Introduction
Vascular smooth muscle cells (VSMCs) and endothelial
cells (ECs) are the main cell types that constitute the blood
vessels, with their function being to control blood vessel pressure
and tension (1). VSMCs maintain
homeostasis in a contractile form with little proliferation under
physiological conditions (2,3).
However, when ECs are damaged, inflammatory factors secreted in
response to pathological conditions cause abnormal proliferation of
VSMCs, initiating their transformation to the synthetic phenotype
due to internal and external changes (3-5).
This abnormal proliferation of VSMCs is known to cause
arteriosclerosis and restenosis (6,7).
Classical surgical therapies for treating these vascular diseases,
such as coronary artery bypass grafting or stent-grafted
angioplasty, are effective for a short period of time. However,
these surgical treatments can cause blood flow disorders due to the
abnormal proliferation of VSMCs over time (8). To overcome this problem, a
drug-coated stent has been developed to treat vascular diseases
more effectively (9). However, the
potential for restenosis due to VSMC proliferation remains a major
long-term threat (8). Therefore,
the development of novel therapeutic agents for the treatment of
vascular diseases is still required.
It has been reported that natural compounds have a
positive effect on cardiovascular diseases and improve some
vascular functions (9). One of the
promising novel treatments of vascular diseases in various natural
products is essential oils (EOs). EOs and their constituents are
promising as therapeutic agents as they have been demonstrated to
functionally improve cardiovascular disease (10,11).
Lime (Citrus aurantifolia) is widely
cultivated worldwide and is an excellent source of vitamins,
particularly vitamin C. Lime EO has previously been used as a
fragrance, an antimicrobial agent and for aromatherapy (12,13).
Lime EO contains limonene, β-pinene, γ-terpinene, citral and
linarul, among other compounds. Lime EO is a volatile complex
mixture and has been used as a means to prevent, improve and treat
diseases (14). Furthermore, the
potential for the use of lime EO as chemotherapy in the prevention
and treatment of inflammatory diseases (15,16),
cancer (17,18) and oxidative stress (19,20),
has been reported in various studies. Regarding the applications in
cardiovascular disease, it has been demonstrated that daily intake
of lemon (Citrus limon), a Citrus species that has a similar
nutritional value to lime, lowers systolic blood pressure (21).
To the best of our knowledge, the effect of
cold-pressed oil (CpO) from limes on VSMC pathological changes is
unclear. It was hypothesized that lime CpO could inhibit the
proliferation of VSMC through known vascular signaling pathways
(22,23) in cardiovascular disease. The
present study investigated whether lime CpO could modulate the MAPK
and PI3K signaling pathways to reduce abnormal cell proliferation
induced by FBS.
Materials and methods
Lime CpO
Cold pressed lime oil was purchased from Sydney
Essential Oil Company. Coupled gas chromatography-mass spectrometry
(GC-MS) analysis was performed using a Thermo Scientific Model ISQ
LT (Thermo Fisher Scientific, Inc.) equipped with both a flame
ionization detector (FID) and a mass spectrometer.
GC-MS analysis
A Durabond-5MS capillary column (60 m x 0.25 mm x
0.25 µm film thickness; Agilent Technologies, Inc.) was used. The
carrier gas, helium, had a constant flow rate of 1 ml/min. The
injection temperature was 250˚C and 1 µl of the sample was injected
with a split ratio of 1:20. The oven temperature was maintained at
50˚C for 5 min, then increased by 10˚C/min to 65˚C and held for 30
min, increased by 5˚C/min to 120˚C and held for 15 min, increased
by 1˚C/min to 140˚C and held for 11 min, increased by 10˚C/min to
250˚C and held for 5 min, and finally increased by 20˚C/min to
325˚C and held for 6 min. For the FID, the temperature was set to
300˚C, air flow was set to 350 ml/min, hydrogen flow was set to 35
ml/min and the make-up gas (helium) flow was set to 40 ml/min. The
mass interface temperature was 250˚C and the ion source temperature
was 250˚C. Mass scan data were acquired in electron ionization (EI)
mode at a 0.2 sec scan time rate with a scan range of 35-550 amu.
The identification of peaks was performed by comparing the peak
average mass spectrum of the peak with an electronic library
database (National Institute of Standards and Technology,
Environmental Protection Agency and National Institutes of Health
Mass Spectral Library; version 2.0 g: https://chemdata.nist.gov/). The identity of the
compounds was assigned by comparison of the Kovats retention index,
determined in relation to a homologous series of n-alkanes
(C7-C30).
Animal care
All animal experiments were approved by the
Institutional Animal Care and Use Committee of Catholic Kwandong
University, International St. Mary's Hospital (Incheon, South
Korea; approval no. CKU 01-2019-008) in cooperation with the
Association for Assessment and Accreditation of Laboratory Animal
Care and performed in accordance with the Guidelines and
Regulations for Animal Care (24).
Rats were housed in a room with a stable temperature of 22.5±1.5˚C,
50-60% humidity and 12 h light-dark cycles with ad libitum
access to food and water. A total of 16 rats were divided into four
groups: control (non-treated), lime CpO, FBS (FBS only treated),
and FBS and lime CpO. Rats were intramuscularly anesthetized with
Zoletil™50 (tiletamine:zolazepam = 1:1; 20 mg/kg; Virbac) and
Rompun 2% (xylazine; 5 mg/kg; Bayer AG), and euthanized via an
intraperitoneal overdose of sodium pentobarbital (100-200 mg/kg).
Euthanasia was confirmed with lack of heartbeat.
Isolation and culture of rat aortic
VSMCs
Rat aortic VSMCs were isolated as previously
described (25,26). Thoracic aortas from male
Sprague-Dawley rats (Orient Bio; n=3; weight, 200-250 g; age, 6-8
weeks) were removed and transferred to incubate in serum-free DMEM
(Invitrogen; Thermo Fisher Scientific, Inc.) containing 100 U/ml
penicillin and 100 µg/ml streptomycin. The aortas were separated
from the connective tissue and transferred to a petri dish
containing 5 ml of an enzyme dissociation mixture, composed of DMEM
with 1 mg/ml collagenase type I (Sigma-Aldrich; Merck KGaA) and 0.5
µg/ml elastase (Thermo Fisher Scientific, Inc.), and incubated for
30 min at 37˚C. The aortas were then transferred to DMEM and the
adventitia was stripped off each aorta with forceps under an
optical microscope. Subsequently, the aortas were transferred to a
conical tube containing 5 ml of enzyme dissociation mixture
containing DMEM with 1 mg/ml of collagenase type I (Sigma-Aldrich;
Merck KGaA) and 0.5 µg/ml of elastase (Thermo Fisher Scientific,
Inc.) and incubated for 2 h at 37˚C. The suspension was centrifuged
at 320 x g for 10 min at room temperature and the pellet was
re-suspended in DMEM with 10% FBS (Atlas Biologicals, Inc) under
room temperature. Rat aortic VSMCs were cultured in DMEM
supplemented with 10% FBS, 1% penicillin-streptomycin in
75-cm2 flasks in a 37˚C incubator with 5% CO2
(Thermo Fisher Scientific, Inc.). Isolated cells from 3 different
animals in passages 5-8 were used in the present study.
