Introduction
One of the most common causes of childhood
intraocular cancer is retinoblastoma (RB) (1). The incidence of RB is constant
worldwide with 1 case per 15,000-20,000 live births, equating to
~9,000 new cases per year (2).
However, the greatest burden of RB is observed in Asia and Africa,
which are regions with higher birth rate compared with Europe, USA
or Canada (1). The regions with
higher prevalence of RB have also been associated with higher
mortality, which may be attributed to delayed diagnosis due to the
lack of familial awareness, access to healthcare and the poor
socioeconomic status (1).
RB is usually initiated by a random mutation in the
RB1 gene of the photoreceptor cells of the retina (2). The study of the RB1 gene, which
was the first tumor suppressor gene to be identified, gave rise to
the famous two-hit hypothesis for cancer development propounded by
Knudson et al (3). The
multifunctional protein pRb (the product of RB1) has been
associated with several tumor suppressive functions (4). Additionally, its role has also been
investigated in the maintenance of genome stability and other
epigenetic modifications (5), for
example, decreases in pRB chromatin are associated with increases
in spontaneous γH2AX deposition and aneuploidy (5).
However, the accuracy of the two-hit hypothesis was
challenged with the genomic revolution, questioning the simplicity
and reliability of RB development on the two-hit hypothesis alone
without taking into consideration chromosomal and epigenetic
alterations (6). This highlighted
the requirement for further investigation in order to understand
the initiation and progression of RB.
Studies in humans and mice have indicated that
biallelic inactivation of RB1 resulted in increased copy
numbers of MYCN, E2F3, DEK, KLF14 and
MDM4 as well as decreased tumor suppressor genes
CDH11 and NFGR (7-9).
These additional alterations were reported to be necessary for the
development and progression of RB (10). While the role of genetics has been
investigated, the role of epigenetic modifications in the molecular
mechanisms of RB remains relatively unexplored (11).
In the current study, the epigenetic modifications
of Notch signaling in RB were investigated, as Notch receptors
serve an important role in the specification and survival of stem
and progenitor cells in retinal development (12). Furthermore, it has been indicated
that Notch1 interacts with pRb via intracellular domains, resulting
in pRb inactivation, which is therefore likely to contribute to the
oncogenic activity of Notch1(13).
In addition, the Notch signaling pathway has been associated with
increased cell proliferation, angiogenesis, recurrence and
maintenance of cancer stem cell population and chemoresistance in
several cancer types, such as hepatocellular carcinoma, breast
cancer and lung cancer (14-18).
Dysregulation of the Notch signaling pathway either
due to overexpression of the ligand or aberrant receptor expression
has been reported in several solid tumor studies, such as thyroid
cancer, lung cancer and intracranial tumors (19). Moreover, activation of the Notch1
gene signature independently of common mutations has also been
reported in chronic lymphocytic leukemia (20). While targeting the Notch pathway has
been proposed as a therapeutic alternative in RB, a deeper
understanding of the epigenetic regulatory mechanisms of Notch
signaling in RB remains elusive (18).
The present study is amongst the first studies, to
the best of our knowledge, to explore microRNA (miR)-mediated
epigenetic modulation of the Notch signaling pathway and its
influence on phenotypes of tumor progression in RB. A previous
study identified a 30-miR core, which was revealed to be
upregulated in RB in 12 patient tissues, using high-throughput
microarray analysis (11). These 30
miRs have been reported to target 182 cancer-associated genes,
albeit none of them was indicated to target the Notch genes.
However, via a literature search, miR-34b-5p was revealed to be
associated with Notch dysregulation in thyroid carcinoma and
uterine cervix adenocarcinoma (21,22).
As the effect of miR-34b-5p-mediated Notch dysregulation in RB
development has not been yet investigated, the current study was
designed to address this question. It was hypothesized that
miR-34b-5p-regulated Notch signaling may drive tumor progression in
RB.
Materials and methods
Patient samples and inclusion
criteria
The current study was performed at The Third
Affiliated Hospital of Qiqihar Medical University (Qiqihar, China)
in accordance with the principles of the Declaration of Helsinki
and was approved by the clinical research Ethics Committee of The
Third Affiliated Hospital of Qiqihar Medical University (Qiqihar,
China). Patients (n=10) aged 4-25 months diagnosed with RB and
undergoing opthalmectomy between January 2014 and January 2017 at
the hospital, with no preoperative chemo- and radiotherapy, were
recruited in the present study and provided written informed
consent. The diagnosis of retinoblastoma was based on the WHO
criteria (23). To avoid RB
contamination, healthy tissue was resected separately from an
opposite quadrant of the tumor tissue (>5 mm from the tumor
margin). Patient sera were also obtained. Briefly, the whole blood
was allowed to clot by leaving it undisturbed at room temperature
for 15-30 min. The clot was removed by centrifuging at 1,000-2,000
x g for 10 min and the supernatant serum was collected.
Simultaneously, age-matched normal sera were also collected from
outpatients of physical examination excluding patients with
infections, tumors and various congenital diseases. All sera were
immediately snap frozen in liquid nitrogen and stored at -80˚C for
further use. The clinical characteristics of the patients with RB
and normal participants are listed in Table S1.
Cell line maintenance and
transfection
Two human-derived RB cell lines, Weri-Rb-1 and Y79,
were obtained from the American Type Culture Collection and
maintained as per the repository's instructions. Briefly, the cells
were seed in 24-well plate and cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) and antibiotics (penicillin and
streptomycin; 100 U/ml each) at 37˚C in a 5% CO2
incubator till 80% confluence. Plasmids (400 ng) containing genes
for Notch1 or Notch2 in pcDNA3.1 vectors (Shanghai GenePharma Co.,
Ltd.) and 0.14 µl 20 µM stock of miR-34b-5p
(5'-AGGCAGUGUAAUUAGCUGAUUGU-3') or control miR
(5'-UUCUCCGAACGUGUCACGUTT-3') in 30 µl medium without serum or
antibiotics were mixed with 0.75 µl Lipofectamine® 2,000
(Invitrogen; Thermo Fisher Scientific, Inc.) for 15 min at room
temperature to form transfection complexes. The complexes were
added into each single well of 24-well plate cultured RB cells.
Assays were performed 48 h post-transfection.
Reverse transcription-quantitative PCR
(RT-qPCR)
As per the manufacturer's protocol, total RNA was
extracted using the TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) from RB cells (Y79 and Weri-Rb-1) and
tissue samples. The RNA was converted to cDNA using a
TaqMan® MicroRNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions were as follows: 16˚C for 30 min, 42˚C for 30 min and
85˚C for 5 min. RT-qPCR was performed using a TaqMan®
Universal PCR Master Mix II (Applied Biosystems; Thermo Fisher
Scientific, Inc.) with small nuclear RNA U6 as the endogenous
control. The RT-qPCR of mRNA was performed with Superscript III
Platinum SYBR Green One-Step qRT-PCR kit (Invitrogen; Thermo Fisher
Scientific, Inc.) using β-actin as the house-keeping gene. The
RT-qPCR were run on an ABI 7500 Real-Time PCR system (Applied
Biosystems) at 95˚C for 2 min, followed by 30 cycles of
amplification at 94˚C for 40 sec, annealing at 55˚C for 40 sec and
extension at 72˚C for 1 min, with a final elongation step at 72˚C
for 10 min, and the relative gene expression was estimated using
the 2-∆∆cq method (24).
