Introduction
Prostate cancer occurs in the prostatic epithelium
and poses a threat to the health of middle-aged and older males
(1). After lung cancer, prostate
cancer is the second most common malignant tumor affecting men in
numerous western countries (2).
Although the prostate cancer mortality rate in China is lower than
that in western countries, the incidence has gradually increased
(3). It is therefore necessary to
explore novel molecular mechanisms for prostate cancer
treatment.
MicroRNAs (miRNAs/miR) are a large class of
endogenous non-coding single chain small molecule RNAs (20-25 nt)
(4,5). Bioinformatics shows that miRNAs, when
specifically combined with target gene mRNAs, can control hundreds
of transcriptional target genes, thus promoting degradation or
inhibiting translation (6). miRNAs
may affect multiple pathways, thus altering the expression levels
of the target mRNA and regulating the signaling pathways of target
genes during the occurrence and development of tumors (7). miRNA expression profiles which vary
depending on tumor type, play a crucial role in early diagnosis and
prognosis as well as being molecular markers in cancer treatment
(8). miRNA expression is abnormal
in various tumors (9). miRNAs play
a role as oncogenes or tumor suppressor genes (4,10).
miR-130b inhibits migration and invasion in breast cancer (11). Exploring the relationship between
miRNA and prostate cancer may provide new therapies for its
treatment.
Delta-like 1 (DLL1), as a canonical ligand of Notch
signaling, participates in cell fate determination, cellular
differentiation and pattern formation (12). Overexpressed DLL1 contributes to the
progression of choriocarcinoma and hepatocellular carcinoma, while
downregulated DLL1 promotes the apoptosis and suppresses the
proliferation of multiple glioma cells (13-15).
In prostate cancer, DLL1 was overexpressed in LNCaP, but decreased
DLL1 promotes the lateral motility of PC-3 cells (16). The roles of DLL1 in prostate cancer
is intriguing and the effects of DLL1 on the progression of
prostate cancer has not been fully elucidated.
Phosphatidylinositol 3 kinase (PI3K)-protein kinase
B (Akt)-mammalian target of rapamycin (mTOR) is one of three major
signaling pathways in cancer. mTOR is a key kinase downstream of
PI3K/Akt, which regulates tumor cell proliferation, growth,
survival and angiogenesis (17-19).
Cancer cells may escape detection and normal biochemical systems
regulating the balance between apoptosis (programmed cell death)
and survival (20). PI3K-Akt-mTOR
generally promotes survival via the inhibition of proapoptotic
factors and activation of anti-apoptotic factors (21). Previous studies suggest that the
PI3K-Akt-mTOR signaling pathway plays an important role in cancer
recurrence and metastasis (22-24).
In the present study, the roles of miR-130b in
prostate cancer were investigated. It was found that miR-130b
regulated migration and invasion of prostate cancer cells via the
PI3K-Akt-mTOR axis.
Materials and methods
Tissue sample collection
A total of 30 prostate cancer tissues and 30
para-carcinoma tissues were obtained from patients (56-87 years;
average age, 71 years) diagnosed with prostate cancer at Yulin
Traditional Chinese Medicine Hospital Yulin, China from September,
2015 to January, 2017. The tissue samples were preserved in liquid
nitrogen at -80˚C. Informed consent was obtained from all patients.
The present study was approved by the Ethics Committee of Yulin
Traditional Chinese Medicine Hospital.
Cell culture
The prostate cancer cell line C4-2B was obtained
from the American Type Culture Collection. The cells were incubated
at 37˚C and cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) including 10% fetal bovine serum (FBS; HyClone;
GE Healthcare Life Sciences) as well as 100 U/ml penicillin and 100
µg/ml streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.). The incubator was humidified with 5% CO2. Cell
propagation was conducted every other day. Cells in the logarithmic
(log) phase were used for experimentation.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted with TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. miRNAs were extracted with a miRNeasy Mini kit (Qiagen,
Inc.). Thereafter, a Primer-Script™ One Step RT-qPCR kit
(Takara Biotechnology, Co., Ltd.) was used for cDNA synthesis at
50˚C. qPCR was performed to amplify the cDNA template using a
SYBR® Premix Dimmer Eraser kit (Takara Biotechnology
Co., Ltd.), according to the manufacturer's protocol. The PCR
process was as follows: Denaturing for 5 min at 95˚C and 40 10-sec
cycles at 95˚C and 1 min at 60˚C. GAPDH was used as a loading
control for DLL1, PI3K, AKT and matrix metalloproteinase (MMP)9,
and mRNA values were normalized against GAPDH. The expression of U6
acted as an internal control for miR-130b. Expression was
quantified using the 2-∆∆Cq method (25). The primer sequences were as follows:
DLL1 forward, 5'-TGCTCCGAAACAAGAGTGTG-3' and reverse,
5'-CAGGCAGAGCAGGTGATACA-3'; MMP9 forward, 5'-CAGAGATGCGTGGAGAGT-3'
and reverse, 5'-TCTTCCGAGTAGTTTTGG-3'; miR-130b forward,
5'-GCCGCCAGTGCAATGATGAA-3' and reverse, 5'-GTGCAGGGTCCGAGGT-3'; U6
forward, 5'-CGCTTCGGCAGCACATATACTA-3' and reverse,
5'-CGCTTCACGAATTTGCGTGTCA-3'; GAPDH forward,
5'-TGAAGGTCGGAGTCAACGGATTTGGT-3' and reverse,
5'-CATGTGGGCCATGAGGTCCACCAC-3'.
Transfection
The log-phase prostate cancer cells
(1x105 cells/well) were divided into groups, namely the
control group, the negative control (NC) group and the miR-130b
mimic group. Cells in the miR-130b mimic group were transfected
with miR-130b mimic using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Cells in the NC group were transfected
with NC mimic. The control group was left untreated. After 72 h
transfection, RT-qPCR was used to detect miR-130b expression in
each group. miR-130b mimics and miR-130b NC were synthesized by
Ribobio and the sequences used were as follow: hsa-miR-130b mimics,
5'-CAGUGCAAUGAUGAAAGGGCAU-3'; hsa-miR-130b NC,
5'-AUCCCCUUUCAUCAUUGCAUUG-3'.
Scratch wound assay
Cell migration was measured using a scratch wound
assay. When cells reached 90% confluency, cells were digested and
suspended at 5x105 cells/ml. Thereafter, cells were
seeded into 6-well plates (5x105 cells/well) for a
monolayer culture. Cells were then scratched with a 10 µl
pipette tip (Thermo Fisher Scientific, Inc.). Cells were then
incubated with RPMI-1640 medium supplemented with 10 g/l bovine
serum albumin (BSA; Gibco; Thermo Fisher Scientific, Inc.) and 1%
FBS for 24 h. Thereafter, the cell migration rate was measured
using a light microscope (magnification, x200; BioTek
Instruments).
