Introduction
Zinc (Zn) is one of the most important
micronutrients, whose deficiency has a great impact on biological,
biochemical and immune functions (1). The direct link between insulin
secretion and function, and Zn concentration was established around
1930, Zn being identified as one of the ions inducing insulin
crystallization as well as its action; later on it was shown that
Zn is essential in the pancreatic β-cell for processing and storage
of insulin (2).
Recent literature data demonstrate that the
dysregulation of Zn metabolism is associated with an increased risk
of diabetes, as well as the impairment of the immune response, the
latter being itself a component of diabetes mellitus (DM)
etiopathology. Zn physiology in the β-cell depends on the function
of ZnT8, a transporter encoded by the SLC30A8 gene, whose
polymorphism is associated with high type 2 DM (T2DM) risk
(3). Among all the trace elements
studied, the plasma Zn concentration seems to be a useful biomarker
of ageing since its concentration has been shown to slowly decline
with age in many different studies (4,5). At
the same time, the intracellular concentration of metallothioneins
(MTs), the main intracellular proteins involved in Zn homeostasis,
was shown to increase during ageing and age-related diseases
(6,7). Zn deficiencies are reported in the
course of ageing and in different diseases, such as DM, obesity,
atherosclerosis, as well as rheumatoid arthritis or respiratory
tract infections, among others (2,8-14).
In fact, the age-related changes of trace element concentrations in
biological fluids/tissues are not only attributed to a deficient
nutritional status, but also to an intrinsic dysregulated
homeostasis (1).
At the systemic level, glycation and oxidative
stress resulting from hyperglycemia and dyslipidemia lead to
accelerated non-enzymatic modification of essential biomolecules,
particularly of proteins (15-19).
In particular, advanced glycation end products (AGEs) are
represented by a heterogenous group of damaging compounds (e.g.,
pentosidine, carboxymethyllysine, and imidazolone) that are formed
by nonenzymatic glycation of macromolecules; some of them have
characteristic fluorescence, with the ability of crosslinking of
protein and interacting with AGE-specific receptor RAGE (20-24).
AGE accumulation in tissues reflects cumulative metabolic stress,
or ‘metabolic memory’ rather than short-term glycemic control, thus
pointing to protein tissue damage resulted from the effects of
exposure to many cardiovascular disease (CVD) risk factors
(17,25-27).
Despite the numerous studies, there is still limited
information concerning the relationships between Zn status,
glycoxidative stress and IR in elderly with impaired glucose
metabolism. The aim of this pilot study was to evaluate serum Zn
levels in elderly subjects with T2DM and analyze the biological and
clinical relevance of this biomarker in terms of glycoxidative
stress, metabolic profile and pancreatic function.
Patients and methods
Subjects
A total of 52 non-smoking elderly subjects (9 men
and 43 women, aged 65-83 years, mean age: 70+5 years) were enrolled
in this cross-sectional study: a T2DM group composed of 27 known
type 2 diabetic patients treated at ‘Prof. Nicolae Paulescu’ INMD
clinic department (during September 2019), and 25 apparently
healthy control subjects selected from the patients admitted at Ana
Aslan National Institute of Gerontology and Geriatrics (during
September 2019), with normal glucose levels and normal lipid
profile, recruited from the outpatient clinical department.
Subjects were included in the T2DM study group, according to the
clinical and biochemical criteria of the American Diabetes
Association (28). Diabetic
patients were on different oral antidiabetic medication, mainly
metformin and gliclazide. Exclusion criteria included acute
infection, acute myocardial infarction, active liver disease or
liver dysfunction, renal impairment, hematological and malignant
overt diseases. Patients who were on insulin or taking any
antioxidant or Zn supplements were also excluded.
