Introduction
Skeletal muscle injury is one of the most common
type of injury in sports, which accounts for ~40% of all
sports-related injuries among the elderly (1). Currently available therapeutic
strategies to promote skeletal muscle healing after an injury
remain unsatisfactory, which is mainly due to skeletal muscle
fibrosis, which frequently hinders full functional recovery
(2-4).
In addition, skeletal muscle fibrosis results in limited movement,
resulting in dysfunction and severely affects the quality of life
(5). Therefore, it is of clinical
importance to study the mechanism underlying fibrosis after
skeletal muscle injury and to explore novel treatment methods.
Skeletal muscle injury repair is regulated by the
balance between muscle fiber regeneration and fibrosis, where
recovery depends on the development of completely regenerated
muscle fibers, extracellular matrix and fibrosis (6). The process of injured skeletal muscle
repair can be divided into the following three stages: Inflammatory
response; repair; and shaping (7).
TGF-β1 is a key factor in the development of kidney, liver, lung
and skeletal muscle fibrosis (8).
In skeletal muscles, TGF-β1 can inhibit myogenic differentiation
and activate MAPK signaling, which eventually lead to skeletal
muscle fibrosis at the injury site (9,10).
Inflammation and immune cells also serve key roles in the
regeneration of the skeletal muscle (11,12).
TGF-β1 inhibits the conversion of M1 macrophages into
the M2 type, where growth factors produced by
M1 macrophages, including TGF-β1, platelet-derived
growth factor, fibroblast growth factor-2 and VEGF, can
subsequently lead to extracellular matrix production (13). Previous studies have shown that the
exogenous treatment of M1 macrophages significantly
reduced muscle fibrosis whilst enhancing muscle fiber regeneration
(14,15).
Acupuncture is an integral aspect of traditional
Chinese medicine and is frequently applied to treat rheumatoid
arthritis, acute gastritis and other immune-related diseases
(16). It can improve immunity,
alleviate inflammation, mediate CD4+ T cell
differentiation and dendritic cell maturation, reduce fibrous
tissue proliferation and inhibit fibrosis (17). Electroacupuncture (EA, weak
electrical stimulation using acupuncture needles) is a type of
acupuncture that (18), when
combined with exercise therapy, has been demonstrated to exert
therapeutic effects on lower back pain due to acute lumbar sprain
(19). However, the effects of EA
on skeletal muscle fibrosis and the possible underlying mechanism
remain unclear. Therefore, in the present study, the potential
effects of EA on inflammatory cell infiltration, injury and
fibrosis in a model of skeletal muscle injury were evaluated. In
addition, the possible mechanism underlying the effects of EA on
skeletal muscle fibrosis was also investigated. It is hoped that
data from the present study can provide a theoretical basis for the
use of EA in treating skeletal muscle fibrosis following
injury.
Materials and methods
Animals
A total of 20 Sprague-Dawley rats (sex, nine male
and nine female; age, 12 weeks; weight, 250±20 g) were obtained
from the Hubei Experimental Animal Research Center (license no.
42000600032492). The animals were housed in a
specific-pathogen-free environment in opaque polypropylene cages in
a standard 12-h light/dark cycle, at 23±3˚C and 50-60% humidity.
Food and water were available ad libitum. The rats were
allowed to adapt to the aforementioned conditions for 7 days before
experimentation.
Following the method previously described by Zhang
et al (20), 100 µl cardiotoxin
(CTX; 10 µM; 0.028 mg/kg) was injected (1 mg; cat. no. SML1754;
Sigma-Aldrich; Merck KGaA) into the anterior tibial muscle of the
rats to establish a model of acute rat tibial anterior muscle
injury. After 24 h of CTX injection, two rats were sacrificed and
the successful model establishment was assessed by H&E staining
and the rats were randomly divided into the following three groups
(n=6 per group): Control; model; and EA. Rats in the control group
was injected with 100 µl normal saline. In the EA group, the
‘Shenyu’ (the lower sides of the second lumbar vertebra) and
‘Housanli’ (posterolateral knee joint, ~5 mm below the fibular
capitulum) acupoints were selected as sites for stimulation
according to ‘Experimental Acupuncture’ and ‘Handbook of
Acupuncture for Experimental Animals’. The rat's limbs were then
fixed whilst the eyes of the rats were covered using a hood and the
acupoints were punctured with a straight stainless-steel
millineedle (0.22x15 mm). The EA therapeutic apparatus (Electronic
acupuncture instrument SDZ II; Suzhou Medical Products Factory Co.,
Ltd.; https://www.hwato-med.com/product/detail.html?id=743)
was then connected to the needles and the acupoints were stimulated
at a frequency of 100-120 times/min at 2 mA under 100 Hz with a
needle retention time of 20 min. The rats were stimulated once a
day and intervention continued for 1 or 2 weeks. During this time,
the rats were assessed for their mental, behavioral (mental:
Excitement and movement; behavioral: Paralysis, flaccid disorder,
spasticity, disorder and claudication), dietary and water intake.
The rats in the model and control groups were not given additional
stimulation. After 7 or 14 consecutive days of EA, nine rats were
anesthetized with 3% sodium pentobarbital (40 mg/kg) before
cervical dislocation under anesthesia. Rat tibialis anterior muscle
tissues and blood samples were then collected and stored at -80˚C.
All experimental procedures in the present study were performed in
accordance with the requirements of the Ethics of Animal
Experiments and approved by the Animal Care and Use Committee of
Wuhan Myhalic Biotechnology Co. Ltd. (approval no.
HLK-20181118-01).
H&E and Masson staining
Anterior tibial muscular tissues were fixed in 4%
paraformaldehyde at 25˚C for 24 h and gradually rehydrated in a
descending ethanol gradient. The tissues were then embedded in
paraffin and sectioned at 3 µm per slice. The sections were baked
in an oven at 60˚C for 40 min, followed by incubation in xylene for
10 min in an oven at 60˚C. The sections were then replaced with
clean xylene and soaked again at 25˚C for 5 min, before being
incubated in a decreasing ethanol gradient. For H&E staining,
the sections were stained with hematoxylin for 5 min at room
temperature and immersed in a 1% ethanolic hydrochloric acid
solution for 30 sec to remove excess hematoxylin. Subsequently, the
sections were counterstained with 5% eosin for 5 min at room
temperature.
For Masson staining, the sections were stained with
a mixture of hematoxylin staining solution and aqueous ferric
chloride solution for 10 min, washed with a hydrochloric
acid-ethanol fractionation solution in water for 15 sec and Masson
bluing solution for 5 min, before being washed with distilled water
for 1 min. They were then stained with Lichon red magenta staining
solution for 5 min, washing with aqueous an acetic acid solution,
aqueous phosphomolybdic acid solution and aqueous acetic acid
solution in turn for 1 min each before staining with aniline blue.