Transwell assay
Cell invasion was measured via Transwell assay with
the 8.0 µm pore size FluoroBlok membrane (Corning which was coated
with BD Matrigel Matrix (Thermo Fisher Scientific, Inc.) in
accordance with the manufacturer's protocol. Transfected cells were
seeded (3x103 cells/well) in the upper chamber with
serum-free medium. The lower chamber contained DMEM (Thermo Fisher
Scientific, Inc.) with 10% FBS. After 48 h incubation, non-migrated
cells were removed with a cotton swab. Invaded cells were fixed
with 100% methanol at room temperature for 10 min. Cells were then
counted under a light microscope (x100 magnification; BioTek
Instruments) in five predetermined fields. Each experiment was
performed in triplicate.
Western blot analysis
Western blot analysis was used to determine protein
levels. Protein samples were lysed using RIPA lysis buffer
(Beyotime Institute of Biotechnology). The supernatant was retained
in order to detect its protein concentration. Total protein was
extracted by using a Total Protein Extraction kit (Phygene Life
Sciences). Thereafter, 40 µg protein was loaded onto a 10% SDS-PAGE
gel. Proteins were then separated by electrophoresis and
transferred to PVDF membranes (Sigma-Aldrich; Merck KGaA). The
membrane was then blocked with 5% skimmed milk in TBST for 1 h at
room temperature. The membrane was then incubated overnight at 4˚C
with primary antibodies: DLL1 (cat. no. 2588; 1:1,000; Cell
Signaling Technology, Inc.), phosphorylated (p)-PI3K (cat. no.
17366; 1:1,000; Cell Signaling Technology, Inc.), PI3K (cat. no.
4255; 1:1,000; Cell Signaling Technology, Inc.), p-Akt (cat. no.
4060; 1:2,000; Cell Signaling Technology, Inc.), Akt (cat. no.
9272; 1:1,000; Cell Signaling Technology, Inc.), GAPDH (cat. no.
5174; 1:1,000; Cell Signaling Technology, Inc.) and MMP9 (cat. no.
3852; 1:1,000; Cell Signaling Technology, Inc.). The membranes were
then incubated with horseradish peroxidase-conjugated secondary
antibodies (cat. no. 7074; 1:1,000; Cell Signaling Technology,
Inc.) for 1 h at 37˚C. Protein bands were visualized using an
enhanced chemiluminescence kit (ECL Prime Western Blotting
Detection Reagent; GE Healthcare) and quantified using ImageJ
software (Fiji version 1.8.0; National Institutes of Health).
Luciferase assay
The target of miR-130b was predicted via Online
database TargetScan (http://www.targetscan.org/vert_72/). The
3'untranslated region (UTR) fragment of DLL1 containing mutant
fragments was cloned into luciferase reporter vectors (Shanghai
GeneChem Co., Ltd.). Prostate cancer cells were cultured in 96-well
plates. miR-130b mimic (60 nM) or NC were co-transfected with 200
ng of the 3'UTR wild-type (WT) or mutant (Mut) plasmid DNA into
cells using Lipofectamine 3000 (Thermo Fisher Scientific, Inc.).
Luciferase activity was measured and normalized to that of
Renilla luciferase after 48 h using a Dual Luciferase
Reporter Assay kit (Thermo Fisher Scientific, Inc.), according to
manufacturer's protocol. All transfection assays were performed
independently in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
software (IBM, Corp.). Data are presented as the mean ± standard
deviation. A Student's t-test (two-tailed) or a one-way analysis of
variance followed by Dunnett's post-hoc test was used to analyze
data. P<0.05 was considered to indicate a statistically
significant difference. All the experiments in the present study
were conducted in triplicate.
Results
The expression of miR-130b and
patients' clinical features
As shown in Table I,
the expression of miR130b was significantly associated with tumor
grades and size; however, there was no significant correlation with
the expression of miR-130 and patients' age, gender, and tumor
metastasis.
 | Table IRelationship between miR-130 and
clinico-pathological parameters of patients with prostate
cancer. |
Table I
Relationship between miR-130 and
clinico-pathological parameters of patients with prostate
cancer.
| Clinical
features | Number | miR-130 high | miR-130 low | P-value |
|---|
| Age (years) | | | | 0.201 |
|
<50 | 9 | 3 | 6 | |
|
>50 | 21 | 7 | 14 | |
| Tumor size | | | | 0.004b |
|
<3
cm | 13 | 8 | 5 | |
|
>3
cm | 17 | 2 | 15 | |
| Histological
grade | | | | 0.018a |
|
Well-intermediately | 12 | 7 | 5 | |
|
Poor | 18 | 3 | 15 | |
| Metastasis | | | | 0.166 |
|
No | 5 | 3 | 2 | |
|
Yes | 25 | 7 | 18 | |
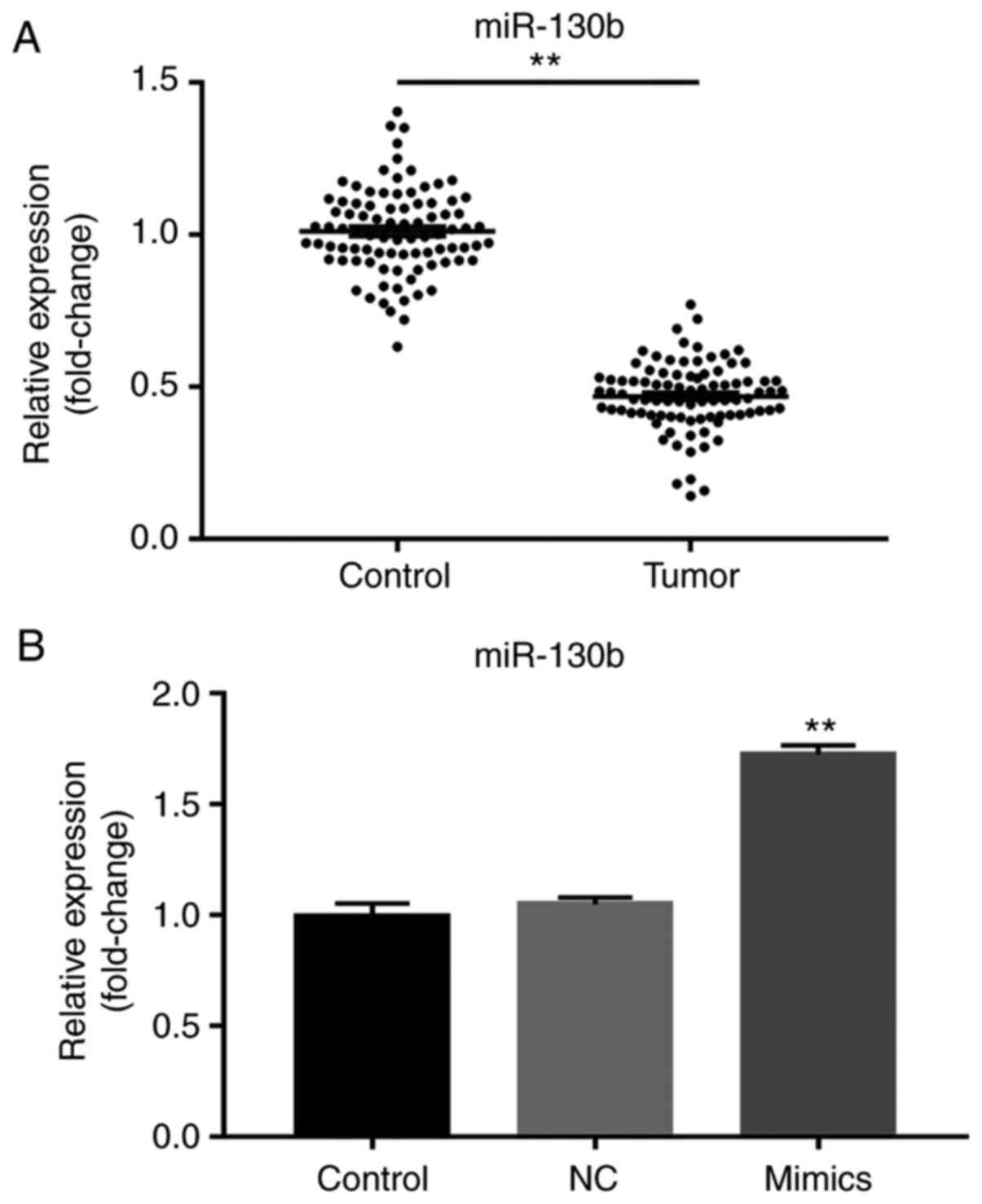
Decreased miR-130b expression in
prostate cancer tissue
RT-qPCR was used to determine miR-130b expression.
