1. Introduction
With the widespread introduction of laparoscopic
cholecystectomy (LC), the incidence of iatrogenic main bile duct
lesions has significantly increased, with incidences ranging from
0.2 to 1.5% according to previous studies (1-3).
Although on a declining trend, with the implementation of critical
view of safety (CVS) in the dissection of the elements that define
Calot's triangle, bile duct injuries (BDI) remain a major concern
during LC. They are a potentially life-threatening complication and
one of the most frequent causes of postoperative morbidity,
associated with increased hospital stay, health-associated costs,
reinterventions or additional procedures for treatment. They are
also one of the main causes of allegations of malpractice in
biliary surgery. The main risk factors for BDI are the severity of
the inflammatory process in acute cholecystitis (AC),
fibro-sclerosing remodeling associated with chronic inflammation,
and the anatomical variability of the bile duct, which are also
risk factors for conversion to open surgery (4). Postoperative adhesions are the leading
cause of conversion from laparoscopy to laparotomy (5). Thus, clinical trials reveal that,
although recommended in current practice protocols, many surgeons
prefer to delay LC in AC until remission of local inflammatory
phenomena to avoid iatrogenic damage to the main bile duct
(6,7).
New safe LC surgery protocols pay special attention
to the prevention of main bile duct lesions (1,2), and
ICG-assisted near-infrared cholangiogram (NIRC) is an emerging
technique that may increase the visualization of the extra biliary
structures (3). Accurate
techniques, understanding of the local anatomy and adequate
exposure of the extra hepatic biliary structures are key factors in
preventing such injuries and providing safe LC.
Intraoperative cholangiography (IOC) provides
important benefits in situations of difficult dissection or
suspicion of main bile duct stones, but has a number of
disadvantages including prolonging surgery, requiring portable
radiology equipment, specialized personnel, irradiation, and
increased costs. Routine IOC may help identify a BDI at the time of
surgery but not necessarily prevent its occurrence (7). Moreover, by requiring the injection of
contrast material into the bile duct, IOC may increase the risk of
BDI.
Ishizawa et al first demonstrated in 2009 the
usefulness of ICG NIRC in liver transplantation, by direct
injection into the bile duct and in open cholecystectomy, by
intravenous preoperative administration (8). This new approach was extremely
attractive, particularly in the context in which the new
laparoscopic systems have incorporated the software function that
allows the acquisition of the image in NIR and the overlap of
information over the image obtained in white light. This allows the
obtaining of additional information without increasing the
operating time or changing the operating time sequence (9).
ICG is a dye currently used in various medical and
surgical specialties (cardiology, ophthalmology and abdominal
surgery) that binds with circulating albumins and lipoproteins and
is excreted into the bile almost unaltered following hepatic
extraction. Its half time in the blood stream is between 3 and 5
min (10). An alternative in
biliary surgery is its direct injection into the gallbladder. The
molecule of ICG has an emission with a spectrum peak at 810-830 nm,
in NIR, that conveniently avoids the endogenous interferences with
water and body proteins. It has an excellent safety profile for
human use; however, it is crystallized using iodized salts which
renders it contraindicated in patients with iodine allergies
(10).
Thus far, given the novelty of the method and the
relatively limited access to laparoscopic equipment with an image
acquisition system for NIR, there is no consensus on the dose,
timing and optimal mode of administration, or the indications in
which NIRC with ICG provides a real benefit through increased
safety in LC.
2. Data and methods
A systematic review was performed on articles in
English published until March 2021, which were identified on
PubMed, Springer Nature, Elsevier and Scopus via specific mesh
terms: ‘Indocyanine green’/‘near-infrared fluorescence’ and
‘laparoscopic cholecystitis’. Criteria for inclusion in this
systematic review were observational studies with non-malignant
pathologies of gallbladder undergoing laparoscopic cholecystitis
using ICG NIRC reporting at least one of the following outcomes:
operative time, biliary anatomy identification time, success rate
of biliary tract imaging, bile duct lesions, conversion to open
surgery, hospital stay, and postoperative complications.
Exclusion criteria included malignancies, studies
with no adequately described surgical procedure, studies evaluating
ICG in open cholecystectomies or other hepato-biliary surgeries.
Editorials, reviews, case-reports, commentaries, letters and book
chapters were not included.
The systematic review analyzed information
regarding: i) dose, timing and administration of ICG for NIRC used
in the previously published studies; ii) the percentage in
increased visualization of extrahepatic biliary structures; iii)
the incidence of main bile duct lesions and conversions in the
NIR-assisted group (vs. the control group, if any); and iv)
secondary reactions associated with ICG administration.
