Introduction
Gastric cancer (GC) is a malignant tumor originating
from the gastric mucosa and is one of the malignant tumors of the
digestive tract that seriously endanger human health (1). Globally in 2012, >70% of new cases
of GC occur in developing countries and ~50% of cases occur in
eastern Asia of which the majority is seen in China (1). According to data released by the China
Cancer Center in 2015, the number of new cases of patients with GC
in China was 679,000 per year, second only to lung cancer (733,000
per year) (2). Notably, the
detection rate of early GC is only 10% due to a lack of specific
early clinical symptoms and diagnostic indicators (3,4).
Therefore, most patients with GC are diagnosed at an advanced stage
and the 5-year survival rate is poor, with a 5-year overall
survival rate of 20% for those treated only by surgery and 30-50%
for those treated with surgery and adjuvant therapy (5). Therefore, the exploration of specific
diagnostic and therapeutic targets is of considerable significance
to develop targeted drugs and improve the survival of patients with
GC.
The transient receptor potential cation channel
subfamily M member 8 (TRPM8) is a vital subtype of the transient
receptor potential (TRP) and was first detected in prostate tissue
(6). The human TRPM8 gene,
which is located on chromosome 2q37.1, is composed of 25 exons,
encodes a protein containing 1,104 amino acids, is mainly expressed
on the cell membrane as a transmembrane protein and is known to be
involved in perceiving temperature and pain (7,8). As a
cold-sensitive channel protein, TRPM8 can be activated by low
temperatures (27˚C) (9). Previous
studies have demonstrated that TRPM8 activation can cause
Ca2+ influx into cells and trigger a series of
biological effects, such as cell proliferation, differentiation and
migration (10,11). TRPM8 was later confirmed to be
abnormally expressed in a number of cancers, including breast
(12), prostate (13) and pancreatic cancer (14). Additionally, TRPM8 is not only
involved in regulating the biological characteristics of tumor
cells (15,16) but also serves as a potential tumor
treatment target (17). In
addition, TRPM8 has also been found to be a receptor for menthol
which is a widely used drug, including for antitumor treatments
(9,18). Li et al (19) found that menthol increased the
Ca2+ concentration in a human bladder cancer cell line
by activating its receptor TRPM8 which reduced the mitochondrial
membrane potential, caused mitochondrial depolarization, induced
cell apoptosis and exerted antitumor effects.
However, the expression of TRPM8 protein and its
clinical significance in GC remains unclear. The present study
aimed to detect TRPM8 expression and to explore its clinical
significance in GC. The findings revealed that TRPM8 protein was
highly expressed in GC tissues and affected the proliferation and
metastasis of GC cells. The present study demonstrates that TRPM8
may be a potential target for the treatment of GC.
Materials and methods
Patient ethics and
characteristics
Tumor and adjacent healthy tissues (>5 cm from
tumor tissue) were collected by surgery from 134 patients with GC
between January and December 2013 in the Beijing Jishuitan Hospital
(Beijing, China). The average age of patients was 60.65±12.38 years
and other clinical data are presented in Table I. Inclusion criteria for the present
study were: i) Patients with GC without any treatment before
surgery; ii) patients with complete information, such as age, sex,
imaging examination, tumor-node-metastasis (TNM) stage; iii)
patients with a complete 5-year follow-up record; and iv) patients
without any other malignant tumor or other chronic infectious
diseases (such as HIV, HBV, HCV). The exclusion criteria for the
present study were: i) Loss to follow-up (Telephone interviews
every quarter, for a period of 5 years) or death from another
illness or accident; ii) pregnant or lactating women, as well as
drug users; and iii) withdrawn from the study halfway. All GC
patients who provided tissues were informed of the content of this
study and signed informed consent. All protocols related to human
tissue samples were reviewed and monitored by the Ethics Committee
at the Beijing Jishuitan Hospital (Beijing, China).
 | Table IAssociation of TRMP8 protein
expression and clinicopathological characteristics of patients with
gastric cancer (n=134). |
Table I
Association of TRMP8 protein
expression and clinicopathological characteristics of patients with
gastric cancer (n=134).
