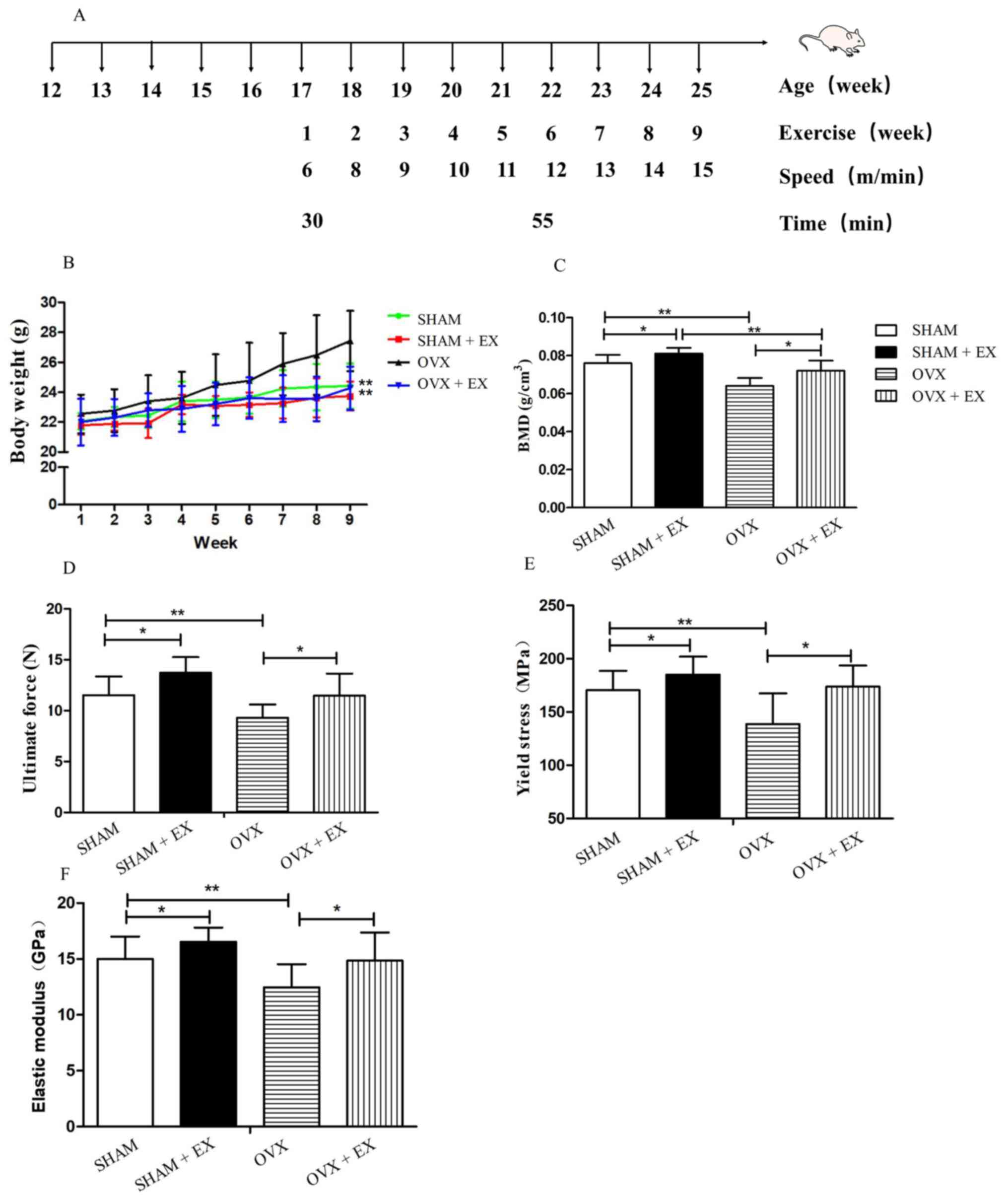
Introduction
Osteoporosis is an age-associated metabolic bone
disease that is characterized by decreasing bone mass and
deteriorating bone microstructure, leading to a general loss in
bone mineral density (BMD) (1).
Appropriate exercise is generally recommended for the prophylaxis
and treatment of osteoporosis, especially high-impact exercise
(2,3). Exercise increases bone formation and
reduces bone resorption, thereby improving bone mass and strength
in aging individuals (4).
Furthermore, exercise has been demonstrated to increase peak bone
mass in growing mice (5). However,
the underlying mechanism has yet to be fully elucidated.
Previous studies have demonstrated that mechanical
loading and hormones are the factors that are predominantly
associated with the effects of exercise on the regulation of bone
homeostasis; and non-coding RNAs, including microRNAs
(miRNAs/miRs), have been reported to be involved in this process
(6-8).
Long non-coding RNAs (lncRNAs) are another type of non-coding RNA,
and these are characterized by having lengths of >200 bp
(9). LncRNAs differ from miRNAs by
having more complex structures and functions due to their longer
sequences (10). An increasing
number of studies have revealed that lncRNAs participate in several
physiological processes of bone cells; in particular, exerting
roles in cell proliferation and differentiation by regulating gene
expression (11-13).
The lncRNA differentiation antagonizing non-protein coding RNA has
been demonstrated to inhibit the differentiation of bone
marrow-derived mesenchymal stem cells (BMSCs) via inactivation of
the p38/mitogen-activated protein kinase (MAPK) signaling pathway
(14). Knockdown of the lncRNA
MIR31HG leads to an increase in the osteogenesis of adipose-derived
stem cells (15). Furthermore,
lncRNAs may regulate gene transcription by interacting with miRNAs
in osteoclasts and osteoblasts, thereby influencing their activity
(16). For example, the lncRNA
potassium voltage-gated channel subfamily KCNQ1 has been indicated
to inhibit osteolysis by inhibiting miR-21a-5p in bone
marrow-derived macrophages (17),
and the lncRNA TSIX transcript, XIST antisense RNA, promotes
osteoblast apoptosis by inhibiting miR-30a-5p (18). These studies have demonstrated that
lncRNAs are involved in bone modeling, and may be key factors
affecting osteoporosis development.
Therefore, the present study aimed to investigate
whether exercise is able to regulate lncRNA expression, thereby
affecting bone remodeling. In addition, evidence was sought after
for an improved understanding of the underlying mechanism by which
osteoporosis is prevented through exercise.
Materials and methods
Animals and establishment of the
animal model
The present study was approved by the Ethics
Committee of Shanghai University of Sport (approval no. 2015030;
Shanghai, China), and all procedures were conducted following the
recommendations of the ARRIVE guidelines (19). The ovariectomized (OVX) mouse was
used to establish osteoporosis model due to estrogen deficiency
(20). A total of 44 C57BL/6
female mice (age, 12 weeks old; weight, 22-24 g; provided by
Changzhou Cavens Laboratory Animal Co., Ltd; http://www.cavens.com.cn/) were used in the study and
randomly divided into four groups (n=11 mice in each group) as
follows: i) The ovariectomy (OVX) group; ii) the sham operation
(SHAM) group; iii) the ovariectomy and exercise (OVX + EX) group;
and iv) the sham operation and exercise (SHAM + EX) group. The mice
in the sham group received a sham operation consisting in the
removal of an equal size of fat around the uterus. The mice in the
OVX group received a bilateral ovariectomy. Before the operations,
mice were anesthetized with avertin (300 mg/kg). All experimental
mice were housed in an environment with a 12-h light/dark cycle at
a temperature of 22±3˚C and a humidity of 55-60%, and they were
provided with food and water ad libitum. The mice were
weighed on Monday each week.