The results showed abnormally low miR-130b expression in tumor
tissues compared with adjacent tissues (P<0.01; Fig. 1A). Furthermore, in cells transfected
with the miR-130b mimic, significantly increased miR-130b
expression was observed compared with the NC and control groups
(P<0.01; Fig. 1B).
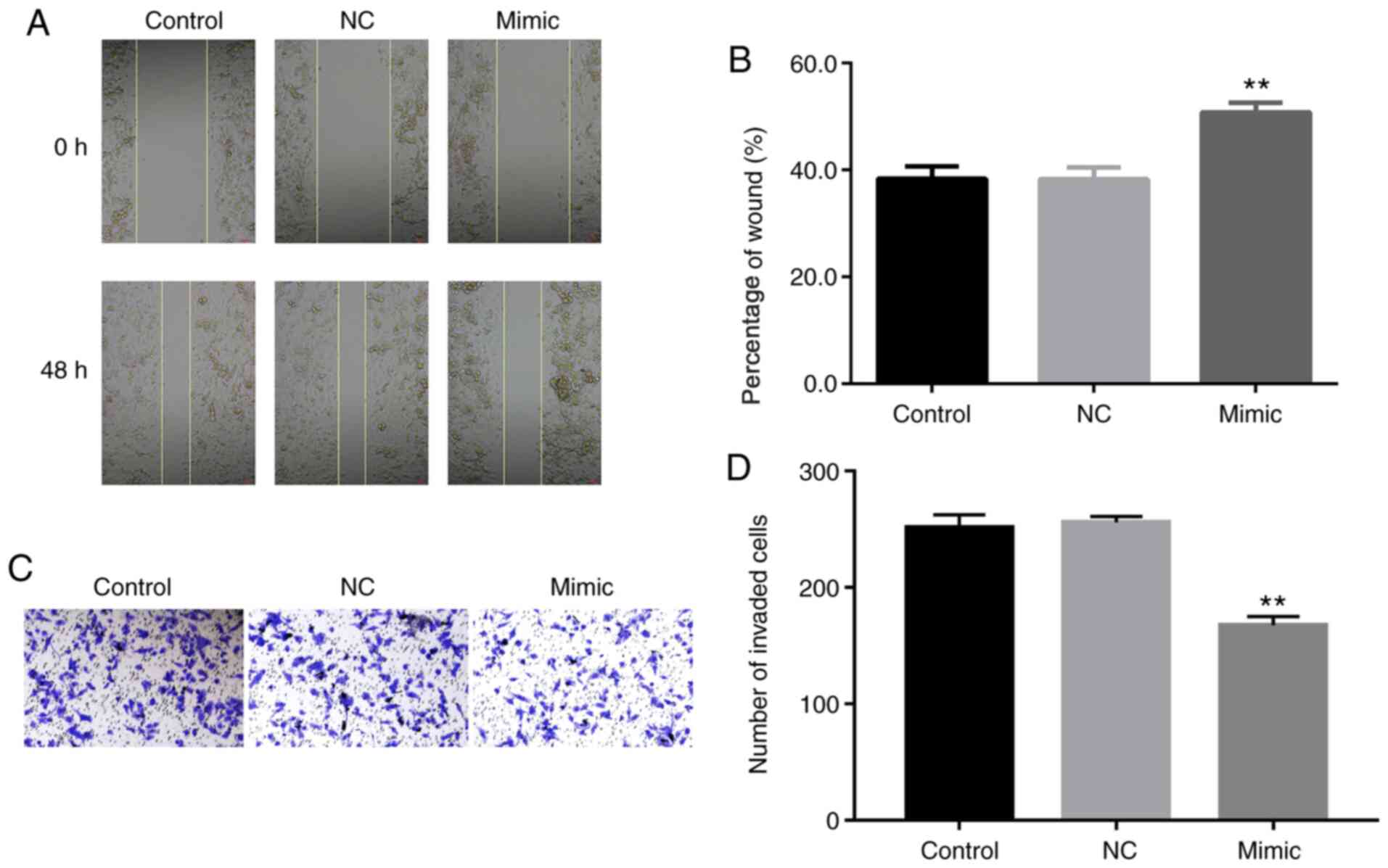
Impact of miR-130b on cell migration
and invasion
Scratch wound and Transwell assays were used to
estimate the effect of miR-130b on the migration of prostate cancer
cells. Wound healing was significantly augmented post-miR-130b
mimic treatment, suggesting that miR-130b suppressed the migration
of prostate cancer cells (P<0.01; Fig. 2A and B). Furthermore, the number of migrated
cells treated with miR-130b mimic significantly decreased compared
with the NC and control groups, thus proving that miR-130b
significantly inhibited prostate cancer cell migration (P<0.01;
Fig. 2C and D).
