Introduction
Mycoplasma pneumoniae
(M. pneumoniae) is the most common pathogen
in pediatric patients with community-acquired pneumonia (1,2) which
breaks out every 3-5 years (3). In
recent years, the number of refractory or severe cases with drug
resistance has been gradually increasing (4-6).
In addition to pulmonary inflammation as the most common
manifestation, M. pneumoniae infection may also cause damage
to multiple systems and organs (7-9).
In the last 20 years, ~60 cases of M. pneumoniae infection
with thrombotic disease in pediatric patients have been reported
worldwide (10-15).
The present study reported on a series of 7 cases of M.
pneumoniae pneumonia (MPP) accompanied by pulmonary embolism
(PE) encountered January 1st, 2016 to August 1st, 2019 at the
Department of Pediatric Intensive Care Unit of The First Hospital
of Jilin University (Changchun, China), and the clinical data of
these cases were reviewed. The present study aimed to improve the
understanding of clinicians regarding the laboratory examinations,
diagnosis and treatments for pediatric patients with MPP-associated
PE.
Case report
Cases
MPP-associated PE was confirmed in 7 cases by
radiological examination combined with serological tests and the
corresponding clinical data were collected and summarized. The
present study had been approved by the Ethics Committee of the
First Hospital of Jilin University (Changchun, China; approval no.
2019-253).
Baseline data
The seven cases were aged between 6 and 11 years
(median, 8.0 years) with a male/female ratio of 4:3, as presented
in Table I. All patients were
otherwise physically healthy. Patients with a family history of
thrombophilia and a history of allergy were excluded.
 | Table IBaseline demographic and clinical
characteristics of the patients (n=7). |
Table I
Baseline demographic and clinical
characteristics of the patients (n=7).
| Characteristic | Value |
|---|
| Demographics | |
|
Age
(years) | 8 (6, 11) |
|
Male
sex | 4 (57.14) |
| Anthropometry | |
|
Body weight
(kg) | 24.2 (21.3, 30) |
|
Body height
(cm) | 123.4 (119.5,
130) |
|
BMI
z-score | 0.5 (-0.5, 1) |
| Clinical
symptoms | |
|
Cough | 7(100) |
|
Fever | 7(100) |
|
Dyspnea | 5 (71.43) |
|
Swelling in
limb | 1 (14.29) |
| Radiological
examination | |
|
Pulmonary
CT | |
|
Extensive
diffuse inflammatory | 6 (85.71) |
|
Subcutaneous
emphysema | 1 (14.29) |
|
Pleural
effusion | 4 (57.14) |
|
Pericardial
effusion | 1 (14.29) |
|
Pulmonary
arterial embolism | |
|
Bilateral
multiple branches | 2 (28.57) |
|
Upper
lobe of the right lung | 2 (28.57) |
|
Lower
lobe of the right lung | 2 (28.57) |
|
Upper
lobe of the left lung | 1 (14.29) |
Clinical symptoms and physical
signs
All of the patients had a cough and fever as the
initial symptoms, typically irritable cough with viscous sputum,
and remittent fever. Among them, 5 cases developed dyspnea within 2
to 6 days and were hospitalized on day 3-12 from onset. These cases
developed PE on day 10-14 and their condition soon deteriorated.
Older pediatric patients complained of chest pain, chest tightness
or sudden dyspnea; younger patients were unable to describe their
symptoms, but physical examinations revealed spiritlessness,
aggravating dyspnea, flapping of nasal wings, reduced respiratory
movement amplitude on the affected side and weak breath sounds. One
case was combined with swelling in the right lower limb.
Results of auxiliary examinations
Laboratory tests
The serum biochemistry results of the cases are
presented in Table II. The serum
M. pneumoniae antibody titers (Particle Agglutination assay;
SERODIA-MYCOII; Fujirebio) were increased by varying degrees [from
negative titer at presentation to positive titer at the 2nd
examination (n=1) or >4-fold increased antibody titers during
admission (n=6)]. All cases had a significant increase in the
platelet count and D-dimer level. The levels of protein C and
protein S first transiently decreased and were then restored to
normal. Furthermore, two cases were weakly positive for
anticardiolipin antibody (ACA). In addition, four cases were
positive for ACA as detected by ELISA (16) (QUANTA Lite ACA IgG III; Inova). A
total of four cases received bronchoscopy, through which
endobronchitis and necrotizing pneumonia were revealed. The
bronchoalveolar lavage fluid was tested positive for M.
pneumoniae (DNA sequence copy number, 7,880-16,343) and
positivity for the macrolide resistance gene was detected in 2
cases.
 | Table IIResults of auxiliary examinations of
the patients. |
Table II
Results of auxiliary examinations of
the patients.
| | Images
included | |
|---|
| Patient no. | WBC
(x109/l) | PLT
(x109/l) | CRP (mg/l) | Serum M.
pneumoniae antibodya | BAL-DNA | Macrolides
resistance gene (BALF) | Other pathogen | ACA | D-dimer (µg/l) | CT | CTPA | Pathology | Treatment | Outcomes |
|---|
| 1 | 34.62 | 755 | 265 | 1:320 | 16343 | Positive | Cpn | Negative | 6883 | Fig. 2 | - | - | OP | Died |
| 2 | 27.55 | 810 | 210 | 1:160 | 14555 | Positive | S.
aureus | Positive | 6500 | Fig. 3 | - | - | OPs, | Alive |
| 3 | 5.93 | 480 | 80 | 1:160 | NA | NA | NA | Positive | 1800 | - | Fig. 5 | - | - | Alive |
| 4 | 12.33 | 550 | 110 | 1:160 | 13880 | NA | Cpn | Positive | 1200 | - | - | Fig. 7 | OPs, | Died |
| 5 | 20.12 | 610 | 164 | 1:160 | NA | NA | Cpn | Weakly
positive | 5868 | Fig. 1 | - | - | OP, | Alive |
| 6 | 9.84 | 490 | 71.4 | 1:40 | 7880 | NA | Cpn | Positive | 1024 | - | - | Fig. 6 | - | Alive |
| 7 | 18.13 | 680 | 123 | 1:160 | NA | NA | S.