CpO treatment
The concentration of CpO on VSMCs culture was
determined as 10-5 dilution after Cell Counting Kit-8 (CCK-8)
assay. For treatment with CpO, VSMCs were seeded into 96-well
plates at a density of 5x103 cells/well. VSMCs were
serum-starved in DMEM containing 0.5% FBS for 24 h and treated with
or without 5% FBS in DMEM for the following 24 h to detect the
effects of lime CpO under in vitro and ex vivo
conditions in a 37˚C incubator. In order to check the cell signal
transduction, MEK1/2 inhibitor U0126 (10 µM; Cell Signaling
Technology, Inc.) and mTOR inhibitor Rapamycin (20 nM; Calbiochem;
Merck KGaA) were treated with 5% FBS for 24 h.
Cell proliferation assay
A CCK-8 (DoGenBio Co., Ltd.) assay was used
according to the manufacturer's protocol. To each well, 10% (v/v)
CCK-8 reagent was added and incubated at 37˚C for 2 h to allow for
the formation of water-soluble tetrazolium salts (WST)-8 formazan.
The absorbance at 450 nm was measured using a microplate reader
(Thermo Fisher Scientific, Inc.).
Cytotoxicity assay
The Cytotoxicity Lactate Dehydrogenase (LDH) Assay
Kit-WST (cat. no. MK401; Takara Bio, Inc.) was used according to
the manufacturer's protocol. Cell suspension and 50 µl DMEM were
sequentially added into a 96-well culture plate (7x103
cells/well) and samples were incubated at 37˚C for 24 h. Samples
were mixed with 100 µl working solution at room temperature for 30
min in the dark. The absorbance at 490 nm was measured using a
microplate reader (Thermo Fisher Scientific, Inc.).
Western blotting
VSMCs were washed once with PBS and lysed using RIPA
lysis buffer (Thermo Fisher Scientific, Inc.). Total protein
concentration was quantified using an albumin standard (bioPLUS™;
BioWORLD). Proteins were then separated by 10% SDS-PAGE by 15-20
mg/ml of protein loading per lane and transferred to a PVDF
membrane (MilliporeSigma). After blocking of the membrane with TBS
with 0.1% Tween-20 (TBS-T; BioPLUS Chemicals) and 5% (w/v) BSA
(bioPLUS™; BioWORLD) in 0.1% TBS-T for 1 h at room temperature, the
membrane was washed twice with TBS-T and incubated with the primary
antibodies diluted in blocking buffer a ratio of 1:1,000 to 1:2,000
overnight at 4˚C. The membrane was washed three times with TBS-T
for 5 min/wash and incubated for 1 h at room temperature with
horseradish peroxidase-conjugated secondary antibodies diluted in
blocking buffer a ratio of 1:4,000. The membrane was subsequently
washed six times with TBS-T for 5 min/wash and bands were detected
with an enhanced chemiluminescence reagent (Abclon, Inc.). Band
intensities were semi-quantified using a Davinch-Western Imaging
System (Davinch-K Co., Ltd.) and ImageJ version 1.44p software
(National Institutes of Health). The following antibodies were used
in these experiments: anti-PI3K (cat. no. sc-7189; Santa Cruz
Biotechnology, Inc.), anti-phosphorylated (p)-PI3K (cat. no. 4228S;
Cell Signaling Technology, Inc.), anti-AKT (cat. no. 9272S; Cell
Signaling Technology, Inc.), anti-p-AKT (cat. no. 9271S; Cell
Signaling Technology, Inc.), anti-mTOR (cat. no. 2972S; Cell
Signaling Technology, Inc.), anti-p-mTOR (cat. no. 2971S; Cell
Signaling Technology, Inc.), anti-MEK (cat. no. 9122; Cell
Signaling Technology, Inc.), anti-p-MEK (cat. no. 9121S; Cell
Signaling Technology, Inc.), anti-ERK (cat. no. 9102S; Cell
Signaling Technology, Inc.), anti-p-ERK (cat. no. sc-7383; Santa
Cruz Biotechnology, Inc.), anti-cyclin D1 (cat. no. 29785; Cell
Signaling Technology, Inc.), anti-proliferating cell nuclear
antigen (PCNA; cat. no. sc-56; Santa Cruz Biotechnology, Inc.),
anti-β-actin (cat. no. ab8227-50; Abcam), anti-caspase-3 (cat. no.
ab13847; Abcam) and anti-cleaved caspase-3 (cat. no. ab49822;
Abcam). Secondary antibodies were used in these experiments as
follows: Goat anti-mouse IgG (H+L)-HRP (cat. no. SA001-500) and
goat anti-rabbit IgG (H+L)-HRP (cat. no. SA002-500) from GenDEPOT,
LLC. The amount of phosphorylation was calculated by dividing the
amount by the total expression amount. This was clarified using
magnification of phosphorylation/expression ratio.
Immunocytochemistry
VSMCs were seeded into four-well plastic cell
culture dishes (1x105 cells/well) and were treated with
CpO for 24 h in a 37˚C incubator. Subsequently, each well was
washed with PBS. Cells were then fixed with 4% paraformaldehyde
diluted in PBS for 10 min at room temperature, after which they
were washed twice with PBS and permeabilized for 10 min at room
temperature with 0.2% Triton X-100 diluted in PBS. After washing
with PBS, the cells were blocked in blocking solution (2% BSA and
10% horse serum (Vector Laboratories, Inc.; Maravai LifeSciences)
in PBS) for 30 min at room temperature and stained for Ki-67 (Dako;
cat. no. M7240; 1:200 dilution) for 1 h at 37˚C. The cells were
incubated with a FITC-conjugated anti-mouse (cat. no. 115-095-003;
1:500 dilution; Jackson ImmunoResearch Laboratories, Inc.)
secondary antibody at room temperature for 1 h and then stained
with a DAPI solution (0.1 µl/ml; Thermo Fisher Scientific, Inc.)
for 5 min. Immunofluorescence was detected via confocal microscopy
(LSM700; Zeiss GmbH) and analyzed using ZEN 2.5 Blue Edition
software (Zeiss GmbH) 1.0 for analysis.