| | TRPM8
expression | |
|---|
| Features | Number of
patients | Low | High | χ2 | P-value |
|---|
| Sex | | | | 0.030 | 0.863 |
|
Female | 94 | 32 | 62 | - | - |
|
Male | 40 | 13 | 27 | | |
| Age (years) | | | | 0.113 | 0.737 |
|
<60 | 48 | 17 | 31 | - | - |
|
≥60 | 86 | 28 | 58 | | |
| Tumor diameter
(cm) | | | | 8.945 | 0.003 |
|
<2.5 | 65 | 30 | 35 | - | - |
|
≥2.5 | 69 | 15 | 54 | | |
| Histological grade
(43) | | | | 0.524 | 0.769 |
|
I | 37 | 14 | 23 | - | - |
|
II | 52 | 16 | 36 | | |
|
III | 45 | 16 | 29 | | |
| Tumor number | | | | 0.179 | 0.672 |
|
Single | 74 | 26 | 48 | - | - |
|
Multiple | 60 | 19 | 41 | | |
| TNM stage (44) | | | | 14.195 | 0.003 |
|
I | 14 | 8 | 6 | - | - |
|
II | 38 | 19 | 19 | | |
|
III | 49 | 13 | 36 | | |
|
IV | 33 | 5 | 28 | | |
| Lymph node
metastasis | | | | 10.269 | 0.001 |
|
Yes | 82 | 19 | 63 | | |
|
No | 52 | 26 | 26 | | |
| Cancer cell remote
metastasis | | | | 6.668 | 0.010 |
|
Yes | 33 | 5 | 28 | | |
|
No | 101 | 40 | 61 | | |
Cell lines and culture
GES-1, SNU-1, AGS, SNU-5, and NCI-N87 were purchased
from the American type culture collection (ATCC) and were cultured
in DMEM medium (cat. no. 11965092; Gibco; Thermo Fisher Scientific
Inc.) supplemented with 10% fetal bovine serum (cat. no. 16140071;
Gibco; Thermo Fisher Scientific Inc.) at 37˚C with 5%
CO2. GES-1 is a normal gastric epithelial cell line and
all others are GC cell lines.
Western blotting
Total protein was extracted from cells using a RIPA
lysate buffer (cat. no. R0010; Beijing Solarbio Science &
Technology Co., Ltd.). Tissues were first broken apart in liquid
nitrogen in a twist bowl and then total protein was extracted from
the cells. The concentration of total protein was detected using a
BCA kit (cat. no. C503021; Sangon Biotech Co. Ltd.). Subsequently,
50 µg of protein per lane was separated by 10% SDS-PAGE under a 90
V constant voltage. The proteins were transferred from the SDS-PAGE
gel to PVDF membranes. Following blocking with 5% skimmed milk at
room temperature for 1 h at room temperature, the membranes were
incubated with primary antibody against TRPM8 (1:500; cat. no.
ab3243; Abcam) overnight at 4˚C. Then Goat Anti-Rabbit IgG H&L
(HRP; 1:2,000; cat. no. ab6721; Abcam) was added at room
temperature for 2 h. After washing 3 times with phosphate-buffered
saline-Tween-20, ECL solution (cat. no. WBKLS0100; Beijing
Xinjingke Biotechnologies Co., Ltd.) was added for detection.
β-actin was used as the loading control. ImageJ v1.8.0 (National
Institute of Health) was used to analyze protein grey value.
Immunohistochemistry (IHC)
TRPM8 protein expression was detected and scored by
IHC as described in a previous study (20). In the present study, the primary
antibody used was an anti-TRPM8 antibody (1:100; cat. no. ab3243;
Abcam) and PBS buffer was used as a negative control. The primary
antibody was incubated overnight at 4˚C and Goat Anti-Rabbit IgG
H&L (HRP; 1:1,000, cat. no. ab6721; Abcam) as the secondary
antibody was incubated at room temperature for 1 h. The number of
positive cells were counted using Leica TCS SP5 microscope
(magnification, x200; Leica Microsystems, Inc.).
Statistical analysis
SPSS 20.0 (IBM Corp.) was used to analyze the data
in the present study. Data in the current study was presented as
the mean ± standard deviation, and three repeats were used for the
same measurement. Both paired and unpaired Student's t-tests and
the χ2 test were used to compare the differences between
2 groups and one-way ANOVA with the post hoc Tukey's test was used
to compare the differences between multiple groups. The log-rank
(Mantel-Cox) test was used to compare the survival of patients with
high and low TRPM8 expression. A Cox regression model was used to
analyze factors affecting the survival of patients with GC.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Upregulation of TRPM8 protein
expression in GC cells and tissues
Firstly, the expression of TRPM8 protein in human
gastric mucosal epithelial cell (GES-1) and human GC cells (SNU-1,
AGS, SNU-5, and NCI-N87) was assessed. The expression of TRPM8
protein in human GC cells was significantly higher compared with
that in normal human gastric mucosal epithelial cells and the level
in the NCI-N87 cell line was highest (Fig. 1A and B). Then, tumor and adjacent healthy
tissues from a 134 patients with GC were measured by western
blotting and IHC. The expression of TRPM8 protein in GC tissues was
significantly higher compared with that noted in the normal
adjacent healthy tissues (Fig. 1C
and D). In addition, IHC
demonstrated that TRPM8 protein was located at the cell membrane
(Fig. 1E) and the IHC score of
TRPM8 protein expression in GC tissues was significantly higher
compared with that observed in the adjacent tissues (Fig. 1F).