Exercise protocol
The two exercise groups of mice (the OVX + EX and
SHAM + EX groups) were subjected to 9 weeks of treadmill running
exercise 4 weeks after the operation was performed (n=11 mice in
each group). The treadmill running training program was adhered to
as described in previous studies (7,21),
with the following modifications. In brief, the speed was set at 6
m/min for 30 min for the 1st week (which comprised 5 days of
exercise followed by 2 days of rest) to allow the mice to become
familiarized with the exercise regime. From the 2nd week, all the
mice performed a 5 min warm-up exercise at a speed of 6 m/min with
a 25˚ slope, followed by a 55 min running exercise at a speed of 8
m/min with a 25˚ slope. Subsequently, the running exercise speed
was increased by 1 m/min each week leading up to the completion of
the treadmill running program after a total of 9 weeks, culminating
in a running speed of 15 m/min with a 25˚ slope for the last
training week (Fig. 1A). The OVX
and SHAM groups were housed under conventional conditions without
the treadmill running training program as a control.
Tissue preparation
All mice were subcutaneously injected with calcein
(0.01 mg/g weight) to fluorescently label the bones at 8 and 2 days
prior to sacrifice. The mice were sacrificed to collect bone
samples 48 h after the last treadmill training session. Briefly,
all mice were anesthetized with avertin (300 mg/kg), and the
animals were sacrificed by CO2 asphyxiation (flow rate
of CO2, 2 l/min; air displacement rate, 20%/min). Death
was confirmed by the absence of breathing, response to a firm toe
pinch, absence of a heartbeat or respiratory sounds, graying of the
mucous membranes and rigor mortis. The animal ethics approval was
obtained in November 2015, and these experiments were performed in
March 2016. Femurs were collected from all the mice to subsequently
perform microarray analysis of the lncRNAs, microcomputed
tomography (µCT), dual-energy X-ray absorptiometry (to determine
the BMD) and bone biomechanics and bone histomorphometry
analyses.
Microarray analysis
A total of three right femurs were selected from
each of the four groups for total RNA extraction using Invitrogen
TRIzol® reagent (Thermo Fisher Scientific, Inc.), and
the extracted RNA was reverse-transcribed into cDNA according to
the manufacturer's protocol (RevertAid First Strand cDNA Synthesis
kit; cat. no. K1622; Thermo Fisher Scientific, Inc.). Subsequently,
a lncRNA Mouse Gene Expression Microarray system V1.0 (microarray
and service provided by Boao Biological Group Co., Ltd.) was used
to detect of the expression levels of lncRNAs. Sample labeling and
array hybridization were performed strictly according to the
manufacturer's protocol (One-Color RNA Spike-In kit; cat. no.
5188-5282; Agilent,). G2565CA Microarray Scanner (Agilent
Technologies, Inc.) was used to scan the chip. Feature Extraction
software v10.7 (Agilent Technologies, Inc.) was used to extract the
data from the resulting images, and these data were subsequently
analyzed using GeneSpring software v11.5 (Agilent Technologies,
Inc.). The filter criteria were set as fold-change ≥2 and P<0.05
for determination of the differential expression of lncRNAs between
groups. The datasets generated and/or analyzed during the current
study are available in the Gene Expression Omnibus repository,
https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE184226.
Gene function analysis
Gene Ontology (GO) (http://www.geneontology.org) and Kyoto Encyclopedia of
Genes and Genomes (KEGG; https://www.kegg.jp/) analyses (consulted in May,
2016) were performed to investigate the underlying ‘molecular
function’, ‘biological process’, ‘cellular component’ and pathway
terms of the genes of interest. The significance of the GO term
enrichment and KEGG pathway correlations were assessed according to
the enrichment score yield based on the P-value.
Six of the 18 co-expressed lncRNAs in the OVX + EX
group and SHAM group (compared with the OVX group) were selected
for target microRNAs analysis. Target miRNA prediction analysis was
performed using the program RegRNA 2.0 (http://regrna2.mbc.nctu.edu.tw/detection.html)
based on gene sequence.
μCT analysis
A total of three left femurs from each of the four
groups were scanned using a µCT device (SkyScan 1174 v2;
Bruker-microCT; Bruker Corporation) using the following settings:
9.26 µm Camera pixel size; 46 kV voltage; and a current of 800 µA.
A total of 125 µCT slices were acquired as regions of interest for
further analysis. The trabecular number (Tb.N; mm), bone
volume/tissue volume (BV/TV, %), trabecular separation (Tb.Sp; mm)
and trabecular thickness (Tb.Th; mm) were then calculated using the
software provided by the µCT system.
BMD and bone biomechanical
analyses
Dual-energy X-ray absorptiometry, using an Osteocore
3 Digital 2D bone densitometer (Medilink), was used to detect the
BMD of the left femurs (eight samples analyzed for each group),
followed by a bone biomechanical test. Femurs were thawed at 4˚C in
normal saline solution and carefully checked under a light
microscope (Leica Microsystems GmbH; magnification, x10) to confirm
the integrity of all bones before the test was performed.
The three-point bending method was used to detect
the mechanical indices of all bones (22). Both proximal and distal areas of
the left femur were fixed on two supports of the machine with a
10-mm span. The front side of the femur was placed facing upwards.
A probe was then moved down at a speed of 1 mm/min with no
pre-load, and the movement of the probe was allowed to proceed
downwards for 1 min after the bone fracture. The software
(LabSANS-Test D30C) enabled calculation of the mechanical indices
of the bones, including the elastic modulus (GPa), yield stress
(MPa) and ultimate force (N).
Bone histomorphometry
The right femurs were fixed with 4% paraformaldehyde
at room temperature for 24 h, and resin embedding was used for the
bone histomorphometry test. Following the procedure outlined in a
previous study (23), the right
distal femurs were dehydrated in an increasing series of ethanol
(percentages of 70, 95 and 100%), cleared using xylene and embedded
in glycol methacrylate. The samples were then cut into 4-µm-thick
slices using a ultra-microtome (Leica Microsystems GmbH). After
which, one of the maximum-profile slices of each sample was
selected for further analysis. The slice was directly examined
using double labeling with calcein by using fluorescence microscopy
(Leica Microsystems GmbH; magnification, x10) to detect the mineral
apposition rate (MAR; percentage of cancellous bone MAR) and, the
bone formation rate/bone volume (BFR/BV) using a digitizing
morphometric system (Osteomeasure High Resolution Color Subsystem;
Osteometrics, Inc.).
Statistical analysis
All data are presented as the mean ± SD. A one-way
ANOVA was performed, followed with a Bonferroni's post hoc test to
analyze the effects of exercise or bilateral ovariectomy on the
bones of mice. All statistical analyses were performed using SPSS
statistical software v20.0 (IBM Corp.), and the figures were
prepared using GraphPad Prism software v5.0 (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of exercise on the animals'
weight, BMD and bone strength
No significant differences in body weight were
observed among the four groups at the beginning of the experiment.