All studies were categorized based on The Oxford
Centre for Evidence-based Medicine, and two reviewers analyzed the
abstracts for inclusion in the systematic review. A PRISMA flow
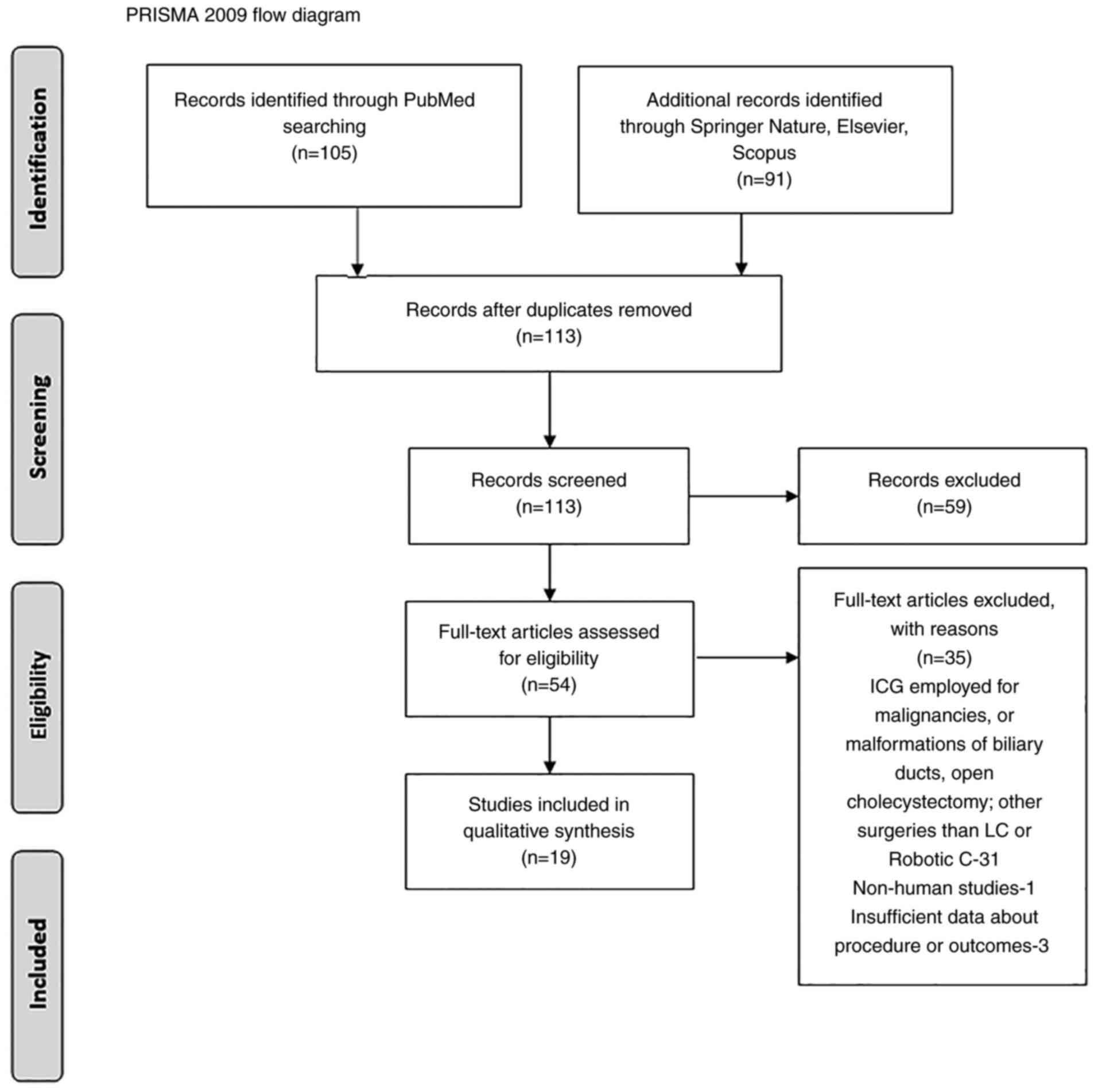
chart was employed to screen studies for eligibility.
Our search resulted in 105 articles identified on
PubMed, and an additional 91 in other databases (Springer Nature,
Elsevier, Scopus). After duplication removal and screening for
eligibility, a total of 19 clinical studies regarding the use of
ICG in LC and/or robotic cholecystectomy (RC), were included in
this review. A PRISMA flow diagram is revealed in Fig. 1.
The ensuing comments are based on the analysis of 19
studies (6,10-27)
documenting the use of ICG NIRC in LCs or RCs, for non-malignant
gallbladder disease (symptomatic biliary lithiasis, acute or
chronic cholecystitis, and gallbladder polyps) in a total of 2,490
patients. Only 2are randomized controlled studies (RCT), 11
prospective and 6 are retrospective studies (7,11-28)
(Table I). The safety of the ICG
was excellent, there was only one report of a self-resolved rash
(0.04%), with no other adverse reactions.
 | Table IClinical studies regarding the role
of NIR-ICG in laparoscopic/robotic cholecystectomies. |
Table I
Clinical studies regarding the role
of NIR-ICG in laparoscopic/robotic cholecystectomies.
