1. Overview
Vitiligo is a multifactorial disease characterized
by a deficiency or absence of skin pigmentation due to the loss or
inactivity of melanocytes in the basal layer of the epidermis,
mucosa or other organs (1,2). The pathogenesis of this disease
involves a convergence of genetic, environmental and metabolic
factors, as well as autoimmune responses (3). The clinical hallmark of vitiligo is
the lack of melanin and the initial manifestation of vitiligo can
appear as the premature whitening or graying of hair (1,4). In
the skin, vitiligo manifests as hypochromic or achromic macules and
patches, which increase in number and size over time (4). These depigmented macules and patches
may occur anywhere on the body. However, they are observed more
frequently around the orifices, genitals and sun-exposed areas
(5). The understanding of how and
why vitiligo lesions are formed and extended is crucial. To
understand these complex processes, it is necessary to understand
how the normal pigmentation mechanism functions and contributes to
the organism's homeostasis.
Melanocytes
Melanocytes are located in the stratum basale (basal
layer) of the epidermis, where they interact through dendrites with
keratinocytes (6). These cells have
two functions: i) the production of melanin; and ii) a role in the
immune system (7). The number of
melanocytes is relatively constant, with between 500 and 2,000
melanocytes/mm2 in the skin. Melanocytes constitute
~5-10% of the cells located in the basal layer of the epidermis
(8).
Melanins, a group of natural pigments produced by
melanocytes, are derived from tyrosine and directly from
dihydroxyphenylalanine (DOPA) (9).
DOPA is the product of the enzyme tyrosinase (diphenol oxidase), a
copper enzyme that utilizes molecular O2 to metabolize
DOPA from tyrosine. Exposure to UV light activates tyrosinase in
melanosomes; this is the initial step of melanin synthesis
(10) that occurs in melanocytes
(11). DOPA is converted to DOPA
quinone and several intermediates are formed until indolequinone is
produced and polymerized to form melanin (12). Eumelanin (brown or black in color)
is the most common product in humans. However, in the presence of
cysteine, eumelanin is converted to pheomelanin (red or yellow in
color) (9,12,13). A
combination, mainly of melanin and carotene, produces color in the
skin, eyes and hair (13). Melanin
can absorb broadband UV light and protects skin cells from UVB
radiation (UVBR) damage, thereby decreasing the risk of
carcinogenesis. Additionally, melanin has antioxidant and radical
scavenging properties (14).
Following synthesis in melanocytes, melanin is
stored in organelles termed melanosomes, which are transported to
nearby keratinocytes to induce pigmentation (15). Additionally, melanocytes serve a
role in the immune system and express various immune molecules,
such as toll-like receptors and proinflammatory cytokines and
chemokines (15). The major
histocompatibility complex (MHC) class II is located only on
professional antigen presenting cells (APCs), including
melanocytes, dendritic cells, macrophages and B cells, as opposed
to the widely distributed MHC class I located on the majority of
vertebrate cells (16).
Cytokine-stimulated melanocytes express surface proteins, including
CD40, a costimulatory protein expressed and required by APCs, and
intercellular adhesion molecule 1 (ICAM1) (15), an endothelial and
leukocyte-associated transmembrane protein. ICAM1 facilitates
leukocyte-endothelial transmigration and increases the risk of
melanoma metastasis (17).
Additionally, melanocytes express numerous proinflammatory
cytokines, including IL-1, -3, -6 and -8, TNF-α and TGF-β (18,19).
Furthermore, melanocytes secrete cytokines following activation by
pattern recognition receptors, which recognize microbe-associated
molecular patterns (MAMPs). MAMPs can be proteins, carbohydrates or
lipids from pathogenic microorganism that are exposed to immune
cells (18). Melanocytes can also
be activated by cytokines secreted by other nearby immune cells
(18).
UVA is mainly responsible for indirect DNA damage by
the generation of reactive oxygen species (ROS) (20). ROS produce single-strand breaks in
DNA and crosslinks in DNA proteins (21,22).
DNA absorbs UVBR at wavelengths of 245-290 nm (23). Therefore, UVBR is a
powerful mutagen (24). The
predominant DNA lesions formed are cyclobutane pyrimidine dimers
(CPDs) and 6-4 pyrimidine-pyrimidones (6-4PPs). CPDs and 6-4PPs are
the most important UVB-induced photoproducts with potential
mutagenic properties, since they can cause highly specific
mutations, such as CC to TT double base substitutions and C to T
substitutions in dipyrimidines (25).
The following sections describe the different types
of vitiligo and which factors intervene in their initiation and
development.
Classification of vitiligo
According to the revised classification of vitiligo
by the Vitiligo Global Issues Consensus Conference (VGICC)
published in 2012(26), there are
three recognized clinical forms of vitiligo: i) non-segmental; ii)
segmental; and iii) unclassified. Each of these categories have
further subclassifications, as described below.
Non-segmental vitiligo
This term is recommended for all non-segmental forms
of vitiligo with the following subclassifications: i) generalized
vitiligo, formerly termed vitiligo vulgaris, is frequently
bilateral and presents with symmetric macules and patches,
affecting any part of the body; however, most commonly the hands,
face and fingers are affected; ii) acrofacial vitiligo, which is
limited to the face, head, hands and feet; ‘lip-tip’ is considered
a subcategory, only affecting the cutaneous lips and fingertips;
iii) vitiligo universalis, which affects 80-90% of the body
surface; iv) mucosal vitiligo, which is the depigmentation of the
oral and genital mucosae; v) mixed vitiligo, which refers to the
simultaneous presentation of segmental and non-segmental vitiligo;
and vi) the rare variants, including minor vitiligo, follicular
vitiligo and vitiligo punctate.
Segmental vitiligo
This subclassification presents unilaterally as an
asymmetric distribution of macules and patches, generally around
the midline. Segmental vitiligo can be monosegmental, bisegmental
or plurisegmental.
Unclassified vitiligo
This term is used for lesions that do not evolve
into segmental or non-segmental vitiligo after presenting for 1-2
years and includes the following variants: i) focal vitiligo,
referring to small patches of skin or mucosal depigmentation; and
ii) single mucosal site involvement, which only affects oral or
genital mucosa.
Koebner phenomenon
The Koebner phenomenon, also known as the isomorphic
response, refers to the development of vitiligo lesions following
trauma. These lesions appear on a site previously unaffected by
vitiligo (27). The Koebner
phenomenon is divided into the following subtypes: i) type 1,
depigmented areas following trauma or injury in the past year; ii)
type 2A, depigmented lesions in areas of repeated pressure or
friction; iii) type 2B, linear, punctiform or crenate depigmented
lesions in areas of repeated pressure or friction; and iv) type 3,
experimentally induced lesions due to trauma, which can be further
subdivided into superficial irritation (level 1), superficial
epidermal trauma (level 2) and dermoepidermal trauma (level 3)
(27).
Further classification of vitiligo:
Stable and active vitiligo
The classification of stable or active vitiligo is
beneficial for dermatologists to specialize treatment strategies
for patients. This classification accounts for the evolution of
skin lesions. Vitiligo is considered active when there is growth of
previous lesions, the appearance of new lesions and/or the presence
of confetti-like depigmentation or trichrome lesions (4,26,28).
The time frame used as a cut-off for vitiligo stability or activity
varies according to different authors, ranging from 6 weeks
(29) to 2 years (30). The VGICC endorses a period of 1 year
(26).
Epidemiology
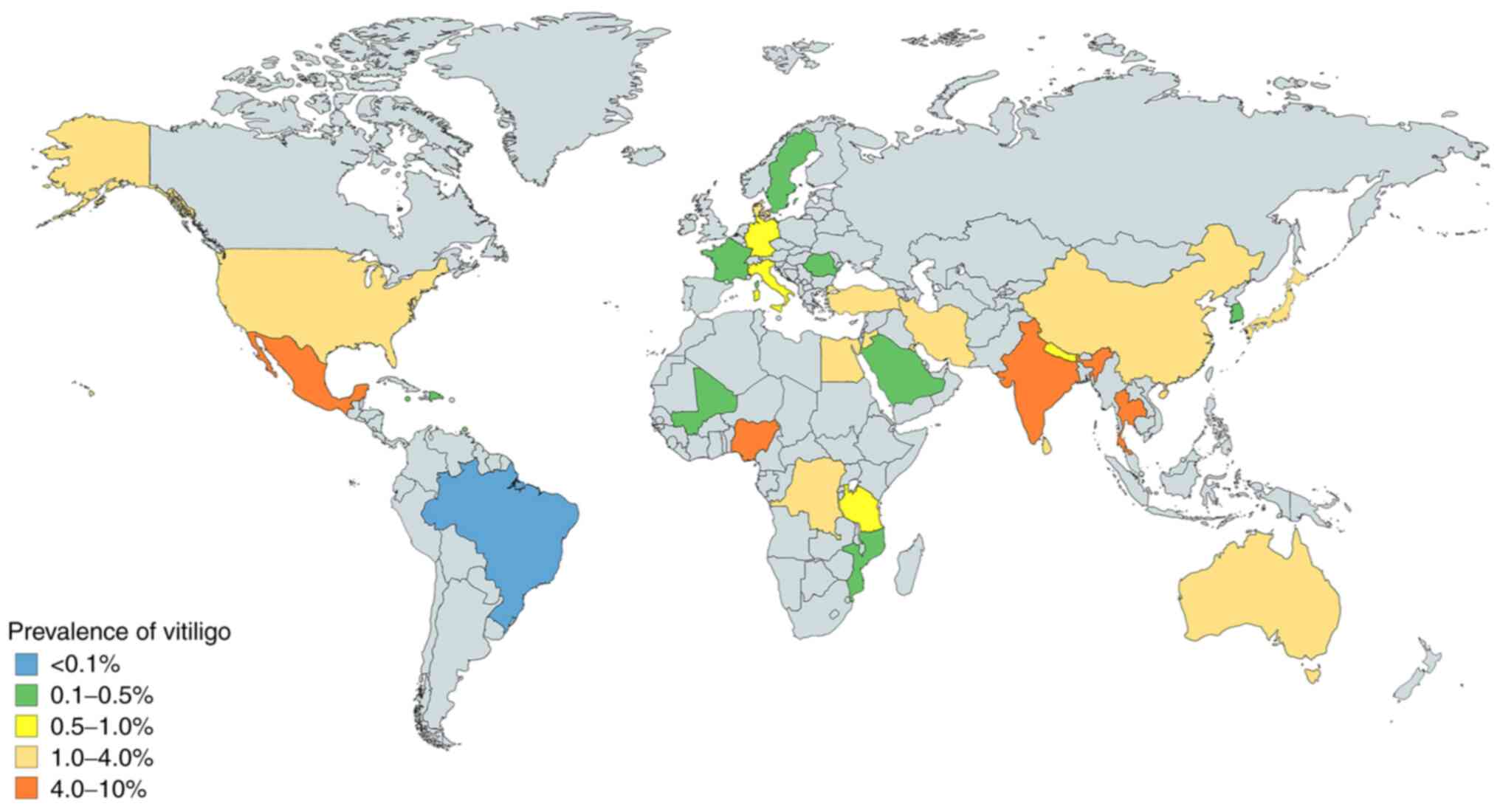
Vitiligo is the most common depigmenting disorder,
with a global prevalence of ~0.06-8.8%, according to data published
between 1964 and 2017 (31-35).