Association of TRPM8 and
clinicopathological features of patients with GC
Patients with GC (n=134) were divided into 2 groups
based on the expression of TRPM8 protein detected using
immunohistochemistry, a TRPM8 protein low expression group (n=45)
with an IHC score of <4 and a high expression group (n=89) with
an IHC score of ≥4. As shown in Table
I, expression of TRPM8 protein was not significantly associated
with sex (P=0.863), age (P=0.737), histological grade (P=0.769) or
tumor number (P=0.672). However, it was significantly associated
with tumor diameter (P=0.003), TNM stage (P=0.003), lymph node
metastasis (P=0.001) and remote metastasis (P=0.010).
TRPM8 protein expression in gastric
tumors of variable sizes
Based on preoperative imaging examination, the
largest diameter of tumor tissue was determined (median diameter of
gastric tissues=2.5 cm) and patients with GC (n=134) were divided
into 2 groups: Tumor diameter <2.5 cm (n=65) and tumor diameter
≥2.5 cm (n=69). As presented in Fig.
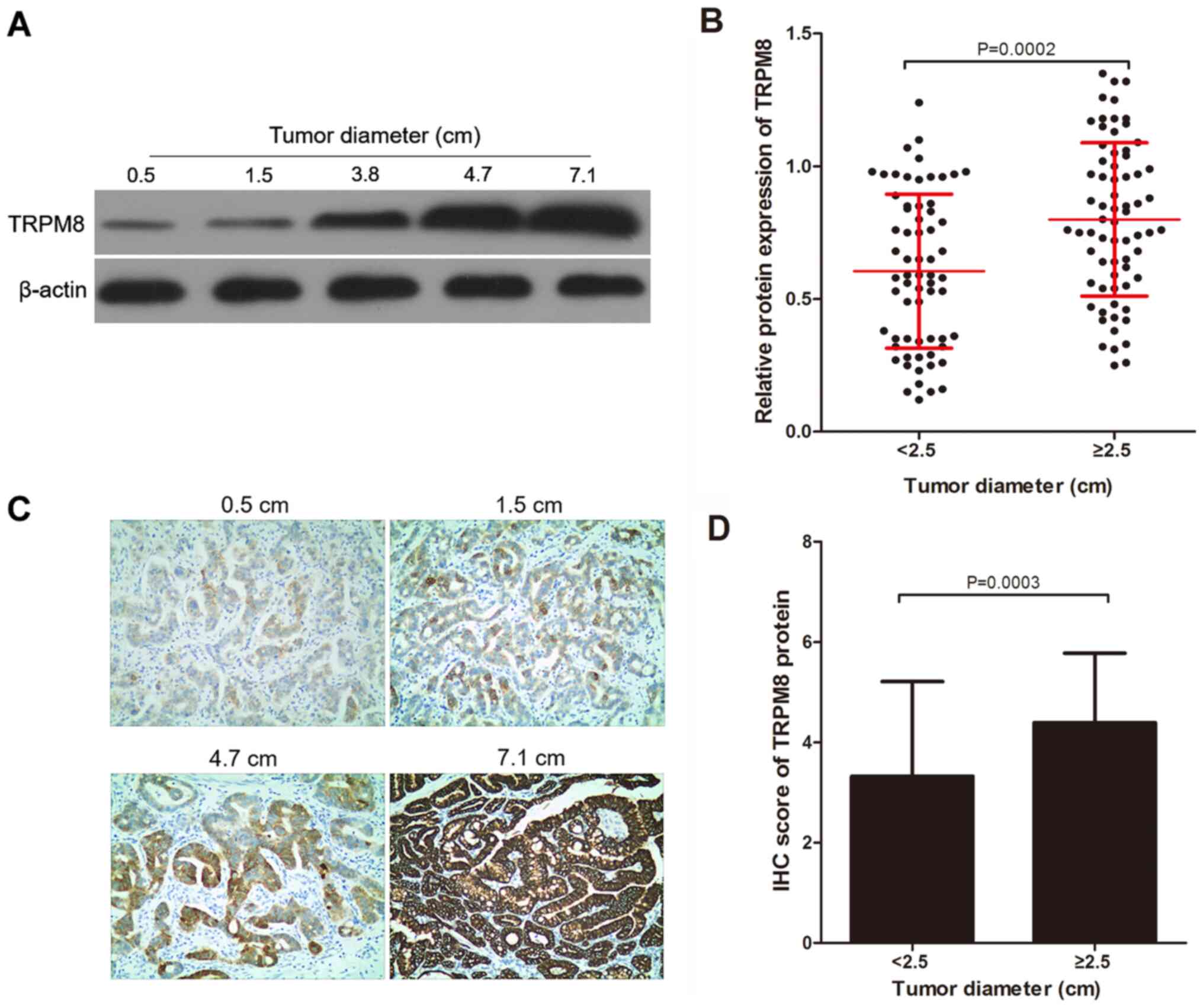
2A, the relative expression levels of TRPM8 in gastric cancer
tissues with tumor diameters of 0.5, 1.5, 3.8, 4.7 and 7.1 cm are
0.12, 0.33, 0.58, 1.18 and 1.42, respectively. TRPM8 protein
expression in the <2.5 cm group was significantly less compared
with that in the ≥2.5 cm group using western blotting (Fig. 2A and B). In addition, the differences in TRPM8
protein expression were detected using IHC in the 2 groups divided
by tumor diameter. Similarly, as indicated by Fig. 2A, the IHC score of TRPM8 in gastric
cancer tissues with tumor diameters of 0.5, 1.5, 4.7 and 7.1 cm are
1, 2, 4 and 6, respectively. It was also indicated that the IHC
score in the <2.5 cm group was significantly lower compared with
the ≥2.5 cm group (Fig. 2C and
D).