However, after 9 weeks of training, the body weight of the OVX
group was significantly increased compared with the SHAM group
(P<0.01), while treadmill running significantly decreased the
body weight of the OVX + EX group (P<0.01). However, no
significant differences in weight were observed between the SHAM
and SHAM + EX groups (Fig.
1B).
The OVX group was revealed to have a significantly
lower BMD compared with the SHAM group (P<0.01; Fig. 1C). The exercise training regime was
demonstrated to increase the BMD, as the SHAM + EX and OVX + EX
groups had significantly higher BMDs compared with the SHAM and OVX
groups, respectively (both P<0.05; Fig. 1C). In addition, the ultimate force,
elastic modulus and yield stress measurements of the OVX group were
all significantly lower compared with those of the SHAM group (all
P<0.01; Fig. 1D-F). The OVX +
EX group also had significantly increased bone strength compared
with the OVX group, as determined from the ultimate force, elastic
modulus and yield stress values (all P<0.05); while the SHAM +
EX group also had significantly increased ultimate force, elastic
modulus and yield stress values compared with the SHAM group
(P<0.05; Fig. 1D-F). In
summary, the OVX group mice were identified as having lower BMDs
and bone strength values than the mice in the SHAM group, which
confirmed that the osteoporosis animal model had been successfully
established in the present study. Exercise increased the BMD and
the bone strength of the OVX group, which indicated that exercise
played a role in the prevention and treatment of osteoporosis.
Effect of exercise on bone mass, bone
formation
The results of the µCT analysis revealed no
significant differences in the values of Tb.N, BV/TV, Tb.Sp and
Tb.Th between the SHAM and SHAM + EX groups. In addition, the BV/TV
ratio was increased in the OVX + EX group compared with the OVX
group (P<0.05; Fig. 2C-F).
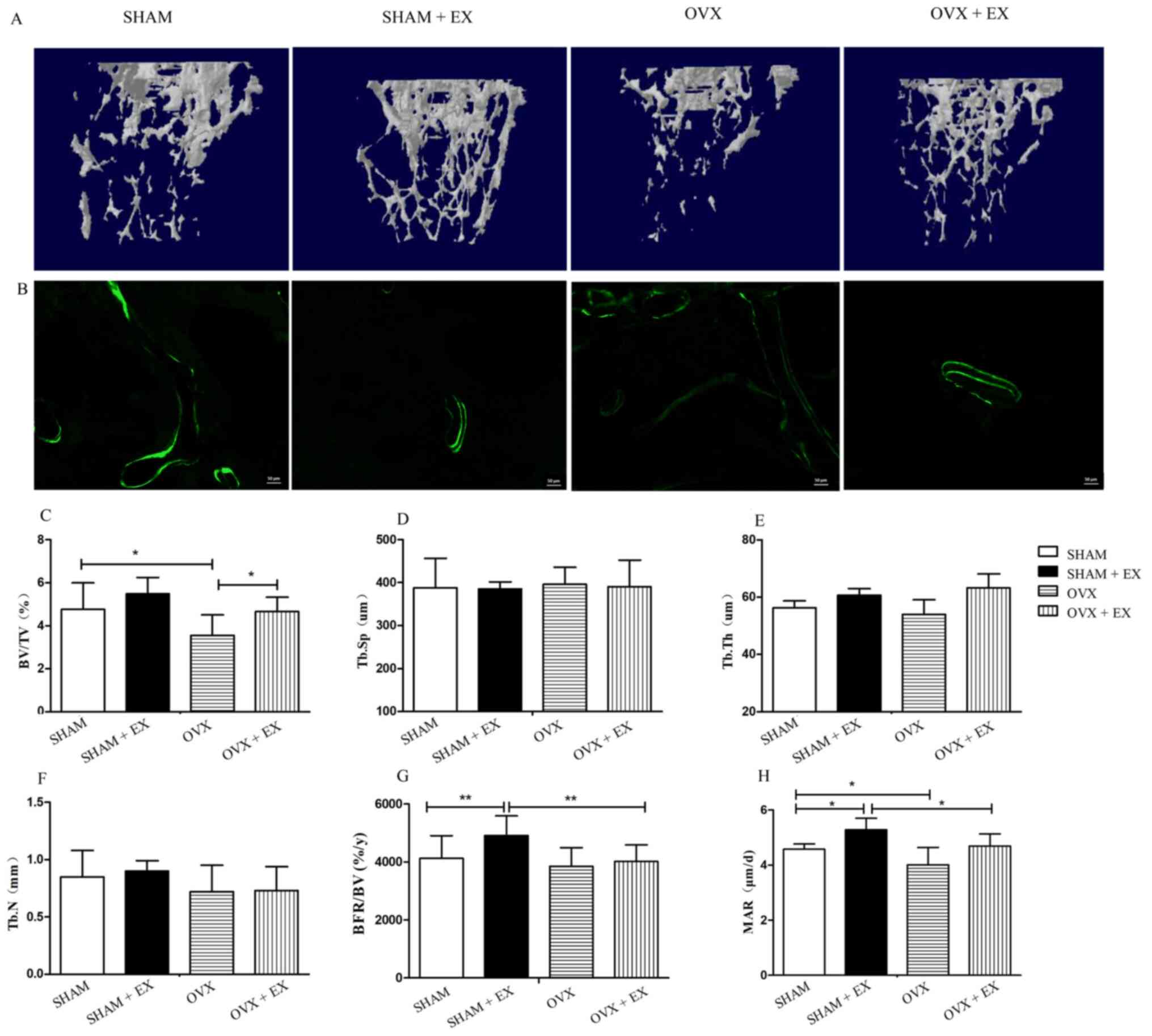 | Figure 2Results of µCT and effect of exercise
on bone histomorphometric parameters. (A) Representative µCT images
demonstrating the three-dimensional trabecular architecture in
mouse femur. (B) Representative images of mouse bones which were
labeled with subcutaneously injected calcein (5 µl/g) at days 1 and
8. (C) Trabecular bone mass (BV/TV), (D) Tb.Sp, (E) Tb.Th and (F)
Tb.N of mouse femur was determined using µCT. (G) Effect of
exercise on BFR/BV. (H) Effect of exercise on the MAR.
*P<0.05; **P<0.01. OVX, bilateral
ovariectomy group; OVX + EX, bilateral ovariectomy and exercise
group; SHAM, sham operation group; SHAM + EX, sham operation and
exercise group; µCT, microcomputed tomography; MAR, mineral
apposition rate; BV/TV, bone volume/tissue volume; BFR/BV, bone
formation rate/bone volume; Tb.N, trabecular number; Tb.Sp,
trabecular separation. Tb.Th, trabecular thickness. |
The results of the bone histomorphometry analysis
revealed that the OVX group had a lower MAR compared with the SHAM
group (P<0.05). However, 9 weeks of exercise training in the
SHAM + EX group led to a significant increase in BFR/BV and MAR
values compared with the SHAM group (both P<0.05; Fig. 2G and H). Though exercise increased the BFR/BV
and MAR values in the OVX + EX group, there was no significant
difference. In summary, the above results indicated that exercise
promoted the bone formation and bone mass of OVX mice, thereby
preventing and treating osteoporosis.