Vitiligo affects men and women equally (33). However, there are differences in the
prevalence of vitiligo according to geographical regions. The
countries with the highest reported prevalence are India (8.8%),
Mexico (2.6-4%) and Japan (≥1.68%; Fig.
1) (31,32,34,35).
Vitiligo affects young and old individuals. The
median age of presentation was 37.6 years in China (range, 5-79
years) (36), while the mean age of
vitiligo presentation was 27.02 years in Pakistan (range, 5.5
months to 82 years) (37) and 26.4
years in Mexico (range, 7 months to 74 years) (38). However, the highest incidence of
vitiligo has been observed in childhood or young adulthood, peaking
at 10-30 years (39).
Comorbidities
The role of autoimmunity in vitiligo is due to the
high prevalence of auto-antibodies against melanocytes and by the
simultaneous presence of other autoimmune diseases, including
Hashimoto's thyroiditis, diabetes mellitus, Addison's disease,
alopecia areata and/or ophthalmic anomalies, such as iritis
(40-42).
These associations vary according to age and sex. For example,
Grave's disease, Hashimoto's thyroiditis, atopic dermatitis,
rheumatoid arthritis, systemic lupus erythematosus and Sjögren's
syndrome have been reported to be associated with vitiligo in
women, while psoriasis with men (43) and myasthenia gravis with young
patients (<40 years old) (44).
Worldwide, thyroid dysfunction in patients with vitiligo ranges
between 0 and 52%, according to data published up to 2012(45). Sedighe et al (46) reported the presence of thyroid
disorders in 17.4% of patients with vitiligo and the most common
presentation was hypothyroidism in Iran. In India, Gopal et
al (47) reported
hypothyroidism in 30% of patients with vitiligo. In 2014, our
previous study reported that hyperthyroidism, hypertension, atopy,
diabetes mellitus and alopecia areata were associated with vitiligo
and that the disorder with the highest frequency was
hyperthyroidism in Mexico (22%) (38). All of the aforementioned disorders
appear to be associated with autoimmunity and genetic factors.
Increased levels of anti-melanocyte and antinuclear
antibodies and complement component 4 have been described in a
recent study in Egyptian patients with vitiligo. These increased
levels had a positive correlation with disease severity (48). Studies on the number of
auto-antibodies in different ethnic groups of patients with
vitiligo are limited. A meta-analysis of 25 case-controlled studies
reported that anti-thyroperoxidase (ATPO), anti-thyroglobulin
(ATG), antinuclear, anti-gastric parietal cell (AGPCA) and
anti-adrenal antibodies were significantly higher in patients with
vitiligo compared with the control group (49). Therefore, it is necessary to
consider the characteristics of diseases, including autoimmune
thyroid disease for ATPO and ATG auto-antibodies, pernicious anemia
and gastric atrophy for AGPCA, in patients with vitiligo (49).
Vitiligo can affect several members of the same
family, indicating a genetic risk. Among patients with vitiligo,
~1/5th of patients have at least one affected close relative
(50). It has been reported that
patients with a family history of vitiligo are at a higher risk of
developing this disease at an early age compared with those without
a family history of vitiligo (38).
Furthermore, Alenizi (51) revealed
that consanguinity is associated with an increased incidence of
vitiligo (51). Nevertheless, the
inheritance pattern of vitiligo is complex as multiple causative
factors are involved (50).
The association of vitiligo with autoimmune
responses is further supported by the presence of lymphocytes in
the dermis of early lesions and auto-antibodies against melanocytes
in numerous patients with active vitiligo (39,44).
Furthermore, the positive response from patients with vitiligo to
treatments with immunomodulatory agents, including corticosteroids
and phototherapy, confirms that a key aspect of vitiligo includes
an autoimmune reaction against factors involved in the pigmentation
of tissues and organs (52).
Risk factors
Certain internal or external factors may be involved
with the ability of melanocytes and keratinocytes to resist harmful
effects.
Internal factors
Two of the most important internal factors,
activation of the immune system and heritability, have been
discussed above. Notably, another internal risk factor for vitiligo
is cancer. Franks and Slansky (53)
demonstrated the risk of cancer development in patients with
autoimmune and chronic inflammatory diseases. Furthermore, Asilian
et al (54) reported a
73-year-old patient with new onset vitiligo who developed
esophageal cancer several years after the diagnosis of vitiligo.
Additionally, Balasubramanian (55)
reported two cases of vitiligo associated with breast cancer.
However, whether vitiligo was a consequence of cancer or whether
both cancer and vitiligo developed in these patients due to genetic
disorders remains unknown. Genetic factors associated with vitiligo
will be described in subsequent sections.
External factors
Multiple external factors, including sunburns,
physical trauma, agricultural and industrial pollution, and
emotional stress, have been reported to induce vitiligo;
consequently, a number of these factors are considered as
environmental risk factors of vitiligo (56,57).
However, sometimes establishing causality and not only association
is difficult, and must be evaluated under the basis of scientific
rigor this.
2. Immunopathogenesis of vitiligo
The immune hypothesis is supported by several
factors, including the association with autoimmune conditions,
organ-specific antibodies, antibodies against antigens in
melanocytes and the participation of immune cells.
Auto-antibodies and antigens
Several melanocyte antibodies are associated with
disease extension. IgG and complement component 3 (C3) deposits
have been observed in the basal membrane zone of skin lesions
(58,59). C3 serves a key role in the
complement system and contributes to innate immunity (58,59).
Additionally, numerous specific autoantigens
associated with pathogenesis have been observed in patients with
vitiligo, including tyrosinase, tyrosinase-related protein,
Melan-A/melanoma antigen recognized by T cells 1 (MART1),
melanosomal matrix glycoprotein (gp100), SOX10 and
melanin-concentrating hormone receptor 1(60).
Cytokines
Multiple studies have investigated the role of
cytokines in vitiligo. IL-6 facilitates leukocyte-melanocyte
interactions and IL-8 attracts neutrophils (60). Additionally, IL-17 has been
associated with vitiligo (61). T
helper 17 (Th17) cells are a subset of pro-inflammatory T helper
cells that infiltrate the upper dermis in active vitiligo (62) and are potent producers of IL-17 and
IL-17F. IL-17 synergizes with local inflammatory mediators,
including IL-1β, IL-6 and TNF-α, and inhibits melanocyte
proliferation (63). Furthermore,
IL-17 expression is upregulated in multiple autoimmune inflammatory
diseases, such as rheumatoid arthritis, systemic lupus
erythematosus, psoriasis and atopic dermatitis (62). The levels of IL-17 in serum have
been associated with the extent and duration of depigmentation and
IL-17 concentration is increased in perilesional skin compared with
in depigmented skin (62,63). IL-17 and TNF-α suppress
melanogenesis by synergistically downregulating genes of the
pigmentation pathway, causing hypopigmentation disorders (64). Additionally, TNF-α contributes to
keratinocyte apoptosis by decreasing the levels of melanogenic
cytokines (65,66). Elucidating these changes in
cytokines has led to the use of methotrexate (MTX), a folate
antagonist, in the treatment of vitiligo (67). A previous study has demonstrated
that MTX decreases the number of T cells producing TNF-α (67). Furthermore, case reports and studies
using oral MTX in vitiligo reported variable results, including
arrested progression, marked skin repigmentation or no significant
changes, and when MTX was compared with oral dexamethasone
minipulse, both were considered equally effective (67-70).
Recently, a topical formula of MTX was used in a case report with
significant improvement in repigmentation of the vitiligo lesion
(71). However, further studies are
required.
Plasmacytoid dendritic cells are part of the
cell-infiltrate in progressive vitiligo and produce human Myxovirus
resistance protein 1, an interferon-induced dynamin-like GTPase,
which is associated with the recruitment of CD4+
chemokine receptor 3+ (CXCR3+) T cells
(72,73). Additionally, plasmacytoid dendritic
cells produce IFN-α, which leads to the production of CXC motif
chemokines, including C-X-C motif chemokine ligand (CXCL) 9 (CXCL9)
and CXCL10, and serves as an initial signal for the recruitment of
effector T cells and the amplification of inflammation (73). Previous studies have reported
increased levels of CXCL9 and CXCL10 in vitiligo (73,74).