TRPM8 protein expression and GC cell
metastasis
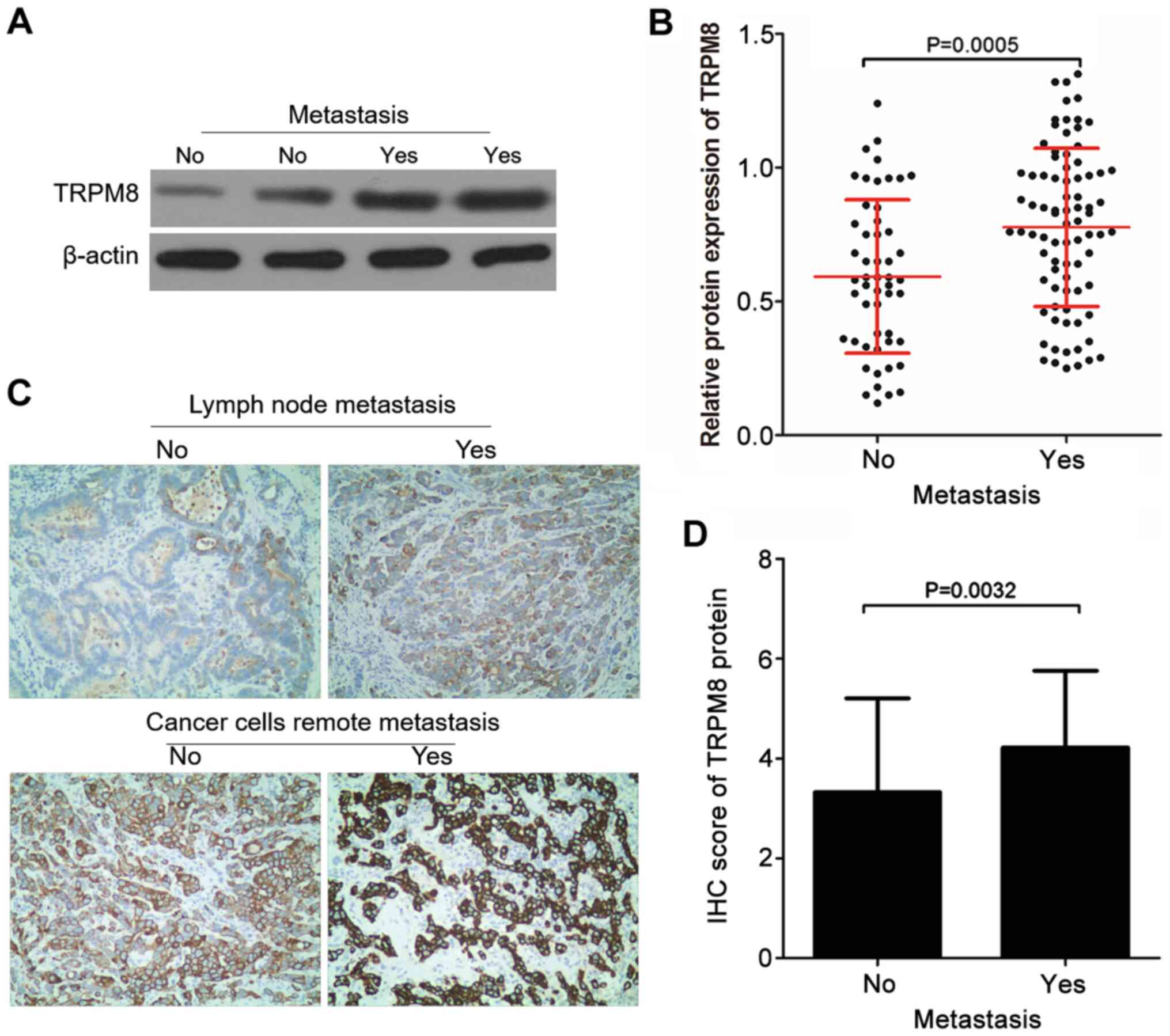
Patients with GC (n=134) were divided into 2 groups
based on whether they had metastasis (lymph node or remote
metastasis) or not and then TRPM8 protein expression was compared
in these 2 groups. A total of 82 GC patients exhibited cancer cell
metastasis (metastasis group) and 52 patients exhibited no cancer
cell metastasis (non-metastasis group). TRPM8 protein expression in
tissues of patients who had metastases was significantly higher
compared with those without metastases as detected by western
blotting (Fig. 3A and B). This was confirmed in the subsequent
IHC analysis. As presented in Fig.