Biochemical parameters
A single blood sample (6 ml) was obtained from each
subject after an 8-12 h overnight fasting. Serum samples were
separated by centrifugation (3,000 x g for 10 min) from whole blood
for the assessment of metabolic profile: glucose, total
cholesterol, high-density lipoprotein cholesterol (HDL-C),
low-density lipoprotein cholesterol (LDL-C), blood urea nitrogen,
creatinine and uric acid, using standard methods on a Cobas C311
Analyzer and Cobas Integra 400 Analyzer for HbA1C (Roche
Diagnostics Ltd., Switzerland). Fasting insulin levels were
assessed with a Sandwich ELISA based assay (EIA-2935, DRG
Diagnostics). The inter-assay coefficient of variation (CV) was
6.0%, and the intra-assay CV was 2.6%. Insulin resistance (IR) was
evaluated using the homeostasis model assessment-insulin resistance
(HOMA-IR): fasting insulin (mU/l) x fasting glucose (mg/dl) divided
by 405(29). The serum AGEs were
assessed by the level of AGE-associated autofluorescence recorded
at 440 nm emission wavelength upon excitation at 350 nm, as
previously described (17). The
measurements were performed on PerkinElmer Spectrofluorimeter (LS
50 B, PerkinElmer, Inc.). Fluorescence intensity was expressed in
relative fluorescence units (RFU). The coefficient of variation
(CV) for replicate measurements was <5%. Zn assessment in serum
samples was performed using a sensitive, standardized, simple and
direct procedure (reagent kit MAK032, Sigma-Aldrich; Merck KGaA)
which measures the colorimetric product (560 nm) resulting from the
binding of Zn ions to a specific ligand, namely 5-Bromo-PAPS
[2-(5-Bromo-2-pyridylazo)-5-[N-propyl-N-(3-sulfopropyl)amino]
phenol disodium salt dihydrate]. Concentration of serum Zn was
expressed in mM Zn (mmol/l serum). The inter-assay and intra-assay
coefficients of variation were 5.4 and 6.7%, respectively.
Biochemical and immunoenzymatic tests were performed on ChemWell
2190 Analyzer (Awareness Technology).
Indices of glycemic control and Zn
status
The glucose-to-zinc ratio (Gly/Zn) was calculated
using the fasting levels of serum glucose and Zn, expressed both as
mmol/l serum. The insulin-to-zinc ratio (Ins/Zn) was calculated
using the fasting levels of serum insulin (mU/l serum) and Zn,
expressed as mmol/l serum. The insulin-zinc resistance index
(HOMA-IR/Zn) was calculated using HOMA-IR individual values and
serum Zn levels.
Statistical analysis
We used the Statistical Package for Social Sciences
software (SPSS, version 15, SPSS, Inc.). Comparison between the
control group and T2DM group was performed using Student's unpaired
t-test. Pearson's two-tailed bivariate correlations (r) were
performed to examine the associations of serum Zn and serum AGEs,
with insulin, glucose, HOMA-IR, as well as the correlations between
AGEs and Gly/Zn, Ins/Zn and HOMA-IR/Zn ratios. Both tests were
two-sided and a P-value <0.05 was considered statistically
significant.
Results
The study population included 52 elderly subjects
divided into two groups: a control group, with normal metabolic
profile (n=25), and a group with T2DM (n=27). The biochemical and
metabolic parameters measured in both groups are presented in
Table I.
 | Table IBiochemical parameters in control and
type 2 diabetes mellitus (T2DM) elderly subjects. |
Table I
Biochemical parameters in control and
type 2 diabetes mellitus (T2DM) elderly subjects.