After staining for 2 min and washing again for 1 min, they were
treated with anhydrous ethanol and xylene and mounted with neutral
resin. All staining processes were performed at 25˚C. All prepared
sections were observed under a light microscope at x200
magnification.
ELISA
Blood was collected and placed at 37˚C for 1-2 h,
centrifuged at 1000 x g for 10 min at 4˚C and the supernatant was
collected as serum. The levels IL-6, IL-4, IL-33, IL-10 and TNF-α
in the serum were evaluated by ELISA. IL-6 (cat. no. RA20607), IL-4
(cat. no. RA20088), IL-33 (cat. no. RA21016), IL-10 (cat. no.
RA20090), and TNF-α (cat. no. RA20035) ELISA kits were obtained
from Bioswamp Life Science Lab; Wuhan Bein Lai Biotechnology Co.,
Ltd. The assay was performed according to the manufacturer's
protocol and the optical density was measured at 450 nm using a
microplate reader (Multiskan MS; Thermo Fisher Scientific,
Inc.).
Immunofluorescence
The formalin-fixed paraffin-embedded tissue blocks
were baked in an oven at 65˚C for 1 h to remove the wax blocks. The
sections were placed in a descending ethanol gradient before being
incubated in citric acid buffer at 125˚C and 103 KPa for 23 min,
naturally cooled and rinsed three times with PBS. The sections were
then blocked in 10% goat serum (cat. no. SL038; Beijing Solarbio
Science & Technology Co., Ltd.) and incubated in a wet box at
25˚C for 10 min. The sections were then incubated overnight at 4˚C
with CD86 (1:50; cat. no. PAB43783; Bioswamp Life Science Lab;
Wuhan Bein Lai Biotechnology Co., Ltd.) and CD163 (1:50; cat. no.
MA5-16656; Invitrogen; Thermo Fisher Scientific, Inc.) primary
antibodies. Sections were then removed and rested at 25˚C for 40
min. After resting, the sections were incubated with Alexa Fluor
594-conjugated goat anti-rabbit IgG (1:20; cat. no. SA00006-4;
Wuhan Sanying Biotechnology) and FITC-conjugated Affinipure Donkey
Anti-Mouse IgG (1:20; cat. no. SA00003-9; Wuhan Sanying
Biotechnology) secondary antibodies for 1 h at 37˚C. The slices
were sealed at 25˚C using a Mounting Medium, antifading (with DAPI)
(cat. no. S2110; Beijing Solarbio Science & Technology Co.,
Ltd.) and left for 30 min to stain the cell nuclei. Images were
captured of 20 fields of view at x200 magnification with a
fluorescence microscope (MD1000; Leica Microsystems GmbH).
Quantification of the images was performed using Image J (v1.53e;
National Institutes of Health)
Immunohistochemistry
The formalin-fixed, paraffin-embedded tissue blocks
were placed at -20˚C for ≥30 min to increase hardness before
slicing at a thickness of 4-μm. Before primary antibody incubation,
the sections were heated and dewaxed using the procedure identical
to that of H&E staining aforementioned. They were then
rehydrated in a descending ethanol gradient and treated with 0.01
mmol/l sodium citrate buffer solution for 23 min at high pressure
(125˚C and 103 kPa) for antigen retrieval. Elimination of
endogenous peroxidase activity was performed by 3% hydrogen
peroxide incubation at 25˚C for 10 min. The sections were then
blocked with 10% goat serum (cat. no. SL038; Beijing Solarbio
Science & Technology Co., Ltd.) for 30 min at 25˚C and
incubated overnight at 4˚C with primary antibodies against Axin2
(1:50; cat. no. PAB40586; Bioswamp Life Science Lab; Wuhan Bein Lai
Biotechnology Co., Ltd.), collagen type II (1:50; cat. no.
PAB43834; Bioswamp Life Science Lab; Wuhan Bein Lai Biotechnology
Co., Ltd.) and β-catenin (1:50; cat. no. PAB30671; Bioswamp Life
Science Lab; Wuhan Bein Lai Biotechnology Co., Ltd.). Afterwards,
the sections were incubated with a MaxVision™
HRP-Polymer anti-Mouse/Rabbit IHC Kit (cat. no. KIT-5020; Fuzhou
Maixin Biotech Co., Ltd.; https://www.maxim.com.cn/sitecn/myzhjcxthsjh/1056.html)
at 4˚C for 45 min, according to manufacturer's protocols. The
sections were then stained using DAB (cat. no. DA1010-2 Beijing
Solarbio Science & Technology Co., Ltd.). Finally, the sections
were counterstained with Harris' hematoxylin at 25˚C for 3 min and
evaluated by visual assessment of the staining intensity using a
light microscope at x200 magnification with 20 of fields of view.
Quantification of immunohistochemical images was performed using
Image J (v1.53e; National Institutes of Health).
Western blotting
The relative protein expression levels were detected
by western blotting. Protein was extracted by lysing muscle tissue
cells using RIPA buffer (cat. no. PAB180006; Wuhan Bein Lai
Biotechnology Co., Ltd.) and 20 µg of protein was quantified using
the BCA protein concentration assay kit. The protein was
resuspended in SDS sample buffer and boiled at 100˚C for 5 min.
Equal amounts of total protein were then separated by 12% SDS-PAGE
and then transferred onto PVDF membranes. The membranes were then
incubated with 5% skimmed milk at 25˚C for 2 h before being
incubated overnight at 4˚C with the following primary antibodies:
TGF-β1 (1:2,000; cat. no. PAB33215; Wuhan Bein Lai Biotechnology
Co., Ltd.), MMP-2 (1:2,000; cat. no. PAB30618; Wuhan Bein Lai
Biotechnology Co., Ltd.), MMP-7 (1:2,000; cat. no. PAB30191; Wuhan
Bein Lai Biotechnology Co., Ltd.), phosphorylated (p-) Smad3
(1:1,000; cat. no. ab193297; Abcam), Smad3 (1:2,000; cat. no.
PAB30705; Wuhan Bein Lai Biotechnology Co., Ltd.), p-ERK1/2
(1:2,000; cat. no. PAB36335-P; Wuhan Bein Lai Biotechnology Co.,
Ltd.), ERK1/2 (1:2,000; cat. no. MAB37327; Wuhan Bein Lai
Biotechnology Co., Ltd.), p-p38 (1:2,000; cat. no. PAB43139-P;
Wuhan Bein Lai Biotechnology Co., Ltd.), p38 (1:2,000; cat. no.
PAB38871; Wuhan Bein Lai Biotechnology Co., Ltd.) and GAPDH
(1:2,000; cat. no. PAB36264; Wuhan Bein Lai Biotechnology Co.,
Ltd.). The membranes were next washed with TBS and incubated with
HRP-conjugated goat anti-rabbit IgG secondary antibody (1:20,000,
cat. no. PAB160011; Bioswamp Life Science Lab; Wuhan Bein Lai
Biotechnology Co., Ltd.) for 2 h at 25˚C. Finally, the
immunoreactivity was visualized by a colorimetric reaction using
the enhanced chemiluminescence substrate buffer (EMD Millipore).