3C, TRPM8 protein IHC scores of patients with GC without and
with lymph node metastasis were 2 and 3, respectively; TRPM8
protein IHC scores of patients with GC without and with distant
cancer cell metastasis were 4 and 6, respectively. The IHC score of
TRPM8 protein in patients with GC who had metastases was
significantly elevated compared with those without metastases
(Fig. 3C and D).
Association between TRPM8 protein
expression and the prognosis of patients with GC
Patients with GC were followed up for 5 years after
surgery to record the time of death and to compare the 5-year
overall survival rate of patients with different TRPM8 protein
expression levels. A total of 64.44% (29/45) of patients with low
TRPM8 protein expression were still alive 5 years after surgery,
while only 12.36% (11/89) of patients with high TRPM8 protein
expression levels were still alive (Fig. 4). The difference in 5-year survival
of patients with variable TRPM8 protein expression levels was
significant (Fig. 4). In addition,
the influencing factors for survival were assessed using the Cox
regression model. According to the results of univariate analysis,
histological grade (OR=2.842; 95% CI=2.342-3.210), TNM stage
(OR=0.854; 95% CI=0.328-1.264), lymph node metastasis (OR=1.964;
95% CI=1.405-3.218), remote metastasis (OR=3.264; 95%
CI=2.501-4.448) and TRPM8 levels (OR=1.032; 95% CI=0.846-2.371).
Multivariate analysis was also performed and it was indicated that
TNM stage [odds ratio (OR)=2.032; 95% CI=0.625-3.102], lymph node
metastasis (OR=3.516; 95% CI=1.653-4.021), remote metastasis
(OR=3.237; 95% CI=1.354-4.021) and TRPM8 protein expression levels
(OR=1.625; 95% CI=0.552-3.128) were independent risk factors that
affected the 5-year survival of patients with GC (Table II).
 | Table IIAnalysis of factors that influence
the overall survival of patients with gastric cancer (n=134). |
Table II
Analysis of factors that influence
the overall survival of patients with gastric cancer (n=134).
| | Univariate
analysis | Multivariate
analysis |
|---|
| Features | 95% CI | OR | P-value | 95% CI | OR | P-value |
|---|
| Sex | 1.864-4.926 | 3.021 | 0.089 | - | - | - |
| Age | 0.792-2.657 | 1.325 | 0.328 | - | - | - |
| Tumor diameter | 1.358-4.328 | 2.682 | 0.068 | - | - | - |
| Histological
grade | 1.969-3.427 | 3.025 | <0.001 | 1.129-3.641 | 2.03 | 0.061 |
| Tumor number | 0.729-3.124 | 1.682 | 0.062 | - | - | - |
| TNM stage | 0.658-2.354 | 1.382 | <0.001 | 0.625-3.102 | 2.032 | 0.001 |
| Lymph node
metastasis | 1.223-3.512 | 2.003 | <0.001 | 1.653-4.021 | 3.561 | 0.042 |
| Remote
metastasis | 2.235-3.126 | 2.658 | <0.001 | 1.354-4.021 | 3.237 | <0.001 |
| TRPM8 levels | 0.635-3.012 | 1.324 | 0.012 | 0.552-3.128 | 1.625 | 0.028 |
Discussion
TRPM8 was initially cloned as a prostate-specific
protein and was found to be activated by cold stimulation and
menthol in a heat-sensitive response (6). TRP family proteins are involved in the
occurrence of various diseases, such as TRPM8 is the principal
mediator of menthol-induced analgesia of acute and inflammatory
pain (21), anti-hyperalgesic
effects of a novel TRPM8 agonist in neuropathic rats (22) and cancer (23,24).
Jiang et al (25) found that
transient receptor potential cation channel subfamily V member 6
knockdown could inhibit the invasion and migration of cancer cells.
Orfanelli et al (26) found
that transient receptor potential cation channel subfamily M member
2 played an essential role in the regulation of biological
functions of tumor cells, such as proliferation, invasion and
migration.