| Aauthors, year | No. patients in the
NIRC group; no. of patients in the WL group | Type of study | Dose, timing,
method of ICG administration in the NIRC group | Adverse
effects | Imaging system | BDI lesion in the
NIRC group; BDI lesions in the WL group | Cases of conversion
to open surgery in the NIRC group; Cases of conversion to open in
the WL group | (Refs.) |
|---|
| Dip et al,
2019 | 321; 318 | RCT | 0.05 mg/kg i.v.,
>45 min | None | Image 1S, Opal 1
(Karl Storz) | 0; 2 (0.62%) | 1 (0.3%,
hemorrhage); 4 (1.2-2% BDI lesions); 2 (insufficient
visibility) | (11) |
| Agnus et al,
2020 | 314 | Prospective | 0.02-0.62 mg/kg
(median 0.3 mg/kg); 30-3,120 min (median 57 min) | 1 self-resolved
rush | ‘D-LIGHT P (Karl
Storz)’, n=238; ‘STRYKER’, n=21; ‘FIREFLY (Surgical Intuitive)’,
n=53 | 0 | N/I | (12) |
| Liu et al,
2018 | 46 | Prospective | 10 ml, 0.125 mg/ml
bolus, direct gallbladder injection (percutaneous
catheter/intraoperatively) | 5 cases of leakage
and impaired visibility | IMAGE1 S, D-Light
P, Karl Storz | 0 | 0 | (13) |
| Bleszynski et
al, 2020 | 108 | Prospective | 1.65 ml, at
anesthetic induction; supplementary 0.3 ml in 30 cases | None | IMAGE1 S, D-Light
P, Karl Storz | 0 | 0 | (7) |
| Daskalaki et
al, 2014 | 184 | Prospective | 2.5 mg of ICG,
i.v., 45 min before | None | Da Vinci
Fluorescence Imaging Vision System | 0 | 0 | (14) |
| Buchs et al,
2012 | 12 | Prospective | 2.5 mg of ICG, i.v.
45 min before | None | Da Vinci
Fluorescence Imaging Vision System | 0 | 0 | (15) |
| Wang et al,
2020 | 34; 36 | Retrospective | 1 ml of ICG (2.5
mg/ml, 30 min before | None | PINPOINT system
(NOVADAQ, Mississauga, Ontario, Canada) | 0 | 0 | (16) |
| Koong et al,
2021 | 30; 33 | RCT | 2.5 mg of ICG,
before induction of anesthesia, 58±23 min | None | IMAGE1 S, D-Light
P, Karl Storz | 0 | 2 (6.25%); 3
(8.35), excluded from the study group | (17) |
| Matsumura et
al, 2021 | 20 | Prospective | 10 patients: 2.5 mg
ICG before surgery 10 patients: 0.25 mg/kg one day before | None | IMAGE1 S, D-Light
P, Karl Storz | 0 | 0 | (18) |
| Yoshiya et
al, 2019 | 39; 81 | Retrospective | 2.5 mg of ICG;
before induction of anesthesia; Repeat 2.5 mg bolus at Calot's
triangle dissection to identify cystic artery and right hepatic
artery | None | IMAGE1 S, D-Light
P, Karl Storz | 0:2 (2.4%) | 1 (2.54% bleeding):
20 (24.69%-16 difficult dissection, 2 BDI lesions, 2 cystic artery
lesions) | (19) |
| Sharma et
al, 2018 | 96; 91 | Retrospective | N/I | None | Firefly, Da Vinci
Fluorescence Imaging Vision system | N/A | 2.1% vs. 8.9%
(P=0.22) | (20) |
| Gangemi et
al, 2017 | 676; 289 | Retrospective | 2.5 mg of the ICG,
45 min before | None | FireFly, Da Vinci
Fluorescence Imaging Vision system | 1:13 (0.15% vs.
4.5%) | 1(0.15%): 4
(1.38%) | (21) |
| Spinoglio et
al, 2013 | 45 | Prospective | 2.5 mg of ICG,
30-45 min before | None | FireFly, Da Vinci
Fluorescence Imaging Vision system | 0 | 0 | (22) |
| Osayi et al,
2015 | 82 | Prospective | 2.5 mg of ICG, 60
min before | None | ‘STRYKER’ | 0 | 0 | (23) |
| Ambe et al,
2019 | 29; 41 | Retrospective | 0.5 ml of ICG, 60
min before | None | PINPOINT,
Novadaq | 0 | 0:1(2.4%) | (24) |
| Quaresima et
al, 2020 | 44; 44 | RCT | 0.1±0.1 mg/kg i.v.,
10.7±8.2 h before (42 cases) 4 cc of 0.5 mg/ml in the gallbladder
(2 cases) | None | Karl Storz Image 1S
D-Light system | 0:0 | 0:0 | (25) |
| Ankersmit et
al, 2017 | 18 | Prospective | 0.2 mg/kg ICG, At
induction (30-70 min before) | None | Olympus (Olympus
Corporation) | 0 | 0 | (26) |
| Broderick et
al, 2021 | 400; 989 | Retrospective | 3 ml of a 25 mg/10
ml solution, >45 min before | None | Stryker 1588 AIM
camera system, or the Stryker 1688 AIM 4K platform | 0.5% vs. 0.91% | 1.5% vs. 8.5% | (27) |
| Dip et al,
2016 | 71 | Prospective | 0.05 mg/kg | None | IMAGE1 S, D-Light
P, Karl Storz | 0 | 0 | (28) |
The gallbladder pathology which required LC or RC,
was in most cases chronic, and was performed as elective
cholecystectomy. Only two studies evaluated the role of ICG NIRC in
AC and complicated cholecystitis. The degree of local inflammation
is an important source of bias when analyzing the results, due to
diminished visualization of the elements of the Calot's
triangle.