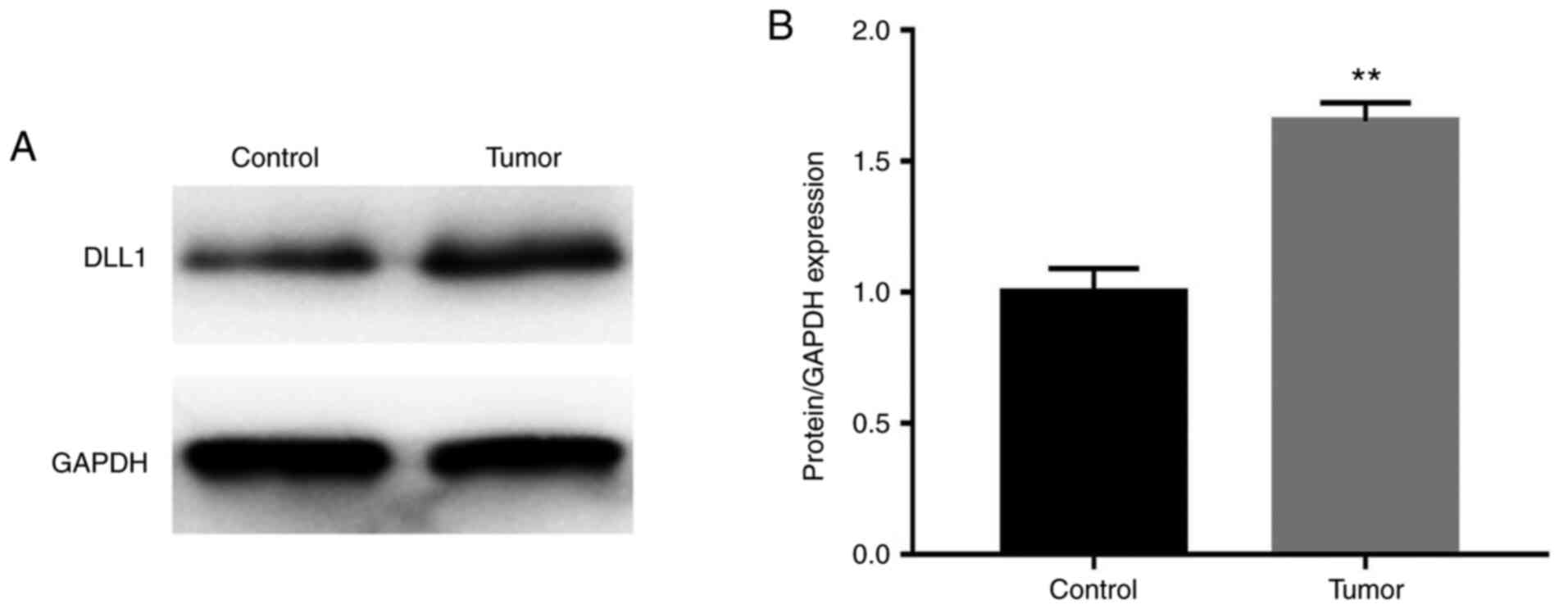
DLL1 expression in prostate
cancer
Western blot analysis was used to examine the DLL1
protein level. The results showed that the DLL1 protein expression
level in prostate cancer tissues was increased compared with in
normal adjacent tissues (P<0.01; Fig. 3).
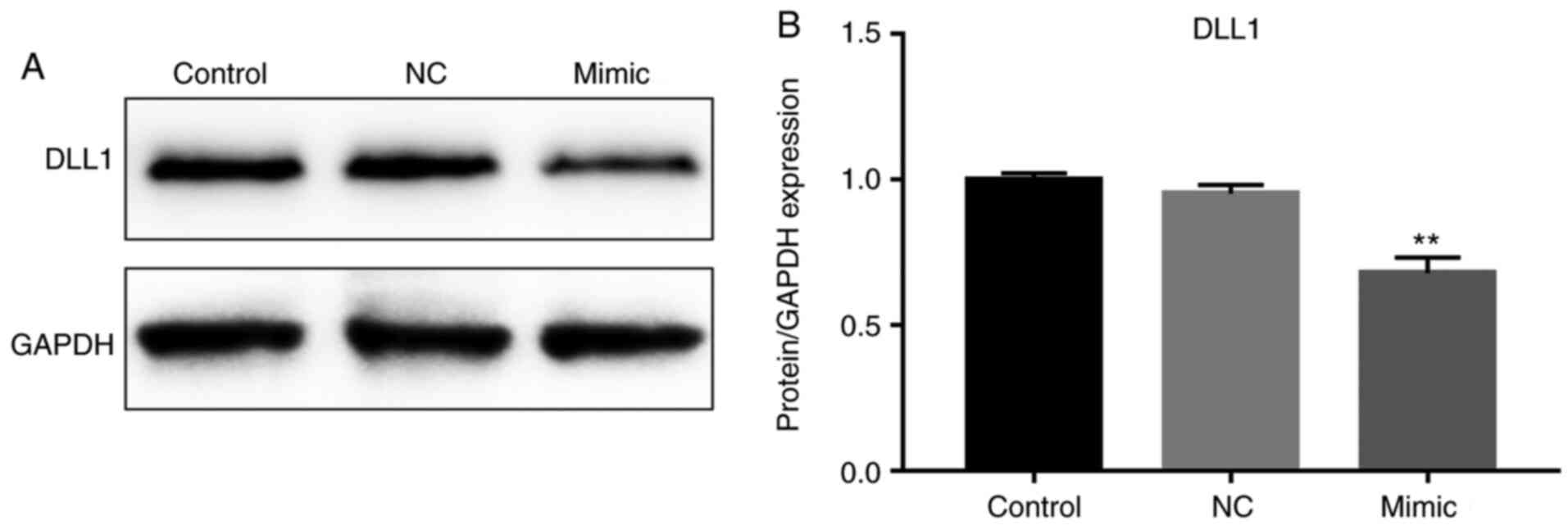
miR-130b inhibits the protein level of
DLL1
The DLL1 protein level in cells treated with
miR-130b mimics was significantly decreased compared with in the NC
and control groups (P<0.01; Fig.
4).