The membranes were scanned using Gel Doz EZ imager (Bio-Rad
Laboratories, Inc.). The gray value of the relevant bands was
quantified using the TANON GIS software (version 4.2; Tanon Science
and Technology Co., Ltd.).
Statistical analysis
Statistical analysis was performed with SPSS 19.0
software (IBM Corp.) using one-way analysis of variance followed by
Tukeyʼs test. Data are expressed as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
General condition
After 7 days of adaptive feeding, a muscle injury
model was constructed by injecting CTX. EA intervention was
performed before the mental state, diet and activity of the rats
were observed. None of the rats died during the experimental
period. The weights of all rats remained stable without significant
difference, and there was no significant difference among the
treatment groups in terms of diet and water intake (data not
shown). Compared with the control rats the mental state of the
model rats gradually deteriorated, where their activity became
limited. Compared with the model rats, the mental state and
activity of the EA-treated rats were improved, which became more
notable with intervention time (data not shown). Subsequently, 24 h
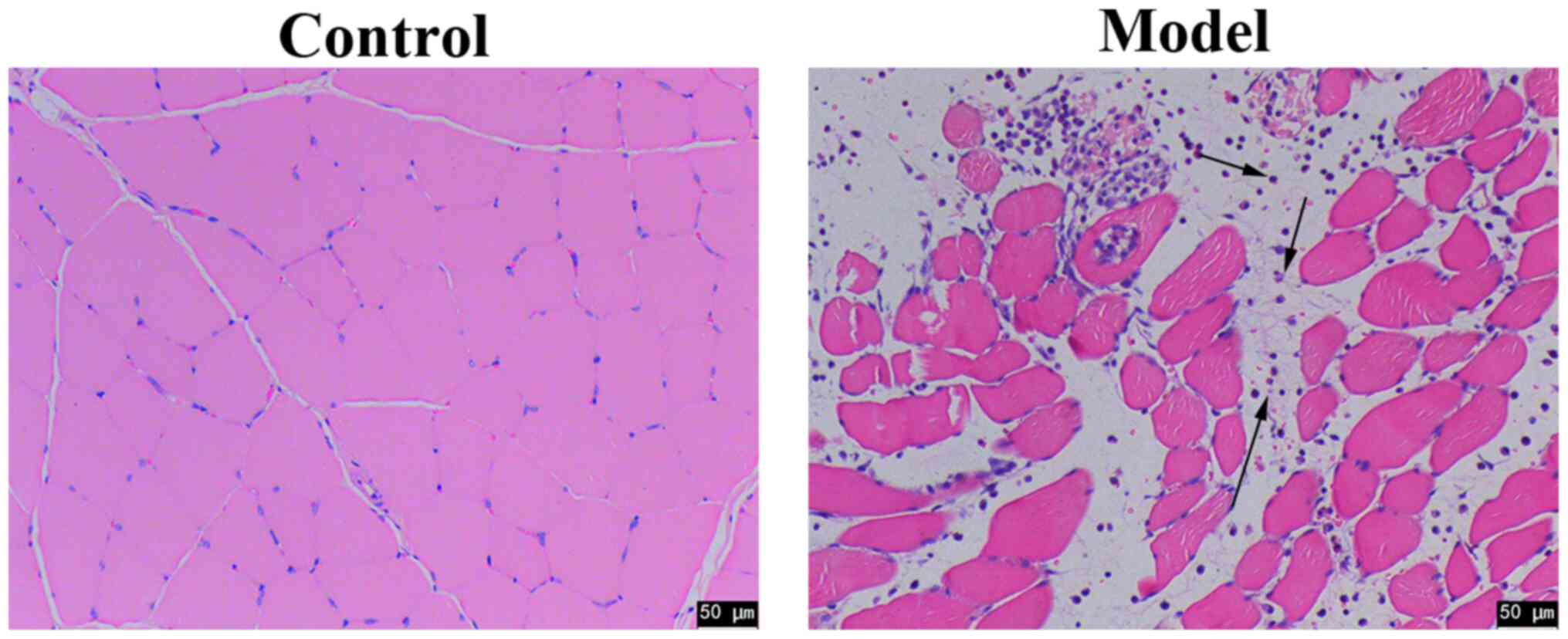
after injection of CTX, HE staining revealed clear inflammatory
cell infiltration in the tissues isolated from rats in the model
group compare with those in the control group (Fig. 1). Since this cell type was present,
this suggests that this skeletal muscle injury model was
successfully constructed.
EA alleviates skeletal muscle
inflammation and fibrosis
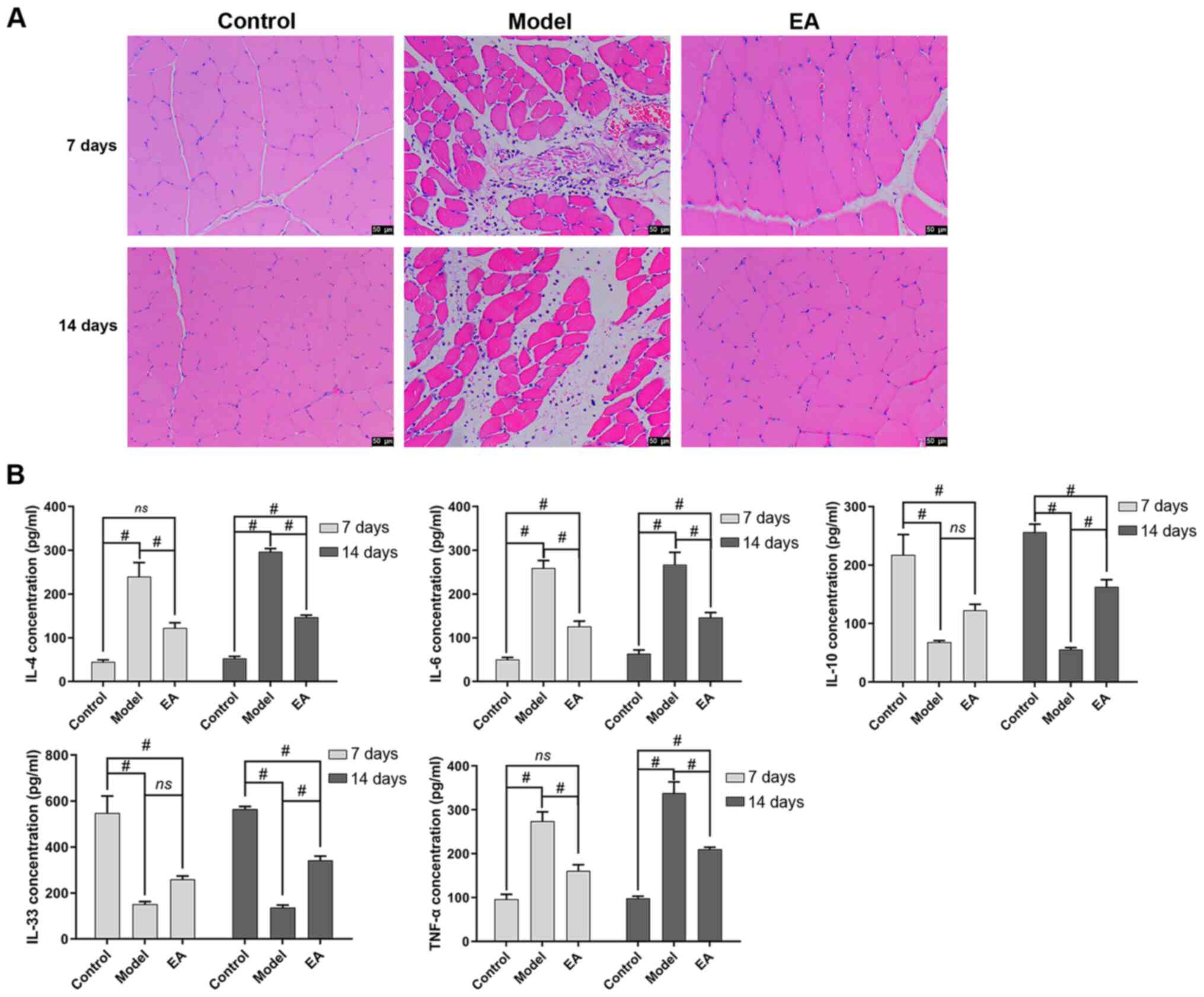
To examine the effect of EA on the inflammatory
response in rats with skeletal muscle injury, the degree of
inflammatory infiltration in skeletal muscle tissues was assessed.