Ex vivo aortic ring assay
Ex vivo sprouting of VSMCs was measured via
an aortic ring assay using Matrigel (BD Biosciences). The thoracic
aortas from the aforementioned 8-week-old Sprague-Dawley rats were
removed and transferred into serum-free DMEM. The endothelial
lining was removed using a 2-Fr Fogarty balloon catheter (Baxter
Healthcare) to minimize the possibility of EC sprouting during the
ring assay. The aorta was washed by gradually passing PBS through
the aorta three times. After removing perivascular adipose tissue,
the aorta was cut into 1-mm-thick segments of aortic ring and
placed in Matrigel. The aortic rings were washed with serum-free
DMEM twice and starved in DMEM supplemented with 0.5% FBS for 24 h
at 37˚C. For the lime CpO group, the medium was changed to DMEM
supplemented with 5% FBS containing 1x10-5 diluted lime
CpO. The cells were cultured at 37˚C and the media were changed
every 3 days and the aortic rings were monitored daily for up to 7
days for sprouting VSMCs. On the 7th day the results were
analyzed.
Statistical analysis
Data are presented as the mean ± SEM of at least
three independent experiments. For statistical analysis, one-way
analysis of variance with Bonferroni's correction was performed for
comparisons among more than two groups. All analyses were performed
using Prism software (version 5.0; GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Suppression of VSMC proliferation by
lime CpO treatment
To evaluate the effect of lime CpO on VSMC
proliferation, cells were treated with lime CpO for 24 h. In the
group treated with FBS only, the cell proliferation rate was
significantly increased by ~30% compared with that of the
FBS-negative and lime CpO-negative control groups. Concentration of
10-5 lime CpO was statistically similar to the control, however
concentration of 10-4 and 10-3 lime CpO was
significantly lower (Fig. 1A).
Based on these results, lime CpO significantly inhibited
FBS-stimulated VSMC proliferation at low concentrations
(10-5). The effect of lime CpO on cell death was
assessed via LDH analysis and it was demonstrated to have no
statistically significant effect on cell death compared with the
FBS-treated only group (Fig. 1B).
Furthermore, caspase-3 and cleaved caspase-3 protein levels were
also not affected by lime CpO treatment compared with the
FBS-treated or FBS-negative and lime CpO-negative control groups
(Fig. 1C). Therefore, the results
suggested that lime CpO may inhibit FBS-induced proliferation of
VSMCs but does not cause cell death.
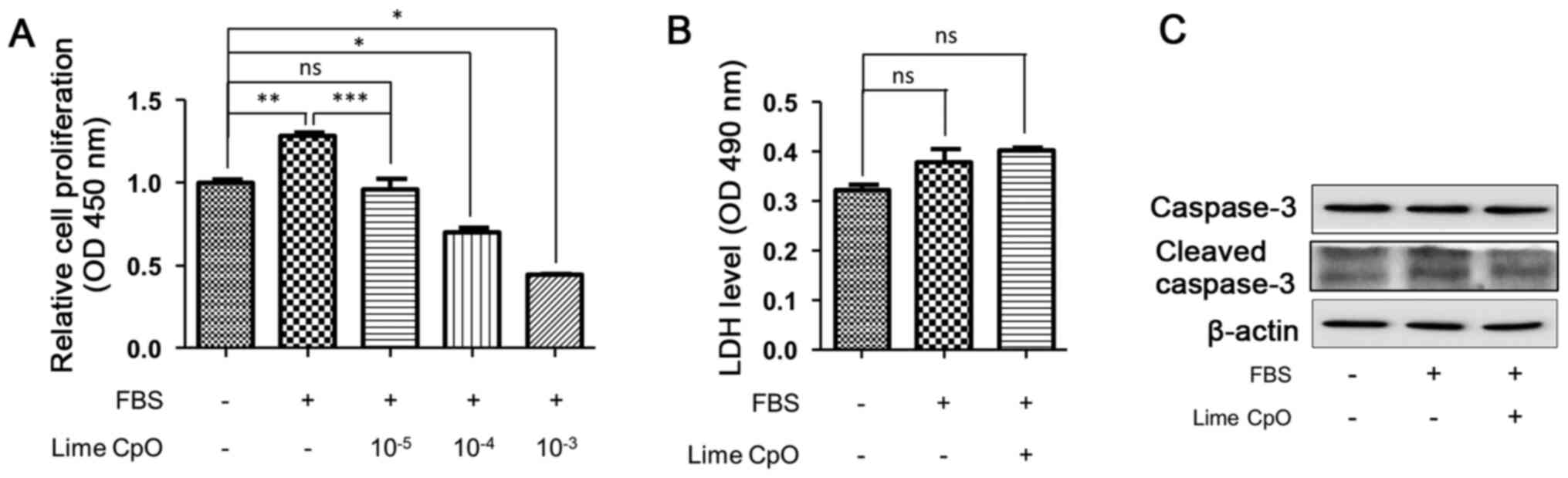 | Figure 1Effects on VSMC proliferation treated
with lime CpO. To examine the effect of lime CpO on VSMC
proliferation, concentration of 10-3 to 10-5
of lime CpO was added to 5% FBS-containing DMEM and VSMCs, and
cells were cultured for 24 h. (A) Cell proliferation was determined
using a CCK-8 assay. (B) To measure cytotoxicity the LDH assay was
performed. (C) Following lime CpO treatment, the protein expression
levels of caspase-3 and cleaved caspase-3 were analyzed via western
blotting. Data are presented as the mean ± SD.