| Parameters | Control group
(n=25) | T2DM group
(n=27) | P-value |
|---|
| Age, mean
(years) | 69±5 | 71±5 | NS |
| Fasting glucose
(mg/dl) | 98.92±10.99 | 152.65±30.23 | <0.001 |
| HbA1c (%) | 5.88±0.62 | 7.46±0.85 | <0.001 |
| Fasting insulin
(µU/ml) | 4.45±1.21 | 11.49±4.31 | <0.001 |
| HOMA-IR | 1.10±0.36 | 4.26±1.57 | <0.001 |
| Total cholesterol
(mg/dl) | 180.67±32.80 | 198.96±33.94 | NS |
| Triglycerides
(mg/dl) | 109.88±69.28 | 167.69±64.52 | <0.01 |
| HDL-cholesterol
(mg/dl) | 55.83±14.73 | 43.73±11.58 | <0.01 |
| LDL-cholesterol
(mg/dl) | 103.83±36.83 | 119.92±30.86 | NS |
| Uric acid
(mg/dl) | 4.42±1.67 | 5.47±1.62 | <0.05 |
| Creatinine
(mg/dl) | 0.86±0.16 | 0.92±0.16 | NS |
| Blood urea nitrogen
(mg/dl) | 38.79±13.50 | 43.27±15.88 | <0.01 |
| AGEs (RFU) | 103.84±13.73 | 120.74±21.47 | <0.01 |
| Serum zinc
(mmol/l) | 14.11±2.06 | 15.71±1.71 | <0.01 |
The mean values of biomarkers indicating the
metabolic dysregulation and cardiovascular risk, namely the values
of fasting glucose, insulin, and triglycerides were significantly
higher in the T2DM group compared with the control group, whereas
HDL-cholesterol levels were significantly lower. Insignificant
differences between groups were observed for creatinine, whereas
uric acid and blood urea nitrogen levels were slightly higher in
the T2DM group. T2DM elderly patients were characterized by a
significant hyperinsulinemia and subsequent significant increases
in insulin resistance index HOMA-IR.
The glycoxidative stress was evaluated as an AGE
biomarker as well as the traditional marker indicating the
persistence of chronic hyperglycemia, HbA1C; both were
significantly higher in elderly subjects with T2DM vs. the control
group.
Serum Zn levels were significantly (P<0.01)
increased in the T2DM group as compared to the control subjects.
The mean Zn value in the T2DM group was 15.7 mmol/l (range:
12.03-19.12 mmol/l), whereas in the control group the average serum
Zn concentration was 14.11 mmol/l (range: 9.89-17.31 mmol/l).
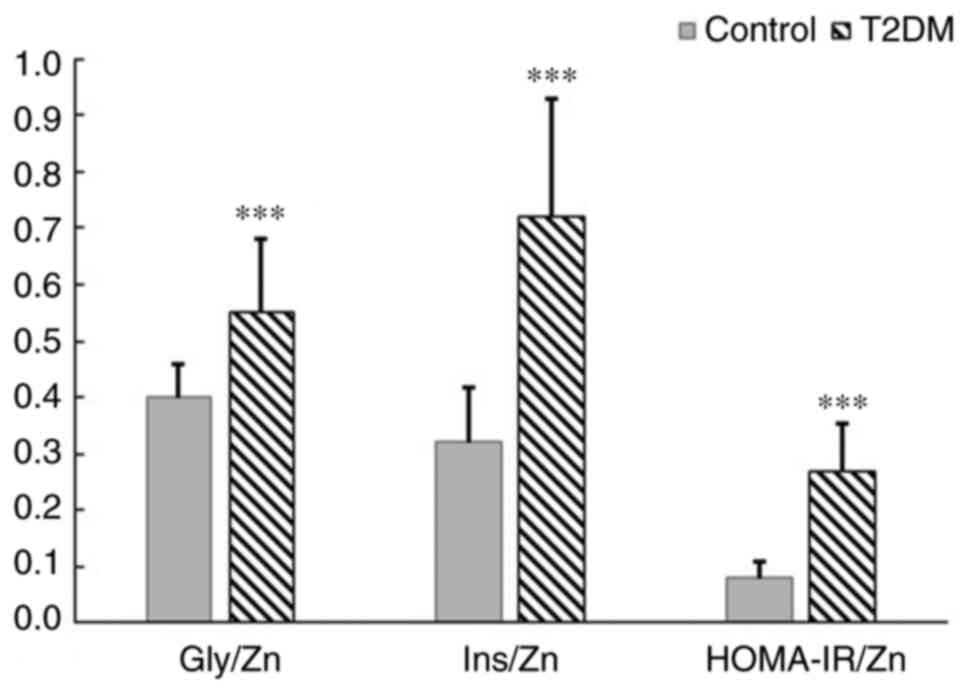
Overall, elderly subjects with T2DM presented
significantly higher serum insulin and Zn levels, as well as
HOMA-IR values, as compared to the control. Therefore, the
calculated Gly/Zn, Ins/Zn and insulin-zinc resistance index
(HOMA-IR/Zn) were further used as surrogate biomarkers to explore
the relationships between serum Zn levels, metabolic profile,
secretory functions of β-pancreatic cells and glycoxidative stress.