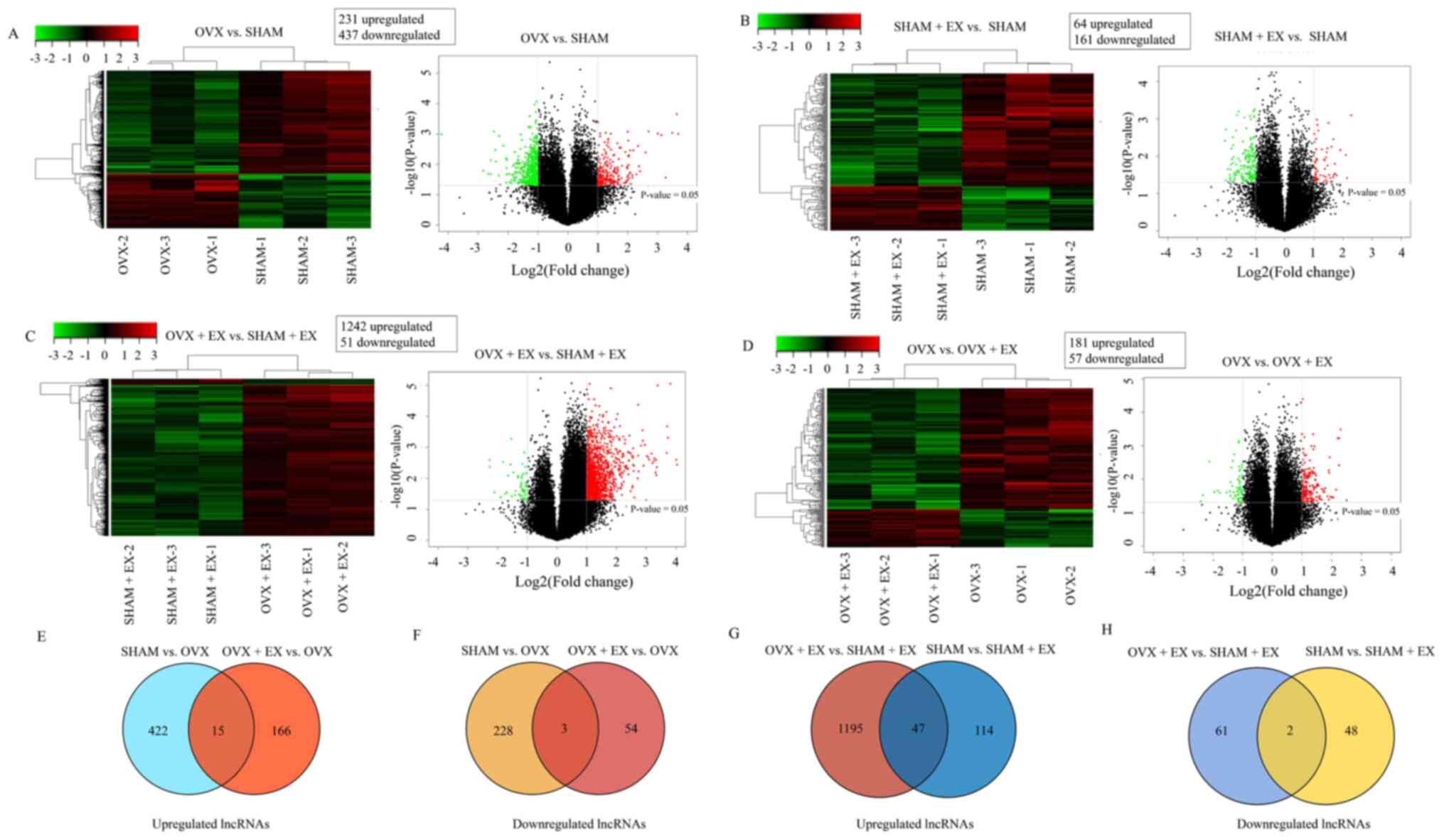
Effect of exercise on the expression
of lncRNAs
The results of the present study indicated that the
establishment of the ovariectomy model altered the patterns of
lncRNA expression in mice. A total of 231 lncRNAs were
significantly upregulated and 437 were significantly downregulated
in the bones of the OVX group compared with the SHAM group
(Fig. 3A). Furthermore, the
treadmill running exercise regime also led to further changes in
the expression of lncRNAs in the bone. A total of 64 lncRNAs were
significantly upregulated and 161 lncRNAs were significantly
downregulated in the bones of the SHAM + EX group compared with the
SHAM group (Fig. 3B). A total of
1,242 lncRNAs were significantly upregulated and 51 lncRNAs were
significantly downregulated in the bones of the OVX + EX group
compared with the SHAM + EX group. (Fig. 3C). Additionally, it was revealed
that 181 lncRNAs were significantly upregulated and 57 lncRNAs were
downregulated in the bones of the OVX + EX group compared with the
OVX group (Fig. 3D), and lncRNA
H19 was among the upregulated lncRNAs (fold change, 2.1; P=0.027).
Finally, it was observed that 15 upregulated and three
downregulated lncRNAs were common amongst the OVX + EX and SHAM
groups when compared against the OVX group (Fig. 3E and F). Moreover, it was also observed that 47
upregulated and two downregulated lncRNAs were common amongst the
OVX + EX and SHAM groups when compared with the SHAM + EX group
(Fig. 3G-H). The above results
demonstrated that exercise altered the expression of some lncRNAs
in the bone of osteoporotic mice, which indicated that exercise may
play a role in preventing and treating osteoporosis by regulating
lncRNAs.