*P<0.05, **P<0.01 and
***P<0.001. CCK-8, Cell Counting Kit-8; CpO,
cold-pressed oil; LDH, lactate dehydrogenase; ns, not significant;
OD, optical density; VSMC, vascular smooth muscle cell. |
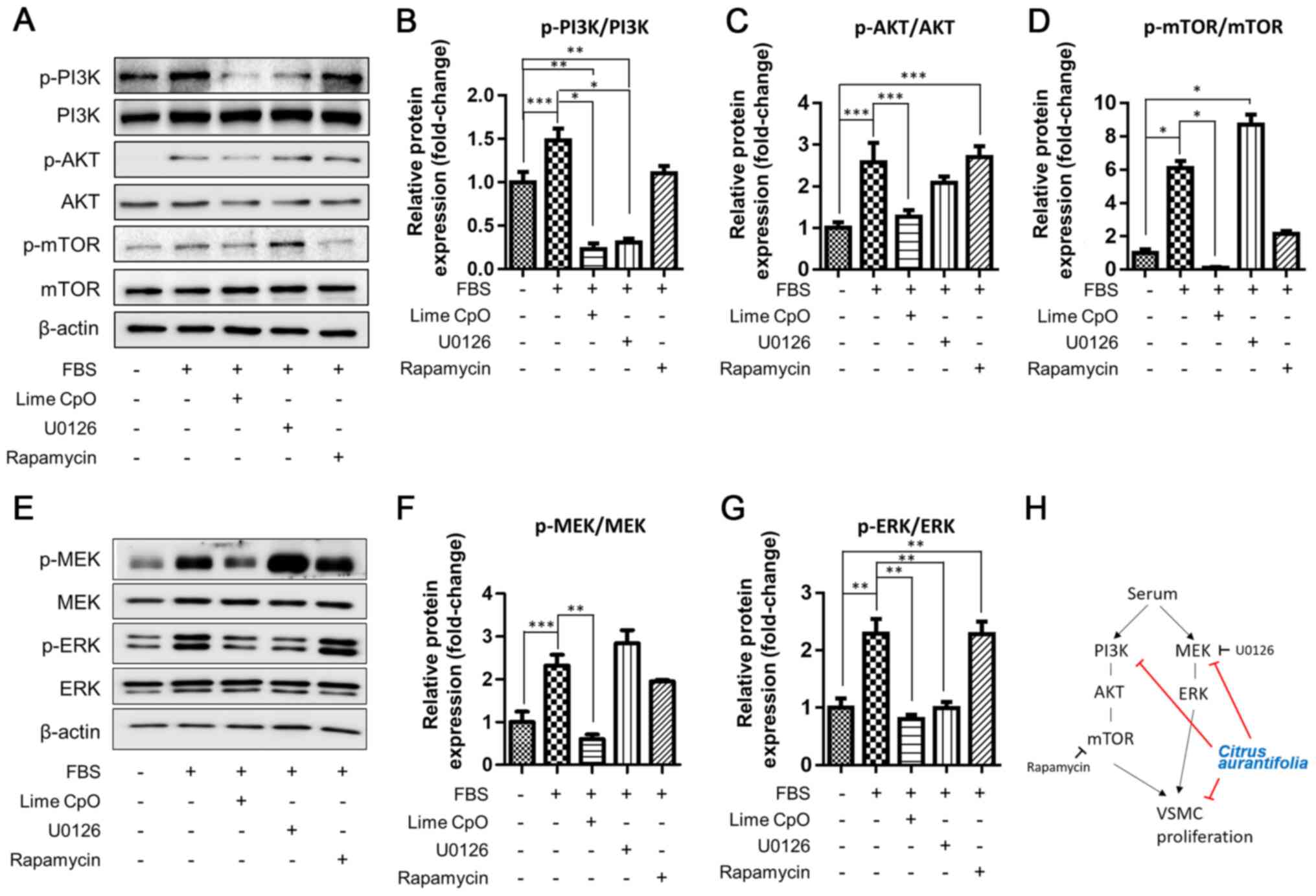
Changes in AKT and ERK signaling
cascades in VSMC proliferation
The mechanism by which lime CpO regulated
proliferation was investigated further. Since phosphorylation of
the PI3K/AKT/mTOR signaling cascade is known to regulate VSMC
proliferation (27), the
phosphorylation of these signaling cascades was analyzed.
Phosphorylation of the PI3K/AKT/mTOR (Fig. 2A-D) and MEK/ERK signaling pathways
(Fig. 2E-G) was significantly
increased by 1.5-6.2-fold in FBS-treated cells compared with the
FBS-negative and lime CpO-negative control group. However, the
signal intensity of each phosphorylated protein band was
significantly reduced in the lime CpO-treated group compared with
the FBS-treated cells or the U0126 or Rapamycin-treated cells.
These results demonstrated that lime CpO inhibited phosphorylation
in a manner similar to that of the MEK and mTOR inhibitors in VSMCs
(Fig. 2H).
Regulation of cell cycle regulators in
VSMCs
It was subsequently demonstrated that the cell
cycle-regulating factors cyclin D1 and PCNA were essential for
regulating proliferation in lime CpO-treated cells. The protein
expression levels of cyclin D1 and PCNA were significantly
increased in FBS-treated cells compared with FBS-negative and lime
CpO-negative control cells; however, the lime CpO-treated cells
displayed significantly reduced protein expression levels compared
with the FBS-treated cells (Fig.
3A-C). Ki-67 was observed in the nucleus during cell division,
indicating cell proliferation (Fig.
3D-E). The number of Ki-67-positive cells was significantly
increased by ~35% in the FBS-treated group compared with the
FBS-negative and lime CpO-negative control group. However, the
number of Ki-67-positive cells was significantly reduced in the
lime CpO-treated group compared with the FBS-treated group
(Fig. 3D and E). This result suggested that lime CpO
had the potential to suppress the cell cycle of VSMCs and regulate
VSMC proliferation.