Furthermore, CXCL10 recruits melanocyte-specific CD8+ T
cells, which migrate to the epidermis due to the interaction of
CXCL10 with CXCR3, which is expressed on T cells in the blood and
skin (74,75).
T cells
Cellular immunity serves an important role in the
pathogenesis of vitiligo and epidermal cells other from melanocytes
can induce an immune response in vitiligo (73). For instance, keratinocytes exposed
to high levels of ROS express CXCL16, which stimulates the
migration and infiltration of CXCR6+ CD8+ T
cells (killer T cells) in the skin (76). These CD8+ T cells detect
specific antigenic proteins derived from melanocytes that are
involved in melanin synthesis, including melanoma antigen
Melan-A/MART1, gp100, tyrosinase, tyrosinase related protein (TYRP)
1-(-a protein specifically produced by melanocytes) -and TYRP2,
also known as dopachrome tautomerase (77,78).
Skin biopsies have revealed CD8+ and
CD4+ T-cell infiltration in the margins of active
lesions, with an increased CD8+ to CD4+ ratio
(79). Additionally, increased
T-cell migration to the skin due to the increased expression of
CD25 and MHC II, and the secretion of IFN-γ have been demonstrated
(80), while increased T-cell
number has been reported to be associated with worse disease extent
(81). Furthermore, CD4+
T cells serve an important role in vitiligo due to their
association with autoimmune diseases (60,80).
IFN-γ-dependent cytokines rely on the JAK-STAT
pathway for signalling in the pathogenesis of vitiligo, and CXCL10
in keratinocytes is an important mediator of depigmentation
(82,83). Tofacitinib, a promising drug that
acts on the JAK-STAT signaling pathway, blocks IFN-γ signaling and
downstream CXCL10 expression, leading to repigmentation in vitiligo
(84). The study by Craiglow et
al (85) was the first to
demonstrate this pathogenesis-based therapy with tofacitinib.
Stressed melanocytes
Melanocytes have poor adaptability to stressors
(73), including high levels of
ROS, which causes an imbalance in the pro-oxidant and antioxidant
levels (86). This increased
oxidative stress damages melanocytes, leading to instability in the
basal layer of the epidermis, lower catalase levels and elevated
superoxide dismutase (SOD) in the blood of patients with vitiligo
(73,86). SOD participates in the degradation
of the O- radical to H2O2 and
O2 (86).
The accumulation of H2O2
inhibits catalase activity, which breaks down
H2O2 to H2O and O2
(87). Catalase is downregulated by
elevated ROS levels in vitiligo, leading to oxidative pathway
imbalance and melanocyte destruction (88). Schallreuter et al (89) reported elevated
H2O2 levels in patients with vitiligo
compared with healthy controls. In addition to the oxidative
imbalance, ROS may impede repigmentation by attenuating
mitochondrial ATP production and impairing melanocyte destruction
(90).
The use of antioxidants remains in study and has
demonstrated variable results. Topic catalase for repigmentation in
vitiligo reduces H2O2 and may prevent
oxidative damage (91). Alshiyab
et al (91) reported that
the combination of topical pseudocatalase/dismutase gel and 0.1%
tacrolimus ointment did not have markedly different results
compared with 0.1% tacrolimus ointment monotherapy in children with
<10% of body surface area affected by vitiligo. However,
clinical trials concerning antioxidants in the treatment of
vitiligo are limited by the number of patients and studies with
larger study populations are warranted.
The aforementioned information on the
immunopathogenesis of vitiligo indicates that vitiligo may be
initiated by metabolic stress due to ROS accumulation, particularly
in the affected areas, suggesting that the use of antioxidants may
have a potential therapeutic implication for the damaged oxidative
pathways (92).
Boniface et al investigated melanocyte
instability and observed that vitiligo melanocytes exhibited
decreased expression of adhesion molecules, including E-cadherin, a
central protein in cell-cell adhesion (73). Furthermore, Rezk et al
(74) and Boniface et al
(73) reported that stressed
melanocytes secrete elevated levels of the chemokine C-C motif
ligand (CCL)-5, CXCL12 and IL-8. The chemokines CXCL12 and CCL5 are
involved in T-cell recruitment, homing (the ability of lymphocytes
to migrate) to the skin, and melanocyte-specific immunity (73,74),
while IL-8 is a potent chemotactic factor for neutrophils and
amplifies the local inflammatory response. Additionally,
melanocyte-derived CXCL12 and CCL5 support APCs, T-cell recruitment
and T-cell activation in early vitiligo, confirming the role of
these chemokines in the activation of melanocyte-specific immunity
(73,74).
Melanocytes and keratinocytes respond to aggressions
by producing cytokines that attract and activate immune cells,
mainly CD8+ T cells and Th17 cells, which recognize
melanocyte surface proteins. Subsequently, CD8+ T cells
and Th17 cells produce several cytokines, including TNF-α, IFN-γ
and IL-17, which cause the most damage to melanocytes (73). Following this, local inflammation is
established and amplified, and melanin production is inhibited, or,
in poorer outcomes, apoptosis and loss of adherence are induced in
melanocytes (73). Additionally,
genetic factors serve a key role in vitiligo and a variety of these
factors are closely associated with the immunologic process, as
discussed in subsequent sections.
3. Genetic factors associated with
vitiligo
Genes involved in the susceptibility
of vitiligo
The participation of different loci of various genes
has been described in families presenting with a high prevalence of
vitiligo (93), including those
located on chromosomes 4q13-q21, 1p31, 7q22, 8p12 and
17p13(94). Table I presents several associations
between genes associated with the susceptibility of vitiligo
(95-100)
and other autoimmune diseases, such as alopecia areata and
Hashimoto thyroiditis (52).
 | Table IMutated genes associated with
depigmentation mechanisms in vitiligo. |
Table I
Mutated genes associated with
depigmentation mechanisms in vitiligo.
| Database | Gene name | Protein | Function | (Refs.) |
|---|
| Genetics Home
Reference | MITF | Melanocyte inducing
transcription factor | Controls the
development of melanin and contributes to the color of hair, eyes
and skin. | (95) |
| Genetics Home
Reference | POMC |
Proopiomelanocortin | Following cleavage
into peptides, POMC serves different functions. The peptides bind
to melanocortin receptors 1, 2, 3 and 4 (MC1R, MC2R, MC3R and
MC4R), triggering signaling pathways and controlling various
important functions, such as regulation of blood sugar levels,
protection of the body from stress and suppression of inflammation,
regulation of pigment production, blood pressure, satiety, energy
expenditure and weight loss. Furthermore, three similar peptides,
α-, β- and γ-(MSH), are derived from POMC. The primary role of
α-MSH is melanocyte stimulation to produce and release
melanin. | (96) |
| UniProtKB | DCT |
Dopachrometautomerase (dopachrome
Δ-isomerase, tyrosine-related protein 2) | Converts dopachrome
to DHICA, which is an intermediate in the biosynthesis of
melanin. | (97) |
| UniProtKB | TYRP1 | Tyrosinase-related
protein 1 | Catalyzes the
oxidation of DHICA in the presence of bound Cu2+ ions.
Additionally, it may regulate or influence the type of melanin
synthesized and, to a lesser extent, is capable of hydroxylating
tyrosine and producing melanin. | (98) |
| UniProtKB | MLANA | Melanoma antigen
recognized by Th1 cells | Serves a vital role
in the expression, stability, trafficking and processing of
melanocyte premelanosome, which is critical for the formation of
stage II melanosomes. | (99) |
| Genetics Home
Reference | CAPN3 | Calpain-3 | Located in the
muscle cells in sarcomeres and its function is not well
understood. | (100) |
Al-Shobaili (101)
reported that the genetics of vitiligo include multiple
susceptibility loci, genetic heterogeneity and incomplete
penetrance with gene-gene and gene-environment interactions, and
that the methods most commonly used for identifying genomic regions
or candidate genes that mediate susceptibility to vitiligo include
two approaches: i) genome-wide linkage analyses, which are
performed by scanning the entire human genome for the
identification of genomic regions associated with the development
of vitiligo; and ii) association analyses of functional candidate
genes with vitiligo onset by detecting specific candidate genes,
which are expected to be associated with the condition considering
their biological functions and performing association studies. Both
of these approaches are based on the comparison of genetic
information from healthy skin with vitiligo biopsies Association
analyses of functional candidate genes better describe the genetic
causes of vitiligo. A description of the genes identified to date
are described in the following sections.
Vacuolar ion transporter 1 (VITI)
Le Poole et al (102) reported that the 3' portion of VIT1
is complementary to the 3' end of MutS Homolog 6 mRNA, enabling the
formation of RNA-RNA hybrids, which may interfere with G/T mismatch
repair function.
Catalase (CAT)
CAT encodes the four subunits of human CAT, which is
bound to the heme group of the functional enzyme (103). Casp et al (104) published a case-control and
family-based association study, which reported a decrease of CAT
enzyme activity in patients with vitiligo. Furthermore, a
concomitant accumulation of excess H2O2 was
observed in the epidermis of the patients (104).
Furthermore, Mosaad et al (105) revealed that the T allele of the
CAT 389 T/C polymorphism may be a susceptibility risk factor for
vitiligo in the Egyptian population. The results observed that TT
genotype carriers of CAT 389 had the lowest levels of CAT, while
the highest level of malondialdehyde was observed in AA genotype
carriers of CAT, indicating that this polymorphism may be
associated with increased oxidative stress in non-segmental
patients with vitiligo (105).
Tenascin C (TNC)
TNC is an extracellular matrix protein implicated in
the guidance of migrating neurons due to stimulation of
angiogenesis in vitro (106). Le Poole et al (107) reported increased expression levels
of TNC in vitiligo lesions. Furthermore, increased TNC has been
demonstrated to be inversely correlated with the duration of the
disease (107). TNC acts as an
anti-adhesive molecule (108).