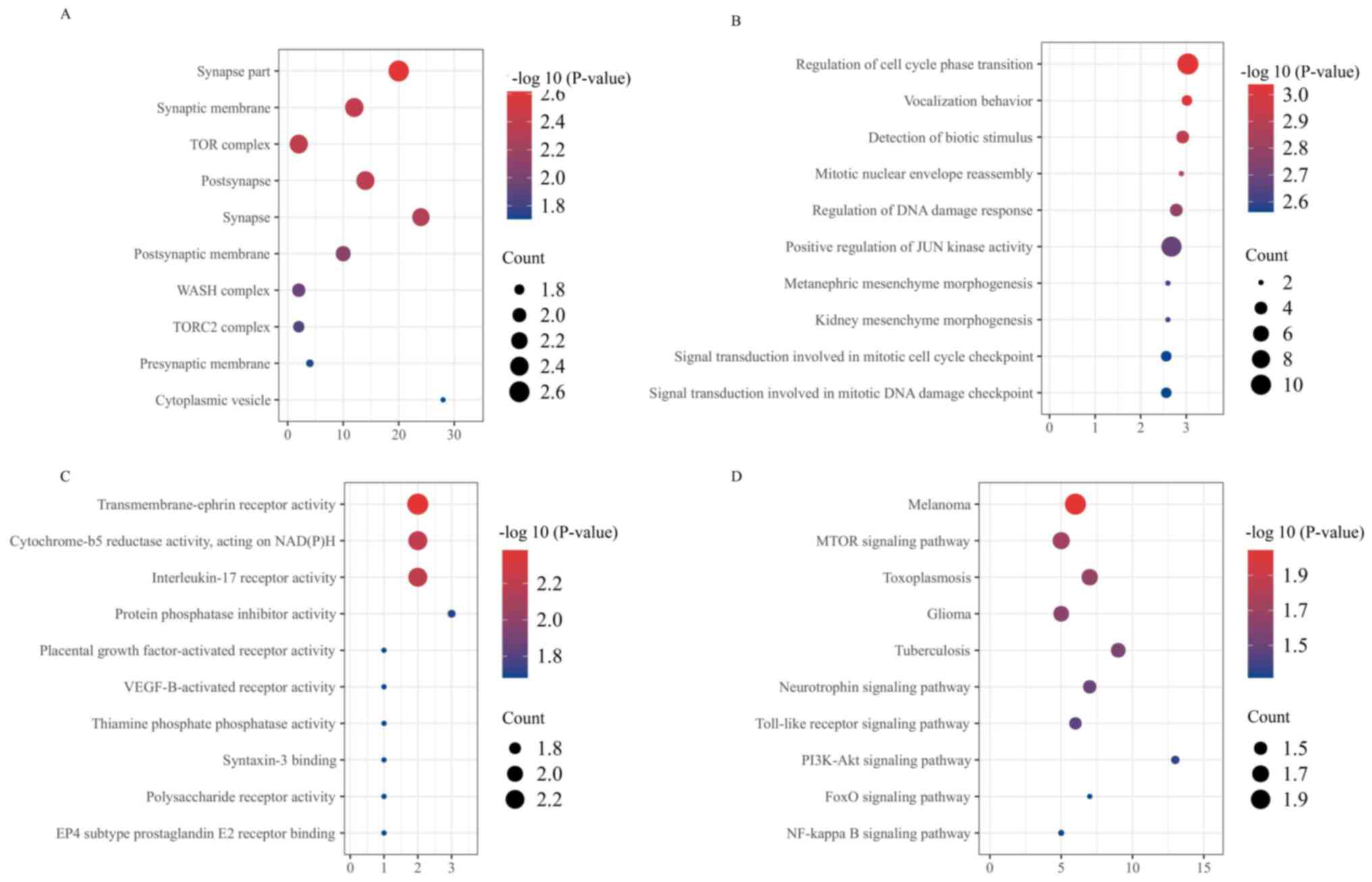
GO enrichment and KEGG analysis
GO analyses of the lncRNAs in the OVX + EX and OVX
groups were performed to compare them, and also to investigate how
lncRNAs regulated gene transcription. The bioinformatics analysis
in the present study revealed that the lncRNAs in these groups were
associated with the ‘cellular components’, ‘biological processes’
and ‘molecular functions’ annotations, the top ten results obtained
from the GO enrichment analysis of the differential genes are
presented in Fig. 4A-C. ‘Synapse
part’ and ‘TOR complex’ were among the top 10 enriched terms of
cellular components; ‘regulation of cell cycle phase transition’
and ‘positive regulation of JUN kinase activity’ were among the top
10 enriched terms of biological processes; and ‘VEGF-B-activated
receptor activity’ and ‘interleukin-17 receptor activity’ were
among the top ten enriched terms of molecular functions (Fig. 4A-C). Subsequently, the KEGG
analysis revealed that the ‘mTOR’, ‘NF-κB’ and ‘PI3K/Akt’ signaling
pathways were likely to be involved in the mechanism through which
the treadmill running exercise regime was able to exert its
influence on the prevention and treatment of the osteoporosis
process (Fig. 4D). The above
results indicated that lncRNAs may promote bone formation through
the above-mentioned factors or signal pathways, such as VEGF-B and
the PI3K/Akt signaling pathway.
Target miRNAs of the lncRNAs
lncRNAs that were downregulated in the OVX group
compared with the SHAM group, and whose expression levels were also
partially reversed in the OVX + EX group, were selected for further
study. Among the 15 upregulated and three downregulated lncRNAs
(Fig. 3E and F; Table
I), four upregulated lncRNAs and two downregulated lncRNAs were
randomly selected to perform miRNA prediction analysis using the
program RegRNA 2.0. A total of 43 miRNAs were identified as being
the potential targets of the six selected lncRNAs (Table II). The present results indicated
that exercise may alter the expression of specific lncRNAs, thereby
enhancing their inhibition of targeted miRNAs to promote bone
formation.
 | Table IFold changes of the co-expressed
lncRNAs in the OVX + EX and SHAM groups that are upregulated or
downregulated compared with the OVX group. |
Table I
Fold changes of the co-expressed
lncRNAs in the OVX + EX and SHAM groups that are upregulated or
downregulated compared with the OVX group.
| A, Upregulated
lncRNAs |
|---|
| Gene symbol | FC of OVX + EX | FC of SHAM |
|---|
| Gm35194 | 2.15 | 2.37 |
| LOC105246953 | 2.44 | 2.33 |
| LOC102637959 | 2.08 | 2.07 |
| Gm30392 | 2.64 | 2.72 |
| NONMMUT014677 | 2.51 | 2.08 |
| NONMMUT016039 | 2.14 | 3.17 |
| NONMMUT027251 | 2.61 | 3 |
| NONMMUT067810 | 2.37 | 4.1 |
|
ri|7330424C03|PX00650H15|3890 | 2.04 | 2.14 |
|
ri|8430440M04|PX00025O03|1121 | 2.05 | 2.49 |
|
ri|9430019C24|PX00108O13|1178 | 2.11 | 2.42 |
|
ri|A230069F12|PX00129C15|2187 | 2.4 | 2.24 |
|
ri|A430104H18|PX00064B06|3523 | 2.05 | 2.39 |
|
ri|B020006F18|PX00325E01|1577 | 2.87 | 3.02 |
|
ri|F930011C10|PL00010K14|3597 | 2.23 | 3 |
| B, Downregulated
lncRNAs |
| Gene symbol | FC of OVX + EX | FC of SHAM |
| NONMMUT006626 | 2.17 | 2.54 |
|
ri|D130079K21|PX00187K16|1491 | 2 | 2.35 |
| uc.mouse.68 | 3.87 | 2.75 |
 | Table IImiRNAs targeted by the dysregulated
lncRNAs. |
Table II
miRNAs targeted by the dysregulated
lncRNAs.
| A, Upregulated
expression |
|---|
| lncRNA | miRNA target
sites |
|---|
| LOC105246953 | mmu-miR-215-3p,
mmu-miR-670-3p, mmu-miR-1186, mmu-miR-1186b |
| LOC102637959 | mmu-miR-15a-5p,
mmu-miR-706, mmu-miR-466i-5p, mmu-miR-1187, mmu-miR-1195 |
| NONMMUT014677 | mmu-miR-669c-3p,
mmu-miR-466f-3p, mmu-miR-466k, mmu-miR-574-5p, mmu-miR-466i-5p,
mmu-miR-466i-3p, mmu-miR-1187, mmu-miR-466m-3p,
mmu-miR-3095-3p |
| NONMMUT027251 | mmu-miR-669c-3p,
mmu-miR-466f-3p, mmu-miR-574-5p, mmu-miR-466i-5p, mmu-miR-466i-3p,
mmu-miR-1187, mmu-miR-466k, mmu-miR-466m-3p, mmu-miR-3095-3p |
| B, Downregulated
expression |
| lncRNA | miRNA target
sites |
|
ri|D130079K21|PX00187K16|1491 | mmu-miR-302b-3p,
mmu-miR-669c-3p, mmu-miR-466i-3p, mmu-miR-669h-3p, mmu-miR-466m-3p,
mmu-miR-466q |
| NONMMUT006626 | mmu-miR-328-3p,
mmu-miR-3473b, mmu-miR-5128, mmu-miR-185-3p, mmu-miR-221-5p,
mmu-miR-221-3p, mmu-miR-452-3p, mmu-miR-466i-5p, mmu-miR-1187,
mmu-miR-3473 |
Discussion
The OVX group mice were identified as having lower
BMDs and bone strength values than the mice in the SHAM group,
which confirmed that the osteoporosis animal model had been
successfully established in the present study. Although 9 weeks of
treadmill running exercise did reverse the bone loss and prevent
osteoporosis in the ovariectomized mice, as was anticipated, the
results revealed that the OVX + EX group had higher BMD and bone
strength values compared with the OVX group, and these findings
were consistent with those of a previous study (7). A previous study also demonstrated
that 10 weeks of treadmill training reduces the bone-resorbing in
ovariectomized mice (24). Other
studies have indicated that exercise promotes osteogenic
differentiation in ovariectomized rats (25,26).
