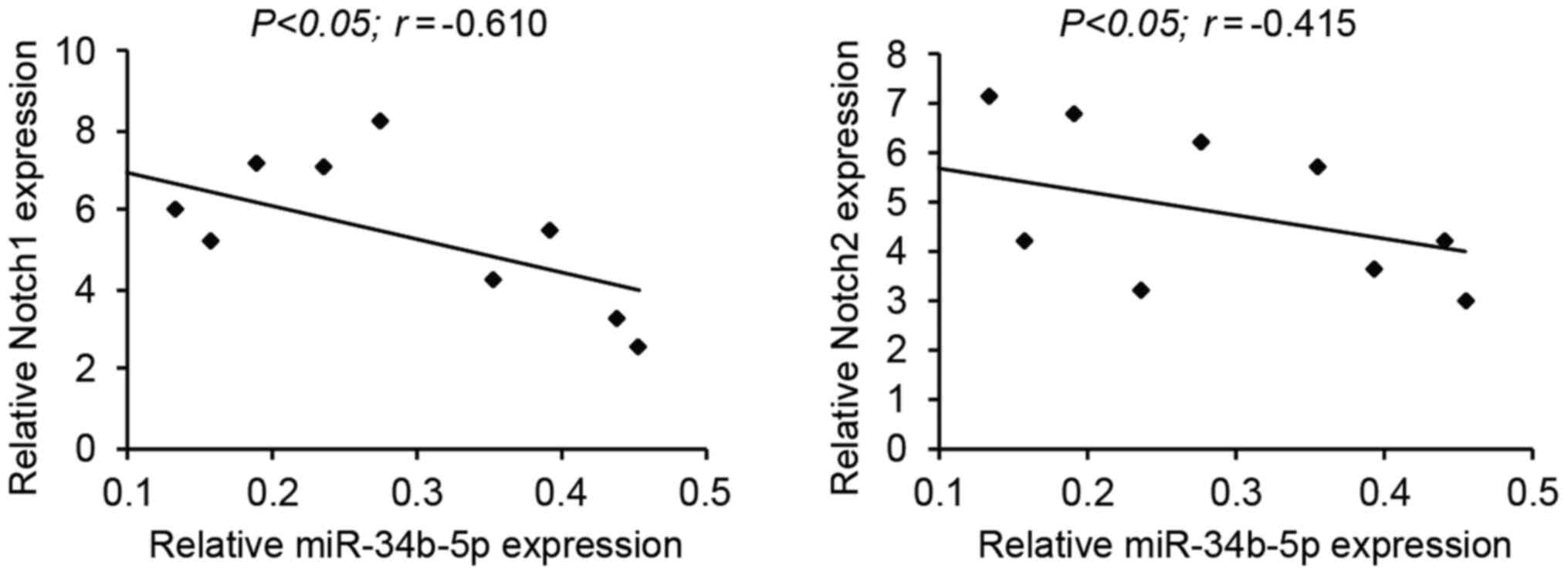
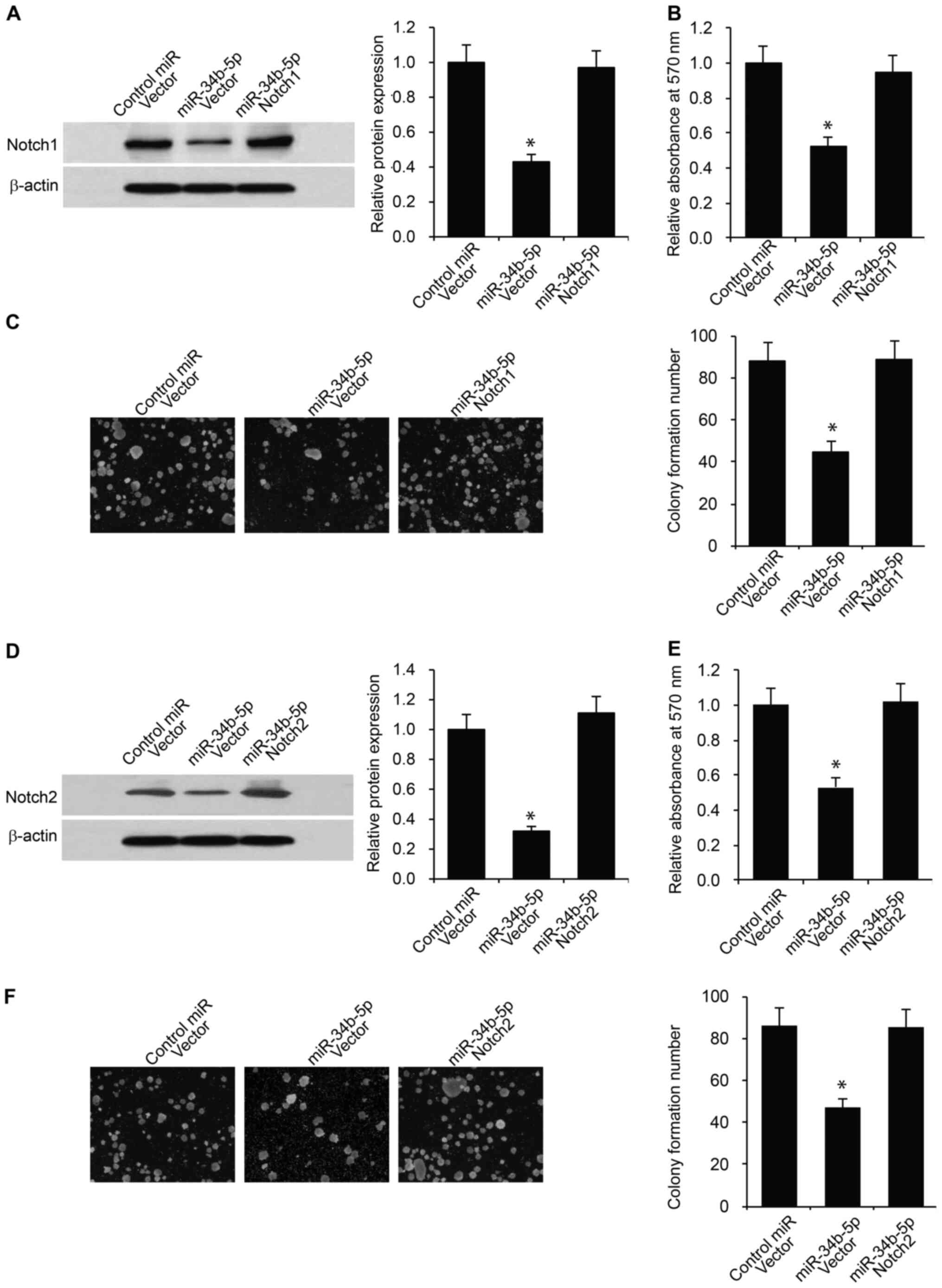
|
1
|
Dimaras H, Kimani K, Dimba EA, Gronsdahl
P, White A, Chan HS and Gallie BL: Retinoblastoma. Lancet.
379:1436–1446. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fabian ID and Sagoo MS: Understanding
retinoblastoma: Epidemiology and genetics. Community Eye Health.
31(7)2018.PubMed/NCBI
|
|
3
|
Knudson AG Jr, Meadows AT, Nichols WW and
Hill R: Chromosomal deletion and retinoblastoma. N Engl J Med.
295:1120–1123. 1976.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chinnam M and Goodrich DW: RB1,
development, and cancer. Curr Top Dev Biol. 94:129–169.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ishak CA, Coschi CH, Roes MV and Dick FA:
Disruption of CDK-resistant chromatin association by pRB causes DNA
damage, mitotic errors, and reduces Condensin II recruitment. Cell
Cycle. 16:1430–1439. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mastrangelo D, De Francesco S, Di Leonardo
A, Lentini L and Hadjistilianou T: Retinoblastoma epidemiology:
Does the evidence matter? Eur J Cancer. 43:1596–1603.