aureus | Weakly
positive | 2109 | Fig. 4 | - | - | OPs, | Alive |
Radiological examination
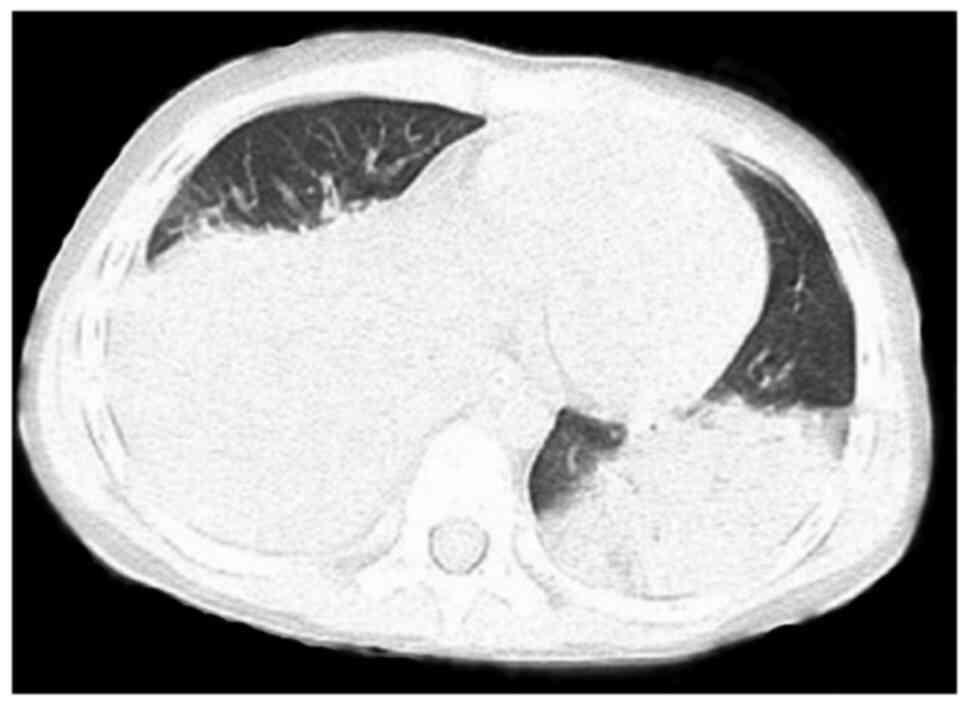
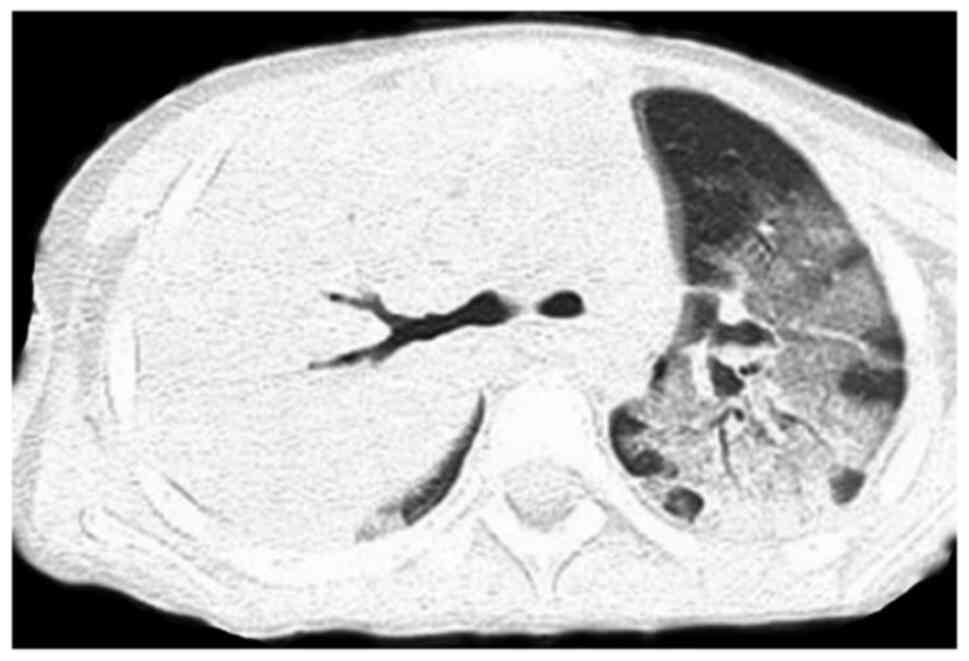
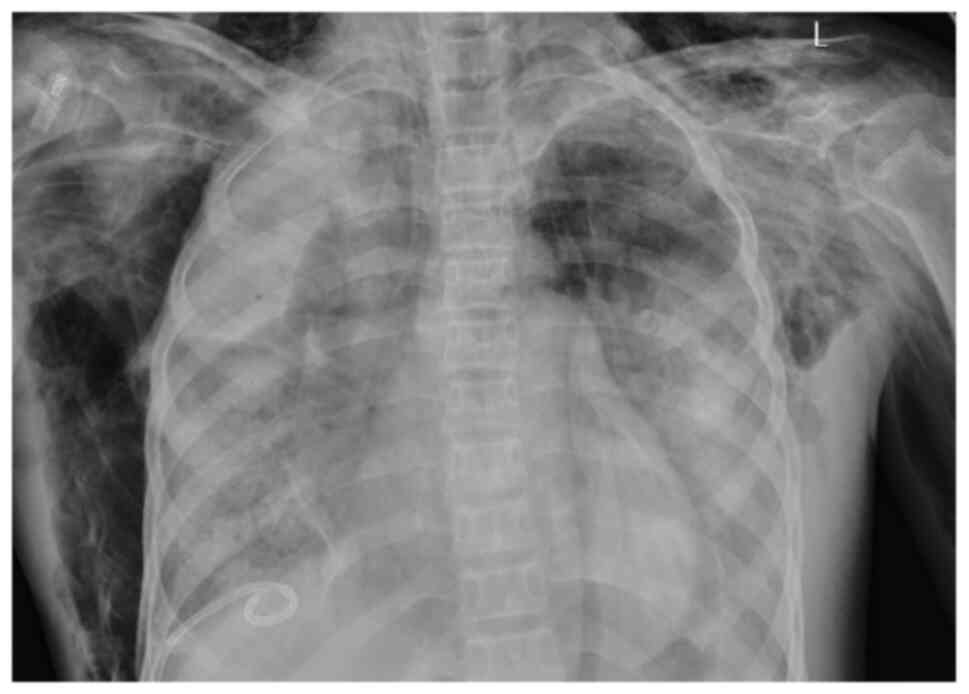
All 7 cases received dynamic monitoring by chest
X-ray, pulmonary CT scan and computed tomographic pulmonary
angiography (CTPA) during hospitalization. Furthermore, 6 cases
were indicated to have extensive diffuse inflammatory changes in
the two lungs upon chest X-ray or pulmonary CT scan (Figs. 1 and 2) and 1 case had subcutaneous emphysema
(Fig. 3). Furthermore, 4 cases were
combined with a moderate amount of pleural effusion and 1 case was
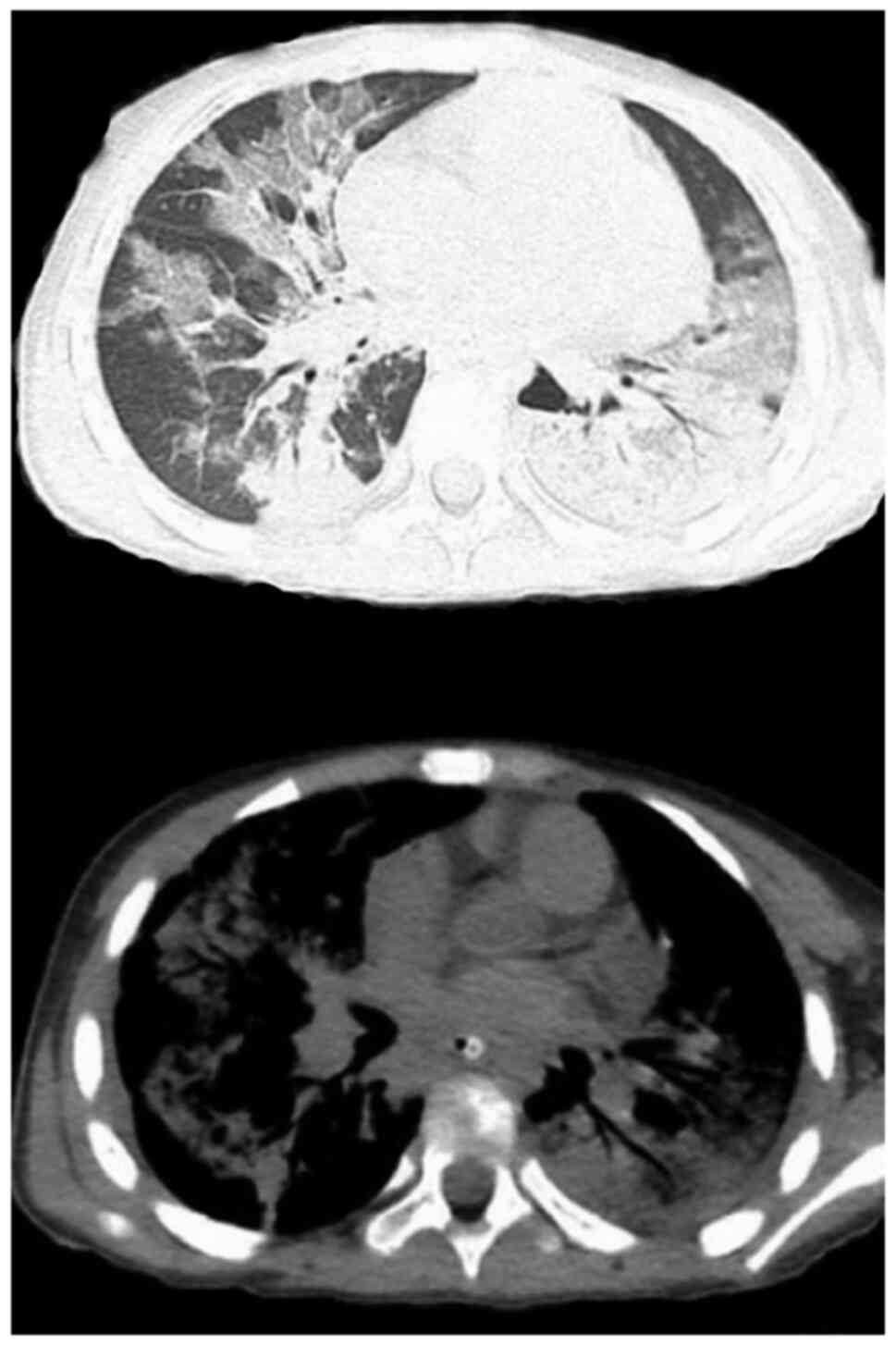
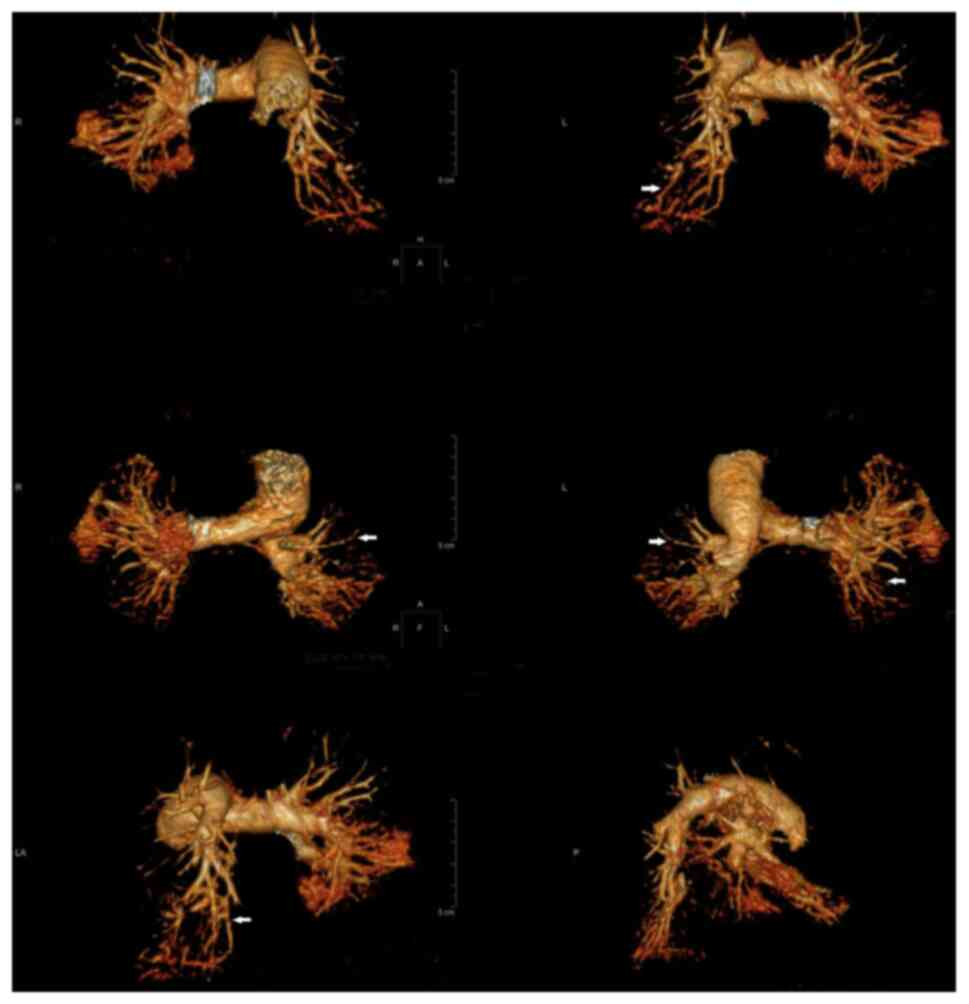
combined with mild pericardial effusion (Fig. 4). CTPA indicated that 2 cases had a
pulmonary arterial embolism in multiple branches bilaterally
(Fig. 5, arrows); 2 cases had a