Compared with that in the control group, the model group showed
notable inflammatory infiltration (Fig. 2A). By contrast, after EA treatment,
inflammatory infiltration was alleviated (Fig. 2A). Subsequently, changes in the
levels of inflammation-related factors in the serum were measured
(Fig. 2B). Compared with those in
the control group, the levels of IL-33 and IL-10 were significantly
decreased in the model group, whilst that of IL-6, IL-4 and TNF-α
was significantly increased on both days 7 and 14 (Fig. 2B). Compared with those in the model
group, EA significantly increased the levels of of IL-33 and IL-10
on day 14 whilst significantly reducing those of IL-6, IL-4 and
TNF-α on both days 7 and 14 (Fig.
2B).
EA promotes the transformation of
macrophages from the M1 into the M2
sub-type
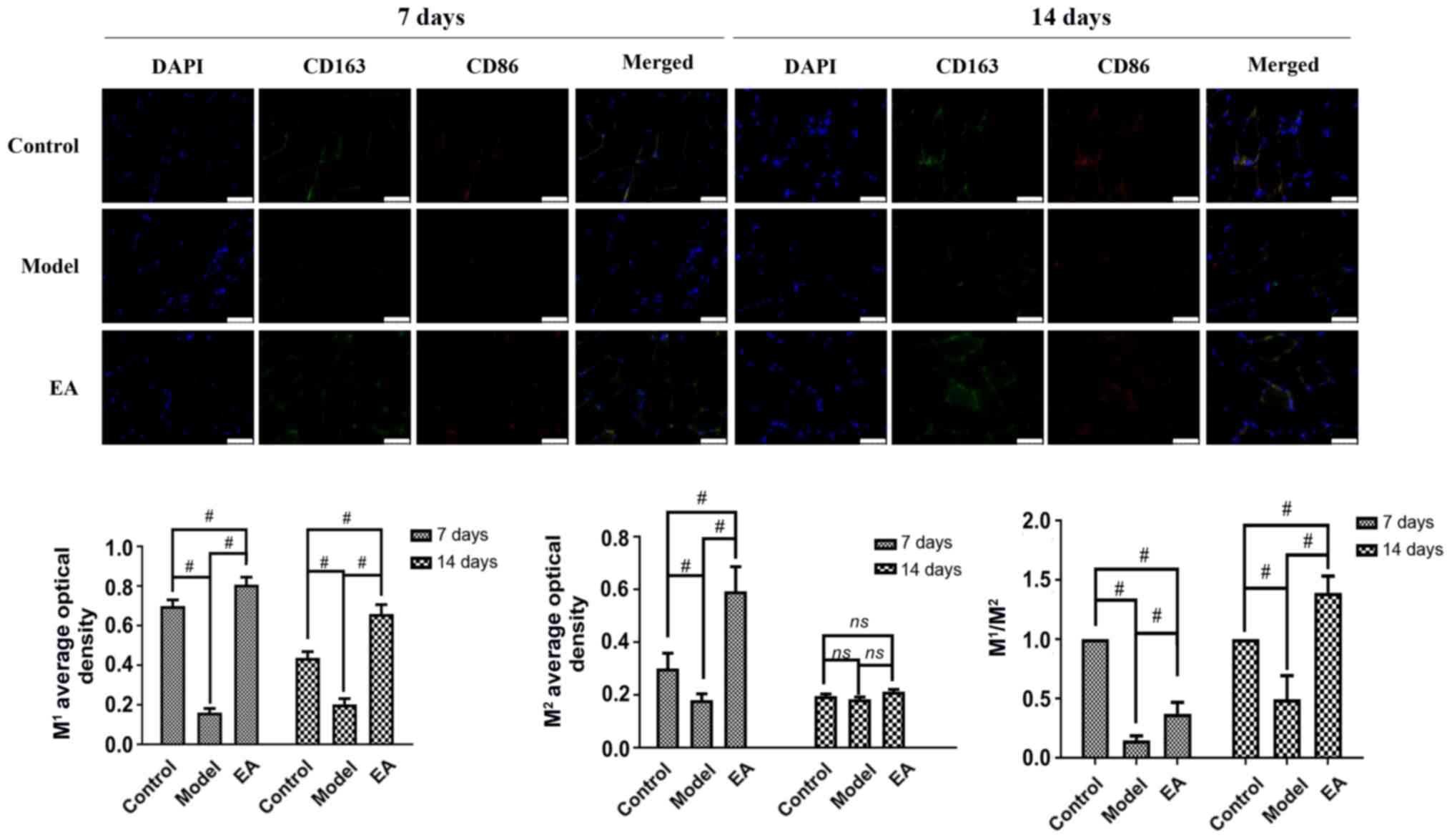
Immunofluorescence was next performed to detect the
expression of surface markers of M1 (CD86) and
M2 (CD163) macrophages in the muscle tissue (Fig. 3). On days 7 and 14, the average
optical density of M1 in the model group was
significantly lower compared with that in the control group and EA
groups (Fig. 3), whereas the trend
of the mean optical density of M2 at day 7 was similar
to that of M1 at day 7 (Fig. 3). In addition, the
M1/M2 ratio was significantly increased by EA
compared with model at both time points (Fig. 3).