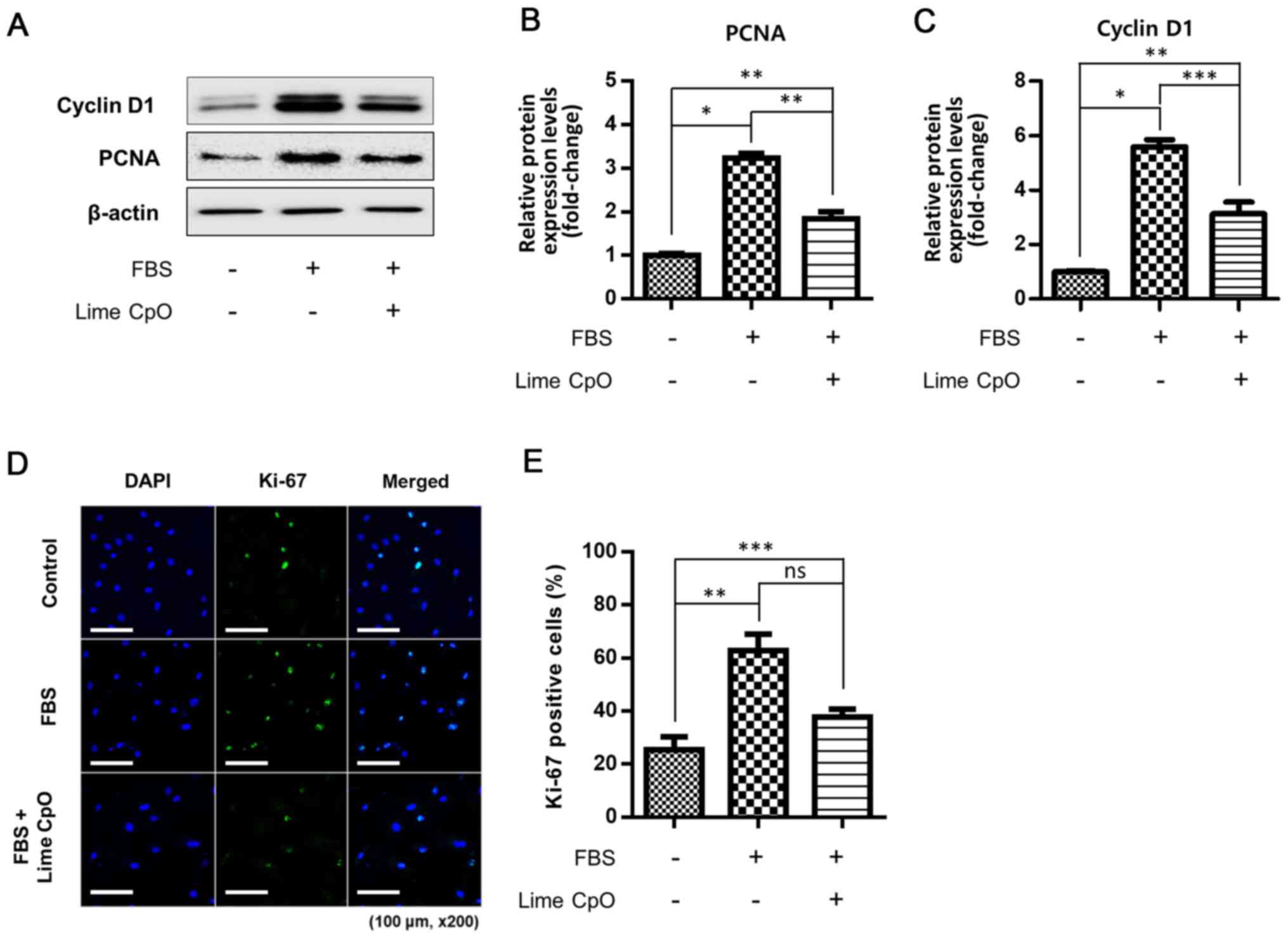 | Figure 3Regulation of the cell cycle in VSMCs
by treating lime CpO. (A) Effects of lime CpO on cell cycle-related
proteins (cyclin D1 and PCNA) were analyzed using western blotting.
Protein expression levels of (B) PCNA and (C) cyclin D1 were
semi-quantified using ImageJ. (D) Following lime CpO treatment,
Ki-67 was immunostained. Green, Ki-67; blue, DAPI. Scale bar, 100
µm. (E) Percentages of Ki-67-positive VSMCs were quantified by
counting the number of Ki-67 (green) labeled cells divided by DAPI
(blue) labeled cells (five individual fields/group).
*P<0.001, **P<0.01 and
***P<0.05. CpO, cold-pressed oil; Ctl, control; ns,
not significant; PCNA, proliferating cell nuclear antigen; VSMC,
vascular smooth muscle cell. |
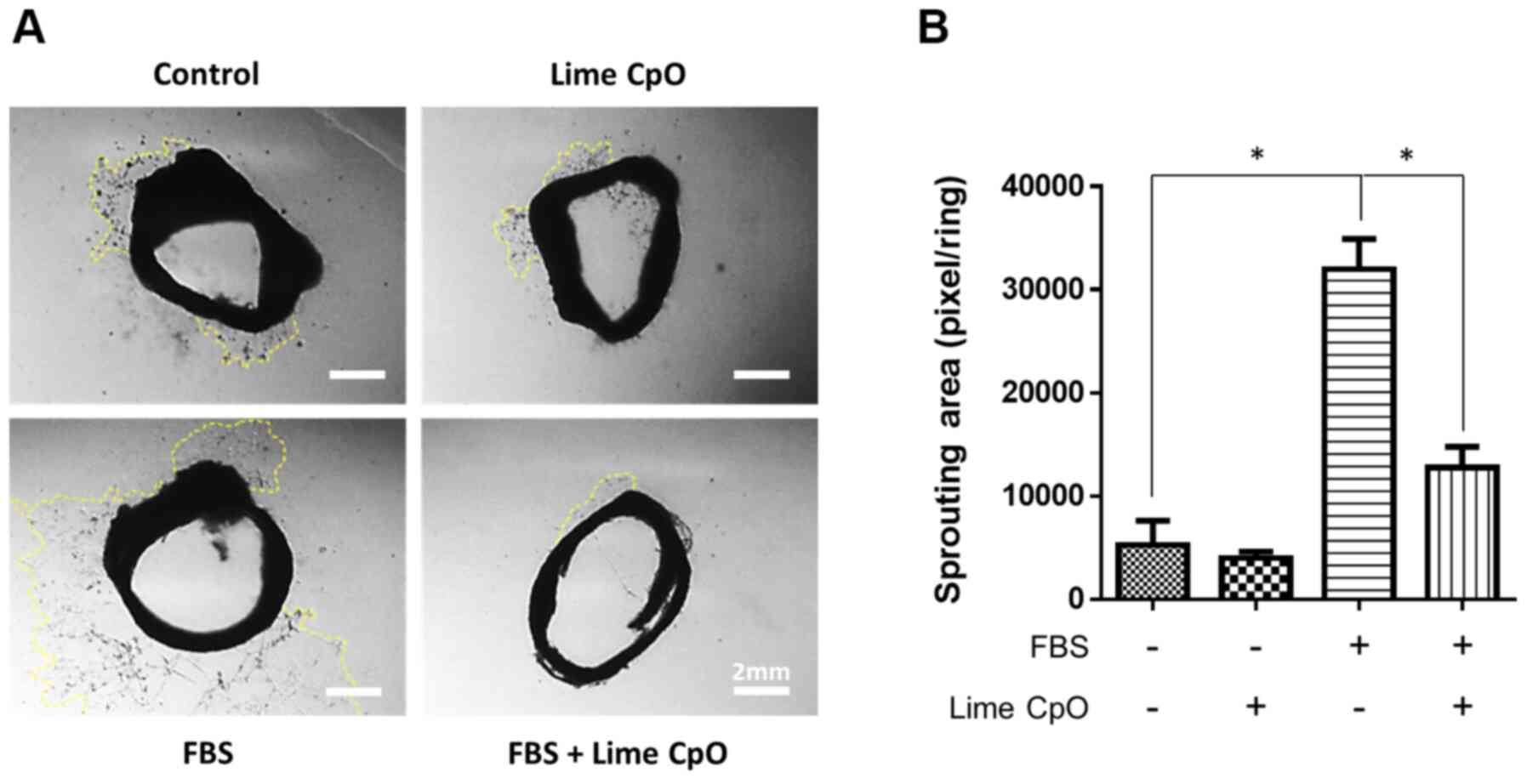
Inhibition of VSMC proliferation in
endothelium-denuded aortic rings
The effect of lime CpO on the proliferation and
migration of VSMCs in tissues was investigated and an ex
vivo aortic ring analysis was performed (Fig. 4A). EC-denuded vascular tissue was
cultured in DMEM containing FBS to observe the proliferation and
migration of VSMCs. Intravascular VSMCs spread to the outside of
the aortic rings and significantly proliferated in response to FBS
compared with the cells in the FBS-negative and lime CpO-negative
control group. However, the degree of spread of the lime CpO and
FBS-treated VSMCs was significantly decreased compared with the
FBS-treated group, to a similar level to that of the control cells
(treated with nether CpO or FBS) (Fig.