All of these three metabolic indices illustrative of the glycemic
control and Zn status displayed significantly higher values in T2DM
patients than in the control subjects (Fig. 1).
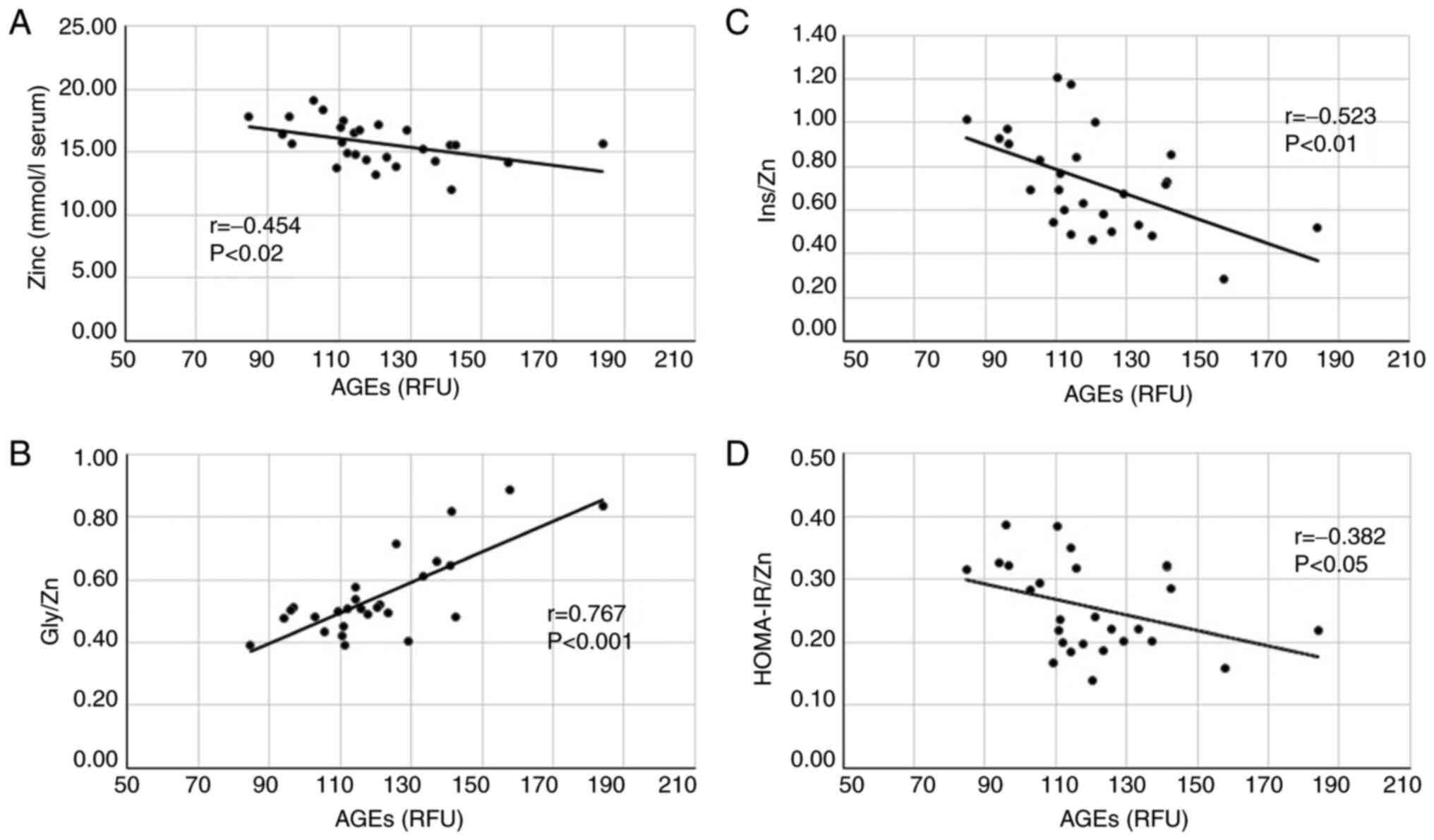
In T2DM patients we aimed to explore the
relationships between the advanced glycation of proteins (AGEs), Zn
systemic status and glucose homeostasis. The correlation analysis
was carried out between levels of AGEs and serum Zn levels, Gly/Zn
ratio, Ins/Zn ratio and HOMA-IR/Zn ratio. Statistical significance
of the Pearson's correlation coefficients was different among the
four study biomarkers as presented in Fig. 2.