2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dimaras H, Khetan V, Halliday W, Orlic M,
Prigoda NL, Piovesan B, Marrano P, Corson TW, Eagle RC Jr, Squire
JA and Gallie BL: Loss of RB1 induces non-proliferative retinoma:
Increasing genomic instability correlates with progression to
retinoblastoma. Hum Mol Genet. 17:1363–1372. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dimaras H and Gallie BL: The p75 NTR
neurotrophin receptor is a tumor suppressor in human and murine
retinoblastoma development. Int J Cancer. 122:2023–2029.
2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dimaras H, Coburn B, Pajovic S and Gallie
BL: Loss of p75 neurotrophin receptor expression accompanies
malignant progression to human and murine retinoblastoma. Mol
Carcinog. 45:333–343. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Kandalam M, Mitra M, Subramanian K and
Biswas J: Molecular pathology of retinoblastoma. Middle East Afr J
Ophthalmol. 17:217–223. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Castro-Magdonel BE, Orjuela M, Camacho J,
García-Chéquer AJ, Cabrera-Muñoz L, Sadowinski-Pine S,
Durán-Figueroa N, Orozco-Romero MJ, Velázquez-Wong AC,
Hernández-Ángeles A, et al: miRNome landscape analysis reveals a 30
miRNA core in retinoblastoma. BMC Cancer. 17(458)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xiao W, Gao Z, Duan Y, Yuan W and Ke Y:
Notch signaling plays a crucial role in cancer stem-like cells
maintaining stemness and mediating chemotaxis in renal cell
carcinoma. J Exp Clin Cancer Res. 36(41)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xiao W, Chen X and He M: Inhibition of the
Jagged/Notch pathway inhibits retinoblastoma cell proliferation via
suppressing the PI3K/Akt, Src, p38MAPK and Wnt/β-catenin signaling
pathways. Mol Med Rep. 10:453–458. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen CY, Chen YY, Hsieh MS, Ho CC, Chen
KY, Shih JY and Yu CJ: Expression of Notch gene and its impact on
survival of patients with resectable non-small cell lung cancer. J
Cancer. 8:1292–1300. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mollen EWJ, Ient J, Tjan-Heijnen VCG,
Boersma LJ, Miele L, Smidt ML and Vooijs MAGG: Moving Breast Cancer
Therapy up a Notch. Front Onco. 8(518)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Baker A, Wyatt D, Bocchetta M, Li J,
Filipovic A, Green A, Peiffer DS, Fuqua S, Miele L, Albain KS and
Osipo C: Notch-1-PTEN-ERK1/2 signaling axis promotes HER2+ breast
cancer cell proliferation and stem cell survival. Oncogene.
37:4489–4504. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sun Q, Wang R, Wang Y, Luo J, Wang P and
Cheng B: Notch1 is a potential therapeutic target for the treatment
of human hepatitis B virus X protein-associated hepatocellular
carcinoma. Oncol Rep. 31:933–939. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Venkatesh V, Nataraj R, Thangaraj GS,
Karthikeyan M, Gnanasekaran A, Kaginelli SB, Kuppanna G, Kallappa
CG and Basalingappa KM: Targeting Notch signalling pathway of
cancer stem cells. Stem Cell Investig. 5(5)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Takebe N, Nguyen D and Yang SX: Targeting
notch signaling pathway in cancer: Clinical development advances
and challenges. Pharmacol Ther. 141:140–149. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fabbri G, Holmes AB, Viganotti M, Scuoppo
C, Belver L, Herranz D, Yan XJ, Kieso Y, Rossi D, Gaidano G, et al:
Common nonmutational NOTCH1 activation in chronic
lymphocytic leukemia. Proc Natl Acad Sci USA. 114:E2911–E2919.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lee H, Kim KR, Cho NH, Hong SR, Jeong H,
Kwon SY, Park KH, An HJ, Kim TH, Kim I, et al: MicroRNA expression
profiling and Notch1 and Notch2 expression in minimal deviation
adenocarcinoma of uterine cervix. World J Surg Oncol.