In the present study, TRPM8 protein expression in
human GC cells was significantly higher compared with that in human
gastric mucosal epithelial cells, and was also elevated in human GC
tissues compared with adjacent healthy tissues. Numerous studies
have demonstrated that TRPM8 plays a vital role in the development
of tumors, suggesting TRPM8 may serve as an oncogene affecting the
progression of a number of types of malignant tumors.
Overexpression of the cation-permeable channel TRPM8 in prostate
cancers promoted cancer progression and menthol inhibited the
proliferation of prostate cancer cells by inhibiting the expression
of TRPM8 protein (16). Knowlton
and McKemy (27) summarized the
emerging role of TRPM8 in a variety of biological systems,
including thermoregulation, cancer, bladder function, and asthma.
Furthermore, Wang et al (15) found that knockdown of TRPM8
suppresses cancer malignancy and enhances epirubicin-induced
apoptosis in human osteosarcoma cells. The findings of the present
study revealed that TRPM8 was highly expressed in GC cells and
tissues, hence it may be involved in the regulation of GC
development as an oncogene.
In the present study, the association between TRPM8
protein expression and clinicopathological data from patients with
GC was assessed and it was found that the expression of TRPM8
protein in GC tissues was significantly associated with the tumor
diameter, TNM stage, lymph node metastasis and remote metastasis.
Notably, in the present study TRPM8 protein was higher expressed in
patients who had larger tumor diameters. The abnormal proliferation
of tumor cells is the root cause of cancer and forms the basis for
distinguishing them from normal cells (28). One of the functions of numerous
antitumor drugs is the suppression of proliferation of tumor cells,
including mechanisms that regulate cell cycle-related protein
expression and mitochondrial toxicity (15,29).
TRPM8 has been found to be involved in promoting cell
proliferation. Bidaux et al (30) found that the epidermal TRPM8 channel
controls the balance between keratinocyte proliferation and
differentiation in a cold-dependent manner and Yee (31) found that overexpression of TRPM8 is
necessary for pancreatic cancer cell proliferation. On the other
hand, TRPM8 has also been found to inhibit the proliferation of
non-cancer cells and topical application of TRPM8 agonists can
reduce epidermal proliferation induced by barrier insult in
vivo (32) and inhibit the
proliferative airway smooth muscle cell phenotype (33). In conclusion, these data indicate
that TRPM8 is a cell proliferation-related protein.
The present study also found that the protein
expression of TRPM8 in patients with GC who had metastases was
significantly higher compared with patients without metastases. The
characteristic that tumor cells are transferred from the primary
site to other sites to continue to grow is not only a criterion for
distinguishing between benign and malignant tumors, but also a
major cause of treatment failure and death in patients with cancer
(34). Numerous previous studies
have found that upregulation of TRPM8 promotes metastasis. Okamoto
et al (35) found that TRPM8
was highly expressed in oral squamous cell carcinomas and reducing
the activity of TRPM8 could significantly decrease the activity of
invasion in oral squamous carcinoma cells in vitro. TRPM8
has also been found to promote metastasis in lung and prostate
cancer (36,37). The results of the present study
suggest that TRMP8 protein is a metastasis-related protein in
GC.
In addition, the present study found that high
expression of TRPM8 was associated with a poorer prognosis for
patients with GC, this may be due to the promotion and
proliferation of GC cells induced by TRPM8. Similarly, studies of
TRPM8 expression in pancreatic cancer (38), urothelial carcinoma of the bladder
(39) and osteosarcoma (40) also found that high expression of
TRMP8 leads to poor prognosis. This indicates that TRMP8 could be
used to evaluate the efficacy of treatment by comparing the
expression level of TRPM8 in GC tumor tissue before and after
treatment. In addition, since the TRPM8 protein expression is
associated with the prognosis of patients with GC, modulating the
expression of TRPM8 in patients with GC through treatment may
affect the prognosis. In conclusion, TRPM8 may be a potential
target for treatment or a parameter for tumor screening in GC. As
TRPM8 is a menthol receptor, it has been used as a target for
menthol-induced apoptosis of human bladder cancer cell line
T24(19) and human leukemia cell
HL-60(41). TRPM8 has been widely
used in the development of drugs for the treatment of malignant
tumors, such as menthol and cannabigerol (10,42).
The present study had several limitations, no cell
proliferation and metastatic assays were performed to investigate
the effects of TRPM8 expression in GC cells. The findings of this
study were based on in vivo data, functional in vitro
mechanistic studies are required to verify the findings of the
present study. In conclusion, the present study found that the
TRPM8 protein was highly expressed in GC tissues and is associated
with the development of GC and may promote GC cell proliferation
and metastasis as an oncogene. The current study demonstrated that
TRPM8 is a potential target for the treatment of gastric cancer,
and some drugs used to treat cancer that target TRPM8 can be used
for the treatment of gastric cancer.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by a grant from Beijing JST
Research Funding (grant no. ZR-201919).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
WL was responsible for the conception and design of
the study. QX drafted the manuscript and revised the final draft
for important intellectual content; QX, NK, JZ, NB and JB performed
the experiments and analyzed the data. All authors have read and
approved the manuscript.