Since fibroblasts secrete TNC, fibroblasts may malfunction in
vitiligo (109) and, therefore,
TNC upregulation may impair the adhesion of melanocytes to
surrounding keratinocytes and facilitate the process of
melanocytorrhagy (109).
Forkhead box D3 (FOXD3)
This gene belongs to the forkhead family of
transcription factors, which are characterized by a distinct
forkhead domain (110). Mutations
in this gene cause autoimmune susceptibility (111). FoxD3 has been indicated to
coordinate a lineage switch between neural/glial and pigment
phenotype in neural crest stem cells via melanocyte inducing
transcription factor (MITF), a key regulator in melanin production
and melanogenesis (111).
TNF-α (-308G/A) GA genotype
This genotype has been associated with the active
form of generalized vitiligo (2).
TNF-α serves an important role in vitiligo by destroying
melanocytes through the induction of apoptosis via a caspase
3-dependent pathway (66).
Additionally, TNF-α inhibits melanocyte stem cell differentiation
(112). In the specific case of
TNF-α (-308G/A), Wilson et al (113) reported a strong association
between the TNF2 allele (-308A) and the human leukocyte antigen
(HLA) A1, B8 and DR3 alleles, indicating that the haplotype may
contribute to numerous autoimmune diseases, such as
insulin-dependent diabetes mellitus, systemic lupus erythematosus,
Graves' disease and celiac disease. Using a human B-cell line, a
previous study used reporter genes under the control of two allelic
TNF promoters to demonstrate that the TNF2 allele (-308A) was a
stronger transcriptional activator compared with the TNF1 common
allele (-308G) (114). Since
patients with active vitiligo usually present with increased levels
of several proinflammatory cytokines, including TNF-α (115), patients with vitiligo with the
TNF2 allele produce higher levels of TNF-α and, consequently, have
a higher risk of more severe and active depigmentation compared
with patients who do not carry this allele (114).
Protein tyrosine phosphatase
non-receptor type 22 (PTPN22) (+1858 C/T)
Our previous study reported that patients who carry
the heterozygous CT genotype have an increased risk of developing
the active form of vitiligo (116). PTPN22 is a protein tyrosine
phosphatase, non-receptor type 22 lymphoid (LYP). The PTPN22 single
nucleotide polymorphism, rs2476601, results in an amino acid change
from arginine to tryptophan at the 620 codon position. This
variant, which is associated with increased vitiligo risk, prevents
the interaction of LYP with the negative regulatory tyrosine kinase
C-Src. As a result, the T-cell receptor-associated kinases may be
able to induce T-cell activation in an uncontrolled manner and
increase the reactivity of the overall immune system, leading to a
predisposition for autoimmune disorder susceptibility (116). Therefore, it has been hypothesized
that individuals lacking the C allele of PTPN22 may have a
decreased capacity to downregulate T-cell responses (117).
Arora and Kumaran (118) reported that, in addition to
PTPN22, genes that encode complexes and proteins involved in the
regulation of immunity, including MHC, angiotensin-converting
enzyme, cytotoxic T lymphocyte antigen-4,
catechol-O-methyltransferase, estrogen receptor, mannan-binding
lectin, HLA, NACHT leucine-rich repeat protein, X-box binding
protein 1, FOXP1 and IL-2 receptor A, are involved in the
immunopathogenesis of vitiligo. Alterations in the encoding genes,
alongside other risk factors, cause melanocytes to be susceptible
to apoptosis and induce the creation of melanocyte-reactive
auto-antibodies and T cells (74).
Furthermore, Arora and Kumaran (118) pointed out that HLA haplotypes,
particularly HLA-A2, -DR4, -DR7 and -DQB1*0303, serve an important
role in vitiligo predisposition. Additionally, our previous studies
investigated variant genes for TNF-α (308G/A variant) (2) and PTPN22 (+1858 C/T variant) (116).
Unbalanced expression of non-immune
genes in vitiligo
In addition to genes involved in
immunopathogenesis, numerous other genes may present altered
expression once vitiligo lesions are clinically detectable. For
instance, genes associated with the regulation of melanocyte
development, function and survival have been identified (119-122).
Furthermore, Strömberg et al (121) analyzed the gene expression profile
of melanocytes in a cell culture isolated from a skin biopsy of a
patient with vitiligo. The results identified the following five
processes involved in the progression of vitiligo: i) development
of melanocytes; ii) intracellular processing and trafficking of
tyrosinase family proteins; iii) packaging and transport of
melanosomes; iv) cell adhesion; and v) processing and presentation
of antigens (121). In regard to
genes associated with the depigmentation mechanism, Kingo et
al (119,120) reported lower expression levels of
MITF and proopiomelanocortin (POMC) in vitiligo skin biopsies
compared with healthy skin. Additionally, our previous study
demonstrated that the upregulation of dopachrometautomerase (DCT),
melanoma antigen recognized by Th1 cells (MLANA), calpain-3 (CAPN3)
and tyrosinase-related protein 1 (TYRP1) expression was associated
with depigmentation in patients with active vitiligo (123).
Downregulation of MITF and POMC expression is
associated with lower skin pigmentation (119,120). However, the mechanism by which the
upregulation of DCT, TYRP1 and MLANA expression causes skin
depigmentation in patients with vitiligo remains to be elucidated
(123). Upregulation of these
genes may be due to a compensatory mechanism in melanocytes;
however, normal melanin production is not achieved. Another
possibility is that the depigmentation of the skin is due to
mutations in DCT, TYRP1 or MLANA, which may clarify why, despite
being upregulated, depigmentation still occurs. Additionally, the
association of vitiligo with CAPN3 overexpression is intriguing and
should be further investigated, as the mechanism by which this
enzyme affects skin pigmentation remains unknown.
Genes associated with apoptosis or
homeostasis loss of melanocytes
The molecular mechanisms involved in the
development of vitiligo have been investigated. Over the last
decade, a genome-wide profiling approach was established to examine
the expression levels of genes involved in the pathogenesis of
vitiligo. Strömberg et al (121) identified 859 differentially
expressed genes in the melanocytes of patients with vitiligo. These
genes are mainly associated with melanocyte development,
intracellular processing and transport of tyrosinase, packaging and
transport of melanosomes, cell adhesion and antigen presentation.
Furthermore, it has been hypothesized that the development of
autoimmunity against melanocytes may be a secondary event caused by
the abnormal functioning of melanocytes in vitiligo (121). However, Shi et al (124) published a study in 2012 using an
mRNA microarray from an avian model of human autoimmune vitiligo
and demonstrated that the inflammatory/innate immune activity,
oxidative stress and the adaptive immune response serve a
predominant role in the loss of melanocytes, providing valuable
information supporting the multifactorial etiology of vitiligo.
Mansuri et al (125)
identified microRNA (miRNA or miR) signatures associated with
vitiligo. miRNAs are small, non-coding RNA molecules that
post-transcriptionally regulate gene expression by binding to
target mRNAs, resulting in translational repression and gene
silencing (125). miRNAs serve
important roles in numerous aspects of homeostasis and disease.
Patients with vitiligo presented with significantly increased
expression levels of miR-1, miR-184, miR-383 and miR-577, while
miR-328 expression was significantly downregulated in patients with
vitiligo compared with controls (125). In silico analysis of these
results indicated possible genetic targets affected by these
miRNAs, including genes involved in inflammation (IL1B), immune
response (PTPN22), oxidative stress (HSP60, HSP70) and skin
pigmentation (TYRP1), and, therefore, demonstrated the crucial role
of these molecules in the development of vitiligo (125).
In 2017, Dey-Rao et al (126) used in silico
bioinformatics-based analyses to examine the vitiligo-blood
transcriptome and identified several transcriptional ‘hot spots’,
which were prioritized targets for identifying disease risk genes.
Five molecules were identified: i) STAT1; ii) protein kinase CΔ;
iii) PTPN6; iv) MYC; and v) fibroblast growth factor receptor
2(126), which all have the
potential to be targeted by drugs for future therapies. Therefore,
these and other molecular targets should be further explored due to
their subtle contributions to vitiligo development, and may provide
novel therapeutic targets.
Table II presents
a group of downregulated genes associated with the loss of cell
homeostasis in vitiligo (127-133),
which were reported by Kingo et al (119,120). The products of p38 and PIK3CB are
protein kinases, which activate or deactivate proteins responding
to stress and are implicated in cell apoptosis and differentiation
(134). Therefore, a decrease in
the expression levels of these genes can result in various
detrimental effects on melanocyte differentiation, melanin
production or the transportation of melanin granules to
keratinocytes. The proteins encoded by RPS6KB1 (ribosomal) and
Bcl-2 are apoptotic suppressors and their downregulation may
explain melanocyte depletion in depigmented areas (120,131,132). Fig.
2 presents the altered gene expression profiles and the
affected pathways in vitiligo.