The present study revealed that treadmill running led to an
increase in the MAR and BFR/BV values in the OVX + EX and SHAM + EX
groups compared with the OVX and SHAM groups, respectively;
however, the difference between the OVX + EX and OVX groups was not
significant. These findings indicated that the running exercise
increased both the BMD and the bone mass in osteoporotic mice
through an increase in bone formation.
lncRNAs, a recently discovered class of regulatory
RNAs, have been demonstrated to have a notable role in bone
metabolism (14,16,27,28).
The expression of lncRNAs is responsive to different physiological
and pathological stimuli (29).
Previous studies have suggested that clusters of lncRNAs are
aberrantly expressed during osteogenic differentiation of stem
cells (30) and in ovariectomized
mice (31). The pairwise
comparison results [OVX vs. OVX + EX; OVX vs. SHAM; SHAM vs. SHAM +
EX; OVX + EX vs. SHAM + EX] of the present study identified a total
of 2,424 significantly differently expressed lncRNAs (1718
upregulated lncRNAs and 706 downregulated lncRNAs). Among them,
lncRNA H19 was significantly upregulated in the OVX group following
exercise training. Previous studies have indicated that lncRNA H19
is involved in tension-induced osteogenesis of stem cells (28), and that its levels are decreased in
the distal femur of disuse osteoporosis rats, where it targets
Dickkopf (DDK) 4 to activate the Wnt signaling pathway (32). This suggests that lncRNA H19 may
also play a notable role in the functions of bone metabolism under
the exercise stimulus.
lncRNAs are transcribed by RNA polymerase II and end
up being localized in the nucleus, cytoplasm or in both. They have
been demonstrated to regulate genome imprinting and gene
expression, and also to participate in X-chromosome-silencing and
other biological processes (33).
Evidence suggests that lncRNAs may fulfill important regulatory
roles during the development of osteoporosis (31,34).
LncRNA colorectal neoplasia differentially expressed is
significantly upregulated in the osteoclasts of patients with
postmenopausal osteoporosis (35).
LncRNA maternally expressed 3 is expressed at a significantly high
level in the BMSCs of postmenopausal women with osteoporosis, which
leads to the upregulated expression of miR-133a-3p to inhibit
osteogenic differentiation (36).
Considering that estrogen deficiency increases bone turnover
(37), these findings suggest that
the markedly increased activity of osteoclasts observed in the
present study after exercise mainly resulted from an inhibition of
bone resorption in osteoporosis.
lncRNAs have a secondary structure that provides
binding sites for proteins or RNAs that are involved in the
regulation of transcription, translation, cell differentiation and
other biological processes (38).
In the present study, the enriched GO pathway terms were associated
with ‘cellular components’ ‘molecular functions’ and ‘biological
processes’; therefore, these processes were implicated in the
treatment and prevention of osteoporosis by exercise. IL-17A and
VEGF were revealed to be enriched molecules. c-Jun N-terminal
kinase is another MAPK that is involved in TNF superfamily member
11-induced osteoclast formation (39). VEGF is an angiogenesis factor
expressed in osteoblast precursor cells (40), which has a key role in bone
angiogenesis and osteoblastogenesis (41). The mTOR signaling pathway is one of
the most important enriched pathways, and this pathway has been
reported to promote osteoblast differentiation and bone
mineralization in older animals (42). The NF-κB and PI3K signaling
pathways were also featured in the top 20 significantly enriched
pathways. The loss or inhibition of NF-κB results in insufficient
levels of osteoclastogenesis, which thereby increases bone mass in
mice (43,44), and PI3K is required to regulate
osteoblastic differentiation of mesenchymal stem cells (45). The KEGG pathway analysis results
also revealed that the expression of lncRNAs regulated by exercise
were associated with the ‘mTOR’, ‘PI3K’ and ‘NF-κB’ signaling
pathways, indicating that, in terms of explaining how exercise may
exert its influence on osteoporosis, lncRNAs may regulate the
differentiation of osteoblasts and osteoclasts mainly through these
signaling pathways.
lncRNAs have been reported to act as competing
endogenous RNAs for miRNAs (46),
indicating that they may have an important role in miRNA-associated
biological processes. The results of the present study identified
that 18 lncRNAs (15 upregulated and three downregulated) were held
in common between the SHAM and OVX + EX treatment groups when
compared with the OVX group. Exercise might have reversed the
expression of lncRNAs in the OVX group to reduce the effects of
bone loss in osteoporosis. Target miRNAs were then predicted
through miRNA prediction analysis to postulate the functional roles
of lncRNAs in the bone. It was hypothesized that one lncRNA may
interact with multiple miRNAs and, vice versa, one miRNA may
connect with two or more lncRNAs. miR-15a was identified as a
potential target of lncRNA LOC102637959. miR-15a is a member of the
miR-15 family, which has been demonstrated to have an important
role in exercise-induced bone formation by inhibiting bone
morphogenetic protein (BMP) signaling in bone cells (47). This finding indicated that lncRNA
LOC102637959 may be the positive regulators of osteoporosis under
an exercise stimulus. In addition, a previous study revealed that
silencing miR-221-3p promotes osteogenic differentiation of BMSCs
through targeting insulin-like growth factor-1 to activate the ERK
signaling pathway (48). Another
study revealed that the knockout of miR-185-3p increases the
osteogenic differentiation of osteoblasts and BMSCs, and also
causes a reduction in the bone loss of osteoporotic mice by
enhancing BMP signaling (49).
miR-302b was also reported to activate Wnt/β-catenin by degrading
DKK1 to promote the differentiation of the osteoblastic cell line
MC3T3-E1(50). miR-221-3p,
miR-185-3p and miR-302b were identified as the target miRNAs of
downregulated lncRNAs in osteoporotic mice following exercise
training, indicating that exercise may alter the expression of
specific lncRNAs, thereby enhancing their inhibition of targeted
miRNAs to promote bone formation.
However, there were several limitations that must be
considered both in terms of the context of the experimental design
of the present study and in terms of the explanation of the
results. First, only three bone samples were selected for each
group for lncRNA array analysis, and the expression levels of
associated lncRNAs were not verified. Therefore, the possible
presence of false-negative or positive results could not be
discounted. Additionally, potential roles of lncRNAs identified by
microarray analysis and the association of molecules, proteins or
miRNAs needs to be further confirmed. Thirdly, bone homeostasis is
mediated by several types of bone cells, and therefore further
studies are required to determine the function and mechanism of
lncRNAs on specific cell types in the bone.