Ethics approval and consent to
participate
This study was performed with the approval of the
Ethics Committee of Beijing Jishuitan Hospital (Beijing, China).
All aspects of the study complied with the Declaration of Helsinki.
All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shiraishi N, Yasuda K and Kitano S:
Laparoscopic gastrectomy with lymph node dissection for gastric
cancer. Gastric Cancer. 9:167–176. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jackson C, Cunningham D and Oliveira J:
ESMO Guidelines Working Group. Gastric cancer: ESMO clinical
recommendations for diagnosis, treatment and follow-up. Ann Oncol.
20 (Suppl 4):S34–S36. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jiang L, Yang KH, Guan QL, Chen Y, Zhao P
and Tian JH: Survival benefit of neoadjuvant chemotherapy for
resectable cancer of the gastric and gastroesophageal junction: A
meta-analysis. J Clin Gastroenterol. 49:387–394. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tsavaler L, Shapero MH, Morkowski S and
Laus R: Trp-p8, a novel prostate-specific gene, is up-regulated in
prostate cancer and other malignancies and shares high homology
with transient receptor potential calcium channel proteins. Cancer
Res. 61:3760–3769. 2001.PubMed/NCBI
|
|
7
|
Liu SC, Lu HH, Cheng LH, Chu YH, Lee FP,
Wu CC and Wang HW: Identification of the cold receptor TRPM8 in the
nasal mucosa. Am J Rhinol Allergy. 29:e112–e116. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Borowiec AS, Sion B, Chalmel F, D Rolland
A, Lemonnier L, De Clerck T, Bokhobza A, Derouiche S, Dewailly E,
Slomianny C, et al: Cold/menthol TRPM8 receptors initiate the
cold-shock response and protect germ cells from cold-shock-induced
oxidation. FASEB J. 30:3155–3170. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bautista DM, Siemens J, Glazer JM, Tsuruda
PR, Basbaum AI, Stucky CL, Jordt SE and Julius D: The menthol
receptor TRPM8 is the principal detector of environmental cold.
Nature. 448:204–208. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yudin Y and Rohacs T: Regulation of TRPM8
channel activity. Mol Cell Endocrinol. 353:68–74. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yamamura H, Ugawa S, Ueda T, Morita A and
Shimada S: TRPM8 activation suppresses cellular viability in human
melanoma. Am J Physiol Cell Physiol. 295:C296–C301. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chodon D, Guilbert A, Dhennin-Duthille I,
Gautier M, Telliez MS, Sevestre H and Ouadid-Ahidouch H: Estrogen
regulation of TRPM8 expression in breast cancer cells. BMC Cancer.
10(212)2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shan Y, Xu Z, Chang Z, Wu D, Wang Y, Yao
X, Ng CF and Chan FL: Ion channel TRPM8 promotes hypoxic growth of
prostate cancer cells via an O2-independent and RACK1-mediated
mechanism of HIF-1α stabilization. J Pathol. 234:514–525.
2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yee NS, Chan AS, Yee JD and Yee RK: TRPM7
and TRPM8 ion channels in pancreatic adenocarcinoma: Potential
roles as cancer biomarkers and targets. Scientifica (Cairo).
2012(415158)2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang Y, Yang Z, Meng Z, Cao H, Zhu G, Liu
T and Wang X: Knockdown of TRPM8 suppresses cancer malignancy and
enhances epirubicin-induced apoptosis in human osteosarcoma cells.
Int J Biol Sci. 10:90–102. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Valero ML, Mello de Queiroz F, Stühmer W,
Viana F and Pardo LA: TRPM8 ion channels differentially modulate
proliferation and cell cycle distribution of normal and cancer
prostate cells. PLoS One. 7(e51825)2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu Z, Wu H, Wei Z, Wang X, Shen P, Wang
S, Wang A, Chen W and Lu Y: TRPM8: A potential target for cancer
treatment. J Cancer Res Clin Oncol. 142:1871–1881. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Namer B, Seifert F, Handwerker HO and
Maihöfner C: TRPA1 and TRPM8 activation in humans: Effects of
cinnamaldehyde and menthol. Neuroreport. 16:955–959.
2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li Q, Wang X, Yang Z, Wang B and Li S:
Menthol induces cell death via the TRPM8 channel in the human
bladder cancer cell line T24. Oncology. 77:335–341. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shimada K, Uzawa K, Kato M, Endo Y, Shiiba
M, Bukawa H, Yokoe H, Seki N and Tanzawa H: Aberrant expression of
RAB1A in human tongue cancer. Br J Cancer. 92:1915–1921.