12(334)2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Maroof H, Islam F, Dong L, Ajjikuttira P,
Gopalan V, McMillan NAJ and Lam AK: Liposomal delivery of
miR-34b-5p induced cancer cell death in thyroid carcinoma. Cells.
7(265)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Singh L and Kashyap S: Update on pathology
of retinoblastoma. Int J Ophthalmol. 11:2011–2016. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Phillips PC, Levow C, Catterall M, Colvin
OM, Pastan I and Brem H: Transforming growth
factor-alpha-Pseudomonas exotoxin fusion protein (TGF-alpha-PE38)
treatment of subcutaneous and intracranial human glioma and
medulloblastoma xenografts in athymic mice. Cancer Res.
54:1008–1015. 1994.PubMed/NCBI
|
|
26
|
Zhao D and Cui Z: MicroRNA-361-3p
regulates retinoblastoma cell proliferation and stemness by
targeting hedgehog signaling. Expe Ther Med. 17:1154–1162.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jadhav AP, Mason HA and Cepko CL: Notch 1
inhibits photoreceptor production in the developing mammalian
retina. Development. 133:913–923. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang L, Liao Y and Tang L: MicroRNA-34
family: A potential tumor suppressor and therapeutic candidate in
cancer. J Exp Clin Cancer Res. 38(53)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Misso G, Di Martino MT, De Rosa G, Farooqi
AA, Lombardi A, Campani V, Zarone MR, Gullà A, Tagliaferri P,
Tassone P and Caraglia M: Mir-34: A new weapon against cancer? Mol
Ther Nucleic Acids. 3(e194)2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Engkvist ME, Stratford EW, Lorenz S,
Meza-Zepeda LA, Myklebost O and Munthe E: Analysis of the miR-34
family functions in breast cancer reveals annotation error of
miR-34b. Sci Rep. 7(9655)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jiang L and Hermeking H: miR-34a and
miR-34b/c suppress intestinal tumorigenesis. Cancer Res.
77:2746–2758. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Majid S, Dar AA, Saini S, Shahryari V,
Arora S, Zaman MS, Chang I, Yamamura S, Tanaka Y, Chiyomaru T, et
al: miRNA-34b inhibits prostate cancer through demethylation,
active chromatin modifications, and AKT pathways. Clin Cancer Res.
19:73–84. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li Z, Luo Q, Xu H, Zheng M, Abdalla BA,
Feng M, Cai B and Zhang X, Nie Q and Zhang X: MiR-34b-5p suppresses
melanoma differentiation-associated gene 5 (MDA5) signaling pathway
to promote avian leukosis virus subgroup J (ALV-J)-infected cells
proliferaction and ALV-J replication. Front Cell Infect Microbiol.
7(17)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4(e05005)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kakuda S, LoPilato RK, Ito A and
Haltiwanger RS: Canonical Notch ligands and Fringes have distinct
effects on NOTCH1 and NOTCH2. J Biol Chem. 295:14710–14722.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Joshi I, Minter LM, Telfer J, Demarest RM,
Capobianco AJ, Aster JC, Sicinski P, Fauq A, Golde TE and Osborne
BA: Notch signaling mediates G1/S cell-cycle progression in T cells
via cyclin D3 and its dependent kinases. Blood. 113:1689–1698.
2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Dai MY, Fang F, Zou Y, Yi X, Ding YJ, Chen
C, Tao ZZ and Chen SM: Downregulation of Notch1 induces apoptosis
and inhibits cell proliferation and metastasis in laryngeal
squamous cell carcinoma. Oncol Rep. 34:3111–3119. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Abel EV, Kim EJ, Wu J, Hynes M, Bednar F,
Proctor E, Wang L, Dziubinski ML and Simeone DM: The Notch pathway
is important in maintaining the cancer stem cell population in
pancreatic cancer. PLoS One. 9(e91983)2014.PubMed/NCBI View Article : Google Scholar
|