As final outcomes, certain studies focused on the
assessment of the visibility rate of the extrahepatic biliary
structures, while others focused on the clinical results concerning
the rate of BDI and the rate of conversion or both.
3. Dose, timing and administration
NIRC has been reported as a simple, feasible, safe
and cost-effective procedure, which may improve safety in difficult
cases of LC (7,8). Its use in combination with white light
has been demonstrated to be superior to white light alone in
identifying extrahepatic biliary anatomy, Calot's triangle,
anatomical variations, accessory hepatic ducts, thus decreasing the
risk of intraoperative BDI (11,16,17).
The most used method of administration of ICG was
intravenously, and only one study (13) evaluated the efficiency of NIRC when
ICG was administered directly into the gallbladder, either via a
preexisting catheter after percutaneous gallbladder evacuation, or
by direct puncture of the gallbladder intraoperatively. In order to
avoid spillage, a pouch was previously created to seal the orifice.
The images obtained by Liu et al were qualified to be of
good quality, ensuring an adequate view of the structure during
dissection, with no cases of conversion and BDI. However, in 5
cases (10.86%) the authors reported leakage of the dye at the level
of the gallbladder puncture, which impaired vision (13).
In most of the cases, ICG was administered shortly
before or at the induction of anesthesia, with a time range of
30-60 min to the intervention. The doses in these cases were either
a fixed dose varying between 1.25-7.5 mg of diluted ICG solution
(14-24,27),
or an adjusted dose of 0.02-0.62 mg/kg (11,12,28).
The majority of the studies included in the review used 2.5 mg
administered within 1 h before imaging (7,14-23).
A supplemental bolus was used intraoperatively in selected cases,
to improve observation of the cystic and right hepatic arteries, if
necessary. In the studies using this timing of administration of
ICG, the main drawback was related to the hyperfluorescence of the
liver in the background.
Other authors attempted to describe an optimal
administration to reduce the fluorescence ratio, by comparing early
with delayed administration. Boogerd et al (29) revealed that the highest bile
duct-to-liver ratio was achieved 3 to 7 h after administration of 5
mg and 5-25 h after administration of 10 mg ICG, and recommended
administering 5 mg ICG at least 3 h before imaging. Similar
conclusions were revealed by Matsumura et al (18) and Chen et al (30), when comparing early to delayed
administration of ICG. The drawbacks of delayed administration are
the relative difficulty of using it in emergency or day care
surgery.
4. Factors influencing the intensity of the
fluorescence signal and visibility of the extrahepatic biliary
structures
The intensity of the NIRC fluorescence signal was
revealed to depend on several factors, including the device used,
the distance from the tip of the laparoscope to its target and the
amount of covering tissue. It is also important to set the tip of
the 0˚ or 30˚ laparoscope vertically to Calot's triangle to
directly irradiate exciting light on the bile ducts and efficiently
obtain fluorescence signals (31).
A disadvantage of NIRC is that its tissue penetration ability is
limited to 5-10 mm (16,17). The major limitation of an ICG
cholangiogram is that it may fail to delineate the deeply located
bile ducts during LC (31). This
finding explains the lower visualization rate for the common
hepatic duct (CHD) in comparison to the more superficial located CD
and the common bile duct (CBD), identified by several authors, and
presented in Table II.
 | Table IIVisualization of extrahepatic biliary
structures with NIRC. |
Table II
Visualization of extrahepatic biliary
structures with NIRC.
| | Before dissection
of Calot's triangle | After dissection of
Calot's triangle | |
|---|
| First author,
year | CD (%) | CBD (%) | CHD (%) | CD (%) | CBD (%) | CHD (%) | (Refs.) |
|---|
| Dip et al,
2019 | 66.6 (ICG) | 49.4 (ICG) | 28.9 (ICG) | 96.9 (ICG) | 75.7 (ICG) | 52.3 (ICG) | (11) |
| | 36.2 (WL) | 20.6 (WL) | 10.9 (WL) | 97.2 (WL) | 50 (WL) | 30.5 (WL) | |
| Agnus et al,
2020 | 88.2 | 76.4 | 59.3 | 97.1 | 86.6 | 69.3 | (12) |
| Liu et al,
2018 | 32 (ICG) | 52 (ICG) | 44 (ICG) | 84 (ICG) | 76 (ICG) | 68 (ICG) | (13) |
| | 8 (WL) | 24 (WL) | 16 (WL) | 44 (WL) | 28 (WL) | 16 (WL) | |
| Bleszynski et
al, 2020 | 90 | 84.3 | 48.1 | N/I | N/I | N/I | (7) |
| Daskalaki et
al, 2014 | 97.8 | 96.1 | 94 | N/I | N/I | N/I | (14) |
| Buchs et al,
2012 | 91.7 | 50 | 33.3 | 100 | 83.3 | 66.7 | (15) |
| Wang et al,
2020 | 91 (vs. 74 non
ICG) | 53 (vs. 21 non
ICG) | 79 (vs. 47 non
ICG) | N/I | N/I | N/I | (16) |
| Spinoglio et
al, 2013 | 93 | 91 | 88 | 97 | 97 | 97 | (22) |
| Ankersmit et
al, 2017 | 30.7 (vs. 16.6 in
WL) | 15.3 (vs. 0 in
WL) | N/I | 72 (vs. 100
WL) | 38.8 (vs. 16.6 in
WL) | N/I | (26) |
| Dip et al,
2016 | 100 (BMI <30
kg/m2) 100 (BMI >30 kg/m2) | 93.9 (BMI <30
kg/m2) 81.6 (BMI >30 kg/m2) | 81.8 (BMI <30
kg/m2) 60.5 (BMI >30 kg/m2) | N/I | N/I | N/I | (28) |
The light emitted may not penetrate through the
tissues of patients with thick peritoneal fat or patients with
peritoneal scarring secondary to inflammation. Thus, in patients
with severe cholecystitis and/or obesity, ICG near-infrared
fluorescent cholangiography may fail to reveal the whole anatomy of
the extrahepatic bile ducts buried in thick connective tissues
prior to dissection of Calot's triangle. Wang et al
(16) revealed that a BMI >25
kg/m2 reduced the visibility of biliary structures in
NIRC. Similar results were observed by Dip et al (28), but the decrease met statistical
significance only for the most profound structure, the CHD.