The primer sequences used for the gene expression analysis were as
follows: Notch1 forward, 5'-GGGCTAACAAAGATATGCAG-3' and reverse,
5'-ACTGAACCTGACCGTACAGTTGGCAAAGTGGTCCAG-3'; Notch2 forward,
5'-AATCCCTGACTCCAGAACG-3' and reverse,
5'-TGGTAGACCAAGTCTGTGATGAT-3'; CD133 forward,
5'-GAAAAGTTGCTCTGCGAACC-3' and reverse, 5'-CTCGACCTCTTTTGCAATCC3';
SOX-2 forward, 5'-GGGAAATGGAGGGGTGCAAAAGAGG-3' and reverse,
5'-TTGCGTGAGTGTGGATGGGATTGGTG-3'; Nanog forward,
5'-TCCTCCTCTTCCTCTATACTAAC-3' and reverse,
5'-CCCACAATCACAGGCATAG-3'; and β-actin forward
5'-AAGGGACTTCCTGTAACAATGCA-3' and reverse,
5'-CTGGAACGGTGAAGGTGACA-3', miR-34b-5p forward
5'-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCAATCA-3' and
reverse, 5'-GCCTAGGCAGTGTCATTAGC-3'; and U6 forward
5'-CTCGCTTCGGCAGCACA-3' and reverse,
5'-AACGCTTCACGAATTTGCGT-3'.
MTT assay
RB cell (Weri-Rb-1 and Y79) proliferation was
assessed using an MTT assay (Beyotime Institute of Biotechnology).
Briefly, for each cell line, 5,000 cells were seeded in a 96-well
plate. MTT was added to each well on day 0, 1, 2, 3 and 4
post-transfection at a final concentration of 0.5 mg/ml, and
incubated for 4 h at 37˚C. The formation of formazan crystals was
assessed under the microscope and the crystals were dissolved using
DMSO. The absorbance was measured in a microplate reader at 450
nm.
Cell counting kit-8 (CCK-8) assay
Cellular proliferation was also measured using a
commercial CCK-8 assay (Beyotime Institute of Biotechnology). A
total of 5,000 cells of the respective RB cell lines were seeded in
a 96-well plate. Following transfection (day 0, 1, 2, 3 and 4), CCK
reagent was added to each well at a final dilution of 1:10. The
cells were subsequently incubated at 37˚C for 2 h and the
absorbance was measured at 450 nm in a microplate reader.
Western blotting
The transfected cells (Y79 and Weri-Rb-1 cells) were
harvested using a commercial RIPA lysis buffer (Beyotime Institute
of Biotechnology) and total protein content was estimated using a
BCA assay (Beyotime Institute of Biotechnology). A total of 20 µg
total protein/lane was separated using 10% SDS-PAGE and
electroblotted to a nitrocellulose membrane (Bio-Rad Laboratories,
Inc.). Following transfer, the membranes were blocked for 1 h at
room temperature with 5% non-fat dried milk. The excess buffer was
removed by washing in PBS buffer and the membranes were incubated
with primary antibodies including Notch1 (cat. no. sc-376403;
1:1,000), Notch2 (cat. no. sc-518049; 1:500), CD133 (cat. no.
sc-19365; 1:500), SOX-2 (cat. no. sc-365823; 1:500), Nanog (cat.
no. sc-374103; 1:500) and β-actin (cat. no. sc-47778; 1:1,000).
overnight at 4˚C. After washing using TBST (Tris Buffered Saline
with 0.1% Tween 20), the membranes were incubated for 1 h with
HRP-conjugated species-specific secondary antibodies (horseradish
peroxidase conjugated goat anti-rabbit Immunoglobulin G, cat. no.
sc-2004; and horseradish peroxidase conjugated goat anti-mouse
Immunoglobulin G, cat. no. sc-2005; each at 1:10,000) at room
temperature. All antibodies were purchased from Santa Cruz
Biotechnology, Inc. Subsequently, the blots were developed using an
ECL kit (Beyotime Institute of Biotechnology). The blots were
developed on an X-ray film (Kodak) and densitometry analysis was
performed using ImageJ software (version 1.48; National Institutes
of Health).
Tumor sphere formation assay
For this assay, 24-well plates with an ultra-low
attachment surface (Corning Inc.) were used. Transfected and
non-transfected Y79 and Weri-Rb-1 cells were seeded at a density of
5,000 cells/well. All cells were cultured in RPMI-1640 medium
without serum, but supplemented with 20 ng/ml epidermal growth
factor and 10 ng/ml basic fibroblast growth factor (both from
Invitrogen; Thermo Fisher Scientific, Inc.). The cells were
maintained for 7 days at 37˚C in a 5% CO2 incubator.
Using a light microscope (Nikon Corporation; magnification, x200)
tumor spheres were imaged and only spheres with diameter over 50 µm
were counted using imageJ software (version 1.48; National
Institutes of Health).
Xenograft tumor model
A total of 10 BALB/c nude mice (male, 4-week-old,
15-20 g; Charles River Laboratories, Inc.) were randomly divided
into two groups. One group was subcutaneously injected with
1x107 Y79 cells with miR-34b-5p overexpression, and the
other group was injected with cells transfected with control miR.
All mice were injected subcutaneously on their right flank. During
the experimental phase, all mice had free access to food and water.
To minimize any suffering of the animals, anesthetics and
analgesics were used for all surgical experiments. Animals were
anesthetized with an intraperitoneal injection of 40 mg/kg sodium
pentobarbital. When lack of movement and absence of corneal reflex,
but presence of heartbeat and respiration were observed, the
surgical experiments were performed. All mice were given Meloxicam
(2.5 mg/kg, s.c.) every 12 h for three days after surgery. The mice
were maintained in an atmosphere of 60% humidity at a temperature
of 26-28˚C with a uniform dark-light cycle of 12 h each of light
and darkness. After 24 days, all mice were sacrificed by
CO2 inhalation (20% of the cage vol/min) followed by
cervical dislocation. Following confirmation of the animals' death,
including presence of rigor mortis, lack of heartbeat, respiration
and corneal reflex, tumor lesions were excised and photographed.
The tumor weight (g), length (mm) and width (mm) was measured.
Tumor volume was calculated using the following formula:
Width2 x length/2(25).
The animal study was approved by the Animal Care and Use Committee
of The Third Affiliated Hospital of Qiqihar Medical University
(Qiqihar, China), complied with Guidelines for the ethical review
of laboratory animal welfare People's Republic of China National
Standard GB/T 35892-2018.
Luciferase assay
The 3'-untranslated (3'-UTR) regions of Notch1 and
Notch2 bearing the putative binding sites of miR-34b-5p were cloned
into the psiCHECK-2 plasmid (Promega Corporation). Mutant 3'-UTR
luciferase reporter vectors containing 5 mutated nucleotides on the
miR-34b-5p binding sites were generated using the QuikChange Multi
Site-Directed Mutagenesis kit (Agilent Technologies, Inc.). The
plasmids were co-transfected with miR-34b-5p or control miR in RB
cells using Lipofectamine 2,000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Using the Dual-Luciferase® Reporter
(DLR™) Assay System (Promega Corporation), firefly and
Renilla luciferase signals were measured at 48 h
post-transfection and the firefly signal was normalized to that of
Renilla, as per the manufacturer's instructions.