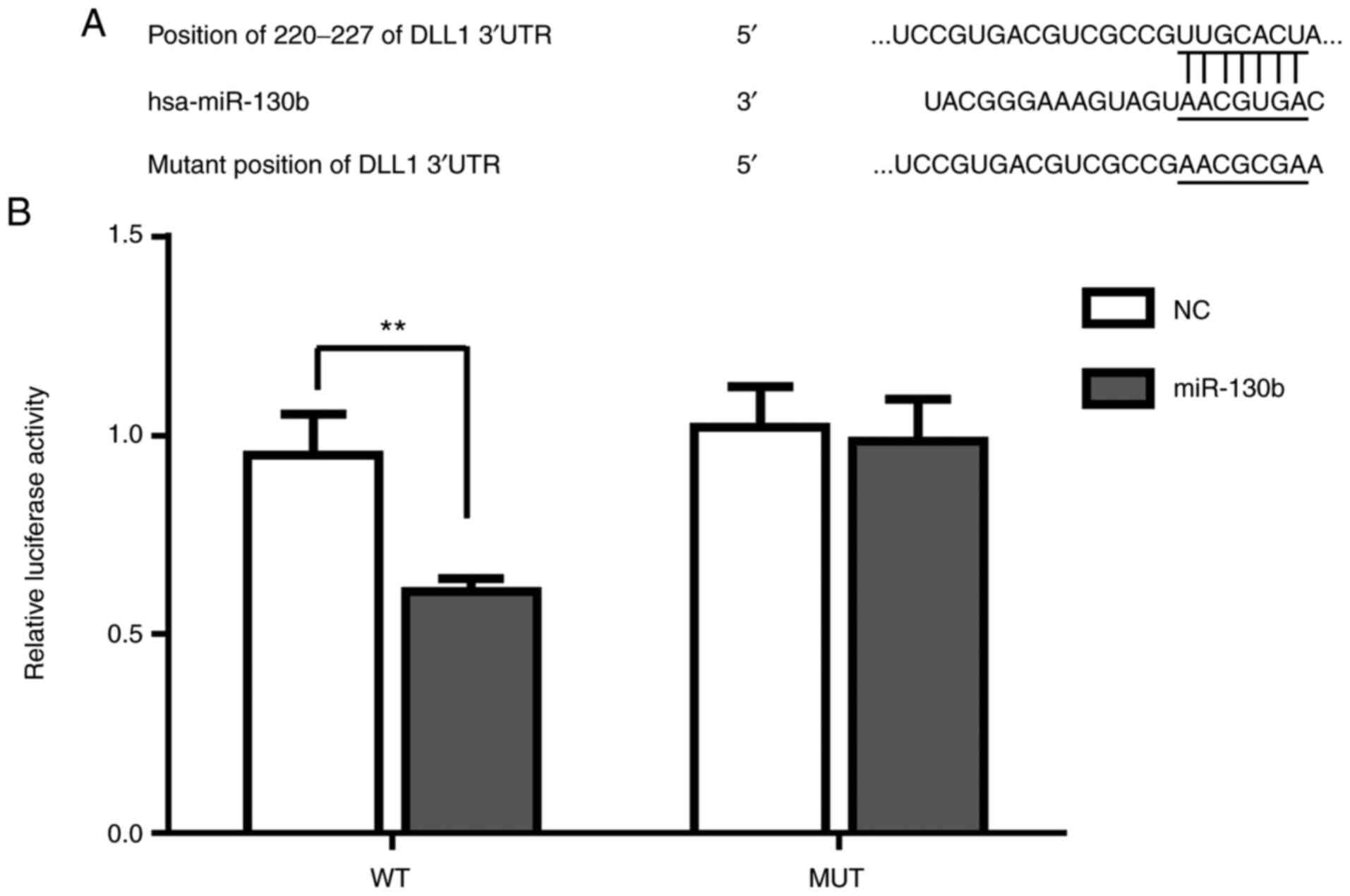
miR-130b targets DLL1
Online software (TargetScan) suggested that DLL1 was
a target gene of miR-130b (http://www.targetscan.org/vert_72/) (Fig. 5A). The results of the luciferase
assay suggested that relative luciferase activity of WT DLL1 was
significantly reduced after miR-130b mimic treatment (P<0.01),
while the luciferase activity of Mut DLL1 in the NC and miR-130b
mimic groups showed no significant difference (Fig. 5B), thus proving that DLL1 was the
direct target of miR-130b.
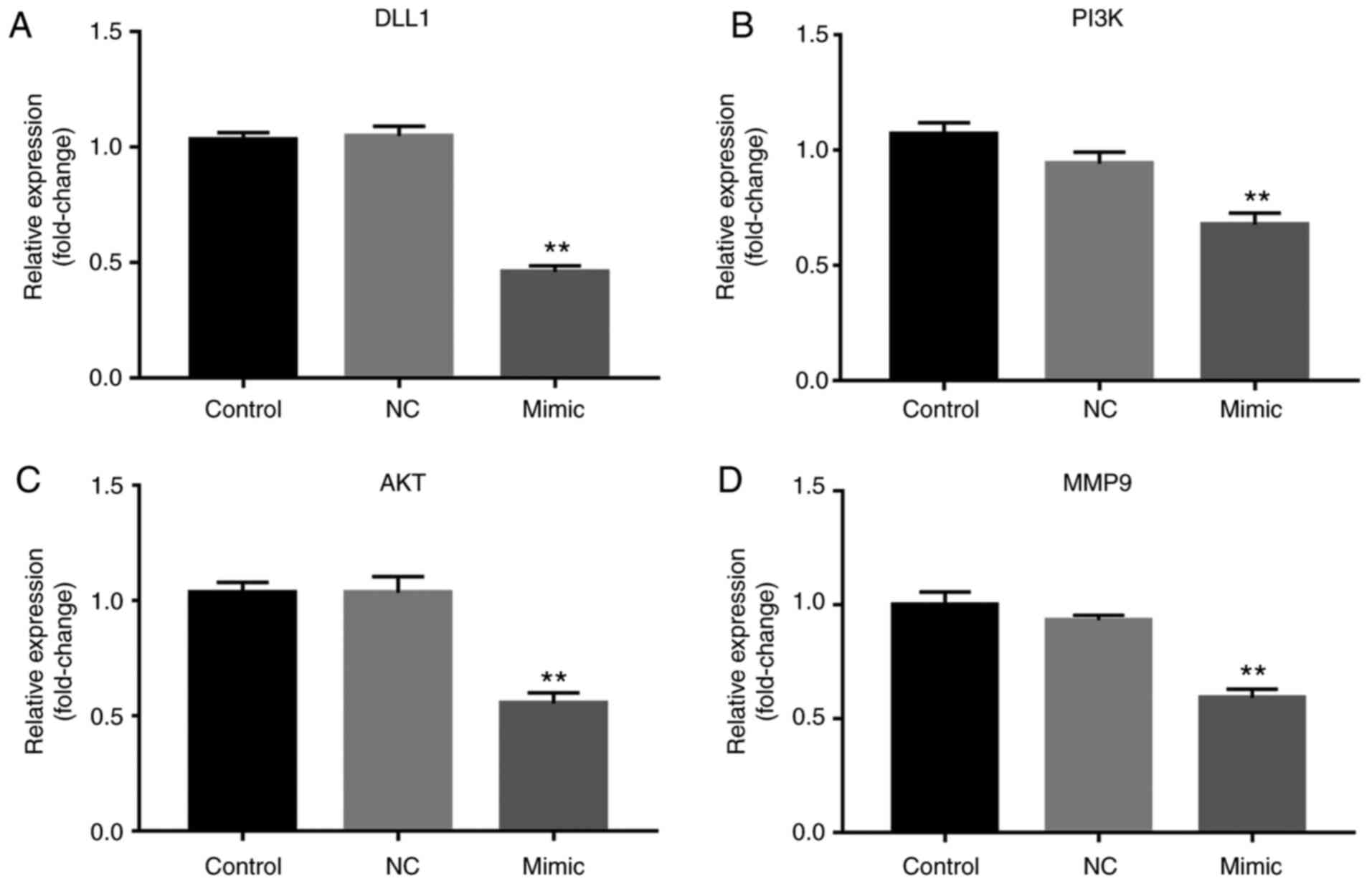
miR-130b suppresses the mRNA level of
PI3K, AKT, MMP9 and DLL1 expression
The RT-qPCR results showed that the mRNA expression
of PI3K, AKT, MMP9 and DLL1 was significantly downregulated after
miR-130b mimic treatment (P<0.01; Fig. 6).
miR-130b suppresses the mRNA level of
p-PI3K, p-AKT, MMP9 and DLL1
Protein levels of p-PI3K, p-AKT, MMP9 and DLL1 were
then checked. The results showed that miR-130b mimic or DLL1 siRNA
significantly decreased p-PI3K, p-AKT, MMP9 and DLL1 protein levels
relative to GAPDH while there were no significant differences in
PI3K and AKT levels (P<0.01; Fig.
7).