AGEs values showed a significant negative
correlation (r=-0.454; P<0.02) with Zn serum levels and a strong
positive association (r=0.767; P<0.001) with the Gly/Zn ratio
(Fig. 2A and B). Regarding the association between AGEs
and β-cell secretory function, the correlation analysis identified
significant negative relationships between AGEs and insulin
(r=-0.459; P<0.02) and significantly higher between AGEs and
Ins/Zn ratio (r=-0.523; P<0.01) (Fig. 2C). Despite the elevated levels of
circulating AGEs found out in hyperglycemic and insulin-resistant
subjects, the association with HOMA-IR was not significant
(r=-0.145; NS), whereas the correlation between AGEs and HOMA-IR/Zn
ratio was significant (r=-0.382; P<0.05) (Fig. 2D).
Discussion
The results of the present showed that elderly
diabetic patients had significantly higher levels of serum Zn in
comparison with the control group subjects. These results are in
agreement with a recent study conducted in postmenopausal women
with diabetes, characterized by significantly higher Zn levels as
compared to the controls (30).
However, these data are in contrast with many studies, conducted
mainly on adult population, in which T2DM individuals displayed a
reduced Zn status correlated with a poor glycemic control (31-33).
In a recent systematic review and meta-analysis, Yin et al
showed that the serum Zn concentration was lower in polycystic
ovary syndrome (PCOS) women with IR than in healthy women. However,
there was no significant difference in serum Zn concentration when
compared between healthy control and PCOS women without IR
(34).
Understanding the complex role of Zn in glucose
homeostasis and subsequent IR and T2DM is at present limited by
controversial findings in the literature and the absence of ‘gold
standard’ animal models that could help elucidate the role of Zn
and Zn transporters (35). It is
acknowledged that, at the pancreatic level, Zn participates in
mechanisms involving insulin secretion and action, as a catalytic
cofactor for carboxypeptidase H, an enzyme which catalyzes the
conversion from proinsulin into insulin. Zn also promotes
phosphorylation of insulin receptor by enhancing glucose transport
into cells (36). Previous studies
have demonstrated the role of higher serum concentration of Zn in
improving insulin sensitivity in T2DM (37). Regarding the roles of Zn in blood
insulin levels, recent experimental studies conducted on mice with
β-cell-specific genetic deficiency for ZnT8 transporter found an
enhanced insulin secretion but also an enhanced insulin clearance
in the liver, due to reduced Zn secretion from these cells
(38).
In the present study, we also pointed out a
significant inverse correlation between Zn levels and AGEs, and a
strong positive correlation between the Gly/Zn ratios and AGEs,
suggesting that both Zn and AGEs are biomarkers that could reflect
the persistence of hyperglycemia. These results could also suggest
that Zn could exert at the systemic level some anti-glycation
actions, as confirmed in studies conducted in vitro
(39). Therefore, we also made an
attempt to ‘normalize’ the traditional indices of β-cells secretory
function and IR to serum Zn levels and obtain new surrogate
biomarkers, namely Ins/Zn and HOMA-IR/Zn ratios, and study their
relationships with AGEs. In the elderly diabetic subjects, Ins/Zn
and HOMA-IR/Zn ratios were significantly inversely correlated with
the glycoxidative stress evaluated as AGEs.
The main limitation of the study was the reduced
number of subjects and the absence of evaluation of proteins
involved in Zn metabolism such as Zn-α2-glycoprotein and Zn-induced
metallothionein, that could help to elucidate the mechanisms
involved in the interplay between pancreatic
β-cells/Zn/glycoxidative stress.