pulmonary arterial embolism in the upper lobe of the right lung and
one of the two lesions was located at the distal end of the right
upper lung; 1 case had filling defects in the pulmonary artery
branches in the upper lobe of the left lung; 2 cases had distal
pulmonary artery embolism in the lower lobe of the right lung.
Furthermore, 1 case with swelling in the lower limbs received local
vascular ultrasound examination, through which thrombosis in the
common femoral vein was detected.
Treatment
After admission, all of the cases were given the
standard anti-infection therapy of macrolides and certain patients
received concurrent antibiotic therapy with third-generation
cephalosporins or carbapenems. Over the same period, moxifloxacin
was selected for the 2 cases with positivity for the drug
resistance gene. Those patients with dyspnea were treated by
tracheal intubation and mechanical ventilation. In the meantime,
other systemic treatments, such as organ protection and nutritional
support, were given. Risk stratification was performed based on the
guidelines of the American College of Chest Physicians (17), along with the thrombolysis and
anti-coagulation therapies. Low-molecular-weight heparin calcium
was injected subcutaneously at the dose of 50 IU/kg per time, twice
daily. During the treatment, the pediatric patients were properly
immobilized to avoid violent cough and movement. Since these
pediatric patients did not have a basic history of congenital heart
disease and presented with no pulmonary thrombosis, no shock due to
PE and no deep vein thrombosis, thrombophilia was excluded and
thrombolytic therapy was therefore not selected. However,
anticoagulant therapy had no curative effect in 5 cases and the
disease progressed to pulmonary infarction; thus, surgical
resections were conducted.