Migration and invasion
Cell migration and invasion was measured using
Transwell chambers (BD Biosciences) containing 24-well inserts with
8-µm pores with or without Matrigel coating (BD Biosciences)
according to the manufacturer's protocol. Briefly, The Matrigel
matrix aliquot was thawed on ice. Matrigel was mixed with coating
buffer thoroughly on ice. A total of 0.1 ml diluted Matrigel matrix
coating solution was added to each insert using a sterile pipet. RB
cells (2x105/well) were seeded in the upper chamber and
incubated in RPMI-1640 medium without FBS for 12 and 24 h for the
migration (without Matrigel coating) and invasion (with Matrigel
coating) assays at 37˚C in a 5% CO2 incubator,
respectively. Cells in the upper chamber were then removed, and the
remaining cells were fixed in 4% paraformaldehyde for 5 mins and
stained with 0.4% crystal violet for 1 mins at room temperature.
Cells were quantified in five randomly selected fields for each
membrane using a light microscope (Nikon Corporation;
magnification, x400), and the average cell count of three
individual membranes was defined as the migration or invasion
index.
TargetScan online tool analysis
The TargetScan online tool (http://www.targetscan.org/vert_72/) was utilized to
predict the targets of the miRNAs being assessed.
Statistical analysis
All experiments were performed in triplicate. Data
are presented as the mean ± SD of the three independent sets of
experiments. Statistical significance was examined using unpaired
Student's t-test or one-way ANOVA followed by Tukey's multiple
comparisons test. The correlation between miR-34b-5p and
Notch1/Notch2 expression levels was determined using Pearson's
correlation analysis. All statistical tests were performed using a
licensed copy of SPSS (version 16; SPSS, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
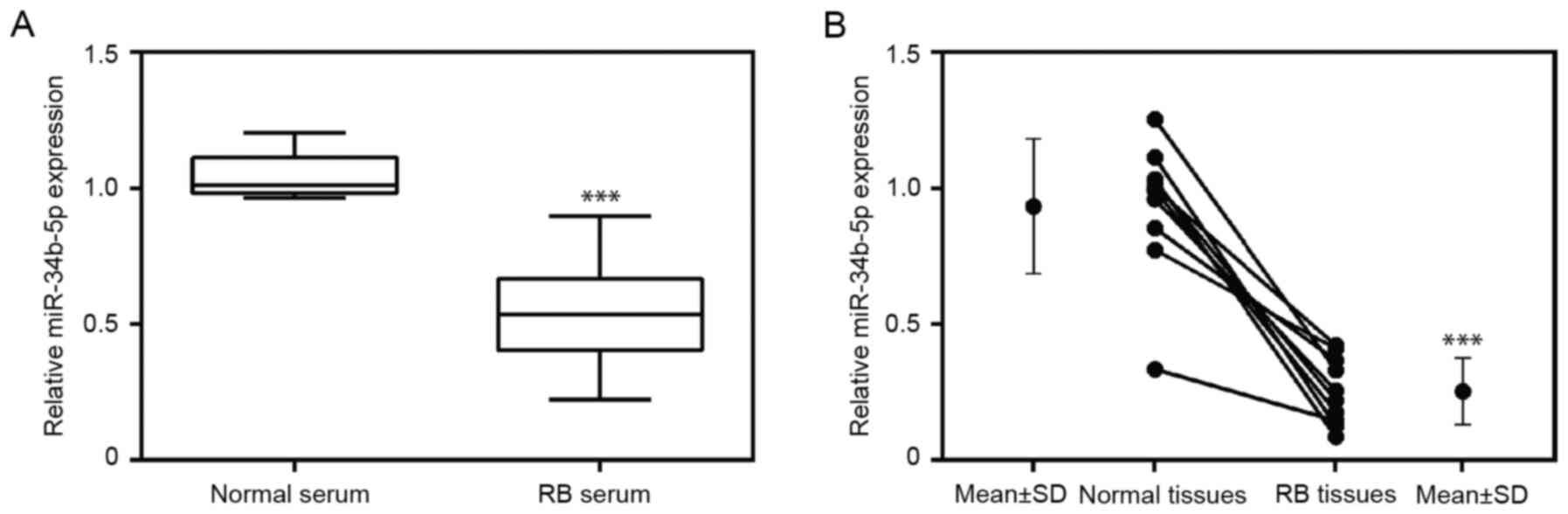
miR-34b-5p is downregulated in tumor
tissues and serum of patients with RB
The expression level of miR-34b-5p was examined in
serum obtained from 10 patients with RB and 10 healthy
participants. Additionally, the levels of miR-34b-5p were assessed
in tumor tissues and paired adjacent non-tumor tissues. The results
indicated that the expression level of miR-34b-5p was significantly
downregulated in RB serum (Fig. 1A)
in comparison to serum from healthy participants. Moreover,
compared with healthy adjacent tissues, miR-34b-5p levels were
significantly reduced in RB tissues (Fig. 1B). These results revealed the
decreased expression of miR-34b-5p levels in both serum and tumor
samples from patients with RB compared with healthy serum and
tissues.
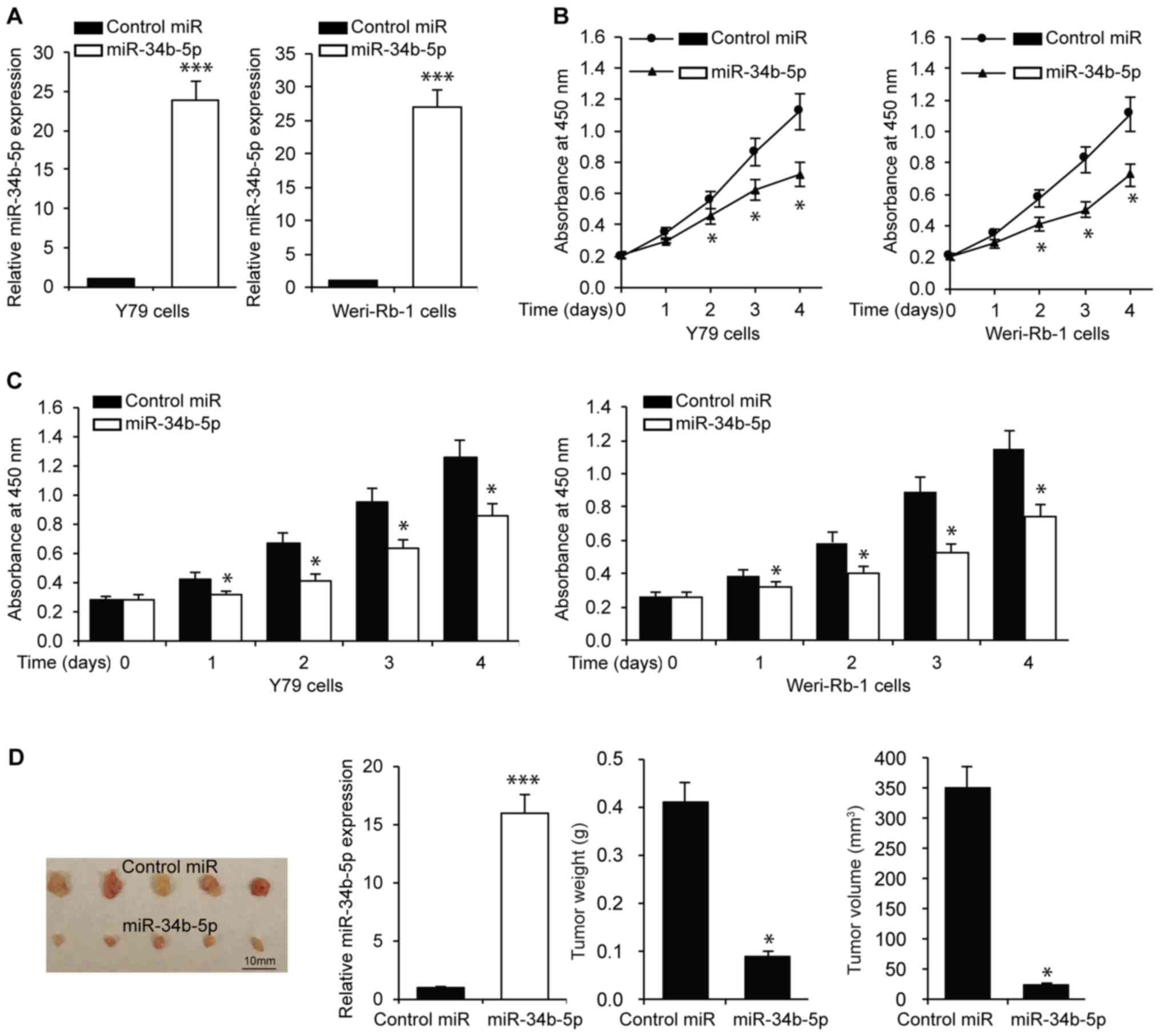