4B). These results suggested that lime CpO inhibited abnormal
VSMC proliferation in injured vascular tissues and possibly
contributed to tissue homeostasis.
Analysis of lime CpO components
In the present study, GC-MS analysis was performed
to evaluate the characteristics and components of lime CpO
(Table I). Table I displays the results of the
component analysis of lime CpO by GC-MS. In total, ~36 types of
volatile compounds were detected as main materials, including 23
monoterpenes, 9 sesquiterpenes and 4 other components. Monoterpenes
(M) and sesquiterpenes (S) accounted for 94.6 and 4.6% of EOs,
respectively, in summed area % of GC-MS spectrometry (Table I). D-limonene (41.40%), γ-terpinene
(13.19%), terpinolene (10.41%) and α-terpineol (8.61%) were the
major monoterpenes among the detected volatile components, overall
accounting for 73.61% of the total volatile components (Table I).
 | Table IChemical Composition of Lime CpO by
MS and KI identification in GC-MS spectrometry. |
Table I
Chemical Composition of Lime CpO by
MS and KI identification in GC-MS spectrometry.
| RT | Constituent | Area, % | KIa | Classification |
|---|
| 24.55 | α-Pinene | 0.90 | 927 | (M) |
| 27.08 | Camphene | 0.26 | 943 | (M) |
| 30.36 |
Linalool-3,7-oxide | 0.10 | 963 | (M) |
| 31.89 | β-Pinene | 1.48 | 972 | (M) |
| 34.15 | β-Myrcene | 0.36 | 986 | (M) |
| 39.22 | 1,4-Cineole | 1.39 | 1026 | (M) |
| 39.52 | α-Terpinene | 1.05 | 1029 | (M) |
| 40.61 | ρ-Cymene | 4.23 | 1039 | (M) |
| 41.27 | D-Limonene | 41.40 | 1045 | (M) |
| 41.50 | Eucalyptol | 1.11 | 1048 | (M) |
| 42.77 | Ocimene
quintoxide | 0.12 | 1059 | (O) |
| 42.98 | (Z)-β-Ocimene | 0.32 | 1061 | (M) |
| 44.08 | γ-Terpinene | 13.19 | 1072 | (M) |
| 46.19 | Terpinolene | 10.41 | 1092 | (M) |
| 46.57 | ρ-Cymenene | 0.33 | 1095 | (M) |
| 47.14 | Linalool | 0.13 | 1101 | (M) |
| 48.49 | Fenchyl
alcohol | 0.48 | 1121 | (M) |
| 49.53 | 1-Terpineol | 0.58 | 1136 | (M) |
| 50.48 | β-Terpineol | 0.72 | 1150 | (M) |
| 51.79 | Decanal | 0.25 | 1169 | (O) |
| 52.22 | endo-Borneol | 0.18 | 1175 | (M) |
| 52.77 | Terpinen-4-ol | 0.44 | 1183 | (M) |
| 53.24 | ρ-Cymen-8-ol | 0.20 | 1190 | (M) |
| 53.92 | α-Terpineol | 8.61 | 1200 | (M) |
| 54.26 | γ-Terpineol | 1.63 | 1204 | (M) |
| 68.01 |
trans-Caryophyllene | 0.47 | 1434 | (S) |
| 68.33 | α-Bergamotene | 0.78 | 1442 | (S) |
| 68.89 | β-Farnesene | 0.10 | 1457 | (S) |
| 69.39 | α-Humulene | 0.13 | 1471 | (S) |
| 69.95 | α-Selinene | 0.14 | 1486 | (S) |
| 70.42 | β-Cadinene | 0.17 | 1498 | (S) |
| 70.69 | α-Farnesene | 0.75 | 1507 | (S) |
| 70.95 | β-Bisabolene | 1.52 | 1516 | (S) |
| 71.94 | β-Maaliene | 0.26 | 1548 | (S) |
| 89.83 | Butyl
palmitate | 0.24 | 2188 | (O) |
| 91.85 | Butyl stearate | 0.20 | 2389 | (O) |
Discussion
The present study investigated the effect of lime
CpO, a natural substance, on VSMC proliferation. To examine whether
lime CpO was involved in VSMC regulation, cellular signaling
pathways were analyzed using western blotting. Lime CpO was
demonstrated to modulate cell cycle regulators in VSMCs.
Furthermore, in ex vivo conditions, lime CpO negatively
regulated VSMC proliferation. Among 36 types of volatile compounds
identified in lime CpO, four molecules (D-limonene, γ-terpinene,
terpinolene and α-terpineol) were specifically detected as the main
components.
In the past decade, natural products have been
demonstrated to be mostly non-toxic and have been used successfully
as therapeutics for numerous diseases (10,28-30).