In the present study, we propose, for the first
time, the evaluation of these new Zn status surrogate biomarkers
involved in glucose homeostasis, as numerous clinical markers and
quotients are routinely used to evaluate the metabolic impairment.
Similarly, a recent study on patients with polycystic ovary
syndrome (PCOS), T2DM and impaired glucose tolerance (IGT)
identified the natural logarithm of Zn-α2-glycoprotein/HOMA-IR as a
better predictor of insulin sensitivity than the product of
triglycerides and glucose and the other lipid ratios (40). Therefore, we evaluated the
biochemical and clinical relevance serum AGEs and the newly
introduced ratios of Ins/Zn and HOMA-IR/Zn, which could be useful
in clinical practice in the diagnosis of IR associated with Zn
deficiency and oxidative stress.
In conclusion, in the present study conducted on
elderly diabetic subjects, we identified new significant
associations between Zn status, glycoxidative stress, pancreatic
β-cell impairment and IR. Similar approaches could help in the
future for the development of preventive strategies for
personalized nutrition and treatment, that would increase the
longevity and quality of life of patients with diabetes.
Acknowledgements
Not applicable.
Funding
The experiments carried out in this study and the publication of
the article were supported by regular financial resources provided
by ‘Carol Davila’ University of Medicine and Pharmacy within the
general framework for the development of scientific research
collaboration.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author.
Authors' contributions
DG and DM conceived and designed the study,
contributed to the experiments and drafted the manuscript. AU, GN
and CMP performed all the experiments and the statistical analysis.
RDM performed the clinical data analysis. CMP, CIT and RDM
contributed to the conception and designed the study. All the
authors have read and approved the final manuscript for
publication.
Ethics approval and consent to
participate
The study protocol was approved by ‘Nicolae
Paulescu’ Institute of Diabetes, Metabolic and Nutrition Diseases
(no. 10403/2019) and ‘Ana Aslan’ National Institute of Gerontology
and Geriatrics (no. 12660/2019) Ethics Committees and all subjects
provided their informed consent before participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mocchegiani E, Romeo J, Malavolta M,
Costarelli L, Giacconi R, Diaz LE and Marcos A: Zinc: Dietary
intake and impact of supplementation on immune function in elderly.
Age (Dordr). 35:839–860. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Maret W: Zinc in pancreatic islet biology,
insulin sensitivity, and diabetes. Prev Nutr Food Sci. 22:1–8.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huang Q, Du J, Merriman C and Gong Z:
Genetic, functional, and immunological study of ZnT8 in diabetes.
Int J Endocrinol. 2019(1524905)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Giacconi R, Costarelli L, Piacenza F,
Basso A, Rink L, Mariani E, Fulop T, Dedoussis G, Herbein G,
Provinciali M, et al: Main biomarkers associated with age-related
plasma zinc decrease and copper/zinc ratio in healthy elderly from
ZincAge study. Eur J Nutr. 56:2457–2466. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Malavolta M, Piacenza F, Basso A, Giacconi
R, Costarelli L and Mocchegiani E: Serum copper to zinc ratio:
Relationship with aging and health status. Mech Ageing Dev.
151:93–100. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Giacconi R, Costarelli L, Piacenza F,
Basso A, Burkle A, Moreno-Villanueva M, Grune T, Weber D, Stuetz W,
Gonos ES, et al: Zinc-induced metallothionein in centenarian
offspring from a large European population: The MARK-AGE Project. J
Gerontol A Biol Sci Med Sci. 73:745–753. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Giacconi R, Cai L, Costarelli L, Cardelli
M, Malavolta M, Piacenza F and Provinciali M: Implications of
impaired zinc homeostasis in diabetic cardiomyopathy and
nephropathy. Biofactors. 43:770–784. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kloubert V and Rink L: Zinc as a
micronutrient and its preventive role of oxidative damage in cells.