EA suppresses collagen deposition and
fibrosis in skeletal muscle
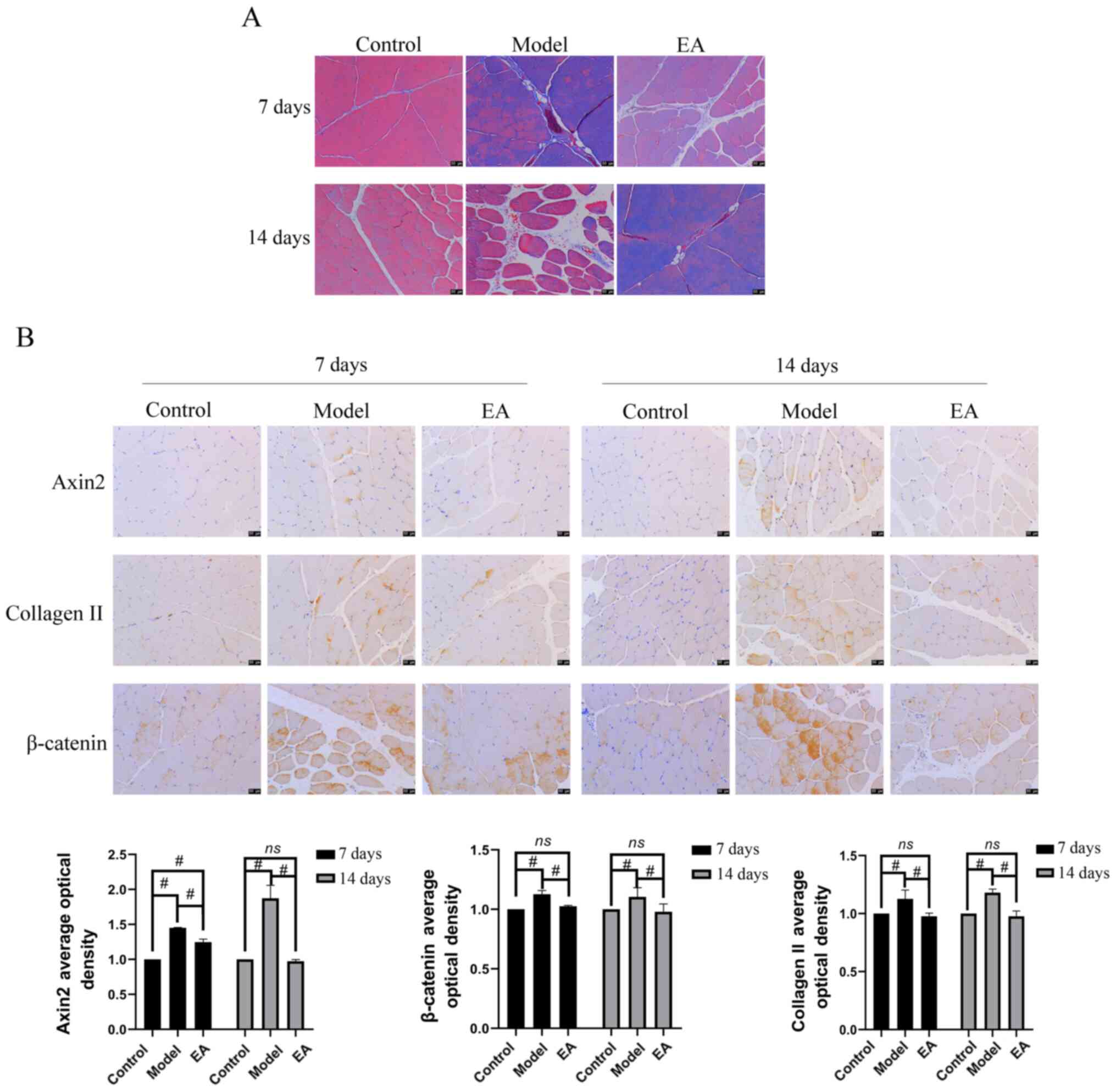
As shown in Fig.
4A, the model group showed greater levels of collagen II
deposition in muscle tissues compared with that in the control and
EA groups, suggesting that EA inhibited collagen deposition in
muscle tissues. Immunohistochemistry was then performed to detect
the expression of muscle fibrosis-related proteins Axin2, collagen
II, β-catenin in muscle tissues (Fig.
4B). Compared with those in the control group, the expression
levels of Axin 2, β-catenin and collagen II were significantly
increased in the model group at both days 7 and 14 (Fig. 4B). However, compared with those in
the model group, the expression levels of these muscle
fibrosis-related proteins were significantly decreased after the EA
intervention both at day 7 and day 14.
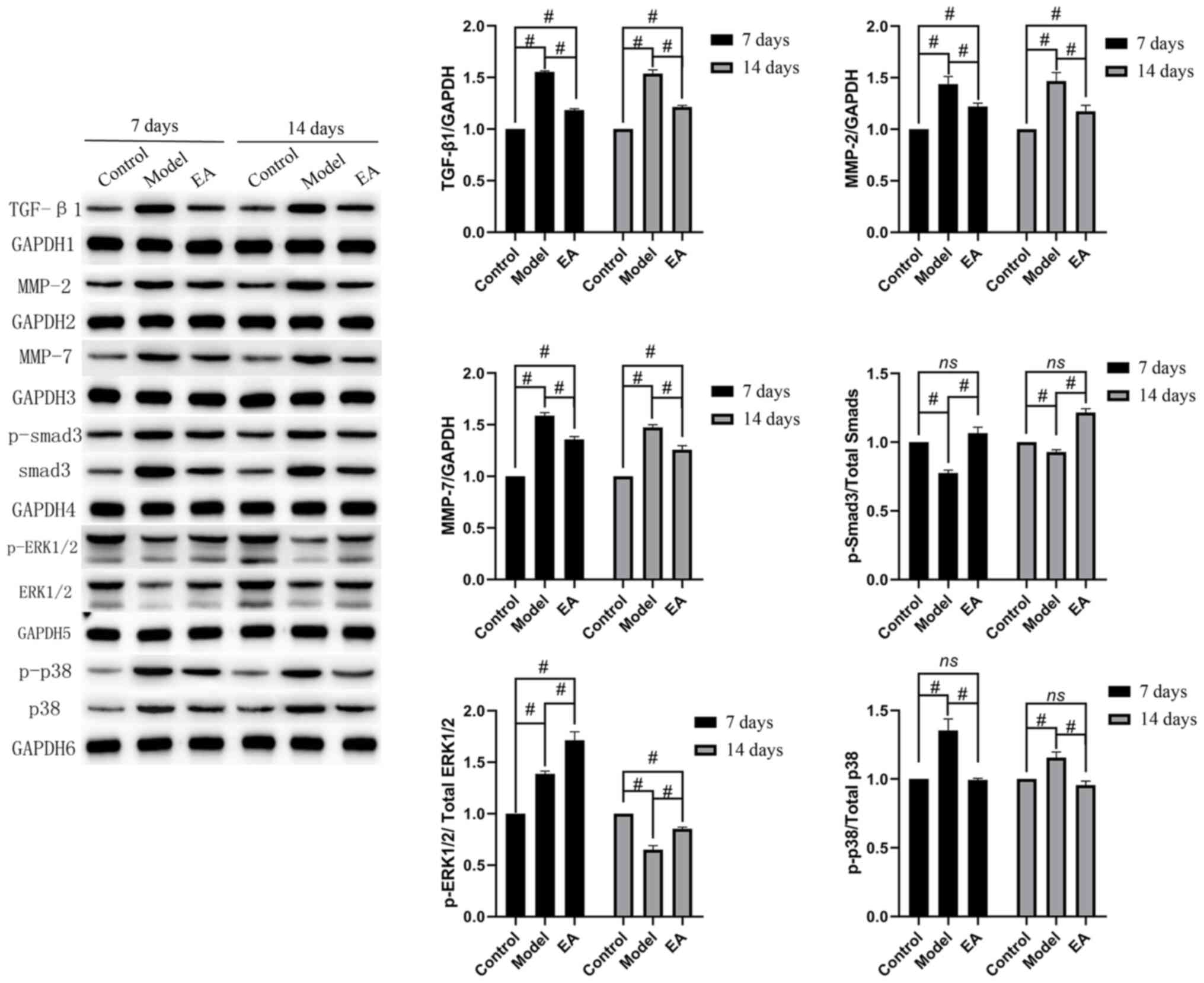
EA reduces skeletal muscle fibrosis
through TGF-β1/Smad3/p38/ERK1/2 signaling
Western blotting was then performed to measure the
protein expression of TGF-β1, MMP-2, MMP-7 and the activation of
Smad3, p38 and ERK1/2 (Fig. 5).