miR-34b-5p inhibits RB cell
proliferation, migration and invasion
To understand the role of miR-34b-5p in RB,
functional assays were performed in RB cell lines, namely Y79 and
Weri-Rb-1. The efficiency of transfection was confirmed via
RT-qPCR, which indicated an increase in the expression of
miR-34b-5p in RB cells, in comparison to cells transfected with miR
controls (Fig. 2A). A comparison of
the tumorigenic capacities between the miR-34b-5p and control miR
groups was also performed. Cell proliferation was assessed using an
MTT assay on Y79 and Weri-Rb-1 cells transfected with miR-34b-5p or
control miR. The results indicated that miR-34b-5p transfection
significantly reduced RB cell proliferation compared with cells
transfected with control miR (Fig.
2B). The proliferative capacity of the cells was also verified
using a CCK-8 assay. The results confirmed the findings of the MTT
assay, indicating reduced proliferation of Y79 and Wer-Rb-1 cells
in the presence of miR-34b-5p (Fig.
2C). In addition, in vivo experiments were performed by
subcutaneous transplantation of Y79 cells overexpressing miR-34b-5p
which were verified using qRT-PCR (Fig.
2D). Tumor xenografts induced by RB cells overexpressing
miR-34b-5p exhibited a slower growth rate and lower weight and
volume compared with cell expressing control miR. The largest tumor
diameter was 9 mm, and tumor volume was 364.5 mm3 in the
control group, while the largest tumor diameter was 3.8 mm, and
tumor volume was 27.4 mm3 in the miR-34b-5p group
(Fig. 2D). Subsequently, it was
investigated whether miR-34b-5p affected RB cell migration and
invasion using a Transwell assay. As presented in Fig. S1, miR-34b-5p significantly
inhibited RB cell migration and invasion compared with control miR.
These results revealed that miR-34b-5p inhibited RB cell
proliferation, migration and invasion.
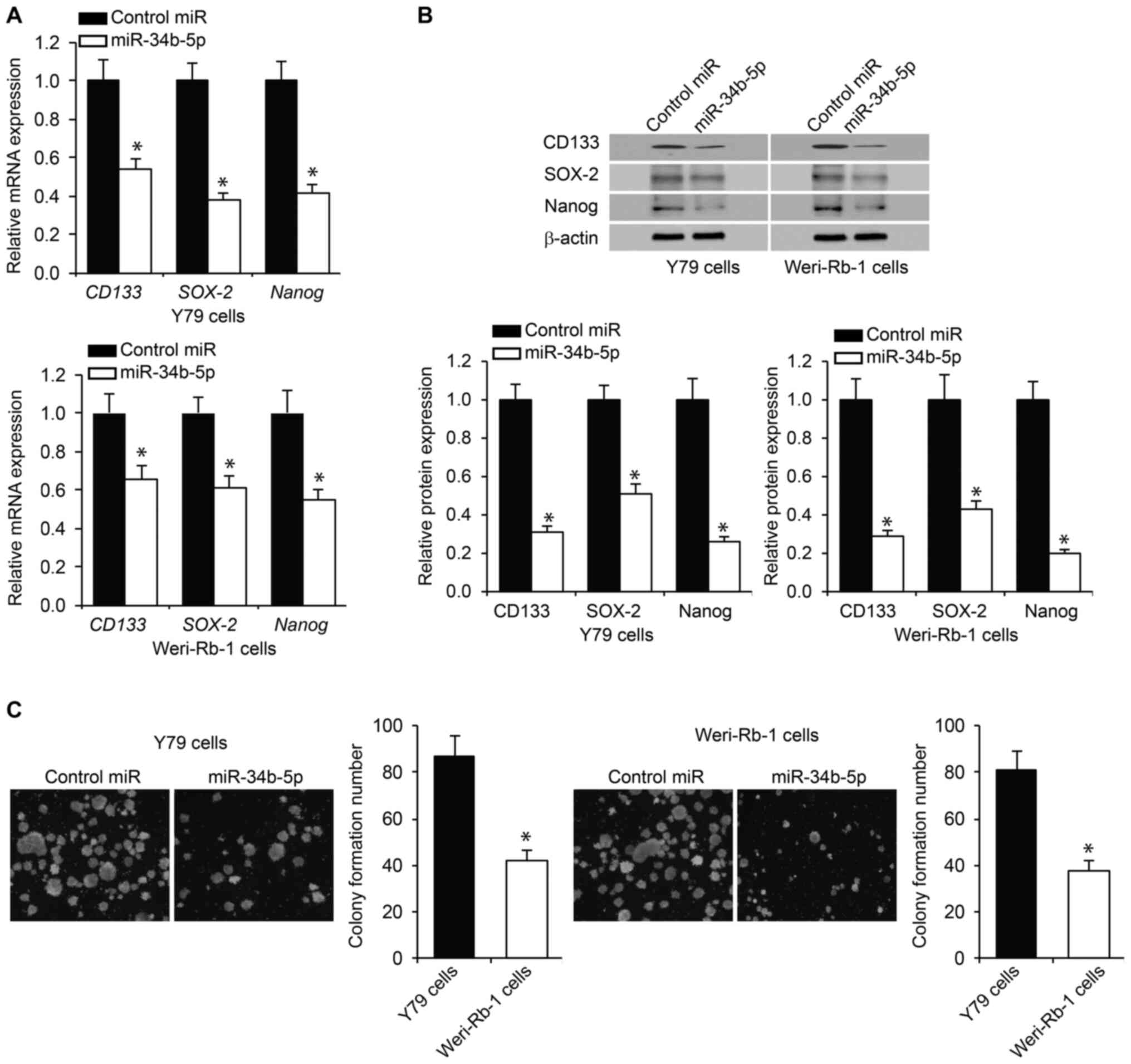
miR-34b-5p reduces RB cell
stemness
To assess the effect of miR-34b-5p on RB cell
stemness, the expression of CD133, SOX-2 and Nanog, which are
sentinel markers of stemness (26),
was examined using RT-qPCR. The expression levels of the
aforementioned markers were significantly decreased in
miR-34b-5p-overexpressing RB cells compared with control cells
(Fig. 3A). The alteration in the
expression levels was verified via western blotting (Fig. 3B), indicating a change in both mRNA
and protein expression. RB stem cell renewal was assessed using a
tumor sphere assay. It was observed that the presence of miR-34b-5p
significantly reduced RB cell self-renewal ability compared with
control miR (Fig. 3C). These
results indicated the ability of miR-34b-5p to inhibit stemness in
RB cells.