Food Funct. 6:3195–3204. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Skalny AV, Rink L, Ajsuvakova OP, Aschner
M, Gritsenko VA, Alekseenko SI, Svistunov AA, Petrakis D, Spandidos
DA, Aaseth J, et al: Zinc and respiratory tract infections:
Perspectives for COVID19 (Review). Int J Mol Med. 46:17–26.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rios-Lugo MJ, Madrigal-Arellano C,
Gaytán-Hernández D, Hernández-Mendoza H and Romero-Guzmán ET:
Association of serum zinc levels in overweight and obesity. Biol
Trace Elem Res. 198:51–57. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rajaee E, Mowla K, Ghorbani A,
Dargahi-Malamir M, Zarei M and Rahimikhah FA: The relationship
between serum zinc levels and rheumatoid arthritis activity. Front
Biol. 13:51–55. 2018.
|
|
12
|
Choi S, Liu X and Pan Z: Zinc deficiency
and cellular oxidative stress: Prognostic implications in
cardiovascular diseases. Acta Pharmacol Sin. 39:1120–1132.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Farhood HB, Balas M, Gradinaru D, Margina
D and Dinischiotu A: Hepatoprotective effects of chlorogenic acid
under hyperglycemic conditions. Rom Biotechnol Lett. 24:301–307.
2019.
|
|
14
|
Farhood HB, Balas MR, Gradinaru D, Margina
D and Dinischiotu A: Effects of chlorogenic acid on the liver cell
metabolism under high glucose conditions. Rom Biotechnol Lett.
24:883–892. 2019.
|
|
15
|
Ungurianu A, Seremet O, Gradinaru D,
Ionescu-Tirgoviste C, Margina D and Danciulescu Miulescu R:
Spectrophotometric versus spectrofluorometric assessment in the
study of the relationships between lipid peroxidation and metabolic
dysregulation. Chem Biol Drug Des. 93:1026–1035. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ungurianu A, Margina D, Gradinaru D,
Bacanu C, Ilie M, Tsitsimpikou C, Tsarouhas K, Spandidos DA and
Tsatsakis AM: Lipoprotein redox status evaluation as a marker of
cardiovascular disease risk in patients with inflammatory disease.
Mol Med Rep. 15:256–262. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gradinaru D, Borsa C, Ionescu C and
Margina D: Advanced oxidative and glycoxidative protein damage
markers in the elderly with type 2 diabetes. J Proteomics.
92:313–322. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Margina D, Ilie M and Gradinaru D:
Quercetin and epigallocatechin gallate induce in vitro a
dose-dependent stiffening and hyperpolarizing effect on the cell
membrane of human mononuclear blood cells. Int J Mol Sci.
13:4839–4859. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gradinaru D, Margina D and Borsa C: In
vitro studies regarding the antioxidant effects of procaine,
Gerovital H3 and Aslavital. Rev Roum Chim. 54:761–766. 2009.
|
|
20
|
Gradinaru D, Khaddour H, Margina D,
Ungurianu A, Borsa C, Ionescu C, Prada GI, Usher J and Elshimali Y:
Insulin-leptin axis, cardiometabolic risk and oxidative stress in
elderly with metabolic syndrome. Exp Clin Endocrinol Diabetes: Feb
8, 2018. (Epub ahead of print). doi: 10.1055/s-0043-123825
2018.
|
|
21
|
Gradinaru D, Margina D, Borsa C, Ionescu
C, Ilie M, Costache M, Dinischiotu A and Prada GI: Adiponectin:
Possible link between metabolic stress and oxidative stress in the
elderly. Aging Clin Exp Res. 29:621–629. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gradinaru D, Margina D, Ilie M, Borsa C,
Ionescu C and Prada GI: Correlation between erythropoietin serum
levels and erythrocyte susceptibility to lipid peroxidation in
elderly with type 2 diabetes. Acta Physiol Hung. 102:400–408.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Borșa C, Gradinaru D, Margina D, Prada GI
and Pena C: Receptor of advanced glycation end products and
cardiovascular risk in elderly with type 2 diabetes mellitus. J
Biol Res. 90:81–86. 2017.
|
|
24
|
Margina D, Gradinaru D and Mitrea N:
Development of a potentiometric method for the evaluation of redox
status in human serum. Rev Roum Chim. 54:45–48. 2009.
|
|
25
|
Zanfirescu A, Cristea AN, Nitulescu GM,
Velescu BS and Gradinaru D: Chronic monosodium glutamate
administration induced hyperalgesia in mice. Nutrients.