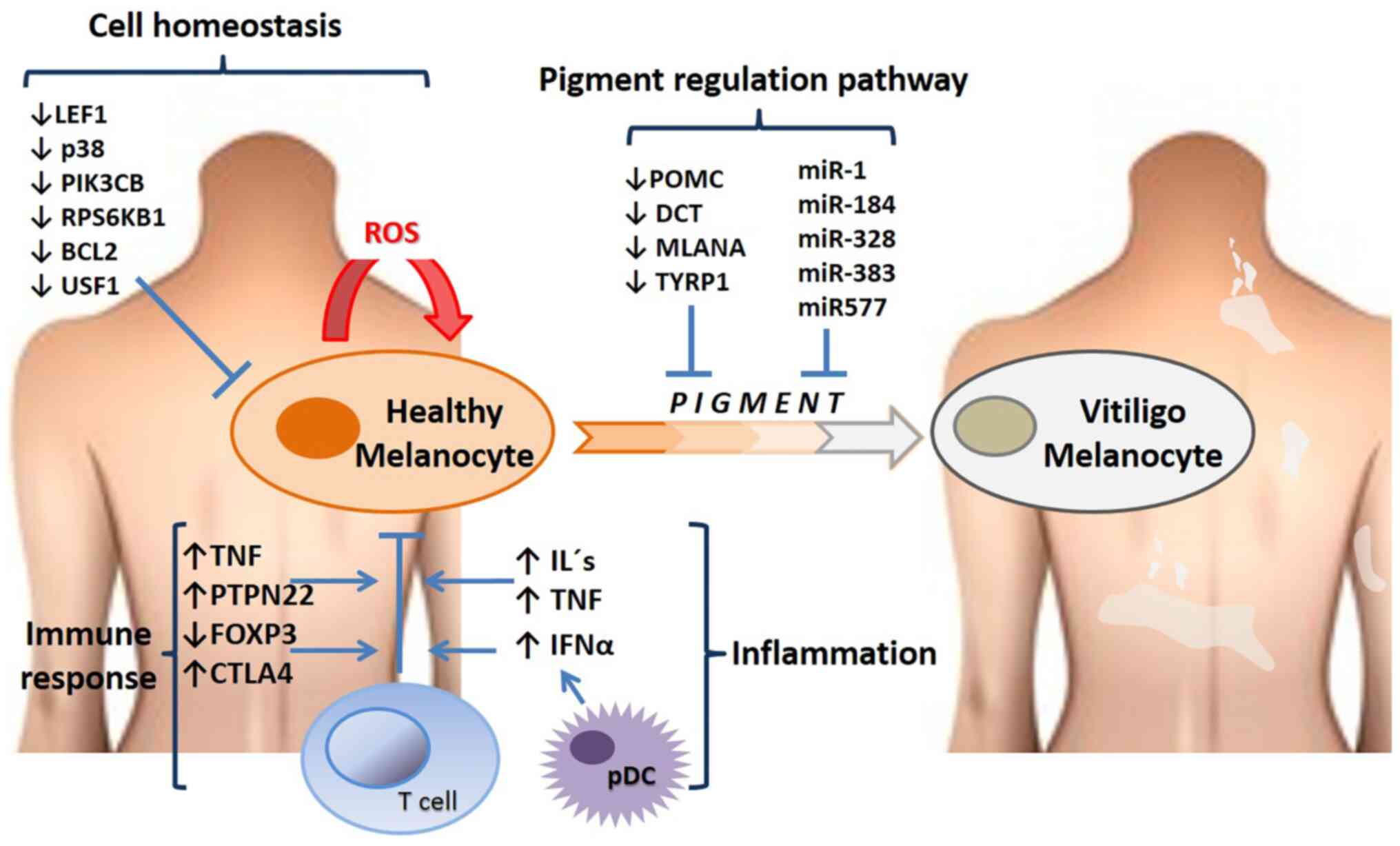 | Figure 2Altered gene expression profiles and
signaling pathways in vitiligo. The figure highlights some of the
genes and RNA levels that are upregulated or downregulated in
patients with vitiligo, and some of the main processes in which
these molecules participate, such as cell homoeostasis, the pigment
pathway (genes that regulate pigmentation such as POMC, DCT and
some microRNAs that regulate translation of genes in skin cells),
immune response and inflammation. miR, microRNA; pDCs, plasmacytoid
dendritic cells; ROS, reactive oxygen species; CTLA4, cytotoxic
T-lymphocyte associated protein 4; FOXP3, forkhead box protein P3;
LEF1, lymphoid enhancer-binding factor-1; PIK3CB,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit β;
PTPN22, protein tyrosine phosphatase non-receptor type 22; RPS6KB1,
ribosomal protein S6 kinase β-1; USF1, upstream stimulatory factor
1; POMC, proopiomelanocortin; DCT, dopachrometautomerase; MLANA,
melanoma antigen recognized by Th1 cells; TYRP1, tyrosinase-related
protein 1. |
 | Table IIDownregulated genes associated with
the loss of melanocyte homeostasis. |
Table II
Downregulated genes associated with
the loss of melanocyte homeostasis.
| First author, year,
or database | Gene name | Protein | Function | (Refs.) |
|---|
| Regazzetti et
al, 2015 | LEF1 | Lymphoid
enhancer-binding factor-1 | Key transducer of
the Wnt signaling pathway and downstream effectors, including
cadherin 2/3 and interferon regulatory factor 4 (IRF4). | (127) |
| Segalés et
al, 2016 Wei and Siegal, 2008 | p38 MAPK | P38
mitogen-activated protein kinase | The p38 MAPK
signaling pathway serves roles in stress stimuli, including
inflammatory cytokines, UV radiation, heat shock and osmotic shock.
In addition, it is involved in cell differentiation, apoptosis and
autophagy. Compared with normal physiological activity, increased
and decreased activity has been associated with pathological events
in several tissues, such as inflammation and altered muscle
regeneration. | (128,129) |
| National Center for
Biotechnology Information | PI3KCB |
Phosphatidylinositol-4,5- bisphosphate
3-kinase catalytic subunit β isoform | Encodes an isoform
of the catalytic subunit of PI3K, which participates in the
signaling pathways of eukaryotic cells. The encoded protein is the
catalytic subunit for PI3Kβ, which serves a role in the neutrophil
activation pathway. Additionally, these cells act in sites of
injury and infection. | (130) |
| UniProtKB | RPS6KB1 | Ribosomal protein
S6 kinase β-1 | Promotes cell
proliferation, growth and cell cycle progression, and the
initiation of protein synthesis, a process mediated by cap-binding
protein. Regulates protein synthesis and mediates cell survival by
repressing the pro-apoptotic function of Bcl-2. The active form
acts on several substrates in the pre-initiation complex.
Additionally, it activates translation elongation. | (131) |
| UniProtKB | Bcl-2 | B-cell lymphoma
2 | Promotes cell
survival, blocks dexamethasone-induced apoptosis and mediates the
survival of post-mitotic Sertoli cells by suppressing the apoptotic
activity of Bax, an apoptosis regulator. Isoforms α and σ are
expressed in various types of cancer cells, as prostate, breast and
gastric cancer among others. | (132) |
| National Center for
Biotechnology Information | USF1 | Upstream
stimulatory factor 1 | Binds to a
symmetrical DNA sequence (E-box; 5'-CACGTG-3') and is expressed in
various viral and cellular promoters. This gene encodes a cellular
transcription factor that regulates various biological processes
mediated by p38. USF1 disorders produce elevated levels of total
serum cholesterol and/or triglycerides, or cause premature coronary
heart disease. | (133) |
The gene descriptions in Table II suggest that p38, PIK3CB,
upstream stimulatory factor 1 and lymphoid enhancer-binding
factor-1 may exert pleiotropic effects. Therefore, it can be
expected that alterations in the expression levels of these genes,
whether combined or separately, may have several consequences for
skin depigmentation as well as in other cells and organs.
4. Conclusions
Vitiligo is a multifactorial disease that can be
activated by both external and internal factors. Among these,
genetic and autoimmune factors are the principal factors for the
initiation and progression of vitiligo lesions. Notably,
melanocytes exhibit biological properties of immune cells and
produce and release cytokines. Therefore, melanocytes can induce an
autoimmune response in susceptible individuals. Vitiligo presents
in several clinical forms and evolves in various ways, indicating
that each of these forms have different etiologies and
physiopathologies. Furthermore, a wide variety of genes have been
reported to contribute to the risk of vitiligo. A number of these
genes participate in key processes of melanocyte metabolism, skin
homeostasis and apoptosis regulation, and encode mediators of the
immune response. Vitiligo has a very complex pathogenesis and its
treatment represents a challenge for dermatologists. The current
review aimed to improve the understanding of the novel aspects of
vitiligo pathogenesis and the molecular mechanisms involved in
melanocyte destruction leading to hypopigmentation, allowing to
identify future therapeutic targets to improve management efficacy
and the quality of life of patients with vitiligo. However, further
studies are required to further clarify the pathogenesis of
vitiligo.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
JOC, SLSF, CNSD, MASS, HGMR, DEKL, NAZS and OTVM
performed the literature review, the data collection and drafted
the manuscript. JOC, SLSF, CNSD, MASS, HGMR, DEKL, NAZS, OTVM, UW
and TL improved and critically revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Le Poole IC, van den Wijngaard RM,
Westerhof W, Dutrieux RP and Das PK: Presence or absence of
melanocytes in vitiligo lesions: An immunohistochemical
investigation. J Invest Dermatol. 100:816–822. 1993.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Salinas-Santander M, Díaz-García D,
Rojas-Martínez A, Cantú-Salinas C, Sánchez-Domínguez C, Reyes-López
M, Cerda-Flores RM, Ocampo-Candiani J and Ortiz-López R: Tumor
necrosis factor-α-308G/A polymorphism is associated with active
vitiligo vulgaris in a northeastern Mexican population. Exp Ther
Med. 3:893–897. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Le Poole IC, Das PK, van den Wijngaard RM,
Bos JD and Westerhof W: Review of the etiopathomechanism of
vitiligo: A convergence theory. Exp Dermatol. 2:145–153.
1993.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rodrigues M, Ezzedine K, Hamzavi I, Pandya
AG and Harris JE: Vitiligo Working Group. New discoveries in the
pathogenesis and classification of vitiligo. J Am Acad Dermatol.
77:1–13. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Matin R: Vitiligo. BMJ Clin Evid.