Compared with that in the control, the protein levels of TGF-β1,
MMP-2, MMP-7 and p-p38 were significantly increased in the model at
7 and 14 days. By contrast, p-ERK1/2 levels were significantly
higher in the model compared with those in the control at 7 days,
whilst the opposite trend was observed at 14 days. The levels of
p-Smad3 were decreased at both time points in the model compared
with those in the control. The EA group had higher levels of
p-ERK1/2 and p-Smad3 activation compared with those in the model
group (Fig. 5), whilst the
expression of TGF-β1, MMP-2, MMP-7 and p-p38 activation was
significantly lower. These results remained consistent at day 7 and
14 (Fig. 5). This suggest that EA
reduced skeletal muscle fibrosis through the TGF-β1/Smad3/p38/
ERK1/2 pathway.
Discussion
Acute skeletal muscle injury is common in sports and
is typically caused by blunt trauma or stretch-induced injury
(21). Previous studies have
reported the ability of skeletal muscle fibers to regenerate and
repair after acute skeletal muscle injury. However, muscle cell
death would occur and the ability to regenerate would be lost if a
blunt trauma injury causes the skeletal muscle fibers to break,
which can cause connective tissue proliferation, which in turn
leads to bruise repair and skeletal muscle fibrosis (22,23).
Due to the continuous infiltration by inflammatory cells, myoblasts
can laterally differentiate into myofibroblasts, the excessive
activation and proliferation of which can result in the production
of a large quantities of extracellular matrix (24). This can lead to the continuous
aggregation of collagen fibers, an important feature of skeletal
muscle fibrosis (24). In the
present study, it was revealed that EA significantly reduce
collagen deposition in the skeletal muscle. Skeletal muscle
fibrosis is generally irreversible and can lead to varying degrees
of functional impairment and decreased exercise capacity (23). Therefore, targeting fibrosis has
been a major research focus in the field of sports medicine.
As important cells in the innate immune system,
macrophages exist in different subtypes based on the surrounding
environment, which can in turn exert different roles (25). The most important subtypes are the
M1 and M2 types (26). M1 macrophages are classically
activated and mainly mediate immune functions (26). They can induce inflammatory damage
by secreting proinflammatory mediators, including IL-6, monocyte
chemoattractant protein-1 and TNF-α (25). By contrast, M2
macrophages serve an anti-inflammatory role by secreting
anti-inflammatory cytokines, such as IL-10(14). Acupuncture has been found to
selectively regulate the phagocytic function of macrophages
(27). Under normal physiological
conditions, EA has little effect on the phagocytic function of
macrophages (28). However, under
pathological conditions, such as obesity, it can enhance
phagocytosis, but when phagocytosis becomes excessive, the
phagocytic index is reduced (28).
Zhang et al (29) observed
that in acupuncture-treated mice, the body mass was reduced, blood
lipid levels and proinflammatory mediator release were decreased,
whilst anti-inflammatory mediator release was promoted, iNOS
expression was decreased and M2 marker (CD206)
expression was increased, compared with those in the model group.
This suggests that acupuncture promoted the transformation of
macrophages from M1 to the M2 subtype and
reduced the inflammatory response in the epididymal white fat
tissues (29). Data in the present
study found that EA effectively upregulated expression of the
M1 and M2 markers. In addition, the levels of
IL-33 and IL-10 in the serum were increased by EA but the serum
levels of IL-6, IL-4 and TNF-α were reduced.
MMPs are matrix-degrading enzymes that exert a
variety of effects on the extracellular matrix. In total, 26
members of the MMP family have been identified to date, the
majority of which share similar structures (30-33).
The increased expression of MMPs assists in the formation of new
muscle fibers at the injured site (33). According to its substrates, MMPs
can be divided into the following four categories: Collagenase
(MMP-1, -8, -13 and -18), gelatinase (MMP-2 and -9), interstitial
lysin (MMP-3, -7, -10, -11 and -12), and membrane metalloproteinase
(MMP-14, -15, -16 and -17) (34).
A number of studies have shown that MMP-2 in skeletal muscle
satellite cell migration and differentiation both in cultured
muscle cells in vitro and in animal models in vivo
(35,36), promoting tissue regeneration
further (37). In addition,
numerous studies have indicated that MMP-2 and MMP-7 serve an
important role in myotube formation, such that they can regulate
the degeneration and regeneration of muscle fibers in dystrophic
muscle (37,38). Studies have also shown that MMP-2
participates in the migration of muscle-specific stem cells and
myoblasts (39), where the
elimination of MMP-2 from the skeletal muscle of Mdx-mice led to
reduced angiogenesis and impaired muscle regeneration (40). Zheng et al (41) found that the expression of MMP-2 is
related to CD206, suggesting that inhibiting the expression of
MMP-2 can delay the progression of skeletal muscle fibrosis. In the
present study, the expression of MMP-2 and TGF-β1 decreased
significantly after EA. TGF-β1 inhibits myogenic cell proliferation
in vitro and promotes the lateral differentiation of muscle
cells into myofibroblasts (42).