However, the method remains a helpful tool in comparison to white
light only, during dissection, avoiding BDI and bile leakage from
the bile duct stump (16,30,31).
The smallest rate of visualization of the biliary anatomy prior to
Calot's triangle dissection was encountered by Ankersmit et
al (26), and this rate could
be explained by the fact that the authors included only patients
with complicated cholecystitis in the study group, with increased
risk of BDI.
5. ICG NIRC and the rate of BDI and
conversion in the study groups
In the reviewed studies, the incidence of conversion
to open surgery varied widely between 0 and 6.25% in the ICG groups
and 0 to 24.69% in the non-ICG groups, and the higher rates were
associated with acute inflammation and complicated cholecystitis.
Overall, the conversion rate was of 0.52% in the 2,490 patients in
the IGC group and 2.52% in the patients who underwent LC without
ICG NIRC.
The incidence of BDI was 0.12% in the ICG group
(with variations between 0 and 0.5%) and 1.31% in the non-ICG
patients, varying from 0 to 4.5%. Studies with a low number of
patients and elective surgeries reported similar incidence of
complications (16,17,24).
Wang et al and Koong et al (16,17)
observed no significant decrease in the time necessary to achieve
CVS during dissection and blood loss.
Yoshiya et al (19) revealed that the ICG group had a
significantly shorter operative time (129±46 vs. 150±56 min;
P=0.0455), markedly lower conversion rate (2.6 vs. 22.0%;
P=0.0017), and lower proportion of subtotal cholecystectomy (0 vs.
6.6%; P=0.0359) than the non-ICG group. Similar results were
revealed by Sharma et al (20) and Gangemi et al (21), when comparing RC with ICG and LC.
The evidence supports the theory that fluorescent cholangiography
during RC may contribute to proper identification of biliary
structures and may reduce the rates of open conversion. However,
the authors admit that there was an increased percentage of AC in
the LC group. It is debatable whether the results were consequences
of an improved visualization of the ICG dye or it may be a result
of the increased visualization and ergonomics of the robotic
platform. At this stage of development, the literature on
intraoperative ICG in LC focused primarily on the efficacy of
technology and its ability to identify structures and less on
clinical outcomes, while a direct comparison of ICG-aided RC and LC
was not performed (21).
Broderick et al (27) revealed that overall BDI were
decreased with the use of an ICG cholangiogram, suggesting that
improved visualization of the biliary tree via ICG as standard of
care during LC may decrease the rate of iatrogenic injury.
6. Challenges in laparoscopic
cholecystectomy using ICG NIR
Optimal gallbladder dissection requires direct
visualization in order to obtain the CVS and reduce the risk of
BDI. ICG cholangiography is unable to completely replace direct
visualization; however, it provides an alternative perspective in
identifying Calot's triangle. This enhanced biliary visualization
with ICG could provide comfort for the operating surgeon, in that
the CD, CBD, and CHD have been properly identified prior to
clipping of the CD and cystic artery (7). With the development of the imaging
systems and software of the most used devices for laparoscopy, NIR
ICG cholangiography is becoming more readily available to the
surgeon, in every day practice. This may be an option for increased
safety of new surgeons, that are learning the LC technique. In
addition, it may improve the early cholecystectomy rates in AC and
decrease the rate of BDI and conversion in complicated cases.
Drawbacks of NIRF-C lie in the need to inject a
fluorophore, the inability to detect retained stones, and the noise
fluorescence signal from the liver. In a comparative study, the
relatively high fluorescence liver background led surgeons to
assign a lower score to the image quality obtained with NIRF-C,
when compared with X-ray intraoperative cholangiography (32). Conversely, in a systematic review by
Vlek et al (33), the
results revealed similar results for biliary tract visualization
with near-infrared imaging with ICG during LC compared with
conventional intraoperative cholangiography, but more
standardization and optimization are needed in the ICG technique.