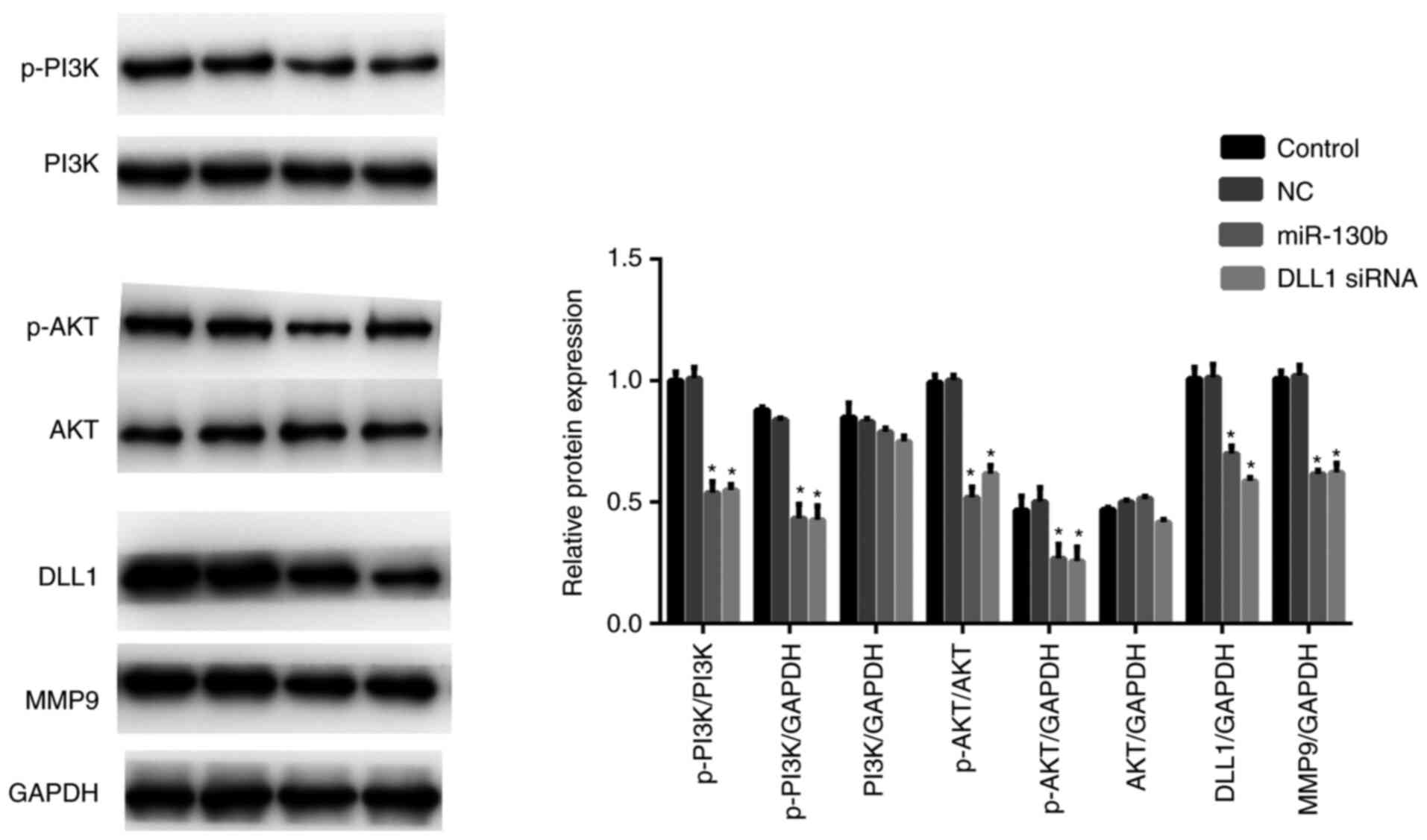 | Figure 7miR-130b downregulates the protein
level of p-PI3K, p-AKT, MMP9 and DLL1. miR-130b mimic or DLL1 siRNA
significantly decreased the protein level of p-PI3K, p-AKT, MMP9
and DLL1. Data are presented as the mean ± standard deviation.
*P<0.05, mimic group vs. NC group. p-AKT,
phosphorylated-protein kinase B; MMP9, matrix metalloproteinase-9;
miR, microRNA; PI3K, phosphatidylinositol 3 kinase; DLL, Delta-like
1. |
Discussion
Paralogous miRNA sequences of the miR130 family,
miR-130a and miR-130b are situated on chromosomes 11 and 22,
respectively (26-28).
miR-130b inhibits cell proliferation and induces apoptosis in
gastric cancer cells by targeting CYLD (29). Moreover, miR-130b is upregulated in
triple-negative breast cancer and participates in its progression
(30). Furthermore, miR-130b
inhibits the cell proliferation and invasion of pancreatic cancer
by targeting signal transducer and activator of transcription
3(31). By contrast, overexpression
of miR-130b promotes cell proliferation in esophageal squamous cell
carcinoma (32) and in
hepatocellular carcinoma (33). The
roles of miR-130b vary across cancer types. However, evidence for
the function of miR-130b in prostate cancer is not clear. In the
present study, it was found that the expression of miR-130b was
suppressed in prostate cancer tissues. It was also suggested that
miR-130b may act as an antitumor gene in prostate cancer. To fully
understand the role of miR-130b in prostate cancer, scratch wound
and Transwell assays were performed, the results of which suggested
that miR-130b inhibited the migration and invasion rates of
prostate cancer cells.
To understand the mechanisms underlying the
regulatory effect of miR-130b on the migration and invasion of
prostate cancer cells, the present study investigated the possible
target genes of miR-130b. It was identified that miR-130b may
directly target DLL1. DLL1 expression was suppressed in the
miR-130b mimic group. Furthermore, downregulated expression of DLL1
promotes lateral motility of PC-3 cells (16). DLL1-activated Notch1 stimulates
C4-2B differentiation (34).
Therefore, it was concluded that miR-130b may affect prostate
cancer cell migration and invasion by targeting DLL1.
To further understand the potential mechanisms of
miR-130b in prostate cancer, the present study assessed related
pathways. The PI3K/Akt/mTOR signaling pathway is one of the most
abnormal pathways in human cancer. PI3K/Akt/mTOR plays a role in
controlling the migration and invasion of glioblastoma cells
(35). Key gene mutations, such as
PI3K, PTEN, Akt, TSC, LKB and mTOR may lead to the abnormal
activation of certain pathways including PI3K/Akt, thus resulting
in tumorigenesis (36). Akt is the
switching locus for the cancer phenotype and a key factor in the
downstream regulation of PI3K (37). Previous studies have shown that the
PI3K/Akt pathway plays a crucial role in the migration and invasion
of prostate cancer cells (38-40).
PI3K/Akt expression was increased post-DLL1 treatment (41). Moreover, previous studies revealed
that miR-130b mediates the progression of breast cancer and
epithelial ovarian cancer via regulating the PI3K/Akt and MMP9
signaling pathway (42,43). In the present study, it was shown
that miR-130b downregulated PI3K/Akt expression. miR-130b mimic
significantly inhibited PI3K/Akt expression, thus restricting
migration and invasion abilities. Moreover, knockout of DLL1
inhibited the expression of p-PI3K/Akt. Therefore, the inhibition
of the PI3K/Akt pathway may be important in tumor treatment.