In conclusion, the present study demonstrated that
the expression levels of certain lncRNAs were regulated by exercise
in osteoporotic mice, and also revealed the putative functions of
these lncRNAs in preventing osteoporosis. Specific lncRNAs, such as
lncRNA H19 and LOC102637959, may be involved in this process. These
findings have improved the current understanding of the role of
exercise in regulating lncRNA expression and provided some evidence
on the underlying mechanism, which should prove to be useful in
terms of designing novel therapeutic interventions for the
prevention and treatment of osteoporosis.
Acknowledgements
Not applicable.
Funding
Funding: The present work was supported by The National Natural
Science Foundation of China (grant nos. 81572242 and 81702235), the
Shanghai Key Lab of Human Sport Competence Development and
Maintenance (Shanghai University of Sport; grant no.
11DZ2261100).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the Gene Expression Omnibus
repository, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE184226.
Authors' contributions
JG, LL, MZ and HL performed the experiments; JG and
XC wrote the manuscript; YY, XT and LZ analyzed the data and
prepared the figures; MW performed the target microRNA analysis and
prepared the tables; XC and JZ contributed to the design of the
work and supervised this entire program. JZ and XC confirm the
authenticity of all the raw data. All authors reviewed, read and
approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Ethics
Committee of Shanghai University of Sport (approval no. 2015030),
and all procedures were conducted in accordance with the
recommendations of the ARRIVE guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bliuc D, Alarkawi D, Nguyen TV, Eisman JA
and Center JR: Risk of subsequent fractures and mortality in
elderly women and men with fragility fractures with and without
osteoporotic bone density: The Dubbo Osteoporosis Epidemiology
Study. J Bone Miner Res. 30:637–646. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Moreira LD, Oliveira ML, Lirani-Galvão AP,
Marin-Mio RV, Santos RN and Lazaretti-Castro M: Physical exercise
and osteoporosis: Effects of different types of exercises on bone
and physical function of postmenopausal women. Arq Bras Endocrinol
Metabol. 58:514–522. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ma D, Wu L and He Z: Effects of walking on
the preservation of bone mineral density in perimenopausal and
postmenopausal women: A systematic review and meta-analysis.
Menopause. 20:1216–1226. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hamilton CJ, Swan VJ and Jamal SA: The
effects of exercise and physical activity participation on bone
mass and geometry in postmenopausal women: A systematic review of
pQCT studies. Osteoporos Int. 21:11–23. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Weaver CM, Gordon CM, Janz KF, Kalkwarf
HJ, Lappe JM, Lewis R, O'Karma M, Wallace TC and Zemel BS: The
National Osteoporosis Foundation's position statement on peak bone
mass development and lifestyle factors: A systematic review and
implementation recommendations. Osteoporos Int. 27:1281–1386.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yuan Y, Chen X, Zhang L, Wu J, Guo J, Zou
D, Chen B, Sun Z, Shen C and Zou J: The roles of exercise in bone
remodeling and in prevention and treatment of osteoporosis. Prog
Biophys Mol Biol. 122:122–130. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen X, Li L, Guo J, Zhang L, Yuan Y, Chen
B, Sun Z, Xu J and Zou J: Treadmill running exercise prevents
senile osteoporosis and upregulates the Wnt signaling pathway in
SAMP6 mice. Oncotarget. 7:71072–71086. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li L, Chen X, Lv S, Dong M, Zhang L, Tu J,
Yang J, Zhang L, Song Y, Xu L, et al: Influence of exercise on bone
remodeling-related hormones and cytokines in ovariectomized rats: A
model of postmenopausal osteoporosis. PLoS One.
9(e112845)2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Brosnan CA and Voinnet O: The long and the
short of noncoding RNAs. Curr Opin Cell Biol. 21:416–425.
2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Krol J, Krol I, Alvarez CP, Fiscella M,
Hierlemann A, Roska B and Filipowicz W: A network comprising short
and long noncoding RNAs and RNA helicase controls mouse retina
architecture. Nat Commun. 6(7305)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wu QY, Li X, Miao ZN, Ye JX, Wang B, Zhang
F, Xu RS, Jiang DL, Zhao MD and Yuan FL: Long non-coding RNAs: A
new regulatory code for osteoporosis. Front Endocrinol (Lausanne).
9(587)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gong YY, Peng MY, Yin DQ and Yang YF: Long
non-coding RNA H19 promotes the osteogenic differentiation of rat
ectomesenchymal stem cells via Wnt/β-catenin signaling pathway. Eur
Rev Med Pharmacol Sci. 22:8805–8813. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liao J, Xiao H, Dai G, He T and Huang W:
Recombinant adenovirus (AdEasy system) mediated exogenous
expression of long non-coding RNA H19 (lncRNA H19) biphasic
regulating osteogenic differentiation of mesenchymal stem cells
(MSCs). Am J Transl Res. 12:1700–1713. 2020.PubMed/NCBI
|
|
14
|
Zhang J, Tao Z and Wang Y: Long non-coding
RNA DANCR regulates the proliferation and osteogenic
differentiation of human bone-derived marrow mesenchymal stem cells
via the p38 MAPK pathway. Int J Mol Med. 41:213–219.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jin C, Jia L, Huang Y, Zheng Y, Du N, Liu
Y and Zhou Y: Inhibition of lncRNA MIR31HG Promotes Osteogenic
Differentiation of Human Adipose-Derived Stem Cells. Stem Cells.
34:2707–2720. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xiaoling G, Shuaibin L and Kailu L:
MicroRNA-19b-3p promotes cell proliferation and osteogenic
differentiation of BMSCs by interacting with lncRNA H19. BMC Med
Genet. 21(11)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gao X, Ge J, Li W, Zhou W and Xu L: LncRNA
KCNQ1OT1 ameliorates particle-induced osteolysis through inducing
macrophage polarization by inhibiting miR-21a-5p. Biol Chem.
399:375–386. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bu Y, Zheng D, Wang L and Liu J: LncRNA
TSIX promotes osteoblast apoptosis in particle-induced osteolysis
by down-regulating miR-30a-5p. Connect Tissue Res. 59:534–541.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Percie Du Sert N, Hurst V, Ahluwalia A,
Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl
U, et al: The ARRIVE guidelines 2.0: updated guidelines for
reporting animal research. BMJ Open Sci. 4(e100115)2020.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Inada M, Matsumoto C and Miyaura C: Animal
models for bone and joint disease. Ovariectomized and
orchidectomized animals. Clin Calcium. 21:164–170. 2011.PubMed/NCBI(In Japanese).
|
|
21
|
Høydal MA, Wisløff U, Kemi OJ and
Ellingsen O: Running speed and maximal oxygen uptake in rats and
mice: Practical implications for exercise training. Eur J
Cardiovasc Prev Rehabil. 14:753–760. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Deckard C, Walker A and Hill BJF: Using
three-point bending to evaluate tibia bone strength in
ovariectomized young mice. J Biol Phys. 43:139–148. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang L, Chen X, Wu J, Yuan Y, Guo J,
Biswas S, Li B and Zou J: The effects of different intensities of
exercise and active vitamin D on mouse bone mass and bone strength.