2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu B, Fan L, Balakrishna S, Sui A, Morris
JB and Jordt SE: TRPM8 is the principal mediator of menthol-induced
analgesia of acute and inflammatory pain. Pain. 154:2169–2177.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Patel R, Gonçalves L, Leveridge M, Mack
SR, Hendrick A, Brice NL and Dickenson AH: Anti-hyperalgesic
effects of a novel TRPM8 agonist in neuropathic rats: A comparison
with topical menthol. Pain. 155:2097–2107. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bödding M: TRP proteins and cancer. Cell
Signal. 19:617–624. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shapovalov G, Ritaine A, Skryma R and
Prevarskaya N: Role of TRP ion channels in cancer and
tumorigenesis. Semin Immunopathol. 38:357–369. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jiang Y, Gou H, Zhu J, Tian S and Yu L:
Lidocaine inhibits the invasion and migration of TRPV6-expressing
cancer cells by TRPV6 downregulation. Oncol Lett. 12:1164–1170.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Orfanelli U, Wenke AK, Doglioni C, Russo
V, Bosserhoff AK and Lavorgna G: Identification of novel sense and
antisense transcription at the TRPM2 locus in cancer. Cell Res.
18:1128–1140. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Knowlton WM and McKemy DD: TRPM8: From
cold to cancer, peppermint to pain. Curr Pharm Biotechnol.
12:68–77. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Colston KW, Berger U and Coombes RC:
Possible role for vitamin D in controlling breast cancer cell
proliferation. Lancet. 333:188–191. 1989.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bidaux G, Borowiec AS, Gordienko D, Beck
B, Shapovalov GG, Lemonnier L, Flourakis M, Vandenberghe M,
Slomianny C, Dewailly E, et al: Epidermal TRPM8 channel isoform
controls the balance between keratinocyte proliferation and
differentiation in a cold-dependent manner. Proc Natl Acad Sci USA.
112:E3345–E3354. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yee NS: Roles of TRPM8 Ion channels in
cancer: Proliferation, survival, and invasion. Cancers (Basel).
7:2134–2146. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Denda M, Tsutsumi M and Denda S: Topical
application of TRPM8 agonists accelerates skin permeability barrier
recovery and reduces epidermal proliferation induced by barrier
insult: Role of cold-sensitive TRP receptors in epidermal
permeability barrier homoeostasis. Exp Dermatol. 19:791–795.
2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang L, An X, Wang Q and He M: Activation
of cold-sensitive channels TRPM8 and TRPA1 inhibits the
proliferative airway smooth muscle cell phenotype. Lung.
194:595–603. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Brooks SA, Lomax-Browne HJ, Carter TM,
Kinch CE and Hall DM: Molecular interactions in cancer cell
metastasis. Acta Histochem. 112:3–25. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Okamoto Y, Ohkubo T, Ikebe T and Yamazaki
J: Blockade of TRPM8 activity reduces the invasion potential of
oral squamous carcinoma cell lines. Int J Oncol. 40:1431–1440.
2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Du GJ, Li JH, Liu WJ, Liu YH, Zhao B, Li
HR, Hou XD, Li H, Qi XX and Duan YJ: The combination of TRPM8 and
TRPA1 expression causes an invasive phenotype in lung cancer.
Tumour Biol. 35:1251–1261. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yang ZH, Wang XH, Wang HP and Hu LQ:
Effects of TRPM8 on the proliferation and motility of prostate
cancer PC-3 cells. Asian J Androl. 11:157–165. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Du JD, Zheng X, Chen YL, Huang ZQ, Cai SW,
Jiao HB, Zhu ZM and Hu B: Elevated transient receptor potential
melastatin 8 (TRPM8) expression is correlated with poor prognosis
in pancreatic cancer. Med Sci Monit. 24:3720–3725. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xiao N, Jiang LM, Ge B, Zhang TY, Zhao XK
and Zhou X: Over-expression of TRPM8 is associated with poor
prognosis in urothelial carcinoma of bladder. Tumour Biol.
35:11499–11504. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhao W and Xu H: High expression of TRPM8
predicts poor prognosis in patients with osteosarcoma. Oncol Lett.
12:1373–1379. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lu HF, Hsueh SC, Yu FS, Yang JS, Tang NY,
Chen SC and Chung JG: The role of Ca2+ in (-)-menthol-induced human
promyelocytic leukemia HL-60 cell death. In Vivo. 20:69–75.
2006.PubMed/NCBI
|
|
42
|
Bhatia SP, Mcginty D, Letizia CS and Api
AM: Fragrance material review on l-menthol. Food Chem Toxicol. 46
(Suppl 11):S224–S227. 2008.PubMed/NCBI View Article : Google Scholar
|