2008(1717)2008.PubMed/NCBI
|
|
6
|
Costin GE and Hearing VJ: Human skin
pigmentation: Melanocytes modulate skin color in response to
stress. FASEB J. 21:976–994. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hara M, Toyoda M, Yaar M, Bhawan J, Avila
EM, Penner IR and Gilchrest BA: Innervation of melanocytes in human
skin. J Exp Med. 184:1385–1395. 1996.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Reemann P, Reimann E, Ilmjärv S, Porosaar
O, Silm H, Jaks V, Vasar E, Kingo K and Kõks S: Melanocytes in the
skin-comparative whole transcriptome analysis of main skin cell
types. PLoS One. 9(e115717)2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Slominski A, Zmijewski MA and Pawelek J:
L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators
of melanocyte functions. Pigment Cell Melanoma Res. 25:14–27.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Solano F: On the metal cofactor in the
tyrosinase family. Int J Mol Sci. 19(633)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sturm RA, Teasdale RD and Box NF: Human
pigmentation genes: Identification, structure and consequences of
polymorphic variation. Gene. 277:49–62. 2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
D'Mello SA, Finlay GJ, Baguley BC and
Askarian-Amiri ME: Signaling pathways in melanogenesis. Int J Mol
Sci. 17(1144)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ortonne JP: Normal and abnormal skin
color. Ann Dermatol Venereol. 139 (Suppl 4):S125–S129.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Brenner M and Hearing VJ: The protective
role of melanin against UV damage in human skin. Photochem
Photobiol. 84:539–549. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hong Y, Song B, Chen HD and Gao XH:
Melanocytes and skin immunity. J Investig Dermatol Symp Proc.
17:37–39. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Elgendi A, Eslam A, Eman A, Nancy W, Karem
K, Osama A and Ahmed E: Association of HLA Class I and II Antigens
with Vitiligo in Egyptian Population. Molecular Enzymology and Drug
Targets, 2016 Vol 02. DOI: 10.21767/2572-5475.10011.
|
|
17
|
Kirnbauer R, Charvat B, Schauer E, Köck A,
Urbanski A, Förster E, Neuner P, Assmann I, Luger TA and Schwarz T:
Modulation of intercellular adhesion molecule-1 expression on human
melanocytes and melanoma cells: Evidence for a regulatory role of
IL-6, IL-7, TNF beta, and UVB light. J Invest Dermatol. 98:320–326.
1992.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gasque P and Jaffar-Bandjee MC: The
immunology and inflammatory responses of human melanocytes in
infectious diseases. J Infect. 71:413–421. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Plonka PM, Passeron T, Brenner M, Tobin
DJ, Shibahara S, Thomas A, Slominski A, Kadekaro AL, Hershkovitz D,
Peters E, et al: What are melanocytes really doing all day long...?
Exp Dermatol. 18:799–819. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fisher GJ, Kang S, Varani J, Bata-Csorgo
Z, Wan Y, Datta S and Voorhees JJ: Mechanisms of photoaging and
chronological skin aging. Arch Dermatol. 138:1462–1470.
2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kvam E and Tyrrell RM: Induction of
oxidative DNA base damage in human skin cells by UV and near
visible radiation. Carcinogenesis. 18:2379–2384. 1997.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sander CS, Chang H, Hamm F, Elsner P and
Thiele JJ: Role of oxidative stress and the antioxidant network in
cutaneous carcinogenesis. Int J Dermatol. 43:326–335.
2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tornaletti S and Pfeifer GP: UV damage and
repair mechanisms in mammalian cells. Bioessays. 18:221–228.
1996.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Linge C: Relevance of in vitro melanocytic
cell studies to the understanding of melanoma. Cancer Surv.
26:71–87. 1996.PubMed/NCBI
|
|
25
|
Vink AA and Roza L: Biological
consequences of cyclobutane pyrimidine dimers. J Photochem
Photobiol B. 65:101–104. 2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ezzedine K, Lim HW, Suzuki T, Katayama I,
Hamzavi I, Lan CC, Goh BK, Anbar T, Silva de Castro C, Lee AY, et
al: Revised classification/nomenclature of vitiligo and related
issues: The vitiligo global issues consensus conference. Pigment
Cell Melanoma Res. 25:E1–E13. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
van Geel N, Speeckaert R, Taieb A, Picardo
M, Böhm M, Gawkrodger DJ, Schallreuter K, Bennett DC, van der Veen
W, Whitton M, et al: Koebner's phenomenon in vitiligo: European
position paper. Pigment Cell Melanoma Res. 24:564–573.
2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Taïeb A and Picardo M: Clinical practice.
Vitiligo. N Engl J Med. 360:160–169. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Moellmann G, Klein-Angerer S, Scollay DA,
Nordlund JJ and Lerner AB: Extracellular granular material and
degeneration of keratinocytes in the normally pigmented epidermis
of patients with vitiligo. J Invest Dermatol. 79:321–330.
1982.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Falabella R, Arrunategui A, Barona MI and
Alzate A: The minigrafting test for vitiligo: Detection of stable
lesions for melanocyte transplantation. J Am Acad Dermatol. 32 (2
Pt 1):228–232. 1995.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Krüger C and Schallreuter KU: A review of
the worldwide prevalence of vitiligo in children/adolescents and
adults. Int J Dermatol. 51:1206–1212. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sehgal VN and Srivastava G: Vitiligo:
Compendium of clinico-epidemiological features. Indian J Dermatol
Venereol Leprol. 73:149–156. 2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Martis J, Bhat R, Nandakishore B and
Shetty JN: A clinical study of vitiligo. Indian J Dermatol Venereol
Leprol. 68:92–93. 2002.PubMed/NCBI
|
|
34
|
Cesar Silva de Castro C and Miot HA:
Prevalence of vitiligo in Brazil-A population survey. Pigment Cell
Melanoma Res. 31:448–450. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang Y, Cai Y, Shi M, Jiang S, Cui S, Wu
Y, Gao XH and Chen HD: The prevalence of vitiligo: A meta-analysis.
PLoS One. 11(e0163806)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang X, Du J, Wang T, Zhou C, Shen Y, Ding
X, Tian S, Liu Y, Peng G, Xue S, et al: Prevalence and clinical
profile of vitiligo in China: A community-based study in six
cities. Acta Derm Venereol. 93:62–65. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Habib A and Raza N: Clinical pattern of
vitiligo. J Coll Physicians Surg Pak. 22:61–62. 2012.PubMed/NCBI
|
|
38
|
Salinas-Santander M, Sanchez-Dominguez C,
Cantú-Salinas C, Ocampo-Garza J, Cerda-Flores R, Ortiz-López R and
Ocampo-Candiani J: Vitiligo: Factores asociados con su aparición en
pacientes del Noreste de México. Dermatol Rev Mex. 232–238.
2014.(In Spanish).
|
|
39
|
Yaghoobi R, Omidian M and Bagherani N:
Vitiligo: A review of the published work. J Dermatol. 38:419–431.
2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Huggins RH, Janusz CA and Schwartz RA:
Vitiligo: A sign of systemic disease. Indian J Dermatol Venereol
Leprol. 72:68–71. 2006.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jin Y, Mailloux CM, Gowan K, Riccardi SL,
LaBerge G, Bennett DC, Fain PR and Spritz RA: NALP1 in
vitiligo-associated multiple autoimmune disease. N Engl J Med.
356:1216–1225. 2007.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Vázquez-Martínez OT, Velásquez-Arenas L,
Méndez-Olvera N and Ocampo-Candiani J: Vitiligo. Overview and
current therapeutics. Dermatología CMQ. 4:187–192. 2006.
|
|
43
|
Chen YT, Chen YJ, Hwang CY, Lin MW, Chen
TJ, Chen CC, Chu SY, Lee DD, Chang YT and Liu HN: Comorbidity
profiles in association with vitiligo: A nationwide
population-based study in Taiwan. J Eur Acad Dermatol Venereol.
29:1362–1369. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Dahir AM and Thomsen SF: Comorbidities in
vitiligo: Comprehensive review. Int J Dermatol. 57:1157–1164.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bae JM, Lee JH, Yun JS, Han B and Han TY:
Vitiligo and overt thyroid diseases: A nationwide population-based
study in Korea. J Am Acad Dermatol. 76:871–878. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sedighe M and Gholamhossein G: Thyroid
dysfunction and thyroid antibodies in Iranian patients with
vitiligo. Indian J Dermatol. 53:9–11. 2008.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Gopal KV, Rao GR and Kumar YH: Increased
prevalence of thyroid dysfunction and diabetes mellitus in Indian
vitiligo patients: A case-control study. Indian Dermatol Online J.
5:456–460. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
El-Gayyar MA, Helmy ME, Amer ER, Elsaied
MA and Gaballah MA: Antimelanocyte antibodies: A possible role in
patients with vitiligo. Indian J Dermatol. 65:33–37.
2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Liu CW and Huang YC: Vitiligo and
autoantibodies: A systematic review and meta-analysis. J Dtsch
Dermatol Ges. 16:845–851. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Genetics Home Reference. Vitiligo.
Inheritance Pattern. Available at: https://ghr.nlm.nih.gov/condition/vitiligo#inheritance
(last accessed 8 July 2019).
|
|
51
|
Alenizi DA: Consanguinity pattern and
heritability of Vitiligo in Arar, Saudi Arabia. J Family Community
Med. 21:13–16. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Allam M and Riad H: Concise review of
recent studies in vitiligo. Qatar Med J. 2013:1–19. 2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Franks AL and Slansky JE: Multiple
associations between a broad spectrum of autoimmune diseases,
chronic inflammatory diseases and cancer. Anticancer Res.
32:1119–1136. 2012.PubMed/NCBI
|
|
54
|
Asilian A, Momeni I and Khosravani P:
Vitiligo associated with esophageal adenocarcinoma. Int J Prev Med.
4:489–490. 2013.PubMed/NCBI
|
|
55
|
Balasubramanian A: Vitiligo associated
with breast cancer-a report of two cases. Int J Cur Res Rev.