Among these natural products, EOs are promising pharmaceutical
agents as they are clinically applicable and can be properly
managed and industrialized for human diseases (31). Depending on the EO extraction
method, such as steam distillation, expression (for example cold
compression) and solvent extraction, the properties of the active
compounds may show different distribution ratio in total
ingredients, but the main active substances remain unchanged
(32). According to a review by
Narang and Jiraungkoorskul (17),
lime and its related byproducts have been suggested to have
potential therapeutic effects in colon cancer, pancreatic cancer,
breast cancer, skin cancer and lymphoma. Lime and its related
byproducts have also been demonstrated to inhibit the cell cycle
and cell proliferation (17).
Patil et al (18) reported
that lime inhibited cancer cell proliferation by modulating Bax,
Bcl-2, caspase-3 and p53 in pancreatic cancer cells. Furthermore,
lime has been reported to improve obesity, the atherogenic index
and fatty liver disease by inducing antioxidant capacity and
hypolipidemic effects (33).
Despite the various previous studies on lime CpO, to the best of
our knowledge, there are currently no reports on the inhibition of
VSMC proliferation, a major cause of occlusive vascular
disease.
The present study demonstrated that lime CpO
treatment at 10-5 concentrations inhibited FBS-induced
VSMC proliferation. Furthermore, lime CpO reduced FBS-induced
phosphorylation of the MAPK/ERK and PI3K/AKT/mTOR signaling
pathways. Lime CpO inhibited the MAPK/ERK and PI3K/AKT/mTOR
signaling pathways at comparable or higher degree. However, there
was no change in the expression or activity of caspase-3 under the
experimental conditions of the present study. A possible reason for
why these results differed from previous reports is that the lime
CpO dose is modifiable for different cytotoxic effects under
various cell types. In the present study, preliminary experiments
were conducted to determine concentrations that were not cytotoxic
(Fig. 1A). Compared with
conventional chemical drugs, lime CpO is a natural compound with
little to no toxicity and controllable doses that can be used in
the body (34). The use of natural
products as therapeutics is advantageous as they are traditionally
used by human and therefore less likely to cause an adverse
reaction when developed as medicines (9). However, in future work, lime CpO
toxicity will be tested in other vascular constituent cells besides
VSMCs, such as vascular ECs and vascular fibroblasts. Furthermore,
whether the same effect is shown in other batches of lime CpO and
identification of the active compounds via selection experiments
with various combinations of constituents from lime CpO should be
investigated in future studies.
A previous report suggested that neroli (Citrus
aurantium L.) EO is an endothelium and smooth muscle-dependent
vasodilator, which alleviates cardiovascular disease (35). It has been reported that neroli EO
modulated intracellular Ca2+ concentrations via the
inhibition of cation channel-mediated extracellular Ca2+
influx and store-operated Ca2+ release mediated by the
ryanodine receptor signaling pathway (35). Among the proposed constituent
compounds of neroli EO, D-limonene and α-terpineol are included in
the data from the present study, suggesting that lime CpO may also
act as a smooth muscle-dependent vasodilator (35). Furthermore, neroli EO have also
reported to possess 100% singlet oxygen scavenging activity as a
strong antioxidant (12,36,37).
Therefore, future work may examine the endothelium and/or smooth
muscle-mediated vasodilator effect of lime CpO in a cardiovascular
disease model. In the present study, lime CpO inhibited excess VSMC
proliferation, which causes occlusive vascular disease, via the
regulation of MAPK and PI3K signaling pathways. These results
suggest that lime CpO may be developed as a potential therapeutic
or health supplement for the treatment of cardiovascular disease.
Future studies may explore the different effects of lime CpO
compared with lime EO and validate its pharmacological effects on
classified compounds including D-limonene, γ-terpinene, terpinolene
and α-terpineol to investigate the proposed molecules of in
vivo cardiovascular disease.
Supplementary Material
Gas chromatography-mass spectrometry
traces of lime cold-pressed oil.
Acknowledgements
Not applicable.
Funding
The present study was supported by the following grants from the
Korea Forest Service (Seoul, Korea): Forest Science Technology
Research and Development Project (grant no. 2017026B10-1719-BA01)
and the National Institute of Forest Science, Forest Science
Technology Research and Development Project (grant no.
FP0900-2016-01).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BWS, CYL and IKK were involved in the
conceptualization, writing, editing and data analysis. JHP, BK,
SLe, SLi, SWK, JWC, MK, JHK, SSL, MJP, HM and KCH performed and
analyzed the experiments, and edited the manuscript. BWS and IKK
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed according to a
protocol approved by the Institutional Animal Care and Use
Committee of Catholic Kwandong University (approval no. CKU
01-2019-008; Incheon, South Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rensen SSM, Doevendans PAFM and van Eys
GJJM: Regulation and characteristics of vascular smooth muscle cell
phenotypic diversity. Neth Heart J. 15:100–108. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hao H, Gabbiani G and Bochaton-Piallat ML:
Arterial smooth muscle cell heterogeneity: Implications for
atherosclerosis and restenosis development. Arterioscler Thromb
Vasc Biol. 23:1510–1520. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Morgan JP, Perreault CL and Morgan KG: The
cellular basis of contraction and relaxation in cardiac and
vascular smooth muscle. Am Heart J. 121:961–968. 1991.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sprague AH and Khalil RA: Inflammatory
cytokines in vascular dysfunction and vascular disease. Biochem
Pharmacol. 78:539–552. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gomez D and Owens GK: Smooth muscle cell
phenotypic switching in atherosclerosis. Cardiovasc Res.
95:156–164. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Anwar MA, Shalhoub J, Lim CS, Gohel MS and
Davies AH: The effect of pressure-induced mechanical stretch on
vascular wall differential gene expression. J Vasc Res. 49:463–478.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Owens GK, Kumar MS and Wamhoff BR:
Molecular regulation of vascular smooth muscle cell differentiation
in development and disease. Physiol Rev. 84:767–801.
2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Clowes AW and Reidy MA: Prevention of
stenosis after vascular reconstruction: Pharmacologic control of
intimal hyperplasia - a review. J Vasc Surg. 13:885–891.
1991.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Garg S and Serruys PW: Coronary stents:
Current status. J Am Coll Cardiol. 56 (Suppl 10):S1–S42.
2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
de Andrade TU, Brasil GA, Endringer DC, da
Nóbrega FR and de Sousa DP: Cardiovascular activity of the chemical
constituents of essential oils. Molecules. 22(E1539)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Amorati R, Foti MC and Valgimigli L:
Antioxidant activity of essential oils. J Agric Food Chem.