Outcomes and follow-up
Anticoagulant therapy is the first choice for all
pediatric patients with PE if there is no contraindication
(18). A total of 2 patients
achieved significant improvement after 15-21 days of treatment as
detected by chest radiological examination and D-dimer test. They
were discharged after the symptoms improved, and the PE disappeared
3-5 months later as detected during the follow-up. The 5 remaining
cases exhibited no improvement in the local PE, which was confirmed
by pulmonary CT scan on day 14-21 during the anticoagulation
therapy. Pulmonary infarction was considered in certain patients
who had local nodular solid lesions in the lungs with cavitation
and the condition deteriorated to high dependence on oxygen. Among
them, 2 patients had considerable pleural effusion (pus) and
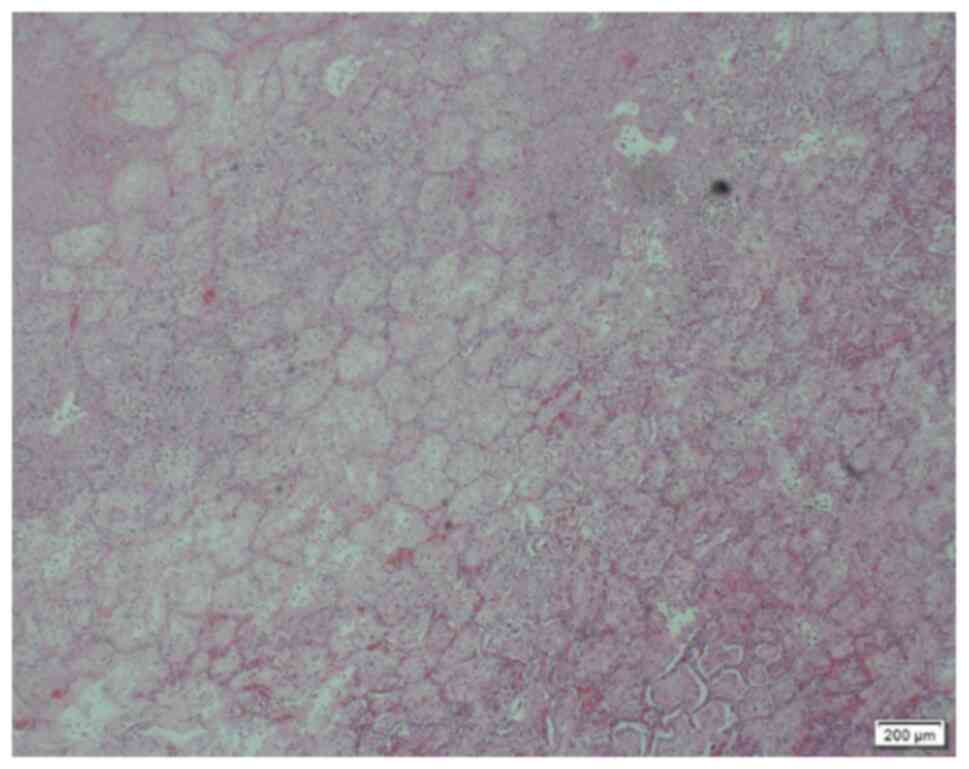
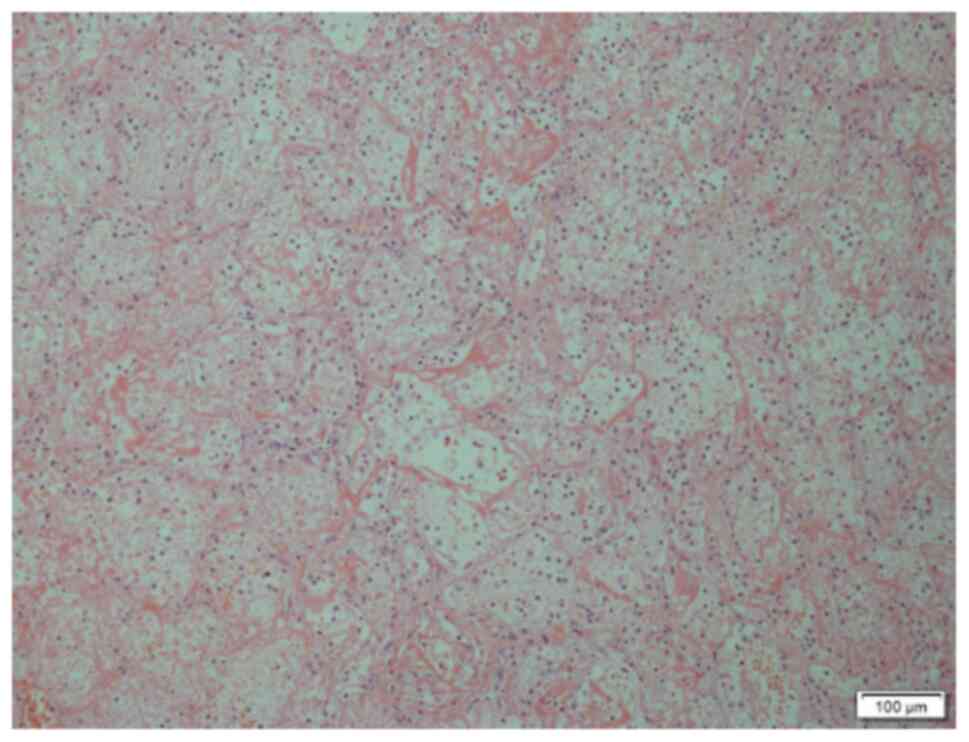
required surgical resection. Intraoperative findings included
dark-red resected pulmonary tissues or yellowish-white infarct-like
changes. These tissues had no contraction and dilation functions,
with high tension and pus coating on their surface. The
pathological diagnosis of the resected pulmonary tissues was
pulmonary infarction (Figs. 6 and
7). Among them, 2 cases were
combined with Acinetobacter baumannii infection after
surgery and Acute Respiratory Distress Syndrome occurred 3-8 days
later. Although the patients were treated with extracorporeal
membrane oxygenation, their condition did not improve and they
eventually died. The remaining surviving pediatric patients
underwent 6-12 months of follow-up and respiratory rehabilitation
and they recovered to a normal state.