7:56–58. 2015.
|
|
56
|
Manga P, Elbuluk N and Orlow SJ: Recent
advances in understanding vitiligo. F1000Res 5: F1000 Faculty
Rev-2234, 2016.
|
|
57
|
Patel S, Rauf A, Khan H, Meher BR and
Hassan SSU: A holistic review on the autoimmune disease vitiligo
with emphasis on the causal factors. Biomed Pharmacother.
92:501–508. 2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Giang J, Seelen MAJ, van Doorn MBA,
Rissmann R, Prens EP and Damman J: Complement activation in
inflammatory skin diseases. Front Immunol. 9(639)2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ricklin D, Reis ES, Mastellos DC, Gros P
and Lambris JD: Complement component C3-The ‘Swiss Army Knife’ of
innate immunity and host defense. Immunol Rev. 274:33–58.
2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Sandoval-Cruz M, García-Carrasco M,
Sánchez-Porras R, Mendoza-Pinto C, Jiménez-Hernández M,
Munguía-Realpozo P and Ruiz-Argüelles A: Immunopathogenesis of
vitiligo. Autoimmun Rev. 10:762–765. 2011.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Basak PY, Adiloglu AK, Ceyhan AM, Tas T
and Akkaya VB: The role of helper and regulatory T cells in the
pathogenesis of vitiligo. J Am Acad Dermatol. 60:256–260.
2009.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Kotobuki Y, Tanemura A, Yang L, Itoi S,
Wataya-Kaneda M, Murota H, Fujimoto M, Serada S, Naka T and
Katayama I: Dysregulation of melanocyte function by Th17-related
cytokines: Significance of Th17 cell infiltration in autoimmune
vitiligo vulgaris. Pigment Cell Melanoma Res. 25:219–230.
2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Bassiouny DA and Shaker O: Role of
interleukin-17 in the pathogenesis of vitiligo. Clin Exp Dermatol.
36:292–297. 2011.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Wang CQF, Akalu YT, Suarez-Farinas M,
Gonzalez J, Mitsui H, Lowes MA, Orlow SJ, Manga P and Krueger JG:
IL-17 and TNF synergistically modulate cytokine expression while
suppressing melanogenesis: Potential relevance to psoriasis. J
Invest Dermatol. 133:2741–2752. 2013.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Moretti S, Fabbri P, Baroni G, Berti S,
Bani D, Berti E, Nassini R, Lotti T and Massi D: Keratinocyte
dysfunction in vitiligo epidermis: Cytokine microenvironment and
correlation to keratinocyte apoptosis. Histol Histopathol.
24:849–857. 2009.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Webb KC, Tung R, Winterfield LS, Gottlieb
AB, Eby JM, Henning SW and Le Poole IC: Tumour necrosis factor-α
inhibition can stabilize disease in progressive vitiligo. Br J
Dermatol. 173:641–650. 2015.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Alghamdi K and Khurrum H: Methotrexate for
the treatment of generalized vitiligo. Saudi Pharm J. 21:423–424.
2013.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Sandra A, Pai S and Shenoi SD: Unstable
vitiligo responding to methotrexate. Indian J Dermatol Venereol
Leprol. 64(309)1998.PubMed/NCBI
|
|
69
|
Garza-Mayers AC and Kroshinsky D: Low-dose
methotrexate for vitiligo. J Drugs Dermatol. 16:705–706.
2017.PubMed/NCBI
|
|
70
|
Singh H, Kumaran MS, Bains A and Parsad D:
A randomized comparative study of oral corticosteroid minipulse and
low-dose oral methotrexate in the treatment of unstable vitiligo.
Dermatology. 231:286–290. 2015.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Abdelmaksoud A, Dave DD, Lotti T and
Vestita M: Topical methotrexate 1% gel for treatment of vitiligo: A
case report and review of the literature. Dermatol Ther.
32(e13013)2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Haller O and Kochs G: Human MxA protein:
An interferon-induced dynamin-like GTPase with broad antiviral
activity. J Interferon Cytokine Res. 31:79–87. 2011.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Boniface K, Seneschal J, Picardo M and
Taïeb A: Vitiligo: Focus on clinical aspects, immunopathogenesis,
and therapy. Clin Rev Allergy Immunol. 54:52–67. 2018.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Rezk AF, Kemp DM, El-Domyati M, El-Din WH,
Lee JB, Uitto J, Igoucheva O and Alexeev V: Misbalanced CXCL12 and
CCL5 chemotactic signals in vitiligo onset and progression. J
Invest Dermatol. 137:1126–1134. 2017.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Harris JE: Cellular stress and innate
inflammation in organ-specific autoimmunity: Lessons learned from
vitiligo. Immunol Rev. 269:11–25. 2016.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Li S, Zhu G, Yang Y, Jian Z, Guo S, Dai W,
Shi Q, Ge R, Ma J, Liu L, et al: Oxidative stress drives
CD8+ T-cell skin trafficking in patients with vitiligo
through CXCL16 upregulation by activating the unfolded protein
response in keratinocytes. J Allergy Clin Immunol. 140:177–189.e9.
2017.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Wańkowicz-Kalińska A, van den Wijngaard
RM, Tigges BJ, Westerhof W, Ogg GS, Cerundolo V, Storkus WJ and Das
PK: Immunopolarization of CD4+ and CD8+ T
cells to type-1-like is associated with melanocyte loss in human
vitiligo. Lab Invest. 83:683–695. 2003.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Xie H, Zhou F, Liu L, Zhu Li Q, Li C and
Gao T: Vitiligo: How do oxidative stress-induced autoantigens
trigger autoimmunity? J Dermatol Sci. 81:3–9. 2016.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Le Poole IC, Wañkowicz-Kaliñska A, van den
Wijngaard RM, Nickoloff BJ and Das PK: Autoimmune aspects of
depigmentation in vitiligo. J Investig Dermatol Symp Proc. 9:68–72.
2004.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Le Poole IC, van den Wijngaard RM,
Westerhof W and Das PK: Presence of T cells and macrophages in
inflammatory vitiligo skin parallels melanocyte disappearance. Am J
Pathol. 148:1219–1228. 1996.PubMed/NCBI
|
|
81
|
Palermo B, Campanelli R, Garbelli S,
Mantovani S, Lantelme E, Brazzelli V, Ardigó M, Borroni G,
Martinetti M, Badulli C, et al: Specific cytotoxic T lymphocyte
responses against Melan-A/MART1, tyrosinase and gp100 in vitiligo
by the use of major histocompatibility complex/peptide tetramers:
The role of cellular immunity in the etiopathogenesis of vitiligo.
J Invest Dermatol. 117:326–332. 2001.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Relke N and Gooderham M: The use of janus
kinase inhibitors in vitiligo: A review of the literature. J Cutan
Med Surg. 23:298–306. 2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Rashighi M, Agarwal P, Richmond JM, Harris
TH, Dresser K, Su MW, Zhou Y, Deng A, Hunter CA, Luster AD and
Harris JE: CXCL10 is critical for the progression and maintenance
of depigmentation in a mouse model of vitiligo. Sci Transl Med.
6(223ra23)2014.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Ciechanowicz P, Rakowska A, Sikora M and
Rudnicka L: JAK-inhibitors in dermatology: Current evidence and
future applications. J Dermatolog Treat. 30:648–658.
2019.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Craiglow BG and King BA: Tofacitinib
citrate for the treatment of vitiligo: A pathogenesis-directed
therapy. JAMA Dermatol. 151:1110–1112. 2015.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Speeckaert R, Dugardin J, Lambert J,
Lapeere H, Verhaeghe E, Speeckaert MM and van Geel N: Critical
appraisal of the oxidative stress pathway in vitiligo: A systematic
review and meta-analysis. J Eur Acad Dermatol Venereol.
32:1089–1098. 2018.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Laddha NC, Dwivedi M, Mansuri MS, Gani AR,
Ansarullah M, Ramachandran AV, Dalai S and Begum R: Vitiligo:
Interplay between oxidative stress and immune system. Exp Dermatol.
22:245–250. 2013.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Dell'Anna ML, Urbanelli S, Mastrofrancesco
A, Camera E, Iacovelli P, Leone G, Manini P, D'Ischia M and Picardo
M: Alterations of mitochondria in peripheral blood mononuclear
cells of vitiligo patients. Pigment Cell Res. 16:553–559.
2003.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Schallreuter KU, Salem MA, Holtz S and
Panske A: Basic evidence for epidermal H2O2/ONOO(-)-mediated
oxidation/nitration in segmental vitiligo is supported by
repigmentation of skin and eyelashes after reduction of epidermal
H2O2 with topical NB-UVB-activated pseudocatalase PC-KUS. FASEB J.
27:3113–3122. 2013.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Xu P, Xue YN, Ji HH, Tan C and Guo S:
H2 O2 -induced oxidative stress disrupts
mitochondrial functions and impairs migratory potential of human
epidermal melanocytes. Exp Dermatol. 29:733–741. 2020.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Alshiyab DM, Al-Qarqaz FA, Muhaidat JM,
Alkhader YS, Al-Sheyab RF and Jafaar SI: Comparison of the efficacy
of Tacrolimus 0.1% ointment and Tacrolimus 0.1% plus topical
pseudocatalase/superoxide dismutase gel in children with limited
vitiligo: A randomized controlled trial. J Dermatolog Treat. 1–4.
2020.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
92
|
Mathachan SR, Khurana A, Gautam RK,
Kulhari A, Sharma L and Sardana K: Does oxidative stress correlate
with disease activity and severity in vitiligo? An analytical
study. J Cosmet Dermatol, 2020 (Epub ahead of print).
|
|
93
|
Spritz RA: The genetics of generalized
vitiligo and associated autoimmune diseases. J Dermatol Sci.
