such as lin-28 homolog B
(57) and run-related transcription
factor 2 (RUNX2) (58). By
contrast, LGR6+ cancer cells display self-renewal and
differentiation capacities, alongside higher oncogenic potential in
lung cancer (59). Therefore,
whether the HOXB7/LGR6 axis is involved in regulating glioma stem
cells requires further investigation.
In conclusion, to the best of our knowledge, the
present study identified the essential roles of LGR6 in glioma for
the first time. In addition, the results indicated a functional
mechanism underlying LGR6 and suggested that the
miR-1236-3p/LGR6/Akt signaling axis regulated the sensitivity of
GBM cells to TMZ. The results of the present study indicated a
potential mechanism underlying the recurrence and resistance to
glioma therapies and suggested a potential cellular and molecular
therapeutic target for GBM.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC, XY and FX designed the present study. YC, XY,
XG, SS and MY collected, analyzed and interpreted data. YC, XY, XG,
FX and MY drafted and reviewed the manuscript. MY, FX and SS
revised the manuscript and provided material support. All authors
agreed to be accountable for all aspects of the current work. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jia B, Liu W, Gu J, Wang J, Lv W, Zhang W,
Hao Q, Pang Z, Mu N, Zhang W and Guo Q: MiR-7-5p suppresses
stemness and enhances temozolomide sensitivity of drug-resistant
glioblastoma cells by targeting Yin Yang 1. Exp Cell Res.
375:73–81. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
McLendon R, Friedman AH, Bigner D, Van
Meir EG, Brat DJ, Mastrogianakis G, Olson JJ, Mikkelsen T, Lehman
N, Aldape K, et al: Comprehensive genomic characterization defines
human glioblastoma genes and core pathways. Nature. 455:1061–1068.
2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shi F, Guo H, Zhang R, Liu H, Wu L, Wu Q,
Liu J, Liu T and Zhang Q: The PI3K inhibitor GDC-0941 enhances
radiosensitization and reduces chemoresistance to temozolomide in
GBM cell lines. Neuroscience. 346:298–308. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Schreck KC and Grossman SA: Role of
temozolomide in the treatment of cancers involving the central
nervous system. Oncology (Williston Park). 32:555–560, 569.
2018.PubMed/NCBI
|
|
5
|
Stephen ZR, Kievit FM, Veiseh O, Chiarelli
PA, Fang C, Wang K, Hatzinger SJ, Ellenbogen RG, Silber JR and
Zhang M: Redox-responsive magnetic nanoparticle for targeted
convection-enhanced delivery of O6-benzylguanine to brain tumors.
ACS Nano. 8:10383–10395. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Stavrovskaya AA, Shushanov SS and
Rybalkina EY: Problems of glioblastoma multiforme drug resistance.
Biochemistry (Mosc). 81:91–100. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Choi S, Yu Y, Grimmer MR, Wahl M, Chang SM
and Costello JF: Temozolomide-associated hypermutation in gliomas.
Neuro Oncol. 20:1300–1309. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang F, Dai CQ, Zhang LR, Bing C, Qin J
and Liu YF: Downregulation of Lgr6 inhibits proliferation and
invasion and increases apoptosis in human colorectal cancer. Int J
Mol Med. 42:625–632. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang W, Ding S and Zhang H, Li J, Zhan J
and Zhang H: G protein-coupled receptor LGR6 is an independent risk
factor for colon adenocarcinoma. Front Med. 13:482–491.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ke J, Ma P, Chen J, Qin J and Qian H: LGR6
promotes the progression of gastric cancer through PI3K/AKT/mTOR
pathway. Onco Targets Ther. 11:3025–3033. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kazanskaya O, Glinka A, del Barco
Barrantes I, Stannek P, Niehrs C and Wu W: R-Spondin2 is a secreted
activator of Wnt/beta-catenin signaling and is required for xenopus
myogenesis. Dev Cell. 7:525–534. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kim KA, Zhao J, Andarmani S, Kakitani M,
Oshima T, Binnerts ME, Abo A, Tomizuka K and Funk WD: R-Spondin
proteins: A novel link to beta-catenin activation. Cell Cycle.
5:23–26. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nam JS, Turcotte TJ, Smith PF, Choi S and
Yoon JK: Mouse cristin/R-spondin family proteins are novel ligands
for the Frizzled 8 and LRP6 receptors and activate
beta-catenin-dependent gene expression. J Biol Chem.
281:13247–13257. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Raslan AA and Yoon JK: R-spondins:
Multi-mode WNT signaling regulators in adult stem cells. Int J
Biochem Cell Biol. 106:26–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang Y, Guo L, Lu X, Cheng C, Sun S, Li
W, Zhao L, Lai C, Zhang S, Yu C, et al: Characterization of Lgr6+
cells as an enriched population of hair cell progenitors compared
to Lgr5+ cells for hair cell generation in the neonatal mouse
cochlea. Front Mol Neurosci. 11(147)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Szenker-Ravi E, Altunoglu U, Leushacke M,
Bosso-Lefèvre C, Khatoo M, Thi Tran H, Naert T, Noelanders R,
Hajamohideen A, Beneteau C, et al: RSPO2 inhibition of RNF43 and
ZNRF3 governs limb development independently of LGR4/5/6. Nature.