Literature search and review
Using ‘Pulmonary Embolism’ and ‘Mycoplasma
pneumoniae pneumonia’ with ‘pediatric’ as the keywords, relevant
articles were searched in the PubMed, MEDLINE, Update, Web of
Science and Embase databases. The Chinese subject heading terms
used in the Wanfang, Chinese National Knowledge Infrastructure and
Chongqing VIP databases were the same as those above. The inclusion
criteria were as follows: i) Pediatric patients with a confirmed
diagnosis of MPP and PE during the treatment process; and ii)
Studies published within the last 20 years. Exclusion criteria was
incomplete clinical information. Ultimately, the clinical data of
10 pediatric cases with MPP and PE were reported and their details
are listed in Table III (19-26).
 | Table IIIDetails of previous studies. |
Table III
Details of previous studies.
| Author (year) | Case no. | Sex | Age (years) |
Intervala (days) | Antibody to M.
pneumoniae | Agglutination
test | D reg | Treatment | Outcome | (Refs.) |
|---|
| Graw-Panzer
(2009) | 1 | M | 13 | 5 | ELISA IgM
(1:128) | Increased D-dimer,
protein S deficiency and positive ACA. | Left popliteal vein
embolism and PE. | Heparin +
warfarin | Radiographic chest
findings returned to normal after 3 months and the anemia resolved
gradually over 5 months. | (19) |
| Chen (2013) | 2 | F | 12 | 12 | PA (1:160) | Increased D-dimer,
positive ACA | Thrombosis in right
lower limb and PE (left lower lobe). | Low-molecular-
weight heparin + warfarin | The chest X-ray was
almost normal at follow-up after 6 months. | (20) |
| Brown (2008) | 3 | M | 6 | 16 | Complement binding
(1:640) | Positive ACA and
acquired activated protein C resistance. | Femoral vein
embolism and PE (left lower lobe) | Not mentioned | Alive. | (21) |
| Su (2012) | 4 | M | 6 | 17 | ELISA (1:128) CA
(1:1,024) | Increased D-dimer,
positive ACA and decreased activity of plasma protein C. | PE (left lower
lobe) | Heparin +
warfarin | At the 3-month
follow-up, Aca was negative, plasma protein C activity recovered
and lung lesions were absorbed. | (22) |
| Wei (2015) | 5 | M | 9 | 23 | Not mentioned
(1:1,280) | Increased D-dimer
and positive ACA. | PE (right lower
lobe). | Heparin +
warfarin | Chest radiographic
findings returned to normal after 3 months. | (23) |
| Zhuo (2015) | 6 | M | 9 | 10 | PA (1:160) | Increased
D-dimer. | PE (mainly on the
right side). |
Low-molecular-weight heparin +
warfarin | Died on the eighth
day after admission. | (24) |
| Qin (2019) | 7 | F | 10 | 14 | Not mentioned
(1:320) | Increased D-dimer,
anticardiolipin IgM antibody was positive, plasma protein C/S
activity was not mentioned. | PE (bilateral
lung). | Low-molecular-
weight heparin calcium + warfarin | At the 8-month
follow-up, chest CT indicated old lung lesions in both lungs,
segmental atelectasis of the right upper lung accompanied by
bilateral lower lung filaments and a small amount of pleural
lesions in the left lung. | (25) |
| Zhang (2019) | 8 | F | 8 | 20 | ELISA
(1:1,280) | Increased D-dimer,
the activities of antithrombin III, protein C/S were normal. | Thrombosis of
posterior tibial vein in both lower limbs and PE (bilateral
lung). | Methylprednisolone
+ Nadroparin calcium | The total course of
treatment was 5.5 months. The lesions were absorbed. | (26) |
| Zhang (2019) | 9 | F | 5 | 6 | ELISA
(1:1,280) | Increased D-dimer,
the activities of antithrombin III, protein C were normal. Protein
S was decreased. | Thrombophlebitis of
the great saphenous vein in right lower limb and PE (lower lobe in
the bilateral lung). | Methylprednisolone
+ Nadroparin calcium | The total course of
treatment was 4.5 months. The lesions were absorbed. | (26) |
These cases were aged between 6 and 13 years (median
age, 9.0 years) with a male/female ratio of 5:1. The levels of
M. pneumoniae antibody were significantly increased, along
with a transient decrease in protein S and protein C. The lesions
were located at the lower lobe close to the hilus of the lung.
After receiving anti-infective and anti-coagulant treatments, 8
cases improved but 1 patient died.
Discussion
Pediatric patients with critical MPP complicated
with PE was rarely reported. Cases with mild PE may be
asymptomatic, while severe cases may suffer from pulmonary arterial
hypertension, unstable hemodynamics or even sudden death (27,28).
The common symptoms include shortness of breath, chest pain and
even dyspnea (19-26).
Missed diagnosis may occur if young pediatric patients are not able
to properly describe their symptoms. Therefore, if PE is not
discovered in a timely manner, the anti-coagulation treatment is
delayed and the disease may progress into acute pulmonary
infarction or even death. When encountering pediatric cases with
MPP, the patient or the parents should be asked whether there is a
family history of protein C/protein S deficiency, recent history of
surgeries or presence of congenital vascular malformation, so as to
preliminarily assess the risk of PE. In the present study,
evaluation at the early stage of admission indicated a low risk of
thrombosis in all cases. However, their symptoms kept on
deteriorating during the treatment and chest radiological
examination indicated poor recovery. In combination with laboratory
tests and chest radiological examination, the diagnosis of PE was
confirmed and the standard treatment was provided.
For such pediatric cases, physicians should begin
early dynamic monitoring and examination, which may be able to
effectively control disease progression, reduce surgical rates and
mortality. However, with the current technological standards
available, it is still limited to perform the interventional
treatment and implement thrombolysis therapy for young pediatric
patients with PE. More work, such as a more appropriate design
using more sophisticated instruments, still needs to be done to
overcome this deficiency in the future.
The pathogenesis of M. pneumoniae infection
with thrombosis remains to be fully elucidated, but it may be
associated with immune damage mediated by infection (7,29-31).
Since the membrane proteins and glycolipids of M. pneumoniae
have certain common antigens in the heart, liver, lung, brain,
kidney and smooth muscle tissues of the human body, upon infection
of the host with M. pneumoniae, the corresponding antibodies
are produced and the immune complex is formed to activate
complement, which produces neutrophils. Previous studies reported
that embolism may affect multiple sites of the body after M.
pneumoniae infection, including the brain, lower extremity
veins, spleen and pulmonary arteries (10-15).