10(1)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zanfirescu A, Ungurianu A, Tsatsakis AM,
Nitulescu GM, Kouretas D, Veskoukis A, Tsoukalas D, Engin AB,
Aschner M and Margina D: A review of the alleged health hazards of
monosodium glutamate. Compr Rev Food Sci Food Saf. 18:1111–1134.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ungurianu A, Seremet O, Gagniuc E, Olaru
OT, Gutu C, Gradinaru D, Ionescu-Tirgoviste C, Margina D and
Danciulescu-Miulescu R: Preclinical and clinical results regarding
the effects of a plant-based antidiabetic formulation versus well
established antidiabetic molecules. Pharmacol Res.
150(104522)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
American Diabetes Association. Improving
care and promoting health in populations: Standards of medical care
in diabetes-2020. Diabetes Care. 43 (Suppl 1):S7–S13.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Matthews DR, Hosker JP, Rudenski AS,
Naylor BA, Treacher DF and Turner RC: Homeostasis model assessment:
Insulin resistance and beta-cell function from fasting plasma
glucose and insulin concentrations in man. Diabetologia.
28:412–419. 1985.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Skalnaya MG, Skalny AV and Tinkov AA:
Serum copper, zinc, and iron levels, and markers of carbohydrate
metabolism in postmenopausal women with prediabetes and type 2
diabetes mellitus. J Trace Elem Med Biol. 43:46–51. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bandeira VDS, Pires LV, Hashimoto LL,
Alencar LL, Almondes KGS, Lottenberg SA and Cozzolino SMF:
Association of reduced zinc status with poor glycemic control in
individuals with type 2 diabetes mellitus. J Trace Elem Med Biol.
44:132–136. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sobczak AIS, Stefanowicz F, Pitt SJ, Ajjan
RA and Stewart AJ: Total plasma magnesium, zinc, copper and
selenium concentrations in type-I and type-II diabetes. Biometals.
32:123–138. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Farooq DM, Alamri AF, Alwhahabi BK,
Metwally AM and Kareem KA: The status of zinc in type 2 diabetic
patients and its association with glycemic control. J Family
Community Med. 27:29–36. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yin J, Hong X, Ma J, Bu Y and Liu R: Serum
trace elements in patients with polycystic ovary syndrome: A
systematic review and meta-analysis. Front Endocrinol (Lausanne).
11(572384)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Pearsey HM, Henson J, Sargeant JA, Davies
MJ, Khunti K, Suzuki T, Bowden-Davies KA, Cuthbertson DJ and Yates
TE: Zinc-alpha2-glycoprotein, dysglycaemia and insulin resistance:
A systematic review and meta-analysis. Rev Endocr Metab Disord.
21:569–575. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Capdor J, Foster M, Petocz P and Samman S:
Zinc and glycemic control: A meta-analysis of randomised placebo
controlled supplementation trials in humans. J Trace Elem Med Biol.
27:137–142. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Vashum KP, McEvoy M, Milton AH, Islam MR,
Hancock S and Attia J: Is serum zinc associated with pancreatic
beta cell function and insulin sensitivity in pre-diabetic and
normal individuals? Findings from the Hunter Community Study. PLoS
One. 9(e83944)2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Watada H and Tamura Y: Impaired insulin
clearance as a cause rather than a consequence of insulin
resistance. J Diabetes Investig. 8:723–725. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kheirouri S, Alizadeh M and Maleki V: Zinc
against advanced glycation end products. Clin Exp Pharmacol
Physiol. 45:491–498. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Qu C, Zhou X, Yang G, Li L, Liu H and
Liang Z: The natural logarithm of zinc-α2-glycoprotein/HOMA-IR is a
better predictor of insulin sensitivity than the product of
triglycerides and glucose and the other lipid ratios. Cytokine.
79:96–102. 2016.PubMed/NCBI View Article : Google Scholar
|