41:3–10. 2006.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Zhang XJ, Chen JJ and Liu JB: The genetic
concept of vitiligo. J Dermatol Sci. 39:137–146. 2005.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Genetics Home Reference. MITF gene.
Available at: https://ghr.nlm.nih.gov/gene/MITF. (last accessed 8
July 2019).
|
|
96
|
Genetics Home Reference. POMC gene.
Available at: https://ghr.nlm.nih.gov/gene/POMC. (last accessed 8
July 2019).
|
|
97
|
UniProtKB. UniProtKB-P40126 (TYRP2_HUMAN).
Available at: https://www.uniprot.org/uniprot/P40126. (last
accessed 8 July 2019).
|
|
98
|
UniProtKB. UniProtKB-P17643 (TYRP1_HUMAN).
Available at: https://www.uniprot.org/uniprot/P17643. (last
accessed 8 July 2019).
|
|
99
|
UniProtKB. UniProtKB-Q16655 (MAR1_HUMSN).
Available at: https://www.uniprot.org/uniprot/Q16655. (last
accessed 8 July 2019).
|
|
100
|
Genetics Home Reference. CAPN3 gene.
Available at: https://ghr.nlm.nih.gov/gene/CAPN3. (last accessed 8
July 2019).
|
|
101
|
Al-Shobaili HA: Update on the genetics
characterization of vitiligo. Int J Health Sci (Qassim). 5:167–179.
2011.PubMed/NCBI
|
|
102
|
Le Poole IC, Sarangarajan R, Zhao Y,
Stennett LS, Brown TL, Sheth P, Miki T and Boissy RE: ‘VIT1’, a
novel gene associated with vitiligo. Pigment Cell Res. 14:475–484.
2001.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Genetics Home Reference. CAT gene.
Available at: https://ghr.nlm.nih.gov/gene/CAT. (last accessed 8
July 2019).
|
|
104
|
Casp CB, She JX and McCormack WT: Genetic
association of the catalase gene (CAT) with vitiligo
susceptibility. Pigment Cell Res. 15:62–66. 2002.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Mosaad YM, Sallam M, Elsaied MA, Fathy H,
Fawzy Z, Elzehery R, Shaat RM and El-Gilany AH: Association of CAT
389 T/C and -89 T/A gene polymorphisms with vitiligo: Relation with
oxidative stress. J Egypt Women Dermatol Soc. 14:121–127. 2017.
|
|
106
|
UniProtKB. UniProtKD-P24821 (TENA_HUMAN).
Available at: https://www.uniprot.org/uniprot/P24821. (last
accessed 8 July 2019).
|
|
107
|
Le Poole IC, van den Wijngaard RM,
Westerhof W and Das PK: Tenascin is overexpressed in vitiligo
lesional skin and inhibits melanocyte adhesion. Br J Dermatol.
137:171–178. 1997.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Murphy-Ullrich JE: The de-adhesive
activity of matricellular proteins: Is intermediate cell adhesion
an adaptive state? J Clin Invest. 107:785–790. 2001.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Esmat SM, Hadidi HHE, Hegazy RA, Gawdat
HI, Tawdy AM, Fawzy MM, AbdelHalim DM, Sultan OS and Shaker OG:
Increased tenascin C and DKK1 in vitiligo: Possible role of
fibroblasts in acral and non-acral disease. Arch Dermatol Res.
310:425–430. 2018.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Schunter JA, Löffler D, Wiesner T, Kovacs
P, Badenhoop K, Aust G, Tönjes A, Müller P, Baber R, Simon JC, et
al: A novel FoxD3 variant is associated with vitiligo and elevated
thyroid auto-antibodies. J Clin Endocrinol Metab. 100:E1335–E1342.
2015.PubMed/NCBI View Article : Google Scholar
|
|
111
|
National Center for Biothechnology
Information. Genes and Expression. Gene. FOXD3 forkhead box D3
[Homo sapiens (human)]. Available at: https://www.ncbi.nlm.nih.gov/gene/27022?report=full_report%202017%20Caption:%201588786844.
(last accesed 8 July 2019).
|
|
112
|
Alghamdi KM, Khurrum H, Taieb A and
Ezzedine K: Treatment of generalized vitiligo with anti-TNF-α
Agents. J Drugs Dermatol. 11:534–539. 2012.PubMed/NCBI
|
|
113
|
Wilson AG, de Vries N, Pociot F, di
Giovine FS, van der Putte LB and Duff GW: An allelic polymorphism
within the human tumor necrosis factor alpha promoter region is
strongly associated with HLA A1, B8, and DR3 alleles. J Exp Med.
177:557–560. 1993.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Wilson AG, Symons JA, McDowell TL,
McDevitt HO and Duff GW: Effects of a polymorphism in the human
tumor necrosis factor alpha promoter on transcriptional activation.
Proc Natl Acad Sci USA. 94:3195–3199. 1997.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Mitra S, De Sarkar S, Pradhan A, Pati AK,
Pradhan R, Mondal D, Sen S, Ghosh A, Chatterjee S and Chatterjee M:
Levels of oxidative damage and proinflammatory cytokines are
enhanced in patients with active vitiligo. Free Radic Res.
51:986–994. 2017.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Garcia-Melendez ME, Salinas-Santander M,
Sanchez-Dominguez C, Gonzalez-Cardenas H, Cerda-Flores RM,
Ocampo-Candiani J and Ortiz-López R: Protein tyrosine phosphatase
PTPN22 +1858C/T polymorphism is associated with active vitiligo.
Exp Ther Med. 8:1433–1437. 2014.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Vang T, Miletic AV, Bottini N and Mustelin
T: Protein tyrosine phosphatase PTPN22 in human autoimmunity.
Autoimmunity. 40:453–461. 2007.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Arora A and Kumaran M: Pathogenesis of
vitiligo: An update. Pigment Int. 4:65–77. 2017.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Kingo K, Aunin E, Karelson M, Philips MA,
Ratsep R, Silm H, Vasar E, Soomets U and Koks S: Gene expression
analysis of melanocortin system in vitiligo. J Dermatol Sci.
48:113–122. 2007.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Kingo K, Aunin E, Karelson M, Ratsep R,
Silm H, Vasar E and Koks S: Expressional changes in the
intracellular melanogenesis pathways and their possible role in the
pathogenesis of vitiligo. J Dermatol Sci. 52:39–46. 2008.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Strömberg S, Bjorklund MG, Asplund A,
Rimini R, Lundeberg J, Nilsson P, Ponten F and Olsson MJ:
Transcriptional profiling of melanocytes from patients with
vitiligo vulgaris. Pigment Cell Melanoma Res. 21:162–171.
2008.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Salinas Santander MA: Análisis del perfil
de expresión de pacientes con vitiligo. Universidad Autónoma de
Nuevo Leon, Repositorio Académico Digital, p 143, 2012. http://eprints.uanl.mx/3248/.
|
|
123
|
Salinas-Santander M, Trevino V, De la
Rosa-Moreno E, Verduzco-Garza B, Sánchez-Domínguez CN,
Cantú-Salinas C, Ocampo-Garza J, Lagos-Rodríguez A, Ocampo-Candiani
J and Ortiz-López R: CAPN3, DCT, MLANA and TYRP1 are overexpressed
in skin of vitiligo vulgaris Mexican patients. Exp Ther Med.
15:2804–2811. 2018.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Shi F, Kong BW, Song JJ, Lee JY,
Dienglewicz RL and Erf GF: Understanding mechanisms of vitiligo
development in Smyth line of chickens by transcriptomic microarray
analysis of evolving autoimmune lesions. BMC Immunol.
13(18)2012.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Mansuri MS, Singh M and Begum R: MiRNA
signatures and transcriptional regulation of their target genes in
vitiligo. J Dermatol Sci. 84:50–58. 2016.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Dey-Rao R and Sinha AA: Vitiligo blood
transcriptomics provides new insights into disease mechanisms and
identifies potential novel therapeutic targets. BMC Genomics.
18(109)2017.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Regazzetti C, Joly F, Marty C, Rivier M,
Mehul B, Reiniche P, Mounier C, Rival Y, Piwnica D, Cavalié M, et
al: Transcriptional analysis of vitiligo skin reveals the
alteration of WNT pathway: A promising target for repigmenting
vitiligo patients. J Invest Dermatol. 135:3105–3114.
2015.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Segalés J, Perdiguero E and Muñoz-Cánoves
P: Regulation of muscle stem cell functions: A focus on the p38
MAPK signaling pathway. Front Cell Dev Biol. 4(91)2016.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Wei S and Siegal GP: Mechanisms modulating
inflammatory osteolysis: A review with insights into therapeutic
targets. Pathol Res Pract. 204:695–706. 2008.PubMed/NCBI View Article : Google Scholar
|
|
130
|
National Center of Biotechnology
Information. Genes and Expression. Gene. PIK3CB
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit
beta [Homo sapiens (human)]. Available at: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=ShowDetailView&TermToSearch=5291.
(last accessed 8 July 2019).
|
|
131
|
UniProtKB. UniProtKB-P23443 (KS6B1_HUMAN).
Available at: https://www.uniprot.org/uniprot/P23443. (last
accessed 8 July 2019).
|
|
132
|
UniProtKB. UniProtKB-Q07812 (BAX_HUMAN).
Available at: https://www.uniprot.org/uniprot/Q07812. (last
accessed 8 July 2019).
|
|
133
|
National Center of Biotechnology
Information. Genes and Expression. Gene. USF1 upstream
transcription factor 1 [Homo sapiens (human)] Available at:
https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=ShowDetailView&TermToSearch=7391.
(last accessed 8 July 2019).
|
|
134
|
Manning G, Whyte DB, Martinez R, Hunter T
and Sudarsanam S: The protein kinase complement of the human
genome. Science. 298:1912–1934. 2002.PubMed/NCBI View Article : Google Scholar
|