557:564–569. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Schindler AJ, Watanabe A and Howell SB:
LGR5 and LGR6 in stem cell biology and ovarian cancer. Oncotarget.
9:1346–1355. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bin BH, Bhin J, Takaishi M, Toyoshima KE,
Kawamata S, Ito K, Hara T, Watanabe T, Irié T, Takagishi T, et al:
Requirement of zinc transporter ZIP10 for epidermal development:
Implication of the ZIP10-p63 axis in epithelial homeostasis. Proc
Natl Acad Sci USA. 114:12243–12248. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Guinot A, Oeztuerk-Winder F and Ventura
JJ: miR-17-92/p38α dysregulation enhances Wnt signaling and selects
Lgr6+ cancer stem-like cells during lung adenocarcinoma
progression. Cancer Res. 76:4012–4022. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Roos A, Dhruv HD, Peng S, Inge LJ, Tuncali
S, Pineda M, Millard N, Mayo Z, Eschbacher JM, Loftus JC, et al:
EGFRvIII-stat5 signaling enhances glioblastoma cell migration and
survival. Mol Cancer Res. 16:1185–1195. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Huang W, Ding X, Ye H, Wang J, Shao J and
Huang T: Hypoxia enhances the migration and invasion of human
glioblastoma U87 cells through PI3K/Akt/mTOR/HIF-1α pathway.
Neuroreport. 29:1578–1585. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang L, Wang J, Jin T, Zhou Y and Chen Q:
FoxG1 facilitates proliferation and inhibits differentiation by
downregulating FoxO/Smad signaling in glioblastoma. Biochem Biophys
Res Commun. 504:46–53. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Vainchenker W, Leroy E, Gilles L, Marty C,
Plo I and Constantinescu SN: JAK inhibitors for the treatment of
myeloproliferative neoplasms and other disorders. F1000Res.
7(82)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Berendsen S, Spliet WGM, Geurts M, Van
Hecke W, Seute T, Snijders TJ, Bours V, Bell EH, Chakravarti A and
Robe PA: Epilepsy associates with decreased HIF-1α/STAT5b signaling
in glioblastoma. Cancers (Basel). 11(41)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shen X, Zhang J, Zhang X, Wang Y, Hu Y and
Guo J: Retinoic acid-induced protein 14 (RAI14) promotes
mTOR-mediated inflammation under inflammatory stress and chemical
hypoxia in a U87 glioblastoma cell line. Cell Mol Neurobiol.
39:241–254. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ke XX, Pang Y, Chen K, Zhang D, Wang F,
Zhu S, Mao J, Hu X, Zhang G and Cui H: Knockdown of arsenic
resistance protein 2 inhibits human glioblastoma cell proliferation
through the MAPK/ERK pathway. Oncol Rep. 40:3313–3322.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cheng P, Ma Y, Gao Z and Duan L: High
mobility group box 1 (HMGB1) predicts invasion and poor prognosis
of glioblastoma multiforme via activating AKT signaling in an
autocrine pathway. Med Sci Monit. 24:8916–8924. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nadkarni A, Shrivastav M, Mladek AC,
Schwingler PM, Grogan PT, Chen J and Sarkaria JN: ATM inhibitor
KU-55933 increases the TMZ responsiveness of only inherently TMZ
sensitive GBM cells. J Neurooncol. 110:349–357. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sun Z, Xue H, Wei Y, Wang C, Yu R, Wang C,
Wang S, Xu J, Qian M, Meng Q and Li G: Mucin O-glycosylating enzyme
GALNT2 facilitates the malignant character of glioma by activating
the EGFR/PI3K/Akt/mTOR axis. Clin Sci (Lond). 133:1167–1184.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wen YT, Wu AT, Bamodu OA, Wei L, Lin CM,
Yen Y, Chao TY, Mukhopadhyay D, Hsiao M and Huang HS: A novel
multi-target small molecule, LCC-09, inhibits stemness and
therapy-resistant phenotypes of glioblastoma cells by increasing
miR-34a and deregulating the DRD4/Akt/mTOR signaling axis. Cancers
(Basel). 11(1442)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kim EH, Jo Y, Sai S, Park MJ, Kim JY, Kim
JS, Lee YJ, Cho JM, Kwak SY, Baek JH, et al: Tumor-treating fields
induce autophagy by blocking the Akt2/miR29b axis in glioblastoma
cells. Oncogene. 38:6630–6646. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
An JX, Ma MH, Zhang CD, Shao S, Zhou NM
and Dai DQ: miR-1236-3p inhibits invasion and metastasis in gastric
cancer by targeting MTA2. Cancer Cell Int. 18(66)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li C, Ge Q, Liu J, Zhang Q, Wang C, Cui K
and Chen Z: Effects of miR-1236-3p and miR-370-5p on activation of
p21 in various tumors and its inhibition on the growth of lung
cancer cells. Tumour Biol. 39(1010428317710824)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bian T, Jiang D, Liu J, Yuan X, Feng J, Li
Q, Zhang Q, Li X, Liu Y and Zhang J: miR-1236-3p suppresses the
migration and invasion by targeting KLF8 in lung adenocarcinoma
A549 cells. Biochem Biophys Res Commun. 492:461–467.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Torre LA, Bray FI, Siegel RL, Ferlay J,
Lortettieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Huang PY, Kandyba E, Jabouille A, Sjolund
J, Kumar A, Halliwill K, McCreery M, DelRosario R, Kang HC, Wong
CE, et al: Lgr6 is a stem cell marker in mouse skin squamous cell
carcinoma. Nat Genet. 49:1624–1632. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
de Lau W, Barker N, Low TY, Koo BK, Li VS,
Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M,
et al: Lgr5 homologues associate with Wnt receptors and mediate
R-spondin signalling. Nature. 476:293–297. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Glinka A, Dolde C, Kirsch N, Huang YL,
Kazanskaya O, Ingelfinger D, Boutros M, Cruciat CM and Niehrs C:
LGR4 and LGR5 are R-spondin receptors mediating Wnt/β-catenin and
Wnt/PCP signalling. EMBO Rep. 12:1055–1061. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lebensohn AM and Rohatgi R: R-spondins can
potentiate WNT signaling without LGRs. Elife.