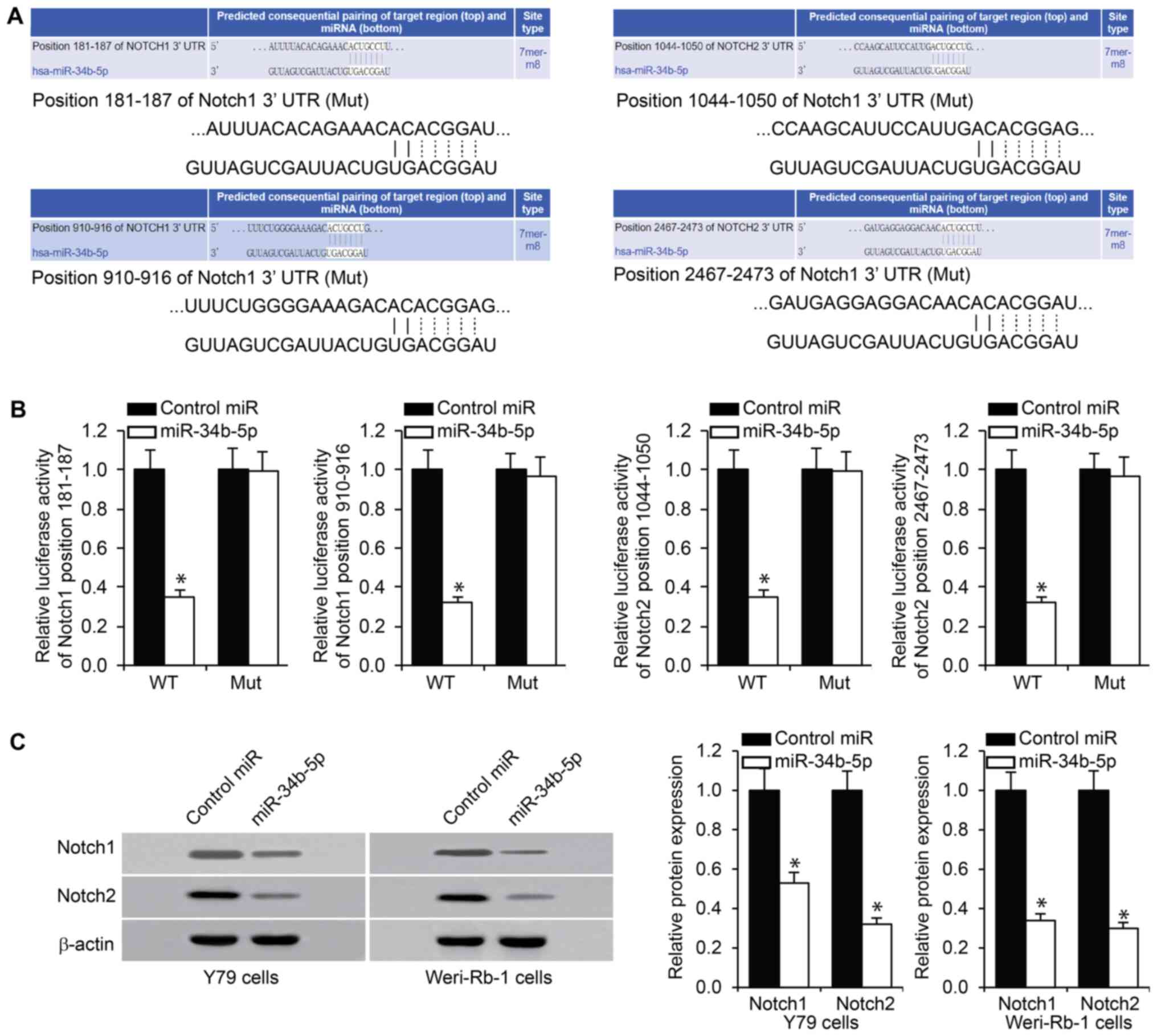
miR-34b-5p directly targets Notch1 and
Notch2
Prior to assessing the molecular mechanism that may
account for the impact of miR-34b-5p on RB cells, putative targets
of miR-34b-5p were predicted using TargetScan online tool. This
bioinformatic analysis identified the 3'-UTR of Notch1 and Notch2
mRNA as potential miR-34b-5p binding targets (Fig. 4A), and further indicated that these
sites were conserved. It was hypothesized that Notch1 and Notch2
were potential targets of miR-34b-5p and their dysregulation by
miR-34b-5p may serve a role in RB development. This was verified
using a luciferase reporter assay involving a luciferase reporter
bearing either wild-type or mutant Notch1 or Notch2 3'-UTR. The
results indicated that co-transfection with miR-34b-5p
significantly repressed luciferase activity compared with control
miR (Fig. 4B). Notch1 and Notch2
protein levels were determined in Y79 and Weri-Rb-1 cells following
miR-34b-5p transfection. As presented in Fig. 4C, a reduction in the levels of
Notch1 and Notch2 expression was observed following transfection
with miR-34b-5p in Y79 and Weri-Rb-1 cells, compared with cells
transfected with control miR. These observations suggested that
Notch1 and Notch2 were possible targets of miR-34b-5p.