Macrophages M1 can secrete TGF-β1, and the mRNA
expression of which is increased after skeletal muscle injury
(43). In the absence of
macrophages, fewer new muscle fibers are formed, which then
increases the fibrotic area (34).
Therefore, macrophages may regulate the lateral differentiation of
myoblasts by secreting TGF-β1, inhibiting the proliferation of
muscle cells and participating in the occurrence and development of
skeletal muscle fibrosis. EA may interfere with these processes and
inhibit fibrosis.
TGF-β1 is the core regulatory factor in skeletal
muscle fibrosis, such that the inhibition of TGF-β1 signaling has
been shown to effectively inhibit skeletal muscle fibrosis
(44). The upregulation of
periostin can activate TGF-β signaling, where knocking out the
periostin gene in mice with muscular dystrophy has been shown to
significantly reduce skeletal muscle fibrosis (45). Bedair et al (46) used decorin to interfere with the
function of TGF-β and observed that post-injury, regeneration of
the skeletal muscle was promoted and the formation of fibrous
tissues was reduced. Halofuginone is an antagonist of Smad3
phosphorylation that has been demonstrated to significantly
suppress the expression of collagen and promote myogenesis in the
skeletal muscle of mdx-mice whilst enhancing regeneration and
functional recovery (47,48). In the present study, EA
significantly reduced the activity of TGF-β1 and p38 and
upregulated the expression of ERK1/2 and Smad3, suggesting that EA
may exert therapeutic effects by regulating the activity of the
TGF-β1/Smad3/p38/ERK1/2 signaling axis. However, there are some
limitations in the present study, since no inhibitor of the TGF-β
pathway was added, whether the regulation of this pathway by EA is
influenced by other factors cannot be ruled out. Therefore, in
further studies, this aspect should be considered to make the
mechanistic role of EA in this signaling pathway clearer.
In conclusion, inhibit collagen deposition in
skeletal muscle and alleviate inflammatory responses post-injury.
The effect of EA may be achieved by regulating the
TGF-β1/Smad3/p38/ERK1/2 signaling pathway.
Acknowledgements
Not applicable.
Funding
The present study is supported by the Wuhan Health and Family
Planning Commission Research Project (grant no. WZ18D20).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HH and ML conceptualized and designed the study. HuL
analyzed data. HaL performed the experiments. All authors have read
and approved the final version of the manuscript. HH and ML confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
All experimental procedures in the present study
were performed in accordance with the requirements of the Ethics of
Animal Experiments and approved by the Animal Care and Use
Committee of Wuhan Myhalic Biotechnology Co. Ltd. (approval no.
HLK-20181118-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liao CH, Lin LP, Yu TY, Hsu CC, Pang JS
and Tsai WC: . Ibuprofen inhibited migration of skeletal muscle
cells in association with downregulation of p130cas and CrkII
expressions. Skelet Muscle. 9(23)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Huard J, Li Y and Fu FH: Muscle injuries
and repair: Current trends in research. J Bone Joint Surg Am.
84:822–832. 2002.PubMed/NCBI
|
|
3
|
Baoge L, Van Den Steen E, Rimbaut S,
Philips N, Witvrouw E, Almqvist KF, Vanderstraeten G and Vanden
Bossche LC: Treatment of skeletal muscle injury: A review. ISRN
Orthop. 2012(689012)2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Garg K, Corona BT and Walters TJ:
Therapeutic strategies for preventing skeletal muscle fibrosis
after injury. Front Pharmacol. 6(87)2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
McDermott MM, Dayanidhi S, Kosmac K, Saini
S, Slysz J, Leeuwenburgh C, Hartnell L, Sufit R and Ferucci L: .
Walking Exercise Therapy Effects on Lower Extremity Skeletal Muscle
in Peripheral Artery Disease. Circ Res. 128:1851–1867.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mahdy MAA: Skeletal muscle fibrosis: An
overview. Cell Tissue Res. 375:575–588. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tsai WC, Yu TY, Chang GJ, Lin LP, Lin MS
and Pang JS: Platelet-Rich Plasma Releasate Promotes Regeneration
and Decreases Inflammation and Apoptosis of Injured Skeletal
Muscle. Am J Sports Med Jul. 46:1980–1986. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Border WA and Noble NA: Transforming
growth factor beta in tissue fibrosis. N Engl J Med. 331:1286–1292.
1994.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Delaney K, Kasprzycka P, Ciemerych MA and
Zimowska M: The role of TGF-β1 during skeletal muscle regeneration.
Cell Biol Int. 41:706–715. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fang H and Judd RL: Adiponectin Regulation
and Function. Compr Physiol. 8:1031–1063. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Delos D, Leineweber MJ, Chaudhury S,
Alzoobaee S, Gao Y and Rodeo SA: The effect of platelet-rich plasma
on muscle contusion healing in a rat model. Am J Sports Med.
42:2067–2074. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li H, Hicks JJ, Wang L, Oyster N,
Philippon MJ, Hurwitz S, Hogan MV and Huard J: Customized
platelet-rich plasma with transforming growth factor β1
neutralization antibody to reduce fibrosis in skeletal muscle.
Biomaterials. 87:147–156. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wehling-Henricks M, Jordan MC, Gotoh T,
Grody WW, Roos KP and Tidball JG: Arginine metabolism by
macrophages promotes cardiac and muscle fibrosis in mdx muscular
dystrophy. PLoS One. 5(e10763)2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nozaki M, Ota S, Terada S, Li Y, Uehara K,
Gharaibeh B, Fu FH and Huard J: Timing of the administration of
suramin treatment after muscle injury. Muscle Nerve. 46:70–79.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ifrim Chen F, Antochi AD and Barbilian AG:
Acupuncture and the retrospect of its modern research. Rom J
Morphol Embryol. 60:411–418. 2019.PubMed/NCBI
|
|
16
|
Martins L, Gallo CC, Honda TSB, Alves PT,
Stilhano RS, Rosa DS, Koh TJ and Han SW: Skeletal muscle healing by
M1-like macrophages produced by transient expression of exogenous
GM-CSF. Stem Cell Res Ther. 11(473)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li JP, Xu T and Xu SS: Expression and
significance of TNF-α and MMP-1 in acupuncture to inhibit skeletal
muscle fibrosis. Annual Meeting of Sports Physiology Committee of
Chinese Physiological Society and Academic Seminar on ‘Sports and
Health’, 2013.
|
|
18
|
Chen JDZ, Ni M and Yin J:
Electroacupuncture treatments for gut motility disorders.
Neurogastroenterol Motil. 30(e13393)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Han H and Li M: Clinical observation of
acupuncture combined with exercise therapy for acute lumbar sprain.