To enhance the contrast between the fluorescence of the
extrahepatic biliary ducts and the liver in the background, various
strategies have been employed including delayed intravenous
administration (3-24 h prior to surgery), or, alternatively
administration of ICG directly into the gallbladder, either by a
preplaced drain tube or by direct intraoperative cystic injection.
In the second case, there is some risk of dye spillage, which could
impair the visibility by contaminating the operative field
(13). The dose is smaller, and the
technique has been applied in AC, after puncture, when the local
inflammation decreases the visibility of the CVS. CD permeability
is essential to obtain the image of the CBD and the intraoperative
injection requires LC suturing skills, to create the sealing pouch
at the level of the gallbladder, which is also a time-consuming
step.
There was an increased variability in dose and
timing of the ICG administration reported in the studies included
in the present review, rendering a meta-analysis unreliable due to
increased risk of bias. Further standardization and training in
this technique are necessary for a comprehensive approach, but
differences in fluorescence imaging systems also hinder comparisons
between fluorescence cholangiography studies (29). In a previous study by Kono et
al (31), 5 different
fluorescence laparoscopic imaging systems for fluorescence
cholangiography were compared including a prototype and an improved
version of the Hamamatsu Photonics laparoscope, the fluorescence
imaging system of Olympus Medical Systems, the Karl Storz HD
fluorescence laparoscope, and the fluorescence imaging system of
Novadaq. The results indicated that the contrast of ICG was
significantly different among all the used laparoscopic imaging
systems, which makes the outcomes difficult to compare.
The European registry on fluorescence image-guided
surgery (FIGS) (www.euro-figs.eu) aims to obtain a snapshot of the
current practices of FIGS and is a valuable tool in promoting and
monitoring FIGS-related educational and consensus activities in
Europe (12).
As both BDI and conversion are low-frequency
complications of LC, the differences between the ICG group and the
non-ICG group become statistically significant with a high number
of patients. In smaller groups, these complications may not be
encountered naturally, or vice versa, the presence of 1-2 separate
cases may significantly increase the incidence rate. Although
certain authors, such as Koong et al (17) did not reveal a statistical advantage
in the use of ICG near-infrared fluorescent cholangiography for
establishing CVS, they did demonstrate that an ICG NIR
cholangiogram was safe and clinically not inferior. Moreover, it
may be a helpful tool for residents or new surgeons, on the
learning curve of LC (17).
In a systematic review and meta-analysis performed
by Liu et al (34),
including 11 studies, with a total of 2,221 patients, the ICG group
was revealed to benefit from statistically significant shorter
operative time, shorter biliary anatomy identification time, lower
blood loss, higher success rate of biliary tract imaging, lower
rate of conversion to open surgery and shorter hospital stay
(34).
In a review performed by Pesce et al
(35), the frequencies of detection
of the extrahepatic biliary system ranged from 71.4 to 100% for the
CD, 33.3 to 100% for the CHD, 50 to 100% for the CBD, and 25 to
100% for the CD-CHD junction, with a significant decreased rate of
visualization in patients with a BMI >35 kg/m2. In
the present review, a significant lower rate of visualization of
extrahepatic biliary structures was revealed in only one study
(26), in which ICG was employed
for numerous patients with complicated cholecystitis, when compared
with that reported by Pesce et al (35). Although in patients with a higher
BMI and/or cholecystitis, fluorescence intensity is lower, NIRF
appears to be particularly more helpful (36) in performing a safe dissection and
reaching CVS.
7. Conclusions
In conclusion, ICG NIRC is considered a promising
tool to increase safety in LC. Its use in combination with white
light has been demonstrated to be superior to white light alone in
identifying the extrahepatic biliary anatomy, thus decreasing the
risk of intraoperative BDI. The intensity of the NIRC fluorescence
signal was revealed to depend on several factors, with obesity and
inflammation as the most clinically significant. Large-scale use of
ICG NIRC is necessary in order to obtain data to confirm its
clinical value and specific indications.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DCB, DS, AMD, DD, CTu and BS conceived and designed
the study. CGS, CTa, ADS, AZ, LCT, AMD, SAB, ACC, DOC and BS
performed the data collection and analysis. DS, DCB, CGS, CTa and
MST drafted the manuscript. DS, MST, CGS, SAB, CTu, DD, AZ, ACC,
DOC and ADS revised the study from a critical perspective for
important intellectual content. DS, LCT and AMD confirm the
authenticity of the raw data. The final version of the manuscript
was read and approved by all authors.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gupta V and Jain G: Safe laparoscopic
cholecystectomy: Adoption of universal culture of safety in
cholecystectomy. World J Gastrointest Surg. 11:62–84.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
van de Graaf FW, Zaïmi I, Stassen LPS and
Lange JF: Safe laparoscopic cholecystectomy: A systematic review of
bile duct injury prevention. Int J Surg. 60:164–172.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Conrad C, Wakabayashi G, Asbun HJ,
Dallemagne B, Demartines N, Diana M, Fuks D, Giménez ME, Goumard C,
Kaneko H, et al: IRCAD recommendation on safe laparoscopic
cholecystectomy. J Hepatobiliary Pancreat Sci. 24:603–615.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Serban D, Socea B, Balasescu SA, Badiu CD,
Tudor C, Dascalu AM, Vancea G, Spataru RI, Sabau AD, Sabau D and
Tanasescu C: Safety of laparoscopic cholecystectomy for acute
cholecystitis in the elderly: A multivariate analysis of risk
factors for intra and postoperative complications. Medicina
(Kaunas). 57(230)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fometescu SG, Costache M, Coveney A,
Oprescu SM, Serban D and Savlovschi C: Peritoneal fibrinolytic
activity and adhesiogenesis. Chirurgia (Bucur). 108:331–340.