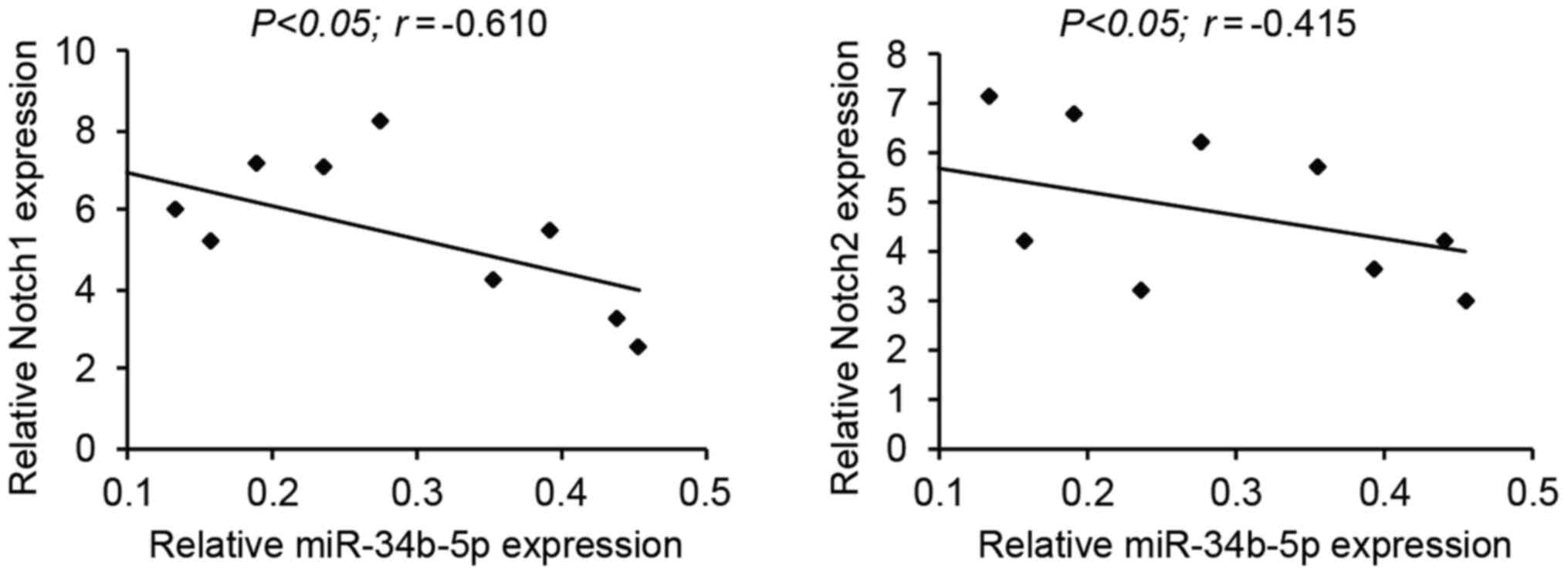
Negative correlation between
miR-34b-5p and Notch1/2 in RB samples
Pearson's correlation analysis was performed to
assess the correlation between miR-34b-5p and its targets, Notch1
and Notch2, in the tumor tissues of patients with RB. A negative
correlation was observed between miR-34b-5p and Notch1, and
miR-34b-5p and Notch2 expression levels (Fig. 5).
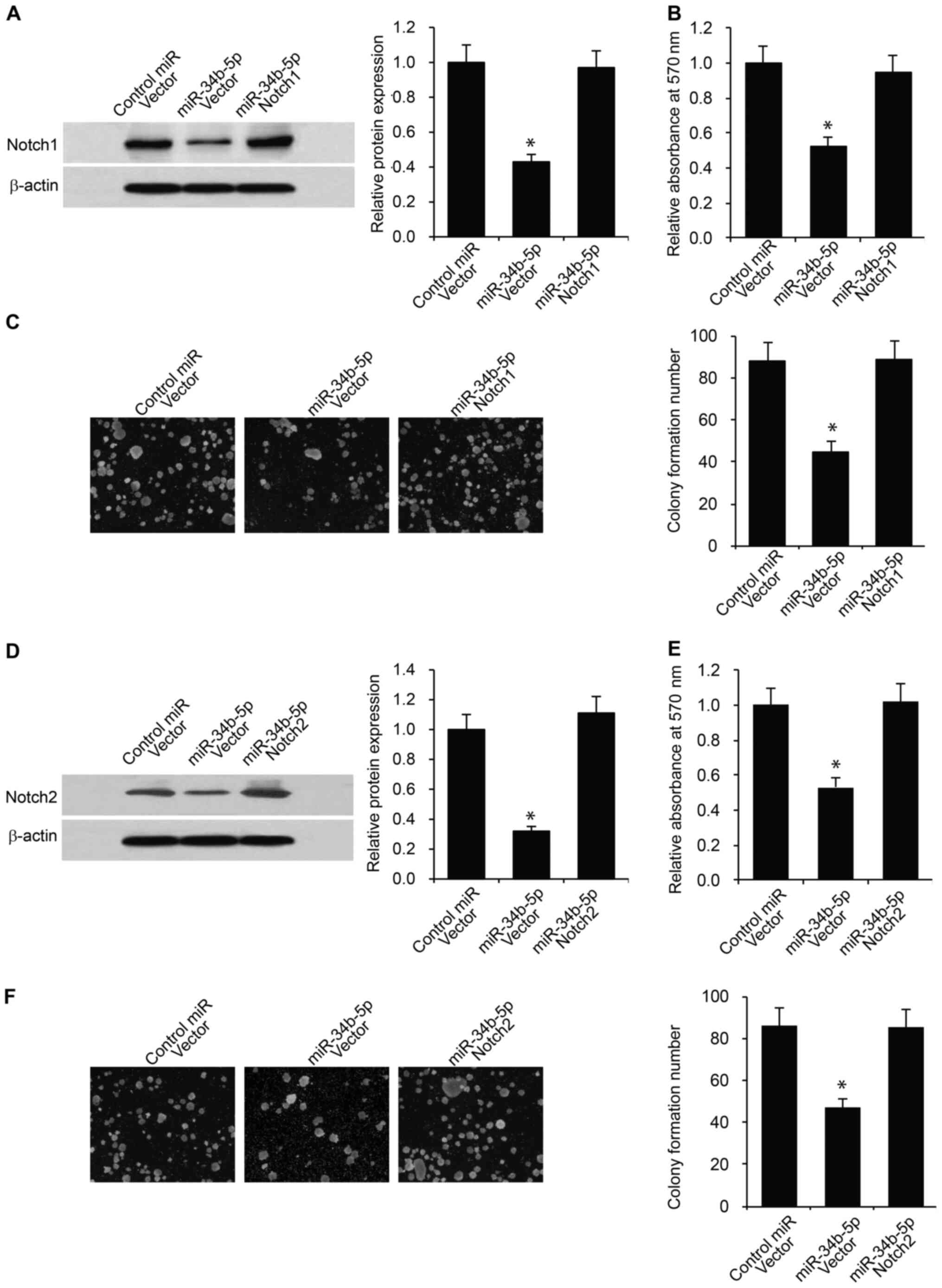
miR-34b-5p inhibits RB cell
proliferation and stemness via Notch1 and Notch2
To determine whether miR-34b-5p inhibits RB cell
proliferation and stemness via Notch1 and Notch2, rescue
experiments were performed by overexpressing Notch1 or Notch2 in
Y79 RB cells transfected with miR-34b-5p. Overexpression of Notch1
or Notch2 was confirmed via western blotting in Y79 RB cells
following Notch1 or Notch2 plasmid transfection in comparison to
empty vector controls (Fig. S2).
Firstly, Notch1 and miR-34b-5p were co-transfected into Y79 RB
cells. The overexpression of Notch1 was confirmed in Y79 RB cells
co-transfected with miR-34b-5p compared with cells transfected with
empty vector (Fig. 6A). These cells
were subjected to CCK-8 proliferation and tumor sphere assays. As
indicated in Fig. 6B and C, overexpression of Notch1 rescued the
decrease in cell proliferation and stemness, which was inhibited by
miR-34b-5p. Similarly, overexpression of Notch2 significantly
reversed the decrease in RB cell proliferation and stemness induced
by miR-34b-5p (Fig. 6D-F).
Discussion
The present study aimed to assess the role of
miR-mediated regulation of Notch1 and Notch2, thereby influencing
the initiation and progression of RB. Notch1 was selected as a
target gene, owing to its significance in retinal development
(27). Additionally, targets beyond
RB1, which may or may not be regulating RB1, were
sought. The association of Notch1 dysregulation with several other
malignancies along with RB justified the target choice. While the
impact of common mutational and non-mutational events on
Notch1-induced dysregulation and cancer has been previously studied
(20), the present study aimed to
elucidate the role of miR-mediated epigenetic modifications in RB.
miR-34b-5p as a potential miR candidate in RB with Notch1 and
Notch2 as its gene targets was identified.
miR-34b-5p is a member of the miR-34 family
consisting of miR-34a, miR-34b and miR-34c. It has been previously
reported that endogenous expression of miR-34b-5p exhibited tumor
suppressive characteristics (28).