MMPs are family members of extracellular proteinases
that regulate cellular biological processes, including cell
proliferation, invasion and migration. MMPs are best known for
their role in the degradation and removal of extracellular matrix
molecules (44). Therefore, MMP
expression was shown to be related to the invasive ability of
prostate cancer. DLL1 and MMP9 play an important role in breast
cancer cell invasion (45). The
present study showed that miR-130b and knockdown of DLL1 suppressed
MMP9 activity. This finding was consistent with previous studies
(46-49).
However, there are some limitations in this study.
Firstly, an miRNA may have several targets and a gene may be
targeted by several miRNAs. Secondly, the present study was limited
to the number of participants enrolled and lack of in vivo
study. Although PI3K, Akt and MMP9 were suppressed in the mimic
group, the current study did not confirm these molecules were a
target of miR-130b directly or indirectly. Moreover, the use of 1%
FBS in wound healing assays is also a limitation of the study.
In conclusion, the present study identified a novel
pathway, interlinking PI3K/Akt, miR-130b, DLL1 and MMP9, p-Akt and
p-PI3K, which regulates the migration and invasion of prostate
cancer cells. It was found that miR-130b expression in prostate
cancer was decreased. Furthermore, miR-130b reduced the migration
of prostate cancer cells by targeting DLL1 and inhibiting the
PI3K/Akt and MMP9 pathways. These findings provide an intriguing
biomarker and treatment strategy for patients with prostate
cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LiJ was involved in the preparation of the
manuscript and worked with BL and HG to perform the experiments and
collect the data; and cooperated with LinJ, DL and JH to interpret
the data. LinJ and BJ were responsible for design of this study.
LiJ and BJ confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from all patients and
the study was approved by Yulin Traditional Chinese Medicine
Hospital Yulin.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen DQ, Yu C, Zhang XF, Liu ZF, Wang R,
Jiang M, Chen H, Yan F, Tao M, Chen LB, et al: HDAC3-mediated
silencing of miR-451 decreases chemosensitivity of patients with
metastatic castration-resistant prostate cancer by targeting NEDD9.
Ther Adv Med Oncol. 10(1758835918783132)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhu JG, Pan C, Jiang J, Deng M, Gao H, Men
B, McClelland M, Mercola D, Zhong WD and Jia Z: Six stroma-based
RNA markers diagnostic for prostate cancer in European-Americans
validated at the RNA and protein levels in patients in China.
Oncotarget. 6:16757–16765. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Paliouras AR, Monteverde T and Garofalo M:
Oncogene-induced regulation of microrna expression: Implications
for cancer initiation, progression and therapy. Cancer Lett.
421:152–160. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang C, Qian D, Zhao H, Lv N, Yu P and
Sun Z: miR-17 improves insulin sensitivity through inhibiting
expression of ASK1 and anti-inflammation of macrophages. Biomed
Pharmacother. 100:448–454. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wu N, Han Y, Liu H, Jiang M, Chu Y, Cao J,
Lin J, Liu Y, Xu B and Xie X: miR-5590-3p inhibited tumor growth in
gastric cancer by targeting DDX5/AKT/m-TOR pathway. Biochem Biophys
Res Commun. 503:1491–1497. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sun L, Guo Z, Sun J, Li J, Dong Z, Zhang
Y, Chen J, Kan Q and Yu Z: MiR-133a acts as an anti-oncogene in
Hepatocellular carcinoma by inhibiting FOSL2 through TGF-β/Smad3
signaling pathway. Biomed Pharmacother. 107:168–176.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kaalund SS, Venø MT, Bak M, Møller RS,
Laursen H, Madsen F, Broholm H, Quistorff B, Uldall P, Tommerup N,
et al: Aberrant expression of mir-218 and mir-204 in human mesial
temporal lobe epilepsy and hippocampal sclerosis-convergence on
axonal guidance. Epilepsia. 55:2017–2027. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shen J and Li M: Microrna-744 inhibits
cellular proliferation and invasion of colorectal cancer by
directly targeting oncogene notch1. Oncol Res: Feb 22, 2018 (Epub
ahead of print). doi: 10.3727/096504018X15188747585738.
|
|
11
|
Chen Q, Zhao X, Zhang H, Yuan H, Zhu M,
Sun Q, Lai X, Wang Y, Huang J, Yan J and Yu J: miR-130b suppresses
prostate cancer metastasis through down-regulation of MMP2. Mol
Carcinog. 54:1292–1300. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Zhang JP, Li N, Bai WZ, Qiu XC, Ma BA,
Zhou Y, Fan QY and Shan LQ: Notch ligand Delta-like 1 promotes the
metastasis of melanoma by enhancing tumor adhesion. Braz J Med Biol
Res. 47:299–306. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pang RT, Leung CO, Lee CL, Lam KK, Ye TM,
Chiu PC and Yeung WS: MicroRNA-34a is a tumor suppressor in
choriocarcinoma via regulation of Delta-like1. BMC Cancer.
13(25)2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yu F, Hao X, Zhao H, Ge C, Yao M, Yang S
and Li J: Delta-like 1 contributes to cell growth by increasing the
interferon-inducible protein 16 expression in hepatocellular
carcinoma. Liver Int. 30:703–714. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Purow BW, Haque RM, Noel MW, Su Q, Burdick
MJ, Lee J, Sundaresan T, Pastorino S, Park JK, Mikolaenko I, et al:
Expression of notch-1 and its ligands, delta-like-1 and jagged-1,
is critical for glioma cell survival and proliferation. Cancer Res.
65:2353–2363. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Scorey N, Fraser SP, Patel P, Pridgeon C,
Dallman MJ and Djamgoz MB: Notch signalling and voltage-gated Na+
channel activity in human prostate cancer cells: Independent
modulation of in vitro motility. Prostate Cancer Prostatic Dis.
9:399–406. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Adimonye A, Stankiewicz E, Kudahetti S,
Trevisan G, Tinwell B, Corbishley C, Lu YJ, Watkin N and Berney D:
Analysis of the pi3k-Akt-mtor pathway in penile cancer: Evaluation
of a therapeutically targetable pathway. Oncotarget. 9:16074–16086.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cretella D, Ravelli A, Fumarola C, La
Monica S, Digiacomo G, Cavazzoni A, Alfieri R, Biondi A, Generali
D, Bonelli M and Petronini PG: The anti-tumor efficacy of cdk4/6
inhibition is enhanced by the combination with pi3k/Akt/mtor
inhibitors through impairment of glucose metabolism in tnbc cells.
J Exp Clin Cancer Res. 37(72)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li H, Xu W, Ma Y, Zhou S and Xiao R: Milk
fat globule membrane protein promotes c2c12 cell proliferation
through the pi3k/Akt signaling pathway. Int J Biol Macromol.