2013.PubMed/NCBI
|
|
6
|
Song GM, Bian W, Zeng XT, Zhou JG, Luo YQ
and Tian X: Laparoscopic cholecystectomy for acute cholecystitis:
Early or delayed?: Evidence from a systematic review of discordant
meta-analyses. Medicine (Baltimore). 95(e3835)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bleszynski MS, DeGirolamo KM, Meneghetti
AT, Chiu CJ and Panton ON: Fluorescent cholangiography in
laparoscopic cholecystectomy: An updated canadian experience. Surg
Innov. 27:38–43. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ishizawa T, Tamura S, Masuda K, Aoki T,
Hasegawa K, Imamura H, Beck Y and Kokudo N: Intraoperative
fluorescent cholangiography using indocyanine green: A biliary road
map for safe surgery. J Am Coll Surg. 208:e1–e4. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dip F, Lo Menzo E, White KP and Rosenthal
RJ: Does near-infrared fluorescent cholangiography with indocyanine
green reduce bile duct injuries and conversions to open surgery
during laparoscopic or robotic cholecystectomy? -A meta-analysis.
Surgery. 169:859–867. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
van Manen L, Handgraaf HJM, Diana M,
Dijkstra J, Ishizawa T, Vahrmeijer AL and Mieog JSD: A practical
guide for the use of indocyanine green and methylene blue in
fluorescence-guided abdominal surgery. J Surg Oncol. 118:283–300.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dip F, LoMenzo E, Sarotto L, Phillips E,
Todeschini H, Nahmod M, Alle L, Schneider S, Kaja L, Boni L, et al:
Randomized trial of near-infrared incisionless fluorescent
cholangiography. Ann Surg. 270:992–999. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Agnus V, Pesce A, Boni L, Van Den Bos J,
Morales-Conde S, Paganini AM, Quaresima S, Balla A, La Greca G,
Plaudis H, et al: Fluorescence-based cholangiography: Preliminary
results from the IHU-IRCAD-EAES EURO-FIGS registry. Surg Endosc.
34:3888–3896. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu YY, Liao CH, Diana M, Wang SY, Kong
SH, Yeh CN, Dallemagne B, Marescaux J and Yeh TS: Near-infrared
cholecystocholangiography with direct intragallbladder indocyanine
green injection: Preliminary clinical results. Surg Endosc.
32:1506–1514. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Daskalaki D, Fernandes E, Wang X, Bianco
FM, Elli EF, Ayloo S, Masrur M, Milone L and Giulianotti PC:
Indocyanine green (ICG) fluorescent cholangiography during robotic
cholecystectomy: Results of 184 consecutive cases in a single
institution. Surg Innov. 21:615–621. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Buchs NC, Hagen ME, Pugin F, Volonte F,
Bucher P, Schiffer E and Morel P: Intra-operative fluorescent
cholangiography using indocyanin green during robotic single site
cholecystectomy. Int J Med Robot. 8:436–440. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Wang C, Peng W, Yang J, Li Y, Yang J, Hu
X, Xia L, Zhang L, Zhong Y, Qiao L and Pan W: Application of
near-infrared fluorescent cholangiography using indocyanine green
in laparoscopic cholecystectomy. J Int Med Res.
48(300060520979224)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Koong JK, Ng GH, Ramayah K, Koh PS and
Yoong BK: Early identification of the critical view of safety in
laparoscopic cholecystectomy using indocyanine green fluorescence
cholangiography: A randomised controlled study. Asian J Surg.
44:537–543. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Matsumura M, Kawaguchi Y, Kobayashi Y,
Kobayashi K, Ishizawa T, Akamatsu N, Kaneko J, Arita J, Kokudo N
and Hasegawa K: Indocyanine green administration a day before
surgery may increase bile duct detectability on fluorescence
cholangiography during laparoscopic cholecystectomy. J
Hepatobiliary Pancreat Sci. 28:202–210. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Yoshiya S, Minagawa R, Kamo K, Kasai M,
Taketani K, Yukaya T, Kimura Y, Koga T, Kai M, Kajiyama K and
Yoshizumi T: Usability of intraoperative fluorescence imaging with
indocyanine green during laparoscopic cholecystectomy after
percutaneous transhepatic gallbladder drainage. World J Surg.