J Bone Miner Metab. 35:265–277. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang M, Ishikawa S, Inagawa T, Ikemoto H,
Guo S, Sunagawa M and Hisamitsu T: Influence of mechanical force on
bone matrix proteins in ovariectomised mice and osteoblast-like
MC3T3-E1 Cells. In Vivo. 31:87–95. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bu S, Chen Y, Wang S, Zhang F and Ji G:
Treadmill training regulates β-catenin signaling through
phosphorylation of GSK-3β in lumbar vertebrae of ovariectomized
rats. Eur J Appl Physiol. 112:3295–3304. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu M, Zhong C, He RX and Chen LF: Icariin
associated with exercise therapy is an effective treatment for
postmenopausal osteoporosis. Chin Med J (Engl). 125:1784–1789.
2012.PubMed/NCBI
|
|
27
|
Shang G, Wang Y, Xu Y, Zhang S, Sun X,
Guan H, Zhao X, Wang Y, Li Y and Zhao G: Long non-coding RNA
TCONS_00041960 enhances osteogenesis and inhibits adipogenesis of
rat bone marrow mesenchymal stem cell by targeting miR-204-5p and
miR-125a-3p. J Cell Physiol. 233:6041–6051. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wu J, Zhao J, Sun L, Pan Y, Wang H and
Zhang WB: Long non-coding RNA H19 mediates mechanical
tension-induced osteogenesis of bone marrow mesenchymal stem cells
via FAK by sponging miR-138. Bone. 108:62–70. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang Y, Yujiao W, Fang W, Linhui Y, Ziqi
G, Zhichen W, Zirui W and Shengwang W: The roles of miRNA, lncRNA
and circRNA in the development of osteoporosis. Biol Res.
53(40)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Huang G, Kang Y, Huang Z and Zhang Z, Meng
F, Chen W, Fu M, Liao W and Zhang Z: Identification and
characterization of long non-coding RNAs in osteogenic
differentiation of human adipose-derived stem cells. Cell Physiol
Biochem. 42:1037–1050. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hao L, Fu J, Tian Y and Wu J: Systematic
analysis of lncRNAs, miRNAs and mRNAs for the identification of
biomarkers for osteoporosis in the mandible of ovariectomized mice.
Int J Mol Med. 40:689–702. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li B, Liu J, Zhao J, Ma JX, Jia HB, Zhang
Y, Xing GS and Ma XL: LncRNA-H19 modulates Wnt/β-catenin signaling
by targeting Dkk4 in hindlimb unloaded rat. Orthop Surg. 9:319–327.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Wei CW, Luo T, Zou SS and Wu AS: The role
of long noncoding RNAs in central nervous system and
neurodegenerative diseases. Front Behav Neurosci.
12(175)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhao N, Zeng L, Liu Y, Han D, Liu H, Xu J,
Jiang Y, Li C, Cai T, Feng H, et al: DLX3 promotes bone marrow
mesenchymal stem cell proliferation through H19/miR-675 axis. Clin
Sci (Lond). 131:2721–2735. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li W, Zhu HM, Xu HD, Zhang B and Huang SM:
CRNDE impacts the proliferation of osteoclast by estrogen
deficiency in postmenopausal osteoporosis. Eur Rev Med Pharmacol
Sci. 22:5815–5821. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang Q, Li Y and Zhang Y, Ma L, Lin L,
Meng J, Jiang L, Wang L, Zhou P and Zhang Y: LncRNA MEG3 inhibited
osteogenic differentiation of bone marrow mesenchymal stem cells
from postmenopausal osteoporosis by targeting miR-133a-3p. Biomed
Pharmacother. 89:1178–1186. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cauley JA: Estrogen and bone health in men
and women. Steroids. 99:11–15. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361.
2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Linder M, Hecking M, Glitzner E, Zwerina
K, Holcmann M, Bakiri L, Ruocco MG, Tuckermann J, Schett G, Wagner
EF, et al: EGFR controls bone development by negatively regulating
mTOR-signaling during osteoblast differentiation. Cell Death
Differ. 25:1094–1106. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ruocco MG, Maeda S, Park JM, Lawrence T,
Hsu LC, Cao Y, Schett G, Wagner EF and Karin M: I{kappa}B kinase
(IKK){beta}, but not IKK{alpha}, is a critical mediator of
osteoclast survival and is required for inflammation-induced bone
loss. J Exp Med. 201:1677–1687. 2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Franzoso G, Carlson L, Xing L, Poljak L,
Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF and Siebenlist U:
Requirement for NF-kappaB in osteoclast and B-cell development.
Genes Dev. 11:3482–3496. 1997.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Youssef A and Han VKM: Regulation of
osteogenic differentiation of placental-derived mesenchymal stem
cells by insulin-like growth factors and low oxygen tension. Stem
Cells Int. 2017(4576327)2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kang H, Yan Y, Jia P, Yang K, Guo C, Chen
H, Qi J, Qian N, Xu X, Wang F, et al: Desferrioxamine reduces
ultrahigh-molecular-weight polyethylene-induced osteolysis by
restraining inflammatory osteoclastogenesis via heme oxygenase-1.
Cell Death Dis. 7(e2435)2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Mayr-Wohlfart U, Waltenberger J, Hausser
H, Kessler S, Günther KP, Dehio C, Puhl W and Brenner RE: Vascular
endothelial growth factor stimulates chemotactic migration of
primary human osteoblasts. Bone. 30:472–477. 2002.PubMed/NCBI View Article : Google Scholar
|
|
45
|
HaDuong JH, Blavier L, Baniwal SK, Frenkel
B, Malvar J, Punj V, Sposto R and DeClerck YA: Interaction between
bone marrow stromal cells and neuroblastoma cells leads to a
VEGFA-mediated osteoblastogenesis. Int J Cancer. 137:797–809.
2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Xia T, Liao Q, Jiang X, Shao Y, Xiao B, Xi
Y and Guo J: Long noncoding RNA associated-competing endogenous
RNAs in gastric cancer. Sci Rep. 4(6088)2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Grünhagen J, Bhushan R, Degenkolbe E,
Jäger M, Knaus P, Mundlos S, Robinson PN and Ott CE: MiR-497~195
cluster microRNAs regulate osteoblast differentiation by targeting
BMP signaling. J Bone Miner Res. 30:796–808. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Gan K, Dong GH, Wang N and Zhu JF:
miR-221-3p and miR-222-3p downregulation promoted osteogenic
differentiation of bone marrow mesenchyme stem cells through
IGF-1/ERK pathway under high glucose condition. Diabetes Res Clin
Pract. 167(108121)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Cui Q, Xing J, Yu M, Wang Y, Xu J, Gu Y,
Nan X, Ma W, Liu H and Zhao H: Mmu-miR-185 depletion promotes
osteogenic differentiation and suppresses bone loss in osteoporosis
through the Bgn-mediated BMP/Smad pathway. Cell Death Dis.
10(172)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wu Z, Zhang Y, Yang Z, Zhu Y, Xie Y, Zhou
F and Cai L: Elevation of miR-302b prevents multiple myeloma cell
growth and bone destruction by blocking DKK1 secretion. Cancer Cell
Int. 21(187)2021.PubMed/NCBI View Article : Google Scholar
|