61:10835–10847. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dosoky NS and Setzer WN: Biological
activities and safety of Citrus spp. essential oils. Int J
Mol Sci. 19(1966)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Reis D and Jones T: Aromatherapy: Using
Essential Oils as a Supportive Therapy. Clin J Oncol Nurs.
21:16–19. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Spadaro F, Costa R, Circosta C and
Occhiuto F: Volatile composition and biological activity of key
lime Citrus aurantifolia essential oil. Nat Prod Commun.
7:1523–1526. 2012.PubMed/NCBI
|
|
15
|
Amorim JL, Simas DLR, Pinheiro MMG, Moreno
DSA, Alviano CS, da Silva AJR and Fernandes PD: Anti-inflammatory
properties and chemical characterization of the essential oils of
four Citrus species. PLoS One. 11(e0153643)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kummer R, Fachini-Queiroz FC,
Estevão-Silva CF, Grespan R, Silva EL, Bersani-Amado CA and Cuman
RKN: Evaluation of anti-inflammatory activity of Citrus
latifolia Tanaka essential oil and limonene in experimental
mouse models. Evid Based Complement Alternat Med.
2013(859083)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Narang N and Jiraungkoorskul W: Anticancer
Activity of Key Lime, Citrus aurantifolia. Pharmacogn Rev.
10:118–122. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Patil JR, Chidambara Murthy KN,
Jayaprakasha GK, Chetti MB and Patil BS: Bioactive compounds from
Mexican lime (Citrus aurantifolia) juice induce apoptosis in
human pancreatic cells. J Agric Food Chem. 57:10933–10942.
2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tosukhowong P, Yachantha C,
Sasivongsbhakdi T, Ratchanon S, Chaisawasdi S, Boonla C and
Tungsanga K: Citraturic, alkalinizing and antioxidative effects of
limeade-based regimen in nephrolithiasis patients. Urol Res.
36:149–155. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Boshtam M, Moshtaghian J, Naderi G, Asgary
S and Nayeri H: Antioxidant effects of Citrus aurantifolia
(Christm) juice and peel extract on LDL oxidation. J Res Med Sci.
16:951–955. 2011.PubMed/NCBI
|
|
21
|
Kato Y, Domoto T, Hiramitsu M, Katagiri T,
Sato K, Miyake Y, Aoi S, Ishihara K, Ikeda H, Umei N, et al: Effect
on blood pressure of daily lemon ingestion and walking. J Nutr
Metab. 2014(912684)2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Muslin AJ: MAPK signalling in
cardiovascular health and disease: Molecular mechanisms and
therapeutic targets. Clin Sci (Lond). 115:203–218. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Muto A, Fitzgerald TN, Pimiento JM,
Maloney SP, Teso D, Paszkowiak JJ, Westvik TS, Kudo FA, Nishibe T
and Dardik A: Smooth muscle cell signal transduction: implications
of vascular biology for vascular surgeons. J Vasc Surg. 45 (Suppl
A):A15–A24. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals, 8th
edition. National Academies Press (US), Washington, DC, 2011.
|
|
25
|
Chang W, Lim S, Song H, Song BW, Kim HJ,
Cha MJ, Sung JM, Kim TW and Hwang KC: Cordycepin inhibits vascular
smooth muscle cell proliferation. Eur J Pharmacol. 597:64–69.
2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lim S, Lee SY, Seo HH, Ham O, Lee C, Park
JH, Lee J, Seung M, Yun I, Han SM, et al: Regulation of
mitochondrial morphology by positive feedback interaction between
PKCδ and Drp1 in vascular smooth muscle cell. J Cell Biochem.
116:648–660. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mathew OP, Ranganna K, Mathew J, Zhu M,
Yousefipour Z, Selvam C and Milton SG: Cellular effects of butylate
on vascular smooth muscle cells are mediated through disparate
actions on dual targets, histone deacetylase (HDAC) activity and
PI3K/Akt signaling network. Int J Mol Sci. 20(2902)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Suen J, Thomas J, Kranz A, Vun S and
Miller M: Effect of flavonoids on oxidative stress and inflammation
in adults at risk of cardiovascular disease: a systematic review.
Healthcare (Basel). 4(69)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li D, Wu H, Dou H, Guo L and Huang W:
Microcapsule of sweet orange essential oil changes gut microbiota
in diet-induced obese rats. Biochem Biophys Res Commun.
505:991–995. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jiang D, Li D and Wu W: Inhibitory effects
and mechanisms of luteolin on proliferation and migration of
vascular smooth muscle cells. Nutrients. 5:1648–1659.
2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lv X, Zhao S, Ning Z, Zeng H, Shu Y, Tao
O, Xiao C, Lu C and Liu Y: Citrus fruits as a treasure trove of
active natural metabolites that potentially provide benefits for
human health. Chem Cent J. 9(68)2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Aziz ZAA, Ahmad A, Setapar SHM, Karakucuk
A, Azim MM, Lokhat D, Rafatullah M, Ganash M, Kamal MA and Ashraf
GM: Essential oils: extraction techniques, pharmaceutical and
therapeutic potential - a review. Curr Drug Metab. 19:1100–1110.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lin LY, Chuang CH, Chen HC and Yang KM:
Lime (Citrus aurantifolia (Christm.) swingle) essential
oils: volatile compounds, antioxidant capacity, and hypolipidemic
effect. Foods. 8(E398)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Adokoh CK, Asante DB, Acheampong DO,
Kotsuchibashi Y, Armah FA, Sirikyi IH, Kimura K, Gmakame E and
Abdul-Rauf S: Chemical profile and in vivo toxicity evaluation of
unripe Citrus aurantifolia essential oil. Toxicol Rep.
6:692–702. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kang P, Ryu K-H, Lee J-M, Kim H-K and Seol
GH: Endothelium- and smooth muscle-dependent vasodilator effects of
Citrus aurantium L. var. amara: Focus on Ca(2+) modulation.
Biomed Pharmacother. 82:467–471. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ammar AH, Bouajila J, Lebrihi A, Mathieu
F, Romdhane M and Zagrouba F: Chemical composition and in vitro
antimicrobial and antioxidant activities of Citrus aurantium
L. flowers essential oil (Neroli oil). Pak J Biol Sci.
15:1034–1040. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ao Y, Satoh K, Shibano K, Kawahito Y and
Shioda S: Singlet oxygen scavenging activity and cytotoxicity of
essential oils from rutaceae. J Clin Biochem Nutr. 43:6–12.
2008.PubMed/NCBI View Article : Google Scholar
|