Chemokines, which attract a large number of white blood cells to
invade the lesion, release a large number of inflammatory mediators
and lysosomal enzymes, causing inflammatory damage to target
organs. It was reported that patients with MPP and thrombus were
positive for ACA (32,33). ACA is an autoantibody that targets
antigens in platelets and cardiolipin on endothelial membranes and
is associated with thrombogenesis. The present study concluded that
M. pneumoniae infection may cause vascular endothelial cell
injury and ACA positivity, leading to a temporary
hypercoagulability state that induces thrombosis. Under severe
conditions, M. pneumoniae infection further affects the
synthesis of coagulation factors and thrombin (e.g., protein C,
protein S and antithrombin III), resulting in embolism. Certain
patients may acquire protein C or protein S deficiency/resistance
(21,34). The D-dimer test is an important
screening method for PE and a negative result may exclude PE with
100% certainty (35,36). While CTPA is considered as the gold
standard for PE diagnosis (37).
Based on the above points, in a child with severe MPP, the D-dimer
test, ACA test, Protein C test, Protein S test and CTPA should be
considered to prevent the occurrence of PE.
At present, the major treatment for pediatric
patients with acute PE is anticoagulant therapy, the purpose of
which is to prevent acute thrombosis and expansion. If available,
local thrombus therapy or interventional thrombus therapy may be
performed. During the anticoagulation therapy with
low-molecular-weight heparin, dynamic monitoring of the coagulation
status and treatment outcome is required to avoid bleeding, whose
risk is considerable. Furthermore, cooperation between the
pediatric thoracic surgery department and the vascular surgery
department is preferred and surgical intervention may be provided
if necessary.
Critical M. pneumoniae infection in pediatric
patients is associated with a high risk of PE. During clinical
treatment, such cases should be screened for high-risk factors and
patients could be closely monitored for any manifestations of PE.
Necessary examinations, particularly CTPA, should be performed to
confirm the diagnosis and to initiate standard treatment as early
as possible. Surgical intervention is another important salvage to
reduce poor prognosis, if the disease progresses to pulmonary
infarction, and therefore results in serious and life threatening
complications.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CQS conceived the current study and drafted and
revised the manuscript. CFY collected the literature and reviewed
and revised the manuscript. YA and ZYZ collected the data and
performed initial analyses. YML coordinated and supervised data
collection, and critically reviewed the manuscript. CQS and YML
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The research followed international and national
regulations in accordance with the Declaration of Helsinki. The
study was approved by the Ethics Committee of the First Hospital of
Jilin University. (Changchun, China; approval no. 2019-253).
Patient consent for publication
Written informed consents were obtained from the
patients' legal guardians for the publication of any accompanying
images prior to submission.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jain S, Williams DJ, Arnold SR, Ampofo K,
Bramley AM, Reed C, Stockmann C, Anderson EJ, Grijalva CG, Self WH,
et al: Community-acquired pneumonia requiring hospitalization among
U.S children. N Engl J Med. 372:835–845. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tashiro M, Fushimi K, Kawano K, Takazono
T, Saijo T, Yamamoto K, Kurihara S, Imamura Y, Miyazaki T,
Yanagihara K, et al: Comparison of efficacy of antimicrobial agents
among hospitalized patients with Mycoplasma pneumoniae
pneumonia in Japan during large epidemics of macrolide-resistant
M. pneumoniae infections: A nationwide observational study.
Clin Infect Dis. 65:1837–1842. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Omori R, Nakata Y, Tessmer HL, Suzuki S
and Shibayama K: The determinant of periodicity in Mycoplasma
pneumoniae incidence: An insight from mathematical modelling.
Sci Rep. 5(14473)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tanaka T, Oishi T, Miyata I, Wakabayashi
S, Kono M, Ono S, Kato A, Fukuda Y, Saito A, Kondo E, et al:
Macrolide-resistant Mycoplasma pneumoniae infection, Japan,
2008-2015. Emerg Infect Dis. 23:1703–1706. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Huang L, Chen H and Peng S: Spontaneous
pneumomediastinum, emphysema, and pulmonary bullae associated with
refractory Mycoplasma pneumoniae pneumonia in a child.
Pediatr Pulmonol. 52:E77–E80. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pereyre S, Goret J and Bébéar C:
Mycoplasma pneumoniae: Current knowledge on macrolide
resistance and treatment. Front Microbiol. 7(974)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Narita M: Classification of extrapulmonary
manifestations due to Mycoplasma pneumoniae infection on the
basis of possible pathogenesis. Front Microbiol.
7(23)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Olson D, Watkins LK, Demirjian A, Lin X,
Robinson CC, Pretty K, Benitez AJ, Winchell JM, Diaz MH, Miller LA,
et al: Outbreak of Mycoplasma pneumoniae-associated
Stevens-Johnson syndrome. Pediatrics. 136:e386–e394.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dhaliwal K and Enright K: Rare
extrapulmonary complications of Mycoplasma pneumoniae
infection. BMJ Case Rep. 2016(bcr2015214044)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Witmer CM, Steenhoff AP, Shah SS and
Raffini LJ: Mycoplasma pneumoniae, splenic infarct, and
transient antiphospholipid antibodies: A new association?
Pediatrics. 119:e292–e295. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bao Y, Li X, Wang K, Zhao C, Ji X and
Jiang M: Central retinal artery occlusion and cerebral infarction
associated with Mycoplasma pneumonia infection in children. BMC
Pediatr. 16(210)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kang B, Kim DH, Hong YJ, Son BK, Lim MK,
Choe YH and Kwon YS: Complete occlusion of the right middle
cerebral artery associated with Mycoplasma pneumoniae
pneumonia. Korean J Pediatr. 59:149–152. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Oeser C, Andreas M, Rath C, Habertheuer A
and Kocher A: Left ventricular thrombus in a patient with cutaneous
T-cell lymphoma, hypereosinophilia and Mycoplasma pneumoniae
infection-a challenging diagnosis: A case report. J Cardiothorac
Surg. 10(21)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bakshi M, Khemani C, Vishwanathan V, Anand
RK and Khubchandani RP: Mycoplasma pneumonia with antiphospholipid
antibodies and a cardiac thrombus. Lupus. 15:105–106.