Although the targets of miR-34a are well characterized, the targets
of miR-34b/c have received little attention, essentially because of
the differential expression of the members of the miR-34 family
(29). While miR-34a is
ubiquitously and highly expressed in the brain tissue, miR-34b and
miR-34c are predominantly expressed in the lung tissue. Thus, the
significance of miR-34b-5p-mediated epigenetic modifications in
different cancers has been less explored (28). Furthermore, the correction of an
annotation error of miR-34b-5p has resulted in the identification
of its function as a tumor suppressor, when endogenously expressed
(30). Therefore, while the targets
of miR-34a are well characterized (29), the targets of miR-34b are only
currently beginning to be explored (31-33).
The present study focused on Notch1 and Notch2, which were
identified as targets of miR-34b-5p via a bioinformatics-based
analysis tool (34), essentially
because of the significance of Notch1 and Notch2 in retinal
development via the suppression of photoreceptor differentiation
and the maintenance of cells in their progenitor states (34). While activation of Notch1 and Notch2
via the upregulation of the ligands Jagged-2 and Delta-like protein
4 is well known, the epigenetic regulation of Notch1 and Notch2 is
less studied, to the best of our knowledge (35).
The present study firstly indicated that miR-34b-5p
levels were reduced in RB tissues compared with adjacent non-tumor
healthy tissues. Moreover, the serum levels of miR-34b-5p were
decreased in patient sera as compared with those in age-matched
controls. This observation is the first demonstration of
dysregulation of miR-34b-5p in RB, to the best of our
knowledge.
The overexpression of miR34-b-5p in the RB cell
lines Y79 and Weri-Rb-1 was associated with reduced cell growth,
expression of sentinel markers of cancer stemness (Nanog, Sox-2 and
CD133) and formation of colonies. Additionally, the association of
miR-34b-5p with its proposed targets (Notch1 and Notch2) was
examined in tissues from patients with RB. The negative correlation
that was observed between the levels of miR34b-5p and that of
Notch1 or Notch2 suggested an inhibitory role of miR34b-5p in
Notch1 and Notch2 expression.
The reduced cell proliferation upon miR34b-5p
overexpression in the RB cell lines may be attributed to the role
of Notch signaling in G1/S progression of the cell
cycle, as it has been demonstrated in T cells (36). Canonical and non-canonical Notch
signaling pathways may induce the expression of cyclin D3, CDK 4
and CDK 6(36). Another study in
laryngeal squamous cell carcinoma indicated that knockdown of
Notch1 was associated with decreased phosphorylation of ERK, AKT,
as well as decreased expression of c-Myc, p21, Bcl-2, cyclin D1,
CDK4 and Cyclin E, along with increased expression of Bax (37).
The significance of Notch signaling in cancer stem
cells (CSC) has been elucidated. Targeting of the Notch signaling
pathways via gamma secretase inhibitors (GSIs) has been revealed to
decrease cancer cell stemness and is being assessed for its
therapeutic benefits (38). In
pancreatic cancers, inhibition of Notch activation, via either GSI
or Hes-1 short hairpin RNA, resulted in a significant decrease in
the proportion of cancer stem cells and tumor sphere formation
(38). Hes-1 is a downstream target
of Notch1 and influences the maintenance and differentiation of
certain stem cells in pancreatic cancer (38). Other mechanisms of Notch-induced CSC
include overexpression of the C-X-C chemokine receptor type 4,
which is known to be responsible for stemness-like properties and
promotion of chemotaxis via the stromal cell-derived factor 1 axis
(12).
In conclusion, the present study demonstrated that
the downregulation of miR-34b-5p in RB resulted in the upregulation
of Notch1 and Notch2 and was subsequently associated with oncogenic
properties, such as dysregulation of cell growth, induction of
cancer cell stemness and promotion of tumor sphere formation. The
current preliminary investigation suggested a potential therapeutic
role for miR-34b-5p in the treatment of RB via regulating the Notch
signaling pathway. However, the findings of the present study are
preliminary and require further verification via in vivo
studies, alongside a more detailed understanding of the regulation
of miR-34b-5p in RB.
Supplementary Material
miR-34b-5p reduces RB cell migration
and invasion. (A) Migration and (B) invasion assays were performed
in Y79 and Weri-Rb-1 RB cells transfected with miR-34b-5p or
control miR (magnification, x400). *P<0.05 vs.
control miR. miR, microRNA; RB, retinoblastoma.
Expression of Notch1 and Notch2 in RB
cells after plasmid transfection. Overexpression of Notch1 and
Notch2 in Y79 RB cells following transfection with Notch1 or Notch2
plasmid was verified by western blotting. RB, retinoblastoma. The
density of CD133, Sox-2 and Nanog was quantified relative to
β-actin. *P<0.05 vs. Vector.
Clinical characteristics of the
patients with retinoblastoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Project of
Qiqihar Science and Technology (SFGG-201946)
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SZ and ZC performed experiments, and collected and
analyzed the data. SZ wrote the manuscript and ZC designed the
experiments. Each author read the manuscript and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The current study performed on human samples was
approved by the clinical research Ethics Committee of The Third
Affiliated Hospital of Qiqihar Medical University (Qiqihar, China)
and each patient or healthy subject provided a written informed
consent. The animal study was approved by the Animal Care and Use
Committee of The Third Affiliated Hospital of Qiqihar Medical
University (Qiqihar, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dimaras H, Kimani K, Dimba EA, Gronsdahl
P, White A, Chan HS and Gallie BL: Retinoblastoma. Lancet.
379:1436–1446. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fabian ID and Sagoo MS: Understanding
retinoblastoma: Epidemiology and genetics. Community Eye Health.
31(7)2018.PubMed/NCBI
|
|
3
|
Knudson AG Jr, Meadows AT, Nichols WW and
Hill R: Chromosomal deletion and retinoblastoma. N Engl J Med.
295:1120–1123. 1976.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chinnam M and Goodrich DW: RB1,
development, and cancer. Curr Top Dev Biol. 94:129–169.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ishak CA, Coschi CH, Roes MV and Dick FA:
Disruption of CDK-resistant chromatin association by pRB causes DNA
damage, mitotic errors, and reduces Condensin II recruitment. Cell
Cycle. 16:1430–1439. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mastrangelo D, De Francesco S, Di Leonardo
A, Lentini L and Hadjistilianou T: Retinoblastoma epidemiology:
Does the evidence matter? Eur J Cancer. 43:1596–1603.
2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dimaras H, Khetan V, Halliday W, Orlic M,
Prigoda NL, Piovesan B, Marrano P, Corson TW, Eagle RC Jr, Squire
JA and Gallie BL: Loss of RB1 induces non-proliferative retinoma:
Increasing genomic instability correlates with progression to
retinoblastoma. Hum Mol Genet. 17:1363–1372. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dimaras H and Gallie BL: The p75 NTR
neurotrophin receptor is a tumor suppressor in human and murine
retinoblastoma development. Int J Cancer. 122:2023–2029.