Lishizhen Med Mater Med Res. 23:244–245. 2012.
|
|
20
|
Zhang J, Xiao Z, Qu C, Cui W, Wang X and
Du J: CD8 T cells are involved in skeletal muscle regeneration
through facilitating MCP-1 secretion and Gr1(high) macrophage
infiltration. J Immunol. 193:5149–5160. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bayer ML, Magnusson SP and Kjaer M: Tendon
Research Group Bispebjerg. Early versus Delayed Rehabilitation
after Acute Muscle Injury. N Engl J Med. 377:1300–1301.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tidball JG: Mechanisms of muscle injury,
repair, and regeneration. Compr Physiol. 1:2029–2062.
2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Smith RS and Chang FC: Traumatic rupture
of the aorta: Still a lethal injury. Am J Surg. 152:660–663.
1986.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Deng XY, Wu ZB and Yang Z: Fibrosis in
skeletal muscle:celluar and molecular mechanism. Chin J RHP.
20:142–147. 2014.
|
|
25
|
DeNardo DG and Ruffell B: Macrophages as
regulators of tumour immunity and immunotherapy. Nat Rev Immunol
Jun. 19:369–382. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xia Y, Rao L, Yao H, Wang Z, Ning P and
Chen X: Engineering Macrophages for Cancer Immunotherapy and Drug
Delivery. Adv Mater. 32(e2002054)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang J, Xiao Z, Qu C, Cui W, Wang X and
Du J: CD8 T cells are involved in skeletal muscle regeneration
through facilitating MCP-1 secretion and Gr1(high) macrophage
infiltration. J Immunol. 193:5149–5160. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhou SN, Xu YY and Hu HT: Research
progress of acupuncture therapy on immune cells. Zhejiang JTradit
Chin Med. 55:74–75. 2020.
|
|
29
|
Zhang SY, Hu X and Tang H: Effect of
acupuncture on white adipose tissue macrophage polarization induced
by high fat diet in obese mice. Chin Acupunc. 37:1205–1211.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ding J, Chang YY, Li WJ, Guo B, Lu M and
Collage XM: The effects of DAPT intervention on the expression of
α-SMA and MMP-1/TIMP-1 in rats cardiac fibroblasts. J Clin Cardiol.
32:735–738. 2016.
|
|
31
|
Wang SQ, Chang Y, Ma XW and Wang F:
Effects of endurence exercise of different intensity on cardiac
collagen of rats and regulation of MMP-1/TIMP-1. Chi Sport Sci
Technol. 51:60–66. 2015.
|
|
32
|
Wang QY, Wang WJ, Xi J, Cai K, Wang P,
Liang JD, Han J and He GZ: Effect of polysaccharides from eucommiae
cortex on expressions of genes of I, III collagen, MMP-1, TIM MP-1
and TGF-β1 from hepatic fibrosis in rat models. Chin J Exp Trad Med
Formu. 24:153–158. 2018.
|
|
33
|
Spinale FG: Myocardial matrix remodeling
and the matrix metalloproteinases: Influence on cardiac form and
function. Physiol Rev. 87:1285–1342. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xiao W, Liu Y and Chen P: Macrophage
depletion impairs skeletal muscle regeneration: The roles of
pro-fibrotic factors, inflammation, and oxidative stress.
Inflammation. 39:2016–2028. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gihring A, Gärtner F, Liu C, Hoenicka M,
Wabitsch M, Knippschild U and Xu P: Influence of Obesity on the
Organization of the Extracellular Matrix and Satellite Cell
Functions After Combined Muscle and Thorax Trauma in C57BL/6J Mice.
Front Physiol. 11(849)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dong G, Wang M, Gu G, Li S, Sun X, Li Z,
Cai H and Zhu Z: MACC1 and HGF are associated with survival in
patients with gastric cancer. Oncol Lett. 15:3207–3213.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nalbandian M, Radak Z and Takeda M:
Lactate Metabolism and Satellite Cell Fate. Front Physiol.
11(610983)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chen X and Li Y: Role of matrix
metalloproteinases in skeletal muscle: Migration, differentiation,
regeneration and fibrosis. Cell Adhes Migr. 3:337–341.
2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zheng LF, Chen LF, Chen PJ and Xiao WH:
Fat deposition in skeletal muscle and its regulatory mechanism.
Acta Physiologica Sinicaa. 69:344–350. 2017.PubMed/NCBI(in Chinese).
|
|
40
|
Miyazaki D, Nakamura A, Fukushima K,
Yoshida K, Takeda S and Ikeda S: Matrix metalloproteinase-2
ablation in dystrophin-deficient mdx muscles reduces angiogenesis
resulting in impaired growth of regenerated muscle fibers. Hum Mol
Genet. 20:1787–1799. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zheng LF: Study on the role and mechanism
of macrophages in the repair of skeletal muscle contusion. Shanghai
university of sport, 2018 (in Chinese).
|
|
42
|
Carlson ME, Conboy MJ, Hsu M, Barchas L,
Jeong J, Agrawal A, Mikels AJ, Agrawal S, Schaffer DV and Conboy
IM: Relative roles of TGF-beta1 and Wnt in the systemic regulation
and aging of satellite cell responses. Aging Cell. 8:676–689.
2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Fjeldborg K, Pedersen SB, Møller HJ,
Christiansen T, Bennetzen M and Richelsen B: Human adipose tissue
macrophages are enhanced but changed to an anti-inflammatory
profile in obesity. J Immunol Res. 2014(309548)2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Rana T, Jiang C, Liu G, Miyata T, Antony
V, Thannickal VJ and Liu RM: PAI-1 Regulation of TGF-β1-induced
Alveolar Type II Cell Senescence, SASP Secretion, and SASP-mediated
Activation of Alveolar Macrophages. Am J Respir Cell Mol Biol.
62:319–330. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lorts A, Schwanekamp JA, Baudino TA,
McNally EM and Molkentin JD: Deletion of periostin reduces muscular
dystrophy and fibrosis in mice by modulating the transforming
growth factor-β pathway. Proc Natl Acad Sci USA. 109:10978–10983.
2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bedair HS, Karthikeyan T, Quintero A, Li Y
and Huard J: Angiotensin II receptor blockade administered after
injury improves muscle regeneration and decreases fibrosis in
normal skeletal muscle. Am J Sports Med. 36:1548–1554.
2008.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Roffe S, Hagai Y, Pines M and Halevy O:
Halofuginone inhibits Smad3 phosphorylation via the PI3K/Akt and
MAPK/ERK pathways in muscle cells: Effect on myotube fusion. Exp
Cell Res. 316:1061–1069. 2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Huebner KD, Jassal DS, Halevy O, Pines M
and Anderson JE: Functional resolution of fibrosis in mdx mouse
dystrophic heart and skeletal muscle by halofuginone. Am J Physiol
Heart Circ Physiol. 294:H1550–H1561. 2008.PubMed/NCBI View Article : Google Scholar
|