7(e33126)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chong ZZ, Li F and Maiese K: Employing new
cellular therapeutic targets for Alzheimer's disease: A change for
the better? Curr Neurovasc Res. 2:55–72. 2005.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Hers I, Vincent EE and Tavaré JM: Akt
signalling in health and disease. Cell Signal. 23:1515–1527.
2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Luo J, Manning BD and Cantley LC:
Targeting the PI3K-Akt pathway in human cancer: Rationale and
promise. Cancer Cell. 4:257–262. 2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Angers S and Moon RT: Proximal events in
Wnt signal transduction. Nat Rev Mol Cell Biol. 10:468–477.
2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yi GZ, Liu YW, Xiang W, Wang H, Chen ZY,
Xie SD and Qi ST: Akt and β-catenin contribute to TMZ resistance
and EMT of MGMT negative malignant glioma cell line. J Neurol Sci.
367:101–106. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Acunzo M, Romano G, Wernicke D and Croce
CM: MicroRNA and cancer-a brief overview. Adv Biol Regul. 57:1–9.
2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Gregory RI and Shiekhattar R: MicroRNA
biogenesis and cancer. Cancer Res. 65:3509–3512. 2005.PubMed/NCBI View Article : Google Scholar
|
|
50
|
An JX, Ma ZS, Ma MH, Shao S, Cao FL and
Dai DQ: MiR-1236-3p serves as a new diagnostic and prognostic
biomarker for gastric cancer. Cancer Biomark. 25:127–132.
2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhu XP, Wang XL, Ma J, Fang YF, Zhang HJ,
Zhang C and Feng MC: Down-regulation of miR-1236-3p is correlated
with clinical progression and unfavorable prognosis in gastric
cancer. Eur Rev Med Pharmacol Sci. 22:5914–5919. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Li QH, Liu Y, Chen S, Zong ZH, Du YP,
Sheng XJ and Zhao Y: circ-CSPP1 promotes proliferation, invasion
and migration of ovarian cancer cells by acting as a miR-1236-3p
sponge. Biomed Pharmacother. 114(108832)2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zhang Q, Miao S, Li C, Cui K, Ge Q and
Chen Z: S-phase kinase-associated protein 2 impairs the inhibitory
effects of miR-1236-3p on bladder tumors. Am J Transl Res.
10:731–743. 2018.PubMed/NCBI
|
|
54
|
Wang Z, Liu L, Guo X, Guo C and Wang W:
microRNA-1236-3p regulates DDP resistance in lung cancer cells.
Open Med (Wars). 14:41–51. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Carè A, Felicetti F, Meccia E, Bottero L,
Parenza M, Stoppacciaro A, Peschle C and Colombo MP: HOXB7: A key
factor for tumor-associated angiogenic switch. Cancer Res.
61:6532–6539. 2001.PubMed/NCBI
|
|
56
|
Duan X, Liu D, Wang Y and Chen Z: Circular
RNA hsa_circ_0074362 Promotes Glioma Cell Proliferation, Migration,
and invasion by attenuating the inhibition of miR-1236-3p on HOXB7
expression. DNA Cell Biol. 37:917–924. 2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Monterisi S, Lo Riso P, Russo K, Bertalot
G, Vecchi M, Testa G, Di Fiore PP and Bianchi F: HOXB7
overexpression in lung cancer is a hallmark of acquired stem-like
phenotype. Oncogene. 37:3575–3588. 2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Gao RT, Zhan LP, Meng C, Zhang N, Chang
SM, Yao R and Li C: Homeobox B7 promotes the osteogenic
differentiation potential of mesenchymal stem cells by activating
RUNX2 and transcript of BSP. Int J Clin Exp Med. 8:10459–10470.
2015.PubMed/NCBI
|
|
59
|
Cortesi E and Ventura JJ: Lgr6: From
stemness to cancer progression. J Lung Health Dis. 3:12–15.
2019.PubMed/NCBI
|