114:1305–1314. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014(150845)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shi X, Yang L, Xie J, Zhao Y, Cong J, Li
Z, Li H, Cheng X and Fan J: UNBS5162 inhibits proliferation of
human melanoma cells by inducing apoptosis via the PI3K/Akt
pathway. Mol Med Rep. 18:3382–3388. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang XR, Wang SY, Sun W and Wei C:
Isoliquiritigenin inhibits proliferation and metastasis of MKN28
gastric cancer cells by suppressing the PI3K/AKT/mTOR signaling
pathway. Mol Med Rep. 18:3429–3436. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Paplomata E and O'Regan R: The
PI3K/AKT/mTOR pathway in breast cancer: Targets, trials and
biomarkers. Ther Adv Med Oncol. 6:154–166. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang HY, Zhang CY, Xu LT, Zang K, Ning ZY,
Jiang F, Chi HY, Zhu XY and Meng ZQ: Bufalin suppressed
hepatocellular carcinoma invasion and metastasis by targeting
HIF-1α via the PI3K/AKT/mTOR pathway. Oncotarget. 7:320193–20208.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen Y, Zhao F, Cui D, Jiang R, Chen J,
Huang Q and Shi J: Hoxd-as1/mir-130a sponge regulates glioma
development by targeting e2f8. Int J Cancer. 142:2313–2322.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tang C, Yang Z, Chen D, Xie Q, Peng T, Wu
J and Qi S: Downregulation of mir-130a promotes cell growth and
epithelial to mesenchymal transition by activating hmgb2 in glioma.
Int J Biochem Cell Biol. 93:25–31. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wei H, Cui R, Bahr J, Zanesi N, Luo Z,
Meng W, Liang G and Croce CM: Mir-130a deregulates pten and
stimulates tumor growth. Cancer Res. 77:6168–6178. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen H, Yang Y, Wang J, Shen D, Zhao J and
Yu Q: miR-130b-5p promotes proliferation, migration and invasion of
gastric cancer cells via targeting RASAL1. Oncol Lett.
15:6361–6367. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang HD, Jiang LH, Sun DW, Li J and Ji
ZL: The role of mir-130a in cancer. Breast Cancer. 24:521–527.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhao G, Zhang JG, Shi Y, Qin Q, Liu Y,
Wang B, Tian K, Deng SC, Li X, Zhu S, et al: miR-130b is a
prognostic marker and inhibits cell proliferation and invasion in
pancreatic cancer through targeting STAT3. PLoS One.
8(e73803)2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yu T, Cao R, Li S, Fu M, Ren L, Chen W,
Zhu H, Zhan Q and Shi R: Mir-130b plays an oncogenic role by
repressing PTEN expression in esophageal squamous cell carcinoma
cells. BMC cancer. 15(29)2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chang RM, Xu JF, Fang F, Yang H and Yang
LY: Microrna-130b promotes proliferation and emt-induced metastasis
via pten/p-Akt/hif-1alpha signaling. Tumour Biol. 37:10609–10619.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zayzafoon M, Abdulkadir SA and McDonald
JM: Notch signaling and ERK activation are important for the
osteomimetic properties of prostate cancer bone metastatic cell
lines. J Biol Chem. 279:3662–3670. 2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Carmen M, Ileana G, Stefano B, Oxana B,
Bernard F, Carlo R, Rosario D and Cataldo A: PP242 counteracts
glioblastoma cell proliferation, migration, invasiveness and
stemness properties by inhibiting mTORC2/AKT. Front Cell Neurosci.
12(99)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Slattery ML, Herrick JS, Lundgreen A,
Fitzpatrick FA, Curtin K and Wolff RK: Genetic variation in a
metabolic signaling pathway and colon and rectal cancer risk: mTOR,
PTEN, STK11, RPKAA1, PRKAG2, TSC1, TSC2, PI3K and Akt1.
Carcinogenesis. 31:1604–1611. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sun DM, Tang BF, Li ZX, Guo HB, Cheng JL,
Song PP and Zhao X: miR-29c reduces the cisplatin resistance of
non-small cell lung cancer cells by negatively regulating the
PI3K/Akt pathway. Sci Rep. 8(8007)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhou Y, Gu P, Li J, Li F, Zhu J, Gao P,
Zang Y, Wang Y, Shan Y and Yang D: Suppression of STIM1 inhibits
the migration and invasion of human prostate cancer cells and is
associated with PI3K/Akt signaling inactivation. Oncol Rep.
38:2629–2636. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xu S, Ge J, Zhang Z and Zhou W: miR-129
inhibits cell proliferation and metastasis by targeting ETS1 via
PI3K/AKT/mTOR pathway in prostate cancer. Biomed Pharmacother.
96:634–641. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Dhupkar P, Zhao H, Mujoo K, An Z and Zhang
N: Crk II silencing down-regulates IGF-IR and inhibits migration
and invasion of prostate cancer cells. Biochem Biophys Rep.
8:382–388. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhang W, Hsu P, Zhong B, Guo S and Zhang
C, Wang Y, Luo C, Zhan Y and Zhang C: MiR-34a enhances chondrocyte
apoptosis, senescence and facilitates development of osteoarthritis
by targeting DLL1 and regulating PI3K/AKT pathway. Cell Physiol
Biochem. 48:1304–1316. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Miao Y, Zheng W, Li N, Su Z, Zhao L, Zhou
H and Jia L: MicroRNA-130b targets PTEN to mediate drug resistance
and proliferation of breast cancer cells via the PI3K/Akt signaling
pathway. Sci Rep. 7(41942)2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhou D, Zhang L, Sun W, Guan W, Lin Q, Ren
W, Zhang J and Xu G: Cytidine monophosphate kinase is inhibited by
the TGF-β signalling pathway through the upregulation of
miR-130b-3p in human epithelial ovarian cancer. Cell Signal.
35:197–207. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Elizabeth IH, Emma FS and Stack MS: With
great age comes great metastatic ability: Ovarian cancer and the
appeal of the aging peritoneal microenvironment. Cancers (Basel).
10(230)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Shui Y, Yu X, Duan R, Bao Q, Wu J, Yuan H
and Ma C: miR-130b-3p inhibits cell invasion and migration by
targeting the Notch ligand Delta-like 1 in breast carcinoma. Gene.
609:80–87. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Duellman T, Chen X, Wakamiya R and Yang J:
Nucleic acid-induced potentiation of matrix metalloproteinase-9
enzymatic activity. Biochem J. 475:1597–1610. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Murawala H, Patel S, Ranadive I, Desai I
and Balakrishnan S: Variation in expression and activity pattern of
mmp2 and mmp9 on different time scales in the regenerating caudal
fin of poecilia latipinna. J Fish Biol. 92:1604–1619.
2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sahay AS, Jadhav AT, Sundrani DP, Wagh GN,
Mehendale SS and Joshi SR: Matrix metalloproteinases-2 (mmp-2) and
matrix metalloproteinases-9 (mmp-9) are differentially expressed in
different regions of normal and preeclampsia placentae. J Cell
Biochem. 119:6657–6664. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Weiler J, Mohr M, Zänker KS and Dittmar T:
Matrix metalloproteinase-9 (mmp9) is involved in the
tnf-alpha-induced fusion of human m13sv1-cre breast epithelial
cells and human mda-mb-435-pfdr1 cancer cells. Cell Commun Signal.
16(14)2018.PubMed/NCBI View Article : Google Scholar
|