2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dimaras H, Coburn B, Pajovic S and Gallie
BL: Loss of p75 neurotrophin receptor expression accompanies
malignant progression to human and murine retinoblastoma. Mol
Carcinog. 45:333–343. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Kandalam M, Mitra M, Subramanian K and
Biswas J: Molecular pathology of retinoblastoma. Middle East Afr J
Ophthalmol. 17:217–223. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Castro-Magdonel BE, Orjuela M, Camacho J,
García-Chéquer AJ, Cabrera-Muñoz L, Sadowinski-Pine S,
Durán-Figueroa N, Orozco-Romero MJ, Velázquez-Wong AC,
Hernández-Ángeles A, et al: miRNome landscape analysis reveals a 30
miRNA core in retinoblastoma. BMC Cancer. 17(458)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xiao W, Gao Z, Duan Y, Yuan W and Ke Y:
Notch signaling plays a crucial role in cancer stem-like cells
maintaining stemness and mediating chemotaxis in renal cell
carcinoma. J Exp Clin Cancer Res. 36(41)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xiao W, Chen X and He M: Inhibition of the
Jagged/Notch pathway inhibits retinoblastoma cell proliferation via
suppressing the PI3K/Akt, Src, p38MAPK and Wnt/β-catenin signaling
pathways. Mol Med Rep. 10:453–458. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen CY, Chen YY, Hsieh MS, Ho CC, Chen
KY, Shih JY and Yu CJ: Expression of Notch gene and its impact on
survival of patients with resectable non-small cell lung cancer. J
Cancer. 8:1292–1300. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mollen EWJ, Ient J, Tjan-Heijnen VCG,
Boersma LJ, Miele L, Smidt ML and Vooijs MAGG: Moving Breast Cancer
Therapy up a Notch. Front Onco. 8(518)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Baker A, Wyatt D, Bocchetta M, Li J,
Filipovic A, Green A, Peiffer DS, Fuqua S, Miele L, Albain KS and
Osipo C: Notch-1-PTEN-ERK1/2 signaling axis promotes HER2+ breast
cancer cell proliferation and stem cell survival. Oncogene.
37:4489–4504. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sun Q, Wang R, Wang Y, Luo J, Wang P and
Cheng B: Notch1 is a potential therapeutic target for the treatment
of human hepatitis B virus X protein-associated hepatocellular
carcinoma. Oncol Rep. 31:933–939. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Venkatesh V, Nataraj R, Thangaraj GS,
Karthikeyan M, Gnanasekaran A, Kaginelli SB, Kuppanna G, Kallappa
CG and Basalingappa KM: Targeting Notch signalling pathway of
cancer stem cells. Stem Cell Investig. 5(5)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Takebe N, Nguyen D and Yang SX: Targeting
notch signaling pathway in cancer: Clinical development advances
and challenges. Pharmacol Ther. 141:140–149. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fabbri G, Holmes AB, Viganotti M, Scuoppo
C, Belver L, Herranz D, Yan XJ, Kieso Y, Rossi D, Gaidano G, et al:
Common nonmutational NOTCH1 activation in chronic
lymphocytic leukemia. Proc Natl Acad Sci USA. 114:E2911–E2919.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lee H, Kim KR, Cho NH, Hong SR, Jeong H,
Kwon SY, Park KH, An HJ, Kim TH, Kim I, et al: MicroRNA expression
profiling and Notch1 and Notch2 expression in minimal deviation
adenocarcinoma of uterine cervix. World J Surg Oncol.
12(334)2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Maroof H, Islam F, Dong L, Ajjikuttira P,
Gopalan V, McMillan NAJ and Lam AK: Liposomal delivery of
miR-34b-5p induced cancer cell death in thyroid carcinoma. Cells.
7(265)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Singh L and Kashyap S: Update on pathology
of retinoblastoma. Int J Ophthalmol. 11:2011–2016. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Phillips PC, Levow C, Catterall M, Colvin
OM, Pastan I and Brem H: Transforming growth
factor-alpha-Pseudomonas exotoxin fusion protein (TGF-alpha-PE38)
treatment of subcutaneous and intracranial human glioma and
medulloblastoma xenografts in athymic mice. Cancer Res.
54:1008–1015. 1994.PubMed/NCBI
|
|
26
|
Zhao D and Cui Z: MicroRNA-361-3p
regulates retinoblastoma cell proliferation and stemness by
targeting hedgehog signaling. Expe Ther Med. 17:1154–1162.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jadhav AP, Mason HA and Cepko CL: Notch 1
inhibits photoreceptor production in the developing mammalian
retina. Development. 133:913–923. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang L, Liao Y and Tang L: MicroRNA-34
family: A potential tumor suppressor and therapeutic candidate in
cancer. J Exp Clin Cancer Res. 38(53)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Misso G, Di Martino MT, De Rosa G, Farooqi
AA, Lombardi A, Campani V, Zarone MR, Gullà A, Tagliaferri P,
Tassone P and Caraglia M: Mir-34: A new weapon against cancer? Mol
Ther Nucleic Acids. 3(e194)2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Engkvist ME, Stratford EW, Lorenz S,
Meza-Zepeda LA, Myklebost O and Munthe E: Analysis of the miR-34
family functions in breast cancer reveals annotation error of
miR-34b. Sci Rep. 7(9655)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jiang L and Hermeking H: miR-34a and
miR-34b/c suppress intestinal tumorigenesis. Cancer Res.
77:2746–2758. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Majid S, Dar AA, Saini S, Shahryari V,
Arora S, Zaman MS, Chang I, Yamamura S, Tanaka Y, Chiyomaru T, et
al: miRNA-34b inhibits prostate cancer through demethylation,
active chromatin modifications, and AKT pathways. Clin Cancer Res.
19:73–84. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li Z, Luo Q, Xu H, Zheng M, Abdalla BA,
Feng M, Cai B and Zhang X, Nie Q and Zhang X: MiR-34b-5p suppresses
melanoma differentiation-associated gene 5 (MDA5) signaling pathway
to promote avian leukosis virus subgroup J (ALV-J)-infected cells
proliferaction and ALV-J replication. Front Cell Infect Microbiol.
7(17)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4(e05005)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kakuda S, LoPilato RK, Ito A and
Haltiwanger RS: Canonical Notch ligands and Fringes have distinct
effects on NOTCH1 and NOTCH2. J Biol Chem. 295:14710–14722.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Joshi I, Minter LM, Telfer J, Demarest RM,
Capobianco AJ, Aster JC, Sicinski P, Fauq A, Golde TE and Osborne
BA: Notch signaling mediates G1/S cell-cycle progression in T cells
via cyclin D3 and its dependent kinases. Blood. 113:1689–1698.
2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Dai MY, Fang F, Zou Y, Yi X, Ding YJ, Chen
C, Tao ZZ and Chen SM: Downregulation of Notch1 induces apoptosis
and inhibits cell proliferation and metastasis in laryngeal
squamous cell carcinoma. Oncol Rep. 34:3111–3119. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Abel EV, Kim EJ, Wu J, Hynes M, Bednar F,
Proctor E, Wang L, Dziubinski ML and Simeone DM: The Notch pathway
is important in maintaining the cancer stem cell population in
pancreatic cancer. PLoS One. 9(e91983)2014.PubMed/NCBI View Article : Google Scholar
|