2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jin X, Zou Y, Zhai J, Liu J and Huang B:
Refractory Mycoplasma pneumoniae pneumonia with concomitant
acute cerebral infarction in a child: A case report and literature
review. Medicine (Baltimore). 97(e0103)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tincani A, Balestrieri G, Allegri F,
Cinquini M, Vianelli M, Taglietti M, Sanmarco M, Ichikawa K, Koike
T, Meroni P and Boffa MC: Overview on anticardiolipin ELISA
standardization. J Autoimmun. 15:195–197. 2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kearon C, Akl EA, Ornelas J, Blaivas A,
Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, et
al: Antithrombotic therapy for VTE disease: CHEST guideline and
expert panel report. Chest. 149:315–352. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Monagle P, Cuello CA, Augustine C, Bonduel
M, Brandão LR, Capman T, Chan AKC, Hanson S, Male C, Meerpohl J, et
al: American society of hematology 2018 Guidelines for management
of venous thromboembolism: Treatment of pediatric venous
thromboembolism. Blood Adv. 2:3292–3316. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Graw-Panzer KD, Verma S, Rao S, Miller ST
and Lee H: Venous thrombosis and pulmonary embolism in a child with
pneumonia due to Mycoplasma pneumoniae. J Natl Med Assoc.
101:956–958. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen Y, Huang P, Chen Q, Lin Z and Tian W:
Two separated thrombi in deep veins associated with pulmonary
embolism after Mycoplasma pneumoniae infection: A case in
adolescent female. Transl Pediatr. 2:198–201. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Brown S, Padley S, Bush A, Cummins D,
Davidson S and Buchdahl R: Mycoplasma pneumonia and pulmonary
embolism in a child due to acquired prothrombotic factors. Pediatr
Pulmonol. 43:200–202. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Su HY, Jin WJ, Zhang HL and Li CC:
Clinical analysis of pulmonary embolism in a child with
Mycoplasma pneumoniae pneumonia. Zhonghua Er Ke Za Zhi.
50:151–154. 2012.PubMed/NCBI(In Chinese).
|
|
23
|
Wei H, Chang Y and Lu S: A case report of
pulmonary embolism associated with Mycoplasma pneumoniae
pneumonia. Zhonghua Er Ke Za Zhi. 53:143–144. 2015.PubMed/NCBI(In Chinese).
|
|
24
|
Zhuo Z, Li F, Chen X, Jin P, Guo Q and
Wang H: Mycoplasma pneumonia combined with pulmonary infarction in
a child. Int J Clin Exp Med. 8:1482–1486. 2015.PubMed/NCBI
|
|
25
|
Qin Y, Wang H, Wang Y, Zhang W, He L and
Liu C: Mycoplasma pneumoniae pneumonia complicated with
pulmonary embolism in children: A case report. J Clin Pediatr.
37:765–768. 2019.(In Chinese).
|
|
26
|
Zhang J, Liu F, Guo C, et al: A report of
2 cases of pediatric refractory Mycoplasma pneumonia complicated
with pulmonary embolism. Chin J Pract Pediatr. 34:1043–1045.
2019.(In Chinese). doi: 10.19538/j.ek2019120617.
|
|
27
|
Dijl FN, Curtin J, Lord D and Fitzgerald
DA: Pulmonary embolism in children. Paediatr Respir Rev.
13:112–122. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Simmons BP and Aber RC: Mycoplasma
pneumoniae pneumonia. Symptoms mimicking pulmonary embolism
with infarction. JAMA. 241:1268–1269. 1979.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li T, Yu H, Hou W, Li Z, Han C and Wang L:
Evaluation of variation in coagulation among children with
Mycoplasma pneumoniae pneumonia: A case-control study. J Int
Med Res. 45:2110–2118. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Narita M: Pathogenesis of neurologic
manifestations of Mycoplasma pneumoniae infection. Pediatr
Neurol. 41:159–166. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sotgiu S, Pugliatti M, Rosati G, Deiana GA
and Sechi GP: Neurological disorders associated with Mycoplasma
pneumoniae infection. Eur J Neurol. 10:165–168. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nagashima M, Higaki T, Satoh H and Nakano
T: Cardiac thrombus associated with Mycoplasma pneumoniae
infection. Interact Cardiovasc Thorac Surg. 11:849–851.
2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Senda J, Ito M, Atsuta N, Watanabe H,
Hattori N, Kawai H and Sobue G: Paradoxical brain embolism induced
by Mycoplasma pneumoniae infection with deep venous
thrombus. Intern Med. 49:2003–2005. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ascer E, Marques M and Gidlund M: M
pneumoniae infection, pulmonary thromboembolism and
antiphospholipid antibodies. BMJ Case Rep.
2011(bcr1220103561)2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
van Es N, van der Hulle T, van Es J, den
Exter PL, Douma RA, Goekoop RJ, Mos IC, Galipienzo J, Kamphuisen
PW, Huisman MV, et al: Wells rule and d-Dimer testing to rule out
pulmonary embolism: A systematic review and individual-patient data
meta-analysis. Ann intern Med. 165:253–261. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
36
|
Konstantinides SV, Barco S, Lankeit M and
Meyer G: Management of pulmonary embolism: An update. J Am Coll
Cardiol. 67:976–990. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Moore AJE, Wachsmann J, Chamarthy MR,
Panjikaran L, Tanabe Y and Rajiah P: Imaging of acute pulmonary
embolism: An update. Cardiovasc Diagn Ther. 8:225–243.
2018.PubMed/NCBI View Article : Google Scholar
|