43:127–133. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sharma S, Huang R, Hui S, Smith MC, Chung
PJ, Schwartzman A and Sugiyama G: The utilization of fluorescent
cholangiography during robotic cholecystectomy at an inner-city
academic medical center. J Robot Surg. 12:481–485. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gangemi A, Danilkowicz R, Elli FE, Bianco
F, Masrur M and Giulianotti PC: Could ICG-aided robotic
cholecystectomy reduce the rate of open conversion reported with
laparoscopic approach? A head to head comparison of the largest
single institution studies. J Robot Surg. 11:77–82. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Spinoglio G, Priora F, Bianchi PP, Lucido
FS, Licciardello A, Maglione V, Grosso F, Quarati R, Ravazzoni F
and Lenti LM: Real-time near-infrared (NIR) fluorescent
cholangiography in single-site robotic cholecystectomy (SSRC): A
single-institutional prospective study. Surg Endosc. 27:2156–2162.
2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Osayi SN, Wendling MR, Drosdeck JM,
Chaudhry UI, Perry KA, Noria SF, Mikami DJ, Needleman BJ,
Muscarella P II, Abdel-Rasoul M, et al: Near-infrared fluorescent
cholangiography facilitates identification of biliary anatomy
during laparoscopic cholecystectomy. Surg Endosc. 29:368–375.
2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ambe PC, Plambeck J, Fernandez-Jesberg V
and Zarras K: The role of indocyanine green fluoroscopy for
intraoperative bile duct visualization during laparoscopic
cholecystectomy: An observational cohort study in 70 patients.
Patient Safe Surg. 13(2)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Quaresima S, Balla A, Palmieri L, Seitaj
A, Fingerhut A, Ursi P and Paganini AM: Routine near infra-red
indocyanine green fluorescent cholangiography versus intraoperative
cholangiography during laparoscopic cholecystectomy: A case-matched
comparison. Surg Endosc. 34:1959–1967. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ankersmit M, van Dam DA, van Rijswijk AS,
van den Heuvel B, Tuynman JB and Meijerink WJHJ: Fluorescent
imaging with indocyanine green during laparoscopic cholecystectomy
in patients at increased risk of bile duct injury. Surg Innov.
24:245–252. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Broderick RC, Lee AM, Cheverie JN, Zhao B,
Blitzer RR, Patel RJ, Soltero S, Sandler BJ, Jacobsen GR, Doucet JJ
and Horgan S: Fluorescent cholangiography significantly improves
patient outcomes for laparoscopic cholecystectomy. Surg Endosc.
35:5729–5739. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dip F, Nguyen D, Montorfano L, Szretter
Noste ME, Lo Menzo E, Simpfendorfer C, Szomstein S and Rosenthal R:
Accuracy of near infrared-guided surgery in morbidly obese subjects
undergoing laparoscopic cholecystectomy. Obes Surg. 26:525–530.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Boogerd LSF, Handgraaf HJM, Huurman VAL,
Lam HD, Mieog JSD, van der Made WJ, van de Velde CJH and Vahrmeijer
AL: The best approach for laparoscopic fluorescence
cholangiography: Overview of the literature and optimization of
dose and dosing time. Surg Innov. 24:386–396. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chen Q, Zhou R, Weng J, Lai Y, Liu H,
Kuang J, Zhang S, Wu Z, Wang W and Gu W: Extrahepatic biliary tract
visualization using near-infrared fluorescence imaging with
indocyanine green: Optimization of dose and dosing time. Surg
Endosc. 35:5573–5582. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kono Y, Ishizawa T, Tani K, Harada N,
Kaneko J, Saiura A, Bandai Y and Kokudo N: Techniques of
fluorescence cholangiography during laparoscopic cholecystectomy
for better delineation of the bile duct anatomy. Medicine
(Baltimore). 94(e1005)2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Diana M, Soler L, Agnus V, D'Urso A, Vix
M, Dallemagne B, Faucher V, Roy C, Mutter D, Marescaux J and
Pessaux P: Prospective evaluation of precision multimodal
gallbladder surgery navigation: Virtual reality, near-infrared
fluorescence, and X-ray-based intraoperative cholangiography. Ann
Surg. 266:890–897. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Vlek SL, van Dam DA, Rubinstein SM, de
Lange-de Klerk ESM, Schoonmade LJ, Tuynman JB, Meijerink WJHJ and
Ankersmit M: Biliary tract visualization using near-infrared
imaging with indocyanine green during laparoscopic cholecystectomy:
Results of a systematic review. Surg Endosc. 31:2731–2742.
2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu Y, Peng Y, Su S, Fang C, Qin S, Wang
X, Xia X, Li B and He P: A meta-analysis of indocyanine green
fluorescence image-guided laparoscopic cholecystectomy for benign
gallbladder disease. Photodiagnosis Photodyn Ther.
32(101948)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Pesce A, Piccolo G, La Greca G and Puleo
S: Utility of fluorescent cholangiography during laparoscopic
cholecystectomy: A systematic review. World J Gastroenterol.
21:7877–7883. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
van den Bos J, Wieringa FP, Bouvy ND and
Stassen LPS: Optimizing the image of fluorescence cholangiography
using ICG: a systematic review and ex vivo experiments. Surg
Endosc. 32:4820–4832. 2018.PubMed/NCBI View Article : Google Scholar
|